Abstract
Objective
The aim of the study was to identify whether erosive lichen sclerosus (LS) is a distinct clinicopathologic subtype.
Materials and Methods
The pathology database was searched for “erosion,” “erosive,” “ulcer,” and “lichen sclerosus.” Inclusion criteria were histopathologic diagnosis of LS and erosion or ulcer overlying a band of hyalinization and/or fibrosis. Exclusions were concurrent neoplasia and insufficient tissue. Histopathologic review documented site, epithelial thickness, adjacent epidermal characteristics, infiltrate, and dermal collagen abnormality. Clinical data included demographics, comorbidities, examination findings, microbiologic results, treatment, and response.
Results
Ten examples of erosive LS and 15 of ulcerated LS occurred in 24 women with a mean age of 67 years. Ulcerated LS was associated with diabetes and nontreatment at time of biopsy. Clinicians identified red patches in all but 1 case of erosive LS. Ulcerated LS was documented as fissure, ulcer, or white plaque, with 8 (53%) described as lichenified LS with epidermal breaches. Erosive LS favored hairless skin with normal adjacent stratum corneum sloping gently into erosion, whereas most ulcers in LS had an abrupt slope from hair-bearing skin. All cases were treated with topical steroids; 2 patients with erosive LS and 10 with ulcerated LS also had oral antifungals, topical estrogen, antibiotics, and/or lesional excision. Treatment yielded complete resolution in 50%.
Conclusions
Erosive LS is an unusual clinicopathologic subtype characterized by red patches on hairless skin seen microscopically as eroded epithelium overlying a band of hyalinized or fibrotic collagen. In contrast, ulcerated LS is usually a traumatic secondary effect in an uncontrolled dermatosis.
Key Words: lichen sclerosus, vulva, erosive, erosion, ulcer, ulcerated, sclerosis, lichen planus
Lichen sclerosus (LS) is a chronic T-cell–mediated inflammatory dermatosis with a predilection for vulvar skin and a spectrum of clinicopathologic manifestations.1,2 Multiple studies describe erosions and ulcers as markers of severity; these features were included in a proposed LS severity scale and its subsequent validation attempt.3,4 However, little is known about the prevalence, histopathology, favored location, duration, and treatment response of erosions and ulcers. Moreover, it is unclear whether epithelial loss within LS is due to the damage-repair cycle inherent to lichenoid dermatitis or is a secondary effect of trauma from rubbing and scratching. Two case reports presented a phenomenon of “erosive LS” recalcitrant to standard treatments.5,6 The accompanying clinical photographs suggested red patches within the pallor and textural change of LS, an appearance not typical of trauma or excoriation. This may represent a distinct clinicopathologic subtype of LS in which erosion reflects the underlying disease process, as in erosive lichen planus (LP).
The aim of this study is to compare the features of erosive and ulcerated LS in an effort to identify whether erosive LS is a distinct clinicopathologic subtype.
METHODS
Search of the local pathology database for the terms “erosive” or “erosion” and “lichen sclerosus” yielded 32 cases with reports describing (1) erosion and (2) dermal hyaline, dermal fibrosis, and/or a thickened basement membrane. Search with the terms “ulcer” and “lichen sclerosus” encountered 22 reports of ulcer or fissure overlying abnormal collagen. Study inclusion required a histopathologic diagnosis of LS and specimen with erosion or ulcer overlying dermal homogenization and/or fibrosis. Exclusions were concurrent neoplasia, prior radiation therapy, and insufficient tissue for evaluation. The definition of erosion was thinned epithelium lacking stratum corneum and granular layers; the presence of neutrophils aided diagnosis at sites near mucocutaneous junction. The label of ulcer was applied for complete loss of epithelium adjacent to intact or eroded epidermis. Mean epithelial thickness in normal epidermis is 0.17 mm with an SD of 0.7, so thin was defined as 0.1 mm or less and thick as 0.24 mm or greater.7 The histopathologic definition of LS was a band-like lymphocytic infiltrate accompanied by dermal hyalinization with evidence of basal layer damage seen as vacuolar change, apoptotic bodies, and/or squamatization; this could be in current, previous, or subsequent specimen(s). Diagnosis of erosive LP, lichenoid dermatitis, and vulvar aberrant maturation were in accordance with the International Society for the Study of Vulvovaginal Disease consensus statements.8,9 The Hunter New England Research Ethics and Governance Unit approved this retrospective histopathological case series (HREC 15/11/18/5.02), and signed written consent was obtained for clinical photographs.
Slide review yielded data on epidermal type, stratum corneum in adjacent epithelium, presence of neutrophils in epidermis and dermis, fragmentation, exocytosis, spongiosis, basal layer features, and thicknesses of the epidermis and abnormal collagen band. The inflammatory infiltrate under intact and breached epidermis was assessed as semiquantitatively as sparse, moderate, or dense; cell types were recorded in descending frequency. Review of medical records provided demographics, body mass index, symptoms, lesion appearance, relevant comorbidities, biopsy location, microbiologic results, treatment, response, and follow-up.
RESULTS
Ten examples of erosive LS and 15 of ulcerated LS occurred in 24 women with a median age of 67 years (range = 44–93 y); 1 woman had 2 biopsies of ulcerated LS taken 7 years apart. Of 30 cases excluded via slide review, 14 (47%) showed erosive LP, 7 (23%) were unevaluable because of insufficient epithelium, 5 (17%) showed a lichenoid dermatitis without specific features of LS, 2 (7%) were LS but lacked ulcer or erosion, and 2 had concurrent neoplasia.
Vulvar specialists provided care to 18 (75%) affected women, and the remainder saw gynecologists. All cases had a clinical impression of LS, and 13 (54%) had previous or subsequent vulvar biopsy diagnostic of LS. Symptoms, tobacco history, and microbiologic results were similar between erosive and ulcerated LS (see Table 1). Diabetes was more common in women with ulcerated LS (6/14 [43%] vs 0, p = .002), and severe urinary incontinence was noted in 4 (28.5%) of 14 cases. Among those with erosive LS, 1 had previous differentiated vulvar intraepithelial neoplasia (VIN), 1 had subsequent usual VIN, and 1 developed human papillomavirus–independent squamous cell cancer (SCC). One woman with ulcerated LS had previous vulvar aberrant maturation.
TABLE 1.
Demographics and Clinical Features of Erosive and Ulcerated LS Cases
| Total (N = 24) | Erosive LS (n = 10) | Ulcerated LS (n = 14) | |
|---|---|---|---|
| Age, median (range), y | 67 (44–93) | 69.5 (44–76) | 65 (44–93) |
| Previous or subsequent histopathologic diagnosis of LS, n (%) | 13 (54) | 8 (80) | 5 (36) |
| Primary symptom, n (%) | |||
| Itch | 17 (71) | 7 (70) | 10 (71) |
| Pain or dyspareunia | 6 (25) | 2 (20) | 4 (29) |
| None | 1 (4) | 1 (10) | 0 |
| Body mass index, mean (range) | 33 (22–49) | 31 (22–40) | 34 (23–49) |
| <25, n (%) | 6 (25) | 4 (40) | 2 (14) |
| 25–39, n (%) | 10 (42) | 3 (30) | 7 (50) |
| ≥40 | 5 (21) | 3 (30) | 5 (36) |
| Diabetes mellitus, n (%)a | 6 (25) | 0 | 6 (43) |
| Tobacco use, n (%) | |||
| Former | 5 (21) | 4 (40) | 1 (7) |
| Never | 19 (79) | 6 (60) | 13 (93) |
| Vaginal culture results, n (%) | |||
| Negative | 5 (21) | 0 | 4 (29) |
| Not done | 13 (54) | 7 (70) | 6 (43) |
| Candida albicans | 4 (17) | 2 (20) | 2 (14) |
| Other | 3 (12.5) | 1 (10) | 2 (14) |
| Location of biopsy, n (%)b | 14/26 (54) | 7 (70%) | 7/16 (44) |
| Labium minus | 6/26 (23) | 2 (20%) | 4/16 (29) |
| Labium majus | 6/26 (23) | 1 (10%) | 5/16 (31) |
| Perineum or perianus | |||
| Description of biopsy site, n (%) | |||
| Red patch | 6 (25) | 6 (60) | 0 |
| White plaque | 3 (12.5) | 1 (10) | 2 (14) |
| Erosion | 3 (12.5) | 3 (30) | 0 |
| Ulcer | 9 (37.5) | 0 | 9 (64) |
| Fissure | 3 (12.5) | 0 | 3 (21) |
| Steroid ointment at time of biopsy, n (%)a | 11 (46) | 8 (80) | 5 (36) |
| Steroid ointment potency prescribed, n (%) | |||
| Superpotent to ultrapotent | 15 (62.5) | 6 (60) | 9 (64) |
| Medium | 8 (33) | 3 (30) | 5 (36) |
| Low | 1 (4) | 1 (10) | 0 |
| Response to treatment, n (%) | |||
| Complete | 12 (50) | 4 (40) | 8 (57) |
| Partial | 10 (42) | 5 (50) | 5 (36) |
| Nil or unknown | 2 (8) | 1 (10) | 1 (7) |
| Follow-up, median (range), y | 3 (1–16) | 5.5 (1–16) | 2 (1–10) |
ap < .05.
bOne case had 3 different biopsy sites.
Clinicians identified red patches or erosions in all but 1 case of erosive LS, expressing concern for VIN in 5 (50%) and erosive LP in 1 (10%; see Figures 1, 2). Descriptions of biopsied areas in ulcerated LS included fissure, ulcer, and white plaque, with clinicians noting severely lichenified LS with epidermal breaches in 8 (57%) of 14 cases (see Figures 3, 4). Patients with ulcerated LS were less likely to be using steroid ointment at the time of biopsy than those with erosive LS (5/14 [36%] vs 8/10 [80%], p = .05). Clinicians prescribed topical steroid ointment to all patients, selecting a superpotent or ultrapotent product in 15 (62.5%) with application frequency ranging from twice weekly to twice daily. Additional measures in 2 patients with erosive LS and 10 with ulcerated LS included topical estrogen in 5 (21%) of 21, oral antifungals in 5 (21%), excision of persistent ulcers or fissures in 3 (12.5%), oral antibiotics for 1 case each of Staphylococcus aureus and bacterial vaginosis, and topical antibiotics for desquamative inflammatory vaginitis in 1. These measures yielded resolution of ulcers or erosions in 50%. Complete response was more likely in women seen by a vulvar specialist (12/18 [67%] vs 0/6, p = .02). Three patients with incomplete response of erosive LS were compliant with daily mid- to high-potency topical steroid, whereas 3 cases of partial or nil response occurred in the setting of twice weekly or low-potency topical steroids. The SCC occurred 20 months after biopsy in the single patient prescribed low-potency steroid ointment. Of 5 ulcerated LS cases with partial resolution, 2 involved noncompliance with daily ultrapotent steroid, 2 had severe LS complicated by morbid obesity, incontinence, and uncontrolled diabetes, and 1 died of other causes shortly after the consultation.
FIGURE 1.

A, Erosive LS: red macules and patches dispersed over residual labia minora on a background of pallor and architectural alteration; B, Hairless skin with compact stratum corneum, reactive nuclear change, band of edematous and hyalinized collagen, and scant lymphocytic infiltrate; there is gradual slope into erosion with epithelial neutrophils and vacuolar change (hematoxylin-eosin, ×100).
FIGURE 2.

A, Erosive LS: scattered pink-red glazed macules and patches over labia minora and posterior fourchette on a background of pallor. B, Parakeratotic hairless skin with a gradual slope into erosion, basal layer with reactive nuclei, vacuolar change and apoptotic bodies, band of hyalinized collagen, and lymphoplasmacytic infiltrate (hematoxylin-eosin, ×100). C, Erosion with neutrophils in epithelium and stroma (hematoxylin-eosin, ×200.).
FIGURE 3.
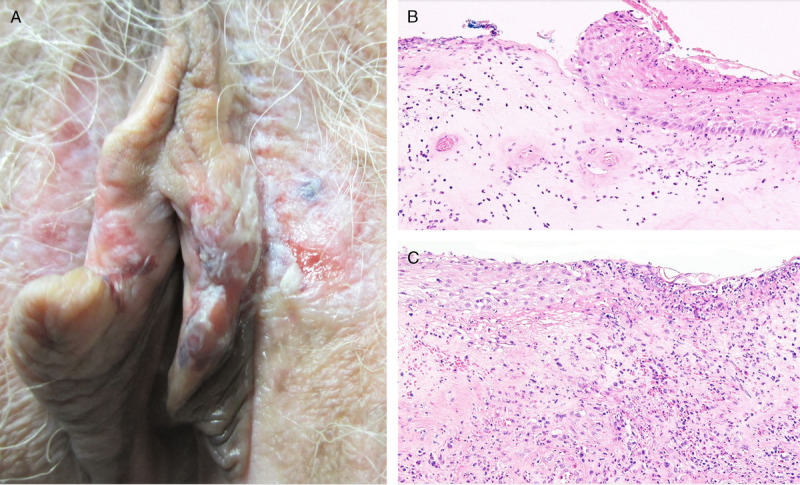
A, Ulcerated LS: pallor, erythema, textural change, ecchymoses, abnormal texture, and multiple erosions and ulcers. B, Abrupt transition between ulcer and eroded epidermis with neutrophils, spongiosis, reactive nuclear change, and thick band of hyalinized and edematous collagen (hematoxylin-eosin, ×100). C, Edge of other side of the ulcer with neutrophil-dominant inflammation across epithelium and stroma accompanied by dermal fibrinous exudate (hematoxylin-eosin, ×100).
FIGURE 4.

A, Ulcerated LS: ulcer at lateral perineum on a background of pallor and abnormal texture. B, Hair-bearing skin with parakeratosis, spongiosis, reactive nuclear change, band of hyalinized collagen, and lymphocytic infiltrate; there is a steep slope into ulcer with underlying neutrophil-dominant infiltrate (hematoxylin-eosin, ×100). C, Resolution of ulcer with improvement in skin color and texture after treatment with clobetasol propionate ointment.
Erosive LS was more likely to occur on hairless skin (9/10 [90%] vs 3/15 [20%], p = .001; see Table 2) with normal adjacent stratum corneum (4/7 [57%] vs 0/15, p = .005). Erosive LS was characterized by a gentle slope between intact and eroded epithelium (see Figures 1, 2). Ulcerated LS favored hair-bearing skin and more often showed an abrupt slope (62% vs 27%, p = .008) with normal to thick adjacent epidermis (14/15 [93%] vs 4/7 [57%], p = .08; see Figures 3, 4). Compared with ulcerated LS, erosive LS was more likely to show exocytosis (9/10 [90%] vs 2/15 [13%], p = .002) and epithelial neutrophils (10/10 [100%] vs 8/15 [53%], p = .02). In both forms, the infiltrate under intact epithelium was lymphocyte dominant and varied from sparse to dense, but underneath ulcers, it was more likely to be neutrophil dominant (5/15 [33%] vs 1/10 [10%], p = .04). Abnormal collagen thickness was similar to epithelial thickness in both erosive and ulcerated LS.
TABLE 2.
Histopathologic Features of Erosive and Ulcerated LS Specimens
| Total (N = 25) | Erosive LS (n = 10) | Ulcerated LS (n = 15) | |
|---|---|---|---|
| Site, n (%)a | |||
| Hairless skin | 12 (48) | 9 (90) | 3 (20) |
| Hair-bearing skin | 13 (52) | 1 (10) | 12 (80) |
| Epithelial thickness, mean (range), mm | |||
| Within erosion | NA | 0.04 (0.02–0.08) | NA |
| Adjacent to erosion or ulcerb | 0.37 (0.1–2) | 0.3 (0.1–0.8) | 0.4 (0.15–2) |
| Epithelial thickness category, n (%)b | |||
| Thin ≤0.1 mm | 4 (16) | 3 (43) | 1 (7) |
| Normal 0.11–0.24 mm | 9 (36) | 2 (29) | 7 (47) |
| Thick ≥0.25 | 9 (36) | 2 (29) | 7 (47) |
| Thickness of abnormal collagen, mean (range), mm | 0.42 (0.05–1) | 0.3 (0.05–0.6) | 0.48 (0.1–2.1) |
| Slope of transition from intact epithelium to erosion or ulcer, mean% (range)a,b | 51 (10–110) | 27 (10–70) | 62 (20–110) |
| Adjacent stratum corneum, n (%)b | |||
| Normal | 4/22 (18) | 4 (57) | 0 |
| Parakeratosis | 10/22 (45) | 1 (14) | 9 (60) |
| Hyperkeratosis | 5/22 (23) | 1 (14) | 4 (27) |
| Combination of features | 3/22 (14) | 1 (14) | 2 (13) |
| Exocytosis, n (%)a | 11 (44) | 9 (90) | 2 (13) |
| Spongiosis, n (%) | 20 (80) | 9 (90) | 11 (73) |
| Epithelial neutrophils, n (%)a | 18 (72) | 10 (100) | 8 (53) |
| Epithelial fragmentation, n (%) | 7 (47) | 1 (10) | 6 (40) |
| Dominant collagen abnormality, n (%) | |||
| Hyalinized | 21 (84) | 9 (90) | 12 (80) |
| Fibrotic | 4 (16) | 1 (10) | 3 (20) |
| Density of infiltrate under intact epithelium, n (%)b | |||
| Sparse | 10/22 (45) | 3/7 (43) | 7 (47) |
| Moderate | 8/22 (36) | 2/7 (29) | 6 (40) |
| Dense | 4/22 (18) | 2/7 (29) | 2 (13) |
| Commonest cell type under intact epithelium, n (%)b | |||
| Lymphocyte | 16/22 (73) | 7 (100) | 9 (60) |
| Neutrophil | 5/22 (23) | 0 | 5 (33) |
| Histiocyte | 1/22 (5) | 0 | 1 (7) |
| Density of infiltrate under erosion or ulcer, n (%) | |||
| Sparse | 5 (20) | 4 (40) | 1 (7) |
| Moderate | 9 (36) | 3 (30) | 6 (60) |
| Dense | 11 (44) | 3 (30) | 8 (53) |
| Most common cell type under erosion or ulcer, n (%)a | |||
| Lymphocyte | 14 (56) | 9 (90) | 5 (33) |
| Neutrophil | 10 (40) | 1 (10) | 9 (60) |
| Plasma | 1 (4) | 0 | 1 (7) |
ap < .05.
bThree cases of erosive LS had no adjacent intact epithelium.
NA indicates not applicable.
DISCUSSION
Erosive LS seems to be an uncommon distinct clinicopathologic subtype. It usually presents as a red patch on hairless skin in patients with known LS managed with topical steroid ointment. Features of gradual slope into erosion, epithelial neutrophils, and nonacanthotic adjacent epidermis are inconsistent with a traumatic process. Fewer patients with erosive LS had diabetes or incontinence and more used regular potent topical steroids at time of biopsy, features suggesting that erosive LS is a recalcitrant subtype. This is consistent with observations from the 2 case reports that described similar clinical appearances with persistent symptoms and signs despite oral steroids, daily superpotent to ultrapotent topical steroids, topical calcineurin inhibitors, and hydroxychloroquine; the authors reported improvement after photodynamic therapy.5,6
In contrast, ulcerated LS usually presents on hair-bearing skin of patients with globally uncontrolled or untreated LS and those with comorbidities affecting skin integrity. A less common scenario is an isolated chronic fissure that resolves with enhanced localized medical or surgical treatment. Abrupt transition between skin and ulcer, adjacent acanthosis or abnormal stratum corneum, and fragmentation all suggest traumatic etiologies of epidermal loss. Ulcerated LS resolved in women who used daily mid- to high-potency steroid ointment, unless disease was complicated by intractable conditions associated with skin breakdown.
The leading clinical differential diagnosis for erosive LS is erosive LP and its mimics: graft versus host disease and drug reactions.10,11 All have similar appearance, although erosive LP more often is symmetric or circumferential at the vestibule. Other alternate diagnoses include superinfection, usual VIN, and differentiated VIN; these may be excluded through a combination of microbiology and biopsy.
The main histopathologic differential diagnosis of erosive LS is bullous LS. Bullous LS shows a subepidermal split, the result of a weakened dermoepidermal junction due to the lichenoid reaction. Rarely, acantholytic dyskeratosis or immunobullous disease is comorbid with LS and isolated to the vulva. If one of these is suspected, the clinician may consider biopsy of adjacent noneroded skin to be sent fresh alongside a formalin-fixed specimen, so direct immunofluorescence may be peformed.12,13
In poorly controlled LS with many fissures and ulcers, biopsies obtained from multiple morphologically distinct sites may be required to exclude differentiated VIN and SCC. There are many etiologies of genital ulcers to include pressure, infectious, reactive, granulomatous, immunobullous, and manifestations of systemic autoimmune disease.10,11 If an ulcer occurs in a field of well-controlled LS, a comprehensive history, examination, and investigations may be required to determine the cause and direct therapy. Focal fissures in LS may arise from sexual trauma, infection, estrogen deficiency, and undertreatment. Rarely, they persist despite maximal medical treatment but respond to excision of lichenified edges with reapproximation of well-treated adjacent skin.10,14 Fissures also may occur within vestibulovaginal sclerosis. In contrast to ulcerated LS, this usually occurs in the space between urethra and clitoral frenulum, has nil to scant lymphocytic infiltrate, and does not respond to topical steroid ointment.15,16
The centralized referral and expert review of biopsy specimens permitted identification and description of this infrequent erosive subtype of LS. Limitations of this study are those inherent to the retrospective design, to include missing data, practice differences across clinicians, and low rates of clinical photography and microbiologic assessment. The small number of cases and variable follow-up preclude comment on erosion recurrence and association with human papillomavirus, VIN, and SCC. The prevalence of erosions and ulcers in treated LS is unknown, and its utility in proposed severity scoring systems remains unclear. The finding that outcome correlates with provider type likely relates to underprescribing of topical steroids by nonspecialists and highlights the need for referral pathways to vulvar clinics and enhanced education on vulvovaginal diseases in training programs.17–19
CONCLUSIONS
Erosive LS is an unusual clinicopathologic subtype of LS characterized by red patches on hairless skin with biopsy showing eroded epithelium overlying a band of hyalinized or fibrotic collagen. It is distinct from ulcerated LS, which is usually a traumatic secondary effect from rubbing and scratching an uncontrolled dermatosis.
Footnotes
The authors have declared they have no conflicts of interest.
This study was approved by the Hunter New England Research Ethics and Governance Unit (HREC 15/11/18/5.02).
Written permission was provided for publication of clinical photos.
Contributor Information
Geoffrey Otton, Email: geoff.otton@health.nsw.gov.au;geoff@newcolpcentre.com;gotton65@gmail.com.
Graeme Dennerstein, Email: gragrazdenn@smartchat.net.au.
Hong Tran, Email: drhongtran@gmail.com.
James Scurry, Email: jim.scurry@health.nsw.gov.au.
REFERENCES
- 1.Terlou A Santegoets LA van der Meijden WI, et al. An autoimmune phenotype in vulvar lichen sclerosus and lichen planus: a Th1 response and high levels of microRNA-155. J Invest Dermatol 2012;132:658–66. [DOI] [PubMed] [Google Scholar]
- 2.Lee A, Bradford J, Fischer G. Long-term management of adult vulvar lichen sclerosus: a prospective cohort study of 507 women. JAMA Dermatol 2015;15:1061–7. [DOI] [PubMed] [Google Scholar]
- 3.Sheinis M, Selk A. Development of the adult vulvar lichen sclerosus severity scale—a Delphi consensus exercise for item generation. J Low Genit Tract Dis 2018;22:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sheinis M Green N Vieira-Baptista P, et al. Adult vulvar lichen sclerosus: can experts agree on the assessment of disease severity? J Low Genit Tract Dis 2020;24:295–8. [DOI] [PubMed] [Google Scholar]
- 5.Vano-Galvan S Fernandez-Guarino M Bea-Ardebol S, et al. Successful treatment of erosive vulvar lichen sclerosus with methylaminolaevulinic acid and laser-mediated photodynamic therapy. J Eur Acad Dermatol Venerol 2009;23:71–2. [DOI] [PubMed] [Google Scholar]
- 6.Romero A Hernandez-Nunez A Cordoba-Guijarro S, et al. Treatment of recalcitrant erosive vulvar lichen sclerosus with photodynamic therapy. J Am Acad Dermatol 2007;57:S46–7. [DOI] [PubMed] [Google Scholar]
- 7.Day T, Holland SM, Scurry J. Normal vulvar histology: variation by site. J Low Genit Tract Dis 2016;20:64–9. [DOI] [PubMed] [Google Scholar]
- 8.Day T Wilkinson E Rowan D, et al. Clinicopathologic diagnostic criteria for vulvar lichen planus. J Low Genit Tract Dis 2020;24:317–29. [DOI] [PubMed] [Google Scholar]
- 9.Heller DS Day T Allbritton JI, et al. , ISSVD Difficult Pathologic Diagnoses Committee. Diagnostic criteria for differentiated vulvar intraepithelial neoplasia and vulvar aberrant maturation. J Low Genit Tract Dis 2021;25:57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohl TG. Fissures, herpes simplex virus, and drug reactions: important erosive vulvar disorders. Obstet Gynecol Clin North Am 2017;44:421–43. [DOI] [PubMed] [Google Scholar]
- 11.Bohl TG. Vulvar ulcers and erosions: a clinical approach. Clin Obstet Gynecol 2015;58:492–502. [DOI] [PubMed] [Google Scholar]
- 12.Haddadeen C Theaker J Rowen D, et al. Acantholytic dyskeratosis of the vulva presenting with clinical features of vulval lichen sclerosus—a possible rare collision entity. J Cutan Pathol 2020;47:61–4. [DOI] [PubMed] [Google Scholar]
- 13.Walsh ML Leonard N Shawki H, et al. Lichen sclerosus and immunobullous disease. J Low Genit Tract Dis 2012;16:468–70. [DOI] [PubMed] [Google Scholar]
- 14.Kennedy CM, Dewdney S, Galask RP. Vulvar granuloma fissuratum: a description of fissuring of the posterior fourchette and the repair. Obstet Gynecol 2005;105:1018–23. [DOI] [PubMed] [Google Scholar]
- 15.Croker BA Scurry JP Petry FM, et al. Vestibular sclerosis: is this a new, distinct clinicopathological entity? J Low Genit Tract Dis 2018;22:260–3. [DOI] [PubMed] [Google Scholar]
- 16.Day T Burston K Dennerstein G, et al. Vestibulovaginal sclerosis versus lichen sclerosus. Int J Gynecol Pathol 2018;37:356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones RW Scurry J Neill S, et al. Guidelines for the follow-up of women with vulvar lichen sclerosus in specialist clinics. Am J Obstet Gynecol 2008;198:496.e1–3. [DOI] [PubMed] [Google Scholar]
- 18.Edwards C Dogra N Antanrajakumar A, et al. Vulvovaginal disease education in Canadian and American gynecology residency programs: a survey of program directors. J Low Genit Tract Dis 2018;22:242–50. [DOI] [PubMed] [Google Scholar]
- 19.Comstock JR, Endo JO, Kornik RI. Adequacy of dermatology and ob-gyn graduate medical education for inflammatory vulvovaginal skin disease: a nationwide needs assessment survey. Int J Womens Dermatol 2020;6:182–5. [DOI] [PMC free article] [PubMed] [Google Scholar]


