Abstract
Simple Summary
Several studies have described the bacterial composition in the intestines of horses, and several factors of influence have been detected. Variation in the results between studies, however, is substantial. Therefore, the current study aimed to study the bacterial composition in the faeces of healthy horses and ponies kept under standard housing and management condition in The Netherlands. Seventy-nine horses and ponies originating from two farms were included. Several factors, such as location, age, the season of sampling, horse type (horses vs. ponies) and pasture access significantly affected the bacterial composition. The current study provides important baseline information on variation in the bacterial composition in healthy horses and ponies under standard housing and management conditions. The aforementioned factors identified in this study to affect the bacterial population of the gut should be considered in future studies regarding the bacterial population of the equine gut.
Abstract
Several studies have described the faecal microbiota of horses and the factors that influence its composition, but the variation in results is substantial. This study aimed to investigate the microbiota composition in healthy equids in The Netherlands under standard housing and management conditions and to evaluate the effect of age, gender, horse type, diet, pasture access, the season of sampling and location on it. Spontaneously produced faecal samples were collected from the stall floor of 79 healthy horses and ponies at two farms. The validity of this sampling technique was evaluated in a small pilot study including five ponies showing that the microbiota composition of faecal samples collected up to 6 h after spontaneous defaecation was similar to that of the samples collected rectally. After DNA extraction, Illumina Miseq 16S rRNA sequencing was performed to determine microbiota composition. The effect of host and environmental factors on microbiota composition were determined using several techniques (NMDS, PERMANOVA, DESeq2). Bacteroidetes was the largest phylum found in the faecal microbiota (50.1%), followed by Firmicutes (28.4%). Alpha-diversity and richness decreased significantly with increasing age. Location, age, season, horse type and pasture access had a significant effect on beta-diversity. The current study provides important baseline information on variation in faecal microbiota in healthy horses and ponies under standard housing and management conditions. These results indicate that faecal microbiota composition is affected by several horse-related and environment-related factors, and these factors should be considered in future studies of the equine faecal microbiota.
Keywords: equine, faecal, microbiota, age, gender, pony, diet, pasture, season, location
1. Introduction
A well-functioning intestinal tract and intestinal microbiota are considered essential for maintaining health in horses [1]. Disturbances of the microbiota are associated with diseases in horses, such as colitis, equine metabolic syndrome and colic [2,3,4], although it is difficult to assess what comes first, disease or altered microbiota. Several studies have described the faecal microbiota of healthy horses, but the variation in results is substantial [5,6,7,8,9,10,11,12,13,14,15,16,17]. Therefore, our understanding of what can be considered normal variation and truly abnormal is currently limited. Inter-individual variation has been demonstrated to be larger than intra-individual variation [7,18]. The single main predictor of microbiota composition is individual identity, and it was suggested that this explains about 50% of the variation [19], meaning that other factors also affect microbiota composition. Several research groups studied the effects of different factors such as age, breed, band, maternal relationship, social behaviour, environmental conditions (such as diet, pasture access, fasting, transportation, exercise and season) and the use of pre- and probiotics and antimicrobials on the equine gut microbiota [6,8,9,10,13,14,15,19,20,21]. Geographic variation in microbiota composition has been demonstrated in humans [22], and the same might be true for horses, highlighting the need for studies from different geographic regions assessing faecal microbiota composition in horses. So far, most studies have compared relatively homogenous groups of horses exposed to one changing variable. This has the advantage of detecting subtle differences attributable to the tested variable, but at the same time, limits the extent to which results can be extrapolated to other populations of horses managed differently. Therefore, more knowledge about the normal faecal microbiota of horses and ponies kept under standard housing and management conditions is needed, for example, to study potential associations between microbiota composition and disease status. This study aimed to describe the microbiota composition in healthy horses and ponies in The Netherlands kept under standard housing and management conditions and assess which factors influence faecal microbiota composition.
2. Materials and Methods
2.1. Study Design, Population and Metadata Collection
The horses and ponies (n = 79) included in the study were client-owned animals housed at two locations in The Netherlands and sampled once between April 2015 and February 2016. At the main location, farm I, 61 animals (aged 5–31 years) were included. All animals were kept in individual stables with straw bedding, and some had pasture access. An additional 18 Warmblood horses (age 4–16 years) were sampled at a second farm (II). These horses were also kept in individual stables on straw or sawdust and some of the horses had pasture access. For each animal, information regarding age (in years), gender (male/female), horse type (horse/pony), diet (type of roughage and type and amount of concentrates), pasture access (yes/no) and season of sampling (summer/winter) was recorded. The minimum and maximum environmental temperatures at the moment of sample collection in the summer were 8 to 18 °C and 3 to 10 °C in the winter. None of the animals included in the study were treated with antimicrobials within the last six months, and none of the horses had any health problems in the past six months, according to the owner.
2.2. Ethical Considerations
No procedures had to be performed on the animals included in this study. Therefore, ethical approval was not required for this study. Informed consent was obtained from all the owners.
2.3. Faecal Sampling
Faecal samples were collected from the stall floor of individually housed horses and ponies. To ensure fresh samples were collected, sampling occurred within 6 h after the stables had been cleaned. Faeces were collected from the centre of a faecal ball. The samples were stored at −80 °C within 2 h of collection. To evaluate the validity of the sampling technique described above, a pilot study was conducted to investigate the effect of air exposure on the equine faecal microbiota when using this convenience sampling technique. For this purpose, faecal samples were collected rectally (t = 0) from five Shetland ponies (that underwent a rectal exam for reproductive purposes), after which these samples were exposed to room air (temperature: 18.2–23.9 °C; humidity 55.0–68.0%) and aliquots were sampled at 1 h, 3 h, 6 h, 12 h and 24 h.
2.4. DNA Extraction & 16S rRNA Amplicon Sequencing
DNA extraction was performed using the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) following a previously published protocol [23]. However, the samples were not treated in the TissueLyser at 30 Hz for 3 × 30 s with cooling on ice in between treatments but were bead-beaten for 5 min on a Vortex-Genie 2 (Scientific Industries, Bohemia, NY, USA). The variable V3 and V4 regions of the 16S rRNA amplicon were amplified, and libraries were prepared following the 16S Metagenomic Sequencing Library Preparation protocol (Illumina, San Diego, CA, USA). Next, each library was normalised, pooled and loaded onto the Illumina MiSeq platform for paired-end sequencing using the 600 cycles MiSeq Reagent Kit V3 (Illumina, San Diego, CA, USA), generating 2 × 300 base pair paired-end reads.
2.5. Bioinformatics Processing
Data preparation was performed using Jupyter notebook version 5.7.8, running on Python 3.7.3. utilising R version 3.4.4. Raw reads (250 bp) obtained from Illumina 16S rRNA amplicon sequencing provided input for the denoising pipeline DADA2. DADA2 models and corrects Illumina-sequenced amplicon errors with high precision [24]. First, the forward and reverse reads were sorted, and the quality profile was plotted. Trimming parameters were derived from the quality plots, maintaining a minimum quality score of 20. Forward-reads contained higher quality compared to reverse reads, as is common among Illumina data. Truncations were set at 15–290 for forward, 15–210 for reverse. Post filter and trimming the reads were merged and the merged data was used to create a sequence table. After the removal of chimeras, taxonomy was assigned using v. 132 of the Silva database [25].
2.6. Data Analysis
The DADA2 object was imported into the Phyloseq package [26]. All samples with less than 5000 reads were excluded. All data analyses and visualisations were performed with R version 4.0.2 [27] using vegan [28] and ggplot [29] packages. For alpha-diversity, data was rarefied to the sample with the lowest read counts (14,665 reads). All analyses were performed on the data of 61 horses and ponies from farm I unless mentioned otherwise.
2.6.1. Relative Abundance and Alpha-Diversity
Relative abundances at the phylum level were assessed for each sample, and phylum and class level bar plots were produced. Alpha-diversity (observed richness and Shannon diversity) was calculated from rarefied data. The effect of potential determinants (age, gender, horse type, roughage, concentrates, pasture access, season and location) on sample alpha-diversity was univariably tested with Wilcoxon rank-sum test (two groups), Kruskal–Wallis test (multiple groups) or linear regression (continuous variable). To evaluate the effect of location (farm) on the faecal microbiota, horses of farm II were compared to a comparable group of 17 Warmblood horses in the same age group from farm I.
2.6.2. Microbiota Composition (Beta-Diversity)
Between sample Bray–Curtis dissimilarity was computed on relative abundance data and used for Non-Metric Dimensional Scaling (NMDS). To determine if significant differences in microbiota composition were present between groups based on the previously mentioned factors, permutational multivariate analysis of variance (PERMANOVA), including beta-dispersion analysis, was performed (vegan package function Adonis2 and betadisper).
2.6.3. Differential Abundance Analysis
To determine taxa that differ in abundance for the tested factors, the DESeq2 package [30] was used. With a Wald test, the DESeq2 package determines if a significant fold change is present. The p-values were adjusted for the FDR (Benjamini–Hochberg approach [31]) with alpha set at 0.05. For this analysis, only factors associated with a significant difference in beta-diversity were considered. Raw count data was used as input, taxa not seen more than three times in at least 20% of the samples were filtered beforehand. Percentages of interesting taxa were calculated to reveal abundances in the entire microbiome.
3. Results
Faecal samples from 79 equids were included in the study. Sixty-one animals located at farm I were included in the analysis to study the effects of age, gender, horse type, roughage, concentrates, pasture access and season on faecal microbiota composition. See Table 1 for descriptive characteristics of the study population. Eighteen horses originating from farm II were compared to 17 horses of the same breed and age from farm I to study the effect of location/farm on faecal microbiota composition. A total of 5,015,145 high quality non-chimeric bacterial 16S reads were generated and annotated to 25,011 OTUs. The distribution of the number of reads across samples was as follows: a minimum of 14,665 reads, a maximum of 87,169 reads and a median of 32,949 reads.
Table 1.
Descriptive data of the study population.
| Age | n | Mean (Years) | SD (Years) | Range (Years) |
|---|---|---|---|---|
| 61 | 15.6 | 6.7 | 5–31 | |
| Gender | ||||
| Male | 37 | |||
| Female | 24 | |||
| Horse type | ||||
| Pony | 29 | |||
| Horse | 32 | |||
| Roughage | ||||
| Hay | 10 | |||
| Haylage | 33 | |||
| Mixed | 18 | |||
| Concentrates | ||||
| <2 kg | 25 | |||
| ≧2 kg | 36 | |||
| Pasture access | ||||
| None | 20 | |||
| Daily | 41 | |||
| Season of sampling | ||||
| Summer | 30 | |||
| Winter | 31 | |||
| Location 1 | ||||
| Farm I | 17 | |||
| Farm II | 18 | |||
1 Only Warmblood horses 4–16 years included.
3.1. Effect of Air Exposure on the Equine Faecal Microbiota
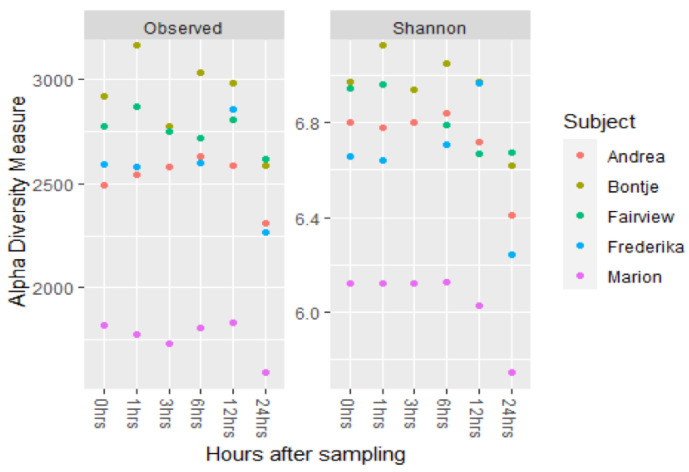
To evaluate the validity of the used sampling technique of collecting fresh faecal samples from the stall floor, a pilot study was conducted to investigate the effect of air exposure on the equine faecal microbiota. Faecal balls were collected rectally from 5 ponies and exposed to room air for different times. Richness and alpha-diversity were stable up to 12 h, but decreased between 12 and 24 h of air exposure, see Figure 1. Significant shifts in relative abundance for different phyla were observed at t = 12 h and t = 24 h compared to t = 0. Bacteroidetes decreased in relative abundance, while Firmicutes increased. At the class level, Bacilli increased after 12 h of air exposure (Supplementary Figure S1).
Figure 1.
Observed richness and alpha-diversity (Shannon diversity index) of the faecal microbiota over time of faecal samples collected from five ponies after exposure to room air. A decrease in observed richness alpha-diversity is visible after 12 h of air exposure (p = 0.062).
3.2. Faecal Microbiota Composition
A relative abundance of taxa in the faecal microbiota of 79 horses and ponies at class level and phylum level is presented in Supplementary Figure S2. Phyla, classes and families with a relative abundance of >1% are presented in Table 2. Bacteroidetes was the phylum with the largest mean relative abundance in our study (50.1%), followed by Firmicutes (28.4%), Spirochaetes (7.1%), Verrucomicrobia (6.5%), Fibrobacteres (5.0%) and Cyanobacteria (1.0%).
Table 2.
Phyla, classes and families with a relative abundance of >1% in the faecal microbiota.
| Phylum | Class | Family | Relative Abundance (%) |
|---|---|---|---|
| Bacteroidetes | 50.1 | ||
| Bacteroidia | 50.1 | ||
| Rikenellaceae | 12.9 | ||
| p-251-o5 | 11.7 | ||
| Prevotellaceae | 9.6 | ||
| F082 | 4.1 | ||
| Bacteroidales_UCG_001 | 3.4 | ||
| Bacteroidales_RF16 | 2.3 | ||
| Firmicutes | 28.4 | ||
| Clostridia | 22.8 | ||
| Lachnospiraceae | 9.8 | ||
| Oscillospiraceae | 3.5 | ||
| UCG-010 | 2.5 | ||
| Ruminococcaceae | 1.6 | ||
| Anaerovoracaceae | 1.2 | ||
| Bacilli | 3.0 | ||
| Erysipelatoclostridiaceae | 1.2 | ||
| Negativicutes | 2.6 | ||
| Acidaminococcaceae | 2.2 | ||
| Spirochaetes | 7.1 | ||
| Spirochaetia | 6.7 | ||
| Spirochaetaceae | 6.7 | ||
| Verrucomicrobia | 6.5 | ||
| Kiritimatiellae * | 6.0 | ||
| Fibrobacteres | 5.0 | ||
| Fibrobacteria | 5.0 | ||
| Fibrobacteraceae | 5.0 | ||
| Cyanobacteria | 1.0 | ||
| Vampirivibrionia | 1.0 |
* Further classification down to family level currently unavailable.
3.3. Factors Affecting Faecal Microbiota Composition
We evaluated the effects of age, gender, horse type, diet (roughage type, concentrates), pasture access, the season of sampling and location on microbiota composition by comparing the alpha-diversity, beta-diversity and differential abundance of taxa between samples.
3.3.1. Age
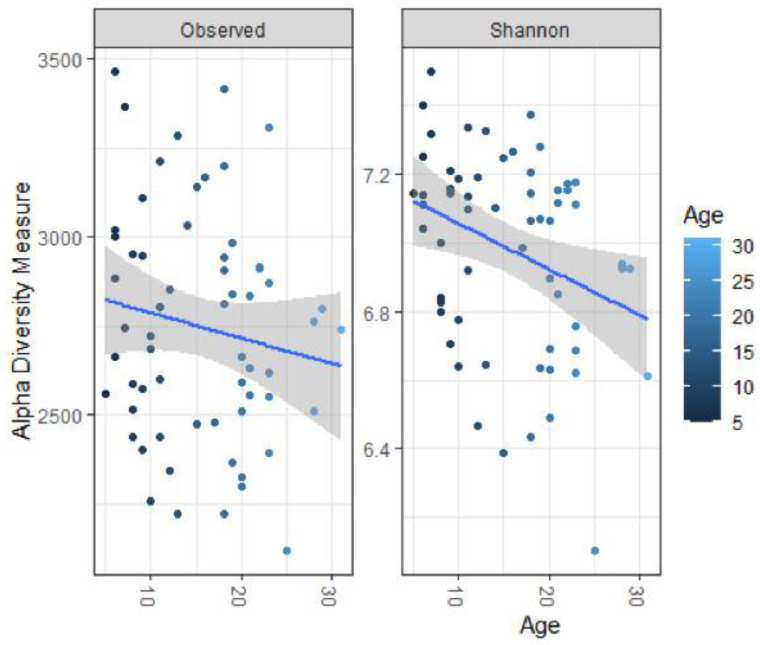
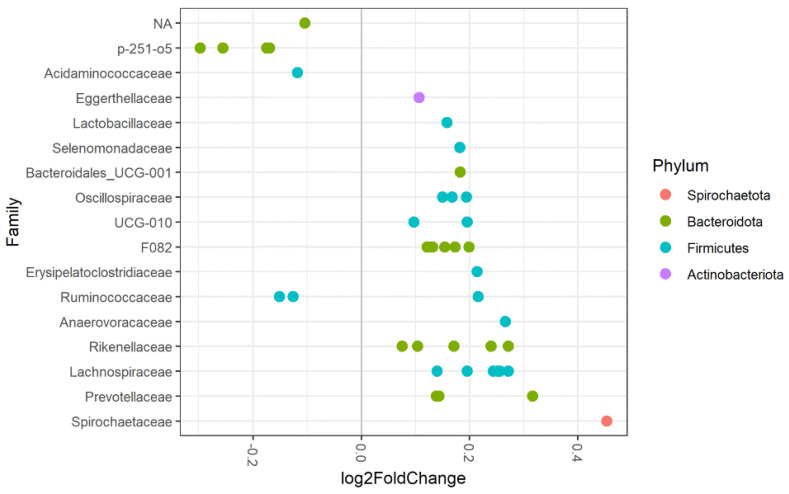
Increasing age led to decreased observed richness (p < 0.001) and alpha-diversity (p = 0.015), see Figure 2. Age also significantly affected beta-diversity, suggesting that the microbiota composition changes as animals age (R2 = 0.031, p = 0.002). Several amplicon sequence variants (ASVs) were significantly less abundant in older horses, including ASVs assigned to the bacterial families Acidaminococcaceae, Ruminococcaceae, p-251-o5 and an unidentified taxon belonging to the phylum of Bacteroidetes, see Figure 3. Other ASVs were more abundant in older horses. These were assigned to the families Eggerthellaceae, Lactobacillaceae, Selenomonadaceae, Oscillospiraceae, Erysipelatoclostridiaceae, Ruminococcaceae, Anaerovoracaceae, Rikenellaceae, Lachnospiraceae, Prevotellaceae, Spirochaetaceae, Bacteroidales_UCG-001, F082 and UCG-010.
Figure 2.
The effect of age on the observed richness and alpha-diversity (Shannon diversity index) on the faecal microbiota of 61 horses and ponies. A significant decrease, determined with linear modelling, in observed richness and alpha-diversity is visible in older horses.
Figure 3.
Differentially abundant ASVs grouped by family for increasing age (by year) of the faecal microbiota in 61 horses and ponies. The log2 fold change (per year) in ASV abundance is shown on the x-axis. ASVs assigned to bacterial families on the left side of the plot are less abundant in horses with increasing age. ASVs assigned to families depicted on the right side of the plot are more abundant in horses with increasing age. NA = ASV belonging to an unknown family (colours indicate the phylum).
3.3.2. Gender
No significant differences in observed richness, alpha- or beta-diversity were observed for gender in this study.
3.3.3. Horse Type
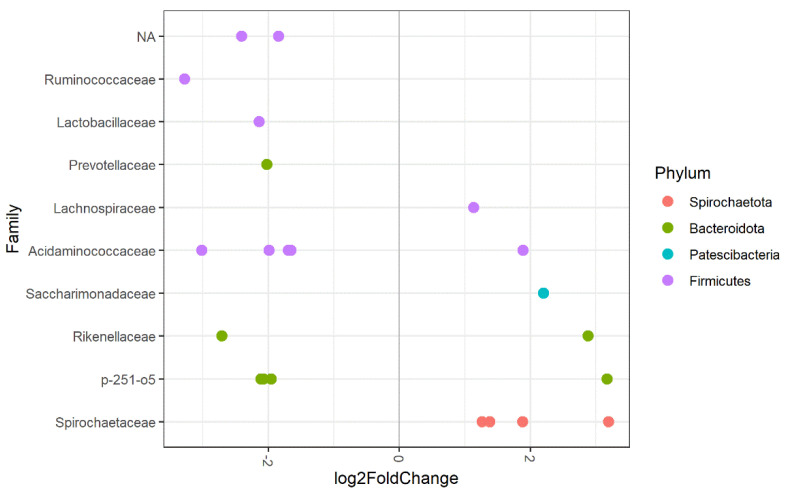
Thirty-two horses (Dutch Warmblood, Rheinlander, Oldenburger and Standardbred horses) and 29 ponies (Haflinger, Tinker, Irish Cobs, Welsh, Appaloosa, New Forest, Fjord, Icelandic, Shetland and mini-Shetland ponies) were included to evaluate for differences in faecal microbiota between horses and ponies. No significant differences in observed richness and alpha-diversity were observed for horses compared to ponies. Beta-diversity was significantly different when horses and ponies were compared. Horse type determined 2.4% of the variation (PERMANOVA p = 0.015 betadisper p = 0.530). ASVs assigned to the following families of bacteria were significantly less abundant in ponies compared to horses: Ruminicoccaceae, Lactobacillaceae, Prevotellaceae, Acidaminococcaceae, Rikenellaceae, p-251-o5 and some unidentified taxa within the Firmicutes phylum. Other ASVs were more abundant in ponies compared to horses. These belonged to the bacterial families of Lachnospiraceae, Acidaminococcaceae, Saccharimonadaceae, Rikenellaceae, Spirochaetaceae and p-251-o5, see Figure 4.
Figure 4.
Differentially abundant ASVs grouped by family for horse type (ponies vs. horses) of the faecal microbiota in 61 horses and ponies. The log2 fold change in species abundance is shown on the x-axis. ASVs assigned to bacterial families on the left side of the plot are less abundant in ponies than horses, ASVs assigned to families depicted on the right side of the plot are more abundant in ponies than horses. NA = ASV belonging to an unknown family (colours indicate the phylum).
3.3.4. Diet
No significant differences in observed richness, alpha- or beta-diversity were observed for the type of roughage fed. Also, no significant differences in richness, alpha- or beta-diversity were observed for animals fed more than 2 kg of concentrates compared to animals fed less than 2 kg of concentrates.
3.3.5. Pasture Access
No significant differences in observed richness and alpha-diversity were observed for pasture access. However, a significant difference in beta-diversity was observed when horses with pasture access were compared to horses that did not have pasture access. Pasture access explained 2.3% of the variation in microbiota composition (PERMANOVA p = 0.035 betadisper p = 0.034). Only a few ASVs were significantly less abundant in animals with pasture access. These belonged to the family of Lachnospiraceae and an unidentified taxon within the phylum of Bacteroidetes. Other ASVs were more abundant in animals with pasture access, belonging to the bacterial families of Prevotellaceae, Rikenellaceae, UCG-010 and two unidentified taxa within the phylum of Bacteriodetes.
3.3.6. Season
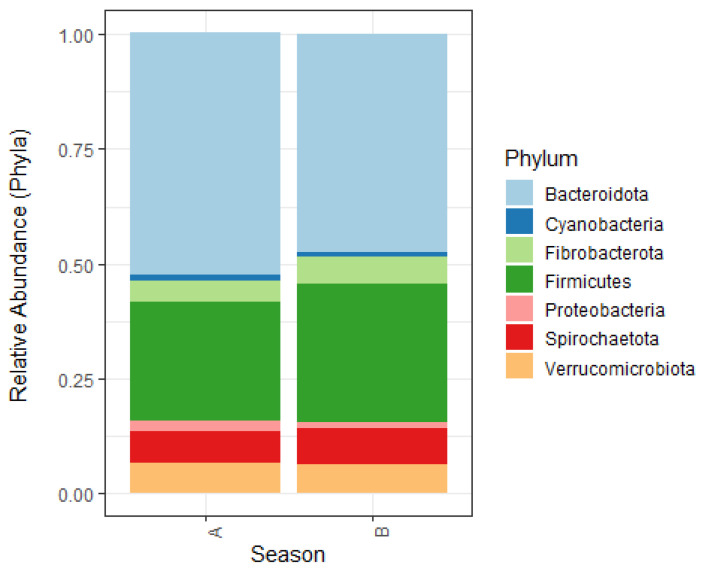
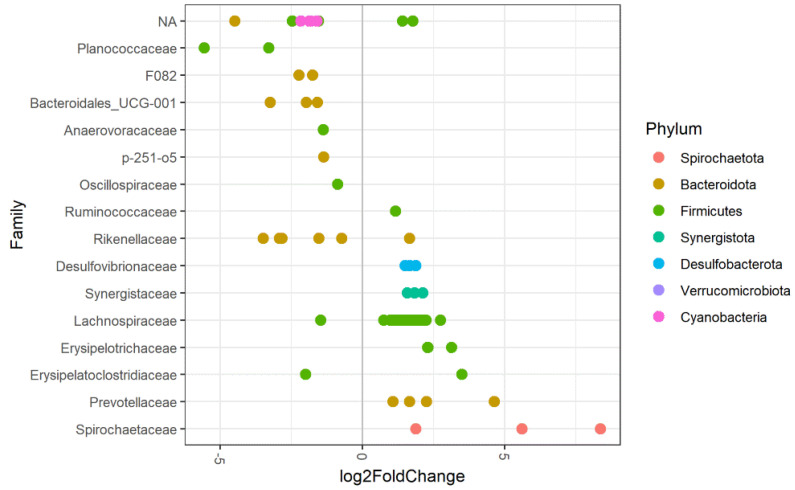
No significant differences in observed richness and alpha-diversity were identified for the season of sampling. However, the microbiota composition of samples collected in summer differed from that of samples collected in winter, evidenced by a significant difference in beta-diversity. The season of sampling explained 2.8% of the variation (PERMANOVA p = 0.002, betadisper p = 0.589). Firmicutes were significantly less abundant in samples collected in summer, Bacteroidetes were significantly less abundant in samples collected in winter (Figure 5). On a more detailed level, several ASVs were significantly less abundant in samples collected in the summer than samples collected in the winter. These were assigned to the bacterial families Planococcaceae, Anaerovoraceae, Oscillospiraceae, Rikenellaceae, Lachnospiraceae, Erysipelatoclostridiaceae, F082, Bacteroidales_UCG-001, p-251-o5 and several unidentified taxa within the phyla of Bacteroidetes, Firmicutes and Cyanobacteria. Other ASVs were more abundant in samples collected in the summer than samples collected in the winter, belonging to the bacterial families Ruminococcaceae, Rikenellaceae, Desulfovibrionaceae, Synergistaceae, Lachnospiraceae, Erysipelotrichaceae, Erysipelatoclostridiaceae, Prevotellaceae, Spirochaetaceae and unidentified taxa within the Firmicutes phylum; see Figure 6.
Figure 5.
Relative abundance of phyla in the faecal microbiota of 61 horses and ponies in The Netherlands. (A) Summer (B) Winter.
Figure 6.
Differentially abundant ASVs grouped by family for season of sampling (winter vs. summer) of the faecal microbiota in 61 horses and ponies. The log2 fold change in species abundance is shown on the x-axis. ASVs assigned to bacterial families on the left side of the plot are less abundant in samples collected in winter compared to samples collected in summer, ASVs assigned to families depicted on the right side of the plot are more abundant samples collected in the winter compared to sampled collected in the summer. NA = ASV belonging to an unknown family (colours indicate the phylum).
3.3.7. Location
To evaluate the effect of location (farm) on microbiota composition, data from 17 Warmblood horses aged 4 to 16 years from farm I were compared to data from 18 Warmblood horses of the same age from farm II. No significant differences in observed richness and alpha-diversity were seen for horses from farm I compared to horses from farm II. However, apparent clustering of samples from horses according to farm/location can be observed in the NMDS plot, see Figure 7, explaining 6.4% of the variation (PERMANOVA p = 0.001, betadisper p = 0.779).
Figure 7.
NMDS plot of beta-diversity showing the effect of location (farm) on the faecal microbiota composition of 35 horses. Red = Farm II, Blue = Farm I. NMDS stress level = 0.17.
4. Discussion
The equine faecal microbiota, as well as factors that shape it, have been studied and described in several publications in recent years but the reported results vary significantly [5,6,7,8,9,10,11,12,13,14,15,16,17]. These differences might, in part, be a result of methodological differences such as DNA extraction and sequencing techniques used. The large differences in reported results regarding the composition of the intestinal microbiota of healthy horses reduces our ability to draw conclusions from studies evaluating associations between specific equine diseases or physiological factors and the intestinal microbiota. The many uncertainties regarding the intestinal microbiota of healthy horses that still exist today support the publication of new studies evaluating the faecal microbiota of healthy subjects and factors that influence its composition.
4.1. Effect of Air Exposure on the Equine Faecal Microbiota
To validate the use of collecting spontaneously produced faecal samples as a form of convenience sampling in the current study, we studied the effect of air exposure on microbiota composition. Our results show that the observed richness and the α-diversity of the microbiota in faecal samples remain stable for up to 6 h, after which they decrease at 12 h of air exposure. The microbiota composition of faecal samples collected up to 6 h after spontaneous defaecation is the same as that of samples collected rectally. Therefore, these samples can be used for faecal microbiota analysis, which validates the faecal sample collection technique applied in the current study. This corresponds to previous reports in which parameters were also reported to be stable up to 6 h after defecation [17,32]. In our study, we found the relative abundance of Bacteroidetes to decrease and Firmicutes to increase over time. At the class level, Bacilli increased significantly in our study, which is in agreement with another study that identified Bacillaceae, Planococcaeae and Enterococcaceae, all families within the class of Bacilli, as bloom taxa [32].
4.2. Faecal Microbiota Composition
In the current study, Bacteroidetes was the most abundant phylum in the faecal microbiota, followed by Firmicutes, Spirochaetes and Fibrobacteres. Our results closely resemble those of some other studies in horses [7,14,17,19]. However, Firmicutes is the main bacterial phylum found in most studies of the faecal microbiota in healthy horses to date [2,3,5,6,8,9,10,12,13,15,16,21,33,34,35,36]. The relative abundance of Bacteroidetes in those studies varies significantly, ranging from 0% to 42% (mean 19%) [3,13]. Of the studies reporting high levels of Bacteroidetes (range 33–52%; mean 43%), similar to our results, almost all used the same DNA extraction kit (QiaAmp Fast DNA Stool Mini Kit) and protocol [7,8,13,14,19,35]. Studies using another commonly used DNA extraction kit and protocol (E.N.Z.A. Stool DNA Kit, Omega Bio-tek, Norcross, GA, USA) consistently report a lower relative abundance of Bacteroidetes ranging from 0% to 25% (mean 7%) [2,3,5,10,15,21]. This indicates that DNA extraction kit and protocol might be a major factor of influence on the results in equine faecal microbiota studies. Several studies have shown significant effects of DNA extraction methods (and sample type) on the results of microbiota analysis [23,37]. However, a study comparing the two DNA extraction kits most commonly used in equine microbiota studies, as mentioned above, is currently lacking. The use of different 16S primers for sequencing might also affect results [38]. Studies evaluating the effect of 16S primer selection on results in equine studies are also currently lacking. The large effect of several critical steps in obtaining microbiota data, as mentioned above, complicates the comparison of taxa abundance and microbiota composition between studies when different protocols are used.
4.3. The Effect of Different Factors on Faecal Microbiota Composition
4.3.1. Age
Our results show a reduction in the number of species (observed richness) and alpha-diversity with the increasing age of horses. The current reports in the literature on horses are ambiguous on this topic. Similar results were obtained in a study comparing the faecal microbiota of mature horses (5–12 years) to that of elderly horses (19–28 years) [8]. In other studies, no effect of age on alpha-diversity was observed [11,39] and in specific breeds (Anglo-Arabian horses and wild Prezwalski horses), the opposite trend was observed where alpha-diversity increased with age [10,40]. In adult humans, alpha-diversity decreases with age, similar to the trend observed in our study in horses [41]. In our study, we identified several ASVs whose relative abundance changed with increasing age. Little is known about the role these taxa play in the gut and how this might affect host health. Of interest is the decrease of two taxa within the family of Ruminococcaceae with increasing age in our study, as Ruminococcaceae have been associated with gut health in mammals and lower relative abundance has been associated with colitis, diarrhoea, colic and equine metabolic syndrome in equids [2,4,36,42]. Older horses, therefore, might be more prone to the development of specific diseases. In humans, increasing age, especially in the group of centenarians, leads to a compromised microbiota with increased levels of pathobionts and a heightened inflammatory state, known as inflammageing, and an increase in peripheral blood inflammatory markers can be observed. [41] If the same process occurs in horses is currently unknown. A marked decrease in Faecalibacterium prauznitzii (a member of the Ruminococcaceae family) was observed in humans. Our study also observed a decrease of specific ASVs assigned to two families of Ruminococcaceae in horses with increasing age, but we were unable to further specify genus/species.
4.3.2. Gender
In the current study, no effect of gender was found on the faecal microbiota. This is in line with reports from other studies in horses and ponies [5,19]. However, one study did report gender differences in Przewalski horses kept under similar circumstances [43]. Gender differences in faecal microbiota are also observed in humans [44]. Gender differences in microbiota composition in horses might be subtle.
4.3.3. Horse Type
Horses and ponies differ in metabolism, and as a result, ponies are more prone to obesity and related disorders such as laminitis and equine metabolic syndrome [45,46,47]. Differences in the prevalence of several gastrointestinal disorders have also been described for horses and ponies [48]. Therefore, we evaluated differences in microbiota composition between horses and ponies in our study. We found no differences in alpha-diversity between horses and ponies, which is in line with a recently published study [49]. However, a significant difference in beta-diversity was observed when horses and ponies were compared, demonstrating significant differences in microbiota composition. Ruminococcaceae (fibrolytic bacteria), Lactobacillaceae (carbohydrate fermentation, production of lactic acid) and Prevotellaceae (breakdown of carbohydrates and protein) were less abundant in ponies compared to horses. Lachnospiraceae (plant polysaccharide fermentation and short-chain fatty acid production), Saccharimonadaceae and Spirochaetaceae were more abundant in ponies. For Acidaminococcaceae, Rikenellaceae and P-251-o5, we observed that specific genera/species within these families increase or decrease according to horse type, suggesting that bacterial shifts occur within these families. In the study by Langner et al. (2020), Ruminococcaceae, Rikenellaceae, F082 and Bacteroidales UCG-001 were found to be more abundant in ponies compared to horses, but only after all subjects included in the study were kept on a 200% net energy requirement diet for two years [49]. The differences, therefore, not only reflect differences between ponies and horses, as they were not present at the start of the study but are heavily influenced by the high energy diet. Breed differences are also described in other studies comparing the microbiota of several European sport horse breeds and Thoroughbred horses and Mongolian horses [10,35]. Most of the identified differences were subtle or were seen only in low abundant phyla and genera. A study comparing the microbiota composition of Quarter Horses, Morgan, Paint and Tennessee Walking found no differences [50]. The differences in microbiota composition between horses and ponies observed in the current study indicate that horse type should be considered in experimental studies of the faecal microbiota.
4.3.4. Diet
Our study did not observe a significant effect of diet (roughage and/or concentrates) on microbiota composition. This contrasts with previous studies in which dietary effects on the intestinal microbiota have been demonstrated [51,52,53]. However, in these studies, larger contrasts in diet between studied groups were tested (e.g., no concentrate feeding vs. high level of concentrate feeding). In our study, horses and ponies living under normal circumstances at a farm were sampled and, therefore, for example, received varying amounts and varying types of concentrates (with varying levels of nutrients) leading to less contrast when groups were compared and limiting our ability to detect differences. Also, the studies mentioned above evaluated the effect of a sudden change in diet instead of assessing the microbiota from equids on a long-term stable diet. Sudden changes in diet might disrupt the microbiota composition and therefore lead to more pronounced differences that are not present while on a steady diet, as was the case for the horses and ponies included in our study.
4.3.5. Pasture Access
In contrast to roughage and concentrates, pasture access did significantly affect microbiota composition in the current study. Lachnospiraceae were less abundant in horses with pasture access in our study, whereas Prevotellaceae, Rikenellaceae and UCG-010 were more abundant. In a study performed in New Zealand, specific taxa were identified to be more abundant in horses abruptly transitioned to pasture: an unclassified genus within the order of Clostridiales, an unclassified genus within the order of Lachnospiraceae, CF231 and BF311 [9]. Another recent study reported an increase in Lactobacillaceae in horses that were abruptly changed from a hay-only diet to a grass diet [54]. The horses included in our study did not undergo abrupt dietary changes and had the same level of pasture access over a prolonged period prior to sampling, which might explain the differences observed when the results of our study are compared to that of previous reports. A recent study from Switzerland also detected significant differences in faecal microbiota composition depending on the number of hours horses had pasture access [55]. Based on the current study and the previously published data, pasture access seems to affect microbiota composition in equids.
4.3.6. Season
We observed a seasonal effect on the faecal microbiota in our study, as evidenced by differences in beta-diversity. Genera/species within the same families increased or decreased with the season of sampling, suggesting that shifts occur within bacterial families. We observed this trend for Rikenellaceae, Lachnospiraceae and members of the family of Erysipelatoclostridiaceae. In a longitudinal study by Salem et al., a biphasic change in microbiota composition was observed over a 12-month period, and alpha-diversity showed a significant non-linear trend [14]. In our study, we did not observe any differences in alpha-diversity. In our study and the study by Salem et al., pasture access varied according to season and was higher in the summer months. Therefore, it is difficult to determine which of the observed differences resulted from dietary changes because of pasture access and which of the differences resulted from the change in season. Nevertheless, our results suggest that season should be included as a co-variant in future studies.
4.3.7. Location
We demonstrated an effect of farm/location on microbiota composition in our study. A previously published study showed relatively low variation in microbiota composition between horses housed in the same environment, indicating interaction patterns across horses housed in the same environment [51]. The effects of location on microbiota composition have been reported previously in domesticated and (semi-) feral horses [19,34,39,56,57]. However, these differences might also in part be explained by dietary or management differences. The location effect should be included in future studies concerning the equine faecal microbiota.
4.4. Strengths and Limitations
As demonstrated in the current study and previous studies, many factors contribute to shaping the gut microbiota in individual animals. However, explained variation by individual factors is generally low, in our study up to 6.4%, suggesting that other (unidentified) factors also play a role. This complexity of involved (and potentially interrelated) determinants is a challenge for suitable study designs. The current broad assessment of the faecal microbiota in horses and ponies living under standard housing conditions at equine farms has the advantage that the results represent the actual situation in the field. The difficulty with a non-homogenous study population is that differences between groups of horses might be less pronounced than in experimental studies in a more homogenous study population with only one variable being studied. By including a more significant number of equids than most equine studies, we have tried to overcome this limitation. In the current study, horses from only two farms were included, limiting the extent to which results can be extrapolated to other equine populations. However, given the relatively large number of equids included in the study and the variation in horse characteristics and management conditions it does provide important baseline information on variation in faecal microbiota in healthy horses and ponies under field conditions. Larger, and preferably international, population-based studies are needed to further investigate and understand the complexity of the equine faecal microbiota and the factors that shape it.
5. Conclusions
The current study provides essential baseline information on variation in faecal microbiota in a group of horses and ponies managed under normal management circumstances and the factors that influence its composition. Bacteroidetes is the largest phylum found in the faecal microbiota of the horses and ponies included in this study, followed by Firmicutes. Alpha-diversity and richness decreased significantly with increasing age. Location, age, season, horse type and pasture access had a significant effect on the composition of the microbiome, explaining 2.3% to 6.4% of the observed variation. These results indicate that faecal microbiota composition is affected by several horse-related and environment-related factors. Studies investigating potential relationships between environmental factors or disease status and faecal microbiota should take these factors into account when interpreting observed differences to avoid the risk of overinterpretation.
Acknowledgments
We would like to thank Jurgen Welmers (Paardenpraktijk Stal Wennekers, Aalsmeer, The Netherlands) and Vanessa Visser (Dierenarts Visser, Soest, The Netherlands) for their assistance in recruiting horses for this study. Also, we would like to thank Arjen Timmerman (Utrecht University, Utrecht, The Netherlands) and Maarten van Putten (University Medical Center Groningen, Groningen, The Netherlands) for their magnificent help with the DNA extraction and 16S rRNA sequencing.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11061762/s1, Figure S1: Relative abundance of bacterial communities at phylum- and class level in the microbiota of faecal samples collected from ponies at different time points after exposure to room air; Figure S2: Relative abundance of taxa at phylum- and class level in the faecal microbiota of 79 horses and ponies at two farms in The Netherlands.
Author Contributions
M.J.P.T., A.L.Z., J.A.W. and M.M.S.v.O.-O. designed the study. M.J.P.T. performed the sample collection and the DNA extraction. J.W.A.R. performed the 16s rRNA sequencing. A.L.Z. and R.E.C.L. analysed the sequencing data and performed the statistical analysis and data visualization. All authors contributed to data interpretation. M.J.P.T., A.L.Z. and R.E.C.L. prepared the manuscript with input and contributions from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as only spontaneous produced faecal samples were used for this study and no procedures had to be performed on the animals included in this study. Informed consent was obtained from all owners.
Informed Consent Statement
Informed consent was obtained from all horse owners of the subjects involved in the study.
Data Availability Statement
The datasets supporting this article have been uploaded to the sequence read archive as part of the supplementary electronic material and are available under accession PRJEB44895.
Conflicts of Interest
J.W.A.R. is currently employed by IDbyDNA Inc. The other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Costa M.C., Weese J.S. Understanding the Intestinal Microbiome in Health and Disease. Vet. Clin. North Am. Equine Pract. 2018;34:1–12. doi: 10.1016/j.cveq.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Costa M.C., Arroyo L.G., Allen-Vercoe E., Stämpfli H.R., Kim P.T., Sturgeon A., Weese J.S. Comparison of the Fecal Microbiota of Healthy Horses and Horses with Colitis by High Throughput Sequencing of the V3-V5 Region of the 16S rRNA Gene. PLoS ONE. 2012;7:e41484. doi: 10.1371/journal.pone.0041484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weese J.S., Holcombe S.J., Embertson R.M., Kurtz K.A., Roessner H.A., Jalali M., Wismer S.E. Changes in the faecal microbiota of mares precede the development ofpost partumcolic. Equine Vet. J. 2014;47:641–649. doi: 10.1111/evj.12361. [DOI] [PubMed] [Google Scholar]
- 4.Elzinga S.E., Weese J.S., Adams A.A. Comparison of the Fecal Microbiota in Horses With Equine Metabolic Syndrome and Metabolically Normal Controls Fed a Similar All-Forage Diet. J. Equine Vet. Sci. 2016;44:9–16. doi: 10.1016/j.jevs.2016.05.010. [DOI] [Google Scholar]
- 5.Costa M., Silva G., Ramos R., Staempfli H., Arroyo L., Kim P., Weese J. Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. Vet. J. 2015;205:74–80. doi: 10.1016/j.tvjl.2015.03.018. [DOI] [PubMed] [Google Scholar]
- 6.De Almeida M.L.M., Feringer W.H., de Carvalho J.R.G., Rodrigues I.M., Jordão L.R., Fonseca M.G., Rezende A.S.C., Neto A.D.Q., Weese J.S., Da Costa M.C., et al. Intense Exercise and Aerobic Conditioning Associated with Chromium or L-Carnitine Supplementation Modified the Fecal Microbiota of Fillies. PLoS ONE. 2016;11:e0167108. doi: 10.1371/journal.pone.0167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dougal K., Harris P.A., Girdwood S.E., Creevey C.J., Curtis G.C., Barfoot C.F., Argo C., Newbold C.J. Changes in the Total Fecal Bacterial Population in Individual Horses Maintained on a Restricted Diet Over 6 Weeks. Front. Microbiol. 2017;8:1502. doi: 10.3389/fmicb.2017.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougal K., De La Fuente G., Harris P.A., Girdwood S.E., Pinloche E., Geor R.J., Nielsen B.D., Ii H.C.S., Elzinga S., Newbold C.J. Characterisation of the Faecal Bacterial Community in Adult and Elderly Horses Fed a High Fibre, High Oil or High Starch Diet Using 454 Pyrosequencing. PLoS ONE. 2014;9:e87424. doi: 10.1371/journal.pone.0087424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes K.A., Kittelmann S., Rogers C.W., Gee E.K., Bolwell C.F., Bermingham E., Thomas D.G. Faecal Microbiota of Forage-Fed Horses in New Zealand and the Population Dynamics of Microbial Communities following Dietary Change. PLoS ONE. 2014;9:e112846. doi: 10.1371/journal.pone.0112846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massacci F.R., Clark A., Ruet A., Lansade L., Costa M., Mach N. Inter-breed diversity and temporal dynamics of the faecal microbiota in healthy horses. J. Anim. Breed. Genet. 2019;137:103–120. doi: 10.1111/jbg.12441. [DOI] [PubMed] [Google Scholar]
- 11.Morrison P.K., Newbold C.J., Jones E., Worgan H.J., Grove-White D.H., Dugdale A.H., Barfoot C., Harris P.A., Argo C.M. The Equine Gastrointestinal Microbiome: Impacts of Age and Obesity. Front. Microbiol. 2018;9:3017. doi: 10.3389/fmicb.2018.03017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnell M.O., Harris H., Jeffery I., Claesson M., Younge B., Toole P.O., Ross R. The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Lett. Appl. Microbiol. 2013;57:492–501. doi: 10.1111/lam.12137. [DOI] [PubMed] [Google Scholar]
- 13.Proudman C., Hunter J.O., Darby A., Escalona E.E., Batty C., Turner C. Characterisation of the faecal metabolome and microbiome of Thoroughbred racehorses. Equine Vet. J. 2014;47:580–586. doi: 10.1111/evj.12324. [DOI] [PubMed] [Google Scholar]
- 14.Salem S.E., Maddox T.W., Berg A., Antczak P., Ketley J.M., Williams N., Archer D.C. Variation in faecal microbiota in a group of horses managed at pasture over a 12-month period. Sci. Rep. 2018;8:8510. doi: 10.1038/s41598-018-26930-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoster A., Mosing M., Jalali M., Staempfli H.R., Weese J.S. Effects of transport, fasting and anaesthesia on the faecal microbiota of healthy adult horses. Equine Vet. J. 2015;48:595–602. doi: 10.1111/evj.12479. [DOI] [PubMed] [Google Scholar]
- 16.Shepherd M.L., Swecker J.W.S., Jensen R.V., Ponder M.A. Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol. Lett. 2011;326:62–68. doi: 10.1111/j.1574-6968.2011.02434.x. [DOI] [PubMed] [Google Scholar]
- 17.Stewart H.L., Pitta D., Indugu N., Vecchiarelli B., Engiles J.B., Southwood L.L. Characterization of the fecal microbiota of healthy horses. Am. J. Vet. Res. 2018;79:811–819. doi: 10.2460/ajvr.79.8.811. [DOI] [PubMed] [Google Scholar]
- 18.Blackmore T.M., Dugdale A., Argo C.M., Curtis G., Pinloche E., Harris P.A., Worgan H.J., Girdwood S.E., Dougal K., Newbold C.J., et al. Strong Stability and Host Specific Bacterial Community in Faeces of Ponies. PLoS ONE. 2013;8:e75079. doi: 10.1371/journal.pone.0075079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antwis R.E., Lea J.M.D., Unwin B., Shultz S. Gut microbiome composition is associated with spatial structuring and social interactions in semi-feral Welsh Mountain ponies. Microbiome. 2018;6:207. doi: 10.1186/s40168-018-0593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faubladier C., Chaucheyras-Durand F., Da Veiga L., Julliand V. Effect of transportation on fecal bacterial communities and fermentative activities in horses: Impact of Saccharomyces cerevisiae CNCM I-1077 supplementation1. J. Anim. Sci. 2013;91:1736–1744. doi: 10.2527/jas.2012-5720. [DOI] [PubMed] [Google Scholar]
- 21.Costa M.C., Stämpfli H.R., Arroyo L.G., Allen-Vercoe E., Gomes R.G., Weese J.S. Changes in the equine fecal microbiota associated with the use of systemic antimicrobial drugs. BMC Vet. Res. 2015;11:19. doi: 10.1186/s12917-015-0335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yatsunenko T., Rey F.E., Manary M.J., Trehan I., Dominguez-Bello M.G., Contreras M., Magris M., Hidalgo G., Baldassano R.N., Anokhin A.P., et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knudsen B.E., Bergmark L., Munk P., Lukjancenko O., Priemé A., Aarestrup F.M., Pamp S.J. Impact of Sample Type and DNA Isolation Procedure on Genomic Inference of Microbiome Composition. mSystems. 2016;1:e00095-16. doi: 10.1128/mSystems.00095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Callahan B.J., Mcmurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McMurdie P.J., Holmes S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.R Core Team . R: A language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2020. [Google Scholar]
- 28.Oksanen J., Blanchet F.G., Friendly M., Kindt R., Legendre P., McGlinn D., Minchin P.R., O’Hara R.B., Simpson G.L., Solymos P., et al. Vegan: Community Ecology Package. R package version 2.5-6, 2019. [(accessed on 1 October 2020)]; Available online: https://CRAN.R-project.org/package=vegan.
- 29.Wickham H. ggplot2: Elegant Graphics for Data Analysis. Springer Nature; Cham, Switzerland: 2016. [Google Scholar]
- 30.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 32.Beckers K.F., Schulz C.J., Childers G.W. Rapid regrowth and detection of microbial contaminants in equine fecal microbiome samples. PLoS ONE. 2017;12:e0187044. doi: 10.1371/journal.pone.0187044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart H.L., Southwood L.L., Indugu N., Vecchiarelli B., Engiles J.B., Pitta D. Differences in the equine faecal microbiota between horses presenting to a tertiary referral hospital for colic compared with an elective surgical procedure. Equine Vet. J. 2019;51:336–342. doi: 10.1111/evj.13010. [DOI] [PubMed] [Google Scholar]
- 34.Biddle A.S., Tomb J.-F., Fan Z. Microbiome and Blood Analyte Differences Point to Community and Metabolic Signatures in Lean and Obese Horses. Front. Vet. Sci. 2018;5:225. doi: 10.3389/fvets.2018.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y., Li B., Bai D., Huang J., Shiraigo W., Yang L., Zhao Q., Ren X., Wu J., Bao W., et al. Comparison of Fecal Microbiota of Mongolian and Thoroughbred Horses by High-throughput Sequencing of the V4 Region of the 16S rRNA Gene. Asian-Australas. J. Anim. Sci. 2015;29:1345–1352. doi: 10.5713/ajas.15.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez C., Taminiau B., Brévers B., Avesani V., Van Broeck J., Leroux A., Gallot M., Bruwier A., Amory H., Delmée M., et al. Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC Microbiol. 2015;15:181. doi: 10.1186/s12866-015-0514-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janabi A.H., Kerkhof L.J., McGuinness L.R., Biddle A.S., McKeever K.H. Comparison of a modified phenol/chloroform and commercial-kit methods for extracting DNA from horse fecal material. J. Microbiol. Methods. 2016;129:14–19. doi: 10.1016/j.mimet.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 38.Klindworth A., Pruesse E., Schweer T., Peplies J., Quast C., Horn M., Glöckner F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013;41:e1. doi: 10.1093/nar/gks808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney C.A., Oliveira B.C.M., Bedenice D., Paradis M.-R., Mazan M., Sage S., Sanchez A., Widmer G. The fecal microbiota of healthy donor horses and geriatric recipients undergoing fecal microbial transplantation for the treatment of diarrhea. PLoS ONE. 2020;15:e0230148. doi: 10.1371/journal.pone.0230148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metcalf J.L., Song S.J., Morton J.T., Weiss S., Seguin-Orlando A., Joly F., Feh C., Taberlet P., Coissac E., Amir A., et al. Evaluating the impact of domestication and captivity on the horse gut microbiome. Sci. Rep. 2017;7:15497. doi: 10.1038/s41598-017-15375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Biagi E., Nylund L., Candela M., Ostan R., Bucci L., Pini E., Nikkïla J., Monti D., Satokari R., Franceschi C., et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/annotation/df45912f-d15c-44ab-8312-e7ec0607604d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daly K., Proudman C.J., Duncan S.H., Flint H.J., Dyer J., Shirazi-Beechey S. Alterations in microbiota and fermentation products in equine large intestine in response to dietary variation and intestinal disease. Br. J. Nutr. 2011;107:989–995. doi: 10.1017/S0007114511003825. [DOI] [PubMed] [Google Scholar]
- 43.Hu D., Chao Y., Li Y., Peng X., Wang C., Wang Z., Zhang D., Li K. Effect of Gender Bias on Equine Fecal Microbiota. J. Equine Vet. Sci. 2021;97:103355. doi: 10.1016/j.jevs.2020.103355. [DOI] [PubMed] [Google Scholar]
- 44.Scepanovic P., Hodel F., Mondot S., Partula V., Byrd A., Hammer C., Alanio C., Bergstedt J., Patin E., Touvier M., et al. Milieu Interieur Consortium A comprehensive assessment of demographic, environmental, and host genetic associations with gut microbiome diversity in healthy individuals. Microbiome. 2019;7:130. doi: 10.1186/s40168-019-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schedlbauer C., Blaue D., Gericke M., Blüher M., Starzonek J., Gittel C., Brehm W., Vervuert I. Impact of body weight gain on hepatic metabolism and hepatic inflammatory cytokines in comparison of Shetland pony geldings and Warmblood horse geldings. PeerJ. 2019;7:e7069. doi: 10.7717/peerj.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adolph S., Schedlbauer C., Blaue D., Schöniger A., Gittel C., Brehm W., Fuhrmann H., Vervuert I. Lipid classes in adipose tissues and liver differ between Shetland ponies and Warmblood horses. PLoS ONE. 2019;14:e0207568. doi: 10.1371/journal.pone.0207568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wylie C.E., Collins S.N., Verheyen K.L., Newton J.R. Risk factors for equine laminitis: A case-control study conducted in veterinary-registered horses and ponies in Great Britain between 2009 and 2011. Vet. J. 2013;198:57–69. doi: 10.1016/j.tvjl.2013.08.028. [DOI] [PubMed] [Google Scholar]
- 48.Dunkel B., Buonpane A., Chang Y.-M. Differences in gastrointestinal lesions in different horse types. Vet. Rec. 2017;181:291. doi: 10.1136/vr.104098. [DOI] [PubMed] [Google Scholar]
- 49.Langner K., Blaue D., Schedlbauer C., Starzonek J., Julliand V., Vervuert I. Changes in the faecal microbiota of horses and ponies during a two-year body weight gain programme. PLoS ONE. 2020;15:e0230015. doi: 10.1371/journal.pone.0230015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ericsson A.C., Johnson P.J., Lopes M.A., Perry S.C., Lanter H.R. A Microbiological Map of the Healthy Equine Gastrointestinal Tract. PLoS ONE. 2016;11:e0166523. doi: 10.1371/journal.pone.0166523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kristoffersen C., Jensen R.B., Avershina E., Austbø D., Tauson A.-H., Rudi K. Diet-Dependent Modular Dynamic Interactions of the Equine Cecal Microbiota. Microbes Environ. 2016;31:378–386. doi: 10.1264/jsme2.ME16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hansen N.C.K., Avershina E., Mydland L.T., Næsset J.A., Austbø D., Moen B., Måge I., Rudi K. High nutrient availability reduces the diversity and stability of the equine caecal microbiota. Microb. Ecol. Health Dis. 2015;26:27216. doi: 10.3402/mehd.v26.27216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warzecha C.M., Coverdale J.A., Janecka J.E., Leatherwood J.L., E Pinchak W., Wickersham T.A., McCann J.C. Influence of short-term dietary starch inclusion on the equine cecal microbiome. J. Anim. Sci. 2017;95:5077–5090. doi: 10.2527/jas2017.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garber A., Hastie P., McGuinness D., Malarange P., Murray J.-A. Abrupt dietary changes between grass and hay alter faecal microbiota of ponies. PLoS ONE. 2020;15:e0237869. doi: 10.1371/journal.pone.0237869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaiser-Thom S., Hilty M., Gerber V. Effects of hypersensitivity disorders and environmental factors on the equine intestinal microbiota. Vet. Q. 2020;40:97–107. doi: 10.1080/01652176.2020.1745317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Zhang K., Liu Y., Li K., Hu D., Wronski T. Community Composition and Diversity of Intestinal Microbiota in Captive and Reintroduced Przewalski’s Horse (Equus ferus przewalskii) Front. Microbiol. 2019;10:1821. doi: 10.3389/fmicb.2019.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De La Torre U., Henderson J.D., Furtado K., Pedroja M., Elenamarie O., Mora A., Pechanec M.Y., Maga E.A., Mienaltowski M.J. Utilizing the fecal microbiota to understand foal gut transitions from birth to weaning. PLoS ONE. 2019;14:e0216211. doi: 10.1371/journal.pone.0216211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting this article have been uploaded to the sequence read archive as part of the supplementary electronic material and are available under accession PRJEB44895.