Abstract
Few instances of treatment-emergent resistance to bictegravir have been reported in the literature. We describe a case of treatment-emergent resistance to bictegravir in a person recently diagnosed with human immunodeficiency virus who developed M184V and R263K mutations while on therapy.
Keywords: bictegravir, HIV, R263K, resistance
Current human immunodeficiency virus (HIV) guidelines for initial therapy in most treatment-naive individuals recommend therapy with an integrase inhibitor (INI) in combination with at least 1, but generally 2, nucleoside reverse transcriptase (RT) inhibitors [1]. Bictegravir (BIC)/emtricitabine (FTC)/tenofovir alafenamide (TAF) is a once-daily, single-tablet option for treatment-naive individuals, and this medication has high potency, low potential for drug–drug interactions, and a high barrier to resistance [2]. It demonstrated noninferiority to active comparators in treatment-naive and treatment-experienced individuals, and there have been no cases of treatment-emergent resistance documented in treatment-naive or treatment-experienced individuals in clinical trials of BIC/FTC/TAF [3]. BIC/FTC/TAF has also been proven efficacious in sustaining viral suppression in individuals with documented M184V/I mutations [4]. As of the time of this brief report, only a few instances of treatment-emergent resistance to BIC have been identified in the literature [5, 6]. We describe a case of treatment-emergent resistance to BIC in a person recently diagnosed with HIV who developed M184V (RT) and R263K (INI) mutations while on BIC therapy.
CASE REPORT
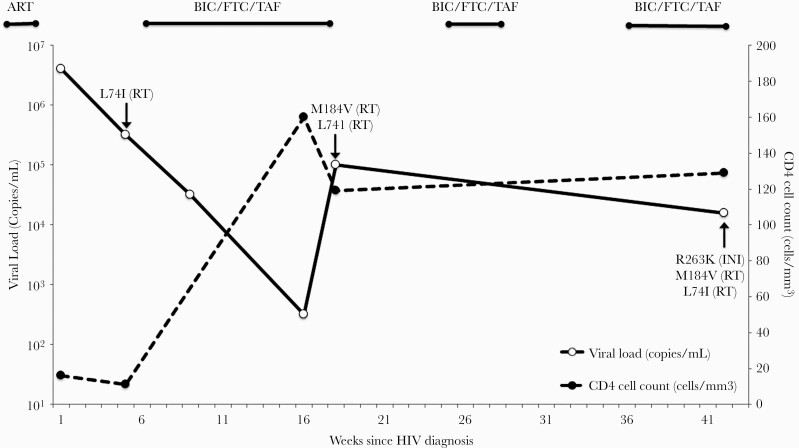
A 51-year-old man was diagnosed with advanced HIV infection complicated by cryptococcal meningitis. Baseline laboratory tests revealed an absolute CD4 count of 16 cells/mm3 (3%) with a viral load of 3 700 000 copies/mL. A pretreatment genotype, which included INI testing, revealed an HIV-1 subtype B along with an L74I (RT) mutation conferring high-level resistance to didanosine and intermediate-level resistance to abacavir. The pretreatment L741 (RT) mutation was not present 27 days earlier on genotype with integrase testing performed at the time of HIV diagnosis at an outside health center. The person was started on BIC/FTC/TAF around 40 days after HIV diagnosis once he had completed 2 weeks of induction therapy with intravenous (IV) amphotericin B lipid complex (5 mg/kg daily) and oral flucytosine (25 mg/kg every 6 hours) followed by 4 weeks of consolidation therapy with oral fluconazole (400 mg daily) for his cryptococcal infection.
After 4 weeks of therapy, his viral load was 30 000 copies/mL, and after 11 weeks, his viral load was 319 copies/mL. The absolute CD4 count increased from 11 cells/mm3 (3%) pretreatment to 160 cells/mm3 (8%) after 11 weeks of therapy. He reported to providers that he was “mostly” taking his medications. At week 13, he was admitted to the hospital for acute encephalopathy with concerns for recurrent cryptococcal meningitis. According to his family, he had been off antiretroviral therapy (ART) and antifungal therapy for “several weeks” at that time. A repeat viral load was 98 000 copies/mL, and an updated genotype revealed an M184V (RT) mutation conferring resistance to lamivudine and FTC in addition to the previously seen L74I (RT) mutation. Given his poor adherence and recurrent central nervous system (CNS) infection, ART was not restarted until he had completed an additional 2-week course of IV amphotericin B lipid complex (5 mg/kg daily) and oral flucytosine (25 mg/kg every 6 hours) induction therapy and was transitioned to oral fluconazole (400 mg daily) for the consolidation phase of treatment for his CNS infection.
At week 20, BIC/FTC/TAF was restarted but later withheld at week 23 due to poor medication adherence resulting from untreated mental illness complicated by substance abuse, followed by a prolonged hospitalization for inpatient psychiatric care. After 8 weeks of inpatient psychiatric care, he was released and BIC/FTC/TAF was resumed at week 31. At follow-up the next month (week 37), he had an HIV RNA load of 14 095 copies/mL and a newly acquired R263K mutation (conferring low-level resistance to BIC) along with the previously documented M184V (RT) and L74I (RT) mutations. ART was adjusted to darunavir/cobicistat and FTC/rilpivirine/TAF. At multiple subsequent follow-up visits, he continued to report inconsistent medication adherence with documented persistent viremia in the range of 400–13 000 copies/mL. Figure 1 provides an illustration of his viral loads, CD4 cell counts, and resistance mutations developed over time in response to ART exposure while on BIC/FTC/TAF.
Figure 1.
Viral loads, CD4 cell counts, antiretroviral therapy, and detected mutations over time. Abbreviations: ART, antiretroviral therapy; BIC, bictegravir; FTC, emtricitabine; HIV, human immunodeficiency virus; INI, integrase inhibitor; RT, reverse transcriptase; TAF, tenofovir alafenamide.
DISCUSSION
Integrase strand transfer inhibitors (INSTIs), which include raltegravir (RAL), elvitegravir (EVG), dolutegravir (DTG), and BIC, are the one of the newest classes of antiretroviral drugs to be approved for treatment of HIV and inhibit the HIV protein integrase from inserting the viral DNA genome into the host cell [7, 8]. Resistance to RAL and EVG has been well-characterized in the literature with the accumulation of primary resistance substitutions along several pathways including the N155H, Q148, and G140A/G148R/H/Q pathways conferring high-level cross-resistance to RAL and EVG [2]. However, second-generation INSTIs like DTG and BIC have demonstrated higher barriers to resistance and maintain potency against primary mutations that confer resistance to RAL or EVG [2, 7, 8].
Treatment-emergent resistance to second-generation INIs is uncommon but has been documented [9]. One mutation of particular interest is the R263K (INI) mutation, which confers low-level (<3-fold reduced susceptibility) resistance to DTG and BIC and is associated with diminished HIV DNA integration and viral fitness [2, 8, 9]. In treatment-naive individuals, the R263K (INI) mutation is very rare and has most commonly been observed by ultra-deep sequencing [8]. Most cases of treatment failure with development of R263K (INI) mutations have been documented in the context of DTG administration, with failure most often linked to low serum drug levels from drug–drug interactions with rifampin or rifabutin [9]. However, a recent 2020 case report did document treatment-emergent resistance in a treatment-experienced individual who had received crushed BIC/FTC/TAF tablets through a nasogastric tube, which the authors speculate may have resulted in inadequate blood levels of the drug that led to the R263K mutation [5].
To the best of our knowledge, this case represents one of very few instances of documented treatment-emergent resistance to BIC in a person with no prior HIV treatment history. Prior to treatment initiation, he had 2 genotypes with integrase testing performed: one at diagnosis revealing a wild-type virus and the other 4 weeks later revealing an L74I (RT) mutation. He experienced an initial (albeit suboptimal) response to ART followed by prolonged viremia in the setting of frequent treatment interruptions and suboptimal adherence due to recurrent CNS infections requiring ART to be held, inpatient treatment for poorly controlled mental health issues, and ongoing alcohol and substance use disorder. Despite suboptimal viral suppression, he experienced a significant CD4 cell reconstitution as denoted by the increase of his CD4 cell count from 11 cells/mm3 to 160 cells/mm3 and 129 cells/mm3 after 11 and 37 weeks of therapy, respectively. The M184V (RT) mutation was detected after 17 weeks of therapy, and at week 37 the R263KR (INI) mutation was detected via bulk sequencing (Viracor Lab, Missouri) necessitating a change in ART. Neither the M184V (RT) mutation nor the R263KR (INI) mutation was observed in the 2 pretreatment genotypes, suggesting that these were treatment-emergent mutations and were not transmitted or present at baseline. This combination of mutations has been shown to decrease viral infectiousness and replicative capacity in vitro when compared to the effects of either individual substitution alone, suggesting that this combination of substitutions has a strong effect on viral load; however, these findings have not been evaluated in clinical settings [10]. Two other reported cases of the R263KR (INI) mutation have been in individuals with non–subtype B HIV type 1, both with advanced HIV disease, one in a treatment-experienced individual and another under first-line treatment with BIC/FTC/TAF [5, 6].
Identifying individuals at highest risk for treatment failure and utilizing strategies to prevent treatment-emergent resistance from developing are paramount to HIV management. As treatment-emergent resistance is heavily linked to ART exposure, barriers to optimal ART adherence must be considered when prescribing a regimen, which include (1) regimen characteristics (ie, pill size and number, frequency, requirement of ingestion with food, and side effects) and (2) unaddressed mental health and substance use issues that may complicate an individual’s ability to adhere to medications [11]. This individual’s treatment course was made difficult by suspected recurrent cryptococcal meningitis that prevented reinitiation of ART due to the potentially devastating long-term neurological consequences. Another consideration given the person’s robust CD4 cell count recovery was immune reconstitution inflammatory syndrome, which is managed by continuing both ART and antifungal therapy and reducing elevated intracranial pressure, if present [1]. Finally, this person’s poorly managed mental health issues and struggles with substance abuse further complicated his care. Substance use disorders are prevalent among people with HIV and contribute to poor medication adherence and poor health outcomes. Second-generation INIs (DTG and BIC) and protease inhibitors provide a higher genetic barrier to resistance and should be given consideration when starting ART in individuals who may have poor adherence [1]. In this case, the better side-effect profile and fewer drug–drug interactions of the INIs were favored given this person’s advanced HIV disease.
A holistic approach to HIV management should include not only ART, but also social support; screening for mental health and substance use disorders; counseling; and, when needed, evidence-based pharmacotherapy, psychiatric treatment, and even rehabilitation to address psychosocial factors that, when controlled, may result in better health outcomes. Individuals starting ART may benefit from more frequent follow-up and laboratory monitoring of viral loads and genotypes (when applicable) until they reach viral suppression. In this case, there were several months during which this individual had no recorded visits or laboratory work that might have allowed for earlier recognition of his adherence challenges and impending development of HIV drug resistance.
Notes
Patient consent statement. This case report does not include factors necessitating patient consent.
Potential conflicts of interest. L. M. reports grants from Gilead Sciences, GSK/ViiV Healthcare, Janssen, MSD, Visby Medical, ThaiMed, Evofem Biosciences, Prosoft Clinical, SpeedDx Pty Ltd, and Lupin Pharmaceuticals, and personal fees from Gilead Sciences, GSK/ViiV Healthcare, and MSD, outside the submitted work. All other authors report no potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Department of Health and Human Services, Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. 2019. https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf. Accessed 1 November 2020.
- 2. Oliveira M, Mesplède T, Quashie PK, et al. . Resistance mutations against dolutegravir in HIV integrase impair the emergence of resistance against reverse transcriptase inhibitors. AIDS 2014; 28:813–9. [DOI] [PubMed] [Google Scholar]
- 3. Gilead Sciences, Inc. BIKTARVY [package insert]. Foster City, CA: Gilead Sciences, Inc; 2019. [Google Scholar]
- 4. Andreatta K, Willkom M, Martin R, et al. . Switching to bictegravir/emtricitabine/tenofovir alafenamide maintained HIV-1 RNA suppression in participants with archived antiretroviral resistance including M184V/I. J Antimicrob Chemother 2019; 74:3555–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lozano AB, Chueca N, de Salazar A, et al. . Failure to bictegravir and development of resistance mutations in an antiretroviral-experienced patient. Antiviral Res 2020; 179:104717. [DOI] [PubMed] [Google Scholar]
- 6. Stoll M, Braun P, Wiesmann F, Knechten H. Development of integrase inhibitor resistance under first-line treatment with bictegravir [abstract P125]. In: HIV Glasgow, Virtual Conference, 5–8 October 2020. J Int AIDS Soc 2020; 23:e25616.33015953 [Google Scholar]
- 7. Anstett K, Brenner B, Mesplede T, Wainberg MA. HIV drug resistance against strand transfer integrase inhibitors. Retrovirology 2017; 14:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tsiang M, Jones GS, Goldsmith J, et al. . Antiviral activity of bictegravir (GS-9883), a novel potent HIV-1 integrase strand transfer inhibitor with an improved resistance profile. Antimicrob Agents Chemother 2016; 60:7086–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pena MJ, Chueca N, D’Avolio A, et al. . Virological failure in HIV to triple therapy with dolutegravir-based firstline treatment: rare but possible. Open Forum Infect Dis 2019; 6:ofy332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singhroy DN, Wainberg MA, Mesplède T. Combination of the R263K and M184I/V resistance substitutions against dolutegravir and lamivudine decreases HIV replicative capacity. Antimicrob Agents Chemother 2015; 59:2882–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nel A, Kagee A. Common mental health problems and antiretroviral therapy adherence. AIDS Care 2011; 23:1360–5. [DOI] [PubMed] [Google Scholar]