Supplemental digital content is available in the text.
Key Words: fatigue, inflammatory bowel disease, influencing factors, path analysis
Abstract
Background
Fatigue is a common symptom in adults with inflammatory bowel disease (IBD) and is influenced by many physiological, psychological, and situational factors. However, the influencing factors of fatigue associated with IBD have not been evaluated.
Objective
This study aims to examine factors associated with fatigue during IBD and develop a parsimonious model that describes the influencing factors of fatigue.
Methods
The study was a secondary analysis of cross-sectional data obtained from IBD Partners, an online cohort of adults with the disease, including 12,053 eligible participants. Data were collected using the Patient-Reported Outcomes Measurement Information System short-form scales measuring fatigue, sleep disturbances, pain interference, anxiety, depression, and satisfaction with social roles. Physical activity was measured using a single question. Demographic and clinical variables were collected. Path analysis was computed to identify the direct and indirect effects of situational, physiological, and psychological factors on IBD–fatigue based on the middle range theory of unpleasant symptoms’ conceptual framework.
Results
Most of the participants were White females. The data best fit a model with situational factors (physical activity and satisfaction with social roles as the mediators). The direct effect of IBD activity, age, sleep disturbances, pain interference, anxiety, and depression on IBD–fatigue was significant. Significant indirect effects were noted on IBD–fatigue from sleep disturbances, pain interference, and depression via physical activity and satisfaction with social roles.
Discussion
The study identified two important intervening variables from the tested model. In addition, other symptoms such as sleep, pain, anxiety, and depression are essential and also influence IBD–fatigue.
Inflammatory bowel disease (IBD) is an autoimmune disorder of the gastrointestinal tract and is characterized by chronic inflammation. Ulcerative colitis (UC) and Crohn’s disease (CD) are the two major forms of IBD (Ananthakrishnan, 2015). The inflammation of IBD is a classic example of symptomology associated with a relapsing–remitting disorder, in which adults with IBD experience both remission as an inactive form of IBD, and relapse, which is a form of active disease (Ananthakrishnan, 2015).
Fatigue is one of the most common aggravating and challenging symptoms reported by adults with IBD (Chavarría et al., 2019; Cohen et al., 2014). Fatigue is a subcomponent of quality of life (QOL) and is inversely associated with QOL in adults with IBD (Cohen et al., 2014). It is estimated that approximately 22%–77% of adults with IBD experience fatigue, affecting their QOL (Bazilchuk, 2016). Fatigue also differs by disease activity, with 22%–41% of adults with IBD in remission and 44%–86% of adults with the active disease experiencing fatigue (Czuber-Dochan, Ream, & Norton, 2013; van Langenberg & Gibson, 2010).
Several factors are associated with fatigue in adults with IBD (hereinafter referred to as IBD–fatigue). The major contributors are disease activity, such as active inflammation (Cohen et al., 2014; Kappelman et al., 2014; van Langenberg & Gibson, 2010); psychological stress (van Langenberg & Gibson, 2010); anxiety and depression (Chavarría et al., 2019; Cohen et al., 2014); and IBD medications, which may play a role such as azathioprine, thiopurine, anti-tumor necrosis factor-α, corticosteroids, and methotrexate (Artom et al., 2017; Villoria et al., 2017). Other clinical correlates of IBD–fatigue includes disease duration and surgical history. An inverse association between IBD duration and fatigue has been reported (Kappelman et al., 2014). In addition, adults who had a prior surgical resection for IBD reported reduced fatigue experience (Kappelman et al., 2014; van Langenberg & Gibson, 2014). Moreover, age was recognized as another factor that influences IBD–fatigue. More specifically, younger adults with IBD (age < 60 years, Bager et al., 2012; age < 65 years, Pellino et al., 2014) were found to have higher fatigue scores than older adults with IBD.
Fatigue often manifests with other symptoms as a cluster in which two or more symptoms co-occur (Arnett & Clark, 2012). Because fatigue presentation because of inflammation is connected to the neurophysiology of cytokine-mediated sickness behavior symptoms, it is vital to examine these symptoms when studying IBD–fatigue. Symptoms that cluster with fatigue include anxiety, depressive behavior, sleep disturbances, increased sensitivity to pain, and psychomotor slowing (Arnett & Clark, 2012).
Physiological (age, disease duration, surgical history, disease activity, sleep disturbances, pain interference, and current medications) and psychological (anxiety and depression) symptoms are contributors to fatigue. Other contributors include physical activity and limited satisfaction with social roles (SSR). Previous studies (Klare et al., 2015; Ng et al., 2007; van Langenberg & Gibson, 2014) and reviews (Engels et al., 2018) have supported the benefits of physical activity and exercise for improving intestinal inflammation, as well as QOL and fatigue, in adults with IBD. In addition, IBD–fatigue negatively influences the social lives of adults with IBD. Adults with IBD–fatigue reported a lack of energy to continue their social lives, pointing out that their fatigue experiences influenced their disconnection from society (Beck et al., 2013).
According to 2015 data, about 1.3% (3 million) of adults in the United States are affected by IBD, representing an increase from 0.9% (2 million) adults in 1999 (Centers for Disease Control and Prevention, 2020). Of concern is that a limited number of studies have been conducted in the United States, with IBD–fatigue as the outcome variable (Borren et al., 2020; Cohen et al., 2014; Hashash et al., 2018; Tinsley et al., 2011). To date, few researchers have evaluated the efficacy of different interventions to manage IBD–fatigue, with most focused on improving adults’ coping skills with IBD in managing their symptoms. These studies tested various interventions such as solution-focused therapy, cognitive behavioral therapy, and psychoeducational intervention to address fatigue (Artom et al., 2019; O'Connor et al., 2019; Vogelaar et al., 2011, 2014). Although other interventions such as acupuncture (Horta et al., 2019) and high-intensity interval training and moderate-intensity continuous training (Tew et al., 2019) on IBD–fatigue have shown positive results, these studies had small sample sizes or did not focus primarily on IBD–fatigue. Thus, findings do not provide strong evidence of effective interventions to mitigate IBD–fatigue, a significant symptom in those with IBD. This lack of evidence may be related to interventions not addressing physiological, psychological, and social factors contributing to IBD–fatigue.
Because of increasing prevalence rates of IBD and known physiological, psychological, and social burdens, it is essential to understand the complex interplay between fatigue and covariates of anxiety, depression, sleep disturbances, pain, physical activity, and SSR. It is necessary to examine these associated factors within a biopsychosocial model of care that integrates various aspects, as supported by Artom et al. (2017). These data will inform the development of nursing interventions to reduce IBD–fatigue. Thus, the purpose of this study was to examine factors associated with IBD–fatigue to develop a parsimonious model that describes the influencing factors of IBD–fatigue.
The middle range theory of unpleasant symptoms (MRTOUS) provided a framework to guide the study (Lenz et al., 1997). Influencing factors, symptoms, and performance outcomes are the three major concepts included in the MRTOUS (Lenz & Pugh, 2014; Lenz et al., 1997); this study focused on influencing factors and symptoms. These three factors are interrelated and can influence the symptom experience individually or in combination (Lenz et al., 1997). Physiological factors usually include functioning body systems, the presence of any pathology, and any treatment-related variables (Lenz & Pugh, 2014). Psychological factors include an individual’s mental states of anxiety and depression. Lastly, situational factors include social and physical environments, which influence the experience of symptoms (Lenz et al., 1997). It is essential to understand that the influencing factors can also mediate the symptom experience (Lenz & Pugh, 2014). Figure 1 depicts the symptom experience of fatigue in the adapted conceptual framework.
FIGURE 1.

Conceptual framework.
Based on the definition of the MRTOUS, physiological factors included in this analysis are sleep disturbances and pain interference, along with demographic and clinical factors (age, disease duration, surgical history, disease activity, and current medications). The psychological factors included in the analysis are anxiety and depression. The situational factors included in the analysis are SSR and physical activity. Therefore, this study was designed to answer the research question: What are the direct and indirect effects of physiological (age, disease duration, surgical history, disease activity, sleep disturbance, pain interference, and current medications), psychological (anxiety and depression), and situational (physical activity and satisfaction with the social roles) factors on IBD–fatigue in adults with IBD?
METHODS
We conducted a secondary analysis of cross-sectional data obtained from IBD Partners, an online cohort of adults with IBD initiated in 2011 by the Crohn’s & Colitis Foundation and the University of North Carolina at Chapel Hill (Long et al., 2012). The online cohort includes participants from all 50 states in the United States (IBD Partners, n.d.). The parent organization’s institutional review boards (University of North Carolina at Chapel Hill and East Carolina University) approved the study.
Sample
Participants were drawn from the Internet-based cohort of IBD Partners based on the inclusion criteria of adults 18 years of age or older with a self-reported IBD diagnosis. This longitudinal descriptive study began enrollment in 2011, and symptom data were collected every 6 months. Adults with IBD with ostomies or J-pouches were excluded from the study because of the lack of criteria to assess disease activity after these surgical procedures. Data were extracted on September 13, 2019, and the final sample size consisted of 12,053 eligible participants.
Variables and Measurements
The Patient-Reported Outcomes Measurement Information System (PROMIS) short-form scales, funded by the National Institutes of Health (NIH), were used to measure fatigue, sleep disturbances, pain interference, anxiety, depression, and SSR. The PROMIS scales were recomputed to a normalized t score, where the mean of the U.S. general population was 50 and the standard deviation (SD) was 10 (NIH, 2019). To assess each variable, its corresponding four-item PROMIS short-form scale was used to measure the occurrence of the symptom over the past 7 days. The PROMIS measures were rated on a 5-point Likert scale that ranged from not at all to very much. Higher scores indicated higher measurements or worse health outcomes in fatigue, sleep disturbance, pain interference, anxiety, and depression. However, higher scores showed better health outcomes for positively worded concepts, such as SSR (NIH, 2019). The PROMIS scores were dichotomized into two categories. For the purpose of this study, the clinical significance of the PROMIS tools was set at clinically significant (score ≥ 55) and nonsignificant (score < 55), except for SSR, based on the norms suggested by the developing team (HealthMeasures, 2020). The PROMIS scores for SSR were categorized in the reverse order, with a score of ≤45 indicating clinical significance and a score of >45 indicating nonsignificance (HealthMeasures, 2020). These categories were based on a clinical significance definition of a difference of at least half SD from the population norm.
Fatigue
The PROMIS fatigue short-form scale was used to measured fatigue (NIH, 2019). This scale had demonstrated optimum reliability with a value of more than .91 for scores with 2 SDs below the mean to 4 SDs above the mean. The scale demonstrated acceptable convergent validity (r = .95) with Functional Assessment of Chronic Illness Therapy–Fatigue Scale (Cella et al., 2010) in a prior study.
Sleep Disturbances
The PROMIS sleep disturbance short-form scale was used to measure sleep disturbance (NIH, 2019). The PROMIS sleep disturbances scale’s reliability was greater than .88 for most of the score distributions. The scale demonstrated acceptable convergent validity (r = .85) with the Pittsburgh Sleep Quality Index in a prior study (Cella et al., 2010).
Pain Interference
The PROMIS pain interference short-form scale was used to measure pain interference (NIH, 2019). The scale had acceptable reliability with a range of .77 for scores 1 SD lower than mean to .97 (for scores ≥ mean). The scale demonstrated acceptable convergent validity (r = .85) with the Brief Pain Inventory interference subscale in a prior study (Cella et al., 2010).
Anxiety
The PROMIS anxiety short-form scale was used to measure anxiety (NIH, 2019). The scale had acceptable reliability (> .89) for the majority of the score distributions. The scale demonstrated acceptable convergent validity (r = .80) with the general distress (anxiety) scale from the Mood and Anxiety Symptom Questionnaire in a prior study (Cella et al., 2010).
Depression
The PROMIS depression short-form scale was used to measure depression (NIH, 2019). The reliability of the PROMIS depression scale was more than .92 for most of the score distributions. The scale demonstrated acceptable convergent validity (r = .83) with the Center for Epidemiological Studies Depression Scale in a prior study (Cella et al., 2010).
Satisfaction With Social Roles
The PROMIS SSR short-form scale was used to measure SSR (NIH, 2019). The PROMIS SSR scale’s reliability was .96 for scores that were 2 SDs less than the mean and 1 SD more than the mean. The scale demonstrated acceptable convergent validity with the SF-36 Role Physical, Role Emotional, and Social Functioning Scale (r ranged from .57 to .59) and the Functional Assessment of Chronic Illness Therapy–Functional Well-Being Scale (r = .76; Cella et al., 2010) in a prior study.
Physical Activity
The following single question was used to measure physical activity: How often did you participate in one or more physical activities for 20–30 minutes of duration per session during your leisure time within the past 6 months? (Gionet & Godin, 1989). This physical activity question was scored on a 6-point scale, and the scores ranged from 1 to 6. The scores were dichotomized into two categories: Higher levels of physical activity (score ≥ 5) and lower levels of physical activity (score < 5). Scores from 1 to 4 reflected physical activities performed within a month, and scores of ≥5 reflected physical activities performed within a week. A preliminary analysis was conducted to examine how this one question related to the Godin Leisure Time Activity (GLTA). This one question moderately correlated with the GLTA index scale scores of adults with IBD (rs = .686, p < .001). In addition, computation of the polychoric correlation revealed a strong correlation between the GLTA and the physical activity question (r = .74, p < .001; Davis et al., 2020).
Demographic and Clinical Variables
The self-reported demographic and clinical variables included in the study were age, IBD duration in years, history of surgery for IBD, current medications by class (aminosalicylates, corticosteroids, immunomodulators, biologics, and narcotics), and disease activity. Medications were dichotomized by current use (yes/no). Similarly, history of surgery for IBD was categorized by yes or no response (yes = had a history, no = no history). Disease duration was categorized into three categories: <10 years, ≥10–20 years, and ≥20 years. Researchers’ point of interest was to identify the influence of age on IBD–fatigue in terms of younger adults versus older adults, consistent with previous research studies (Bager et al., 2012; Pellino et al., 2014). Therefore, age was dichotomized at <60 (younger adults) and ≥60 (older adults). Disease activity indices of both CD and UC were collected using validated tools. Self-reporting of the Simple Clinical Colitis Activity Index (SCCAI) was used for adults with UC (Jowett et al., 2003). Self-reporting of the short Crohn’s Disease Activity Index (sCDAI) was used for adults with CD (Thia et al., 2011). These variables were categorized into active and inactive diseases based on prior validated definitions (SCCAI ≤ 2 and sCDAI < 150 for remission).
Data Analysis
We used IBM SPSS Statistics for Windows Version 24.0 (IBM Corp., Armonk, NY) software to analyze the descriptive statistics. Proportions were calculated for the dichotomous scores of the PROMIS measures (fatigue, anxiety, depression, sleep disturbances, pain interference, and SSR) and physical activity. Proportions were also calculated for the clinical variables, including surgical history, disease activity, and current medications.
Path analysis was used to test the conceptual framework of the study that was adapted from the MRTOUS. Path analysis was used to examine relationships among numerous variables and test a causal model illustrated in a path diagram based upon logic, theoretically based statements of relationships among variables, and/or experience (Mertler & Reinhart, 2017). We tested three models (see figures in Supplemental Digital Content, http://links.lww.com/NRES/A389) based on the conceptual framework adapted from the MRTOUS to identify the direct and indirect effects of situational, physiological, and psychological factors on IBD–fatigue. The statistical software Mplus (Version 8.4, n.d.) was used to conduct the path analysis using the weighted least square mean and variance adjusted estimation. This estimation is the default estimator in Mplus for path analysis with categorical variables and missing values in the data (Muthén & Muthén, 1998–2017; Wang & Wang, 2012). The primary data met the power and sampling guidelines for path analysis as the final sample size consisted of 12,053 participants. Significance levels were set at p < .01, a more stringent level because of this large sample size.
Before conducting the path analysis, multicollinearity was assessed, and patterns of missing data were examined. Multicollinearity of the variables was tested using polychoric correlation (see Table S1 in Supplemental Digital Content, http://links.lww.com/NRES/A390). Overall, the data set had a very negligible missing value with <2% missing data for all the path analysis variables. The data were complete for seven of the final model variables (PROMIS scores of fatigue, anxiety, depression, sleep disturbances, pain interference, SSR, and physical activity scores). For the final model’s remaining two variables, the missing data were 0.1% for age and 0.6% for disease activity. In addition, the weighted least square estimation of Mplus has a default setup of pairwise deletion to manage missing data (Wang & Wang, 2012).
Model fit was assessed using several goodness-of-fit indices, including the chi-square goodness-of-fit test; the relative goodness-of-fit indices, such as the Tucker–Lewis index (TLI); the comparative fit index (CFI); and the absolute goodness of fit indices, such as the root-mean-square error (RMSE) of approximation and the standardized root-mean-square residual (SRMR). A nonsignificant probability level of the chi-square test is expected for a model that fits the data well (Tabachnick & Fidell, 2013). However, one should be very cautious while looking at the nonsignificance level of a chi-square test, as a significant chi-square value is possible with a large sample (Tabachnick & Fidell, 2013). The CFI and TLI values ranged from 0 to 1; a value close to .95 or higher denotes an acceptable model fit with the data. A root-mean-square error of approximation value of <.05 indicates an excellent fit, a value of <.08 indicates a moderate fit, and a value of >.10 indicates a poor fit of the model with the data. Generally, SRMR values of <.08 are considered a good fit (Tabachnick & Fidell, 2013).
RESULTS
Sample Characteristics
Most of the sample (N = 12,053) was White (92%), and more than two thirds (72%) were women. The mean age was 43 years (SD = 15, minimum–maximum = 18–91). Disease duration ranged from <1 to 75 years, with a mean of 13 years. Most of the participants (85%) were <60 years old. Nearly equal proportions of disease activity were noted, with 51% in remission and 49% having active disease. The majority (70%) had not undergone any surgical procedures to manage IBD. Of those who were taking medications, most were on aminosalicylates (46%), followed by biologics (37%) and immunomodulators (27%). See Table 1 for sample characteristics.
TABLE 1.
Sample Characteristics (N = 12,053)
| Description | n | % |
|---|---|---|
| Gender | ||
| Female | 8,660 | 72 |
| Male | 3,391 | 28 |
| Age in years | ||
| <60 | 10,187 | 85 |
| ≥60 | 1,852 | 15 |
| IBD type | ||
| CD | 7,546 | 63 |
| UC | 4,501 | 37 |
| Disease activity | ||
| Remission | 6,158 | 51 |
| Active disease | 5,826 | 49 |
| Disease duration (years) | ||
| <10 | 6,291 | 52 |
| ≥10–20 | 2,897 | 24 |
| ≥20 | 2,837 | 24 |
| IBD medications (yes) | ||
| Aminosalicylates | 5,583 | 46 |
| Biologics | 4,483 | 37 |
| Immunomodulators | 3,215 | 27 |
| Corticosteroids | 2,449 | 20 |
| Narcotics | 1,308 | 11 |
Note. Disease activity: short Crohn’s Disease Activity Index of <150 for CD remission and ≥150 for CD active, Simple Clinical Colitis Activity Index of ≤2 for UC remission and >2 for UC Active. Biologics: anti-tumor necrosis factor, anti-integrin, anti-IL 12/23. Immunomodulators: azathioprine, mercaptopurine, and methotrexate. IBD = inflammatory bowel disease; CD = Crohn’s disease; UC = ulcerative colitis.
Approximately half of the participants (56%) reported clinically significant fatigue (t ≥ 55), followed by pain interference (51%) and anxiety (50%). Conversely, 44% reported not being satisfied with their social roles (t ≤ 45). Over half of the participants (62%) reported being physically active. Less than half of the participants reported sleep disturbances (34%) and being depressed (40%). See Table 2 for PROMIS and physical activity descriptions.
TABLE 2.
Descriptives of Patient-Reported Outcomes Measurement Information System (PROMIS) Scores and Physical Activity Scores (N = 12,053)
| Description | n | % |
|---|---|---|
| PROMIS fatigue | ||
| <55 | 5,316 | 44 |
| ≥55 | 6,737 | 56 |
| PROMIS anxiety | ||
| <55 | 6,035 | 50 |
| ≥55 | 6,018 | 50 |
| PROMIS depression | ||
| <55 | 7,225 | 60 |
| ≥55 | 4,828 | 40 |
| PROMIS pain interference | ||
| <55 | 5,907 | 49 |
| ≥55 | 6,146 | 51 |
| PROMIS sleep disturbances | ||
| <55 | 7,936 | 66 |
| ≥55 | 4,117 | 34 |
| PROMIS satisfaction with social roles | ||
| >45 | 6,731 | 56 |
| ≤45 | 5,322 | 44 |
| Physical activity | ||
| <5 | 4,627 | 38 |
| ≥5 | 7,426 | 62 |
Path Analysis Model
Three models were tested based on the conceptual framework adapted from the MRTOUS to identify the direct and indirect effects of situational, physiological, and psychological factors on IBD–fatigue and evaluate the fit of the proposed models to the data. We evaluated each model fitting based on the goodness-of-fit indices and conducted a series of model selections to identify the best and most parsimonious model for these data. The data best fit the model with situational factors (physical activity and SSR) as the mediator: fit indices, χ2(5, 11,822) = 205.18, p < .001 (RMSE = 0.058, CFI/TLI = 0.95/0.79, SRMR = 0.028). The final model with all direct effects is shown in Figure 2.
FIGURE 2.
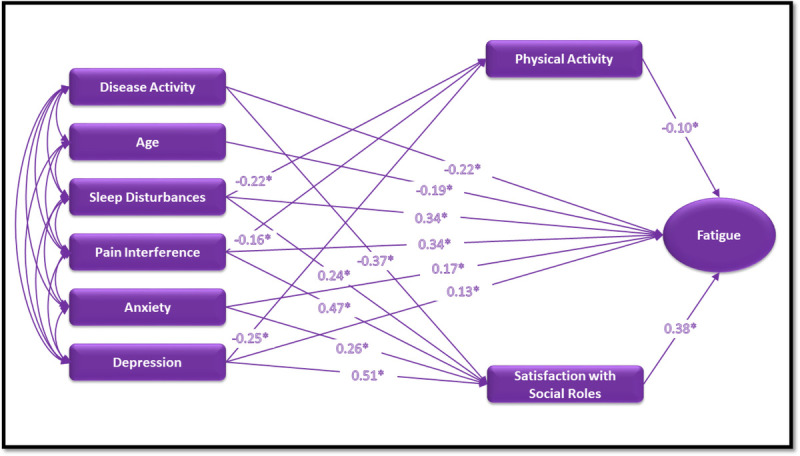
Final model with direct effects. *p < .001.
Decomposition of the Model: Direct Effects
Path analysis results revealed that all direct paths from the physiological, psychological, and situational factors to IBD–fatigue in the final model were significant, as seen in Table 3. We observed the highest direct effect on IBD–fatigue from SSR (β = .38, 99% CI [.33, .42], p < .001), followed by sleep disturbance (β = .34, 99% CI [.28, .40], p < .001) and by pain interference (β = .34, 99% CI [.27, .39], p < .001). SSR is reverse-coded (≤45 = worse SSR and >45 = better SSR) compared to other PROMIS measures (e.g., <55 = better fatigue and ≥55 = worse fatigue/clinically significant fatigue). Thus, lower SSR led to higher levels of fatigue in adults with IBD. Conversely, higher scores of sleep disturbance and pain interference contributed to worse fatigue symptoms. A negative direct effect was noted from disease activity (β = −.22, 99% CI [−.28, −.17], p < .001) to IBD–fatigue, indicating higher fatigue with higher disease activity. A negative direct effect observed from age (β = −.19, 99% CI [−.26, −.14], p < .001) to IBD–fatigue indicates younger adults (<60 years) in this sample experienced higher levels of fatigue.
TABLE 3.
Decomposition of Effects of Final Path Model
| Variables | Direct effects on fatigue β (99% CI) |
Direct effects on physical activity β (99% CI) | Direct effects on SSR β (99% CI) |
Indirect effects on fatigue via physical activity β (99% CI) |
Indirect effects on fatigue via SSR β (99% CI) |
|---|---|---|---|---|---|
| Physiological factors | |||||
| Disease activity | −.22* [−.28, −.17] | — | −.37* [−.42, −.31] | — | — |
| Age | −.19* [−.26, −.14] | — | — | — | — |
| Sleep disturbances | .34* [.28, .40] | −.22* [−.28, −.16] | .24* [.19, .30] | .02* [.01, .03] | .09* [.07, .12] |
| Pain interference | .34* CI [.27, .39] | −.16* [−.23, −.09] | .47* CI [.42, .53] | .02* [.01, .03] | .18* [.15, .21] |
| Psychological factors | |||||
| Anxiety | .17* CI [.11, .24] | — | .26* CI [.20, .33] | — | — |
| Depression | .13* CI [.06, .19] | −.25* [−.33, −.18] | .51* CI [.45, .58] | .03* [.02, .04] | .19* [.16, .22] |
| Situational factors | |||||
| Physical activity | −.10* [−.13, −.07] | — | — | — | — |
| SSR | .38* [.33, .42] | — | — | — | — |
Note. β = standardized coefficients; CI = confidence interval; SSR = satisfaction with social roles.
*p < .001.
Three significant negative direct paths were observed from two of the physiological (sleep disturbance and pain interference) factors and one psychological factor (depression) to physical activity. Depression (β = −.25, 99% CI [−.33, −.18], p < .001), sleep disturbance (β = −.22, 99% CI [−.28, −.16], p < .001), and pain interference had negative (β = −.16, 95% CI [−.23, −.09], p < .001) direct effects on physical activity. Therefore, adults with IBD with higher scores of depression, sleep disturbance, and pain interference engaged in less physical activity.
We noticed a significant direct effect on SSR from both psychological factors (anxiety and depression) and physiological factors of sleep disturbance and pain interference. SSR is revelry coded; higher levels of these measures indicate worse SSR in adults with IBD. In addition, a significant negative direct path was observed from disease activity to SSR (β = −.37, 99% CI [−.42, −.31], p < .001), suggesting improvements in SSR with lower disease activity.
Decomposition of the Model: Indirect Effects
The indirect or mediating effects of physical activity on IBD–fatigue were analyzed. Three significant positive indirect effects on fatigue via physical activity were noted; from sleep disturbance (β = .02, 99% CI [.01, .03], p < .001), pain interference (β = .02, 99% CI [.01, .03], p < .001), and depression (β = .03, 95% CI [.02, .04], p < .001) through physical activity on IBD–fatigue. Similar results were identified where sleep disturbance, pain interference, and depression also had mediating effects on IBD–fatigue via SSR. The indirect effects were from pain interference (β = .18, 99% CI [.15, .21], p < .001), depression (β = .19, 99% CI [.16, .22], p < .001), and sleep disturbances (β = .09, 99% CI [.07, .12], p < .001) through SSR to IBD–fatigue. Because the direct effects of sleep disturbance, pain interference, and depression on IBD–fatigue were more than the indirect or mediating effects of these variables on IBD–fatigue via physical activity and SSR, the mediation is considered as a partial mediation (Tabachnick & Fidell, 2013).
DISCUSSION
To our knowledge, this is the first study to use path analysis based on a model adapted from the MRTOUS in adults with IBD to evaluate the direct and mediating effects of various factors on IBD–fatigue. This study is also one of few studies to evaluate IBD–fatigue as an outcome variable in the United States (Borren et al., 2020; Cohen et al., 2014; Hashash et al., 2018; Tinsley et al., 2011). Although previous studies have evaluated factors that affect IBD–fatigue, none evaluated a combination of physiological, psychological, and situational factors on IBD–fatigue as was done in this study.
Consistent with previous findings (Chavarría et al., 2019), IBD–fatigue was highly prevalent (56%, N = 12,053) in this study cohort. The path analysis results revealed a negative direct effect of disease activity, which confirmed previous studies’ findings where the active disease was a significant contributor to IBD–fatigue (Cohen et al., 2014; Kappelman et al., 2014). Adults with IBD should be encouraged to follow the recommended treatment regimens and practices to manage their disease activity and achieve clinical remission to lower IBD–fatigue.
Consistent with previously published data in which adults <60 years of age with IBD were found to have higher fatigue scores (Bager et al., 2012), our study findings indicate younger adults with IBD report higher levels of fatigue compared to older adults. A direct negative effect was also observed from age to IBD–fatigue in the path analysis, supporting that as age increases, fatigue decreases. One possible explanation is that older adults adjust their lives based on their fatigue more readily than younger ones. Furthermore, older adults’ work and home responsibilities may differ from those of younger adults. Moreover, individuals with chronic illness may adapt to the demands of the disease over time and may eventually accept the illness as they age (Aujoulat et al., 2008). Thus, it is essential for healthcare personnel to understand fatigue related to age when developing strategies to manage IBD–fatigue.
Our study’s findings indicated direct effects of sleep disturbance, pain interference, anxiety, and depression on IBD–fatigue and the mediating variables of physical activity and SSR. These findings support the model of fatigue representing a cytokine-mediated sickness behavior related to IBD inflammation (Arnett & Clark, 2012). Previously published data found that poor sleep quality was more pronounced in adults with IBD–fatigue (Chavarría et al., 2019), and an association was noted between pain and IBD–fatigue (Beck et al., 2013). In addition, adults with higher anxiety and depression scores had higher fatigue impact scores (Ratnakumaran et al., 2018). Therefore, the development of interventions to improve sleep habits and management of pain, anxiety, and depression may influence IBD–fatigue.
Our study highlighted the indirect or mediating effects of physical activity on IBD–fatigue from sleep disturbance and pain interference, as well as from depression. The indirect effects demonstrate the importance of considering physical activity as an intervention to manage IBD–fatigue. Prior work has used the terms exercise and physical activity interchangeably. These studies have demonstrated the role of physical activity/exercise to manage IBD–fatigue (Klare et al., 2015; Ng et al., 2007; van Langenberg & Gibson, 2014). However, none of these intervention studies were conducted in the United States. In addition, physical activity may improve other symptoms, such as sleep disturbances (Chan et al., 2014), mood (Nathan et al., 2013), and overall QOL (Klare et al., 2015; Ng et al., 2007). Considering the multiple contributors to IBD–fatigue and the close association of IBD–fatigue with other symptoms, such as anxiety, depression, sleep disturbances, and pain interference, more studies are warranted among adults with IBD to demonstrate the effectiveness of physical activity on IBD–fatigue and other symptoms.
An important finding of this study was the association between IBD–fatigue and SSR, an understudied area of IBD–fatigue research. Results indicate the intervening or mediating effects of SSR on IBD–fatigue from sleep disturbance, pain interference, and depression. About 44% of the participants reported not being satisfied in SSR. Previous qualitative studies reported the disruption of social roles because of IBD symptoms and fatigue (Czuber-Dochan, Dibley et al., 2013; Devlen et al., 2014). Assessing social roles in adults with IBD may be important as an indicator of IBD–fatigue. Further research is needed to understand IBD–fatigue and social roles.
In summary, this study demonstrates the direct effects of physiological factors, psychological factors, and situational factors on IBD–fatigue. In addition, the study highlights the mediating effects of physical activity and SSR, potentially modifiable factors, on IBD–fatigue.
Therefore, the data from IBD Partners were collected online and may not be sufficiently diversified to reflect various socioeconomic backgrounds. Because the primary data did not include zip codes, it was impossible to divide the participants based on urban or rural status. In addition, adults with IBD who are literate and who have Internet access may be overrepresented in this online cohort. Therefore, external generalization of the results to unrepresented groups cannot be supported. Finally, we could not include any biomarkers of anemia, nutritional markers, or biomarkers of inflammation to evaluate their effects on IBD–fatigue as that information was not available in the IBD Partners cohort. The study’s strengths include a large sample size, use of validated instruments, and sound statistical approaches for data analysis.
Conclusion
Because of the multifactorial nature of IBD–fatigue, the integration of all influencing factors should be considered to provide a holistic approach to IBD–fatigue management. We tested a path model adapted from the MRTOUS to evaluate factors associated with IBD–fatigue. We identified two critical mediating variables from this model. These variables, physical activity and SSR, may be effective interventions to manage IBD–fatigue.
Supplementary Material

ORCID iDs
Suja P. Davis https://orcid.org/0000-0001-9024-3089
Ding-Geng Chen https://orcid.org/0000-0002-1123-027X
Patricia B. Crane https://orcid.org/000-0002-4710-3133
Linda P. Bolin https://orcid.org/0000-0002-2889-3459
Lee Ann Johnson https://orcid.org/0000-0002-7372-4996
Millie D. Long https://orcid.org/0000-0001-5983-2758
Footnotes
The parent study was funded by a grant from Crohn’s & Colitis Foundation and the Patient-Centered Outcomes Research Institute and by the National Institutes of Health Center Grant P30DK03498. This study is funded by the Alpha Alpha chapter of Sigma Theta Tau International. The content is solely the authors’ responsibility and does not necessarily represent the official views of the Crohn’s & Colitis Foundation, the Patient-Centered Outcomes Research Institute, the National Institutes of Health, or the Sigma Theta Tau International. The authors thank IBD Partners for granting the data for analysis.
The institutional review boards of the University of North Carolina at Chapel Hill and East Carolina University approved the study. After receiving approval, a data use agreement form was completed by the first author.
The authors (Davis, Chen, Crane, Bolin, and Johnson) have no conflicts of interest to report. Dr. Long reports the following conflicts of interest: Consulting: AbbVie, Takeda, Janssen, Pfizer, Salix, Prometheus, Valeant, Target PharmaSolutions, UCB. Research support: Takeda, Pfizer.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.nursingresearchonline.com).
Contributor Information
Ding-Geng Chen, Email: dinchen@email.unc.edu.
Patricia B. Crane, Email: pcrane2@uncc.edu.
Linda P. Bolin, Email: bolinl@ecu.edu.
Lee Ann Johnson, Email: lj6gd@virginia.edu.
Millie D. Long, Email: millie_long@med.unc.edu.
REFERENCES
- Ananthakrishnan A. (2015). Why did this happen to me? Epidemiology, genetics, and pathophysiology of IBD. In Stein D. J., & Shaker R. (Eds.), Inflammatory bowel disease: A point of clinical care guide (pp. 1–6). : Springer. 10.1007/978-3-319-14072-8_1 [DOI] [Google Scholar]
- Arnett S. V., & Clark I. A. (2012). Inflammatory fatigue and sickness behaviour—Lessons for the diagnosis and management of chronic fatigue syndrome. Journal of Affective Disorders, 141, 130–142. 10.1016/j.jad.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Artom M. Czuber-Dochan W. Sturt J. Murrells T., & Norton C. (2017). The contribution of clinical and psychosocial factors to fatigue in 182 patients with inflammatory bowel disease: A cross-sectional study. Alimentary Pharmacology & Therapeutics, 45, 403–446. 10.1111/apt.13870 [DOI] [PubMed] [Google Scholar]
- Artom M. Czuber-Dochan W. Sturt J. Proudfoot H. Roberts D., & Norton C. (2019). Cognitive–behavioral therapy for the management of inflammatory bowel disease-fatigue: A feasibility randomized controlled trial. Pilot and Feasibility Studies, 5, 145. 10.1186/s40814-019-0538-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aujoulat I. Marcolongo R. Bonadiman L., & Deccache A. (2008). Reconsidering patient empowerment in chronic illness: A critique of models of self-efficacy and bodily control. Social Science & Medicine, 66, 1228–1239. 10.1016/j.socscimed.2007.11.034 [DOI] [PubMed] [Google Scholar]
- Bager P. Befrits R. Wikman O. Lindgren S. Moum B. Hjortswang H. Hjollund N. H., & Dahlerup J. F. (2012). Fatigue in outpatients with inflammatory bowel disease is common and multifactorial. Alimentary Pharmacology and Therapeutics, 35, 133–141. 10.1111/j.1365-2036.2011.04914.x [DOI] [PubMed] [Google Scholar]
- Bazilchuk N. (2016, November 26). Fatigue an underestimated aspect of inflammatory bowel disease. http://sciencenordic.com/fatigue-underestimated-aspect-inflammatory-bowel-disease
- Beck A. Bager P. Jensen P. E., & Dahlerup J. F. (2013). How fatigue is experienced and handled by female outpatients with inflammatory bowel disease. Gastroenterology Research and Practice, 2013, 153818. 10.1155/2013/153818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borren N. Z. Tan W. Colizzo F. P. Luther J. Garber J. J. Khalili H. van Der Woude C. J., & Ananthakrishnan A. N. (2020). Longitudinal trajectory of fatigue with initiation of biologic therapy in inflammatory bowel diseases: A prospective cohort study. Journal of Crohn's and Colitis, 14, 309–315. 10.1093/ecco-jcc/jjz148 [DOI] [PubMed] [Google Scholar]
- Cella D. Riley W. Stone A. Rothrock N. Reeve B. Yount S. Amtmann D. Bode R. Buysse D. Choi S. Cook K. DeVellis R. DeWalt D. Fries J. F. Gershon R. Hahn E. A. Lai J.-S. Pilkonis P. Revicki D., PROMIS Cooperative Group (2010). The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. Journal of Clinical Epidemiology, 63, 1179–1194. 10.1016/j.jclinepi.2010.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . (2020, August 11). Data and statistics: Inflammatory bowel disease presence (IBD) in the United States. https://www.cdc.gov/ibd/data-statistics.htm
- Chan D. Robbins H. Rogers S. Clark S., & Poullis A. (2014). Inflammatory bowel disease and exercise: Results of a Crohn’s and colitis U.K. survey. Frontline Gastroenterology, 5, 44–48. 10.1136/flgastro-2013-100339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarría C., Casanova M. J., Chaparro M., Barreiro-de Acosta M., Ezquiaga E., Bujanda L., Rivero M., Argüelles-Arias F., Martín-Arranz M. D., Martínez-Montiel M. P., Valls M., Ferreiro-Iglesias R., Llaó J., Moraleja-Yudego I., Casellas F., Antolín-Melero B., Cortés X., Plaza R., Navarro-Llavat M., Gisbert J. P. (2019). Prevalence and factors associated with fatigue in patients with inflammatory bowel disease: A multicentre study. Journal of Crohn's and Colitis, 13, 996–1002. 10.1093/ecco-jcc/jjz024 [DOI] [PubMed] [Google Scholar]
- Cohen B. L. Zoëga H. Shah S. A. LeLeiko N. Lidofsky S. Bright R. Law M. Moniz H. Merrick M., & Sands B. E. (2014). Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Alimentary Pharmacology & Therapeutics, 39, 811–822. 10.1111/apt.12659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuber-Dochan W. Dibley L. B. Terry H. Ream E., & Norton C. (2013). The experience of fatigue in people with inflammatory bowel disease: An exploratory study. Journal of Advanced Nursing, 69, 1987–1989. 10.1111/jan.12060 [DOI] [PubMed] [Google Scholar]
- Czuber-Dochan W. Ream E., & Norton C. (2013). Review article: Description and management of fatigue in inflammatory bowel disease. Alimentary Pharmacology and Therapeutics, 37, 505–516. 10.1111/apt.12205 [DOI] [PubMed] [Google Scholar]
- Davis S. Melendez C. Crane P., & Long M. (2020). P094 a comparison of exercise measures among adults with inflammatory bowel disease. Gastroenterology, 158, S13. 10.1053/j.gastro.2019.11.067 [DOI] [Google Scholar]
- Devlen J. Beusterien K. Yen L. Ahmed A. Cheifetz A. S., & Moss A. C. (2014). The burden of inflammatory bowel disease: A patient-reported qualitative analysis and development of a conceptual model. Inflammatory Bowel Diseases, 20, 545–552. 10.1097/01.MIB.0000440983.86659.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engels M. Cross R. K., & Long M. D. (2018). Exercise in patients with inflammatory bowel diseases: Current perspectives. Clinical and Experimental Gastroenterology, 11, 1–11. 10.2147/CEG.S120816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionet N. J., & Godin G. (1989). Self-reported exercise behavior of employees: A validity study. Journal of Occupational Medicine, 31, 969–973. 10.1097/00043764-198912000-00007 [DOI] [PubMed] [Google Scholar]
- Hashash J. G. Ramos-Rivers C. Youk A. Chiu W. K. Duff K. Regueiro M. Binion D. G. Koutrobakis I. Vachon A. Benhayon D. Dunn M. A., & Szigethy E. M. (2018). Quality of sleep and coexistent psychopathology have significant impact on fatigue burden in patients with inflammatory bowel disease. Journal of Clinical Gastroenterology, 52, 423–430. 10.1097/MCG.0000000000000729 [DOI] [PubMed] [Google Scholar]
- HealthMeasures . (2020, August 11). PROMIS® score cut points. http://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/promis-score-cut-points
- Horta D. Lira A. Sanchez-Lloansi M. Villora A. Teggiachi M. García-Rojo D. García-Molina S. Figuerola A. Esteve M., & Calvet X. (2019). A prospective pilot randomized study: Electroacupuncture vs. Sham procedure for the treatment of fatigue in patients with quiescent inflammatory bowel disease. Inflammatory Bowel Diseases, 26, 484–492. 10.1093/ibd/izz091 [DOI] [PubMed] [Google Scholar]
- IBD Partners . (n.d.). Home. https://ccfa.med.unc.edu/
- Jowett S. L. Seal C. J. Phillips E. Gregory W. Barton J. R., & Welfare M. R. (2003). Defining relapse of ulcerative colitis using a symptom-based activity index. Scandinavian Journal of Gastroenterology, 38, 164–171. 10.1080/00365520310000654 [DOI] [PubMed] [Google Scholar]
- Kappelman M. D. Long M. D. Martin C. DeWalt D. A. Kinneer P. M. Chen W. Lewis J. D., & Sandler R. S. (2014). Evaluation of the patient-reported outcomes measurement information system in a large cohort of patients with inflammatory bowel diseases. Clinical Gastroenterology and Hepatology, 12, 1315–1323.e2. 10.1016/j.cgh.2013.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klare P. Nigg J. Nold J. Haller B. Krug A. B. Mair S. Thoeringer C. K. Christle J. W. Schmid R. M. Halle M., & Huber W. (2015). The impact of a ten-week physical activity program on health-related quality of life in patients with inflammatory bowel disease: A prospective randomized controlled trial. Digestion, 91, 239–247. 10.1159/000371795 [DOI] [PubMed] [Google Scholar]
- Lenz E. R., & Pugh L. C. (2014). The theory of unpleasant symptoms. In Smith M. J., & Lieh P. R. (Eds.), Middle range theory for nursing (3rd ed., pp. 165–196). : Springer. [Google Scholar]
- Lenz E. R. Pugh L. C. Milligan R. A. Gift A., & Suppe F. (1997). The middle range theory of unpleasant symptoms: An update. Advances in Nursing Science, 19, 14–27. [DOI] [PubMed] [Google Scholar]
- Long M. D. Kappelman M. D. Martin C. F. Lewis J. D. Mayer L. Kinneer P. M., & Sandler R. S. (2012). Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): Methodology and initial results. Inflammatory Bowel Diseases, 18, 2099–2106. 10.1002/ibd.22895 [DOI] [PubMed] [Google Scholar]
- Mertler C. A., & Reinhart R. V. (2017). Advanced and multivariate statistical methods: Practical application and interpretation (6th ed.). Routledge. [Google Scholar]
- Mplus (Version 8.4) (n.d.). Computer software. Muthén & Muthén. [Google Scholar]
- Muthén L. K., & Muthén B. O. (1998–2017). Mplus user’s guide (8th ed.). Author. [Google Scholar]
- Nathan I. Norton C. Czuber-Dochan W., & Forbes A. (2013). Exercise in individuals with inflammatory bowel disease. Gastroenterology Nursing, 36, 437–442. 10.1097/SGA.0000000000000005 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health . (2019). PROMIS®. http://www.healthmeasures.net/index.php?option=com_content&view=category&layout=blog&id=147&Itemid=806
- Ng V. Millard W. Lebrun C., & Howard J. (2007). Low-intensity exercise improves quality of life in patients with Crohn's disease. Clinical Journal of Sport Medicine, 17, 384–388. 10.1097/JSM.0b013e31802b4fda [DOI] [PubMed] [Google Scholar]
- O'Connor A. Ratnakumaran R. Warren L. Pullen D. Errington A. Gracie D. J. Sagar R. C. Hamlin P. J., & Ford A. C. (2019). Randomized controlled trial: A pilot study of a psychoeducational intervention for fatigue in patients with quiescent inflammatory bowel disease. Therapeutic Advances in Chronic Disease, 10, 2040622319838439. 10.1177/2040622319838439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellino G. Sciaudone G. Caserta V. Candilio G. De Fatico G. S. Gagliardi S. Landino I. Patturelli M. Riegler G. Di Caprio E. L. Canonico S. Gritti P., & Selvaggi F. (2014). Fatigue in inflammatory bowel diseases: Relationship with age and disease activity. International Journal of Surgery, 12, S60–S63. 10.1016/j.ijsu.2014.08.379 [DOI] [PubMed] [Google Scholar]
- Ratnakumaran R. Warren L. Gracie D. J. Sagar R. C. Hamlin P. J. O’Connor A., & Ford A. C. (2018). Fatigue in inflammatory bowel disease reflects mood and symptom-reporting behavior rather than biochemical activity or anemia. Clinical Gastroenterology and Hepatology, 16, 1165–1167. 10.1016/j.cgh.2017.11.030 [DOI] [PubMed] [Google Scholar]
- Tabachnick B. G., & Fidell L. S. (2013). Using multivariate statistics (6th ed.). Pearson Education. [Google Scholar]
- Tew G. A. Leighton D. Carpenter R. Anderson S. Langmead L. Ramage J. Faulkner J. Coleman E. Fairhurst C. Seed M., & Bottoms L. (2019). High-intensity interval training and moderate-intensity continuous training in adults with Crohn's disease: A pilot randomised controlled trial. BMC Gastroenterology, 19, 19. 10.1186/s12876-019-0936-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thia K. Faubion W. A. Jr. Loftus E. V. Jr. Persson T. Persson A., & Sandborn W. J. (2011). Short CDAI: Development and validation of a shortened and simplified Crohn’s disease activity index. Inflammatory Bowel Diseases, 17, 105–111. 10.1002/ibd.21400 [DOI] [PubMed] [Google Scholar]
- Tinsley A. Macklin E. A. Korzenik J. R., & Sands B. E. (2011). Validation of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) in patients with inflammatory bowel disease. Alimentary Pharmacology & Therapeutics, 34, 1328–1336. 10.1111/j.1365-2036.2011.04871.x [DOI] [PubMed] [Google Scholar]
- van Langenberg D. R., & Gibson P. R. (2010). Systematic review: Fatigue in inflammatory bowel disease. Alimentary Pharmacology & Therapeutics, 32, 131–143. 10.1111/j.1365-2036.2010.04347.x [DOI] [PubMed] [Google Scholar]
- van Langenberg D. R., & Gibson P. R. (2014). Factors associated with physical and cognitive fatigue in patients with Crohn’s disease: A cross-sectional and longitudinal study. Inflammatory Bowel Diseases, 20, 115–125. 10.1097/01.MIB.0000437614.91258.70 [DOI] [PubMed] [Google Scholar]
- Villoria A. García V. Dosal A. Moreno L. Montserrat A. Figuerola A. Horta D. Calvet X., & Ramírez-Lázaro M. J. (2017). Fatigue in out-patients with inflammatory bowel disease: Prevalence and predictive factors. PLOS ONE, 12, e0181435. 10.1371/journal.pone.0181435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelaar L. van't Spijker A. Timman R. van Tilburg A. J. P. Bac D. Vogelaar T. Kuipers E. J. van Busschbach J. J. V., & van der Woude C. J. (2014). Fatigue management in patients with IBD: A randomised controlled trial. Gut, 63, 911–918. 10.1136/gutjnl-2013-305191 [DOI] [PubMed] [Google Scholar]
- Vogelaar L. van't Spijker A. Vogelaar T. van Busschbach J. J. Visser M. S. Kuipers E. J., & van der Woude C. J. (2011). Solution focused therapy: A promising new tool in the management of fatigue in Crohn's disease patients psychological interventions for the management of fatigue in Crohn's disease. Journal of Crohn's and Colitis, 5, 585–591. 10.1016/j.crohns.2011.06.001 [DOI] [PubMed] [Google Scholar]
- Wang J., & Wang X. (2012). Structural equation modeling: Applications using Mplus (1st ed.). Wiley. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


