Abstract
Objectives
To summarise the published evidence on the gastrointestinal manifestations of COVID-19 in children and to determine the prevalence of gastrointestinal symptoms.
Methods
In this systematic review and meta-analysis, we searched PubMed, Embase, CINAHL and the WHO’s database of publications on novel coronavirus. We included English language studies that had described original demographic and clinical characteristics of children diagnosed with COVID-19 and reported on the presence or absence of gastrointestinal symptoms. Meta-analysis was conducted using the random-effects model. The pooled prevalence of gastrointestinal symptoms was expressed as proportion and 95% CI.
Results
The search identified 269 citations. Thirteen studies (nine case series and four case reports) comprising data for 284 patients were included. Overall, we rated four studies as having a low risk of bias, eight studies as moderate and one study as high risk of bias. In a meta-analysis of nine studies, comprising 280 patients, the pooled prevalence of all gastrointestinal symptoms was 22.8% (95% CI 13.1% to 35.2%; I2=54%). Diarrhoea was the most commonly reported gastrointestinal symptom followed by vomiting and abdominal pain.
Conclusions
Nearly a quarter of children with COVID-19 have gastrointestinal symptoms. It is important for clinicians to be aware of the gastrointestinal manifestation of COVID-19.
PROSPERO registration number
CRD42020177569.
Keywords: abdominal pain, diarrhoea, infectious disease
Significance of this study.
What is already known on this topic
COVID-19 has been declared as a pandemic by the WHO.
The main features of COVID-19 are respiratory and include cough, sore throat, dyspnoea and pneumonia.
What this study adds
Gastrointestinal symptoms are common in children with COVID-19.
Nearly a quarter of children with COVID-19 have gastrointestinal symptoms.
The most common gastrointestinal symptom is diarrhoea followed by vomiting and abdominal pain.
Background
COVID-19 is a highly contagious disease that was first reported in Wuhan, Hubei Province, China in December 2019. Within weeks of the emergence of the disease, it had spread to several countries and the WHO declared the outbreak as a Public Health Emergency of International Concern in January 2020 and as a pandemic in March 2020.1 According to the dashboard of the Center for Systems Science and Engineering at Johns Hopkins University, Baltimore, USA,2 the disease has been reported in 187 countries, affected over 3 million people worldwide and caused over 230 000 deaths as at 30 April 2020.
COVID-19 is caused by SARS-CoV-2, previously known as the 2019 novel coronavirus (2019-nCoV). SARS-CoV-2 is a novel member of coronaviruses which are a large class of highly diverse, enveloped, positive-sense, single-stranded RNA viruses.3 Most reported cases of the disease are adults, but the disease also affects children, including neonates.4 The first reported paediatric case of COVID-19 was probably a 10-year-old boy from Shenzhen, China, who was diagnosed with the condition in January 2020.5
There have been a number of studies describing the clinical features of COVID-19 since the disease was first reported in December 2019. While the disease typically presents as acute respiratory disease and pneumonia, it has been reported to impact other systems including the gastrointestinal tract. Indeed, the recognition that the gastrointestinal tract may be a source of transmission of SARS-CoV-2 has led to many endoscopy units having had to postpone a large proportion of endoscopic procedures. A number of reports on patients with COVID-19 mention gastrointestinal symptoms such as diarrhoea, nausea, vomiting and abdominal pain.6 Despite these, the gastrointestinal manifestations of the disease are not widely recognised by clinicians. As COVID-19 continues to spread throughout the world, it is essential for its gastrointestinal manifestations to be understood as they have the potential of distracting the clinician from making a diagnosis of COVID-19, especially in situations when the gastrointestinal symptoms precede the typical respiratory symptoms. The aim of this systematic review and meta-analysis is to describe the gastrointestinal manifestations of COVID-19 in children and to determine the prevalence of such symptoms.
Methods
Search strategy and study selection
This study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses7 and was registered with the International Prospective Register of Systematic Reviews (PROSPERO) (https://www.crd.york.ac.uk/prospero/). Considering the date of the earliest confirmed reports of COVID-19, we searched PubMed, Embase and CINAHL from 1 December 2019 to 1 April 2020. The WHO database of publications on novel coronavirus was also searched for potentially relevant publications.8 We also searched reference lists of retrieved articles to identify additional citations that may have been missed through the electronic searches. Eligible studies were published in English language articles that have described original demographic and clinical characteristics of children diagnosed with COVID-19 and reported the presence or absence of gastrointestinal symptoms. Eligible studies included case reports, case series, cohort studies and retrospective studies. Review articles and opinion articles not reporting original data were excluded.
Studies were identified with the following search terms: (coronavirus OR COVID-19 OR COVID 19 OR SARS-CoV-2 OR 2019-nCoV) AND (children OR child* OR pediatric OR paediatrics) AND (symptom OR symptoms OR characteristics OR characteristic* OR data OR features OR clinical). We included both medical subject headings and free text terms. The search strategy of the PubMed search is shown in online supplementary appendix 1S. Two independent reviewers evaluated the titles and abstracts of papers to identify relevant studies. Articles identified were independently assessed by two reviewers using predefined eligibility criteria. Any disagreements between authors regarding study inclusion were resolved through discussion among the reviewers.
flgastro-2020-101529supp001.pdf (80.4KB, pdf)
Risk of bias
All the studies that met our inclusion criteria were case series and case reports. We used the checklist developed by Murad et al 9 to assess risk of bias. This tool was specifically developed to assess risk of bias in case series and case reports and had previously been used in published systematic reviews.10 The checklist was adapted from the Newcastle-Ottawa tool11 by the removal of items that relate to comparability and adjustment (which are not relevant to non-comparative studies) and retained items that focused on selection, representativeness of cases and ascertainment of outcomes and exposure. This resulted in five criteria in the form of questions with a binary response (yes/no), whether the item was suggestive of bias or not. We considered the quality of the report good (low risk of bias) when all five criteria were fulfilled, moderate when four were fulfilled and poor (high risk of bias) when three or fewer were fulfilled.
Severity of COVID-19
We classified the severity of COVID-19 as asymptomatic, mild, moderate or severe based on the degree of respiratory symptoms.12 Asymptomatic cases had no respiratory symptoms. Mild cases had fever, cough, upper respiratory tract symptoms but without pneumonia or intensive care unit (ICU) admission. Moderate cases had mild symptoms together with pneumonia. Severe cases had moderate symptoms in addition to ICU admission, acute respiratory distress syndrome or organ failure.
Data extraction
Data were extracted independently by two reviewers into a Microsoft Excel spreadsheet using a predefined checklist. Extracted data included the following: study design, year of publication, country, author name(s), number of patients, patient demographics, gastrointestinal symptoms and/or signs, other clinical symptoms and timing of gastrointestinal symptoms in relation to respiratory symptoms. Any differences were discussed and resolved by consensus.
Data analysis
Initial statistical analyses were descriptive. Meta-analysis was conducted using the random-effects model. The pooled prevalence of gastrointestinal symptoms was expressed as proportion and 95% CI and was presented in a forest plot. We calculated I2 statistic to measure the proportion of total variation in study estimates attributed to heterogeneity. I2 values of <25%, 25%–75% and >75% indicate low, moderate and high heterogeneity, respectively.13 Meta-analysis was performed using Comprehensive Meta-Analysis Statistical Software (V.3). We planned to perform subgroup analyses based on country of publication and severity of COVID-19.
Results
Overview of the included studies
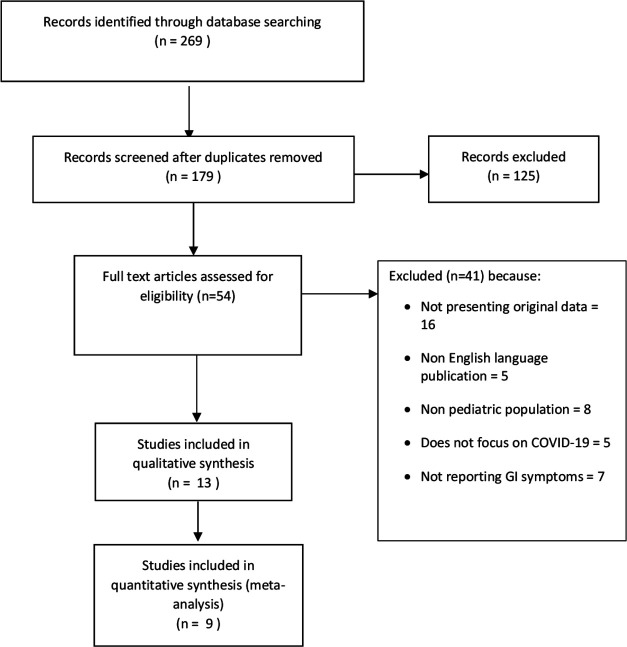
The search strategy identified a total of 269 citations, of which 54 met the criteria for full-text review. A flowchart detailing the studies’ selection process is shown in figure 1. Following full-text review, we included 13 papers comprising data for 284 patients.14–26 The characteristics of the included studies are provided in table 1. Two of the included studies were from Singapore17 and Korea,21 and the other 11 studies were from China. All the included studies had been published between 9 February 2020 and 29 March 2020. Nine of the included papers were case series,14 16 18 20 22–26 and the other four were case reports.15 17 19 21 The nine case series,14 16 18 20 22–26 involving 280 patients, had adequate data to allow estimates of prevalence and these were included in a meta-analysis. The risk of bias assessment in the studies is summarised in online supplementary table 1S. Overall, we rated four studies14 15 19 22 as having a low risk of bias, eight studies16–18 20 21 23 25 26 as moderate and one study24 as high risk of bias.
Figure 1.
Flow diagram of the study selection process. GI, gastrointestinal.
Table 1.
Characteristics of included studies
| Study and country | Sample size | Study type | Mean age | Age range | Severity of Covid-19 | Method of diagnosis |
| Ji et al and China16 |
2 (0 female) | Case series | 12 years | 9–15 years | Mild (n=2) | RT-PCR |
| Liu et al and China18 |
5 (1 female) | Case series | 5.9 years | 7 months to 13 years | Asymptomatic (n=1), mild (n=1), moderate (n=3) | RT-PCR |
| Lu et al and China20 |
171 (67 female) | Case series | 6.7 | 1 day to 15 years | Asymptomatic (n=27), mild (n=33), moderate (n=108), severe (n=3) | RT-PCR |
| Qiu et al and China22 |
36 (13 female) | Case series | 8.3 | 1–16 | Mild (n=17), moderate (n=19) | RT-PCR |
| Sun et al and China23 |
8 (2 female) | Case series | Not stated | 2 months to 15 years | Severe (n=8) | RT-PCR |
| Xia et al and China24 |
20 (7 female) | Case series | 2.1 years | 1 day to 14.7 years | Moderate (n=20) | Nucleic acid test |
| Zhang et al and China25 |
3 (0 female) | Case series | 7.7 years | 6–9 years | Mild (n=1), moderate (n=2) | RT-PCR |
| Zheng et al and China26 |
25 (11 female) | Case series | 3 years (median) | 3 months to 14 years | Mild (n=8), moderate (n=15), severe (n=2) | RT-PCR |
| Cui et al and China15 |
1 (1 female) | Case report | 55 days | N/A | Moderate (n=1) | RT-PCR |
| Kam et al and Singapore17 |
1 (0 female) | Case report | 6 months | N/A | Mild (n=1) | RT-PCR |
| Liu et al and China19 |
1 (0 female) | Case report | 10 years | N/A | Mild (n=1) | RT-PCR |
| Park et al and Korea21 |
1 (1 female) | Case report | 10 years | N/A | Moderate (n=1) | RT-PCR |
| Cai et al and China14 |
10 (6 female) | Case series | 6 years | 3–131 months | Mild (n=6), moderate (n=4) | RT-PCR |
RT, reverse transcription.
flgastro-2020-101529supp002.pdf (93.6KB, pdf)
Diagnosis of COVID-19
All the included studies reported patients admitted to hospital with COVID-19. In 12 of the included studies,14–23 25 26 the diagnosis of COVID-19 was established by reverse transcription polymerase chain (RT-PCR) and in one study,24 by the nucleic acid test. The severity of COVID-19 varied from asymptomatic cases to severe cases (table 1). Reported non-gastrointestinal symptoms/features included fever, cough, dyspnoea, blocked nose, sore throat and rhinorrhoea.
Gastrointestinal symptoms of COVID-19
A variety of gastrointestinal symptoms were reported in children with COVID-19. Reported gastrointestinal symptoms included diarrhoea, vomiting and abdominal pain. One study18 reported that the gastrointestinal symptoms developed after the onset of respiratory symptoms but the other studies did not specify the timing of gastrointestinal symptoms in relation to respiratory symptoms.
Prevalence of gastrointestinal symptoms in COVID-19
The prevalence of gastrointestinal symptoms in the individual primary studies ranged from 0% to 88%. In a meta-analysis of nine studies comprising 280 children, the pooled prevalence of gastrointestinal symptoms was 22.8% (95% CI 12.7% to 37.6%) with a moderate degree of heterogeneity (I2=59%) (figure 2). The pooled prevalence of diarrhoea (figure 3), vomiting (figure 4) and abdominal pain (figure 5) was, respectively, 12.4% (95% CI 7.8% to 19.2%), 10.3% (95% CI 4.9% to 20.3%) and 5.4% (95% CI 2.2% to 12.7%). The I2 statistic for these respective symptoms was 19.6%, 45% and 19.9%.
Figure 2.
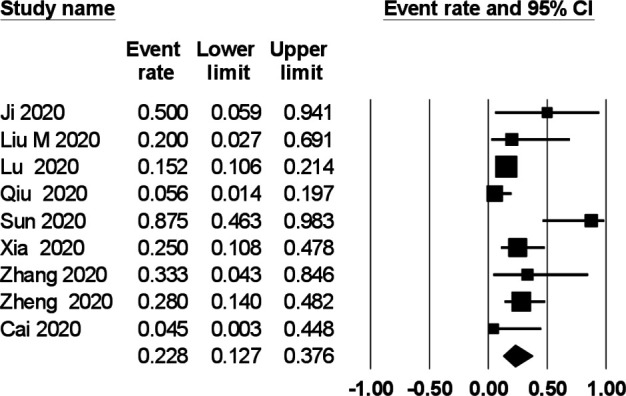
Prevalence of gastrointestinal symptoms in children with COVID-19.
Figure 3.
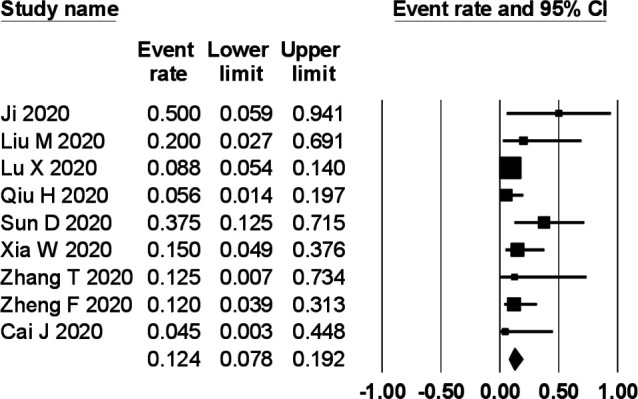
Prevalence of diarrhoea in children with COVID-19.
Figure 4.
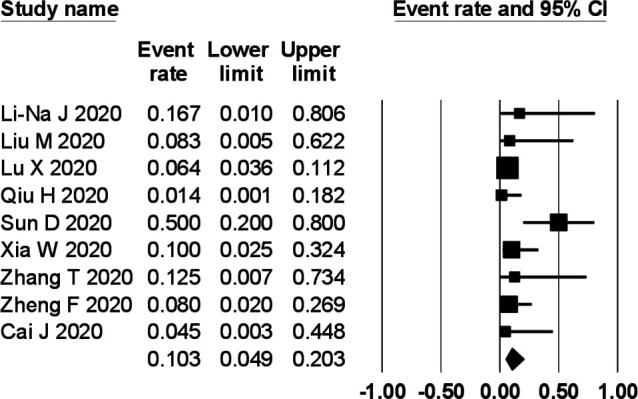
Prevalence of vomiting in children with COVID-19.
Figure 5.
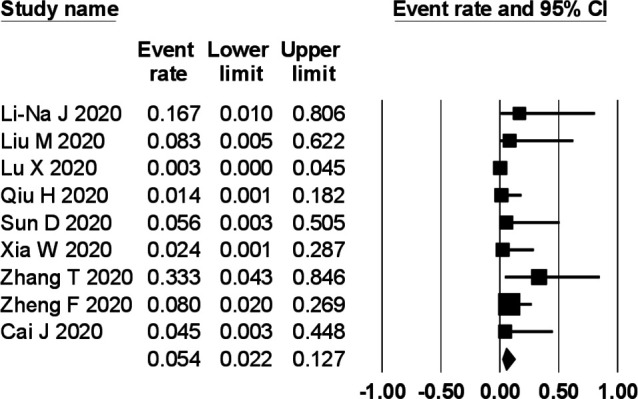
Prevalence of abdominal pain in children with COVID-19.
Subgroup analysis
We planned to perform subgroup analyses based on country of publication and severity of COVID-19 but there was not enough data available in the included studies.
Discussion
As at 30 April 2020, COVID-19 has been reported in 187 countries, affected over 3 million people worldwide and caused about 230 000 deaths.2 The primary manifestations of COVID-19 are mainly respiratory with features such as fever, cough, sore throat, dyspnoea and pneumonia.27 However, a number of studies make mention of gastrointestinal symptoms.6 The first confirmed patient with COVID-19 in the USA reported a 2-day history of nausea and vomiting in addition to a dry cough on admission, and also started to pass loose stools from Day 2 of admission.28
To our knowledge, this is the first systematic review and meta-analysis that has reported the gastrointestinal manifestations of COVID-19 in children and estimated the pooled prevalence of such symptoms. The results of our pooled analyses show that gastrointestinal symptoms are common in children with COVID-19 with nearly a quarter of patients exhibiting at least one gastrointestinal symptom. The most common gastrointestinal symptom was diarrhoea, followed by vomiting and then abdominal pain. The results of our study are generally consistent with a recent meta-analysis in adult patients with COVID-19 which found the prevalence of gastrointestinal symptoms to be about 18%.29 Perhaps it is not surprising that gastrointestinal symptoms are common in patients with COVID-19. A number of studies have suggested a possible faecal–oral transmission.6 30 Xiao et al showed that SARS-CoV-2 RNA can be found in stool samples of patients with COVID-19 and that the positive stool test may persist for up to 12 days and remain positive even after respiratory samples have tested negative.31 While gastrointestinal symptoms in patients with COVID-19 do not lead to mortality, they nonetheless, contribute to the overall picture of the disease. Unfortunately, there was a paucity of information in the primary studies regarding how the presence of gastrointestinal symptoms impact on the overall course of patients’ disease.
Our study has a number of strengths. A comprehensive literature search was performed to identify eligible studies. Two review authors independently assessed studies for inclusion, extracted data and rated study quality. By including data from all available reports, the overall sample size was increased thereby improving the statistical power of our meta-analysis. Although this is a comprehensive review, there are also limitations. The primary studies were either case series or case reports and the limitations of these study designs must be borne in mind. All the included studies were from China, Singapore and Korea so the prevalence of gastrointestinal symptoms in other geographic regions may or may not be similar. The included studies primarily aimed to describe children with COVID-19 and as respiratory symptoms are the most serious symptoms, it is possible that some gastrointestinal symptoms might have been under-reported. Similarly, we excluded studies which did not report the presence or absence of gastrointestinal symptoms, and non-reporting was not assumed to equate to not present.
In conclusion, our review found that gastrointestinal symptoms are common in children with COVID-19 with nearly a quarter of patients developing such symptoms. Diarrhoea, vomiting and abdominal pain were the main gastrointestinal symptoms. It is important for clinicians to be aware of the gastrointestinal manifestation of COVID-19.
Footnotes
Contributors: AA had the idea for the review. CG-C, IA and MG selected studies for inclusion and abstracted data. AKA wrote the first draft. MG, CG-C, IA, ET-B and AKA interpreted data and critically revised the paper for important intellectual content. All authors approved the final version.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Bedford J, Enria D, Giesecke J, et al. COVID-19: towards controlling of a pandemic. Lancet 2020;395:1015–8. 10.1016/S0140-6736(20)30673-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis 2020;20:533–4. 10.1016/S1473-3099(20)30120-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. He F, Deng Y, Li W, et al. Coronavirus disease 2019: what we know? J Med Virol 2020;92:719–25. 10.1002/jmv.25766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lai C-C, Liu YH, Wang C-Y, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect 2020;53:404–12. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514–23. 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology 2020;158:1518–9. 10.1053/j.gastro.2020.02.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006–12. 10.1016/j.jclinepi.2009.06.005 [DOI] [PubMed] [Google Scholar]
- 8. World Health Organization website . Who database of publications on coronavirus disease (COVID-2019). Available: www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov [Accessed 12 Apr 2020].
- 9. Murad MH, Sultan S, Haffar S, et al. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med 2018;23:60–3. 10.1136/bmjebm-2017-110853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bazerbachi F, Leise MD, Watt KD, et al. Systematic review of mixed cryoglobulinemia associated with hepatitis E virus infection: association or causation? Gastroenterol Rep 2017;5:178–84. 10.1093/gastro/gox021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [webpage on the Internet. Ottawa, ON: Ottawa Hospital Research Institute, 2011. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 12. World Health Organization . Clinical management of severe acute respiratory infection when COVID-19 is suspected. Available: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Accessed 17 Apr 2020].
- 13. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 14. Cai J, Xu J, Lin D, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis 2020. 10.1093/cid/ciaa198. [Epub ahead of print: 28 Feb 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cui Y, Tian M, Huang D, et al. A 55-Day-Old female infant infected with 2019 novel coronavirus disease: presenting with pneumonia, liver injury, and heart damage. J Infect Dis 2020;221:1775–81. 10.1093/infdis/jiaa113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ji L-N, Chao S, Wang Y-J, et al. Clinical features of pediatric patients with COVID-19: a report of two family cluster cases. World J Pediatr 2020;16:267–70. 10.1007/s12519-020-00356-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kam K-Q, Yung CF, Cui L, et al. A well infant with coronavirus disease 2019 with high viral load. Clin Infect Dis 2020;71:847–9. 10.1093/cid/ciaa201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu M, Song Z, Xiao K. High-Resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr 2020;44:311–3. 10.1097/RCT.0000000000001023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Liu Y, Yang Y, Zhang C, et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–74. 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu X, Zhang L, Du H, et al. SARS-CoV-2 infection in children. N Engl J Med 2020;382:1663–5. 10.1056/NEJMc2005073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park JY, Han MS, Park KU, et al. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci 2020;35:e124. 10.3346/jkms.2020.35.e124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Qiu H, Wu J, Hong L, et al. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis 2020;20:689–96. 10.1016/S1473-3099(20)30198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sun D, Li H, Lu X-X, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr 2020;16:251–9. 10.1007/s12519-020-00354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xia W, Shao J, Guo Y, et al. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol 2020;55:1169–74. 10.1002/ppul.24718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang T, Cui X, Zhao X, et al. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol 2020;92:909–14. 10.1002/jmv.25795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng F, Liao C, Fan Q-H, et al. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci 2020;40:275–80. 10.1007/s11596-020-2172-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect 2020;80:656–65. 10.1016/j.jinf.2020.03.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020;382:929–36. 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020;159:81–95. 10.1053/j.gastro.2020.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen Y, Chen L, Deng Q, et al. The presence of SARS-CoV-2 RNA in the feces of COVID-19 patients. J Med Virol 2020;92:833–40. 10.1002/jmv.25825 [DOI] [PubMed] [Google Scholar]
- 31. Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020;158:1831–3. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2020-101529supp001.pdf (80.4KB, pdf)
flgastro-2020-101529supp002.pdf (93.6KB, pdf)