Abstract
Antiretroviral therapy effectively prevents sexual and vertical transmission of HIV. Yet, some women living with HIV report having unmet needs for reproductive health care. This study measured the prevalence of women discussing reproductive goals with any current healthcare provider and assessed the effect of the current HIV care provider’s gender on such discussions and whether comfort was a mediator. We analysed baseline and 18-month survey data from 533 women living with HIV enrolled in the Canadian HIV Women’s Sexual and Reproductive Health Cohort Study (CHIWOS) (2013–2017), a community-based participatory study, restricting the analysis to participants aged 16–45 years. We used causal mediation analysis to estimate direct and indirect effects of the gender of one’s HIV care provider on reproductive discussions, incorporating mediating and interaction effects of women having any provider with whom they felt comfortable discussing reproductive goals. Between the baseline and 18-month follow-up surveys, 34.3% (183/533) of women discussed their reproductive goals with a healthcare provider. Having a woman HIV care provider was associated with a 1.18 excess relative risk (ERR) of discussion (95%CI: 0.15, 2.20). The mediating effect of comfort was primarily explained by the fact that those participants with women providers felt more comfortable discussing their reproductive goals compared to participants with men providers, accounting for 66% (95%CI: 32%, 99%) of the total effect. Findings support that HIV provider gender affects women’s comfort and whether they discuss reproductive goals, which must be acknowledged and addressed in care delivery.
Keywords: CHIWOS, women, HIV, family planning services, pre-conception care, reproductive health, patient comfort
Résumé
Le traitement antirétroviral prévient efficacement la transmission sexuelle et verticale du VIH. Pourtant, certaines femmes vivant avec le VIH affirment avoir des besoins insatisfaits de soins de santé reproductrice. Cette étude a mesuré la prévalence de femmes parlant de leurs objectifs reproductifs avec un prestataire de soins de santé en activité; elle a aussi évalué l’effet du genre du prestataire actuel de soins du VIH sur ces discussions et s’est demandé si le sentiment d’aise était un médiateur. Nous avons analysé les données d’une enquête initiale et de suivi après 18 mois auprès de 533 femmes séropositives recrutées dans l’étude sur la santé sexuelle et reproductive des femmes vivant avec le VIH au Canada (CHIWOS) (2013–2017), une étude participative communautaire, en restreignant l’analyse aux participantes âgées de 16 à 45 ans. Nous avons utilisé l’analyse de médiation causale pour estimer les effets directs et indirects du genre du prestataire de soins du VIH sur les discussions reproductives, en intégrant la médiation et les effets de l’interaction de la possibilité pour les femmes de compter sur un prestataire avec qui elles se sentaient à l’aise pour discuter de leurs objectifs reproductifs. Entre l’enquête initiale et l’enquête de suivi après 18 mois, 34,3% (183/533) des femmes avaient évoqué leurs objectifs reproductifs avec un prestataire de soins de santé. Un agent féminin de soins du VIH était associé avec un excès de risque relatif de 1.18 de discussion (IC95%: 0.15, 2.20). L’effet médiateur du confort s’expliquait principalement par le fait que les participantes ayant un prestataire de soins féminin étaient plus à l’aise pour aborder leurs objectifs reproductifs que les participantes traitées par des prestataires masculins, représentant 66% (IC95%: 32%, 99%) de l’effet total. Les conclusions montrent que le genre des prestataires de soins du VIH influe sur le sentiment d’aise des femmes et sur le fait qu’elles parlent ou non de leurs objectifs reproductifs, ce qui doit être pris en compte dans la prestation des soins.
Resumen
La terapia antirretroviral previene eficazmente la transmisión sexual y vertical del VIH. Sin embargo, algunas mujeres que viven con VIH informan tener necesidades insatisfechas de servicios de salud reproductiva. Este estudio midió la prevalencia de mujeres que discuten sus objetivos reproductivos con un prestador de servicios de salud, evaluó el efecto del género del prestador de servicios de VIH en esas conversaciones y determinó si la comodidad era un mediador. Analizamos los datos de la línea de base y de una encuesta administrada a los 18 meses a 533 mujeres que viven con VIH inscritas en el Estudio de Cohorte sobre Salud Sexual y Reproductiva de Mujeres Canadienses con VIH (CHIWOS, por sus siglas en inglés) (2013–2017), estudio participativo comunitario, y restringimos el análisis a participantes de 16 a 45 años. Utilizamos análisis de mediación causal para calcular los efectos directos e indirectos del género del prestador de servicios de VIH en las conversaciones sobre salud reproductiva, incorporando los efectos de mediación e interacción en las mujeres que tenían a un prestador de servicios con quien se sentían cómodas discutiendo sus objetivos reproductivos. Entre la línea de base y la encuesta de seguimiento administrada a los 18 meses, el 34.3% (183/533) de las mujeres discutieron sus objetivos reproductivos con un prestador de servicios de salud. Tener a una mujer como prestadora de servicios de VIH estaba asociado con un exceso de riesgo relativo de 1.18 (ERR) de discusión (IC de 95%: 0.15, 2.20). El efecto mediador de comodidad fue explicado principalmente por el hecho de que las participantes con prestadoras de servicios se sentían más cómodas discutiendo sus metas reproductivas comparadas con las participantes con prestadores de servicios, a lo cual se le atribuye el 66% (IC de 95%: 32%, 99%) del efecto total. Los hallazgos corroboran que el género de quienes prestan servicios de VIH afecta la comodidad de las mujeres y el hecho de que discutan o no sus objetivos reproductivos, lo cual se debe reconocer y abordar en la prestación de servicios.
Introduction
Due to advances in HIV treatment and medical care, people are living longer, healthier lives with HIV compared to the early years of the epidemic.1 Several studies have also established that there is effectively no risk of HIV transmission through sex without a condom when a person living with HIV is on antiretroviral therapy (ART) and has a consistently suppressed viral load.2–4 Additionally, with appropriate treatment and care, women living with HIV can become pregnant and have children with a very low risk of perinatal HIV transmission (0.4% in Canada).5–7
These advances have resulted in changes to the reproductive desires, behaviours, and outcomes of women living with HIV.8–12 Updates to treatment guidelines, including recommendations for safer conception and contraception, have emerged to support the sexual and reproductive health and rights of people living with or affected by HIV.13 In one Canadian province (Ontario), an estimated 63% of women of reproductive age living with HIV intended to give birth in the future.14 These reproductive intentions translate to more pregnancies compared to the earlier years of the HIV epidemic.10,12
Despite these trends, women living with HIV report having unmet needs for reproductive health care.15–17 In a Canadian cohort of women living with HIV, 25% reported becoming pregnant after HIV diagnosis, with 60.8% of these pregnancies being unintended.18 The World Health Organization (WHO) recommends “dual protection” (long-acting, reversible contraception plus condoms) for women living with HIV to prevent sexual and perinatal transmission of HIV.19 Less than 20% of women living with HIV in Canada practise WHO-defined dual protection, and 40% practised an expanded definition of dual protection (long-acting, reversible contraception plus either condoms or a suppressed HIV viral load).17 The range of contraceptive methods used by women living with HIV is also more limited compared to women in the general Canadian population.17 Among women living with HIV, studies suggest that awareness about safer conception methods20,21 and the prevalence of receiving pre-conception counselling are low.21
The Canadian HIV Pregnancy Planning Guidelines,22 the WHO consolidated guideline on sexual and reproductive health and rights of women living with HIV,23 and community-driven guidelines24 offer guidance about reproductive counselling and support for women living with HIV. They recommend that healthcare providers initiate discussions about reproductive goals on a regular basis, asking about women’s preferred number, spacing, and timing of biological children, or whether women prefer to avoid pregnancy altogether. Nevertheless, existing data suggest that such discussions are not routine; a retrospective study of women of reproductive age living with HIV in Ontario found that 51% reported ever discussing pregnancy planning with a healthcare provider since HIV diagnosis.21
A systematic review conducted in 2011 found that the practice of discussing reproductive goals may vary with healthcare provider characteristics such as training, sex, gender, age, and cultural differences affecting provider approaches to sexual health-related discussions with patients.25 More women HIV care providers in the United States reported assessing the reproductive intentions of their female patients compared to men HIV care providers (57% [95% CI: 48-–65%] vs 40% [95% CI: 31-–50%]).26 As reproductive discussions can be initiated by either the healthcare provider or the patient, we hypothesise that such differences may be explained by gender differences in providers prioritising reproductive counselling, and/or by women living with HIV feeling more comfortable initiating reproductive discussions with women healthcare providers. To inform strategies to support discussions between women living with HIV and their healthcare providers about their reproductive goals, the objectives of this study were to (1) estimate the prevalence of women discussing reproductive goals with any current healthcare provider, (2) assess the effect of their current HIV care provider’s gender on discussing reproductive goals, and (3) determine the role of patient comfort as a mediator of the effect of provider gender on reproductive discussions.
Methods
Study design
We analysed baseline and 18-month follow-up survey data from The Canadian HIV Women’s Sexual and Reproductive Health Cohort Study (CHIWOS), a multi-site longitudinal study following 1422 women living with HIV in three Canadian provinces (British Columbia, Ontario, and Quebec). The methodological approach, described in greater detail elsewhere,27 followed the tenets of community-based participatory research. The study was approved by the Research Ethics Boards (REBs) at Women’s College Hospital (Ontario), Simon Fraser University (BC), University of British Columbia/ Providence Health (BC), McGill University Health Centre (Quebec) and the independent REBs of other study sites. All participants provided written informed consent prior to enrolment.
Study sample and recruitment
Eligible participants identified as women (cis- and trans-inclusive), were 16 years of age or older, had been diagnosed with HIV, and were living in one of the three study provinces at the time of recruitment. Recruitment occurred between 2013 and 2015 through HIV clinics, AIDS service organisations, online and “word-of-mouth” peer networks.28 A non-random, purposive sampling approach was used to reflect the geographic distribution of women living with HIV in each study province, and to recruit more trans women and women less engaged in health care and HIV research. This allowed the analysis of health care access and outcomes for these specific vulnerable populations.
Inclusion and exclusion criteria
For this analysis, we restricted the sample to cis- and trans-women who completed both the baseline and 18-month follow-up surveys and who were of reproductive age (16–45 years) at baseline. We further excluded women who reported at baseline that they had not accessed HIV medical care in the past year, as they were missing information about the gender of their HIV care provider. We also excluded those who reported at the 18-month visit that discussing reproductive goals was “not applicable” to them because they were unable to conceive and those who preferred not to answer the question (n = 3).
Data collection
Data collection occurred via peer research associate-administered web-based surveys conducted in-person, by phone or by skype.29 Surveys were administered in English or French using the online software FluidSurveys™. Baseline surveys were administered between 2013 and 2015. The 18-month follow-up survey was completed between 2015 and 2017.
Measures
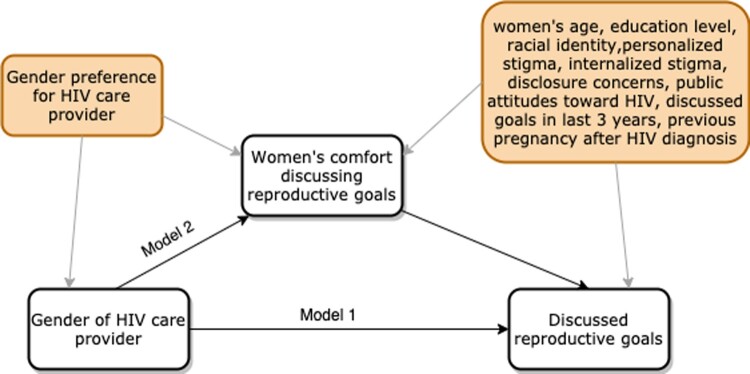
A mediation diagram of the relationship between gender of the HIV care provider (exposure) and discussions (outcome) was constructed based on evidence from the literature and input from HIV care providers (Figure 1). The mediator and confounders of the exposure-outcome relationship were identified from the diagram. We hypothesised that women’s comfort discussing reproductive goals is a mediator (variable in the causal pathway) between provider gender and discussions.
Figure 1.
Mediation diagram for the hypothesised effect of HIV care provider gender on discussing reproductive goals
Notes: Exposure = gender of women’s healthcare provider, measured at baseline (woman vs man); Mediator = comfortable discussing reproductive goals, measured at baseline (yes vs no); Outcome = discussed reproductive goals between the baseline and 18-month follow-up surveys, measured at 18-month follow-up. Confounders of the exposure → mediator relationship: Having a gender preference for one’s HIV care provider; Confounders of the mediator → outcome relationship: women’s age, education level, racial identify, HIV stigma (personalised, internalised, disclosure concerns, public attitudes), had a discussion about reproductive goals with a healthcare provider in last 3 years, having at least one pregnancy since being diagnosed with HIV
Outcome: discussing reproductive goals
The outcome was measured at the 18-month study visit, based on participants’ responses to the question, “Since your last CHIWOS interview, have you discussed your reproductive goals with a healthcare provider?” “Reproductive goals” were defined as women’s preferred number, spacing, and timing of biological children or not wanting children. Responses were dichotomised into “yes” and “no”. We collapsed the responses “no” and “don’t know” (n = 1) as not remembering reproductive discussions was considered equivalent to not having the discussion in terms of information retained from the exchange with a healthcare provider.
Exposure: gender of primary HIV care provider
The exposure was the gender of the primary HIV care provider (healthcare provider who primarily prescribes HIV medicines, follows CD4 count, viral load, etc.), which was identified from participants’ response to the baseline question “What is the gender of your primary HIV care provider?” Response options were “woman”, “man”, “trans person”, “don’t know”, and “prefer not to answer”.
Mediator: women’s comfort discussing reproductive goals
We considered women’s comfort discussing their reproductive goals with a healthcare provider as a mediator (Figure 1) and measured comfort at baseline. Participants were asked, “Do you currently have a healthcare provider with whom you feel comfortable talking to about your reproductive goals?” Responses were dichotomised into “yes” and “no”, combining “no”, “don’t know” (n = 9) and “prefer not to answer” (n = 2). We assumed that women preferring not to answer the question about comfort discussing reproductive goals in a peer-administered survey likely indicated a lack of comfort discussing reproductive goals with a healthcare provider.
Confounders
We measured confounders of the exposure (gender of the HIV care provider)-mediator (women’s comfort discussing their reproductive goals with their healthcare provider) relationship. Women indicated their preference for the gender of their HIV care provider: “I prefer my HIV doctor to be a woman”, “I prefer my HIV doctor to be a man / no preference or other”. As patients in Canada can choose their HIV healthcare provider, this was done to account for women potentially seeking out women HIV care providers due to gender preference and comfort.
We measured confounders of the mediator (comfort)-outcome (discussions) relationship: women’s age (10-year intervals), education level (lower than high school, high school or higher), and race/ethnicity (Indigenous, African/Caribbean/Black, or white), HIV-related stigma, having discussed reproductive goals with a healthcare provider in the three years preceding the baseline survey, and reporting a pregnancy since HIV diagnosis. HIV-related stigma was included in the model to account for its effect on women’s comfort in discussing their reproductive goals and its effect on reproductive discussions. We used the validated shortened HIV Stigma Scale (HSS)30 to measure four HIV-related stigma dimensions: (i) personalised stigma measures experiences of enacted stigma through rejection and isolation; (ii) concerns disclosing HIV status; (iii) negative self-image measures internalised stigma; and (iv) public attitudes measures perceived stigma. HIV-related stigma dimensions were dichotomised to high and low stigma with the sample median as the cut-off. Previous discussions were included in the model to adjust for decreased likelihood of subsequent reproductive discussion if one had occurred recently. All confounders were measured at baseline.
Statistical analysis
Guided by the mediation diagram (Figure 1), we used two multivariable logistic regression models to estimate odds ratios (and 95% confidence intervals): Model 1, a confounder-adjusted model, was fitted to the data to quantify the association of the exposure (provider gender), mediator (comfort), and their interaction on the outcome (discussions). Including an interaction term allowed us to identify whether strategies to intervene on comfort may have a larger impact on women who receive care from providers of one gender over another. Model 2 is a confounder-adjusted model estimating the association between the exposure and mediator.
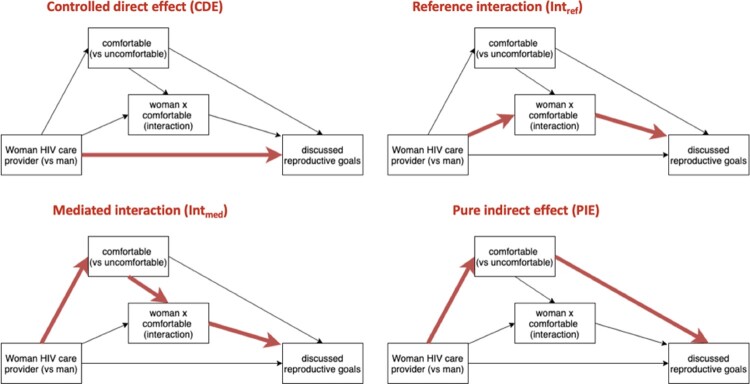
To complement the mediation analysis using the logistic regression models, we used the med4way package31 in Stata version 15.1 (StataCorp, 2017) to quantify the decomposed effect of HIV care provider gender on reproductive discussions, considering mediation (variable in the pathway between exposure and outcome) and interaction (effect of exposure on outcome varying across another variable) effects. The med4way package uses parametric regression models to calculate contrasts in decomposed effects.31 For a binary mediator and outcome, two logistic regression models were fitted: a model for the outcome as a function of the exposure, the mediator, their interaction and confounders and a second model for the mediator as a function of the exposure and confounders. This approach to mediation analysis allowed us to decompose the total effect, while considering interaction effects between the exposure and mediator and nonlinearity in our model. We applied methods described by VanderWeele32 to decompose the effect into four distinct components: the effect due to mediation only (pure indirect effect), the effect due to interaction only (reference interaction), the effect due to mediation and interaction (mediated interaction), and the effect due to neither mediation nor interaction (controlled direct effect). Our model assumes a counterfactual framework.32 The four components of the decomposition are illustrated (Figure 2). Estimates are reported as excess relative risk: Excess Relative Risk (ERR) = Risk Ratio (RR) −1.
Figure 2.
Summary of 4-way decomposition in causal mediation analysis
Notes: Bolded arrows represent each of the four components of the decomposition
Results
Sample characteristics
A total of 1422 women living with HIV completed the baseline CHIWOS survey and 1252 completed the 18-month follow-up survey for a retention rate of 88%. For the present analysis, we excluded participants over the age of 45 years (n = 571), participants who did not access HIV medical care in the previous year (n = 59), participants who responded that discussing reproductive goals was not relevant to them as they could not have children (n = 154) or who preferred not to answer the question about reproductive discussions (n = 3), and participants who were lost to follow-up at the 18-month visit (n = 102), yielding a final analytic sample of 533 participants (37.5% of the enrolled cohort).
Table 1 shows the baseline characteristics of the 533 included participants. Women had a median age of 35 [IQR: 31.40] and identified as Indigenous (21.0%), African, Caribbean or Black (36.4%), white (37.3%), and mixed or other race (5.3%). Most participants (85.2%) identified as heterosexual, were taking ART (78.2%), and reported an undetectable HIV viral load (75.2%). Regarding healthcare provider gender, 40.2% received HIV care from a woman provider and 59.9% from a man. Women’s reported reproductive intentions included not intending to become pregnant in the future (41.3%), intending to become pregnant (28.5%), and being unsure (20.6%).
Table 1.
Baseline characteristics of participants in the analytical sample (n=533)
| Characteristic | Median [IQR] or n (%) | |
|---|---|---|
| Province of residence | British Columbia | 114 (21.39) |
| Ontario | 307 (57.60) | |
| Quebec | 112 (21.01) | |
| Age at baseline (years) | 35 [31, 40] | |
| Racial and/or ethnic background | Indigenous | 112 (21.01) |
| African, Caribbean, Black | 194 (36.40) | |
| White | 199 (37.34) | |
| Mixed / Other | 28 (5.25) | |
| Sex assigned at birth | Male/ Other | 5 (0.94) |
| Female | 531 (99.06) | |
| Gender identity | Woman | 525 (98.50) |
| Transwoman | 4 (0.75) | |
| Other | 4 (0.75) | |
| Sexual orientation | Heterosexual/ Straight | 454 (85.18) |
| LGBTTQ/DK/PNTA | 79 (14.82) | |
| Highest level of formal education | Lower than high school/DK/PNTA | 69 (12.95) |
| High school or higher | 464 (87.05) | |
| Household annual income (CAD) | <$20,000 | 302 (56.66) |
| $20,000-$40,000 | 112 (21.01) | |
| >40,000 | 100 (18.76) | |
| DK/PNTA | 19 (3.56) | |
| Incarceration history | Never | 385 (72.23) |
| Ever/ DK/PNTA | 148 (27.77) | |
| What is your current legal status in Canada | Canadian Citizen | 397 (74.48) |
| Landed Immigrant/Permanent Resident | 80 (15.01) | |
| Refugee | 39 (7.32) | |
| Other/DK/PNTA | 17 (3.19) | |
| Relationship status | Married/Relationship/Common-law | 198 (37.15) |
| Single | 280 (52.53) | |
| Separated/Divorced/Widowed | 55 (10.32) | |
| Gender of current HIV provider | Woman | 214 (40.15) |
| Man | 319 (59.85) | |
| Ever discussed reproductive goals with a healthcare provider since HIV diagnosis | No/DK | 235 (44.09) |
| Yes | 249 (46.72) | |
| Unable/don’t want children | 46 (8.63) | |
| PNTA | 2 (0.38) | |
| Intention to become pregnant in future | No | 220 (41.28) |
| Yes | 152 (28.52) | |
| DK | 110 (20.64) | |
| PNTA/Missing | 51 (9.57) | |
| Number of children | 0 | 233 (43.71) |
| 1 to 2 | 199 (37.34) | |
| 3 to 4 | 80 (15.01) | |
| 5 or more | 21 (3.94) | |
| Pregnancy after HIV diagnosis | Yes | 69 (12.95) |
| No | 464 (87.05) | |
| Country of birth | Canadian born | 315 (59.10) |
| Foreign born/ DK/PNTA | 218 (40.90) | |
| Current ART use | Currently on ART | 417 (78.24) |
| Not currently/ DK/ PNTA | 116 (21.77) | |
| Most recent viral load results | Undetectable (below 50 copies/mL) | 401 (75.23) |
| Detectable (over 50 copies/mL) | 95 (17.82) | |
| DK/PNTA | 37 (6.94) | |
| Most recent CD4 count | <200 cells/mm3 | 22 (4.13) |
| 200-500 cells/mm3 | 132 (24.77) | |
| >500 cells/mm3 | 288 (54.03) | |
| DK/PNTA | 91 (17.07) | |
| LGBTTQ, Lesbian, Gay, Bisexual Transgender, Two-Spirit, Queer; DK/PNTA, don’t know, prefer not to answer; ART, antiretroviral therapy | ||
Reproductive discussions
At baseline, approximately half (46.7%) of women had discussed their reproductive goals with a healthcare provider since being diagnosed with HIV. Subsequently, at the 18-month follow-up survey, 34.3% (183/533) of women reported having discussed their reproductive goals with a healthcare provider since baseline. A quarter of women reported discussing their reproductive goals at both timepoints (136/533), while 21.5% (115/533) reported discussions at baseline only, 7.3% (39/533) at follow-up only, and 37.3% (200/533) at neither baseline nor follow-up. About a quarter (25.9%) of women who had a man as a primary HIV provider discussed their reproductive goals between their baseline and 18-month visits, compared to 46.3% of those who had a woman provider (p < 0.001). Among women whose primary HIV provider was a man, 36.4% reported having a healthcare provider with whom they felt comfortable discussing their reproductive goals, while 70.6% of women with a woman primary HIV provider reported that they had a provider with whom they felt comfortable discussing their reproductive goals (p < 0.0001).
Table 2 presents the confounder-adjusted logistic regression Model 1, with outcome being discussing reproductive goals between the baseline and 18-month follow-up study visits. Having a woman HIV care provider was not associated with discussing reproductive goals when the model was adjusted for comfort and other covariates (aOR = 0.72; 95%CI: 0.33, 1.57). Comfort was associated with higher odds of discussing reproductive goals (aOR = 2.24; 95%CI: 1.30, 3.87). Among women who reported feeling comfortable, having a woman provider was not associated with reproductive discussions (aOR = 0.92; 95%CI: 0.37, 2.29). Among women who reported not feeling comfortable discussing their reproductive goals with a current provider, having a woman provider was associated with lower odds of such discussions (aOR = 0.16; 95%CI: 0.05, 0.47). In Model 2, we analysed factors associated with our hypothesised mediator comfort discussing reproductive goals (Table 3). We observed that women whose primary HIV care provider was a woman had 4.18 times higher odds (95%CI: 2.70, 6.49) of reporting feeling comfortable discussing their reproductive goals with a current care provider after adjusting for covariates.
Table 2.
Multivariate logistic regression results for Model 1 (outcome: discussed reproductive goals with a healthcare provider between baseline and 18-month follow-up)
| Variable | aOR (95%CI) | p-value |
|---|---|---|
| Woman HIV care provider (ref. man) | 0.72 (0.33, 1.57) | 0.415 |
| Comfortable* | 0.92 (0.37, 2.29) | .859 |
| Not comfortable* | 0.16 (0.05, 0.47) | 0.001 |
| Comfortable (ref. not comfortable) | 2.24 (1.30, 3.87) | 0.004 |
| Education (ref. lower than HS) | 1.35 (0.73, 2.50) | 0.339 |
| Personalised stigma (ref. low) | 0.62 (0.39, 0.98) | 0.043 |
| Negative self-image (ref. low) | 0.87 (0.55, 1.38) | 0.547 |
| Disclosure concerns (ref. low) | 1.04 (0.63, 1.75) | 0.861 |
| Public attitudes (ref. low) | 1.30 (0.83, 2.03) | 0.252 |
| Prefer woman provider (ref. man/ no preference) | 1.38 (0.81, 2.35) | 0.231 |
| Agea | 0.76 (0.56, 1.02) | 0.071 |
| Indigenous (ref. white/ACB) | 0.72 (0.40, 1.29) | 0.262 |
| ACB (ref. white/Indigenous) | 0.98 (0.61, 1.57) | 0.923 |
| Previous discussion within last 3 years (ref. no) | 2.13 (1.56, 2.92) | <0.001 |
| Pregnancy after HIV diagnosis (ref. no) | 1.47 (0.84, 2.60) | 0.180 |
*measure of interaction between HIV care provider gender and comfort discussing reproductive goals.
coded at intervals of 10 years.
Note: ACB, African, Caribbean, and/or Black.
Table 3.
Multivariate logistic regression results for Model 2 (mediator modelled as the outcome: comfort discussing reproductive goals with current healthcare provider)
| Variable | aOR (95%CI) | p-value |
|---|---|---|
| Woman HIV care provider (ref. man) | 4.18 (2.70, 6.49) | <0.001 |
| Education (ref. lower than HS) | 1.17 (0.67, 2.06) | 0.579 |
| Personalised stigma (ref. low) | 1.15 (0.74, 1.80) | 0.535 |
| Negative self-image (ref. low) | 0.68 (0.43, 1.07) | 0.093 |
| Disclosure concerns (ref. low) | 0.63 (0.38, 1.04) | 0.069 |
| Public attitudes (ref. low) | 1.17 (0.77, 1.78) | 0.470 |
| Prefer woman provider (ref. man/ no preference) | 0.74 (0.44, 1.24) | 0.249 |
| Agea | 0.91 (0.69, 1.21) | 0.532 |
| Indigenous (ref. white/ ACB) | 0.79 (0.47, 1.31) | 0.359 |
| ACB (ref. white/Indigenous) | 2.20 (1.40, 3.47) | <0.001 |
| Previous discussion within last 3 years (ref. no) | 1.34 (1.00, 1.80) | 0.048 |
| Pregnancy after HIV diagnosis (ref. no) | 1.59 (0.89, 2.85) | 0.116 |
coded at intervals of 10 years.
Notes: ACB, African, Caribbean, and/or Black.
Table 4 presents the total effect of primary HIV care provider gender on discussions decomposed into a controlled direct effect (if everyone was uncomfortable, how much would gender of HIV care provider affect discussions), reference interaction effect (effect of having a woman provider modified by comfort and in the absence of mediation), the mediated interaction (effect of comfort on discussions, where the effect of comfort varies when the provider is woman vs man), and the pure mediated effect (effect of woman provider on discussions due to mediation through comfort only). The total effect of having a woman primary HIV care provider, when the mediator is set to its natural value, corresponded to a 1.18 (95%CI: 0.15, 2.20) excess relative risk (ERR) of reproductive discussion. When fixing the mediator, the controlled direct effect of provider gender is attenuated to −0.18 ERR (95%CI: −0.58, 0.22). The reference interaction between the effects of having a woman provider and comfort was associated with a 0.59 ERR (95%CI: −0.02, 1.19) of reproductive discussion. The mediated interaction was associated with a 0.49 ERR (95%CI: −0.03, 1.02) of reproductive discussion. The pure indirect effect of provider gender through comfort was associated with a 0.28 ERR (95%CI: 0.06, 0.50) of reproductive discussion. Mediation accounted for 66% (95%CI: 32%, 99%) of the total effect of healthcare provider gender on reproductive discussions.
Table 4.
Mediation and interaction of comfort and effect of gender of healthcare provider on reproductive discussions (4-way decomposition)
| Component | Interpretation | Excess relative riska (95%CI) | p-value | Proportion attributable (95%CI) |
|---|---|---|---|---|
| Total effect | Effect of provider gender on discussions | 1.18 (0.15, 2.20) | 0.024 | 100% |
| Controlled direct effect | Effect of provider gender due to neither mediation nor interaction | −0.18 (−0.58, 0.22) | 0.367 | −15% (−55%, 24%) |
| Reference interaction | Effect of provider gender due to interaction only | 0.59 (−0.02, 1.19) | 0.058 | 50% (19%, 81%) |
| Mediated interaction | Effect of provider gender due to mediation and interaction | 0.49 (−0.03, 1.02) | 0.065 | 42% (18%, 66%) |
| Pure indirect effect | Effect of provider gender due to mediation only | 0.28 (0.06, 0.50) | 0.012 | 24% (−5%, 52%) |
| Total % mediated | – | – | 66% (32%, 99%) |
adjusted for education, personalised HIV-related stigma, negative self-image related to HIV stigma, disclosure concerns, public attitudes towards HIV, preferring a woman HIV care provider, age, race/ethnicity, specialty of HIV care provider, previous discussions, previous pregnancy since HIV diagnosis.
Discussion
Among women of reproductive age living with HIV in the cohort, at baseline, 46.7% had discussed their reproductive goals with a healthcare provider since being diagnosed with HIV. Subsequently, 34.3% discussed their reproductive goals over the 18-month observation period. This finding supports previous studies reporting that women living with HIV experience gaps in reproductive health care.15,16,21,26,33,34 Also consistent with previously published research,15,26 women who received HIV care from a woman HIV provider were more likely to have discussed their reproductive goals with a healthcare provider. In our analyses, this included discussions with the primary HIV care provider or any other healthcare provider through referral or other means. We were able to deconstruct the effect of HIV care provider gender, revealing that the effect of HIV care provider gender on reproductive discussions operates principally through an indirect pathway mediated by women’s comfort discussing their reproductive goals.
The estimated controlled direct effect was not significant, implying that, hypothetically, if all women had equal comfort discussing their reproductive goals, there would be no association between the gender of women’s providers and whether or not reproductive discussions occurred. That more discussions were reported by women whose provider was also a woman can be primarily explained by differences in women’s comfort. Given the intersecting challenges associated with sexuality, reproduction, motherhood, trauma, HIV-related stigma,35 as well as racism and other forms of oppression that many women living with HIV face, women may feel more comfortable discussing these topics with an HIV care provider who is a woman36 or asking that provider for a referral to another provider with whom they are more comfortable.
The substantive pathway through women’s comfort highlights a point of interest for future interventions aimed at increasing reproductive discussions between women and their healthcare providers. Promising strategies include integrating women’s reproductive health care in the delivery of HIV care16,37,38 and increasing women’s comfort discussing their reproductive goals. An integrated model of HIV care where allied healthcare providers are easily accessible37 would facilitate reproductive discussions. A pre-post retrospective comparison of attendance at family planning clinics in Nigeria found that attendance at these clinics increased after the implementation of training for providers and formalised referrals between family planning and HIV clinics.39 In general, HIV care providers of all genders should be capable of providing reproductive health care and counselling to women living with HIV. However, women’s comfort discussing their reproductive goals may vary with the gender of their provider and social and cultural experiences.40,41 Targeted training for care providers who identify as men may also help to educate them about initiating these discussions while addressing the comfort needs of women living with HIV.
Strategies to support women’s comfort may include promoting self-efficacy,42 creating a safe and supportive clinic environment, and signalling that reproductive discussions are welcome. Providers should also be aware of provider-patient and gendered power relations that exist in clinical encounters and approach these discussions accordingly.43 The introduction of signs in clinic offices, waiting rooms or online44 with information about pregnancy planning and contraceptives can help to signal that care providers at the clinic support the sexual and reproductive health and rights of women living with HIV. Annual reproductive discussions should also be part of routine HIV care to help normalise these discussions, potentially increasing comfort for both patients and providers.25
This study is not without limitations. First, recall bias and social desirability bias may have led to misreporting of reproductive discussions in the past three years, gender preference for HIV care provider, and comfort discussing reproductive goals. Second, women who reported that they were unable to have children were excluded from the analysis, and information on why they were unable to have children is unknown. Hence, reproductive health counselling may still be relevant. Third, participants lost to follow-up, who represent a more marginalised population, were excluded from our analysis which may have led to an overestimation of the proportion who discussed their reproductive goals between baseline and follow-up. This also limits the generalisability of our findings. Fourth, we did not account for changes in healthcare provider over the study period. Our analysis measured reproductive discussions with any healthcare provider as opposed to with the primary HIV care provider; hence, we cannot conclude whether the effect of having a woman HIV care provider contributes to discussions with that provider or facilitating discussions with other healthcare providers involved in women’s health care. We were, however, able to account for women living with HIV accessing health care in various settings by measuring reproductive discussions that occurred with any healthcare provider. Additionally, we did not measure participant and healthcare provider knowledge about reproductive health care for women living with HIV and safer conception strategies. Healthcare provider specialty or training may have influenced the initiation of reproductive discussions, however, reliable data on provider specialty was not available for inclusion in our model.45 We measured healthcare provider gender and women’s comfort discussing reproductive goals at the same time point; consequently, the direction of association cannot be inferred. Reproductive discussions may still be relevant for women who reported being unable to conceive, leading to an overestimation of the proportion of women living with HIV who discussed their reproductive goals with a healthcare provider. Estimations of excess relative risk may be biased by the rare outcome assumption leading to an underestimation of the indirect effect.46 Finally, there may be unmeasured confounders that were not considered in our model, including post-exposure confounders of the mediator-outcome relationship. We included previous reproductive discussions as a confounder in our model; however, previous discussions may be a post-exposure confounder of the mediator-outcome relationship, which may have led to model bias.
Previous studies have described gaps in the delivery of reproductive health care for women living with HIV. In this study, we empirically assessed the relationships between healthcare provider gender, patient comfort, and discussing reproductive goals. Through the application of components of the causal framework, the longitudinal design of our study, and measured confounders, we are able to estimate the causal mechanism between healthcare provider gender and reproductive discussions. We highlight a potential avenue for interventions aimed at the delivery of reproductive health care. Further research is needed to better understand the concept of comfort and strategies that promote comfort discussing reproductive goals among women living with HIV. These strategies are needed to support the family planning, preconception, contraceptive, abortion, and sexual health needs of this population.
Acknowledgements
The CHIWOS Research Team thanks the women living with HIV for their contributions to this study and the national team of co-investigators, collaborators, and Peer Research Associates. We acknowledge the National Steering Committee; our three provincial community advisory boards; the national CHIWOS Aboriginal Advisory Board-Positive Aboriginal Women; the national CHIWOS African, Caribbean and Black Advisory Board; and all of our partnering organizations and funders.
ϒCHIWOS Research Team: Rahma Abdul-Noor (Women’s College Research Institute), Aranka Anema (Harvard Medical School), Jonathan Angel (Ottawa Hospital Research Institute), Dada Mamvula Bakombo (McGill University Health Centre), Fatimatou Barry (Women’s College Research Institute), Greta Bauer (University of Western Ontario), Kerrigan Beaver (Women’s College Research Institute), Marc Boucher (CHU Ste-Justine), Isabelle Boucoiran (CHU Ste-Justine), Jason Brophy (Children’s Hospital of Eastern Ontario), Lori Brotto (University of British Columbia), Ann N. Burchell (St, Michael’s Hospital), Claudette Cardinal (Simon Fraser University), Allison Carter (Kirby Institute), Lynne Cioppa (Women’s College Research Institute), Tracey Conway (Women’s College Research Institute), José Côté (Centre Hospitalier de l’Université de Montréal), Jasmine Cotnam (Canadian Aboriginal AIDS Network), Cori d’Ambrumenil (AIDS Vancouver Island), Janice Dayle, (McGill University Health Centre), Erin Ding (British Columbia Centre for Excellence in HIV/AIDS), Danièle Dubuc, (McGill University Health Centre), Janice Duddy (Pacific AIDS Network), Mylène Fernet (Université du Québec à Montréal), Annette Fraleigh (Women’s College Research Institute), Peggy Frank (Simon Fraser University), Brenda Gagnier (Women’s College Research Institute), Marilou Gagnon (University of Victoria), Jacqueline Gahagan (Dalhousie University), Claudine Gasingirwa (Women’s College Research Institute), Nada Gataric (British Columbia Centre for Excellence in HIV/AIDS), Rebecca Gormley (British Columbia Centre for Excellence in HIV/AIDS), Saara Greene (McMaster University), Danielle Groleau (McGill University), Charlotte Guerlotté (COCQ- SIDA), Trevor Hart (Ryerson University), Catherine Hankins (McGill University), Emily Heer (Alberta Health Services), Robert S. Hogg (Simon Fraser University), Terry Howard (Glasshouse Consultants), Shazia Islam (Women’s College Research Institute), Joseph Jean-Gilles (GAP-VIES), Hermione Jefferis (AIDS Vancouver Island), Evin Jones (Pacific AIDS Network), Charu Kaushic (McMaster University), Mina Kazemi (Women’s College Research Institute), Mary Kestler (Oak Tree Clinic BCWH) Maxime Kiboyogo (McGill University Health Centre), Marina Klein (McGill University Health Centre), Nadine Kronfli (McGill University Health Center), Gladys Kwaramba (Women’s College Research Institute), Gary Lacasse (Canadian AIDS Society), Ashley Lacombe-Duncan (University of Michigan), Melanie Lee (Simon Fraser University), Rebecca Lee (CIHR Canadian HIV Trials Network), Jenny Li (British Columbia Centre for Excellence in HIV/AIDS), Viviane Lima (British Columbia Centre for Excellence in HIV/AIDS), Elisa Lloyd-Smith (Vancouver General Hospital), Carmen Logie (University of Toronto), Evelyn Maan (Oak Tree Clinic), Valérie Martel-Lafrenière (Centre Hospitalier de l’Université de Montréal), Carrie Martin (Canadian Aboriginal AIDS Network), Renee Masching (Canadian Aboriginal AIDS Network), Lyne Massie (Université du Québec à Montréal), Melissa Medjuck (formerly of the Positive Women’s Network), Brigitte Ménard, (McGill University Health Centre), Cari L. Miller (formerly of Simon Fraser University), Judy Mitchell (Positive Living North), Gerardo Mondragon (British Columbia Centre for Excellence), Deborah Money (Faculty of Medicine at UBC), Ken Monteith (COCQ-SIDA), Marvelous Muchenje (Women’s Health in Women’s Hands CHC), Florida Mukandamutsa (CASM), Mary Ndung’u (African Partnership Against AIDS), Valerie Nicholson (Simon Fraser University), Kelly O’Brien (University of Toronto), Nadia O’Brien (McGill University Health Centre and McGill University), Gina Ogilvie (University of British Columbia), Susanna Ogunnaike-Cooke (Public Health Agency of Canada), Joanne Otis (Université du Québec à Montréal), Rebeccah Parry (Simon Fraser University), Sophie Patterson (Simon Fraser University), Angela Paul (Positive Living North), Doris Peltier (Canadian Aboriginal AIDS Network), Neora Pick (Oak Tree Clinic BCWH), Alie Pierre (McGill University Health Centre), Jeff Powis (Michael Garron Hospital), Karène Proulx-Boucher (McGill University Health Centre), Corinna Quan (Windsor Regional Hospital), Jesleen Rana (Women’s Health in Women’s Hands CHC), Eric Roth (University of Victoria), Danielle Rouleau (Centre Hospitalier de l’Université de Montréal), Geneviève Rouleau (Centre Hospitalier de l’Université de Montréal), Sergio Rueda (Centre for Addiction and Metal Health), Kate Salters (British Columbia Centre for Excellence in HIV/AIDS), Margarite Sanchez (ViVA), Roger Sandre (Haven Clinic), Jacquie Sas (CIHR Canadian HIV Trials Network), Édénia Savoie (McGill University Health Centre), Paul Sereda (British Columbia Centre for Excellence in HIV/AIDS), Stephanie Smith (Women’s College Research Institute), Marcie Summers (formerly of the Positive Women’s Network), Wangari Tharao (Women’s Health in Women’s Hands CHC), Christina Tom (Simon Fraser University), Cécile Tremblay (Centre Hospitalier de l’Université de Montréal), Jason Trigg (British Columbia Centre for Excellence in HIV/AIDS), Sylvie Trottier (Centre Hospitalier Universitaire de Québec), Angela Underhill (Women’s College Research Institute), Anne Wagner (Ryerson University), Sharon Walmsley (University Health Network), Clara Wang (British Columbia Centre for Excellence in HIV/AIDS), Kath Webster (Simon Fraser University), Wendy Wobeser (Queen’s University), Denise Wozniak (Positive Living Society of British Columbia), Mark H. Yudin (St. Michael’s Hospital), Wendy Zhang (British Columbia Centre for Excellence in HIV/AIDS), Julia Zhu (British Columbia Centre for Excellence in HIV/AIDS). All other CHIWOS Research Team Members who wish to remain anonymous.
Funding Statement
CHIWOS is funded by the Canadian Institutes of Health Research (CIHR) [grant number MOP-111041], the CIHR Canadian HIV Trials Network, Canadian Institutes of Health Research [CTN 262], the Ontario HIV Treatment Network (OHTN), and the Academic Health Science Centres (AHSC) Alternative Funding Plans (AFP) Innovation Fund. L.S. was funded by a CIHR Vanier Canada Graduate Scholarship, A.D.P. received salary support from Fonds de la recherche en santé du Québec (FRQS) and Fédération des médecins omnipraticiens du Québec through an LE-250 scholarship. N.O., A.N.B, and A.K. received salary support from CIHR.
References
- 1.Samji H, Cescon A, Hogg RS, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355, DOI: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171–181. DOI: 10.1001/jama.2016.5148. [DOI] [PubMed] [Google Scholar]
- 3.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet. 2019;6736(19). DOI: 10.1016/S0140-6736(19)30418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830–839. DOI: 10.1056/NEJMoa1600693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandelbrot L, Tubiana R, Le Chenadec J, et al. No perinatal HIV-1 transmission from women with effective antiretroviral therapy starting before conception. Clin Infect Dis. 2015;61(11):1715–1725. DOI: 10.1093/cid/civ578. [DOI] [PubMed] [Google Scholar]
- 6.Camacho-Gonzalez AF, Kingbo M-H, Boylan A, et al. Missed opportunities for prevention of mother-to-child transmission in the United States. AIDS. 2015;29(12):1511–1515. DOI: 10.1097/QAD.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forbes JC, Alimenti AM, Singer J, et al. A national review of vertical HIV transmission. AIDS. 2012;26(6):757–763. DOI: 10.1097/QAD.0b013e328350995c. [DOI] [PubMed] [Google Scholar]
- 8.Brown JL, Haddad LB, Gause NK, et al. Examining the contraceptive decisions of young, HIV-infected women: a qualitative study. Women Health. 2019;59(3):305–317. DOI: 10.1080/03630242.2018.1452836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgess A, Purssell E.. What is the relationship between increased access to HAART, relationship status and fertility decisions amongst HIV-positive women? A literature review and meta-analysis. J Clin Nurs. 2017;26(23–24):3800–3810. DOI: 10.1111/jocn.13731. [DOI] [PubMed] [Google Scholar]
- 10.Haddad LB, Wall KM, Mehta CC, et al. Trends of and factors associated with live-birth and abortion rates among HIV-positive and HIV-negative women. Am J Obstet Gynecol. 2017;216(1):71.e1–71.e16. DOI: 10.1016/j.ajog.2016.09.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floridia M, Tamburrini E, Masuelli G, et al. Rate, correlates and outcomes of repeat pregnancy in HIV-infected women. HIV Med. 2017;18(6):440–443. DOI: 10.1111/hiv.12473. [DOI] [PubMed] [Google Scholar]
- 12.Van Ommen CE, Albert AYK, Piske M, et al. Exploring the live birth rates of women living with HIV in British Columbia. Canada. Kowalska JD, ed. PLoS One. 2019;14(2):e0211434. DOI: 10.1371/journal.pone.0211434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Lancet HIV . U = U taking off in 2017. Lancet HIV. 2017;4(11):e475. DOI: 10.1016/S2352-3018(17)30183-2. [DOI] [PubMed] [Google Scholar]
- 14.Loutfy MR, Hart TA, Mohammed SS, et al. Fertility desires and intentions of HIV-positive women of reproductive age in Ontario, Canada: a cross-sectional study. PLoS One. 2009;4(12). DOI: 10.1371/journal.pone.0007925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finocchario-Kessler S, Dariotis JK, Sweat MD, et al. Do HIV-infected women want to discuss reproductive plans with providers, and are those conversations occurring? AIDS Patient Care STDS. 2010;24(5):317–323. DOI: 10.1089/apc.2009.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien N, Greene S, Carter A, et al. Envisioning women-centered HIV care: Perspectives from women living with HIV in Canada. Women’s Heal Issues. 2017;27(6):721–730. DOI: 10.1016/j.whi.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Kaida A, Patterson S, Carter A, et al. Contraceptive choice and use of dual protection among women living with HIV in Canada: priorities for integrated care. Perspect Sex Reprod Health. 2017;49(4):223–236. DOI: 10.1111/psrh.12046. [DOI] [PubMed] [Google Scholar]
- 18.Salters K, Loutfy M, De Pokomandy A, et al. Pregnancy incidence and intention after HIV diagnosis among women living with HIV in Canada. PLoS One. 2017;12(7):e0180524. DOI: 10.1371/journal.pone.0180524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization . Medical eligibility criteria for contraceptive use: A WHO family planning cornerstone. 5th ed. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 20.Steiner RJ, Dariotis JK, Anderson JR, et al. Preconception care for people living with HIV. AIDS. 2013;27:S113–S119. DOI: 10.1097/QAD.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 21.Loutfy MR, Blitz S, Zhang Y, et al. Self-reported preconception care of HIV-positive women of reproductive potential: a retrospective study. J Int Assoc Provid AIDS Care. 2014;13(5):424–433. DOI: 10.1177/2325957413494238. [DOI] [PubMed] [Google Scholar]
- 22.Loutfy M, Kennedy VL, Poliquin V, et al. No. 354-Canadian HIV pregnancy planning guidelines. J Obstet Gynaecol Can. 2018;40(1):94–114. DOI: 10.1016/j.jogc.2017.06.033. [DOI] [PubMed] [Google Scholar]
- 23.World Health Organization . Consolidated guideline on sexual and reproductive health and rights of women living with HIV. Geneva: World Health Organization; 2017. [PubMed] [Google Scholar]
- 24.CATIE . The power of undetectable: what you need to know about HIV treatment as prevention. 2019. Available from: https://www.catie.ca/en/practical-guides/power-undetectable/treatment-prevention.
- 25.Dyer K, das Nair R.. Why don’t healthcare professionals talk about sex? A systematic review of recent qualitative studies conducted in the United Kingdom. J Sex Med. 2013;10(11):2658–2670. DOI: 10.1111/j.1743-6109.2012.02856.x. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale RH, Bradley H, Weiser J.. Reproductive health counseling delivered to women living with HIV in the United States. AIDS Care. 2017;29(7):928–935. DOI: 10.1080/09540121.2017.1280125. [DOI] [PubMed] [Google Scholar]
- 27.Webster K, Carter A, Proulx-boucher K, et al. Strategies for recruiting women living with human immunodeficiency virus in community-based research: lessons from Canada. Prog Commun Health Partnerships: Res Educ Action. 2018;12(1):21–34. [DOI] [PubMed] [Google Scholar]
- 28.Loutfy M, de Pokomandy A, Kennedy VL, et al. Cohort profile: the Canadian HIV Women’s Sexual and Reproductive Health Cohort Study (CHIWOS). PLoS One. 2017;12(9):e0184708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaida A, Carter A, Nicholson V, et al. Hiring, training, and supporting Peer Research Associates: operationalizing community-based research principles within epidemiological studies by, with, and for women living with HIV. Harm Reduct J. 2019;16(47). DOI: 10.1186/s12954-019-0309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wright K, Naar-King S, Lam P, et al. Stigma scale revised: reliability and validity of a brief measure of stigma for HIV+ Youth. Journal of Adolescent Health. 2007;40(1):96–98. DOI: 10.1016/J.JADOHEALTH.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Discacciati A, Bellavia A, Lee JJ, et al. Med4way: a Stata command to investigate mediating and interactive mechanisms using the four-way effect decomposition. Int J Epidemiol. 2018: 1–6. DOI: 10.1093/ije/dyy236. [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ. A unification of mediation and interaction: a four-Way decomposition. Epidemiology. 2014;25:749, DOI: 10.1097/EDE.0000000000000121.A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Badell ML, Lathrop E, Haddad LB, et al. Reproductive healthcare needs and desires in a cohort of HIV-positive women. Infect Dis Obstet Gynecol. 2012;2012, DOI: 10.1155/2012/107878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Squires KE, Hodder SL, Feinberg J, et al. Health needs of HIV-infected women in the United States: insights from The Women Living Positive Survey. AIDS Patient Care STDS. 2011;25(5):279–285. DOI: 10.1089/apc.2010.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ingram D, Hutchinson SA.. Double binds and the reproductive and mothering experiences of HIV-positive women. Qual Health Res. 2000;10(1):117–132. DOI: 10.1177/104973200129118282. [DOI] [PubMed] [Google Scholar]
- 36.Carlsson-Lalloo E, Berg M, Mellgren Å, et al. Sexuality and childbearing as it is experienced by women living with HIV in Sweden: a lifeworld phenomenological study. Int J Qual Stud Health Well-being. 2018;13(1). DOI: 10.1080/17482631.2018.1487760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kestler M, Murray M, Money D, et al. The Oak Tree Clinic: The envisioned model of care for women living with human immunodeficiency virus in Canada. Women’s Heal Issues. 2018;28(2):197–198. DOI: 10.1016/j.whi.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 38.Steiner RJ, Finocchario-Kessler S, Dariotis JK.. Engaging HIV care providers in conversations with their reproductive-age patients about fertility desires and intentions: a historical review of the HIV epidemic in the United States. Am J Public Health. 2013;103(8):1357–1366. DOI: 10.2105/AJPH.2013.301265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chabikuli NO, Awi DD, Chukwujekwu O, et al. The use of routine monitoring and evaluation systems to assess a referral model of family planning and HIV service integration in Nigeria. special issue: family planning and HIV.). AIDS. 2009;23(1):S97–S103. [DOI] [PubMed] [Google Scholar]
- 40.Carlsson-Lalloo E, Rusner M, Mellgren Å, et al. Sexuality and reproduction in HIV-positive women: a meta-synthesis. AIDS Patient Care STDS. 2016;30(2):56–69. DOI: 10.1089/apc.2015.0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norberg A, Nelson J, Holly C, et al. Experiences of HIV-infected adults and healthcare providers with healthcare delivery practices that influence engagement in US primary healthcare settings. JBI Database Syst Rev Implement Reports. 2019;17(6):1154–1228. DOI: 10.11124/jbisrir-2017-003756. [DOI] [PubMed] [Google Scholar]
- 42.Hughes AK, Rostant OS, Curran PG.. Improving sexual health communication between older women and their providers. Res Aging. 2014;36(4):450–466. DOI: 10.1177/0164027513500055. [DOI] [PubMed] [Google Scholar]
- 43.Nimmon L, Stenfors-Hayes T.. The “handling” of power in the physician-patient encounter: perceptions from experienced physicians. BMC Med Educ. 2016;16(1):114. DOI: 10.1186/s12909-016-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingsberg SA, Schaffir J, Faught BM, et al. Female sexual health: barriers to optimal outcomes and a roadmap for improved patient–clinician communications. J Women’s Heal. 2019;28(4):432–443. DOI: 10.1089/jwh.2018.7352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Brien N, Godard-Sebillotte C, Skerritt L, et al. Assessing gaps in comprehensive HIV care across settings of care for women living with HIV in Canada. J Women’s Heal. 2020;29(11):1475–1485. DOI: 10.1089/jwh.2019.8121. [DOI] [PubMed] [Google Scholar]
- 46.VanderWeele TJ, Valeri L, Ananth C V.. Counterpoint: mediation formulas with binary mediators and outcomes and the “Rare Outcome Assumption”. Am J Epidemiol. 2019;188(7):1204–1205. DOI: 10.1093/aje/kwy281. [DOI] [PMC free article] [PubMed] [Google Scholar]