Abstract
Background
Telemedicine (TM) programs can be implemented to deliver specialty care through virtual platforms and overcome geographic/resource constraints. Few data exist to describe outcomes associated with TM-based infectious diseases (ID) management. The purpose of this study was to compare outcomes associated with TM and onsite standard-of-care (SOC) ID consultation after implementation of an antimicrobial stewardship (AMS)-led Staphylococcus aureus bacteremia (SAB) bundle.
Methods
A retrospective cohort study was conducted on the effects of a SAB bundle comparing ID consult delivery (SOC or TM) at 10 US hospitals within Atrium Health in adult patients admitted from September 2016 through December 2017. The type of ID consult provided was based on the admitting hospital; no hospital had both modalities. Bundle components included the following: (1) ID consult, (2) appropriate antibiotics, (3) repeat blood cultures until clearance, (4) echocardiogram obtainment, and (5) appropriate antibiotic duration. The AMS facilitated bundle initiation and compliance. The primary outcome was bundle adherence between groups. Differences in clinical outcomes were also assessed.
Results
We evaluated 738 patients with SAB (576 with SOC, 162 with TM ID). No differences were observed in overall bundle adherence (SOC 86% vs TM 89%, P = .33). In addition, no significant differences resulted between groups for hospital mortality, 30-day SAB-related readmission, persistent bacteremia, and culture clearance. Groups did not differ in 30-day mortality when controlling for demographics, bacteremia source, and physiological measures with multivariable logistic regression.
Conclusions
Our findings provide evidence to support effective use of TM ID consultation and AMS-led care bundles for SAB management in resource-limited settings.
Keywords: antimicrobial stewardship, bacteremia, infectious diseases, Staphylococcus aureus, telemedicine
The use of infectious diseases telemedicine for the management of Staphylococcus aureus bacteremia resulted in similar treatment outcomes compared with traditional onsite consultation supporting the ongoing expansion of telemedicine services using antimicrobial stewardship support in hospitals with limited resources.
Staphylococcus aureus bacteremia (SAB) is an increasingly common infection associated with significant morbidity, mortality, and healthcare costs [1–4]. Several components of SAB care have been described in the literature to augment treatment in patients with SAB; often these components are compiled and implemented through a “care bundle” to systematically facilitate optimal infection-related patient care while ensuring accountability. Because proper care of SAB is complex, adherence to components is paramount to outcomes, which makes antimicrobial stewardship (AMS) programs ideally positioned to help facilitate SAB care. Several studies have demonstrated a significant increase in adherence of quality performance measures from AMS-led SAB care bundles, in collaboration with infectious diseases (ID) consultation, at both large, academic tertiary centers and community hospitals [5–7].
Infectious diseases specialists have been shown to improve identification of infectious foci and disseminated disease, promote adherence to best practices, as well as reduce mortality and shorten hospital length of stay [8–12]. Despite the advantages of these services to improve care of patients with SAB, some institutions lack the resources and funding to support availability of consistent ID consultation services and AMS resources. In these situations, delivering ID consultation by telemedicine (TM) platforms may be a viable option to provide expanded, specialized patient care. Telemedicine programs have been effectively implemented to deliver care through virtual platforms to overcome geographic and resource constraints [13]. Improved communication technologies and patient acceptance have allowed expansion of TM services nationwide, offering more timely and convenient services [14–16]. Although not intended to overshadow in-person care, TM can provide specific services that may otherwise not be available onsite and is expected to offer added benefit compared with no specialty care at all.
The use of TM to deliver ID services has been endorsed by the Infectious Diseases Society of America (IDSA), with aims to increase access to ID specialty care and improve patient outcomes [14]. To date, research on TM-based management of patients with ID has focused on human immunodeficiency virus, tuberculosis, outpatient parenteral antimicrobial therapy (OPAT) management, and, most recently, communicable diseases such as coronavirus [13, 14, 17]. No data currently exist regarding management of SAB from sites using TM for formal ID consultation. Accordingly, the purpose of this study is to compare SAB bundle adherence and other clinical outcomes between 2 ID consultation delivery modes, TM and traditional in-person care, after implementation of a system-wide AMS-led care bundle.
METHODS
Study Design
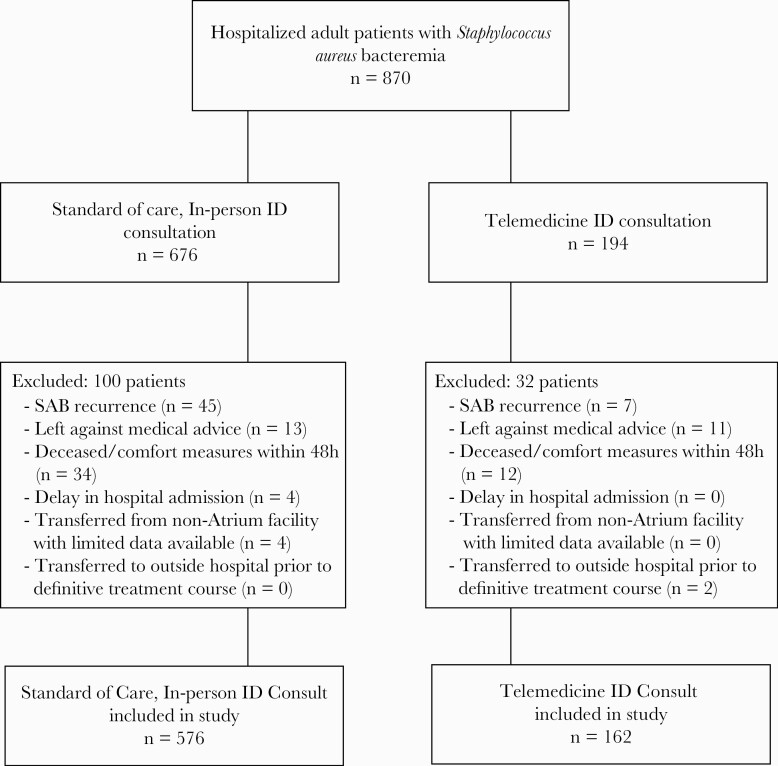
This was a multicenter, retrospective cohort study that included adult hospitalized patients, 18 years and older, admitted to one of the 10 adult acute care Charlotte, NC-based Atrium Health hospitals, or Atrium Health Primary Enterprise (AH-PE), with SAB from September 2016 through December 2017. Patients admitted with SAB received an ID consult either onsite or via TM, depending on the hospital where they were admitted; no hospital provided both ID delivery services. Of the 870 patients who were admitted, 676 patients were admitted into one of the 5 hospitals with onsite ID consultants and received standard-of-care (SOC) in-person ID consultation, 194 patients were admitted to one of the 5 hospitals without an onsite ID consultation and received a TM ID consultation. Bed sizes of each hospital are summarized in Table 2. Based on 2017 admission data (excluding observation stays), hospitals that provided TM ID consults ranged from approximately 2700 to 9800 annual inpatient admissions compared with approximately 7900–52 000 annual inpatient admissions for facilities that provided onsite ID care. Only the patient’s first SAB occurrence during each study period was included in the primary analysis. Patients who left against medical advice, passed away, or transitioned to comfort care within 48 hours of index blood culture, transferred from an outside hospital (OSH) with limited available data, or were transferred to an OSH before definitive treatment course was determined by an ID provider were excluded, leaving 576 patients receiving SOC and 162 receiving TM ID consults (see Figure 1). The SAB bundle was implemented in both groups.
Table 2.
Characteristics of Study Cohort by ID Consult Strategy
| Characteristics | All Patientsa N = 738 |
TM n = 162 |
SOC n = 576 |
P Value |
|---|---|---|---|---|
| Age, years | <.01 | |||
| Median (IQR)b | 58 (45–70) | 62 (50–76) | 57 (43–68) | |
| Gender | .09 | |||
| Male | 475 (64) | 95 (59) | 380 (66) | |
| Female | 263 (36) | 67 (41) | 196 (34) | |
| Race | .01 | |||
| White | 512 (69) | 129 (80) | 383 (66) | |
| Black | 184 (25) | 28 (17) | 156 (27) | |
| Other | 42 (6) | 5 (3) | 37 (6) | |
| Charlson Comorbidity Index | ||||
| Median (IQR)b | 2 (0–5) | 3 (1–5) | 2 (0–5) | .15 |
| History of intravenous drug use | 85 (12) | 17 (10) | 68 (12) | .64 |
| Admitting Hospital (Location, Bed Size) | ||||
| Hospital A (urban, 1132 beds) | - | - | 209 (36) | |
| Hospital B (urban, 173 beds) | - | - | 83 (14) | |
| Hospital C (urban, 457 beds) | - | - | 144 (25) | |
| Hospital D (urban, 235 beds) | - | - | 98 (17) | |
| Hospital E (urban, 100 beds) | - | - | 42 (7) | |
| Hospital F (rural, 241 beds) | - | 45 (28) | - | |
| Hospital G (rural, 67 beds) | - | 6 (4) | - | |
| Hospital H (rural, 101 beds) | - | 31 (19) | - | |
| Hospital I (rural, 109 beds) | - | 17 (10) | - | |
| Hospital J (suburban, 175 beds) | - | 63 (39) | - | |
| Source of Bacteremia | .26 | |||
| Endovascular | 17 (2) | 3 (2) | 14 (2) | |
| Respiratory | 59 (8) | 20 (12) | 39 (7) | |
| Gastrointestinal | 12 (2) | 3 (2) | 9 (2) | |
| Genitourinary | 11 (1) | 2 (1) | 9 (2) | |
| Skin and soft tissue | 284 (39) | 54 (34) | 230 (40) | |
| Bone/joint | 84 (11) | 18 (11) | 66 (11) | |
| Catheter/hardware | 127 (17) | 24 (15) | 103 (18) | |
| Other | 73 (10) | 16 (10) | 57 (10) | |
| Unknown | 71 (10) | 22 (13) | 49 (9) | |
| MRSA bacteremia | 312 (42) | 83 (51) | 229 (40) | .01 |
| Complicated bacteremia | 498 (68) | 96 (60) | 402 (70) | .04 |
| Laboratory and Physiologic Measures | ||||
| Lactate ≥2 mmol/L | 234 (32) | 55 (34) | 179 (31) | .49 |
| WBC >12/µL or <4/µL | 473 (64) | 104 (64) | 369 (64) | .97 |
| Temperature >38°C or <36°C | 167 (23) | 47 (29) | 120 (21) | .03 |
| Quick SOFA ≥2 | 80 (11) | 19 (12) | 61 (11) | .34 |
| SBP ≤100 mmHg | 99 (13) | 24 (15) | 75 (13) | .55 |
| Respiratory rate ≥22 | 162 (22) | 39 (24) | 123 (21) | .46 |
| GCS <15 | 143 (19) | 32 (20) | 111 (19) | .89 |
| BCID use | 474 (64) | 96 (59) | 378 (66) | .14 |
| Hours to ID Consult | ||||
| Median (IQR)b | 23 (14–33) | 26 (18–37) | 22 (13–32) | <.01 |
| Hours to Gram stain | ||||
| Median (IQR)b | 19 (15–23) | 20 (16–25) | 18 (15–23) | .02 |
Abbreviations: BCID, Verigene blood culture identification; CCI, Charlson comorbidity index; GCS, Glasgow Coma Scale; ID, infectious diseases; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; SBP, systolic blood pressure; SOC, standard of care; SOFA, sequential organ failure assessment; TM, telemedicine; WBC, white blood cell.
aDenominator reflects 27 patients excluded due to death before measurement time frame.
bTable values represent n (%) for each group based on χ 2 tests, unless otherwise noted.
Figure 1.
CONSORT Diagram for Study Design. ID, infectious diseases; SAB, Staphylococcus aureus bacteremia.
Study Intervention
Atrium Health has had a robust AMS program since 2013, the Antimicrobial Support Network (ASN), that covers 10 hospitals in AH-PE. By late 2016, all 10 acute inpatient hospitals were able to receive ID consultation either onsite (SOC) or using a TM platform. Telemedicine was offered by live audio-video consultation at least thrice weekly (eg, Monday, Wednesday, and Friday). Computer carts with attached cameras were used to complete TM and operated by nurses onsite using VidyoConnect (Vidyo, Hackensack, NJ). An AGNES-Connect (AMD Global Telemedicine, Chelmsford, MA) stethoscope was used with nurse assistance to complete physical exams; other parts of the physical exam were completed visually via camera. All ID providers worked within the Atrium Health ID division and rotated between hospitals, providing consultation by both delivery platforms.
A system-wide, evidence-based, AMS-led SAB bundle was launched in September 2016 at the AH-PE. Components of the SAB bundle are summarized in Table 1. All patients admitted to any AH-PE hospital with SAB required an ID consult. During the study period, initiation of the SAB bundle was completed by an ASN pharmacist on first shift (7:00 am–4:30 pm), notifying the oncall ID provider of a new blood culture result after real-time notification from the microbiology laboratory. Further communication to the primary team was completed by an ASN pharmacist regarding required ID consultation and any pertinent preliminary recommendations (eg, antibiotic changes). Aside from helping initiate an ID consult, ASN pharmacists assisted with antibiotic optimization (eg, streamline to anti-staphylococcal beta-lactam for methicillin-sensitive S aureus or vancomycin for methicillin-resistant S aureus [MRSA], suggest alternative/combination therapy when deemed appropriate), along with completion of allergy assessments as needed and ordering of repeat blood cultures until culture clearance. Second shift coverage (4:30 pm–10:30 pm) was primarily provided by critical care pharmacists with AMS training that worked virtually to cover all AH-PE facilities during the study period on weekdays and ASN pharmacists on weekends and holidays; only antibiotic escalations (eg, addition of new antibiotic such as intravenous vancomycin) were completed during this time, if warranted. Cultures that returned positive during third shift (10:30 pm–7:00 am) were identified via a clinical surveillance system, Theradoc (Premier Healthcare Solutions, Inc., Charlotte, NC), and addressed by the ASN team the following day. An official ID consultation typically followed within 24 to 48 hours of initial positive culture result. In situations in which a formal consult could not be completed during this time frame, ID physicians communicated recommendations directly with the primary team and documented them in the electronic medical record (EMR); patients were seen formally by an ID physician within 72 hours in these cases. Daily patient monitoring was completed by the ASN team and ID providers to ensure timely completion of the SAB bundle components.
Table 1.
SAB Bundle Components
| 1. Required ID consultation |
| 2. Appropriate IV antibiotics within 24 hours of positive Staphylococcus aureus BCID resulta |
| - IV vancomycin for MRSA bacteremia, unless severe allergy documented or with persistent bacteremia requiring combination/alternative therapy |
| - IV beta-lactam therapy (eg, preferably cefazolin or nafcillin) for MSSA bacteremia, unless severe allergy documented |
| 3. Repeat blood cultures every 48–72 hours until clearance is documented |
| 4. Obtainment of an echocardiogramb |
| 5. Appropriate duration of therapy (from documented clearance of bacteremia) |
| - Uncomplicated bacteremia: ≥2 weeks IV therapy |
| - Complicated bacteremia: ≥4 weeks IV therapy |
Abbreviations: BCID, blood culture identification; ID, infectious diseases; IV, intravenous; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S aureus; SAB, S aureus bacteremia.
aBefore implementation of Verigene BCID at Atrium Health in early 2017, there were 6 total SAB bundle components. Bundle component 2 (appropriate empiric IV antibiotics within 24 hours of positive S aureus blood culture) and 3 (appropriate definitive IV antibiotics within 24 hours of sensitivity results) were consolidated to the second bundle component described above.
bType of echocardiogram obtained was at the discretion of the ID physician.
Access to medical testing and specialties were similar between hospitals, although there were some differences pertaining to cardiac services. All but 2 hospitals (rural, only with ID TM consultation services) were able to complete transesophageal echocardiograms (TEEs). Cardiac thoracic surgery specialists were only available at 3 large, urban hospitals where ID consultation care was provided onsite. If additional specialty services were needed and not offered at their facility, interhospital transfers were completed to another hospital within AH-PE, which were most frequently to urban hospitals with onsite ID consultation. All facilities provided vascular access to facilitate OPAT, and the ID team was primarily responsible at both onsite and TM ID consultation sites for OPAT prescription and follow-up.
Microbiology
Microbiology services were available at each individual site. Further speciation and susceptibility testing were performed at a centralized laboratory within Atrium Health. The MicroScan WalkAway (Beckman Coulter, Pasadena, CA) automated system was used for susceptibility testing. In early 2017, Verigene Blood Culture Identification (BCID) (Nanosphere, Inc., Northbrook, IL) results, which included detection of S aureus and the mecA gene from blood culture broths, became available to all 10 Atrium Health hospitals included in the study.
Data and Outcomes
Baseline demographics and comorbidities, as summarized by the Charlson comorbidity index, were collected [18]. Quick sequential organ failure assessment (qSOFA) score for sepsis was used to assess clinical severity at time of presentation [19]. These laboratories (eg, Glasgow Coma Scale, respiratory rate, systolic blood pressure) were based on initial values upon admission, with maximal values recorded for other laboratories such as lactate and white blood count within the first 24 hours. Other data collected included bacteremia source, microbiology data, along with other specific data points needed for assessment of ID consultation delivery method effects. A portion of the data was extracted from a REDCap data management system that was collected prospectively for the purpose of an AMS patient quality initiative (eg, bundle component adherence, bacteremia source, microbiology data). Remaining data were retrieved directly from the EMR. Patients could be transferred to other hospitals within AH-PE, if needed; in these cases, the assigned hospital for analysis was based on their admitting hospital.
The primary outcome of interest was overall SAB bundle adherence, comparing the 2 modes of ID consultation delivery, SOC versus TM. Separate bundle component adherence rates were also assessed. Adherence was calculated based on treatment requirements for each patient. The final component of the care bundle, appropriate duration of therapy, was based on bacteremia severity, which was classified as uncomplicated or complicated. Uncomplicated bacteremia was defined as the exclusion of endocarditis, no implanted prosthesis, negative follow-up blood cultures drawn within 96 hours of initial set, defervescence within 72 hours of therapy, no evidence of metastatic site infection, and catheter removal if catheter-associated infection [20–22]. Patients who did not meet criteria for uncomplicated bacteremia were considered to have complicated bacteremia. At least 2 weeks of intravenous therapy was recommended for patients with uncomplicated bacteremia compared with a minimum of 4 weeks in patients with complicated bacteremia. Bundle nonadherence was reviewed by a small clinical group of pharmacists and physicians, and continuing education/feedback was provided to AMS and ID teams. Further adjudication was performed by 3 separate ID providers by majority vote, specifically for patients that did not receive recommended durations of therapy based on study definitions for complicated and uncomplicated bacteremia.
Secondary outcomes of interest included in-hospital mortality, 30-day all-cause mortality, 30-day SAB-related readmission rates, incidence of persistent bacteremia, and days to culture clearance. Several time-to-analyses were completed with the initial blood culture date representing time zero for most calculations (eg, time to ID consult, time to Gram stain). The initial positive blood culture was the reference used to determine 30-day all-cause mortality. Date of discharge was the reference used to analyze 30-day SAB-related readmissions. The SAB-related readmissions were defined as rehospitalizations that were due to progressive and/or disseminated SAB, re-emergence of positive blood cultures with S aureus, antibiotic-related adverse effects and/or due to OPAT complications; planned readmissions for source control procedures related to SAB episode were not included. Secondary review of all SAB-related readmissions was performed by 3 separate ID providers, as well. Persistent bacteremia was defined as positive blood culture for 7 days or more. Days to culture clearance was calculated as the difference between the first negative blood culture set and initial positive blood culture with S aureus.
Patient Consent Statement
The study was approved by the Institutional Review Board (IRB) at Atrium Health and with a waiver for informed consent (IRB no. 03-17-08E). Due to the study design, the requirement for signed patient consent was waived.
Statistical Analysis
Standard descriptive statistics were used to compare demographic characteristics between the SOC and TM groups. Continuous variables are reported as medians with interquartile range when appropriate and compared using t test or Wilcoxon Mann-Whitney and analysis of variance tests to assess differences between the SOC and TM groups. Categorical and binary variables are presented as percentages and compared using χ 2 or Fisher’s exact test. A follow-up test of differences in 30-day mortality between the SOC and TM groups was conducted using multivariable logistic regression models and are presented in adjusted odds ratios with 95% confidence interval (CI). P values less than .05 were considered significant. All analyses were conducted using SAS software (SAS Institute Inc., Cary, NC).
RESULTS
We studied 738 patients with SAB, 576 patients who received SOC and 162 receiving TM ID consults. Demographic characteristics were similar between the SOC and TM groups, with the exception of age and race (see Table 2). Patients in the TM were significantly older and more commonly white. The groups did not differ in baseline comorbidity or the source of bacteremia; however, they differed in blood stream infections categorized as complicated and caused by MRSA. Of 312 patients who had a positive MRSA culture (42%), 229 were in the SOC group (40%) and 83 were in the TM group (51%). The SOC group had a higher proportion of complicated bacteremia than the TM group (70% vs 60%, P = .04). Finally, the groups significantly differed in hours to ID consult (TM 26 hours, SOC 22 hours) and hours to Gram stain (TM 20 hours, SOC 18 hours), with the SOC group showing fewer median hours to both than the TM group (see Table 2).
Comparing adherence rates for the SOC and TM groups, no differences resulted between the 2 groups in overall bundle adherence (86% vs 89%, respectively; P = .33), as well as in adherence for each component of the bundle (see Table 3). The type of echocardiogram prescribed was based on the discretion of the ID attending, either obtainment of a transthoracic echocardiogram (TTE) or TEE fulfilled the fourth bundle component. A TTE was most prescribed. Approximately one quarter of patients underwent a TEE (199 of 738 patients or 27%), with 40 patients (25%) in the TM group and 159 patients (28%) in the SOC group (P = .46). The most common reason for nonadherence in both groups was lack of repeat blood cultures within 72 hours of last positive culture. Analysis of the clinical outcomes showed that the SOC and TM groups had similar rates of hospital mortality, 30-day SAB-related readmission, persistent bacteremia, and time to culture clearance (see Table 3). The only clinically meaningful difference observed between the groups was in 30-day mortality, with the TM group showing higher mortality rates than the SOC group (17% vs 11%, P = .08). To assess whether this difference was due to the form of ID consultation or other variables, a follow-up multivariable analysis was conducted (see Table 4). When controlling for demographic, bacteremia source, and physiological (eg, lactate, white cell count, qSOFA, etc) data, the SAB bundle delivery strategy was not associated with 30-day mortality rates (odds ratio = 1.08; 95% CI, 0.62–1.87; P = .79). Age (per 5-year increase) was an independent risk factor for 30-day mortality, as was lactate levels above 2 mmol/L, white blood cell counts greater than 12/µL or less than 4/µL, and qSOFA score equal to or greater than 2. Accordingly, the method of ID consultation was not associated with significant differences in clinical outcomes for those with SAB.
Table 3.
Bundle Adherence and Clinical Outcomes by ID Consult Strategy
| n (%) | All Patientsa N = 738 |
TM n = 162 |
SOC n = 576 |
P Value |
|---|---|---|---|---|
| Bundle Adherence | .33 | |||
| Overall bundle adherence | 639 (86.6) | 144 (88.9) | 495 (85.9) | |
| BC 1: ID consult | 738 (100) | 162 (100) | 576 (100) | |
| BC 2: Appropriate antibiotics | 734 (99.5) | 162 (100) | 572 (99.3) | |
| BC 3: Repeat blood cultures | 683 (92.5) | 152 (93.8) | 531 (92.1) | |
| BC 4: Echocardiogram | 724 (98.1) | 159 (98.1) | 565 (98.1) | |
| BC 5: Appropriate antibiotic duration | 630 (85.4) | 135 (83.3) | 495 (85.9) | |
| Clinical Events | ||||
| Hospital mortality | 57 (8) | 14 (9) | 43 (7) | .62 |
| 30-day mortality | 91 (12) | 27 (17) | 64 (11) | .08 |
| 30-day SAB-related readmission | 39 (5) | 7 (4) | 32 (6) | .53 |
| Persistent bacteremia | 78 (11) | 18 (11) | 60 (11) | .82 |
| Days to culture clearance, median (IQR)b | 3 (2–5) | 3 (2–4) | 3 (2–5) | .94 |
Abbreviations: BC, bundle component; ID, infectious diseases; IQR, interquartile range; SAB, Staphylococcus aureus bacteremia; SOC, standard of care; TM, telemedicine.
aDenominator reflects 27 patients excluded due to death before measurement time frame.
bTable values represent n (%) for each group based on χ 2 tests, unless otherwise noted.
Table 4.
Multivariable Logistic Regression Models Evaluating Associations Between Telemedicine ID Consultation and 30-Day Mortality
| Adjusted | ||
|---|---|---|
| Variable | OR (95% CI) | P Value |
| 30-Day Mortality | ||
| SAB Bundle Delivery Strategy | ||
| SOC | Reference | .79 |
| TM | 1.08 (0.62–1.87) | |
| Age | 1.04 (1.02–1.05) | <.01 |
| Gender | ||
| Female | Reference | |
| Male | 1.02 (0.62–1.69) | .93 |
| Race | ||
| White | Reference | |
| Black | 0.80 (0.44–1.44) | .53 |
| Other | 0.36 (0.08–1.57) | .22 |
| Comorbidity score | 1.04 (0.97–1.12) | .32 |
| Bacteremia Source | ||
| Skin and Soft Tissue | Reference | |
| Respiratory | 1.19 (0.48–2.98) | .86 |
| Bone/Joint | 0.87 (0.34–2.21) | .29 |
| Catheter/Hardware | 1.77 (0.85–3.65) | .22 |
| Other | 1.80 (0.98–3.29) | .10 |
| MRSA | 1.39 (0.86–2.24) | .18 |
| Lactate ≥2 mmol/L | 2.23 (1.40–3.71) | <.01 |
| WBC >12/µL or <4/µL | 1.59 (0.92–2.75) | .09 |
| Temperature >38°C or <36°C | 1.09 (0.63–1.90) | .76 |
| qSOFA ≥2 | 1.65 (1.19–2.29) | <.01 |
Abbreviations: CI, confidence interval; ID, infectious diseases; MRSA, methicillin-resistant Staphylococcus aureus; OR, odds ratio; qSOFA, quick sequential organ failure assessment; SAB, S aureus bacteremia; SOC, standard of care; TM, telemedicine; WBC, white blood cell.
DISCUSSION
Patients with SAB are vulnerable to complications and increased morbidity and mortality. Treatment is complex and requires many different care components to improve outcomes. Although successful treatment of SAB is increased through ID consultations and AMS that improve identification of infectious foci and increase adherence to care components, not all treatment facilities have access to these resources. We found that the use of virtual TM ID consultation may bridge the gap, providing access to specialty care and as a result provide patient outcomes that are not significantly different from similar onsite ID consultation.
With approximately half of US hospitals without access to ID consultations, the use of TM consultation could be an alternative option, avoiding the need to transfer the patient to another hospital and likely minimize delays in care [23]. The results of our cohort add to available literature by further supporting TM use to provide specialty care to underserved patient populations. Although a bulk of current data reports patient satisfaction around TM, we were able to demonstrate similar clinical outcomes for a complex clinical scenario independent of the mode of ID consultation [16, 24, 25]. In addition to clinical outcomes, we identified that adherence to the SAB bundle was not different by mode of care delivery. This highlights the ability to provide consistency in ID care under this model.
A unique aspect to our delivery of TM ID services included the collaboration with our AMS program, which aided seamless follow-up on days TM ID services were not provided. The AMS pharmacists were vital for successful implementation of the SAB bundle, most often facilitating ID consultation initiation, appropriate initial antibiotics, and timely repeat blood cultures. With AMS services becoming required by the Centers for Medicare and Medicaid Services, our approach with partnering with our AMS program can be adopted by other sites establishing new TM ID services.
Our study accompanies several limitations that allow for future research. The retrospective design of our cohort is nonrandomized. The presented analysis attempts to correct for demographic differences seen between groups, but the possibility remains that other nonrandom factors have not been assessed. Although our study showed no meaningful differences in short-term clinical outcomes for SAB treatment under TM ID, conclusions cannot be extended to other TM ID treatments conditions, TM without AMS support, or long-term treatment outcomes. Further validation of ID TM care for treatment of SAB and other ID treatment conditions should be considered.
More than 50% of our cohort was defined as a complicated SAB. Subgroup analysis of specific complications were not feasible with our sample size. Therefore, we are unable to determine any subpopulation differences in SAB care under TM. Endovascular sources of bacteremia only accounted for 2% in each group; however, specific diagnoses of disseminated SAB (eg, endocarditis) were not collected. The TM group comprised higher rates of MRSA, as well as longer time to Gram stain and ID consultation. Patients in the SOC group had higher rates of complicated SAB. These differences were likely due to different patient populations at the specific hospital sites because the type of ID consult received was based on the admitting hospital, as well as the fact that TM ID was only offered thrice weekly compared with daily in the SOC group. Despite these differences, no measured clinical outcomes were different between the groups. The included hospitals with TM ID consultations were mostly small and rural, compared with hospitals with onsite ID services that were generally large, metropolitan, teaching hospitals with expanded specialty services. The comparison of hospitals between groups is helpful to establish a contemporary comparator but is clearly not well matched when considering other available clinical services offered or patient populations as described. Transfers to another AH-PE hospital could have occurred if the patient required specialty services that were not available at their admitting hospital (eg, cardiac thoracic surgery specialists). For analysis, patients were assigned based on the admitting hospital to capture early SAB management, which remains a limitation to our study because other sites may not have the capability of interhospital transfers in a healthcare system, further limiting applicability.
It is notable that our study represents a retrospective analysis conducted within a single, large health system and lacks a historical comparison before the implementation of the AMS-led SAB bundle. We are also unable to delineate the role of the AMS pharmacist to assess the separate influences of the ID TM service and the AMS pharmacist. Without robust AMS services to provide daily oversight of the SAB quality bundle components, it may be hard to replicate our model with the high degree of bundle adherence we demonstrated in an ID TM group. We believe that the success of our SAB bundle is largely dependent on the collaboration between ID and AMS and demonstrates the ability to expand ID services through TM more effectively with AMS involvement.
CONCLUSIONS
In summary, use of ID TM for the management of SAB resulted in similar treatment outcomes compared with SOC. The combination of AMS developed care bundle for SAB and either onsite or telemedicine ID consultation allows for similar SAB bundle compliance, and our analysis demonstrates no difference in short-term clinical outcomes including 30-day mortality and hospital readmissions. This study supports the ongoing expansion and continued evaluation of ID TM consultation in underserved hospitals where resources are limited to offer onsite ID care.
Acknowledgments
We acknowledge the contribution of Atrium Health’s Antimicrobial Support Network and the Division of Infectious Diseases to the success of this project.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Liu C, Bayer A, Cosgrove SE, et al. ; Infectious Diseases Society of America. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 2011; 52:e18–55. [DOI] [PubMed] [Google Scholar]
- 2. van Hal SJ, Jensen SO, Vaska VL, et al. Predictors of mortality in Staphylococcus aureus bacteremia. Clin Microbiol Rev 2012; 25:362–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Naber CK. Staphylococcus aureus bacteremia: epidemiology, pathophysiology, and management strategies. Clin Infect Dis 2009; 48:231–7. [DOI] [PubMed] [Google Scholar]
- 4. Tong SY, Davis JS, Eichenberger E, et al. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 2015; 28:603–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nguyen CT, Gandhi T, Chenoweth C, et al. Impact of an antimicrobial stewardship-led intervention for Staphylococcus aureus bacteraemia: a quasi-experimental study. J Antimicrob Chemother 2015; 70:3390–6. [DOI] [PubMed] [Google Scholar]
- 6. Wenzler E, Wang F, Goff DA, et al. An automated, pharmacist-driven initiative improves quality of care for Staphylococcus aureus bacteremia. Clin Infect Dis 2017; 65:194–200. [DOI] [PubMed] [Google Scholar]
- 7. Smith JR, Frens JJ, Snider CB, Claeys KC. Impact of a pharmacist-driven care package on Staphylococcus aureus bacteremia management in a large community healthcare network: a propensity score-matched, quasi-experimental study. Diagn Microbiol Infect Dis 2018; 90:50–4. [DOI] [PubMed] [Google Scholar]
- 8. López-Cortés LE, Del Toro MD, Gálvez-Acebal J, et al. ; REIPI/SAB group. Impact of an evidence-based bundle intervention in the quality-of-care management and outcome of Staphylococcus aureus bacteremia. Clin Infect Dis 2013; 57:1225–33. [DOI] [PubMed] [Google Scholar]
- 9. Jenkins TC, Price CS, Sabel AL, et al. Impact of routine infectious diseases service consultation on the evaluation, management, and outcomes of Staphylococcus aureus bacteremia. Clin Infect Dis 2008; 46:1000–8. [DOI] [PubMed] [Google Scholar]
- 10. Rieg S, Peyerl-Hoffmann G, de With K, et al. Mortality of S. aureus bacteremia and infectious diseases specialist consultation–a study of 521 patients in Germany. J Infect 2009; 59:232–9. [DOI] [PubMed] [Google Scholar]
- 11. Nagao M, Iinuma Y, Saito T, et al. Close cooperation between infectious disease physicians and attending physicians can result in better management and outcome for patients with Staphylococcus aureus bacteraemia. Clin Microbiol Infect 2010; 16:1783–8. [DOI] [PubMed] [Google Scholar]
- 12. Robinson JO, Pozzi-Langhi S, Phillips M, et al. Formal infectious diseases consultation is associated with decreased mortality in Staphylococcus aureus bacteraemia. Eur J Clin Microbiol Infect Dis 2012; 31:2421–8. [DOI] [PubMed] [Google Scholar]
- 13. Coombes CE, Gregory ME. The current and future use of telemedicine in infectious diseases practice. Curr Infect Dis Rep 2019; 21:41. [DOI] [PubMed] [Google Scholar]
- 14. Young JD, Abdel-Massih R, Herchline T, et al. Infectious Diseases Society of America position statement on telehealth and telemedicine as applied to the practice of infectious diseases. Clin Infect Dis 2019; 68:1437–43. [DOI] [PubMed] [Google Scholar]
- 15. Abdel-Massih RC, Mellors JW. Telemedicine and infectious diseases practice: a leap forward or a step back? Open Forum Infect Dis 2019; 6:ofz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burnham JP, Fritz SA, Yaeger LH, Colditz GA. Telemedicine infectious diseases consultations and clinical outcomes: a systematic review. Open Forum Infect Dis 2019; 6:ofz517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020; 382:1679–81. [DOI] [PubMed] [Google Scholar]
- 18. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol 2011; 173:676–82. [DOI] [PubMed] [Google Scholar]
- 19. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016; 315:801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fowler VG Jr, Sanders LL, Sexton DJ, et al. Outcome of Staphylococcus aureus bacteremia according to compliance with recommendations of infectious diseases specialists: experience with 244 patients. Clin Infect Dis 1998; 27:478–86. [DOI] [PubMed] [Google Scholar]
- 21. Fowler VG Jr, Olsen MK, Corey GR, et al. Clinical identifiers of complicated Staphylococcus aureus bacteremia. Arch Intern Med 2003; 163:2066–72. [DOI] [PubMed] [Google Scholar]
- 22. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 2009; 49:1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McQuillen DP, MacIntyre AT. The value that infectious diseases physicians bring to the healthcare system. J Infect Dis 2017; 216:588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burnham JP, Fritz SA, Yaeger LH, Colditz GA. Telemedicine infectious diseases consultations and clinical outcomes: a systematic review and meta-analysis protocol. Syst Rev 2019; 8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Monkowski D, Rhodes LV, Templer S, et al. A retrospective cohort study to assess the impact of an inpatient infectious disease telemedicine consultation service on hospital and patient outcomes. Clin Infect Dis 2020; 70:763–70. [DOI] [PubMed] [Google Scholar]