Supplemental digital content is available in the text.
Key Words: adults, clinical research, education, participation, race, trust, underrepresented
Abstract
Background
Despite numerous efforts to create more equitable healthcare systems, minority populations face long-standing health disparities compared to White populations. Healthcare research is the necessary foundation for creating equitable health systems and providing patient-centered care. Significant challenges exist, however, with recruiting and engaging underrepresented populations in clinical research.
Objectives
The purpose of this analysis was to determine how research participants' race, trust, and level of education influence participation barriers in clinical research.
Methods
The study used secondary, cross-sectional survey data that were collected between 2014 and 2016 through the former Mid-South Clinical Data Research Network, currently known as the Stakeholders, Technology, and Research Clinical Research Network. Descriptive statistics and Spearman rank correlations were performed between level of education, level of trust, and each attitude statement for each racial category.
Results
A total of 2,190 survey responses were used in the data analysis. The mean age of respondents was 52 years, with majority being women, White, insured, and working full time. Overall, the respondents had favorable attitudes toward research participation. Trust was correlated with agreement in many attitude statements for both White and African American respondents, whereas correlations with education level were more variable depending on racial grouping. Trust level was negatively associated with agreement toward the statement “researchers do not care about me” in White and Native American respondents.
Discussion
The results support the importance of trust to research participation. Generally, education level was not strongly predictive of research participation, although prediction was influenced by race and attitude.
At the turn of the 21st century, the Institute of Medicine released the article Crossing the Quality Chasm, which emphasized the need for an effective, equitable, and patient-centered healthcare delivery system (Agency for Healthcare Research and Quality [AHRQ], 2019). The need for improved healthcare derived from the long-standing systemic health inequities experienced by racial and ethnic minority groups. More than two decades later, amidst a global pandemic, the United States continues to seek care solutions as minority populations persistently face devastating health disparities. To provide effective and equitable care, improved healthcare research is necessary (Beattie et al., 2012).
Healthcare research is a general term that includes a variety of research methods that ultimately develop or provide knowledge regarding disease, risk factors, outcomes of treatment, public health interventions, functional abilities, patterns of care, and healthcare usage (Beattie et al., 2012). Precision health research has been touted as a novel and person-centered method of healthcare research. Precision health approaches disease treatment and prevention by accounting for the individual’s genetic variability, lifestyle, and social determinants for providers to care for patients most effectively and equitably.
Although research is the necessary foundation for healthcare advancement and understanding, significant challenges exist with recruiting and engaging underrepresented populations in healthcare research. The National Institutes of Health recognize African Americans (AAs), American Indians (AIs), Alaskan Natives, Hispanics, Native Hawaiians, and other Pacific Islanders as underrepresented populations in research studies (National Institutes of Health, n.d.). In 2017, the U.S. Food and Drug Administration reported 81% of clinical trial participants as White, 14% as AA, 2.7% as Asian, and the remaining 2.3% as Hispanic, Pacific Islander, AI, Alaskan Native, or Native Hawaiian (U.S. Food and Drug Administration, 2017). Although minority populations are underrepresented in clinical research, they face the greatest health disparities (AHRQ, 2019).
The 2018 National Healthcare Quality and Disparities Report documented that minority populations receive poorer quality of care and face greater barriers in accessing care compared to White populations (AHRQ, 2019). In addition, AAs, AIs, and Hispanics have greater rates of preventable hospitalizations and higher mortality rates compared to Whites (AHRQ, 2019). Incidence of specific diseases, cancers, and reactions to medications and treatments differ between races and ethnicities. For example, AA men have a greater incidence of prostate cancer compared to White men. AA women have the same incidence of breast cancer as White women, but they have higher mortality rates (Reifenstein, 2018). Amid the ongoing pandemic, AAs account for one third of COVID-19 cases and are twice as likely to die from the virus (Garg et al., 2020). Hispanics and AAs have the greatest prevalence of diabetes, adolescent obesity, and asthma compared to Whites (National Center for Health Statistics, 2019). Similarly, almost half of all AA adults have some form of cardiovascular disease compared to about one third of White adults (Reifenstein, 2018). It is necessary to recruit and engage diverse populations to create equitable health systems. However, engaging and recruiting participants to accurately represent the diversity of the population is a challenging process (Erves et al., 2017).
Participation barriers in research and variables that can influence an individual’s willingness to participate have been identified in the literature. Barriers identified include the participant’s level of trust, access to research information, fear of the unknown or adverse effects, inconvenience, and reputation of researchers and research institutions.
Level of Trust
The concept of trust is examined in healthcare literature, specifically because of relationship dynamics between healthcare professionals and patients (Hall et al., 2001). Trust is defined as the degree to which the patient relies and depends on and is confident about the provider (Hall et al., 2001). Trust is present in situations of risk, uncertainty, vulnerability, or unequal status where there is a level of dependence on another individual creating a relationship of vulnerability (Hall et al., 2001). Variables identified throughout literature that act as barriers to participant trust in clinical research include inadequate information regarding research studies, unethical behavior by the research team, and safety concerns (Ceballos et al., 2014; Cortés et al., 2017; Erves et al., 2017; Scharff et al., 2010). Many individuals believe that collected samples like blood, urine, saliva, or stool are unethically disposed of or used after the research study without permission (Ceballos et al., 2014; Kraft et al., 2018). In addition, study participants expressed fear of taking medications that would cause adverse effects, receiving unnecessary surgery, experiencing unintended consequences of the study, having personal information used against them, and being treated like “guinea pigs” or “lab rats” (Cortés et al., 2017; Durant et al., 2011; Erves et al., 2017; Scharff et al., 2010).
Race and Ethnicity
Race and ethnicity are variables that not only influence patient participation in research but also influence trust. Because of historic and recent events of segregation, racism, and unequal civil rights, AAs report less willingness to participate in research compared to Whites (Dunlop et al., 2011; Durant et al., 2011; Kraft et al., 2018; Ma et al., 2013). A variety of studies reference the Tuskegee syphilis study that was conducted from the 1930s to the 1970s that left the AA community fearful and distrusting of research (Alsan & Wanamaker, 2018; Durant et al., 2011; Ma et al., 2013; Scharff et al., 2010). The Tuskegee study permitted hundreds of adult AA men with syphilis to go untreated despite the availability of an effective treatment: penicillin (Alsan & Wanamaker, 2018). In addition, the treatment of Henrietta Lacks and her family in the 1950s continues to alter the perspectives of AAs toward healthcare institutions and American society (Kraft et al., 2018). Henrietta Lacks was an AA woman whose cells were collected from a cervical cancer biopsy and later used to develop HeLa cells, which were commercialized and highly profitable. The Lacks family did not gain any profit, however, from Ms. Lacks cells (Lee et al., 2019).
Hispanic individuals also face specific cultural and racial variables that influence participation in clinical research (Ceballos et al., 2014; Kraft et al., 2018; Ulrich et al., 2013). Some Hispanic study participants have expressed their willingness to participate in research but have limited understanding of the healthcare system because of immigration to the United States later in their lives (Ceballos et al., 2014; Kraft et al., 2018; Ulrich et al., 2013). In addition, Hispanic persons have expressed fear of racial discrimination and misunderstanding because of language barriers (Ceballos et al., 2014; Ulrich et al., 2013).
Education
An individual’s education level may affect an individual’s literacy and understanding (Asare et al., 2017), thus affecting what a participant knows and understands about research. In one study measuring recruitment and participation in clinical research, individuals with increased levels of education, particularly college graduates, were more likely to participate (Baquet et al., 2006). The results were consistent with another study where 97% of participants were college educated and reported favorable views of research and willingness to participate in clinical trials (Brewer et al., 2014). In a study measuring AAs’ willingness to participate in research before and after a preconsent education session, individuals with a high school level of education or less were more likely to participate in a clinical trial after receiving preconsent education (Dunlop et al., 2011). Although past researchers have explored the relationship between education level and an individual’s participation in research, convincing evidence of the influence on education and research participation is lacking.
Although many researchers have identified numerous barriers and facilitators to participation in research, few have examined specific correlations to an individual’s attitude toward participating in research. In addition, geographic variation exists in participation barriers throughout the United States. Individuals living in urban areas report greater distrust of research compared to those living in rural areas, yet rural participants report lack of interest in participating in clinical trials compared to those living in urban areas (Baquet et al., 2006; Friedman et al., 2013).
In 2014, the National Patient-Centered Clinical Research Network (PCORnet) was established by the Patient-Centered Outcomes Research Institute with the goal of transforming the culture of clinical research through patient-centered engagement and recruitment (Unertl et al., 2018). Using the multiple healthcare facilities and millions of patients in the network, the Stakeholders, Technology, and Research Clinical Research Network (STAR-CRN), formally known as the Mid-South Clinical Data Research Network (CDRN)—a subunit of the PCORnet—aims to increase the number of research participants through their diverse patient network. To effectively engage patients in the diverse STAR-CRN network, it is necessary to identify the barriers that specific patients encounter during the research process. The purpose of this analysis was to determine how stakeholders' race, trust, and level of education influence participation barriers in clinical research.
The social cognitive theory (SCT) by Bandura (1971) provided the theoretical framework for the study. The theory illustrates how individuals learn and maintain behaviors in the social context in which they live (see Figure 1). The SCT considers the continual interaction between cognitive, environmental, and behavioral factors to ultimately determine human behavior. Cognitive factors include an individual’s knowledge, expectations, and attitudes. Environmental factors include societal and cultural norms, community access and resources, and the influence of others. Behavioral factors include skills, practice, and an individual’s self-efficacy. The triadic reciprocal relationship between cognitive factors, the environment, and human behavior explains the theorized relationship between variables in the study.
FIGURE 1.
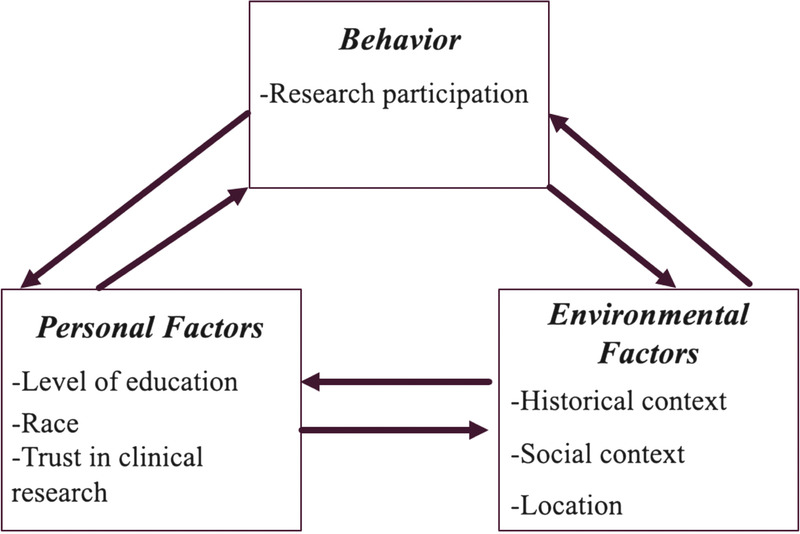
Core factors of the social cognitive theory with variables used in study.
METHODS
The study used secondary, cross-sectional survey data that were collected between 2014 and 2016 through the former CDRN, currently known as the STAR-CRN. The surveys were distributed throughout clinics within the former Mid-South CDRN after receiving approval from the Vanderbilt University Medical Center Institutional Review Board. The Belmont University Institutional Review Board approved the project as exempt in April 2019.
Clinical Setting
Although the former Mid-South CDRN is expansive throughout the Southeast, the survey was specifically distributed to patients visiting Vanderbilt University Medical Center or Nashville General Hospital clinic.
Project Sample
The research participants were adults (18 years old and older) living in the Southeastern United States who received care at least one time from a provider at one of the aforementioned clinical sites. There were no further inclusion or exclusion criteria.
Data Collection Instruments
Between 2014 and 2016, approximately 5,000 patients in the CDRN were surveyed to identify barriers that impede patient involvement in research. Two parallel surveys were administered using a random process (Erves et al., 2017). The surveys differed by tools measuring the concept of trust. One survey included the tool Hall–Trust in Medical Research (Hall et al., 2006), whereas the other included the Mainous–Trust in Medical Research (Mainous et al., 2006). The current analysis only used data collected from the survey containing the Trust in Medical Research by Hall et al. (2006). All surveys were administered using research electronic data capture (Harris et al., 2019).
Race, ethnicity, and level of education were collected in the demographic portion of the survey. The Trust in Medical Research by Hall et al. (2006) was used to measure the respondent’s level of trust in medical research. The trust tool was developed initially through a pilot study with a 25-item questionnaire. It was then simplified by following an item reduction procedure to develop the current 12-item tool (Hall et al., 2006). The Cronbach's alpha coefficient was .87, and the response pattern was normally distributed (Hall et al., 2006). Questions to assess barriers to participation in medical research were taken from a previous study by Mouton et al. (1997) using a 5-point Likert scale for each statement, ranging from strongly agree to strongly disagree. The specific questions were created from a literature review of barriers to participation in research. A panel of four experts reviewed the 12-question instrument for clarity, content validity, and cultural sensitivity. The content validity and cultural sensitivity both scored as 1.0 (Millon-Underwood et al., 1993).
Data Collection Process
Participants were recruited in person at participating clinics. Prior to receiving a survey, participants were informed of the purpose, time commitment, risks and benefits, and compensation, including a $25 gift card. Survey results were stored in a data set through the Meharry–Vanderbilt Alliance.
IBM SPSS Statistics (Version 26) was used for the analysis. Descriptive statistics were performed on the variables of level of education, trust level, race, and each attitude statement in the barriers to participation scale. A Spearman rank correlation was performed between level of education, level of trust, and attitude statement for each racial category.
RESULTS
A total of 2,190 survey responses were used in the analysis. Sociodemographic characteristics of survey respondents are shown in Table 1. The mean age of respondents was 52 years (SD = 15.65), with majority being female (68.3%, n = 1,496), White (77.4%, n = 1,696), insured (73.5%, n = 1,610), and working full time (50.4%, n = 1,103). The mean trust score was 39.85 (SD = 6.7). Trust scores by racial grouping are shown in the Supplemental Digital Content (http://links.lww.com/NRES/A386). Middle Easterners reported the least amount of trust (M = 36.11, SD = 5.8) compared to other groups. Most of the respondents had at least 2 years of college education (85.8%, n = 1,880). Education levels are separated by racial groupings in Table 2. Very few respondents in each racial grouping had less than an eighth-grade education.
TABLE 1.
Sociodemographic Characteristics of Survey Respondents
| Characteristic | n | % |
|---|---|---|
| Gender | ||
| Male | 640 | 29.2 |
| Female | 1496 | 68.3 |
| Other | 54 | 2.5 |
| Race | ||
| White | 1696 | 77.4 |
| African American | 336 | 15.3 |
| Hispanic | 56 | 2.6 |
| Native American | 39 | 1.8 |
| Asian | 23 | 1.05 |
| Prefer not to answer | 27 | 1.23 |
| Middle Eastern | 9 | 0.4 |
| Native Hawaiian | 4 | 0.2 |
| Education | ||
| 8th grade or less | 17 | 0.8 |
| Some high school (did not graduate) | 58 | 2.6 |
| High school graduate or GED | 219 | 10.1 |
| Some college or 2-year degree | 561 | 25.6 |
| College degree | 638 | 29.1 |
| More than a college degree | 681 | 31.1 |
| Prefer not to answer | 16 | 0.7 |
| Employment | ||
| Full time | 1103 | 50.4 |
| Part time (<32 hours) | 193 | 8.8 |
| Unemployed | 108 | 4.9 |
| Volunteer | 22 | 1.0 |
| Stay-at-home parents | 87 | 4.0 |
| Retired | 376 | 17.0 |
| Receiving disability | 158 | 7.2 |
| Other | 143 | 6.5 |
| Insurance | ||
| Insured | 1610 | 73.5 |
| Uninsured | 68 | 3.1 |
| Medicaid | 73 | 3.3 |
| Self-pay | 318 | 14.5 |
| Other | 121 | 5.5 |
| Household Income | ||
| <$10,000 | 142 | 6.5 |
| $10,000–$14,999 | 72 | 3.3 |
| $15,000–$24,999 | 136 | 6.2 |
| $25,000–$34,999 | 197 | 8.9 |
| $35,000–$49,999 | 233 | 10.6 |
| $50,000–$74,999 | 356 | 16.3 |
| $75,000–$99,999 | 288 | 13.2 |
| $100,000–$149,999 | 260 | 11.9 |
| $150,000 or more | 218 | 10.0 |
| Other | 288 | 13.2 |
TABLE 2.
Education Level by Race
| Education level | Race | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total (N = 2,190) |
White (n = 1,696) |
African American (n = 336) |
Hispanic (n = 56) |
Native American (n = 39) |
Asian (n = 23) |
Middle Eastern (n = 9) |
Native Hawaiian (n = 4) |
Prefer not to answer (n = 27) |
||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| 8th grade or less | 17 | 0.8 | 4 | 0.2 | 12 | 3.6 | — | — | — | — | — | — | — | — | — | — | 1 | 3.7 |
| Some high school (did not graduate) | 58 | 2.6 | 19 | 1.1 | 33 | 9.8 | 3 | 5.4 | 1 | 2.6 | — | — | — | — | — | — | 2 | 7.4 |
| High school graduate or GED | 219 | 10.1 | 147 | 8.7 | 60 | 17.9 | 5 | 8.9 | 6 | 15.4 | — | — | — | — | — | — | 1 | 3.7 |
| Some college or 2-year degree | 561 | 25.6 | 438 | 25.8 | 80 | 23.5 | 11 | 19.6 | 16 | 41.0 | 6 | 26.1 | 1 | 11.1 | 1 | 25.0 | 8 | 29.6 |
| College degree | 638 | 29.1 | 514 | 30.3 | 75 | 22.3 | 22 | 39.3 | 7 | 17.9 | 7 | 30.4 | 2 | 22.2 | 3 | 75.0 | 8 | 29.6 |
| More than a college degree | 681 | 31.1 | 567 | 33.4 | 71 | 21.1 | 13 | 23.2 | 9 | 23.0 | 9 | 39.1 | 6 | 66.7 | — | — | 6 | 22.2 |
| Prefer not to answer | 16 | 0.7 | 7 | 0.4 | 5 | 1.5 | 2 | 3.6 | — | — | 1 | 4.3 | — | — | — | — | 1 | 3.7 |
Overall, the respondents had favorable attitudes toward research participation. Percentage of respondent agreement toward attitude statements are displayed in Table 3. Most of the participants agreed that research benefits society, participation in research means better care, and research in the United States is ethical. Attitudes toward researchers were generally positive in that only a few agreed that “researchers do not care about me” (5.2%, n = 114) and “scientists cannot be trusted” (2.2%, n = 48).
TABLE 3.
Percentage of Survey Respondents Reporting Agreement With Each Attitude Statement
| Agreement (N = 2,190) | ||
|---|---|---|
| Research participation attitude statements | n | % |
| Participation in research benefits society | 1,934 | 88.3 |
| Participation in research will mean better care | 1,637 | 74.7 |
| Participation in research is risky | 526 | 24 |
| Researcher do not care about me | 114 | 5.2 |
| Participation in research is enjoyable | 727 | 33.2 |
| Participation in research allows me to socialize | 372 | 17 |
| Participation in research is against my religion | 41 | 1.9 |
| Participation in research is morally wrong | 31 | 1.4 |
| Transportation is a problem for research participants | 351 | 16 |
| Scientists cannot be trusted | 48 | 2.2 |
| Research conducted in the United States is ethical | 1,523 | 69.5 |
| It is better to be treated by doctors who are researchers | 677 | 30.9 |
Note. Agreement combines the responses agree and strongly agree.
Spearman correlations were performed using the racial groupings of White, AA, Hispanic, Native American, Asian, and Middle Eastern. Correlation results are displayed in Table 4. Correlations were not performed for the Native Hawaiian group because of a small sample size (n < 5) and the “prefer not to answer” grouping. White, AA, Hispanic, and Native American respondents displayed positive associations between trust and agreement toward “participation in research will mean better care.” Trust was strongly correlated with agreement for each attitude statement for White respondents, except for “participation is against my religion” (correlation coefficient [CC] = −.005, p = .844) and “participation in research is morally wrong” (CC = −.016, p = .509); however, association with education level was variable. Trust level was negatively associated with agreement toward the statement “researchers do not care about me” in White (CC = −.192, p = .000) and Native American (CC = −.371, p = .020) respondents. Trust level was correlated with specific attitude statements for Native American respondents, but there was less evidence of associations involving their education level. Conversely, in Asian respondents, education level was positively correlated with the statements “participation in research is morally wrong” (CC = .540, p = .008), “scientists cannot be trusted” (CC = .568, p = .005), “research conducted in the United States is ethical” (CC = .453, p = .030), and “it is better to be treated by doctors who are researchers” (CC = .418, p = .047).
TABLE 4.
Spearman Correlations Between Attitude Statements, Trust Score, and Education by Race
| Statement | White (n = 1,696) |
African American (n = 336) |
Hispanic (n = 56) |
Native American (n = 39) | Asian (n = 23) |
Middle Eastern (n = 9) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trust | Education | Trust | Education | Trust | Education | Trust | Education | Trust | Education | Trust | Education | |
| Participation in research benefits society |
CC .096** .000 |
CC .179** .000 |
CC .097 .075 |
CC .309** .000 |
CC .147 .280 |
CC .244 .069 |
CC −.021 .901 |
CC .291 .072 |
CC −.046 .835 |
CC .067 .760 |
CC .348 .359 |
CC .207 .593 |
| Participation in research will mean better care |
CC .243** .000 |
CC.019 .439 |
CC .254** .000 |
CC: .042 .438 |
CC .272* .042 |
CC .185 .171 |
CC .340* .034 |
CC −.103 .533 |
CC .229 .294 |
CC .019 .930 |
CC .550 .125 |
CC .245 .524 |
| Participation in research is risky |
CC −.052* .032 |
CC −.080** .001 |
CC .018 .740 |
CC .131* .016 |
CC .040 .768 |
CC −.217 .108 |
CC .014 .930 |
CC .052 .753 |
CC −.195 .372 |
CC .115 .601 |
CC .719* .029 |
CC .000 1.0 |
| Researchers do not care about me |
CC −.192** .000 |
CC −.032 .182 |
CC .011 .848 |
CC −.087 .112 |
CC −.173 .205 |
CC .141 .301 |
CC −.371* .020 |
CC .079 .632 |
CC .160 .467 |
CC .346 .106 |
CC−.112 .774 |
CC .245 .525 |
| Participation in research is enjoyable |
CC .196** .000 |
CC .052* .032 |
CC .203** .000 |
CC −.003 .957 |
CC .346** .009 |
CC −.082 .549 |
CC .233 .153 |
CC −.185 .259 |
CC −.043 .845 |
CC .123 .575 |
CC −.208 .591 |
CC .371 .325 |
| Participation in research allows me to socialize |
CC .167** .000 |
CC −.107** .000 |
CC .234** .000 |
CC −.092 .092 |
CC .240 .075 |
CC −.082 .547 |
CC .176 .283 |
CC −.288 .076 |
CC −.046 .835 |
CC .028 .897 |
CC −.219 .572 |
CC −.491 .179 |
| Participation in research is against my religion |
CC −.005 .844 |
CC −.145** .000 |
CC.060 .271 |
CC −.134* .014 |
CC −.068 .618 |
CC −.179 .187 |
CC .179 .435 |
CC .079 .634 |
CC −.040 .855 |
CC .235 .280 |
CC .413 .270 |
CC −.546 .129 |
| Participation in research is morally wrong |
CC −.016** .509 |
CC −.151** .000 |
CC .013 .816 |
CC −.135* .013 |
CC −.199 .141 |
CC −.348** .009 |
CC .326* .043 |
CC −.022 .893 |
CC −.011 .959 |
CC .540** .008 |
CC .260 .500 |
CC −.124 .751 |
| Transportation is a problem for research participants |
CC −.090** .000 |
CC .020 .406 |
CC .010 .848 |
CC −.052 .346 |
CC −.109 .423 |
CC −.160 .239 |
CC −.150 .363 |
CC −.180 .274 |
CC −.014 .949 |
CC −.381 .073 |
CC −.070 .858 |
CC −.220 .569 |
| Scientists cannot be trusted |
CC −.068** .005 |
CC −.160** .000 |
CC .002 .974 |
CC −.116* .034 |
CC −.133 .330 |
CC −.183 .177 |
CC −.061 .712 |
CC .082 .620 |
CC −.147 .505 |
CC .568** .005 |
CC .440 .236 |
CC −.161 .680 |
| Research conducted in the United States is ethical |
CC −.123** .000 |
CC −.113** .000 |
CC −.053 .329 |
CC −.227** .000 |
CC −.198 .144 |
CC .142 .298 |
CC .226 .166 |
CC .324* .044 |
CC .272 .209 |
CC .453* .030 |
CC .364 .336 |
CC .371 .325 |
| It is better to be treated by doctors who are researchers |
CC .120** .000 |
CC .042 .089 |
CC .193** .000 |
CC −.005 .928 |
CC .198 .143 |
CC .078 .570 |
CC .185 .259 |
CC −.174 .289 |
CC .032 .884 |
CC .418* .047 |
CC .390 .300 |
CC −.068 .862 |
Note. CC = Spearman correlation coefficient.
*p < .05.
**p < .01.
DISCUSSION
Overall, this cross-sectional survey of adults in the Southeastern United States demonstrates favorable attitudes toward research participation. The data suggest that attitudes are positive regarding perceived societal benefit and the belief that research leads to better medical care. The attitudes toward research are consistent with both the findings of Mouton et al. (1997) and Brewer et al. (2014). In addition, the research of Kraft et al. (2018) displayed similar themes during focus group interviews of AAs, Chinese, Hispanics, Whites, and Asians who agreed that research would benefit society and, in general, improve medical care. Although favorable attitudes toward research participation are seen within the data, the relationship of attitudes and an individual’s race, trust, and education is important to consider in the context of the theoretical framework of the project and implications for future clinical practice.
Race and Ethnicity
The data were separated by racial groupings to consider race in analyses. The study results display variability between racial groupings in education level, trust level, and attitudes toward participating in research. The difference of trust and education between Whites, AAs, Hispanics, Native Americans, Asians, and Middle Easterners support the theoretical underpinnings of the study and the SCT.
Trust
The findings of the study display strong evidence that an individual’s trust in clinical research influences one’s attitude toward research. In White, AA, Hispanic, Native American, and Middle Easterner respondents, trust is more often related to attitudes toward research than an individual’s education level. These findings are consistent with those of other researchers who found that trust is important to research participation (Scharff et al., 2010). In addition, the findings support trust as a predictor of barriers to clinical research. This is seen in inverse relationships between respondents’ agreement with more negative statements about participation and trust. Conversely, respondents’ agreement with more positive statements about participation were associated with positive relationships to trust. These findings support the SCT, providing evidence that greater trust toward research may be predictive of research participation.
Education
There was less evidence of the relationship between level of education and attitude about research participation. This finding is inconsistent with past research regarding barriers to research participation, which indicates that low education levels can contribute to decreased research participation because of the difficulty of understanding the research information or the informed consent process (Asare et al., 2017; Baquet et al., 2006). In a qualitative survey of Hispanic beliefs about biomedical research participation, participants discussed not having a formal education, which acted as a barrier for participating in research from fear of the unknown (Ceballos et al., 2014). This was not supported in the current analysis.
The study results do suggest that education level may influence attitudes toward research for Asian respondents compared to the influence of trust. This finding is different from results in prior research regarding Asian American reverence and respect to healthcare providers (Jayaram, 2020). In addition, earlier research has shown that language and health literacy are common barriers for Asian Americans when navigating the healthcare system (Kim & Keefe, 2010), which may align with an individual’s education level. However, because of the small representation of Asians in this analysis, conclusion cannot be drawn.
Generally, education level was not predictive of research participation, although education influence did differ by race and attitude. Although education can influence behavior, a variety of personal and environmental factors exist that may hold stronger influence on research participation.
The variability in the results of this analysis support the SCT in that numerous factors influence an individual’s decision to participate in research. Researchers must be aware of factors that contribute to an individual’s attitude toward research and educate potential study participants accordingly. As the study results show, an individual may believe that research benefits society while also perceiving research to be unethical. The fear of unethical treatment may outweigh the altruistic motivation and societal benefits, therefore hindering participation.
Using multiple recruitment methods for engaging participants may also be helpful. Researchers may consider engaging community representatives, community networks, and churches to promote research participation (Luebbert & Perez, 2016). Community-based participatory research (CBPR) has shown encouraging recruitment results (Scharff et al., 2010). CBPR operates on long-term community research relationships (Scharff et al., 2010) and can improve knowledge gaps within communities regarding disclosure and transparency, fear of research procedures, and societal effects in relation to research trials (Cortés et al., 2017). For example, Chadiha et al. (2011) used a CBPR framework to build a research volunteer registry, increasing the registry from 102 to 1,273 individuals. In addition, increased ethnographic research, particularly for highly underrepresented races and subgroups, may be helpful in recognizing barriers to participating in research that are not as culturally explicit.
Because of the overall favorable attitudes toward participating in research found in the study, a question is raised about exposure to research in clinical settings. For example, Pinto et al. (2014) found that healthcare providers with greater education and experience were more involved in the recruitment and facilitation of research with underrepresented populations. Patients, although possibly willing to participate in research, may have little to no knowledge of ongoing research if providers themselves have limited knowledge. In outpatient clinical settings, providers state that time constraints, forgetting to recruit, and the small number of eligible individuals act as barriers in recruiting patients into research (Page et al., 2011).
The findings from this analysis can be used to enhance ongoing research recruitment and engagement efforts, especially in underrepresented populations. It may be beneficial to consider clinician’s effectiveness with recruitment efforts based on practice setting, especially those in more rural or primary care settings, which tend to employ more physician assistants and nurse practitioners (AHRQ, 2012). It is possible that providers working within academic or large medical centers in urban areas have greater exposure to research engagement opportunities for patients compared to more community-based providers. In addition, greater exploration of doctor of nursing practice practitioners as facilitators of research participation may be useful (Weierbach et al., 2010).
Limitations
Several limitations are acknowledged. Sampling bias is possible in that individuals holding negative attitudes toward research participation may have been less likely to complete a survey and are therefore underrepresented. In addition, the data were collected between 2014 and 2016, and understanding of research may have changed. In addition, because of use of existing data, a precise response rate is unable to be calculated. To understand the relationship between variables, Spearman’s correlation was used. However, performing a correlation analysis between every attitude score, trust level, and education level for each racial category could have resulted in Type 1 errors. Moreover, because of the cross-sectional design, no causal relationships can be stated. In addition, it is difficult to draw scientific conclusions from small sample sizes like some within the study.
Conclusion
Engaging and recruiting participants, particularly from minority populations, in clinical research is a national priority. Research allows for increased knowledge in healthcare delivery and treatment, which ultimately allows for effective, equitable, and patient-centered care. Without diversity in research participants, the health disparities already occurring within minority populations will only worsen. The results of this analysis support the importance of trust to research participation. Researchers involved in recruiting and engaging participants in research must have heightened awareness, consideration, and appreciation of the complex relationships of personal and environmental factors that make a participant and their attitudes, specifically toward research, unique.
Supplementary Material

Footnotes
This work was supported by the Meharry–Vanderbilt Alliance and the Patient-Centered Outcomes Research Institute under Award Number R-1306-04869. This project was supported by National Institutes of Health/National Center for Advancing Translational Sciences Vanderbilt University Medical Center/Meharry Medical College. CTSA Grant Number UL1 TR000445. Its contents are the authors’ sole responsibility and do not necessarily represent official National Institutes of Health views.
The study used secondary, cross-sectional data from a deidentified data set. Approval to use the data set was obtained through the Belmont University Institutional Review Board.
The authors have no conflicts of interest to report.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.nursingresearchonline.com).
Contributor Information
Linda Wofford, Email: linda.wofford@belmont.edu.
Alecia Fair, Email: alecia.fair@meharry-vanderbilt.org.
David Philippi, Email: david.phillippi@belmont.edu.
REFERENCES
- Agency for Healthcare Research and Quality . (2012, January). Primary care workforce facts and stats no. 3. https://www.ahrq.gov/sites/default/files/publications/files/pcwork3.pdf
- Agency for Healthcare Research and Quality . (2019, September). 2018 National healthcare quality and disparities report. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2018qdr-final-es.pdf
- Alsan M., & Wanamaker M. (2018). Tuskegee and the health of Black men. Quarterly Journal of Economics, 133, 407–455. 10.1093/qje/qjx029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asare M. Flannery M., & Kamen C. (2017). Social determinants of health: A framework for studying cancer health disparities and minority participation in research. Oncology Nursing Forum, 44, 20–23. 10.1188/17.ONF.20-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A. (1971). Social learning theory. http://www.asecib.ase.ro/mps/Bandura_SocialLearningTheory.pdf
- Baquet C. R. Commiskey P. Daniel Mullins C., & Mishra S. I. (2006). Recruitment and participation in clinical trials: Socio-demographic, rural/urban, and health care access predictors. Cancer Detection and Prevention, 30, 24–33. 10.1016/j.cdp.2005.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie M. Shepherd A., & Howieson B. (2012). Do the Institute of Medicine’s (IOM’s) dimensions of quality capture the current meaning of quality in health care?—An integrative review. Journal of Research in Nursing, 18, 288–304. 10.1177/1744987112440568 [DOI] [Google Scholar]
- Brewer L. C. Hayes S. N. Parker M. W. Balls-Berry J. E. Halyard M. Y. Pinn V. W., & Breikopf C. (2014). African American women’s perceptions and attitudes regarding participation in medical research: The Mayo Clinic/The Links, Incorporated partnership. Journal of Women’s Health (Larchmt), 23, 681–687. 10.1089/jwh.2014.4751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos R. M. Knerr S. Scott M. A. Hohl S. D. Malen R. C. Vichis H., & Thompson B. (2014). Latino beliefs about biomedical research participation: A qualitative study on the U.S.–Mexico border. Journal of Empirical Research on Human Research Ethics, 9, 10–21. 10.1177/1556264614544454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadiha L. A. Washington O. G. M. Lichtenberg P. A. Green C. R. Daniels K. L., & Jackson J. S. (2011). Building a registry of research volunteers among older urban African Americans: Recruitment processes and outcomes from a community-based partnership. Gerontologist, 51, S106–S115. 10.1093/geront/gnr034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortés Y. I. Arcia A. Kearney J. Luchsinger J., & Lucero R. J. (2017). Urban-dwelling community members’ views on biomedical research engagement. Qualitative Health Research, 27, 130–137. 10.1177/1049732315627650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop A. L. Leroy Z. C. Logue K. M. Glanz K., & Dunlop B. W. (2011). Preconsent education about research processes improved African Americans’ willingness to participate in clinical research. Journal of Clinical Epidemiology, 64, 872–877. 10.1016/j.jclinepi.2010.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant R. W. Legedza A. T. Marcantonio E. R. Freeman M. B., & Landon B. E. (2011). Different types of distrust in clinical research among whites and African Americans. Journal of the National Medical Association, 103, 123–130. 10.1016/S0027-9684(15)30261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erves J. C. Mayo-Gamble T. L. Malin-Fair A. Boyer A. Joosten Y. Vaughn Y. C. Sherden L. Luther P. Miller S., & Wilkins C. H. (2017). Needs, priorities, and recommendations for engaged underrepresented populations in clinical research: A community perspective. Journal of Community Health, 42, 472–480. 10.1007/s10900-016-0279-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D. B. Bergeron C. D. Foster C. Tanner A., & Kim S.-H. (2013). What do people really know and think about clinical trials? A comparison of rural and urban communities in the South. Journal of Community Health, 38, 642–651. 10.1007/s10900-013-9659-z [DOI] [PubMed] [Google Scholar]
- Garg S. Kim L. Whitaker M. O’Halloran A. Cummings C. Holstein R. Prill M. Chai S. J. Kirley P. D. Alden N. B. Kawasaki B. Yousey-Hindes K. Niccolai L. Anderson E. J. Openo K. P. Weigel A. Monroe M. L. Ryan P. Henderson J., … Fry A. (2020, April 17). Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. Morbidity and Mortality Weekly Report, 69, 458–464. 10.15585/mmwr.mm6915e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall M. A. Camacho F. Lawlor J. S. DePuy V. Sugarman J., & Weinfurt K. (2006). Measuring trust in medical researchers. Medical Care, 44, 1048–1053. [DOI] [PubMed] [Google Scholar]
- Hall M. A. Dugan E. Zheng B., & Mishra A. K. (2001). Trust in physicians and medical institutions: What is it, can it be measured, and does it matter. Milbank Quarterly, 79, 613–639. 10.1111/1468-0009.00223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A. Taylor R. Minor B. L. Elliott V. Fernandez M. O’Neal L. McLeod L. Delacqua G. Delacqua F. Kirby J., & Duda S. N., REDCap Consortium (2019). The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics, 95, 103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaram G. (2020). Working with Asian American patients. Retrieved February 10, 2020, from https://www.psychiatry.org/psychiatrists/cultural-competency/education/best-practice-highlights/working-with-asian-american-patients
- Kim W., & Keefe R. H. (2010). Barriers to healthcare among Asian Americans. Social Work in Public Health, 25, 286–295. 10.1080/19371910903240704 [DOI] [PubMed] [Google Scholar]
- Kraft S. A. Cho M. K. Gillespie K. Halley M. Varsava N. Ormond K. E. Luft H. S. Wilfond B. S., & Lee S. S.-J. (2018). Beyond consent: Building trusting relationships with diverse populations in precision medicine research. American Journal of Bioethics, 18, 3–20. 10.1080/15265161.2018.1431322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S. Cho M. K. Kraft S. A. Varsava N. Gillespie K. Ormond K. E. Wilfond B. S., & Mangus D. (2019). “I don’t want to be Henrietta Lacks”: Diverse patient perspectives on donating biospecimens for precision medicine research. Genetics in Medicine, 21, 107–113. 10.1038/s41436-018-0032-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luebbert R., & Perez A. (2016). Barriers to clinical research participation among African Americans. Journal of Transcultural Nursing, 27, 456–463. 10.1177/1043659615575578 [DOI] [PubMed] [Google Scholar]
- Ma M. Kibler J. L. Vigil-Otero A. Sarpong D. Lally M., & Mayer K. H. (2013). Correlates of willingness to participate in microbicide research among African Americans. Journal of Health Psychology, 18, 65–74. 10.1177/1359105312438108 [DOI] [PubMed] [Google Scholar]
- Mainous A. G. 3rd Smith D. W. Geesey M. E., & Tilley B. C. (2006). Development of a measure to assess patient trust in medical research. Annals of Family Medicine, 4, 247–252. 10.1370/afm.541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millon-Underwood S. Sanders E., & Davis M. (1993). Determinants of participation in state-of-the-art cancer prevention, early detection/screening, and treatment trials among African-Americans. Cancer Nursing, 16, 25–33. [PubMed] [Google Scholar]
- Mouton C. P. Harris S. Rovi S. Solorzano P., & Johnson M. S. (1997). Barriers to Black women’s participation in cancer clinical trials. Journal of the National Medical Association, 89, 721–727. [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics . (2019). Health, United States, 2018. https://www.cdc.gov/nchs/data/hus/hus18.pdf [PubMed]
- National Institutes of Health . (n.d.). Get the facts. Retrieved September 1, 2018, from https://extramural-diversity.nih.gov/diversity-matters/get-the-facts
- Page M. J. French S. D. McKenzie J. E. O’Connor D. A., & Green S. E. (2011). Recruitment difficulties in a primary care cluster randomised trial: Investigating factors contributing to general practitioners' recruitment of patients. BMC Medical Research Methodology, 11, 35. 10.1186/1471-2288-11-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto R. M. Wall M. M., & Spector A. Y. (2014). Modeling the structure of partnership between researchers and front-line service providers: Strengthening collaborative public health research. Journal of Mixed Methods Research, 8, 83–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifenstein K. (2018). Commentary: Will we ever get enough? Strategies to enhance minority participation in research. ABNF Journal, 29, 17–26. [Google Scholar]
- Scharff D. P. Mathews K. J. Jackson P. Hoffsuemmer J. Martin E., & Edwards D. (2010). More than Tuskegee: Understanding mistrust about research participation. Journal of Health Care for the Poor and Underserved, 21, 879–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich A. Thompson B. Livaudais J. C. Espinoza N. Cordova A., & Coronado G. D. (2013). Issues in biomedical research: What do Hispanics think? American Journal of Health Behavior, 37, 80–85. 10.5993/AJHB.37.1.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unertl K. M. Fair A. M. Favours J. S. Dolor R. J. Smoot D., & Wilkins C. H. (2018). Clinicians’ perspectives on and interest in participating in a clinical data research network across the Southeastern United States. BMC Health Services Research, 18, 568. 10.1186/s12913-018-3399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Food and Drug Administration . (2017, July). 2015–2016 Global participation in clinical trials report. https://www.fda.gov/files/drugs/published/2015---2016-Global-Clinical-Trials-Report.pdf
- Weierbach F. M. Glick D. F. Fletcher K. Rowlands A., & Lyder C. H. (2010). Nursing research and participant recruitment: Organizational challenges and strategies. Journal of Nursing Administration, 40, 43–48. 10.1097/NNA.0b013e3181c97afb [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


