Abstract
Background
The effects of cytomegalovirus (CMV)-specific cell-mediated immunity (CMI) on CMV infection in patients with autoimmune diseases receiving immunosuppressants have not been explored.
Methods
Patients with active systemic lupus erythematosus (SLE) were preemptively monitored for clinically significant CMV infection (CsCMVI; defined as plasma CMV DNA loads >3 log10 IU/mL). CMV-specific CMI was assessed using an enzyme-linked immunosorbent assay (QuantiFERON-CMV [QF]) before as well as 1 and 3 months after intense immunosuppressive therapy.
Results
The study included 55 patients with active SLE; patients were a mean age (SD) of 34 (13) years and had a median SLE Disease Activity Index 2000 score (SD) of 14 (8), and 93% were female. Most patients had renal involvement (67%), received methylprednisolone (93%), and were CMV-seropositive (95%). Thirteen (23.6%) patients developed CsCMVI. Among patients with active SLE who were QF-negative (QF–) and QF-positive (QF+) before receiving immunosuppressive therapy, 28.6% and 25% developed CsCMVI, respectively (P = .69). However, 1 month postimmunosuppression, more QF– than QF+ patients developed CsCMVI (44.4% vs 11.8%; P = .03; adjusted hazard ratio, 4.97; 95% CI, 1.07–23.10; P = .04).
Conclusions
Patients with active SLE and low CMV-specific T-cell responses could develop CMV infection after receiving immunosuppressants. Further studies should focus on CMV-specific CMI among patients with autoimmune diseases.
Keywords: autoimmune disease, CMV-specific immunity, immunosuppressant, quantiferon, SLE, viremia
Infectious complications are undesirable consequences of immunosuppressive therapies administered to patients with systemic lupus erythematosus (SLE) and active organ involvement [1]. A retrospective study revealed that 58% of patients with SLE developed up to 5 episodes of infection within the first year of treatment, with 63% of episodes requiring a hospital stay [2]. One-third of the causative pathogens were opportunistic. Infections were likely attributable to the patients’ profoundly immunocompromised condition and resulted in serious consequences, including death [2–4]. Cytomegalovirus (CMV) is an emerging cause of opportunistic infections in HIV-negative, nontransplant immunocompromised patients, especially those with active autoimmune diseases such as SLE who require intensive immunosuppressive therapy for life-threatening organ involvement [5–8]. A case–control study of CMV disease in SLE patients receiving immunosuppressive therapy revealed that CMV tissue-invasive diseases such as pneumonitis, gastritis, colitis, and severe disseminated disease had high mortality rates of up to 60% [6]. A cumulative dose of corticosteroids equivalent to 10 mg of prednisolone per day for 3 months could double the risk of CMV infection [5, 6].
CMV usually infects healthy individuals and causes no or mild symptoms. Immune responses against CMV, including antibodies and T-lymphocytes, control the virus but do not eliminate it [9]. Detectable virus is still present in the host during the latent infection period. When host immunity declines, the latent virus can reactivate and cause renewed infection, which can be asymptomatic or result in symptomatic CMV disease (eg, CMV syndrome, tissue-invasive disease, or disseminated CMV disease) [7]. CMV-specific cell-mediated immunity (CMI) plays an important role in controlling CMV infection in immunocompromised patients, especially among solid organ transplant (SOT) recipients [9]. Prospective studies to preemptively monitor CMV infection in patients with active SLE receiving immunosuppressants have not previously been carried out. Studies of CMV-specific CMI in this population are also scarce. Therefore, we aimed to investigate the roles of CMV-specific CMI, and especially T-cell responses, as predictors of CMV infection outcome among these patients.
METHODS
Study Population
We conducted a prospective study of patients with active SLE between November 2017 and May 2020 at a tertiary care university hospital in Bangkok, Thailand. Patients with active SLE aged 18 years or older receiving intensive immunosuppressive therapy were eligible (Supplementary Figure 1). All patients provided informed consent before enrollment. SLE patients fulfilled Systemic Lupus International Collaborating Clinics 2012 classification criteria [10]. Clinical data including age, sex, CMV serostatus, disease activity, active organ involvement, type of immunosuppressive therapy, and laboratory findings were extracted. All patients included in this study had undetectable or asymptomatic low-level (<3 log10 IU/mL) CMV DNAemia before immunosuppression. CMV-specific immunoglobulin G (IgG) was evaluated using an enzyme-linked fluorescent immunoassay performed on a VIDAS instrument (bioMérieux, Durham, NC, USA). Results were interpreted as follows: negative (<4 AU/mL), equivocal (4–5 AU/mL), or positive (>6 AU/mL). Disease activity was measured using the SLE Disease Activity Index 2000 (SLEDAI-2K) [11]. SLEDAI-2K weighs individual 24 descriptors in 9 organ systems; these scores can be summed to yield a global score ranging from 0 to 105 (Supplementary Figure 2). Disease activity was defined as follows: no activity (SLEDAI-2K score 0), mild activity (SLEDAI-2K score 1–5), moderate activity (SLEDAI-2K score 6–10), high activity (SLEDAI-2K score 11–19), and very high activity (SLEDAI-2K score ≥20). Active SLE was defined as moderate disease activity or greater [12–14] or active disease otherwise determined by a rheumatologist.
At our center, induction immunosuppressive therapies for the majority of patients include intravenous methylprednisolone (500–1000 mg/d for 3–5 days), intravenous cyclophosphamide based on the National Institute of Health regimen (standard dose; 0.5–1 g/m2 every month for 6 months), or mycophenolate mofetil (2–3 g/d). The Euro-Lupus intravenous cyclophosphamide regimen (low dose; 500 mg every 2 weeks for 3 months for a cumulative dose of 3 g) was administered in some patients. Rituximab (1–2 g) was implemented as an adjunct therapy in refractory patients. Maintenance therapy was with either azathioprine, mycophenolate mofetil, and/or calcineurin inhibitors including tacrolimus or cyclosporin.
The primary outcome was incidence of clinically significant CMV infection (CsCMVI) among patients with active SLE receiving intense immunosuppressants. Patients with QuantiFERON-CMV-positive (QF+) and QF-negative (QF–) results were analyzed separately. The secondary objectives were to monitor CMV-specific CMI and investigate other predictors of CsCMVI. The study protocol was approved by the Human Research Ethics Committee of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (approval number: ID 06-61-14).
Clinical Definitions
Patients with active SLE were preemptively monitored for CMV infection. CMV DNA loads in plasma were quantified using a RealTime CMV assay (Abbott Molecular Inc., Des Plaines, IL, USA) and reported in international units (IU)/mL (1 copy/mL = 1.56 IU/mL). CMV DNAemia was defined as detectable plasma CMV DNA of any level. CsCMVI was defined as plasma CMV DNA loads >3 log10 IU/mL. CsCMVI was further subclassified into asymptomatic CMV infection (no symptoms) and CMV disease (any symptoms). CMV disease was further subclassified into CMV syndrome or CMV tissue-invasive diseases. CMV syndrome was defined as CMV infection and at least 2 of the following: fever ≥38°C for at least 2 days, new or increased malaise or fatigue, 2 independent measurements of leukopenia or neutropenia, 5% atypical lymphocytes, thrombocytopenia, or increased hepatic aminotransferases above twice the upper normal limit [9]. CMV tissue-invasive disease was defined as CMV infection with specific organ symptoms such as gastritis, colitis, or pneumonitis [9].
Measurement of CMV-Specific T-cell Responses
The QuantiFERON-CMV assay (Qiagen, Bangkok, Thailand) was used to assess CMV-specific T-cell responses. Aliquots of whole blood (1 mL) were collected into 3 heparinized tubes. Tube 1 contained a mixture of 22 peptides (1 µg/mL each) from a variety of CMV proteins, including phosphoprotein 65 (pp65), immediate early protein (IE)-1, IE-2, pp50, and glycoprotein B. Peptides were selected to be recognized by the most common HLA types present in the general population. Tube 2 served as a positive mitogen control tube and contained phytohemagglutinin. Tube 3 served as a negative control and contained sterile phosphate-buffered saline. The tubes were shaken firmly but not overly vigorously and incubated for 18–24 hours at 37°C. Subsequently, the supernatant was harvested, and interferon (IFN)-γ levels were measured in IU/mL using a standard enzyme-linked immunosorbent assay (ELISA) as per the manufacturer’s instructions. The recommended cutoff value for CMV-specific IFN-γ is 0.2 IU/mL. Results were reported as reactive, nonreactive, and indeterminate [15]. If IFN-γ in Tube 1 was <0.2 IU/mL and the mitogen control (Tube 2) was positive (≥0.5 IU/mL), the test was considered negative. If IFN-γ in Tube 1 was <0.2 IU/mL and the mitogen control (Tube 2) was negative (<0.5 IU/mL), the test was considered indeterminate. Patients with reactive and nonreactive results were considered to be QF+ and QF–, respectively. Those with indeterminate results were considered QF– for comparative analysis.
Statistical Analyses
Categorical variables were reported as frequencies and percentages. Continuous variables were reported as means and SDs or medians and interquartile ranges (IQRs). The chi-square test or Fisher exact test was used to assess differences between categorical variables, as appropriate. The cumulative CsCMVI-free survival of QF+ and QF– patients with active SLE was estimated using Kaplan-Meier methods. Differences between the 2 groups were assessed using the log-rank test. A Cox proportional hazard model was used to analyze predictors of CsCMVI. Variables with P values <.10 in the univariate analysis were included in the multivariate analysis. P values <.05 were considered statistically significant. Statistical analyses were performed using Stata, version 16 (StataCorp, College Station, TX, USA).
RESULTS
Study Population
Fifty-five patients with active SLE were enrolled in the study. Their baseline characteristics are shown in Table 1. The mean age of the patients (SD) was 34 (13) years, and 51 (93%) were female. The mean SLEDAI-2K score (SD) was 14 (8). The most common organ involvements were kidney (67%), hematologic system (49%), skin (44%), musculoskeletal system (24%), neurologic system (16%), and serosa (13%). The most common immunosuppressive therapy was intravenous methylprednisolone (93%), followed by intravenous cyclophosphamide (82%), mycophenolic acid (27%), and rituximab (13%). Forty-seven patients (85.5%) were CMV-seropositive, 3 (5.5%) were CMV-seronegative, and 5 (9.1%) had unknown serological status.
Table 1.
Baseline Characteristic of 55 Patients With Active SLE
| Total (n = 55) | CsCMVI (n = 13) | Non-CsCMVI (n = 42) | P Value | |
|---|---|---|---|---|
| Age, mean (SD), y | 34 (13) | 40 (15) | 33 (12) | .09 |
| Female, No. (%) | 51 (93) | 12 (92) | 39 (93) | .95 |
| SLEDAI-2K score, mean (SD) | 14 (8) | 11 (7) | 16 (9) | .07 |
| Active organ involvement, No. (%) | ||||
| Skin | 24 (44) | 4 (31) | 20 (48) | .28 |
| Musculoskeletal system | 13 (24) | 3 (23) | 10 (24) | .96 |
| Serosa | 7 (13) | 3 (23) | 4 (10) | .20 |
| Kidney | 37 (67) | 7 (54) | 30 (71) | .24 |
| Neurological system | 9 (16) | 4 (31) | 5 (12) | .11 |
| Hematologic system | 27 (49) | 6 (46) | 21 (50) | .81 |
| CMV IgG,a No. (%) | ||||
| Positive | 47 (94) | 11 (85) | 36 (92) | .10 |
| QuantiFERON-CMV,b No. (%) | ||||
| Reactive | 16 (38) | 4 (33) | 12 (40) | .69 |
| Nonreactive | 8 (19) | 4 (33) | 4 (13) | .14 |
| Indeterminate | 18 (43) | 5 (42) | 13 (43) | .92 |
| Immunosuppressants, No. (%) | ||||
| Methylprednisolone | 51 (93) | 9 (69) | 42 (100) | .002c |
| Cyclophosphamide | 45 (82) | 10 (77) | 35 (83) | .60 |
| Mycophenolic acid | 15 (27) | 3 (23) | 12 (29) | .70 |
| Rituximab | 7 (13) | 3 (23) | 4 (10) | .11 |
Abbreviations: BMI, body mass index; CMV, cytomegalovirus; IgG, immunoglobulin G; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus; SLEDAI-2K, SLE disease activity index 2000.
aAssessed in n = 50 patients.
bAssessed in n = 42 patients.
cFisher exact test.
CsCMVI
During a mean follow-up (SD) of 2.87 (1.2) months, CMV DNA was detected in the plasma of 34 (61.8%) patients. Among these patients, 13 (13.6%) had CsCMVI. Among patients with CsCMVI, 9 (69.2%) had asymptomatic CMV infection, 2 (15.4%) had CMV syndrome, 1 (7.7%) had CMV colitis, and 1 (7.7%) had CMV pneumonitis. The mean onset of CsCMVI (SD) was at 1.0 (0.6) months. The median CMV DNA load (IQR) was 2393 (1796–7437) IU/mL. All patients were treated with intravenous ganciclovir (5 mg/kg) every 12 hours (renally adjusted) until at least 1 measurement of undetectable DNA load in plasma in combination with reduced doses of immunosuppressants. One patient with CsCMVI died ~2 months after receiving intensive immunosuppressive therapy. This death was not attributable to CMV infection.
CMV-Specific T-cell Responses Before Administration of Immunosuppressants
CMV-specific T-cell immunity measured was measured using the QuantiFERON-CMV assay. Immune responses against CMV in active SLE patients receiving intense immunosuppressive therapy at different time points are shown in Table 2. Among 42 evaluable patients, 16 (38.1%) and 26 (61.9%) patients were QF+ and QF–, respectively. Among the latter patients, 8 (19%) were QF-nonreactive and 18 (42.9%) were QF-indeterminate. Among CMV-seropositive patients, 12 (33.3%) were QF-reactive, 7 (19.4%) were QF-nonreactive, 8 (22.2%) were QF-indeterminate, and 9 (25.1%) were not assessed. The single CMV-seronegative patient had an indeterminate QF result. Of the 2 patients whose CMV IgG was not measured, 1 was QF-reactive and 1 was QF-indeterminate.
Table 2.
CMV-Specific T-cell Immunity Measured by QuantiFERON-CMV and the Occurrence of CMV Infection in Patients With Active SLE Receiving Intense Immunosuppressants
| Outcomes | CMV-Specific T-cell Immunity, No. (%) | |||
|---|---|---|---|---|
| QF-Positive | QF-Negative | |||
| Reactive | Nonreactive | Indeterminate | Nonreactive and Indeterminate | |
| Before receiving immunosuppressants | ||||
| All patients (n = 42) | n = 16 | n = 8 | n = 18 | n = 26 |
| CMV DNAemia | 10 (62.5) | 7 (87.5) | 8 (44.4) | 15 (57.6) |
| Ref. | P = .20 | P = .29 | P = .75 | |
| CsCMVI | 4 (25) | 4 (50) | 5 (22.2) | 8 (28.6) |
| Ref. | P = .67 | P = .55 | P = .69 | |
| 1 mo after receiving immunosuppressant | ||||
| All patients (n = 35) | n = 17 | n = 11 | n = 7 | n = 18 |
| CMV DNAemia | 10 (58.8) | 11 (100) | 5 (71.4) | 16 (88.9) |
| Ref. | P = .02a | P = .56 | P = .04 | |
| CsCMVI | 2 (11.8) | 6 (54.5) | 2 (28.6) | 8 (44.4) |
| Ref. | P = .01 | P = .72 | P = .03 |
Abbreviations: CMV, cytomegalovirus; CsCMVI, clinically significant CMV infection; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus.
aFisher exact test.
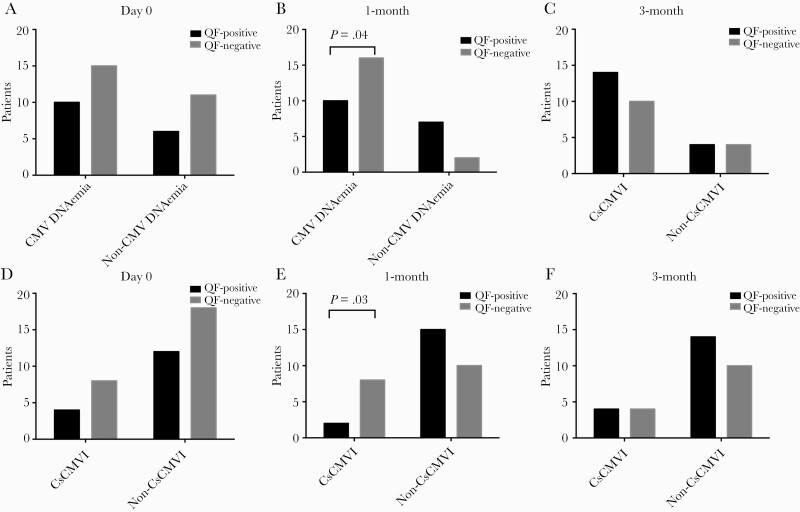
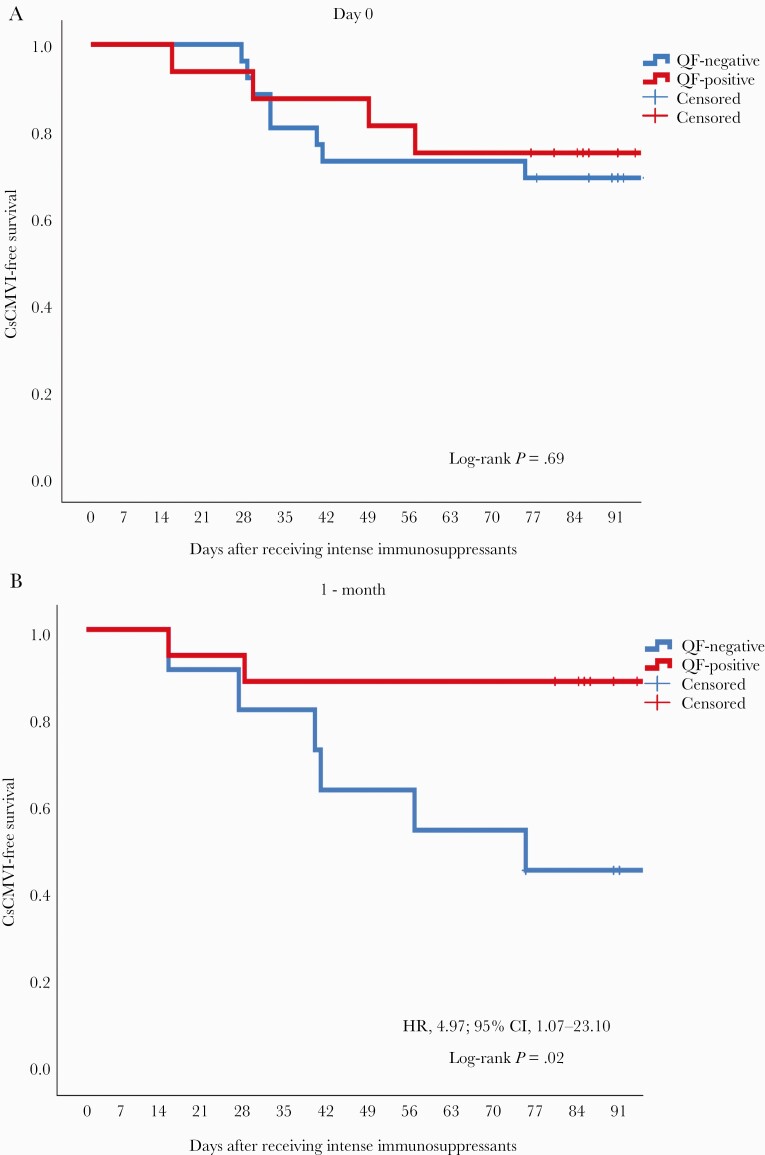
Overall, 15/26 (57.6%) QF– patients and 10/16 (62.5%) QF+ patients developed CMV DNAemia (P = .75). Moreover, 4/14 (25%) QF– patients and 8/26 (28.6%) QF+ patients developed CsCMVI (P = .69) (Figure 1). Kaplan-Meier analysis showed no significant difference in the CsCMVI-free survival of QF+ and QF– patients (log-rank test P = .69) (Figure 2).
Figure 1.
Association between CMV-specific cell-mediated immunity measured by QF before and 1 month after administration of intense immunosuppressants and the occurrence of CMV DNAemia (A–C) and CsCMVI (D–F) among patients with active SLE. Abbreviations: CMV, cytomegalovirus; CsCMVI, clinically significant CMV infection; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus.
Figure 2.
Kaplan-Meier curves showing CsCMVI-free survival among QF+ and QF– patients with active SLE before (A) and 1 month (B) after administration of intense immunosuppressants. Abbreviations: CMV, cytomegalovirus; CsCMVI, clinically significant CMV infection; HR, hazard ratio; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus.
CMV-Specific T-cell Responses Post–Administration of Immunosuppressants
At 1 month postimmunosuppression, CMV-specific T-cell immunity was measured in 35 patients using QuantiFERON-CMV; 17 (48.6%) patients were QF+ and 18 (51.4%) were QF–. Among the latter group of patients, 11 (31.4%) and 7 (20%) patients were QF-nonreactive and QF-indeterminate, respectively. QF– patients developed CMV DNAemia significantly more frequently (16/18, 88.9%) than QF+ patients (10/17, 58.8%; P = .04). QF– patients also developed CsCMVI significantly more frequently (8/18, 44.4%) than QF+ patients (2/17, 11.8%; P = .03) (Figure 1). Kaplan-Meier analysis revealed a significant difference in CsCMVI-free survival between the 2 groups (log-rank P = .02) (Figure 2).
Among the 19 patients whose full data were available before as well as 1 month after immunosuppression, 9 (47.4%) were QF+ and 10 (52.6%) were QF–. Among the latter group of patients, 4 (21.1%) and 6 (31.5%) patients were QF-nonreactive and QF-indeterminate, respectively. Similar proportions of QF– (8/10, 80.0%) and QF+ (7/9, 77.8%) patients developed CMV DNAemia (P > .999). QF– patients showed a nonsignificant trend toward more frequent CsCMVI (4/10, 40%) compared with QF+ patients (2/9, 22.2%; P = .63). CMV-specific T-cell responses measured by QuantiFERON-CMV and the occurrence of CMV infection among 19 active SLE patients receiving intense immunosuppressants at different time points are shown in Supplementary Table 1.
Monitoring of CMV-Specific T-cell Responses
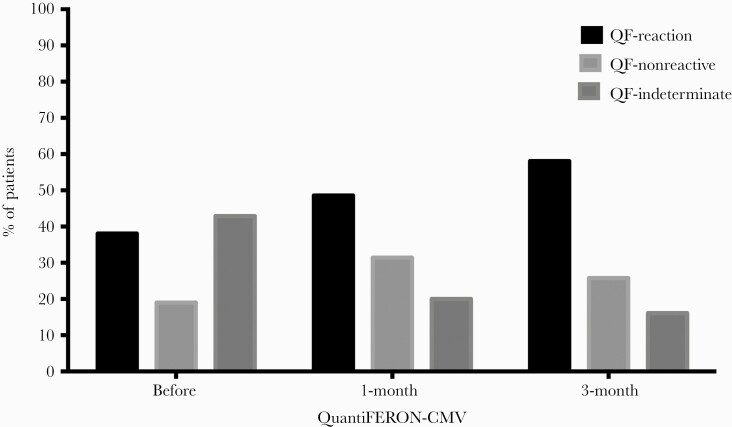
Data for the subset of patients with QuantiFERON-CMV results before as well as 1 and 3 months after receiving immunosuppressants are shown in Figure 3. For 16 patients with QF-reactive results pre-immunosuppression, 12 (75%) remained reactive and 4 (25%) became nonreactive (n = 3) or indeterminate (n = 1) after 1 month of immunosuppressive therapy. Among patients showing QF reversion, 2 (50%) developed CsCMVI. At 3 months postimmunosuppression, 31 patients had QuantiFERON-CMV results; 18 (58.1%) were reactive, 8 (25.8%) were nonreactive, and 5 (16.1%) were indeterminate.
Figure 3.
Proportion of patients with reactive, nonreactive, or indeterminate CMV-specific cell-mediated immunity measured by QF before, 1 month after, and 3 months after administration of intense immunosuppressants in patients with active SLE. Abbreviations: CMV, cytomegalovirus; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus.
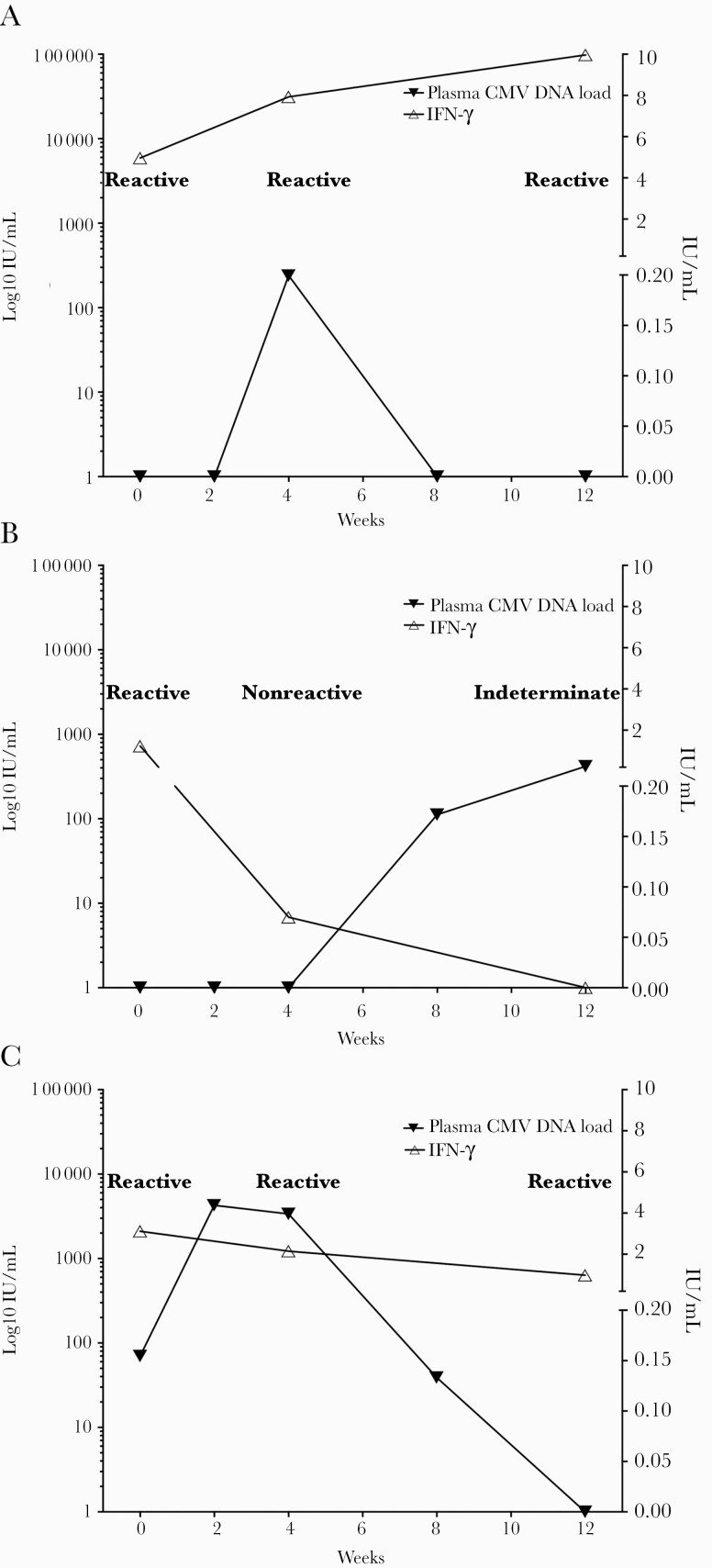
CMV-specific CMI measured by QuantiFERON-CMV (including IFN-γ level) and plasma CMV DNA load were prospectively monitored in 3 representative patients (Figure 4). Patient A developed CMV DNAemia (2 log10 IU/mL) after 4 weeks of immunosuppressive therapy; CMV DNA load was later suppressed, along with an increase in IFN-γ level (in the reactive range). By contrast, patient B developed CMV DNAemia (2 log10 IU/mL) after the initially QF-reactive results became nonreactive. Finally, patient C achieved an undetectable CMV DNA load following CMV DNAemia (3 log10 IU/mL) with consecutive QF-reactive results.
Figure 4.
Monitoring of CMV-specific cell-mediated immunity by QF. IFN-γ levels (right y-axis) and plasma cytomegalovirus DNA loads (left y-axis) were assessed before as well as 1 and 3 months after administration of intense immunosuppressants in 3 representative patients with active SLE (A, B, and C). Abbreviations: CMV, cytomegalovirus; IFN, interferon; QF, QuantiFERON-CMV; SLE, systemic lupus erythematosus.
Predictors of CsCMVI in Patients With Active SLE
Risk factors for CsCMVI were analyzed using a Cox proportional hazard model (Table 3). In univariate analysis, QF− status among patients with active SLE 1 month after receiving intense immunosuppressants was associated with CsCMVI (hazard ratio [HR], 4.89; 95% CI, 1.06–22.74; P = .04). Additionally, neuropsychiatric manifestations of SLE were marginally associated with CsCMVI (HR, 2.73; 95% CI, 0.84–8.88; P = .09). However, age, sex, SLEDAI score, type of immunosuppressant, and lymphopenia (<1000/mm3) were not associated with CsCMVI. In multivariate analysis, QF− status at 1 month postimmunosuppression remained significantly associated with CsCMVI (adjusted HR, 4.97; 95% CI, 1.07–23.10; P = .04).
Table 3.
Cox Proportional Hazard Modeling of Predictors of Clinically Significant CMV Infection Among Patients With Active SLE
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | |
| Age | 1.03 | 0.99–1.07 | .14 | |||
| Female | 0.90 | 0.12–6.96 | .92 | |||
| SLEDAI-2K score | 0.91 | 0.81–1.02 | .10 | |||
| Neuropsychiatric SLE | 2.73 | 0.84–8.88 | .09 | 2.39 | 0.70–8.19 | .17 |
| Methylprednisolone therapy | 0.97 | 0.13–7.47 | .98 | |||
| Cyclophosphamide therapy | 0.58 | 0.16–2.09 | .40 | |||
| Lymphopeniaa | 1.53 | 0.47–4.99 | .48 | |||
| QF-negative | 0.78 | 0.24–2.60 | .69 | |||
| Lymphopeniaa at 1 mo postimmunosuppression | 1.10 | 0.34–3.57 | .88 | |||
| QF-negative at 1 mo postimmunosuppression | 4.89 | 1.06–22.74 | .04 | 4.97 | 1.07–23.10 | .04 |
Abbreviations: CMV, cytomegalovirus; HR, hazard ratio; SLE, systemic lupus erythematosus; SLEDAI, SLE Disease Activity Index 2000; QF, QuantiFERON-CMV.
aAbsolute lymphocyte count <1000 /mm3.
DISCUSSION
Here, we reported one of the first prospective studies to preemptively monitor CMV infection in patients with active SLE receiving intense immunosuppressive therapy. CMV infection with clinical significance occurred in ~1 of 7 patients, with relatively early onset and clinical manifestations ranging from asymptomatic infection to tissue-invasive disease. We found that active SLE patients with low postimmunosuppressant CMV-specific CMI measured by QuantiFERON-CMV were at increased risk of CMV infection after adjusting for other variables.
The incidence of CMV infection is increasing among SLE patients, especially those requiring intensive immunosuppressive therapy for active organ disease [5–8]. We monitored CMV infection for the first 3 months following initiation of intense immunosuppressive therapy based on the results of a previous retrospective case–control study, which found that CMV infection was likely to develop early (median, 1.3 months after initiating immunosuppressive therapy) [6]. The onset of CMV infection occurs relatively earlier in SLE patients than in other immunocompromised populations, such as SOT recipients, in whom the mean onset was 3 months following induction therapy [16]. Approximately two-thirds of the patients studied here developed low-level CMV DNAemia without disease, and there was only 1 non-CMV-related death. These results contrast with those of a previous retrospective case–control study, which found higher rates of CMV tissue-invasive diseases (eg, pneumonitis and gastrointestinal disease) and high mortality rates of up to two-thirds of patients [6]. This discrepancy could be explained by our preemptive monitoring for CMV replication and prompt interventions to prevent disease progression. We also found that neuropsychiatric manifestations of SLE, which potentially suggest the need for more intense immunosuppression, showed a marginal association with increased risk of CMV infection later in the disease course [17]. Other variables did not show any correlation, including age, sex, disease-specific activity score, and type of immunosuppressant.
The incidence of CMV infection among SLE patients receiving intense immunosuppressants is higher compared with other patients with autoimmune diseases. The incidence reported in the literature ranges from 37% to 53% depending on the definition (from asymptomatic CMV DNAemia to tissue-invasive disease) [18, 19]. By contrast, we found that ~13% of active SLE patients receiving immunosuppressants suffered from CMV infection with a plasma CMV DNA load of ≥3 log10 IU/mL, which required treatment. The SLE patients included in this study had high disease activity as determined by SLEDAI-2K. Although CMV infection appears to be associated with autoimmune diseases, whether CMV reactivation triggers autoimmune disease flare-ups or develops because of immunosuppression following therapy for active autoimmune disease is still debated [20].
Deficiency of total and CMV-specific immunity is associated with CMV infection in SOT and hematopoietic stem cell transplant recipients. A previous prospective study of SLE patients revealed that low white blood cell counts were common, with leukopenia and lymphopenia observed in 57.3% and 96.6% of patients, respectively. However, the presence of leukopenia at any time was not a risk factor for severe infection in patients with SLE [21]. Low absolute lymphocyte counts have been associated with several viral infections including CMV [22, 23]. A retrospective study of SLE patients revealed that lymphopenia, especially CD4+ lymphopenia, was associated with CMV disease; however, this association was not detected in our cohort [24].
CMV-specific humoral and cell-mediated immunity is believed to play a major role in controlling viral replication in immunocompromised patients. Anti-CMV IgG has been traditionally and widely used to predict the risk of CMV infection in transplant recipients. However, even among CMV-seropositive patients with preexisting immunity, some risk of CMV infection remains. A study of CMV-seropositive kidney and liver transplant recipients revealed that low pretransplant CMV IgG titer was a risk factor for CMV reactivation after transplant [25, 26]. Although most of the patients in our study had positive CMV serostatus, we identified notable discordant results in several cases for CMV-specific humoral and cellular immunity. These data could identify individuals at higher risk of infection (eg, patients who have positive CMV IgG but are QF-nonreactive). The high heterogeneity of CMV-specific immunity has also been demonstrated in immunocompetent individuals [27].
We used the 2 major targets of CMI against CMV, pp65 and IE-1, to assess CMV-specific T-cell responses in our study [28, 29]. Functional CMV-specific CMI has been shown to control CMV infection in SOT recipients. Limited expansion of CMV-specific CD8+ T cells places kidney transplant recipients at risk of CMV infection [30]. Therefore, assessment of CMV-specific T-cell responses is of interest to improve care of SOT recipients who develop CMV infection [31]. Our study affirmed this association in patients with autoimmune diseases for the first time. Although pre-immunosuppressant CMV-specific CMI status did not predict CMV infection in our study population, patients whose CMV-specific CMI diminishes postimmunosuppression could have up to a 5-times-greater risk of CMV reactivation compared with patients with intact CMV-specific CMI. We found that approximately one-fourth of patients with active SLE had diminished CMV-specific CMI later in their disease course, likely because of the intense immunosuppressive regimens administered in our cohort. Interestingly, a significant proportion patients were QF indeterminate. Patients with indeterminate results may have profound T-cell suppression, as reflected by the inability of T cells to secrete IFN-γ response to superantigen stimulation in a control sample. Hence, an indeterminate QF result is usually considered to confer the highest risk of CMV infection, particularly in CMV-seronegative SOT recipients receiving transplants from CMV-seropositive donors [32]. However, our study did not detect this association, likely because of the predominant CMV-positive serostatus of the study population. Therefore, we decided to combine patients with indeterminate QF results with QF nonreactive patients to increase statistical power.
A previous study reported that the QuantiFERON-CMV assay before and 1 month after immunosuppression had suboptimal accuracy for predicting protective CMV-specific CMI (sensitivity, 77.4%; specificity, 34.3%; positive predictive value, 64.1%; and negative predictive value, 50.0%); there was a nonsignificant difference in 1-year CMV infection rates between QF− (nonreactive or indeterminate) and QF+ patients [33]. A few previous studies suggested a modified cutoff value (≥0.1 IU/mL) to increase the test’s sensitivity for immunocompromised patients, particularly CMV-seropositive SOT recipients [33, 34]. We were able to distinguish those at risk of CMV infection by using the cutoff value suggested by the manufacturer 1 month postimmunosuppression. Using a different IFN-γ quantification technique (the ELISpot assay), low pretransplant CMV-specific CMI also predicted CMV infection among CMV-seropositive kidney transplant recipients who received non-T-cell-depleting antibody induction therapy. Using the ELISpot assay, CMV IE-1 better stratified risk of CMV infection compared with the CMV pp65 antigen. Jarque et al. suggested application of an adjusted cutoff for the ELISpot assay to risk stratification when CMI is measured after transplant [35]. Therefore, a future study should focus on defining optimal cutoff values for specific test methods and specific patient populations.
Overall, we observed a decreasing proportion of patients who were QF-nonreactive and -indeterminate after receiving immunosuppressants, which contradicted our thought that the proportion should be increasing. Instead, we assessed the role of CMV-specific CMI by monitoring responses and plasma CMV DNA loads chronologically in a real-world situation on 3 representative patients. We also further quantified the amount of IFN-γ secreted in the sample tube (after subtracting the negative control) in addition to interpretation as a qualitative test. We found some inverse correlations between IFN-γ levels and CMV DNA loads. However, the usefulness and performance of quantifying IFN-γ levels require further study. Therefore, we encouraged individualized CMV-specific immune monitoring rather than a whole-group interpretation.
Because CMV replication is associated with impaired CMV-specific immune control, CMV-specific T-cell monitoring could be used to explore and optimize CMV management in patients with autoimmune diseases, as it is for transplant recipients [9, 31]. Our study supported a potential role of CMV-specific CMI monitoring in these patients. Further studies are needed to confirm the utility of CMV-specific CMI monitoring in real-world practice.
The limitations of our study include the relatively small number of patients and missing QF data for some patients. In addition, we were unable to explore responses to each CMV-specific protein because the QuantiFERON-CMV assay uses premixed pooled peptides. Finally, a threshold to initiate preemptive therapy has not been established among this specific population. Thus, we tentatively used CMV DNA loads of 3 log10 IU/mL as the threshold; this value distinguished patients with and without symptoms based on a retrospective study at our institution (unpublished data). To our knowledge, our study is one of the first and largest to thoroughly investigate CMV-specific CMI responses in patients with active autoimmune diseases receiving intensive immunosuppressive therapy. Our data may help clinicians caring for patients with active autoimmune disease and major organ involvement. Our results could also increase awareness of CMV prevention in patients with autoimmune diseases scheduled to receive aggressive doses of immunosuppressants.
In conclusion, patients with active SLE could be at risk for CMV infection soon after receiving intensive immunosuppressive therapy. Preemptive CMV monitoring is encouraged to detect early replication of this virus and prevent unfavorable consequences. Impaired CMV-specific T-cell responses after receiving immunosuppressants are independently associated with CMV infection. Intact CMV-specific CMI appears to prevent CMV infection in populations with high rates of CMV seropositivity. A future large-scale study focused on detailed assessment of individualized CMV-specific CMI status among patients with autoimmune diseases is needed.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Chutatip Tongsook, Ekawat Pasomsub, Kanchana Sriwanicharak, and Thanitta Suangtamai for technical support. The QuantiFERON-CMV assay was kindly provided by Qiagen. The RealTime CMV assay was generously supplied by Abbott Molecular.
Financial support. This study was supported by the Faculty of Medicine Ramathobodi Hospital, Mahidol University, Bangkok, Thailand (CF_63001).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. The patients’ written consent was obtained. The study protocol was reviewed and approved by the Institutional Review Board of the Faculty of Medicine at Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (approval number: ID 06-61-14).
Author contributions. Conceptualization: Jackrapong Bruminhent, Suphanan Autto, Prapaporn Pisitkun. Data collation: Jackrapong Bruminhent, Suphanan Autto, Asalaysa Bushyakanist, Suppachok Kirdlarp. Data analysis: Jackrapong Bruminhent, Suphanan Autto. Manuscript writing (original draft): Jackrapong Bruminhent, Suphanan Autto. Manuscript reviewing and editing: Jackrapong Bruminhent, Suphanan Autto, Porpon Rotjanapan, Pintip Ngarmjanyaporn, Asalaysa Bushyakanist, Suppachok Kirdlarp, Pichaya O-charoen, Chavachol Setthaudom, Prapaporn Pisitkun.
References
- 1. Restrepo-Escobar M, Granda-Carvajal PA, Aguirre DC, et al. Predictive models of infection in patients with systemic lupus erythematosus: a systematic literature review. Lupus. 2021; 30:421–30. [DOI] [PubMed] [Google Scholar]
- 2. Phaspinyo K, Ngamjanyaporn P, Fongsrisin S, Nantiruj K. Infections in systemic lupus erythematosus patients: study from Thailand P2N-09. Int J Rheum Dis 2008; 11. [Google Scholar]
- 3. Janwityanuchit S, Totemchokchyakarn K, Krachangwongchai K, Vatanasuk M. Infection in systemic lupus erythematosus. J Med Assoc Thai 1993; 76:542–8. [PubMed] [Google Scholar]
- 4. Wongchinsri J, Tantawichien T, Osiri M, et al. Infection in Thai patients with systemic lupus erythematosus: a review of hospitalized patients. J Med Assoc Thai 2002; 85:S34–9. [PubMed] [Google Scholar]
- 5. Choo HMC, Cher WQ, Kwan YH, Fong WWS. Risk factors for cytomegalovirus disease in systemic lupus erythematosus (SLE): a systematic review. Adv Rheumatol 2019; 59:12. [DOI] [PubMed] [Google Scholar]
- 6. Ponglorpisit P, Watcharananan SP, Ngamjanyaporn P. Clinical characteristic and risk factor of cytomegalovirus disease in systemic lupus erythematosus patients. Presented as poster presentation #1548 at: IDWeek October 5, 2013; 2013; San Francisco, CA. [Google Scholar]
- 7. Bruminhent J, Watcharananan SP. Cytomegalovirus infection in non-HIV patients: a return of the monster. J Infect Dis Antimicrob Agents 2018; 35:111–4. [Google Scholar]
- 8. Sebastiani GD, Iuliano A, Canofari C, Bracci M. Cytomegalovirus infection in systemic lupus erythematosus: report of four cases challenging the management of the disease, and literature review. Lupus 2019; 28:432–7. [DOI] [PubMed] [Google Scholar]
- 9. Razonable RR, Humar A. Cytomegalovirus in solid organ transplant recipients-guidelines of the American Society of Transplantation Infectious Diseases community of practice. Clin Transplant 2019; 33:e13512. [DOI] [PubMed] [Google Scholar]
- 10. Petri M, Orbai AM, Alarcón GS, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012; 64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002; 29:288–91. [PubMed] [Google Scholar]
- 12. Arora S, Isenberg DA, Castrejon I. Measures of adult systemic lupus erythematosus: disease activity and damage. Arthritis Care Res (Hoboken) 2020; 72:27–46. [DOI] [PubMed] [Google Scholar]
- 13. Polachek A, Gladman DD, Su J, Urowitz MB. Defining low disease activity in systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2017; 69:997–1003. [DOI] [PubMed] [Google Scholar]
- 14. Mosca M, Bombardieri S. Assessing remission in systemic lupus erythematosus. Clin Exp Rheumatol 2006; 24:S–99–104. [PubMed] [Google Scholar]
- 15. Walker S, Fazou C, Crough T, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis 2007; 9:165–70. [DOI] [PubMed] [Google Scholar]
- 16. Chiasakul T, Townamchai N, Jutivorakool K, et al. Risk factors of cytomegalovirus disease in kidney transplant recipients: a single-center study in Thailand. Transplant Proc 2015; 47:2460–4. [DOI] [PubMed] [Google Scholar]
- 17. Ahn GY, Kim D, Won S, et al. Prevalence, risk factors, and impact on mortality of neuropsychiatric lupus: a prospective, single-center study. Lupus 2018; 27:1338–47. [DOI] [PubMed] [Google Scholar]
- 18. Lee KY, Yoo BW, Ahn SS, et al. Predictors of mortality in autoimmune disease patients with concurrent cytomegalovirus infections detected by quantitative real-time PCR. PLoS One 2017; 12:e0181590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xue Y, Jiang L, Wan WG, et al. Cytomegalovirus pneumonia in patients with rheumatic diseases after immunosuppressive therapy: a single center study in China. Chin Med J (Engl) 2016; 129:267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Halenius A, Hengel H. Human cytomegalovirus and autoimmune disease. Biomed Res Int 2014; 2014:472978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lertchaisataporn K, Kasitanon N, Wangkaew S, et al. An evaluation of the association of leukopenia and severe infection in patients with systemic lupus erythematosus. J Clin Rheumatol 2013; 19:115–20. [DOI] [PubMed] [Google Scholar]
- 22. Meesing A, Razonable RR. Absolute lymphocyte count thresholds: a simple, readily available tool to predict the risk of cytomegalovirus infection after transplantation. Open Forum Infect Dis 2018; 5:ofy230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bruminhent J, Srisala S, Klinmalai C, et al. BK polyomavirus-specific T cell immune responses in kidney transplant recipients diagnosed with BK polyomavirus-associated nephropathy. BMC Infect Dis 2019; 19:974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qin L, Qiu Z, Hsieh E, et al. Association between lymphocyte subsets and cytomegalovirus infection status among patients with systemic lupus erythematosus: a pilot study. Medicine (Baltimore) 2019; 98:e16997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bruminhent J, Thongprayoon C, Dierkhising RA, et al. Risk factors for cytomegalovirus reactivation after liver transplantation: can pre-transplant cytomegalovirus antibody titers predict outcome? Liver Transpl 2015; 21:539–46. [DOI] [PubMed] [Google Scholar]
- 26. Kirisri S, Vongsakulyanon A, Kantachuvesiri S, et al. Predictors of CMV infection in CMV-seropositive kidney transplant recipients: impact of pre-transplant CMV-specific humoral immunity. Open Forum Infectious Dis 2021; ofab199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valle-Arroyo J, Aguado R, Páez-Vega A, et al. Lack of cytomegalovirus (CMV)-specific cell-mediated immune response using QuantiFERON-CMV assay in CMV-seropositive healthy volunteers: fact not artifact. Sci Rep 2020; 10:7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kern F, Bunde T, Faulhaber N, et al. Cytomegalovirus (CMV) phosphoprotein 65 makes a large contribution to shaping the T cell repertoire in CMV-exposed individuals. J Infect Dis 2002; 185:1709–16. [DOI] [PubMed] [Google Scholar]
- 29. Davignon JL, Clément D, Alriquet J, et al. Analysis of the proliferative T cell response to human cytomegalovirus major immediate-early protein (IE1): phenotype, frequency and variability. Scand J Immunol 1995; 41:247–55. [DOI] [PubMed] [Google Scholar]
- 30. Kumar D, Chin-Hong P, Kayler L, et al. A prospective multicenter observational study of cell-mediated immunity as a predictor for cytomegalovirus infection in kidney transplant recipients. Am J Transplant 2019; 19:2505–16. [DOI] [PubMed] [Google Scholar]
- 31. Sester M, Leboeuf C, Schmidt T, Hirsch HH. The “ABC” of virus-specific T cell immunity in solid organ transplantation. Am J Transplant 2016; 16:1697–706. [DOI] [PubMed] [Google Scholar]
- 32. Manuel O, Husain S, Kumar D, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis 2013; 56:817–24. [DOI] [PubMed] [Google Scholar]
- 33. Fernández-Ruiz M, Rodríguez-Goncer I, Parra P, et al. Monitoring of CMV-specific cell-mediated immunity with a commercial ELISA-based interferon-γ release assay in kidney transplant recipients treated with antithymocyte globulin. Am J Transplant 2020; 20:2070–80. [DOI] [PubMed] [Google Scholar]
- 34. Kumar D, Chernenko S, Moussa G, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant 2009; 9:1214–22. [DOI] [PubMed] [Google Scholar]
- 35. Jarque M, Crespo E, Melilli E, et al. Cellular immunity to predict the risk of cytomegalovirus infection in kidney transplantation: a prospective, interventional, multicenter clinical trial. Clin Infect Dis 2020; 71:2375–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.