ABSTRACT
Background: Most posttraumatic stress disorder (PTSD) sleep disturbances reports have been conducted in male combat veteran populations, usually decades after the disorder’s onset. Given the increase in the prevalence of violence against women and the fact that women are at greater risk for developing PTSD, it is critical to examine sleep abnormalities in this population.
Objectives: To examine subjective and objective sleep quality in young women with PTSD following sexual assault compared with a control group at baseline and after one year of treatment.
Methods: Seventy-four women with PTSD following sexual assault and 64 healthy controls with no history of sexual assault were assessed using the Clinician-Administered PTSD Scale (CAPS-5), the Beck Depression Inventory, the Beck Anxiety Inventory, the Pittsburgh Sleep Quality Index (PSQI), the Modified Fatigue Impact Scale, and the Insomnia Severity Index. Subjects also underwent full in-lab polysomnography. PTSD participants received pharmacological and/or psychological therapy between baseline and one-year follow-up.
Results: The PTSD group had significantly higher scores in the clinical and sleep measurements than the control group. Although the PTSD group reported poorer subjective sleep quality than healthy controls, there were few between-group differences in objective sleep. Analysis of the PTSD group at baseline and one-year follow-up showed that the PSQI global score was a significant predictor of PTSD improvement.
Conclusions: Sleep quality is impaired in young women with PTSD and may impact long-term treatment responses. Better sleep quality is significantly associated with PTSD improvement, independent of depression and anxiety.
KEYWORDS: PTSD, sexual violence, sexual assault, sleep disorder, PSQI
HIGHLIGHTS
Several variables may influence sleep abnormalities in PTSD patients, including the type of trauma, gender, age, and time elapsed since the trauma.
The present study evaluates subjective and objective sleep assessments in young women with PTSD who had suffered sexual assault.
Short abstract
Antecedentes: la mayoría de los reportes de trastornos del sueño en el trastorno por estrés postraumático (TEPT) se han realizado en poblaciones de hombres veteranos de guerra, generalmente décadas después del inicio del trastorno. Dado el aumento en la prevalencia de la violencia contra las mujeres y el hecho de que las mujeres tienen un mayor riesgo de desarrollar TEPT, es fundamental examinar las anomalías del sueño en esta población.
Objetivos: Examinar la calidad del sueño subjetiva y objetiva en mujeres jóvenes con trastorno de estrés postraumático después de una agresión sexual en comparación con un grupo control al inicio y después de un año de tratamiento.
Métodos: Se evaluaron 74 mujeres con TEPT después de agresión sexual y 64 controles sanos sin antecedentes de agresión sexual utilizando la Escala de TEPT administrada por un médico (CAPS-5, en su sigla en inglés), el Inventario de Depresión de Beck, el Inventario de Ansiedad de Beck, la Calidad del Sueño de Pittsburgh. (PSQI, en su sigla en inglés), la escala de impacto de fatiga modificada y el índice de gravedad del insomnio. Los sujetos también se sometieron a una polisomnografía completa en el laboratorio. Los participantes con TEPT recibieron terapia farmacológica y/o psicológica entre el inicio y el seguimiento al año.
Resultados: El grupo de TEPT tuvo puntuaciones significativamente más altas en las mediciones clínicas y del sueño que el grupo de control. Aunque el grupo de TEPT reportó una peor calidad del sueño subjetivo que los controles sanos, hubo pocas diferencias entre grupos en el sueño objetivo. El análisis del grupo de TEPT al inicio y al año de seguimiento mostró que la puntuación global del PSQI fue un predictor significativo de la mejoría del TEPT.
Conclusiones: La calidad del sueño se ve afectada en mujeres jóvenes con TEPT y puede afectar las respuestas al tratamiento a largo plazo. Una mejor calidad del sueño se asocia significativamente con la mejora del TEPT, independientemente de la depresión y la ansiedad.
PALABRAS CLAVE: TEPT, violencia sexual, agresión sexual, trastorno del sueño, PSQI
Short abstract
背景: 大多数创伤后应激障碍 (PTSD) 睡眠障碍的报告是在男性退伍军人群体中进行的, 通常在这种疾病发作后数十年。鉴于对女性的暴力流行率的增加以及女性罹患PTSD的更大风险, 考查该人群的睡眠异常至关重要。
目的: 在基线和治疗一年后, 考查性侵犯后发展出PTSD的年轻女性相较于对照组的主观和客观睡眠质量。
方法: 使用临床用PTSD量表 (CAPS-5), 贝克抑郁量表, 贝克焦虑量表, 匹兹堡睡眠质量指数(PSQI), 改良版疲劳影响量表和失眠严重程度指数评估了74名性侵犯后的PTSD女性患者和64名无性侵犯史的健康对照者。受试者还进行了完整的实验室多导睡眠监测。 PTSD参与者在基线和一年随访之间接受了药物和/或心理治疗。
结果: PTSD组在临床和睡眠测量方面的得分均显著高于对照组。尽管PTSD组比对照组报告了更差的主观睡眠质量, 但客观睡眠的组间差异很小。在基线和一年随访时对PTSD组进行的分析表明, PSQI整体评分是PTSD改善的重要预测因素。
结论: PTSD年轻女性患者的睡眠质量受损, 可能影响长期治疗反应。更好的睡眠质量与PTSD改善显著相关, 与抑郁和焦虑无关。
关键词: PTSD, 性暴力, 性侵犯, 睡眠障碍, PSQI
1. Introduction
Sexual violence is a significant public health concern. A World Health Organization study found that between 19% and 76% of women reported physical or sexual violence worldwide (World Health Organization [WHO], 2005). According to data from the Brazilian Public Security Forum, there were 50,000 reports of sexual assault against women in Brazil in 2016. However, the rate of sexual violence against women is much higher, considering that an immense proportion is underreported (Cerqueira et al., 2018). Sexual violence is one of the most severe traumatic events to afflict women. It is associated with physical, sexual, reproductive, and mental health disorders, including anxiety disorder, depression, substance abuse, and posttraumatic stress disorder (PTSD) (Burnam et al., 1988).
The global increase in violence against women affects the prevalence of PTSD, a mental health disorder triggered by exposure to a traumatic event. Epidemiological studies showed that women are nearly twice as likely to develop PTSD, and between 17% and 65% of women with a lifetime history of sexual assault develop the disorder (Campbell, Dworkin, & Cabral, 2009; Frans, Rimmo, Aberg, & Fredrikson, 2005). Sleep disturbances have a high prevalence and are a hallmark of the disorder (Ross, Ball, Sullivan, & Caroff, 1989); between 70% and 87% of people suffering from PTSD report sleep disturbances, including difficulty initiating and maintaining sleep, nightmares, and interrupted sleep (Germain, 2013). Hyperarousal persists beyond wakefulness and leads to insomnia symptoms, suggesting a bi-directional association between sleep and PTSD development and maintenance (Mellman & Hipolito, 2006).
PTSD treatment includes psychological and pharmacological interventions. Psychological approaches include prolonged exposure, cognitive therapy, and eye movement desensitization and reprocessing. However, exposure-based psychotherapies may be poorly tolerated by patients (Foa, Zoellner, Feeny, Hembree, & Alvarez-Conrad, 2002). Interpersonal psychotherapy for PTSD (IPT-PTSD) is a non-exposure-based therapy that focuses on how trauma affects patients’ current interpersonal functioning (Markowitz, 2016). Markowitz and colleagues found that IPT-PTSD had greater efficacy among sexual assault survivors than prolonged exposure and relaxation therapy (Markowitz, Neria, Lovell, Van Meter, & Petkova, 2017). Although SSRIs are the first line for pharmacological treatment, patients frequently complain of sleep disturbances even when there is an amelioration of PTSD symptoms (Germain, 2013); therefore, assessing sleep disturbances in PTSD is essential for establishing clinical interventions and outcomes.
Even though subjective reports of sleep disruptions are frequent, polysomnographic studies have reported inconsistent results. Three meta-analyses evaluating polysomnographic measured sleep abnormalities in PTSD have been published. In the first meta-analysis, Kobayashi et al. (2007) found that PTSD patients had more stage 1 sleep, less slow-wave sleep, and greater REM density than individuals without PTSD. In the second meta-analysis, Baglioni et al. (2016) reported shortened slow-wave sleep and REM sleep latency but no alterations in REM density or REM duration. More recently, Zhang et al. (2019) showed decreased slow-wave sleep, total sleep time, sleep efficiency, and increased wakefulness after sleep onset in PTSD patients than healthy controls. Furthermore, REM sleep percentage was decreased in PTSD patients in studies including participants with mean age below 30 years old.
Most PTSD sleep studies have been conducted in male combat veteran populations, usually decades after the onset of the disorder (Mellman, Kobayashi, Lavela, Wilson, & Hall Brown, 2014). There are few studies examining sleep parameters in women with PTSD. Otte et al. (2007) found numerically less delta sleep in women with PTSD, although the difference was not statistically significant. Richards et al. (2013) compared objective sleep measures in male and female PTSD subjects and found greater REM sleep in PTSD females than control females; however, they did not find a difference in male subjects. Lipinska and Thomas (2017) conducted a study investigating subjective and objective sleep measures in PTSD women, sexual assault survivors recruited from a rape crisis centre. They reported poorer subjective sleep quality in PTSD women than trauma-exposed and healthy controls; nevertheless, laboratory sleep measures using polysomnography only demonstrated decreased sleep depth. Given the increase in the prevalence of violence against young women and the fact that women are at greater risk for developing PTSD, we emphasize the relevance of studying sleep disturbances in this population.
The current study objectives were to examine PTSD severity and sleep parameters measured subjectively and objectively in young women sexual assault survivors with PTSD compared to healthy women without a history of trauma exposure. We also aimed to compare sleep variables in the PTSD group before and after one year of treatment.
2. Methods
2.1. Participants and protocol
The data were obtained from a more extensive study on PTSD and neuroprogression; full details of the study are published elsewhere (Coimbra et al., 2020). The non-PTSD control group included women who neither had a history of sexual abuse nor psychiatric diagnosis, recruited through advertisements in local newspapers and posters posted in our university and public buildings in the community. Seventy-four sexually-assaulted women with PTSD and sixty-four women without PTSD were evaluated. All sexually -assaulted women were referred from the largest women’s public health facility in São Paulo, Brazil, that offers care for women following sexual violence. Patients participated in this study between January 2016 and February 2020, conducted at the Universidade Federal de São Paulo. Of note, this study was carried out before the COVID-19 pandemic.
The inclusion criteria were as follows: 18 to 45 years of age with a history of sexual assault in the 1 to 6 months before study enrolment and a diagnosis of posttraumatic stress disorder according to DSM-5 criteria. The healthy controls were women 18 to 45 years of age from the community with no PTSD or history of sexual assault. The exclusion criteria for both groups were as follows: menopausal symptomatology or pregnancy, corticosteroid use, positivity for human immunodeficiency virus, sexually transmitted diseases, acute or unstable clinical conditions, neurological disorders, and lifetime history of bipolar, psychotic, or substance dependence or abuse (not in remission for the previous six months). Participants undergoing any psychological or psychiatric treatment or taking psychotropic medication were also excluded.
We only recruited female sexual assault survivors because it is vital to understand PTSD characteristics and sleep abnormalities in this female population. Furthermore, given that exposure to a high impact trauma (such as sexual violence) is more likely to precipitate a wide range of physiopathological and biomarker alterations in women even when they do not develop PTSD, we recruited healthy control participants to differentiate between the exposure to trauma and development of PTSD and conditions in those who have not experienced a traumatic event.
All participants underwent a clinical and psychiatric interview to verify selection criteria. Participants were informed in detail about the nature and purpose of the study approved by the Institutional Review Board of our university; all provided written informed consent.
2.2. Interventions
PTSD participants were randomized to treatment either with sertraline or interpersonal psychotherapy adapted to PTSD (IPT-PTSD).
2.2.1. Sertraline
Sertraline dosage ranged from 50 to 200 mg/daily, depending on clinical presentation and tolerance. Two psychiatrists, experienced in treating PTSD patients, followed the patients over the 14-week treatment. Subjects were evaluated at baseline, week 2, week 4, week 8, and week 14.
2.2.2. Interpersonal psychotherapy adapted to PTSD (IPT-PTSD)
IPT-PTSD was delivered in 14-weekly 50-min sessions with an experienced therapist. Five therapists (psychiatrists), who had at least one year of experience with IPT, were trained and supervised weekly to use IPT-PTSD.
Both the sertraline and IPT-PTSD groups could receive low dosages of quetiapine (25–50 mg), risperidone (1–2 mg), or zolpidem CR (12.5 mg) if necessary.
2.3. Procedures and assessments
Participants were clinically evaluated, assessed using several clinical scales (described below), and underwent one night of polysomnography (PSG) at baseline and after completing one year of enrolment in the study. The healthy control group had a one-time assessment and underwent one night of PSG recording at baseline. More protocol details can be found elsewhere (Coimbra et al., 2020). Ten PTSD and one control participant refused PSG recording at baseline. Two PTSD participants refused PSG recording at one-year follow-up (Figure 1).
Figure 1.
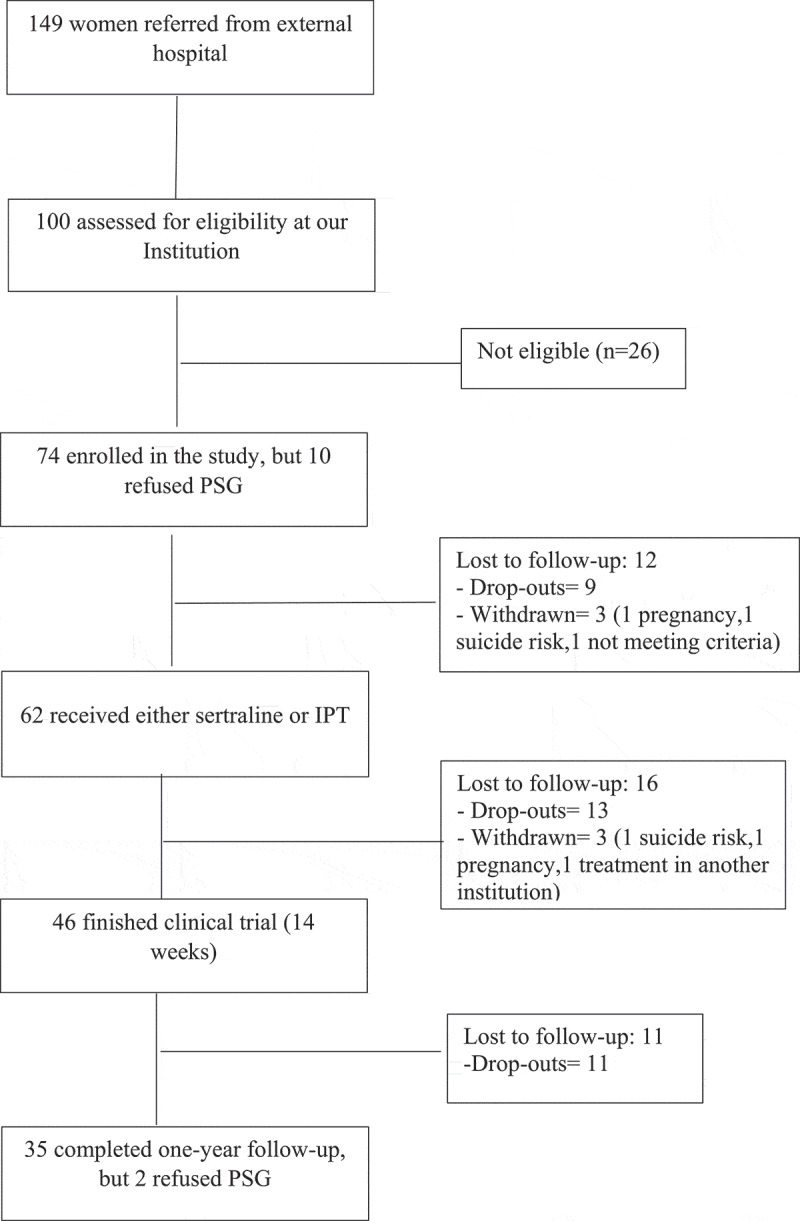
Flowchart
After the initial 14 weeks of treatment, the participants who showed improvement remained in the treatment and were followed until completing one-year of treatment. Those who did not show clinical improvement received standard treatment: sertraline (50–200 mg), quetiapine (25–100 mg), zolpidem CR (6.25–12.50 mg), risperidone (1–4 mg), clonazepam (0.25–2 mg), venlafaxine (75–150 mg), paroxetine (20–60 mg), fluoxetine (20–80 mg), and interpersonal psychotherapy until completing one-year follow-up. A response was considered a > 30% decrease from baseline CAPS-5. Potential remission was defined as CAPS score < 26 (Weathers et al., 2018).
2.4. Measures
The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5) is a structured diagnostic interview containing a 30-item questionnaire capable of assessing both the frequency and intensity of PTSD symptoms and the variables associated with the trauma using a frequency/severity scale varying from 0 to 4. A large-scale psychometric study showed strong evidence of its validity and reliability as a measure for the symptoms of PTSD (Weathers et al., 2018). An adapted Portuguese language version was used (Oliveira-Watanabe, Ramos-Lima, Santos, Mello, & Mello, 2019).
The Mini International Neuropsychiatric Interview (MINI) is a structured diagnostic interview designed for clinical practice and research in psychiatric and primary care settings. The MINI provides the clinician accurate psychiatric diagnoses (psychosis, mood, anxiety, personality, and PTSD) and is widely used. A validated Portuguese language version was used (Amorim, 2000).
The Beck Depression Inventory–Second Edition (BDI-II) is a self-reported instrument composed of a 21-item questionnaire for measuring clinical depression. The participant must evaluate each question referring to depression symptoms on a 0–4 severity scale. The score consists of the sum of the individual items classifying the severity of the depression as minimum 0–13, mild 14–19, moderate 20–28, or severe 29–63 (Beck, Steer, & Brown, 1996). A validated Portuguese language version was used (Gomes-Oliveira, Gorenstein, Neto, Andrade, & Wang, 2012).
The Beck Anxiety Inventory (BAI) is a self-reported instrument composed of a 21-item questionnaire related to anxiety symptoms. The participant must evaluate each question referring to anxiety symptoms on a 0–3 severity scale. The score consists of the sum of the individual items classifying the severity of the anxiety as minimum 0–7, mild 8–15, moderate 16–25, or severe: 26–63 (Beck, Steer, & Carbin, 1988). A validated Portuguese language version was used (Cunha, 2001).
The Pittsburgh Sleep Quality Index (PSQI) is a 19-item self-reported measure that assesses seven components of sleep quality, including sleep latency, duration, efficiency, disturbances, use of sleep medication, and daytime dysfunction. Each component is rated on a 0–3 scale referring to the frequency of each disturbance, and the sum of the components provides a global score ranging from 0 to 21. A PSQI global score higher than 5 indicates clinically significant sleep disturbance (Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). A validated Portuguese language version was used (Bertolazi et al., 2011).
The Pittsburgh Sleep Quality Index addendum for PTSD (PSQI-A) is a self-assessment scale consisting of seven items to assess seven types of disruptive nocturnal behaviours reported by PTSD patients. The seven items assess the frequency of hot flashes, general nervousness, memories or nightmares of the traumatic experience, severe anxiety or panic (not related to traumatic memories), nightmares (not related to traumatic memories), episodes of terror or screaming during sleep without fully awakening, and episodes of acting out dreams. Each item is rated on a 0–3 scale. The global score ranges from 0 to 21. A PSQI-A score higher than 6 indicates disruptive nocturnal behaviours (Germain, Hall, Krakow, Katherine Shear, & Buysse, 2005). A validated Portuguese language version was used (Barbosa Neto et al., 2014).
The Epworth Sleepiness Scale (ESS) is a self-report questionnaire that measures the occurrence and intensity of daytime sleepiness. The ESS evaluates the probability of falling asleep in eight situations involving daily activities. The total score ranges from 0 to 24; scores higher than 10 indicate excessive daytime sleepiness (Johns, 1991). A validated Portuguese language version was used (Bertolazi, 2009).
The Insomnia Severity Index (ISI) is a 7-item self-report questionnaire measuring the patient’s self-perception of insomnia. The total score ranges from 0 to 28; 0–7 indicates the absence of insomnia, 8–14 indicates subthreshold insomnia, 15–21 indicates moderate insomnia and 22–28 indicates severe insomnia (Morin, 1993). A validated Portuguese language version was used (Bastien, Vallières, & Morin, 2001).
The Modified Fatigue Impact Scale (MFIS) consists of 21 questions divided into physical, cognitive, and psychosocial domains. Each item is rated from 0 to 4. The total score of MFIS ranges from 0 to 84 points. Values below 38 indicate the absence of fatigue, and above this value, the higher the score, the greater the degree of fatigue of the individual (Flachenecker et al., 2002). A validated Portuguese language version was used (Pavan et al., 2007).
The control group underwent one night of polysomnography recording without adaptation at baseline. PTSD participants had one night of polysomnography recording without adaptation at baseline and one night of polysomnography recording at 1-year follow-up. PSG recordings were conducted in the Instituto do Sono-AFIP at the Universidade Federal de São Paulo. Recordings were conducted using an EMBLA (EMBLA N7000, EMBLA Systems, Inc., Broomfield, CO, USA). All recordings included a six-channel electroencephalogram (EEG) (F4-M1, C4-M1, 02-M1, F3-M2, C3-M2, 01-M2), a two-channel electrooculogram, four-channel electromyography with electrodes placed in the submental and tibial regions, and a one-channel electrocardiograph (D2-modified). Airflow detection was performed using a thermocouple and nasal cannula. Inductance plethysmography belts assessed respiratory effort. Snoring, body position, SaO2, and pulse rate were evaluated using EMBLA® sensors (EMBLA Systems Inc., Broomfield, CO, USA). All PSGs were performed and scored by two technicians following guidelines for sleep studies and were reviewed by a sleep medicine physician. Sleep stages, EEG arousals, and leg movements were scored according to established criteria (Berry et al., 2007). Apnoea was defined as complete or close to complete airflow cessation for ≥10 s. Hypopnoea was identified as an evident reduction in the breathing amplitude (at least 30% below the baseline) for ≥10 s accompanied by either an EEG arousal or a SaO2 drop of ≥ 3% (Flemons et al., 1999; Jonas et al., 2017).
The following sleep variables were determined: REM sleep latency, sleep onset latency; total sleep time, wake after sleep onset, sleep efficiency, and the percentages of total sleep composed of N1, N2, N3, REM sleep, arousal index (number of arousals per hour), periodic limb movements index with and without arousal, number of limb movements per hour with and without arousal, apnoea-hypopnoea index, number of apnoeas, and hypopneas per hour.
Female PSG-certified technicians attended all participants. All PTSD participants had a relative as co-sleeper for PSG recordings in the laboratory to ensure wellbeing in an unfamiliar sleep environment. Similarly, controls were allowed to have a companion; however, they chose to remain alone, as they felt confident with the sleep lab facilities and care.
2.5. Statistical analyses
Statistical analyses were performed using SPSS software version 24, with an alpha level of 0.05 for all analyses. Continuous variables were expressed as means and standard deviations. Categoric data were represented as absolute and percentage frequencies. The univariate general linear model was used to compare control and PTSD groups.
Fisher exact test and Chi-Square were used to compare frequencies. The generalized estimating equation for gamma distribution was used for data analysis at baseline and after one year of treatment in the PTSD group.
3. Results
3.1. Baseline results
Clinical and demographic characteristics are presented in Table 1. The PTSD group was slightly but significantly younger, and there were more African-Brazilian participants than the control group. No medical or unrelated psychiatric comorbidities occurred in any group. The mean time elapsed between the sexual assault and PSG evaluation for the PTSD group was 2.4 months (standard deviation [SD] = 1.7).
Table 1.
Baseline characteristics
| Characteristics | Controls (n = 64) |
PTSD (n = 74) |
p |
|---|---|---|---|
| Mean Age (year) ± SD | 27.90 ± 7.40 | 24.40 ± 6.75 | 0.004 |
| BMI | 25.05 ± 3.90 | 24.75 ± 4.80 | 0.68 |
| Ethnicity | |||
| - Caucasian | 46 (71.9%) | 33 (44.6%) | 0.001 |
| - African Brazilian | 18 (28.1%) | 41 (55.4%) | |
| Marital Status, n (%) | |||
| - Single/Divorced | 46 (71.9%) | 52 (70.3%) | 0.83 |
| - Married | 18 (28.1%) | 22 (29.7%) | |
| Education Level, n (%) | |||
| - Elementary school | 2 (3.1%) | 5 (6.8%) | 0.33 |
| - High school/College | 62 (96.9%) | 69 (93.2%) | |
| Religion, n (%) | |||
| - Christian | 37 (57.8%) | 48 (64.9%) | 0.66 |
| - Non-Christian | 5 (5.8%) | 4 (5.4%) | |
| - Atheist/No religion | 22 (34.4%) | 22 (29.7%) |
SD = standard deviation; BMI = Body Mass Index.
Psychopathological, sleep quality measures, and PSG parameters are shown in Table 2. As expected, the severity of psychopathological symptoms was higher, and sleep quality was lower in the PTSD group. The latter had significantly higher scores for depression, anxiety, insomnia, fatigue, and worse sleep quality, except for daytime sleepiness. Comparison of objectively measured sleep parameters between groups showed that the PTSD group had less total sleep time and a trend towards lower REM sleep time but a similar proportion of REM sleep. The control group had a higher arousal index.
Table 2.
Psychopathological and sleep scales, and PSG parameters
| Psychopathological and Sleep Scales |
Controls (N = 64) Mean±SD |
PTSD Baseline (N = 74) Mean±SD |
PTSD One-year (N = 35) Mean±SD |
p1 | p1a | p2 |
|---|---|---|---|---|---|---|
| BDI | 3.90 ± 3.95 | 27.89 ± 13.70 | 15.05 ± 12.50 | <0.001 | <0.001 | |
| BAI | 3.60 ± 3.65 | 29.55 ± 11.66 | 21.60 ± 19.90 | <0.001 | 0.038 | |
| MFIS | 16.40 ± 12.85 | 54.50 ± 14.36 | 32.30 ± 22.45 | <0.001 | <0.001 | |
| ISI | 3.70 ± 3.70 | 16.70 ± 6.05 | 11.40 ± 8.80 | <0.001 | 0.005 | |
| ESS | 7.25 ± 4.55 | 7.62 ± 5.12 | 7.52 ± 5.90 | 0.84 | 0.92 | |
| PSQI | 3.02 ± 1.90 | 10.40 ± 3.50 | 7.97 ± 5.20 | <0.001 | 0.006 | |
| PSQI-A | 7.82 ± 4.20 | 5.05 ± 5.45 | 0.008 | |||
| CAPS-5 |
|
42.94 ± 8.90 |
23.88 ± 18.45 |
|
|
<0.001 |
| PSG Parameters |
Controls (N = 63) Mean±SD |
PTSD Baseline (N = 64) Mean±SD |
PTSD One-year (N = 33) Mean±SD |
p1 |
p1a |
p2 |
| TST min | 361.30 ± 51.90 | 341.40 ± 60.0 | 336.00 ± 105.70 | 0.05 | 0.11 | 0.79 |
| SOL min | 36.15 ± 43.90 | 47.10 ± 46.50 | 65.55 ± 96.70 | 0.20 | 0.26 | 0.28 |
| SE % | 84.20 ± 11.30 | 80.70 ± 13.00 | 80.40 ± 15.30 | 0.11 | 0.27 | 0.93 |
| N1% | 6.40 ± 4.60 | 6.30 ± 3.30 | 8.20 ± 6.00 | 0.86 | 0.96 | 0.06 |
| N2% | 49.60 ± 9.20 | 50.60 ± 8.40 | 53.10 ± 8.44 | 0.51 | 0.24 | 0.10 |
| N3% | 23.70 ± 7.00 | 24.10 ± 7.00 | 21.50 ± 9.20 | 0.74 | 0.74 | 0.19 |
| REM % | 20.80 ± 8.30 | 18.80 ± 7.00 | 18.30 ± 6.15 | 0.13 | 0.06 | 0.73 |
| WASO min | 34.40 ± 29.50 | 36.90 ± 38.72 | 36.52 ± 30.00 | 0.69 | 0.91 | 0.95 |
| RDI | 4.20 ± 5.00 | 4.50 ± 5.30 | 4.70 ± 6.14 | 0.73 | 0.16 | 0.82 |
| Min SaO2 | 90.10 ± 3.45 | 90.40 ± 3.76 | 89.20 ± 4.13 | 0.58 | 0.74 | 0.13 |
| Arousal Index | 12.60 ± 6.55 | 10.40 ± 4.00 | 12.60 ± 8.15 | 0.02 | 0.16 | 0.11 |
| AIH | 3.80 ± 5.10 | 4.40 ± 5.20 | 4.50 ± 6.10 | 0.57 | 0.12 | 0.88 |
| REM Latency min | 110.40 ± 54.50 | 126.30 ± 74.80 | 128.20 ± 78.80 | 0.17 | 0.65 | 0.89 |
GEE test, p < 0.05.
BDI = Beck Depression Inventory, BAI = Beck Anxiety Inventory, MFIS = Modified Fatigue Impact Scale, ISI = Insomnia Severity Index, ESS = Epworth Sleepiness Scale PSQI = Pittsburgh Sleep Quality Index, PSQI-A = Pittsburgh Sleep Quality Index addendum for PTSD, CAPS-5 = Clinician Administered PTSD Scale for DSM-5, TST = Total Sleep Time, SOL = Sleep Onset Latency, SE = Sleep Efficiency, WASO = Wake After Sleep Onset, RDI = Respiratory Disturbance Index, Arousal Index = Number of arousals/hour, AHI = Apnoea-hypopnoea Index.
p1 = p-value between the control group and PTSD group, p2 = p-value between PSTD baseline and PTSD after 1-year follow up.
p1a = p-value adjusted for time elapsed between trauma event and PSG baseline measurement.
3.2. One-year follow-up
Analysis of psychopathological and sleep assessments and PSG parameters in the PTSD group at baseline and one-year follow-up are shown in Table 2. Depression, anxiety, fatigue, PTSD symptoms, and sleep questionnaire responses were significantly improved at one-year follow-up.
Twenty-three (65.7%) of 35 PTSD women had significant improvement of PTSD severity (CAPS-5 reduction > 30%), and 20 women (57.1%) had potential remission (CAPS-5 < 26) of symptoms. Of note, eight women (10.81%) were re-exposed to a traumatic event during the study. Although PTSD participants reported significant improvement of subjective measurements of sleep quality, there were no significant changes in PSG parameters after one-year follow-up compared to baseline. None of the subject’s polysomnographic recordings indicated a sleep-related breathing disorder.
Comparing the baseline demographic data, psychopathological and sleep quality measures of the completers (completed one-year follow-up) and the non-completer PTSD groups (i.e. who did not complete one year of treatment), there were no significant differences, except for more severe insomnia and slightly older age in the completer PTSD group (Table 3).
Table 3.
Demographic data, psychopathological, and sleep scales in the PTSD group
| Baseline Demographic Data |
PTSD group Completers (n = 35) |
PTSD group Non completers (n = 39) |
p |
|---|---|---|---|
| Mean Age (year) ± SD | 26.40 ± 7.70 | 23.45 ± 9.40 | 0.05 |
|
Ethnicity -Caucasian -African-Brazilian |
12 (34.3%) 23 (65.7%) |
21 (53.8%) 18 (46.2%) |
0.09 |
|
Marital Status, n (%) -Single/Divorced -Married |
22 (68.6%) 13 (37.1%) |
30 (76.9%) 9 (23.1%) |
0.18 |
|
Education Level, n (%) -Elmentary school -High school/College |
3 (8.6%) 32 (91.4%) |
2 (5.1%) 37 (94.9%) |
0.66 |
|
Religion, n (%) -Christian -Non-Christian -Atheist/No religion |
24 (68.6%) 1 (2.9%) 10 (28.5%) |
24 (61.5%) 3 (7.7%) 12 (30.8%) |
0.61 |
| Baseline Psychopathological and Sleep Scales |
PTSD group Completers (n = 35) |
PTSD group Non completers (n = 39) |
p |
| BDI | 27.90 ± 13.95 | 27.84 ± 9.80 | 0.98 |
| BAI | 29.30 ± 11.95 | 27.75 ± 11.20 | 0.57 |
| MFIS | 54.50 ± 14.60 | 48.40 ± 16.90 | 0.10 |
| ISI | 16.70 ± 6.00 | 14.00 ± 5.65 | 0.05 |
| ESS | 7.60 ± 5.12 | 7.30 ± 5.60 | 0.81 |
| PSQI | 10.40 ± 3.50 | 10.20 ± 3.50 | 0.81 |
| PSQI-A | 7.60 ± 4.30 | 7.70 ± 3.65 | 0.93 |
| CAPS-5 | 42.90 ± 9.00 | 42.20 ± 9.40 | 0.72 |
GLM test, p < 0.05.
BDI = Beck Depression Inventory, BAI = Beck Anxiety Inventory, MFIS = Modified Fatigue Impact Scale, ISI = Insomnia Severity Index, ESS = Epworth Sleepiness Scale, PSQI = Pittsburgh Sleep.Quality Index, PSQI-A = Pittsburgh Sleep Quality Index addendum for PTSD, CAPS-5 = Clinician Administered PTSD Scale for DSM-5.
3.3. Regression results
A power analysis was conducted using the GPower software 3.1.9.2 (Faul, Erdfelder, Buchner, & Lang, 2009) for the linear regression analysis. We calculated the power using our sample size of 35 patients in a linear regression model, including three independent variables in the final model with a significance level of 5% and effect size of 2.25, based on the f-calculation from the R2. Using these parameters, a power level of 0.95 was achieved for the analysis. The final model was obtained from preliminary analysis using bivariate Pearson correlations to examine objectively- and subjectively-measured sleep parameters and psychopathological assessments. The best fit model was chosen using the Wald method. The Durbin–Watson test was used for autocorrelation, and the VIF and tolerance were performed to determine the presence of multicollinearity. We calculated tolerance and VIF values to evaluate multicollinearity between variables, with tolerance > 0.2 and VIF < 10 considered indicative of no collinearity among the independent variables.
Results of multiple linear regression analyses, including ISI, PSQI, PSQI-A, BAI, BDI, MFIS delta values as independent variables and CAPS-5 delta value as a dependent variable, are presented in Table 4. The PSQI delta global score was the variable that was a significant predictor of PTSD improvement. There was a positive correlation between PSQI delta global score and PTSD clinical improvement, indicating an association between PTSD improvement and sleep quality improvement.
Table 4.
Multiple regression analysis with delta CAPS-5 score as a dependent variable
| 95%CI |
Collinearity statistics |
||||||
|---|---|---|---|---|---|---|---|
| Independent Variables | Beta | t | p | Lower bound | Upper bound | Tolerance | VIF |
| Constant | −2.313 | 0.029 | −13.179 | − 0.789 | |||
| Delta PSQI | 0.392 | 2.286 | 0.030 | 0.149 | 2.758 | 0.443 | 2.258 |
| Delta BDI | 0.276 | 1.906 | 0.067 | −0.023 | 0.629 | 0.620 | 1.614 |
| Delta IGI | 0.259 | 1.472 | 0.153 | −0.212 | 1.288 | 0.418 | 2.392 |
R2 = 0.64, DW = 1.56.
B = regression coefficient; CI = confidence interval; VIF = variance inflation factor; DW = Durbin-Watson test.
4. Discussion
Studies reporting sleep disturbances in PTSD patients are characterized by significant variability in their findings. Several variables may influence sleep abnormalities, including the type of trauma, gender, age, and time elapsed since the trauma. The current study analysed sleep findings in young women with PTSD after suffering sexual assault one to six months before the study.
4.1. Control vs. PTSD group
As expected, the PTSD group had significantly higher scores on clinical and sleep measurements than the control group. Women in the PTSD group had severe anxiety, moderate depression, moderate insomnia, sleep disturbances, and fatigue. Although hypervigilance in PTSD individuals causes persistent nightmares and insomnia and might result in daytime sleepiness, women with PTSD did not report daytime somnolence.
In our study, the PTSD group demonstrated a significantly reduced arousal index but did not significantly differ in sleep efficiency percentage compared with the control group. Furthermore, the time elapsed after trauma exposure and PSG recording (mean time of 2.4 months ± 1.7) did not influence REM sleep results (Table 2); however, there was a trend towards an association between the percentage of REM sleep and PTSD duration. Studies have reported fragmentation or reduction of REM sleep in patients in the acute phase after trauma exposure and no REM sleep disruption with chronic PTSD, suggesting that the duration of PTSD may influence patterns of REM sleep (Habukawa, Uchimura, Maeda, Kotorii, & Maeda, 2007; Mellman et al., 2014).
The PTSD group did not show a decrease in slow-wave sleep, and this finding is consistent with other studies. Otte et al. (2007) found that women with PTSD did not have a statistically significant decline in delta sleep than control subjects, and Neylan et al. (2006) reported that a decrease in delta sleep was significant only in males.
Our PTSD participants demonstrated lower REM sleep percentage; however, this was not statistically significant from healthy controls. Although PTSD young women reported poorer subjective sleep quality than healthy controls, there were few between-group differences in objective sleep. Kobayashi et al. (2007) compared sleep parameters measured by PSG conducted in the laboratory with actigraphy at home. They demonstrated PTSD participants’ hypervigilance at home could be attenuated in a sleep laboratory setting. Indeed, to ensure patients’ comfort, we allowed a trusted relative to sleep in the same room and assigned only female technicians to attend to them. We acknowledge that this safety measure may have influenced PSG results.
4.2. PTSD group: baseline vs. one-year follow-up
PTSD symptomatology, fatigue, and PSQI-A showed significant improvement in the PTSD women at one-year follow-up and reached normal scores. Although, depression and anxiety symptoms improved significantly; the participants still had mild depression and moderate anxiety symptoms. Also, despite improved sleep parameters, poor sleep quality and sub-threshold insomnia were maintained at follow-up. Even though there was an improvement in PTSD symptoms after a one-year follow-up, residual symptoms may be a sustained stress syndrome and contribute to the acceleration of cellular ageing and neuroprogression (Miller, Lin, Wolf, & Miller, 2017).
Interestingly, as defined by PSQI global score, sleep quality was a significant predictor of PTSD improvement. PSQI is one of the most commonly used assessments of sleep and insomnia symptoms. Consistent with our finding, several studies found associations between sleep disturbances and PTSD severity. In a study with PTSD participants, Belleville et al. (2009) assessed the impact of sleep disturbances on PTSD symptom severity and perceived health. The authors reported that sleep quality impacted PTSD symptoms even when the effects of other potential confounding variables were controlled. Germain et al. (2004) studied the influence of patient-related characteristics, disorder-related characteristics, and psychiatric comorbidities on the severity of sleep disturbances in PTSD outpatients. The authors found that poor sleep quality and severe sleep disturbance were a clinically significant component of PTSD regardless of age, gender, psychiatric comorbidity, type of trauma, or PTSD chronicity.
Nevertheless, we found that sleep quality significantly affected young women with PTSD, but not PSG sleep structure. There were no differences in PSG results at baseline and one-year follow-up in the PTSD group. Sleep complaints (measured in the current study by PSQI, PSQI-A, and ISI) were not associated with objective polysomnographic reports in laboratory settings. One possible explanation for this discrepancy may be differences in the environmental context (Germain, Hall, Shear, Nofzinger, & Buysse, 2006; Lipinska & Thomas, 2017). Subjects in the PTSD group may have felt safer in the laboratory than in the home environment, where they are exposed to violence and crime, and they slept better in the laboratory. Another possible explanation is that PSG measurement provides an overview of sleep architecture, and subtle abnormalities may not be detected.
Further analysis of sleep disturbances in PTSD may include other assessments such as power spectral analysis, cyclic alternating pattern, and further research on REM fragmentation, REM density, REM theta, and heart rate variability analysis. Such studies might reveal more subtle differences in objective measures of sleep in PTSD subjects and healthy controls. Future investigations using actigraphy or sleep diaries might also reveal more about the relative time course of sleep patterns and clinical improvement.
There are limitations in this study. First, there was a high drop-out rate. Approximately 12% of the women scheduled for initial evaluations did not attend appointments, 21% dropped out before finishing clinical trial, and 24% dropped out before completing one year of follow-up. The high drop-out rate may be due to sexual violence survivors’ tendency to avoid coming into contact with rape memory, which is one of the symptoms of PTSD. Moreover, sexually assaulted women may be reluctant to seek treatment for their symptoms because they often do not even report the assault to their family due to shame (Foa, Rothbaum, Riggs, & Murdock, 1991). Second, we did not have a control group of women exposed to trauma without PTSD. Trauma exposure is more likely to precipitate a wide range of physiopathological alterations in subjects even when they do not develop PTSD.
Given that our study recruited sexual assault survivors 1 to 6 months after the traumatic event, we chose to recruit a healthy control group to differentiate between the exposure to trauma and development of PTSD and those who did not endure a traumatic event. Furthermore, in a study conducted by Lipinska and Thomas (2017) with three groups of women: PTSD, trauma-exposed non-PTSD, and healthy controls, the PTSD group reported poorer subjective sleep quality, and there was no significant difference in subjective sleep quality between the trauma-exposed non-PTSD group and healthy controls. Regarding objective measures of sleep, the PTSD group only had decreased sleep depth, suggesting that those in the PTSD, trauma-exposed non-PTSD, and healthy control groups had relatively similar sleep patterns when indexed by PSG measures. Third, although reliance on single-night PSG assessment is a limitation in our study, both control and PTSD groups were assessed for one night, allowing the same exposure to the possible effects of a single-night PSG. Polysomnographic studies comparing first-night adaptation effects in PTSD subjects and controls reported mixed findings. Woodward et al. (1996) found that PTSD inpatients had decreased first-night effects compared to outpatient controls. In contrast, PTSD outpatients had enhanced first-night effects compared to outpatient controls, suggesting that adaptation effects observed in PTSD may reflect enhanced sensitivity to a novel environment. Ross et al. (1999) did not find differences in adaptation effects in a sample of outpatient and residential treatment PTSD subjects compared to outpatient controls in a laboratory study. Herbst et al. (2010) examined the presence of first-night effects in PTSD outpatients and controls studied in the home and hospital settings and found that the PTSD outpatient group did not show first-night sleep architecture changes at home or in the hospital.
The fourth limitation of the study was that completer and non-completer PTSD groups differed in age and insomnia severity. One possible explanation is that the PTSD group were low-income participants living in distant neighbourhoods and far from the hospital where they had the clinical appointments. We acknowledge that these drop-outs may have introduced bias in our final results.
Despite these limitations, we believe that our study has many strengths, including a homogeneous population of young female samples who suffered the same traumatic event in the acute and subacute stage of PTSD. Moreover, the study included a clinical follow-up of one year and sleep assessments with subjective and objective measurements using complete in-laboratory PSG recordings.
In conclusion, this study contributes to the sparse literature on sleep abnormalities in female survivors of sexual assault. Our findings demonstrated that young women with PTSD subjectively reported more significant sleep disturbances; however, PSG only found less total sleep time than healthy controls. Nevertheless, sleep quality was significantly associated with PTSD improvement at one-year follow-up, independently of depression and anxiety scores, suggesting that sleep quality is impaired in young women with PTSD and may impact long-term responses to treatment.
Funding Statement
This study was supported by grants from FAPESP [2014/12559-5] and from CNPq [303389/2016-8]. There was financing in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) – [Finance Code 001] and Associação Fundo de Incentivo à Pesquisa (Afip). The clinical trial of this study was registered at Brazilian Clinical Trials, number RBR-3z474z, URL: http://www.ensaiosclinicos.gov.br/rg/RBR-3z474z/. Registration Date: March 24th, 2015.
Disclosure of potential conflicts of interest
The authors have no financial conflicts of interest.
Data availability statement
The data that support the findings of this study are openly available in Mendeley Data at http:dx.doi.org/10.17632/wrtd9cy3rw.1; https://data.mendeley.com/.
Ethics statement
All participants provided written informed consent and were informed in detail about the nature and purpose of the study approved by the Universidade Federal de São Paulo Committee Board (CAAE: 84772918.0.0000.5505), following statement of compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki).
References
- Amorim, P. (2000). Mini International Neuropsychiatric Interview (MINI): validação de entrevista breve para diagnóstico de transtornos mentais. Revista Brasileira de Psiquiatria, 22, 106–12. doi: 10.1590/S1516-44462000000300003 [DOI] [Google Scholar]
- Baglioni, C., Nanovska, S., Regen, W., Spiegelhalder, K., Feige, B., Nissen, C., Reynolds, C. F., & Riemann, D. (2016). Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychological Bulletin, 142, 969–990. doi: 10.1037/bul0000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa Neto, J. B., Germain, A., Mattos, P. F., Serafim, P. M., Santos, R. C. M., Martini, L. C., … Mello, M. F. (2014). Psychometric properties of the Brazilian version of the Pittsburgh Sleep Quality Index Addendum for PTSD (PSQI-A). Revista Brasileira de Psiquiatria, 36, 330–335. doi: 10.1590/1516-4446-2013-1225 [DOI] [PubMed] [Google Scholar]
- Bastien, C. H., Vallières, A., & Morin, C. M. (2001). Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Medicine, 2, 297–307. doi: 10.1016/S1389-9457(00)00065-4 [DOI] [PubMed] [Google Scholar]
- Beck, A., Steer, R., & Brown, G. (1996). BDI-II, Beck depression inventory: Manual. San Antonio, TX: Psychological Corp. [Google Scholar]
- Beck, A. T., Steer, R. A., & Carbin, M. G. (1988). Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review, 8, 77–100. doi: 10.1016/0272-7358(88)90050-5 [DOI] [Google Scholar]
- Belleville, G., Guay, S., & Marchand, A. (2009). Impact of sleep disturbances on PTSD symptoms and perceived health. The Journal of Nervous and Mental Disease, 197, 126–132. doi: 10.1097/NMD.0b013e3181961d8e [DOI] [PubMed]
- Berry, R., Brooks, R., Gamaldo, C., Harding, S., Lloyd, R., & Marcus, C. (2007). The AASM manual for the scoring of sleep and associated events: Rules, terminology and technical specifications (1st ed.). Westchester, IL: American Academy of Sleep Medicine, 2007. Journal of Clinical Sleep Medicine. Academy of Sleep. Med, Darien, IL. [Google Scholar]
- Bertolazi, A. N., Fagondes, S. C., Hoff, L. S.,Pedro, V. D., Barreto, S. S. M., & Johns, M. W. (2009). Validação da escala de sonolência de epworth para uso no Brasil. Jornal Brasileiro de Pneumologia, 35(9), 877–883. doi: 10.1590/S1806-37132009000900009 [DOI] [PubMed] [Google Scholar]
- Bertolazi, A. N., Fagondes, S. C., Hoff, L. S., Dartora, E. G., Da Silva Miozzo, I. C., De Barba, M. E. F., & Menna Barreto, S. S. (2011). Validation of the Brazilian Portuguese version of the Pittsburgh Sleep Quality Index. Sleep Medicine, 12, 70–75. doi: 10.1016/j.sleep.2010.04.020 [DOI] [PubMed] [Google Scholar]
- Burnam, M. A., Stein, J. A., Golding, J. M., Siegel, J. M., Sorenson, S. B., Forsythe, A. B., & Telles, C. A. (1988). Sexual assault and mental disorders in a community population. Journal of Consulting and Clinical Psychology, 56, 843–850. doi: 10.1037/0022-006X.56.6.843 [DOI] [PubMed] [Google Scholar]
- Buysse, D. J., Reynolds, C. F., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28, 193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- Campbell, R., Dworkin, E., & Cabral, G. (2009). An ecological model of the impact of sexual assault on women’s mental health. Trauma, Violence, & Abuse, 10, 225–246. doi: 10.1177/1524838009334456 [DOI] [PubMed] [Google Scholar]
- Cerqueira, D., Lima, R., Bueno, S., Neme, C., Ferreira, H., Coelho, D.,Alves, P., Pinheiro, M., Astolfi, R., Marques, D. (2018). Atlas da violência 2018. Rio de Janeiro: Instituto de Pesquisa Econômica Aplicada; Fórum Brasileiro de Segurança Pública. [Google Scholar]
- Coimbra, B. M., Yeh, M., D’Elia, A. T., Maciel, M. R., Carvalho, C. M., Milani, A. C., … Mello, M. F. (2020). Posttraumatic stress disorder and neuroprogression in women following sexual assault: Protocol for a randomized clinical trial evaluating allostatic load and aging process acceleration. JMIR Research Protocols, 9, e19162. doi: 10.2196/19162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha, J. (2001). Manual da versão em português das Escalas Beck. São Paulo: Casa do Psicólogo. [Google Scholar]
- Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41, 1149–1160. doi: 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Flachenecker, P., Kümpfel, T., Kallmann, B., Gottschalk, M., Grauer, O., Rieckmann, P., … Toyka, K. V. (2002). Fatigue in multiple sclerosis: A comparison of different rating scales and correlation to clinical parameters. Multiple Sclerosis (Houndmills, Basingstoke, England), 8, 523–526. doi: 10.1191/1352458502ms839oa [DOI] [PubMed] [Google Scholar]
- Flemons, W. W., Buysse, D., Redline, S., Oack, A., Strohl, K., Wheatley, J., … Romaniuk, J. (1999). Sleep-related breathing disorders in adults: Recommendations for syndrome definition and measurement techniques in clinical research. Sleep. doi: 10.1093/sleep/22.5.667 [DOI] [PubMed]
- Foa, E. B., Rothbaum, B. O., Riggs, D. S., & Murdock, T. B. (1991). Treatment of posttraumatic stress disorder in rape victims: A comparison between cognitive-behavioral procedures and counseling. Journal of Consulting and Clinical Psychology, 59, 715–723. doi: 10.1037/0022-006X.59.5.715 [DOI] [PubMed] [Google Scholar]
- Foa, E. B., Zoellner, L. A., Feeny, N. C., Hembree, E. A., & Alvarez-Conrad, J. (2002). Does imaginal exposure exacerbate PTSD symptoms? Journal of Consulting and Clinical Psychology, 70, 1022–1028. doi: 10.1037//0022-006x.70.4.1022 [DOI] [PubMed] [Google Scholar]
- Frans, O., Rimmo, P.-A., Aberg, L., & Fredrikson, M. (2005). Trauma exposure and post-traumatic stress disorder in the general population. Acta psychiatrica Scandinavica, 111, 290–291. doi: 10.1111/j.1600-0447.2004.00463.x [DOI] [PubMed] [Google Scholar]
- Germain, A. (2013). Sleep disturbances as the hallmark of PTSD: Where are we now? The American Journal of Psychiatry. doi: 10.1176/appi.ajp.2012.12040432 [DOI] [PMC free article] [PubMed]
- Germain, A., Buysse, D. J., Shear, M. K., Fayyad, R., & Austin, C. (2004). Clinical correlates of poor sleep quality in posttraumatic stress disorder. Journal of Traumatic Stress, 17, 477–484. doi: 10.1007/s10960-004-5796-6 [DOI] [PubMed]
- Germain, A., Hall, M., Krakow, B., Katherine Shear, M., & Buysse, D. J. (2005). A brief sleep scale for posttraumatic stress disorder: Pittsburgh Sleep Quality Index Addendum for PTSD. Journal of Anxiety Disorders, 19, 233–244. doi: 10.1016/j.janxdis.2004.02.001 [DOI] [PubMed] [Google Scholar]
- Germain, A., Hall, M., Shear, M. K., Nofzinger, E. A., & Buysse, D. J. (2006). Ecological study of sleep disruption in PTSD: A pilot study. Annals of the New York Academy of Sciences, 1071, 438–441. doi: 10.1196/annals.1364.038 [DOI] [PubMed] [Google Scholar]
- Gomes-Oliveira, M. H., Gorenstein, C., Neto, F. L., Andrade, L. H., & Wang, Y. P. (2012). Validation of the Brazilian Portuguese Version of the Beck Depression Inventory-II in a community sample. Revista Brasileira De Psiquiatria, 34, 389–394. doi: 10.1016/j.rbp.2012.03.005 [DOI] [PubMed] [Google Scholar]
- Habukawa, M., Uchimura, N., Maeda, M., Kotorii, N., & Maeda, H. (2007, November 15). Sleep findings in young adult patients with posttraumatic stress disorder. Biological Psychiatry, 62(10), 1179–1182. doi: 10.1016/j.biopsych.2007.01.007 [DOI] [PubMed] [Google Scholar]
- Herbst, E., Metzler, T. J., Lenoci, M., McCaslin, S. E., Inslicht, S., Marmar, C. R., & Neylan, T. C. (2010). Adaptation effects to sleep studies in participants with and without chronic posttraumatic stress disorder. Psychophysiology, 47, 1127–1133. doi: 10.1111/j.1469-8986.2010.01030.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns, M. W. (1991). A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep, 14, 540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- Jonas, D. E., Amick, H. R., Feltner, C., PalmieriWeber, R., Arvanitis, M., Stine, A., … Harris, R. P. (2017). Screening for obstructive sleep apnea in adults evidence report and systematic review for the US preventive services task force. JAMA: The Journal of the American Medical Association. doi: 10.1001/jama.2016.19635 [DOI] [PubMed]
- Kobayashi, I., Boarts, J. M., & Delahanty, D. L. (2007). Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology, 44, 660–669. doi: 10.1111/j.1469-8986.2007.537.x [DOI] [PubMed] [Google Scholar]
- Lipinska, G., & Thomas, K. G. F. (2017). Better sleep in a strange bed? Sleep quality in South African women with posttraumatic stress disorder. Frontiers in Psychology, 8. doi: 10.3389/fpsyg.2017.01555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz, J. C. (2016). Interpersonal psychotherapy for posttraumatic stress disorder. New York: Oxford University Press. [Google Scholar]
- Markowitz, J. C., Neria, Y., Lovell, K., Van Meter, P. E., & Petkova, E. (2017). History of sexual trauma moderates psychotherapy outcome for posttraumatic stress disorder. Depression and Anxiety, 34, 692–700. doi: 10.1002/da.22619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, T. A., & Hipolito, M. M. S. (2006). Sleep disturbances in the aftermath of trauma and posttraumatic stress disorder. CNS Spectrums, 11(8), 611–615. doi: 10.1017/S1092852900013663 [DOI] [PubMed] [Google Scholar]
- Mellman, T. A., Kobayashi, I., Lavela, J., Wilson, B., & Hall Brown, T. S. (2014). A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep, 37, 1321–1326. doi: 10.5665/sleep.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M. W., Lin, A. P., Wolf, E. J., & Miller, D. R. (2017). Oxidative stress, inflammation, and neuroprogression in chronic PTSD. Harvard Review of Psychiatry, 26, 1. doi: 10.1097/HRP.0000000000000167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin, C. M. (1993). Insomnia: Psychological assessment and management. New York: Guilford Press. [Google Scholar]
- Neylan, T. C., Otte, C., Yehuda, R., & Marmar, C. R. (2006). Neuroendocrine regulation of sleep disturbances in PTSD, in: Annals of the New York academy of sciences. Psychobiology of Posttraumatic Stress Disorder: A Decade of Progress, 1071(1), 203–215. doi: 10.1196/annals.1364.015 [DOI] [PubMed] [Google Scholar]
- Oliveira-Watanabe, T. T., Ramos-Lima, L. F., Santos, R. C., Mello, M. F., & Mello, A. F. (2019). The Clinician-Administered PTSD Scale (CAPS-5): Adaptation to Brazilian Portuguese. Revista Brasileira de Psiquiatria, 41, 92–93. doi: 10.1590/1516-4446-2018-0136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otte, C., Lenoci, M., Metzler, T., Yehuda, R., Marmar, C. R., & Neylan, T. C. (2007). Effects of metyrapone on hypothalamic-pituitary-adrenal axis and sleep in women with post-traumatic stress disorder. Biological Psychiatry, 61, 952–956. doi: 10.1016/j.biopsych.2006.08.018 [DOI] [PubMed]
- Pavan, K., Schmidt, K., Marangoni, B., Mendes, M. F., Tilbery, C. P., & Lianza, S. (2007). Esclerose múltipla: adaptação transcultural e validação da escala modificada de impacto de fadiga. Arquivos de Neuro-Psiquiatria, 65, 669–673. doi: 10.1590/s0004-282x2007000400024 [DOI] [PubMed] [Google Scholar]
- Richards, A., Metzler, T. J., Ruoff, L. M., Inslicht, S. S., Rao, M., Talbot, L. S., & Neylan, T. C. (2013). Sex differences in objective measures of sleep in post-traumatic stress disorder and healthy control subjects. Journal of Sleep Research, 22, 679–687. doi: 10.1111/jsr.12064 [DOI] [PMC free article] [PubMed]
- Ross, R. J., Ball, W. A., Sanford, L. D., Morrison, A. R., Dinges, D. F., Silver, S. M., Kribbs, N. B., Mulvaney, F. D., Gehrman, P. R., & McGinnis, D. E. (1999). Rapid eye movement sleep changes during the adaptation night in combat veterans with posttraumatic stress disorder. Biological Psychiatry, 45(7), 938–941. doi: 10.1016/s0006-3223(98)00233-9 [DOI] [PubMed] [Google Scholar]
- Ross, R. J., Ball, W. A., Sullivan, K. A., & Caroff, S. N. (1989). Sleep disturbance as the Hallmark of posttraumatic stress disorder. The American Journal of Psychiatry, 146, 128-a-129. doi: 10.1176/ajp.146.6.697 [DOI] [PubMed] [Google Scholar]
- Weathers, F. W., Bovin, M. J., Lee, D. J., Sloan, D. M., Schnurr, P. P., Kaloupek, D. G., … Marx, B. P. (2018). The Clinician-Administered PTSD Scale for DSM–5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychological Assessment, 30, 383–395. doi: 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward, S. H., Bliwise, D. L., Friedman, M. J., & Gusman, F. D. (1996). First night effects in post-traumatic stress disorder inpatients. Sleep, 19(4), 312–317. doi: 10.1093/sleep/19.4.312 [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) . (2005). WHO multi-country study on women’s health and domestic violence against women. Geneva: Initial results on Prevalence, Health Outcomes and Women’s Responses. [Google Scholar]
- Zhang, Y., Ren, R., Sanford, L. D., Yang, L., Zhou, J., Zhang, J., … Tang, X. (2019). Sleep in posttraumatic stress disorder: A systematic review and meta-analysis of polysomnographic findings. Sleep Medicine Reviews, 48, 101210. doi: 10.1016/j.smrv.2019.08.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in Mendeley Data at http:dx.doi.org/10.17632/wrtd9cy3rw.1; https://data.mendeley.com/.


