Abstract
Objectives:
Granny Smith is a cultivated hybrid variety of apple with a high antioxidant content relative to all other species of apple. Acute pancreatitis (AP) is an instantly emerging inflammatory condition with a high mortality rate. The preferred treatment is restricted to symptomatic relief and supportive care. The present study was undertaken to evaluate the favorable effects of Granny Smith apple extract (GSAE) as a prophylactic treatment for L-arginine-induced AP in rats.
Materials and Methods:
Male Sprague Dawley rats were divided in to five groups (n=6): Normal control (saline), disease control (a single dose of L-arginine 2.5 g/kg I.P.), positive control (pelatonin 10 mg/kg I.P.), and GSAE I and II (200 mg/kg and 400 mg/kg, orally, respectively). All groups were treated for 7 days. At the end of the study, blood samples were collected from the retro-orbital plexus, serum separated, and subjected to estimation of biomarker enzymes such as amylase, lipase, antioxidant enzymes, etc. The animals were then sacrificed, and the pancreas was isolated and subjected to estimation of tissue biomarkers, DNA fragmentation assay, and histopathological studies.
Results:
Serum levels of amylase and lipase were significantly (p<0.001) reduced in L-arginine-treated rats. Similar results were also observed with tissue inflammatory markers such as malondialdehyde, nitrate, etc. There was a dramatic increase (p<0.001) in the overall antioxidant enzyme levels when compared with disease control rats. Histopathological examination of pancreatic tissue showed an intact structural feature of acinar cells in the extract-treated group of rats, which was further in pact with the intact DNA found in the DNA fragmentation assay.
Conclusion:
Thus, GSAE treatment was found to be beneficial in lowering the inflammatory conditions of AP by improving the overall antioxidant levels, and a further investigation into its exact molecular mechanism is needed.
Keywords: Granny Smith apple, L-arginine, free radicals, pancreatitis
INTRODUCTION
Acute pancreatitis (AP) is a perilous condition, and there has been a spike in its incidence worldwide.1 AP is a mild, self-limiting inflammatory condition of the exocrine pancreatic tissue caused by activation of stress signals and a disparity of protective mechanisms in the pancreatic tissue. The initial symptoms include persistent and recurring epigastric pain, nausea, vomiting, weight loss, fever, chills, and shock, which, if left undiagnosed or untreated, may eventually lead to severe AP, where the pancreatic tissue is displaced by fibrotic cells, and the association of additional organ manifestations leads to mortality.2
Almost 80% of reported pancreatitis cases have familial incidence,3 and the major causes of mortality include cardiovascular and respiratory collapse.4 No specific therapies have been reported for the management of AP, and treatment is primarily based on supportive care to prevent hypoxemia, fluid resuscitation, and a critical component of the disease, malnutrition.
Despite many explorations in regard with pathogenesis and the treatment modalities developed, the pathogenesis of AP still remains vague. The most widely accepted hypothesis is the involvement of oxidative stress and premature activation of zymogens, followed by autodigestion of the tissue and successive activation of local and systemic inflammation.5 The autodigestive process of acinar cells stimulates an inflammatory response (neutrophil and macrophage infiltration, release of cytokines, interleukins 1, 6, and 8, and other inflammatory mediators) within the pancreatic parenchyma.2
Based upon the above hypothesis, a number of experimental animal studies have proposed the administration of antioxidant compounds that deplete reactive oxygen species and show a beneficial effect in the treatment of AP.6 Many medicinal plants have yielded favorable outcomes in the management of the disease course.7 Suggested preventive mechanisms include the presence of phenolics and other phytochemical antioxidants in the eradication and neutralization of free radicals.8
Several epidemiological studies suggest that increased dietary intake of fruits and vegetables is associated with a decreased risk of disease incidence.9 One of the well-known and most produced fruits worldwide is the apple (Malus x domesticus). Apples play a significant role in the dietand are consumed in various forms. They belong to the family Rosaceae and contain high amounts of fiber, pectin, potassium, and vitamins A and C. They also contain different classes of phenolics such as flavonols, dihydro chalcones, p-hydroxy benzoic acid, etc. Many different varieties of apples are available, and the focus of our study is on Granny Smith apples.
The Granny Smith apple is a hybrid variety of Malus domesticus and Malus slyvesterus propagated by Maria Smith in Australia, hence the name. It is reported to have the second highest amount of flavonoids and procyanidins10 among all the varieties of apples. The fruits are crisp, tart, and juicy with a light green, hard skin and a long storage life. The low amount of ethylene production facilitates their preservation, when compared with other apple varieties.11 Granny Smith apple is the preferred fruit in healthy weight loss regimens because of their wealth of dietary fiber and potassium and low caloric content.12 The objective of the current study is to evaluate the shielding potential of Granny Smith apple extract (GSAE) on experimentally induced AP by L-arginine and its potential to overcome malnutrition.
MATERIALS AND METHODS
Chemicals
Sodium phosphate buffer, sodium chloride, starch, chloroform, ethanol, O-dianisidine, phosphate buffer, ethanol, hydrogen peroxide, potassium dihydrogen phosphate, trichloro acetic acid (TCA), dinitro phenyl hydrazine (DNPH) reagent, butanol, ethylene diamine tetra acetic acid (EDTA), pyridine, potassium chloride, sodium dodecyl sulfate, thiobarbituric acid (TBA), 5,5-dithio bis 2-nitrobenzoic acid (DTNB), thiourea, N-(1-naphthyl) ethylene diamide dihydrochloride, and formaldehyde, were purchased from Sigma-Aldrich Inc., Mumbai, India. b-NADH, hexadecyltrimethylammonium bromide (HTAB), and sodium pyruvate were purchased from SD Fine Ltd. Mumbai, India. Commercial kits for estimation of amylase, C-reactive protein from Akray health care Pvt Ltd., Hyderabad, India and Lipase kit from Aggape diagnostics Pvt Ltd. Hyderabad, India. DNA isolation kit from Bioartis Pvt Ltd. Hyderabad, India. L-arginine and melatonin were purchased from Sigma-Aldrich Pvt Ltd. Mumbai, India.
Plant material and extraction
Fresh Granny Smith apples were purchased in the month of March 2017 from the local market in Hyderabad, India. The samples were authenticated for their variety with voucher no.1002 by the Department of Botany, S.V. University, Andhra Pradesh, India. The core of the apple was removed, and the rest of the apple was blended to obtain fresh juice with pulp. The obtained pulp was subjected to extraction with 95% ethanol in an orbital shaker for 24 h. The extracts were filtered and evaporated to dryness with the help of a rotary evaporator. The resultant dry extracts were stored at a cool temperature and resuspended in normal saline immediately before the use.
Animals
In the present study, male Sprague Dawley rats were chosen with a body weight range of 150-200 g. They were housed in pathogen-free polypropylene cages and acclimated for one week before the onset of the study. The temperature was maintained at 25°C±1°C with 55% humidity. Rats were controlled constantly with a 12:12 h light/dark cycle and were givena standard pellet diet with water ad libitum. All the experiments carried out were approved by the Institutional Animal Ethics Committee (1448/PO/Re/S/11/CPCSEA/07/2016) as per CPCSEA guidelines.
Acute toxicity studies
The acute toxicity study was conducted according to the fixed dose method of OECD guideline 420.13 GSAE was dissolved in normal saline and administered orally as per the prescribed doses mentioned in the guidelines, and observations were conducted for the next 14 days. Animals given the test extracts did not manifest any significant abnormal signs, behavioral changes, changes in body weight or macroscopic findings at any time during the observation period. At the end of the study period, no mortality or lethality was observed, and hence, the LD50 was found to be above 2000 mg/kg. Thus 1/10th and 1/5th of the LD50 dose was selected for the present study.
Preparation of L-arginine solution
A 20% L-arginine solution was prepared in normal saline, and the pH was adjusted to 7.0. The solution was filtered through a syringe tube filter in a tissue culture hood before administration to rats at a dose of 2.5 g/kg body weight.14 The prepared solution was used for the induction of AP and was administered intraperitoneally.
Study protocol
The present study involved randomization of animals in to five groups with six animals in each. Group I was considered as the control group and received normal saline p.o. daily for 7 days. Group II, the disease control group, received a single dose of freshly prepared L-arginine solution at a dose level of 2.5 g/kg b.w. on day 5 of the experiment. Group III, a positive-control group, received melatonin at a dose of 10 mg/kg I.P. for 7 days.15,16 Groups IV and V received oral administration of GSAE at doses of 200 mg/kg and 400 mg/kg, respectively. Groups III, IV, and V were induced with AP by single-dose administration of L-arginine (2.5 g/kg b.w.) at an interval of 1 h after administration of the extracts on day 5 of the study.17
At the end of the experimental period, the animals were anesthetized under light ether, and blood samples were obtained from the retro-orbital plexus. Further, these samples were used for evaluation of pancreatic, inflammatory, and antioxidant biomarker enzymes. The rats were then decapitated for isolation of the pancreas. Isolated pancreas were divided into portions for tissue inflammatory marker evaluation, DNA fragmentation assays, and histopathological investigations.
Estimation of biomarker enzymes
Estimation of amylase
Serum amylase was estimated with a commercial kit manufactured by Akray Healthcare Pvt. Ltd. Briefly, 1000 µL of amylase mono reagent was mixed with 20 µL of serum and incubated for 60 seconds. The absorbance was read at 405 nm. Amylase activity was reported in U/L, where one unit was described as the amount of amylase required to generate 1 µ mole of p-nitrophenol per minute at 25°C.
Estimation of lipase
Serum lipase was analyzed by using the commercial kit from Aggape Diagnostics Ltd. According to the manufacturer’s instructions, 1000 µL of reagent 1 was mixed with 20 µL of serum and incubated at 37°C for 5 minutes. To this mixture, 250 µL of reagent 2 was added and held at room temperature for 2 minutes. The absorbance of the resultant mixture was read at 580 nm. Lipase activity was reported in U/L, where one unit was described as the amount of lipase required to generate 1 µ mole of methyl resorufin at 37°C.
Estimation of superoxide dismutase (SOD)
Cold water and a chloroform/ethanol mixture (15:1 ratio) were added to an equal quantity of packed cells, and the mixture was centrifuged at 2000 rpm for 20 minutes. A 0.1 mL aliquot of the supernatant was separated and 0.88 mL of riboflavin and 60 µL of O-dianisidine were added. The absorbance was measured at 460 nm.18 The SOD activity was calculated from a standard curve and expressed in mg/protein/min.
Estimation of glutathione
A tissue homogenate was prepared in 0.1 M phosphate buffer. To the homogenate 20% TCA and 1 mM EDTA were added in equal volumes and allowed to stand for 5 min, which was then centrifuged at 2000 rpm for 10 min. 200 µL of the supernatant was separated and 1.5 mL of DTNB reagent was added. The absorbance was measured at 412 nm.19 The values obtained were articulated as mg/dL which was calculated against a standard curve and the amount of glutathione reduced is directly proportional to the production of 5-thio 2-nitrobenzoic acid from DTNB.
Estimation of catalase
To 0.1 mL of serum, 2.5 mL of phosphate buffer was added, and the mixture was incubated for 30 min at 25°C. The blend was transferred into a cuvette, and 650 µL of hydrogen peroxide solution was added to initiate the reaction. The alteration in absorbance was measured at 240 nm for 3 min.20 The catalase activity was expressed in µmoL H2O2/mg protein/minute, which was calculated against the total amount of protein lysed by the enzyme to degrade 1 µmol hydrogen peroxide per minute.
Estimation of vitamin C
The blood plasma was separated, and 0.6% TCA was added to a volume of 0.5 mL plasma to bring the total volume to 1.5 mL. The mixture was centrifuged for 20 min at 3500 rpm. The clear supernatant was collected, and to it an equal volume of DNPH reagent (2% DNPH and 4% thiourea in 9N H2SO4) was added and incubated for 30 min at room temperature. The resultant mixture was then read at an absorbance of 530 nm.21 The amount of vitamin C was calculated from a standard curve obtained by taking ascorbic acid as the reference standard and was expressed in mg/dL.
Estimation of lactate dehydrogenase (LDH)
To 0.1 mL of tissue homogenate or serum, 3 mL of LDH reagent (2.8 mL of 0.13 mM b-NADH and 0.1 mL of 34 mM sodium pyruvate) was added and incubated at 37°C for 5 minutes. The absorbance of the mixture was tested at 340 nm every minute for 3 minutes. The ΔA activity was measured and represented as U/L.22 The LDH activity was expressed as U/L where one unit was articulated as the reduction of 1 µ mole of pyruvate to L-lactate in 1 minute at 37°C and pH 7.5.
Estimation of myeloperoxidase (MPO)
Pancreatic MPO was estimated according to Bradley et al.23 The homogenized tissue was suspended in a mixture containing 50 mM phosphate buffer (pH 6.0) containing 0.5% HTAB and sonicated in an ice bath for 10 minutes. The suspensions were freeze-thawed three times, and the resultant mixture was centrifuged at 40,000x g for 15 min. The pellet thus obtained was mixed with 2.9 mL of 50 mM phosphate buffer (pH 6.0) containing 0.167 mg/mL of O-dianisidine hydrochloride and 0.0005% of hydrogen peroxide and checked for absorbance at 460 nm. One unit of MPO was defined as the quantity that degraded 1 µ/mol of H2O2 per minute, and the activity was expressed as units/mg of protein.23
Estimation of malondialdehyde (MDA)
The levels of MDA were estimated using 0.4 mL of tissue homogenate to which a reaction mixture of 1.5 mL, containing TBA (0.8%), acetic acid (20%), and distilled water was added and incubated for 1 h at 95°C in a water bath. Following incubation, the mixture was cooled, and to it 5 mL of butanol: pyridine mixture (15:1) was added and centrifuged at 3.000 rpm for 10 minutes. The clear supernatant was collected and checked for absorbance at 532 nm against a blank containing a butanol:pyridine mixture. The quantity of MDA was calculated by a standard graph preparation using 1,1,3,3-tetramethoxypropane in the concentration range of 1-10 nmol in 1 mL distilled water. The results are articulated as nmol of MDA/mg protein.24
Tissue nitrite levels
The tissue nitrite levels were estimated according to Green et al.25 with slight modifications. Briefly, the homogenized pancreatic tissue was centrifuged at 11,000 g for 15 min at 4°C. The obtained supernatant (100 µL) was mixed with 100 µL of Griess reagent and incubated at room temperature for 10 min. The absorbance was checked at 540 nm. Nitrite levels were standardized with using sodium nitrite, and the results obtained were expressed as micromoles of nitrate/nitrite.
Estimation of C-reactive protein (CRP)
Serum CRP levels were estimated by using a commercial kit from Akray Healthcare Ltd. Quantitative estimation was performed by preparing a series of dilutions of the test serum in normal saline (e.g., 1:2, 1:4, 1:8, etc.), to which one drop of CRP latex reagent was added. The formation of agglutination on the glass slide was taken as the highest titer for CRP and represented as a factor of 6 with units in micrograms per milliliter.
DNA fragmentation assay
The DNA fragmentation assay was performed according to the method of Basnakian and James26 using agarose gel electrophoresis. Briefly, DNA from pancreatic tissue was isolated by using a commercial available DNA Isolation kit by Bioartis Pvt Ltd. The isolated DNA pellet was air dried and resuspended in Tris-EDTA buffer (pH 8.0) containing 1mM EDTA. The resuspended DNA was loaded on to the agarose gel electrophoresis for analysis.
Histopathological studies
The isolated pancreases were fixed in formal in and subjected to histopathological studies. The pancreatic tissues were washed and fixed in paraffin blocks, which were sliced in 5-µm sections and stained with hematoxylin and eosin and then evaluated under a microscope with dark field background changes in the pancreatic tissue. Pancreatic tissue injury was reviewed for degeneration of acinar cells, edema, interstitial inflammation, and hemorrhage.
Statistical analysis
All the results are articulated as mean ± standard error of mean. Statistical analysis of the data was performed by One-Way ANOVA followed by Dunett’s multiple comparison test using GraphPad Prism 5 software with the threshold for statistical significance at p<0.001.
RESULTS
Effect of GSAE on AP-induced enzyme production
To examine the effect of GSAE on the development and severity of AP, rats were pretreated with GSAE (200 mg/kg and 400 mg/kg) in the respective groups as described in the experimental design. A few hours after induction of AP, rats in the control and positive-control groups were still active; whereas rats in disease control group were lethargic, with decreased motor activity, reduced reflex action, and reduced intake of food and water.
The rats in disease control group showed a significant increase (p<0.001) in the levels of amylase when compared with the control group rats. Rats treated with melatonin showed marked reduced levels of serum amylase, revealing its protective action. Rats pretreated with GSAE (200 mg/kg and 400 mg/kg b.w.) showed a significant dose-dependent decrease in serum amylase levels (p<0.001) when compared with the L-arginine control group rats (Table 1).
Table 1. Changes in the pancreatic biomarkers in rats treated with L-arginine and GSAE.
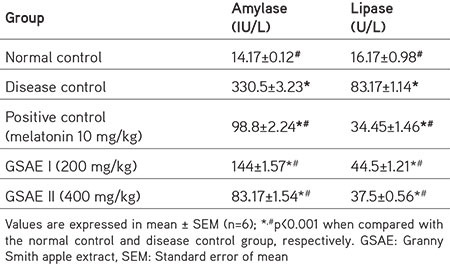
Similar changes were also observed for serum lipase levels. Rats of the disease control group showed a significant increase (p<0.001) in lipase levels when compared with those of the control group; whereas the melatonin and GSAE-treated rats showed a dose-dependent significant decrease (p<0.001) in the lipase enzyme levels when compared with those of the L-arginine control group (Table 1).
Effect of GSAE on antioxidant enzymes in AP
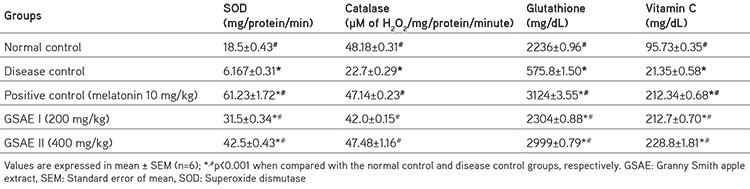
There was a significant decrease (p<0.001) in the SOD, catalase, glutathione, and vitamin C levels in rats of the disease control group when compared with those of the control group. In contrast, the levels were significantly increased in rats treated with melatonin and in the GSAE group of rats (p<0.001) when compared with rats of the disease control group, and no significant change was observed in GSAE group rats when compared with control group rats (Table 2).
Table 2. Changes in the antioxidant biomarkers in rats treated with L-arginine and GSAE.
Effect of GSAE on other inflammatory mediators in AP
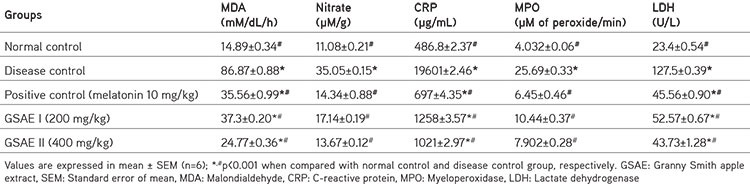
Treatment with L-arginine increased the levels of nitrate, MDA, LDH, CRP, and MPO significantly (p<0.001) in comparison with positive control and control group rats, indicating the incidence of pancreatic damage and inflammation. In contrast, melatonin and GSAE treatment reduced the levels of nitrate, MDA, LDH, CRP, and MPO significantly (p<0.001) in a dose-dependent manner. However, the levels were higher in the GSAE group of rats when compared with the control group (Table 3).
Table 3. Changes in inflammatory mediators in rats treated with L-arginine and GSAE.
Histopathological studies
The isolated pancreas was subjected to histopathological study using hematoxylin and eosin staining. Normal pancreatic architecture was seen in the control group of rats (Figure 1A), whereas L-arginine treatment showed inflammatory changes with vacuolar degeneration and extensive damage to acinar cells (Figure 1B). The positive-control group treated with melatonin showed normal architecture (Figure 1C). Rats treated with GSAE showed reduced inflammatory changes with no degeneration and maintenance of normal structural design (Figure 1D, E).
Figure 1.
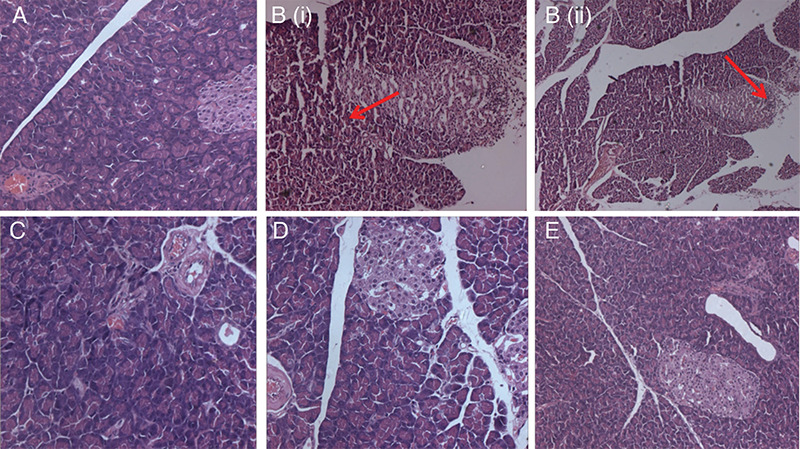
Histopathological changes in the pancreas. A) Normal control group rats with no damage to acinar cells; B (i) and B (ii) disease control group: L-arginine treated rats with vacuolar degeneration and extensive damage of the acinar cells with infiltration of leucocytes-red arrow; C) positive-control melatonin group of rats with normal architecture of acinar cells; D, E) GSAE-treated rats showing mild damage to the normal echotexture of acinar cells
GSAE: Granny Smith apple extract
DNA fragmentation assay
One of the major criteria for DNA fragmentation apoptosis. In the present study, control group rats showed intact DNA (Figure 2A) when compared with L-arginine control group rats, where a smear pattern was observed, representing extensive damage to DNA (Figure 2B). The melatonin and GSAE-treated groups of rats showed intact DNA, indicating their protective effects against DNA damage (Figure 2C-E).
Figure 2.

Gel image of DNA fragmentation assay of the pancreatic tissue. A) Group I: Normal control pancreas; B) group II: Disease control group pancreas showing fragmented DNA in the form of a smear indicating extensive damage; C) group III: Positive-control melatonin-treated group showing intact DNA; D, E) group IV, V: GSAE-treated pancreas showing intact DNA
GSAE: Granny Smith apple extract
DISCUSSION
The results of the study indicate a protective effect of GSAE on experimentally induced AP in rats by L-arginine. Administration of GSAE exhibited beneficial effects by reducing oxidative and nitrosative stress and modulating the inflammatory process.
L-arginine, an essential amino acid, was used for induction of AP in the present study, which is reported as a highly reproducible, non-invasive model of AP that produces dose-dependent acinar necrosis.27 Further, incessant administration of L-arginine for an extended period may also induce chronic pancreatitis.28
The chief indicative markers for the diagnosis of AP include serum amylase and lipase due to their direct release into the circulation, which is accredited to enzymatic activation in pancreatic acinar cells. After the initial attack, the levels of these enzymes usually increase within 4-8 h and peak at 24 h.29 Increased serum lipase levels are considered as a more reliable marker than those of amylase.30 In the present study, rats treated with L-arginine showed a significant elevation of serum amylase and lipase levels, which was observed as acinar cell necrosis (devoid of changes in the Islets of Langerhans) as observed in the histopathological study, indicating the development of AP, which is in accordance with previous reports.31 By comparison, treatment with GSAE decreased the elevated levels of lipase and amylase significantly in a dose-dependent manner. The reduction of enzyme levels by GSAE is consistent with a previously reported in vitro study on inhibition of lipase, a-amylase, and a-glucosidase enzymes.30,32 The results of the GSAE extract groups are synchronous with that of the positive-control group treated with melatonin, which also reduced the elevated levels of amylase and lipase radically, in accordance with previous reports.31
The pathogenesis of AP implicated the generation of free radicals and activation of inflammatory mediators, which contributed to the unfavorable effects.33 Lipid peroxidation provoked by oxidative stress and altered glutathione metabolism has been reported to take place early in the disease course. The rate of MDA production directly signifies lipid membrane peroxidation, which indirectly reflects the association of free-radical generation in mitochondria.34 On the other hand, MPO in the circulation indicates its release from activated neutrophils portraying dominant proinflammatory properties.35 Additionally, the severity and stage of AP show a relationship with the levels of blood MPO. Further, MPO levels correlate with CRP levels. Many studies have reported that these events were neutralized by administration of antioxidants with a favorable effect on reduction of reactive oxygen species.36 Melatonin, a renowned antioxidant, lowered the levels of MDA, MPO, and CRP, in agreement with previous studies.31 Similar effects were observed to that of GSAE administration, where the elevated levels of MDA, MPO, and CRP were reduced dose dependently, owing to the eradication of reactive oxygen species and halting of the lipid peroxidation process and inflammatory cytokine release, which has also been observed as a decreased amount of acinar cell damage in histopathological studies of the pancreatic tissue. The Granny Smith apple stands out as an antioxidant-rich fruit among all apple varieties. The presence of two major metabolite compounds, rutin and catechin, streng then the antioxidative properties of the fruit.37 The presence of these flavonoid compounds along with other compounds such as quercetin and procyanidins contribute to the total antioxidant capacity of many plant products.38,39 Further, Granny Smith peels have demonstrated antioxidant activity in the total oxy-radical scavenging assay and have shown the capacity to inhibit HepG2 cell proliferation.40
The presence of LDH has been reported in tissues under hypoxia conditions, which is a major event in inflammatory processes.41 Increased LDH levels were observed in the L-arginine group of rats, and the opposite was observed in melatonin-and GSAE-treated rats in a dose-dependent manner.
Damage by oxidative and nitrosative stress is mitigated by antioxidant resistance enzymes like SOD, glutathione, catalase, and vitamin C. L-arginine-treated rats showed decreased levels of SOD, glutathione, catalase, and vitamin C, indicating the involvement of reactive oxygen species.42 Melatonin and GSAE administration increased the levels of SOD, glutathione, catalase, and vitamin C, suggesting that it can exert protective effects by modulating defense mechanisms. The favorable effects of GSAE could be due to its antioxidant activity, which is in accordance with the in vitro antioxidant capacity reported by Tzanakis et al.43 and Saxena et al.44 Additionally, there was an increase in the levels of nitrite, indicating the involvement of released inducible nitric oxide synthase (iNOS) from inflammatory cytokines, i.e., the release of iNOS is directly proportional to nitrite levels in the plasma. NO and its metabolic products have a key role in inflammatory processes.45 NO combines with superoxide to form peroxynitrite, a highly reactive oxidant that damages the cell by lipid membrane and sulfhydryl oxidation.46 Administration of antioxidants decreased the release of NO indirectly, indicating its beneficial effect on nitrosative stress.36 GSAE administration reduced the levels of nitrite dose dependently. Granny smith is one of the varieties of apple containing richest polyphenols. Procyanidin B2, catechin, flavanols, quercetin, and vitamin C have been isolated from the whole fruit.47 The antioxidant activity of Granny Smith apples has been ascribed to the presence of these polyphenolic compounds.47 Also, Lotito and Frei48 reported on the antioxidant capacity of apple polyphenolics in human plasma along with their favorable effects on the prevention of many diseases. These facts suggest a role for the eradication of reactive oxygen and nitrogen species in the protection against AP. Furthermore, phenolics have been reported to restrain the NO-induced proinflammatory reaction by obstructing the levels of expression of iNOS dose dependently.49
Accumulating evidence suggests that apoptosis plays a significant role in relation to the severity of AP. The balance between apoptosis and necrosis plays a key role in defense mechanisms against AP and the resolution of disease severity.50 DNA fragmentation is considered as the universal criterion for the detection of apoptosis.51 Mervi et al.52 suggested that inhibition of polyamine synthesis has an effect on protein synthesis; in turn, nucleic acid synthesis is restricted. Further, catabolism of proteins is highly active in acinar cells of the pancreas, and it is probable that the overdose of L-arginine induced necrosis or degradation in these cells initially. Mitochondrial damage initiates the process of apoptosis as a consequence of high calcium loads. The cellular damage in pancreatitis is mostly associated with damage to mitochondria along with succession of the disease in most animal models and in Humans,53 which is in accordance with the results of the current study, where smear pattern of DNA was observed in L-arginine-treated disease control rats. Oxidative stress and protein oxidation in the cells could cause abnormal cross linking and cleavage of DNA leading to death of the cell.54 Acinar cells from the pancreas of rats treated with melatonin and GSAE showed intact DNA, signifying protective effects against stress-mediated DNA damage.
CONCLUSION
The current study suggests that GSAE exerts beneficial effects on L-arginine-induced AP by eradicating stress markers and augmenting antioxidant status. Chemical constituents such as polyphenols and flavonoid compounds present in the Granny Smith apple are responsible for its favorable effects. Further investigations are required to evaluate the exact chemical constituents and their molecular mechanism of action on the disease profile.
The present study was carried out to evaluate the prophylactic action of GSAE on AP. The amount of damage to the pancreas with L-arginine administration was evidenced with increased levels of amylase and lipase and reduction in the levels of antioxidative markers with increased levels of inflammatory markers. The histopathological findings with acinar cell necrosis were also in sync with the biochemical markers. The administration of GSAE prophylactically reversed the diseased conditions by improving antioxidant status and maintaining the normal echo texture of the pancreatic acinar cells. The shielding effect was rational in a dose-dependent manner. Further investigation of its molecular mechanism with extraction of each constituent is needed to determine the precise therapeutic potential of GSAE.
Acknowledgments
The authors are grateful to the Principal and Management of Sri Indu Institute of Pharmacy for providing the facilities to carry out this research work. Similarly, the authors wholeheartedly thank the Principal of SSJ College of Pharmacy for her technical assistance in this research work.
Footnotes
Conflicts of interest: No conflict of interest was declared by the authors. The authors alone are responsible for the content and writing of the paper.
References
- 1.Singh P, Garg PK. Pathophysiological mechanisms in acute pancreatitis: current understanding. Indian J Gastroenterol. 2016;35:153–166. doi: 10.1007/s12664-016-0647-y. [DOI] [PubMed] [Google Scholar]
- 2.Anchi P, Khurana A, Bale S, Godugu C. The role of plant derived products in pancreatitis: Experimental and clinical evidence. Phytother Res. 2017;31:591–623. doi: 10.1002/ptr.5792. [DOI] [PubMed] [Google Scholar]
- 3.Lowenfels AB, Maisonneuve P, DiMagno EP, Elitsur Y, Gates Jr LK, Perrault J, Whitcomb DC; International Hereditary Pancreatitis Study Group. Hereditary pancreatitis and the risk of pancreatic cancer. J Natl Cancer Inst. 1997;89:442–446. doi: 10.1093/jnci/89.6.442. [DOI] [PubMed] [Google Scholar]
- 4.McFadden DW. Organ failure and multiple organ system failure in pancreatitis. Pancreas. 1991;6(Suppl 1):S37–S43. doi: 10.1097/00006676-199101001-00007. [DOI] [PubMed] [Google Scholar]
- 5.Meher S, Mishra TS, Sasmal PK, Rath S, Sharma R, Rout B, Sahu MK. Role of biomarkers in diagnosis and prognostic evaluation of acute pancreatitis. J Biomark. 2015;2015:519534. doi: 10.1155/2015/519534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeurnink SM, Nijs MM, Prins HA, Greving JP, Siersema PD. Antioxidants as a treatment for acute pancreatitis: a meta-analysis. Pancreatol. 2015;15:203–208. doi: 10.1016/j.pan.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Easler JJ, Mounzer R, Papachristou GI. Pharmacological therapy for acute pancreatitis: where are we now? where are we going? Minerva gastroenterologica e dietologica. 2012;58:365–376. [PubMed] [Google Scholar]
- 8.Durazzo A, Lucarini M, Souto EB, Cicala C, Caiazzo E, Izzo AA, Novellino E, Santini A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother Res. 2019;33:2221–2243. doi: 10.1002/ptr.6419. [DOI] [PubMed] [Google Scholar]
- 9.Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 10.Boyer J, Liu RH. Apple phytochemicals and their health benefits. Nutr J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Little CR, Holmes RJ. Storage technology for apples and pears. Melbourne, Vic: Department of Natural Resources and Environment; 2000. [Google Scholar]
- 12.Coffman, Melody Anne. “The Health Benefits of Granny Smith Apples”. Healthy Eating. November 2018. Available from: [Internet] https://healthyeating.sfgate.com/health-benefits-granny-smith-apples-3334.html .
- 13.Organization for Economic Co‑Operation and Development. Test No 420: Acute oral toxicity – Fixed dose procedure. In: OECD Guidelines for the Testing of Chemicals. Sec. 4. Paris: OECD Publishing. 2002. [Google Scholar]
- 14.Dawra R, Saluja AK. L-arginine-induced experimental acute pancreatitis Dawra R, Saluja AK. L-arginine-induced experimental acute pancreatitis. Pancreapedia. 2012. [Google Scholar]
- 15.Szabolcs A, Reiter RJ, Letoha T, Hegyi P, Papai G, Varga I, Jarmay K, Kaszaki J, Sari R, Rakonczay Jr Z, Lonovics J, Takacs T. Effect of melatonin on the severity of L-arginineinduced experimental acute pancreatitis in rats. World J Gastroenterol. 2006;12:251–258. doi: 10.3748/wjg.v12.i2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaur J, Sidhu S, Chopra K, Khan MU. Calendula officinalis ameliorates l-arginine induced acute necrotizing pancreatitis in rats. Pharm Biol. 2016;54:2951–2959. doi: 10.1080/13880209.2016.1195848. [DOI] [PubMed] [Google Scholar]
- 17.Yildiz BD, Hamaloglu E. Basic experimental pancreatitis models for beginners. Surg Sci. 2010;1:31–39. [Google Scholar]
- 18.Misra HP, Fridovich I. Superoxide dismutase: a photochemical augmentation assay. Arch Biochem Biophys. 1977;181:308–312. doi: 10.1016/0003-9861(77)90509-4. [DOI] [PubMed] [Google Scholar]
- 19.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and non protein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 20.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133–140. [PubMed] [Google Scholar]
- 21.Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Meth Enzymol. 1979;62:3–11. doi: 10.1016/0076-6879(79)62181-x. [DOI] [PubMed] [Google Scholar]
- 22.Bergmeyer HU, Bernt E. Methods of Enzymatic Analysis. (2nd ed) New York, NY: Academic Press. 1974:574–579. [Google Scholar]
- 23.Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 25.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 26.Basnakian AG, James SJ. A rapid and sensitive assay for the detection of DNA fragmentation during early phases of apoptosis. Nucleic Acids Res. 1994;22:2714–2715. doi: 10.1093/nar/22.13.2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess arginine on rat pancreas. J Nutri. 1984;114:467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- 28.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford). 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegyi P, Rakonczay Jr Z, Sári R, Góg C, Lonovics J, Takács T, Czakó L. Larginine-induced experimental pancreatitis. W J Gastroenterol. 2004;10:2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abdel-Gawad SK. Therapeutic and protective effect of wheat germ oil on l-arginine induced acute pancreatitis in adult albino rats. J Cell Sci Ther. 2015;S8:S8. [Google Scholar]
- 31.Sidhu S, Pandhi P, Malhotra S, Vaiphei K, Khanduja KL. Melatonin treatment is beneficial in pancreatic repair process in experimental acute pancreatitis. Eur J Pharmacol. 2010;628:282–289. doi: 10.1016/j.ejphar.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 32.Oboh G, Omojokun OS, Oyeleye SI, Akinyemi AJ. Inhibition of α-amylase, α-glucosidase and oxidative stress by some common apple varieties. Nutrafoods. August. 2016. [Google Scholar]
- 33.Armstrong JA, Cash N, Soares PM, Souza MH, Sutton R, Criddle DN. Oxidative stress in acute pancreatitis: lost in translation? Free Radic Res. 2013;47:917–933. doi: 10.3109/10715762.2013.835046. [DOI] [PubMed] [Google Scholar]
- 34.Huang L, Cao J. The protective effects of Shen-Fu injection on experimental acute pancreatitis in a rat model. Oxidative medicine and cellular longevity. 2014;2014:248786. doi: 10.1155/2014/248786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Park BK, Chung JB, Lee JH, Suh JH, Park SW, Song SY, Kim H, Kim KH, Kang JK. Role of oxygen free radicals in patients with acute pancreatitis. World J Gastroenterol. 2003;9:2266–2269. doi: 10.3748/wjg.v9.i10.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simsek O, Kocael A, Kocael P, Orhan A, Cengiz M, Balcı H, Ulualp K, Uzun H. Inflammatory mediators in the diagnosis and treatment of acute pancreatitis: pentraxin-3, procalcitonin and myeloperoxidase. Arch Med Sci. 2018;14:288. doi: 10.5114/aoms.2016.57886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shafi W, Mansoor S, Jan S, Singh DB, Mohsin Kazi M, Raish M, Alwadei M, Mir JI, Ahmad P. Variability in catechin and rutin contents and their antioxidant potential in diverse apple genotypes. Molecules. 2019;24:943. doi: 10.3390/molecules24050943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai X, Zhang H, Ren S. Antioxidant activity and HPLC analysis of polyphenol-enriched extracts from industrial apple pomace. J Sci Food Agric. 2013;93:2502–2506. doi: 10.1002/jsfa.6066. [DOI] [PubMed] [Google Scholar]
- 39.Heo HJ, Lee CY. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J Agric Food Chem. 2004;52:7514–7517. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 40.Wolfe K, Wu X, Liu RH. Antioxidant activity of apple peels. J Agric Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 41.Cui J, Xiong J, Zhang Y, Peng T, Huang M, Lin Y, Guo Y, Wu H, Wang C. Serum lactate dehydrogenase is predictive of persistent organ failure in acute pancreatitis. J Crit Care. 2017;41:161–165. doi: 10.1016/j.jcrc.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 42.Abdin AA, El-Hamid MA, El-Seoud SH, Balaha MF. Effect of pentoxifylline and/or alpha lipoic acid on experimentally induced acute pancreatitis. E J Pharmacol. 2010;643:289–296. doi: 10.1016/j.ejphar.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 43.Tzanakis E, Kalogeropoulos TH, Tzimas ST, Chatzilazarou A, Katsoyannos E. Phenols and antioxidant activity of apple, quince, pomegranate, bitter orange and almond-leaved pear methanolic extracts. E J Sci Tech. 2006;1:16–28. [Google Scholar]
- 44.Saxena S, Verma J, Gautam S. Potential prophylactic properties of apple and characterization of potent bioactive from cv. “Granny Smith” displaying strong antimutagenicity in models including human lymphoblast TK6+/− cell line. J Food Sci. 2016;81:H508–H518. doi: 10.1111/1750-3841.13190. [DOI] [PubMed] [Google Scholar]
- 45.Meher S, Rath S, Sharma R, Rout B, Mishra TS, Sasmal PK, Sinha MK. Pathophysiology of oxidative stress and antioxidant therapy in acute pancreatitis. J Mol Biomark Diagn. 2015;6:257. [Google Scholar]
- 46.Rios EC, Moretti AS, Velasco IT, Souza HP, Abatepaulo F, Soriano F. Hypertonic saline and reduced peroxynitrite formation in experimental pancreatitis. Clinics (Sau Paulo). 2011;66:469–476. doi: 10.1590/S1807-59322011000300019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Biedrzycka E, Amarowicz R. Diet and health: apple polyphenols as antioxidant. Food Rev Int. 2008;24:235–251. [Google Scholar]
- 48.Lotito SB, Frei B. Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Rad Bio Med. 2004;36:201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 49.Heo HJ, Choi SJ, Choi SG, Shin DH, Lee JM, Lee CY. Effects of banana, orange, and apple on oxidative stress-induced neurotoxicity in PC12 cells. J Food Sci. 2008;73:H28–H32. doi: 10.1111/j.1750-3841.2007.00632.x. [DOI] [PubMed] [Google Scholar]
- 50.Shen J, Wan R, Hu G, Wang F, Shen J, Wang X. Involvement of thrombopoietin in acinar cell necrosis in L-arginine-induced acute pancreatitis in mice. Cytokine. 2012;60:294–301. doi: 10.1016/j.cyto.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 51.Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am J Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- 52.Mervi T Hyvonen, Herzig KH, Sinervirta R, Albrecht E, Nordback I, Sand J, Keinanen TA, Vepsalainen J, Grigorenko N, Khomutov AR, Kruger B, Janne J, Alhonen L. Activated polyamine catabolism in acute pancreatitis: α-methylated polyamine analogues prevent trypsinogen activation and pancreatitis-associated mortality. Am J Pathol. 2006;168:115–122. doi: 10.2353/ajpath.2006.050518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao XL, Xiang J, Wana MH, Yu Q, Chen WW, Chen GY, Tang WF. Effect of acute pancreatitis on the pharmacokinetics of Chinese herbal ointment Liu-He-Dan in anaesthetized rats. J Ethnopharmacol. 2013;145:94–99. doi: 10.1016/j.jep.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 54.Deavall DG, Martin EA, Horner JM, Roberts R. Drug-induced oxidative stress and toxicity. J Toxicol. 2012;2012:645460. doi: 10.1155/2012/645460. [DOI] [PMC free article] [PubMed] [Google Scholar]


