Supplemental Digital Content is Available in the Text.
Abstract
BACKGROUND
Flawless skin is one of the most universally desired features, and demand for improvements in skin quality is growing rapidly. Skin quality has been shown to substantially impact emotional health, quality of life, self-perception, and interactions with others. Although skin quality improvements are a common end point in studies of cosmeceuticals, they are rarely assessed in clinical studies of other aesthetic treatments and products. Descriptive terminology for skin quality parameters also varies considerably within the aesthetic field, relying on a range of redundant and occasionally contradictory descriptors. In short, skin quality has not been clearly defined.
OBJECTIVE
The goal of this review is to highlight the importance of skin quality to patients and physicians, explore known and unknown factors comprising skin quality, and provide clarity regarding terminology, descriptors, and evaluation tools for assessing skin quality.
MATERIALS AND METHODS
A review of the literature on skin quality was performed without limitation on publication date. Relevant articles are presented.
RESULTS AND CONCLUSION
We propose a framework of attributes contributing to skin quality rooted in 3 fundamental categories—visible, mechanical, and topographical—with the aim to provide information to help guide clinicians and inform future clinical studies.
According to psychologist Nancy Etcoff,1 identification of beauty is intuitive, whereas definition of beauty is subjective, mutable, and difficult to formulate into words. As the cornerstones of beauty include smooth, healthy looking skin, what comprises desirable skin is similarly difficult to define. Yet, flawless skin is an important component of facial attractiveness and continues to be one of the most universally desired features.1–3 In a recent global survey, 94% of the 14,584 people interviewed desired to improve their facial skin, and terms such as radiance and healthy, glowing skin are requested by patients seeking improvements in their appearance (unpublished data, Allergan Aesthetics). The encompassing term for this collection of desired outcomes is skin quality.
Skin quality as a concept is gaining traction in the aesthetic field worldwide. Rejuvenation procedures, cosmeceuticals, and minimally invasive injectable therapies are increasingly popular. However, reaching a consistent, objective definition of skin quality has been difficult. Current literature focuses heavily on age-related changes in skin quality, rather than skin quality per se, and descriptive terminology has substantial variability between investigators and geographically (See Supplemental Digital Content 1, Table S1, http://links.lww.com/DSS/A795 for levels of evidence of included literature). The lack of a clear, comprehensive definition precludes identification of clinical indicators and evaluation tools necessary for proper assessment and treatment of undesirable skin quality. In short, skin quality has yet to be clearly defined. Thus, the authors' goal is to elucidate the importance of skin quality to patients and aesthetic physicians, explore and understand what factors comprise it, and identify the gaps in the authors' understanding. The authors then propose a novel classification of skin quality attributes to provide clarity for both patients and physicians.
Importance of Skin Quality
Biological and Evolutionary Perspectives
The appearance of one's skin provides a wealth of information about an individual. Skin health is intricately linked to overall well-being, and clear skin is one of the body's “visual certificates of health,”1 reflecting general health and vitality, as well as disease and nutritional state.4–10 The visible condition of skin can also validate reproductive health and fertility.11 Attributes of skin quality (e.g., texture and homogenous coloration) contribute to perceptions of facial attractiveness,2 which may then correlate with mate choice and mating success,12 potentially because of the condition of one's skin indicating the quality of his/her immune system.2 Indeed, increasing evidence supports a link between immune health and facial attractiveness,13–15 although more research is needed to fully understand the contribution of individual skin quality attributes to this relationship. In addition, men may perceive female skin as more attractive and healthier during the fertile (i.e., late follicular) phase of the menstrual cycle,16–18 although data are equivocal and suggest minor variations in chromophore distribution could be the driver.16,19
Present characteristics of skin evolved in consideration of health, disease, and sexual selection. Human's relative hairlessness was an adaptation to ward off parasites1 and combined with the development of sweat glands to allow for efficient heat dissipation.20 A rich vascular network evolved to support skin's sweat glands, hair follicles, and multiplying cells,20 while a diverse symbiotic microbiome that varies across sex, age, skin site, and geographical location influences attributes of skin quality and, in cases of dysbiosis, skin disorders.21–25 Variations in skin pigment evolved in response to geographic differences in ultraviolet (UV) radiation, with increased melanin content in high UV areas as a means of photoprotection, and paler skin evolving for lower-light environments and enhanced vitamin D synthesis.1,20,26,27 Anthropologic data also suggest that, within a society, women evolved with lighter skin than men, so that signs of attraction, such as flushing or blushing, would be more apparent.28
Much of the authors' understanding of biological factors comprising skin quality has been elucidated through studies of aging, although a full discussion is outside the scope of this article. Briefly, intrinsic aging is associated with structural and functional deterioration of the skin, with declines in collagen, elastin, chondroitin, and hyaluronic acid, among other components.29–31 Together with other age-related alterations and damage, these changes lead to decreases in barrier function and hydration and concomitant increases in sagging, pore size, wrinkles and deep expression lines, dullness, blotchiness, rough texture, hyperpigmentation, dryness, and erythema.20,30,32–39
Skin aging literature has also highlighted variation in skin quality among individuals of different ethnicities, which remains understudied within aesthetics. Despite the widely considered protective effect of increased melanocytes and/or melanin, both intrinsic aging and photoaging occur, rendering skin less resilient and elastic.40 In addition, age-related changes in pigmentation, pore size, elasticity, oiliness, and thickness may differ between men and women of the same ethnicity.41–43 The appearance of facial pores also varies among ethnic groups, with Asians having the smallest pores compared with African American, white, and Hispanic subjects, and African Americans having the most severe impairment of the structure surrounding facial pores.43 Furthermore, the effects of photoaging differ across skin phototypes, with lighter skin more prone to depigmentation, atrophic changes, and skin cancers, and darker skin more prone to hypertrophic skin changes, deep wrinkles, and skin thickening.33 In short, the appearance of skin speaks to the biological underpinnings of health and reproductive fitness, with important, but understudied, differences across ethnicities.
Psychosocial Impact
Physical appearance and perceptions of attractiveness are multifactorial and intricately linked.44 As a result, the quality of one's skin has a strong psychosocial influence on individuals. Poor skin quality can result from a myriad of factors and may negatively impact a person's emotional health, quality of life, self-perception, and interactions with others.45–47 Importantly, self-perception is affected by interactions with, and judgements by, others. Multiple studies have shown that skin, as a person's primary interface with their surroundings,26 influences others' judgments of one's health, personality traits, youthfulness, and emotional and psychological well-being.48–50 Studies investigating manipulations of skin surface topography in photographs of middle-aged women found that small topographic skin alterations significantly influenced observers' preferences for specific faces and perceptions of age and attractiveness.19 In another study of facial photographs of women aged 40 to 71 years, removal of age spots, telangiectasia, furrows, lines, and wrinkles significantly impacted raters' perceptions of age and health.51 Furthermore, increasing evidence suggests that noninvasive facial rejuvenation produces sustained improvements in self-esteem, self-ratings of attractiveness, and decreases self-perceived age.52–56 Together, these data highlight the significant psychosocial impact of skin quality and potential for improvements using aesthetic procedures.
Indeed, improvement of skin quality is an increasingly common objective of clinical studies of aesthetic treatments and the primary goal of such treatments in clinical practice.9,37,57–63 According to a recent prospective, multicenter, observational study of 511 subjects seeking cosmetic procedures, approximately 80% of subjects said a desire for a youthful, more attractive appearance and clear skin motivated them to seek aesthetic treatment; other common reasons included improving psychosocial well-being, looking good professionally, and feeling less self-conscious around others.64 In addition, clinical studies use several instruments (e.g., Skindex-16 and FACE-Q) to measure postprocedure subject satisfaction with skin and the psychosocial impact of treatment,65 highlighting critical links between skin's appearance and psychosocial factors.
Attributes of Skin Quality: Approaching a More Rigorous Definition
Defining Skin Quality
Despite the growing awareness and importance of skin quality in human evolution, psychology, aesthetic treatments and practice, and clinical research, there is a dearth of literature and a lack of consistency in descriptive terminology for skin quality parameters. Studies of the effects of cosmetic products and procedures on skin quality rely on a range of often redundant, and sometimes contradictory, descriptors that are rarely defined (See Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A796). It is imperative to establish scientific rigor surrounding the definition and measurement of skin quality to guide the development and implementation of appropriate treatment strategies.
The authors propose a framework of attributes contributing to skin quality in healthy skin rooted in 3 fundamental categories: visual, mechanical, and topographical (Figure 1, Table 1). Visual attributes are purely visible, even after completely smoothing away topographic imperfections on the skin, and are assessed by light's reflection onto the skin. Topographical attributes are perceived by touch and viewed by topographic imagery. Mechanical attributes are related to how skin moves and can be measured by physical manipulation or deformation of the skin. To overcome inconsistencies in terminology currently applied to skin quality, Table 1 defines individual attributes based on the authors' clinical experience and Supplemental Digital Content 2, Table S2, http://links.lww.com/DSS/A796 includes a review of the limited descriptions in the literature; a summary of considerations related to each attribute follows. It is important to note that these categories are not mutually exclusive; individual attributes may fit into multiple categories. Furthermore, scars, which relate to all 3 proposed categories of skin quality (as they are palpable, affect the movement of skin at the scar site, and are readily visible), were not included as a skin quality attribute because they are a secondary skin lesion rather than a primary attribute of skin quality.
Figure 1.
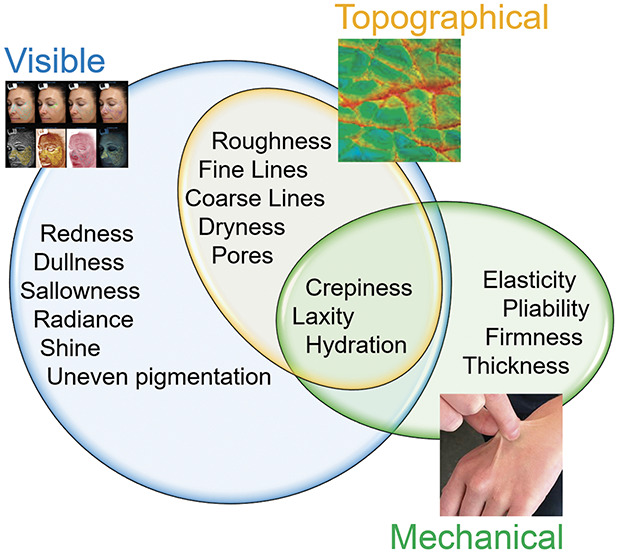
Proposed framework of skin quailty attributes.
TABLE 1.
Proposed Skin Quality Attributes and Their Definitions
| Attribute | Definition |
| Uneven pigmentation | Variation in melanin |
| Redness | Erythema or visible hemoglobin |
| Dullness/sallowness | Absence of glow; yellow or grayish undertone |
| Radiance | Ability of skin to “glow” or reflect light |
| Oiliness/shine | Excess sebum on the skin surface |
| Dryness | Lack of moisture; dehydration |
| Roughness | Uneven, not level texture |
| Fine lines | Light wrinkles |
| Coarse lines | Deep wrinkles |
| Pores | Surface landmark of pilosebaceous unit |
| Crepiness | Fine cigarette paper wrinkling of skin |
| Hydration | Water content; moisturization |
| Laxity | Loose skin |
| Elasticity/pliability | Ability to recoil with manipulation |
| Firmness | Relative ability to be stretched |
| Thickness | Density of the epidermis and dermis |
Visual Attributes
Uneven pigmentation, which often refers specifically to variations in melanin, is a primary visual attribute based on skin's melanin content; darker skin is richer in melanin.1,20 Photoaged skin may show areas of hypopigmentation or hyperpigmentation, whereas melasma appears as hyperpigmented patches in a characteristic distribution, both giving the appearance of mottling or blotchiness.9 Redness (erythema) relates to underlying skin color and relative vascular burden and visibility through the skin.17,66,67 Dullness and sallowness refer to the lack of natural radiance and may be associated with a yellow undertone to the skin. These are additional visual signs of poor skin quality, which may result from myriad causes.9 On the opposite side of the spectrum is the ability of the skin to reflect light, which the authors term radiance or “glow”; radiance is both visual and tactile because it depends on hydration levels and the amount of dead or dry skin accumulation blocking light reflection.68 Oiliness/shine and dryness are similarly visual and tactile attributes. Oiliness, or excess sebum production, may result from intrinsic (hormonal) or extrinsic (oxidative stress) factors.33,39,68 Hydration is perceptible by sight, touch, and biomechanics; that is, the moisture level of the skin can be seen and felt and affects the skin's ability to be manipulated.69
Topographical Attributes
Topographical attributes are also visible, but are measured by topographic methods. These attributes include smoothness or roughness (texture); this component is an important indicator of the presence or absence of aging or photodamage, considering extremely coarse skin may signal elastosis,70 and smooth skin is considered younger-looking.61 Topographical attributes also include the presence of fine or coarse lines or wrinkles.71 Enlarged pores relate to topographical properties of skin and have been correlated to increased sebum production, advancing age, and male sex.39 Skin crepiness may appear where underlying structural support is lost (e.g., fat and/or muscle atrophy, degradation of collagen and elastin fibers), leaving thin skin hanging loosely in its place.32
Mechanical Attributes
Elasticity, or recoilability, is a mechanical property of skin that decreases with compromised integrity of the network of dermal elastic fibers.30,61 Firmness of the skin relates to its pliability and has been an important effectiveness measure in studies of aesthetic treatments.59,68 Thickness and tightness of the skin—overly thick or thin skin and tight or loose skin—are also mechanical properties impacted by aging, whether intrinsic or extrinsic; excessive thickness and sagging skin have been attributed to variations in epidermal and dermal thickness or morphologic changes related to sun exposure and aging.20,72
Skin Quality Assessment and Measurement
Objective measurement of skin quality includes a variety of techniques (See Supplemental Digital Content 3, Table S3, http://links.lww.com/DSS/A797).101-125 Skin elasticity and firmness can be measured by a Cutometer probe, which generates a dislocation/relaxation curve based on manipulation of the skin with the application and release of negative pressure.63,73 Other probe-based techniques for elasticity and firmness assessment are dermal skin torque meter, indentation, or angular rotation techniques.68,74 Corneometers measure hydration by evaluating epidermal capacitance in the stratum corneum, while other instruments measure electrical impedance to assess skin hydration.75 Measurement of changes in pigment uses light absorption and reflectance to assess melanin and hemoglobin with Mexameter or full spectrum color analysis using standard CIEL*a*b* protocol with Chroma Meter.76 Assessment of topography or morphology often rely on high-definition imaging techniques and 3D fringe projection or modeling.68,74,77 The number and precision of measurement tools for skin quality assessment continues to grow (See Supplemental Digital Content 3, Table S3, http://links.lww.com/DSS/A797), but as reviewed below, substantial knowledge gaps remain.
Treatments Targeting Skin Quality Improvements
Current treatments and procedures targeting skin quality improvements include rejuvenation procedures (e.g., chemical peels, microneedling, laser, high-intensity focused ultrasound, and dermabrasion), cosmeceuticals, and oral supplementation (i.e., ‟nutraceuticals”31,78–81), among others. Increasing evidence supports skin quality changes, including improved texture, elasticity, pliability, hydration, and oiliness, from minimally invasive injectable procedures (e.g., botulinum toxin and intradermal fillers).56,82–88 However, larger, well-controlled studies are needed to better understand the effects of neurotoxins and fillers on skin quality. As with measurement and assessment tools, knowledge gaps remain in our understanding of treatments for skin quality improvement.
Gaps in our Understanding and Knowledge
There remain inconsistencies and gaps in the literature regarding how skin quality attributes are described, defined, and tested (See Supplemental Digital Content 2 and 3, Table S2 and Table S3, http://links.lww.com/DSS/A796 and http://links.lww.com/DSS/A797). The rare definition of a specific skin quality characteristic, such as hydration or elasticity, is generally based on mechanical parameters or calculations derived from measurement tools and instruments (e.g., Corneometer). Thus, clinical practice expertise shapes the evaluation and interpretation of most attributes, which may lead to variability in clinical practice and study. Similarly, although skin quality can be broken down into component parts, some attributes may be dependent on others. For example, redness may reflect the degree of microscopic vascularity visible under Fitzpatrick phototype I skin.
Measurement tools for skin quality also have other limitations. Objective measurements are often used in isolation and are allocated mainly to studying the effects of aging, which lack substantiation in assessments of overall skin quality.68,74 Furthermore, since skin properties differ based on the area of the face (e.g., chins have the highest pH89) and probe-based tools can only analyze small portions of facial skin, these measurements may not provide accurate representation of overall facial skin quality. Furthermore, objective measurement tools may only be able to assess one attribute at a time and may identify statistically significant, but nonclinically relevant, changes in skin quality. Future studies should consider use of multiple objective tools, as well as combining objective and subjective photonumeric grading of skin quality parameters. However, subjective assessment tools (e.g., clinical rating scales) are generally not inclusive of all skin types or ethnicities,70,90–92 which needs to be addressed.
Imaging, in particular, is a useful tool for objectively measuring skin quality attributes (See Supplemental Digital Content 3, Table S3, http://links.lww.com/DSS/A797). Image acquisition (e.g., exposure and angle) and analysis parameters are not currently standardized in aesthetics, but the growing availability of increasingly sensitive instruments, computer-aided image analyses, and artificial intelligence with machine learning make these methods promising for obtaining high-quality, objective measurement of skin quality attributes. Finally, aesthetic procedures and a desire for skin quality improvements are gaining in popularity among individuals with skin of color, although we still have a limited understanding of distinctions in skin quality attributes across ethnicities.63,93–98
Conclusion
The importance of facial attractiveness is well documented and undeniable. Since the early 20th century, clinical literature has highlighted the substantial influence of physical appearance on attractiveness and the psychological benefits of cosmetics and aesthetic procedures.44,99 Almost 100 years later, attaining a healthier, more attractive appearance and clear skin is a major motivation in seeking aesthetic procedures.100 The undercurrent in these observations is a desire for impeccable skin quality. Although there is no shortage of literature detailing the effects of visible skin condition on physical, psychological, and emotional well-being and the substantial psychosocial impact of aging skin, limited data are available explicating the topic of skin quality parameters and their rapidly growing importance in clinical settings. This review aims to address this literature gap, focusing on clarity regarding terminology, descriptors, and evaluation tools of skin quality. This information is intended to help guide clinicians who treat subjects concerned about skin appearance and inform future clinical studies.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.dermatologicsurgery.org).
S. Humphrey is a speaker, consultant, and/or investigator for Cutanea, Evolus, Galderma, L'Oreal, Merz, Bonti, Revance, and Allergan Aesthetics, an AbbVie company. S. Manson Brown and R. Mehta are employees of Allergan Aesthetics, an AbbVie company. S. J. Cross is an employee of AbbVie, Inc. Medical writing was provided by S. J. Cross of AbbVie and funded by AbbVie, Inc.
References
- 1.Etcoff NL. Survival of the Prettiest: the Science of Beauty. New York, NY: Anchor Books; 2000. [Google Scholar]
- 2.Fink B, Grammer K, Thornhill R. Human (Homo sapiens) facial attractiveness in relation to skin texture and color. J Comp Psychol 2001;115:92–9. [DOI] [PubMed] [Google Scholar]
- 3.Morris D. The Naked Ape: A Zoologist's Study of the Human Animal. New York, NY: McGraw-Hill; 1967. [Google Scholar]
- 4.Arda O, Göksügür N, Tüzün Y. Basic histological structure and functions of facial skin. Clin Dermatol 2014;32:3–13. [DOI] [PubMed] [Google Scholar]
- 5.Baret M, Bensimon N, Coronel S, Ventura S, et al. Characterization and quantification of the skin radiance through new digital image analysis. Skin Res Technol 2006;12:254–60. [DOI] [PubMed] [Google Scholar]
- 6.Yoon HS, Baik SH, Oh CH. Quantitative measurement of desquamation and skin elasticity in diabetic patients. Skin Res Technol 2002;8:250–4. [DOI] [PubMed] [Google Scholar]
- 7.de Macedo GM, Nunes S, Barreto T. Skin disorders in diabetes mellitus: an epidemiology and physiopathology review. Diabetol Metab Syndr 2016;8:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SY, Ko EJ, Lee YH, Kim BG, et al. Effects of collagen tripeptide supplement on skin properties: a prospective, randomized, controlled study. J Cosmet Laser Ther 2014;16:132–7. [DOI] [PubMed] [Google Scholar]
- 9.Birnbaum JE, McDaniel DH, Hickman J, Dispensa L, et al. A multicenter, placebo-controlled, double-blind clinical trial assessing the effects of a multicomponent nutritional supplement for treating photoaged skin in healthy women. J Cosmet Dermatol 2017;16:120–31. [DOI] [PubMed] [Google Scholar]
- 10.DiBaise M, Tarleton SM. Hair, nails, and skin: differentiating cutaneous manifestations of micronutrient deficiency. Nutr Clin Pract 2019;34:490–503. [DOI] [PubMed] [Google Scholar]
- 11.Swami V, Furnham A, Joshi K. The influence of skin tone, hair length, and hair colour on ratings of women's physical attractiveness, health and fertility. Scand J Psychol 2008;49:429–37. [DOI] [PubMed] [Google Scholar]
- 12.Rhodes G, Simmons LW, Peters M. Attractiveness and sexual behavior: does attractiveness enhance mating success? Evol Hum Behav 2005;26:186–201. [Google Scholar]
- 13.Lie HC, Rhodes G, Simmons LW. Genetic diversity revealed in human faces. Evolution 2008;62:2473–86. [DOI] [PubMed] [Google Scholar]
- 14.Phalane KG, Tribe C, Steel HC, Cholo MC, et al. Facial appearance reveals immunity in African men. Sci Rep 2017;7:7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts SC, Little AC, Gosling LM, Perrett DI, et al. MHC-heterozygosity and human facial attractiveness. Evol Hum Behav 2005;26:213–26. [Google Scholar]
- 16.Samson N, Fink B, Matts P. Does a woman's skin color indicate her fertility level? Swiss J Psychol 2011;70:199–202. [Google Scholar]
- 17.Burriss RP, Troscianko J, Lovell PG, Fulford AJ, et al. Changes in women's facial skin color over the ovulatory cycle are not detectable by the human visual system. PLoS One 2015;10:e0130093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Puts DA, Bailey DH, Cárdenas RA, Burriss RP, et al. Women's attractiveness changes with estradiol and progesterone across the ovulatory cycle. Horm Behav 2013;63:13–9. [DOI] [PubMed] [Google Scholar]
- 19.Samson N, Fink B, Matts PJ, Dawes NC, et al. Visible changes of female facial skin surface topography in relation to age and attractiveness perception. J Cosmet Dermatol 2010;9:79–88. [DOI] [PubMed] [Google Scholar]
- 20.Jablonski NG. The evolution of human skin and skin color. Annu Rev Anthropol 2004;33:585–623. [Google Scholar]
- 21.Ross AA, Müller KM, Weese JS, Neufeld JD. Comprehensive skin microbiome analysis reveals the uniqueness of human skin and evidence for phylosymbiosis within the class Mammalia. Proc Natl Acad Sci USA 2018;115:E5786–E95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhai W, Huang Y, Zhang X, Fei W, et al. Profile of the skin microbiota in a healthy Chinese population. J Dermatol 2018;45:1289–300. [DOI] [PubMed] [Google Scholar]
- 23.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol 2011;9:244–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somboonna N, Wilantho A, Srisuttiyakorn C, Assawamakin A, et al. Bacterial communities on facial skin of teenage and elderly Thai females. Arch Microbiol 2017;199:1035–42. [DOI] [PubMed] [Google Scholar]
- 25.Mukherjee S, Mitra R, Maitra A, Gupta S, et al. Sebum and hydration levels in specific regions of human face significantly predict the nature and diversity of facial skin microbiome. Sci Rep 2016;6:36062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jablonski NG. The Anthropology of Skin Colors: An Examination of the Evolution of Skin Pigmentation and the Concepts of Race and Skin of Color. Dermatoanthropology of Ethnic Skin and Hair. Cham, Switzerland: Springer; 2017; pp. 1–11. [Google Scholar]
- 27.Trivedi A, Gandhi J. The evolution of human skin color. JAMA Dermatol 2017;153:1165. [DOI] [PubMed] [Google Scholar]
- 28.Aoki K. Sexual selection as a cause of human skin colour variation: Darwin's hypothesis revisited. Ann Hum Biol 2002;29:589–608. [DOI] [PubMed] [Google Scholar]
- 29.Ryan T. The ageing of the blood supply and the lymphatic drainage of the skin. Micron 2004;35:161–71. [DOI] [PubMed] [Google Scholar]
- 30.Haydont V, Bernard BA, Fortunel NO. Age-related evolutions of the dermis: clinical signs, fibroblast and extracellular matrix dynamics. Mech Ageing Dev 2019;177:150–6. [DOI] [PubMed] [Google Scholar]
- 31.Di Cerbo A, Laurino C, Palmieri B, Iannitti T. A dietary supplement improves facial photoaging and skin sebum, hydration and tonicity modulating serum fibronectin, neutrophil elastase 2, hyaluronic acid and carbonylated proteins. J Photochem Photobiol B Biol 2015;144:94–103. [DOI] [PubMed] [Google Scholar]
- 32.Donofrio LM. Fat distribution: a morphologic study of the aging face. Dermatol Surg 2000;26:1107–12. [PubMed] [Google Scholar]
- 33.Tobin DJ. Introduction to skin aging. J Tissue Viability 2017;26:37–46. [DOI] [PubMed] [Google Scholar]
- 34.Bonté F, Girard D, Archambault JC, Desmoulière A. Skin changes during ageing. Subcell Biochem 2019;91:249–80. [DOI] [PubMed] [Google Scholar]
- 35.El-Domyati M, Attia S, Saleh F, Brown D, et al. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol 2002;11:398–405. [DOI] [PubMed] [Google Scholar]
- 36.Merinville E, Grennan GZ, Gillbro JM, Mathieu J, et al. Influence of facial skin ageing characteristics on the perceived age in a Russian female population. Int J Cosmet Sci 2015;37(Suppl 1):3–8. [DOI] [PubMed] [Google Scholar]
- 37.Makino ET, Jain A, Tan P, Nguyen A, et al. Clinical efficacy of a novel two-part skincare system on pollution-induced skin damage. J Drugs Dermatol 2018;17:975–81. [PubMed] [Google Scholar]
- 38.Lee DH, Oh JH, Chung JH. Glycosaminoglycan and proteoglycan in skin aging. J Dermatol Sci 2016;83:174–81. [DOI] [PubMed] [Google Scholar]
- 39.Roh M, Han M, Kim D, Chung K. Sebum output as a factor contributing to the size of facial pores. Br J Dermatol 2006;155:890–4. [DOI] [PubMed] [Google Scholar]
- 40.Langton AK, Alessi S, Hann M, Chien AL, et al. Aging in skin of color: disruption to elastic fiber organization is detrimental to skin's biomechanical function. J Invest Dermatol 2019;139:779–88. [DOI] [PubMed] [Google Scholar]
- 41.Colomb L, Flament F, Wagle A, Agrawal D. In vivo evaluation of some biophysical parameters of the facial skin of Indian women. Part I: variability with age and geographical locations. Int J Cosmet Sci 2018;40:50–7. [DOI] [PubMed] [Google Scholar]
- 42.Miyamoto K, Inoue Y, Hsueh K, Liang Z, et al. Characterization of comprehensive appearances of skin ageing: an 11-year longitudinal study on facial skin ageing in Japanese females at Akita. J Dermatol Sci 2011;64:229–36. [DOI] [PubMed] [Google Scholar]
- 43.Sugiyama-Nakagiri Y, Sugata K, Hachiya A, Osanai O, et al. Ethnic differences in the structural properties of facial skin. J Dermatol Sci 2009;53:135–9. [DOI] [PubMed] [Google Scholar]
- 44.Perrin FA. Physical attractiveness and repulsiveness. J Exp Psychol 1921;4:203–17. [Google Scholar]
- 45.Balkrishnan R, McMichael AJ, Hu JY, Camacho FT, et al. Correlates of health-related quality of life in women with severe facial blemishes. Int J Dermatol 2006;45:111–5. [DOI] [PubMed] [Google Scholar]
- 46.Sommer B, Zschocke I, Bergfeld D, Sattler G, et al. Satisfaction of patients after treatment with botulinum toxin for dynamic facial lines. Dermatol Surg 2003;29:456–60. [DOI] [PubMed] [Google Scholar]
- 47.Farage M, Miller K, Berardesca E, Maibach H. Psychological and social implications of aging skin: normal aging and the effects of cutaneous disease. In: Farage M, Miller K, Maibach H, editors. Textbook of Aging Skin. Berlin, Heidelberg: Springer; 2017. [Google Scholar]
- 48.Samson N, Fink B, Matts PJ. Visible skin condition and perception of human facial appearance. Int J Cosmet Sci 2010;32:167–84. [DOI] [PubMed] [Google Scholar]
- 49.Kligman AM, Graham JA. The psychology of appearance in the elderly. Dermatol Clin 1986;4:501–7. [PubMed] [Google Scholar]
- 50.Dayan S, Rivkin A, Sykes JM, Teller CF, et al. Aesthetic treatment positively impacts social perception: analysis of subjects from the HARMONY study. Aesthet Surg J 2019;39:1380–9. [DOI] [PubMed] [Google Scholar]
- 51.Fink B, Matts PJ. The effects of skin colour distribution and topography cues on the perception of female facial age and health. J Eur Acad Dermatol Venereol 2008;22:493–8. [DOI] [PubMed] [Google Scholar]
- 52.Dayan SH, Arkins JP, Patel AB, Gal TJ. A double-blind, randomized, placebo-controlled health-outcomes survey of the effect of botulinum toxin type a injections on quality of life and self-esteem. Dermatol Surg 2010;36(Suppl 4):2088–97. [DOI] [PubMed] [Google Scholar]
- 53.Imadojemu S, Sarwer DB, Percec I, Sonnad SS, et al. Influence of surgical and minimally invasive facial cosmetic procedures on psychosocial outcomes: a systematic review. JAMA Dermatol 2013;149:1325–33. [DOI] [PubMed] [Google Scholar]
- 54.Weinkle SH, Werschler WP, Teller CF, Sykes JM, et al. Impact of comprehensive, minimally invasive, multimodal aesthetic treatment on satisfaction with facial appearance: the HARMONY study. Aesthet Surg J 2018;38:540–56. [DOI] [PubMed] [Google Scholar]
- 55.Ogilvie P, Safa M, Chantrey J, Leys C, et al. Improvements in satisfaction with skin after treatment of facial fine lines with VYC-12 injectable gel: patient-reported outcomes from a prospective study. J Cosmet Dermatol 2020;19:1065–70. [DOI] [PubMed] [Google Scholar]
- 56.Bertossi D, Giampaoli G, Lucchese A, Manuelli M, et al. The skin rejuvenation associated treatment-Fraxel laser, Microbotox, and low G prime hyaluronic acid: preliminary results. Lasers Med Sci 2019;34:1449–55. [DOI] [PubMed] [Google Scholar]
- 57.Humphrey S, Jacky B, Gallagher C. Preventive, cumulative effects of botulinum toxin type A in facial aesthetics. Dermatolog Surg 2017;43:S244–S51. [DOI] [PubMed] [Google Scholar]
- 58.Belmontesi M, De Angelis F, Di Gregorio C, Iozzo I, et al. Injectable non-animal stabilized hyaluronic acid as a skin quality booster: an expert panel consensus. J Drugs Dermatol 2018;17:83–8. [PubMed] [Google Scholar]
- 59.Sundaram H, Cegielska A, Wojciechowska A, Delobel P. Prospective, randomized, investigator-blinded, split-face evaluation of a topical crosslinked hyaluronic acid serum for post-procedural improvement of skin quality and biomechanical attributes. J Drugs Dermatol 2018;17:442–50. [PubMed] [Google Scholar]
- 60.Carruthers A, Carruthers J, Fagien S, Lei X, et al. Repeated onabotulinumtoxinA treatment of glabellar lines at rest over three treatment cycles. Dermatol Surg 2016;42:1094–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ono I. A study on the alterations in skin viscoelasticity before and after an intradermal administration of growth factor. J Cutan Aesthet Surg 2011;4:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dayan SH, Bacos JT, Ho TV, Gandhi ND, et al. Topical skin therapies in subjects undergoing full facial rejuvenation. J Cosmet Dermatol 2019;18:798–805. [DOI] [PubMed] [Google Scholar]
- 63.Maisel A, Waldman A, Furlan K, Weil A, et al. Self-reported patient motivations for seeking cosmetic procedures. JAMA Dermatol 2018;154:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hibler BP, Schwitzer J, Rossi AM. Assessing improvement of facial appearance and quality of life after minimally-invasive cosmetic dermatology procedures using the FACE-Q scales. J Drugs Dermatol 2016;15:62–7. [PMC free article] [PubMed] [Google Scholar]
- 65.Rowland HM, Burriss RP. Human colour in mate choice and competition. Philos Trans R Soc Lond B Biol Sci 2017;372;20160350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stephen ID, Law Smith MJ, Stirrat MR, Perrett DI. Facial skin coloration affects perceived health of human faces. Int J Primatol 2009;30:845–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bielfeldt S, Springmann G, Seise M, Wilhelm KP, et al. An updated review of clinical methods in the assessment of ageing skin - new perspectives and evaluation for claims support. Int J Cosmet Sci 2018;40:348–55. [DOI] [PubMed] [Google Scholar]
- 68.Mayrovitz HN, Corbitt K, Grammenos A, Abello A, et al. Skin indentation firmness and tissue dielectric constant assessed in face, neck, and arm skin of young healthy women. Skin Res Technol 2017;23:112–20. [DOI] [PubMed] [Google Scholar]
- 69.Donofrio L, Carruthers A, Hardas B, Murphy DK, et al. Development and validation of a photonumeric scale for evaluation of facial skin texture. Dermatol Surg 2016;42(Suppl 1):S219–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Callaghan TM, Wilhelm KP. A review of ageing and an examination of clinical methods in the assessment of ageing skin. Part 2: clinical perspectives and clinical methods in the evaluation of ageing skin. Int J Cosmet Sci 2008;30:323–32. [DOI] [PubMed] [Google Scholar]
- 71.Bensouilah J, Buck P. Skin structure and function. In: Bensouilah J, Buck P, editors. Aromadermatology: Aromatherapy in the Treatment and Care of Common Skin Conditions. Abingdon, United Kingdom: Radcliffe Publishing; 2006; pp. 1–11. [Google Scholar]
- 72.Choi JW, Kwon SH, Huh CH, Park KC, et al. The influences of skin visco-elasticity, hydration level and aging on the formation of wrinkles: a comprehensive and objective approach. Skin Res Technol 2013;19:e349–55. [DOI] [PubMed] [Google Scholar]
- 73.Alanen E, Nuutinen J, Nicklén K, Lahtinen T, et al. Measurement of hydration in the stratum corneum with the MoistureMeter and comparison with the Corneometer. Skin Res Technol 2004;10:32–7. [DOI] [PubMed] [Google Scholar]
- 74.van der Wal M, Bloemen M, Verhaegen P, Tuinebreijer W, et al. Objective color measurements: clinimetric performance of three devices on normal skin and scar tissue. J Burn Care Res 2013;34:e187–94. [DOI] [PubMed] [Google Scholar]
- 75.Boone MA, Suppa M, Marneffe A, Miyamoto M, et al. High-definition optical coherence tomography intrinsic skin ageing assessment in women: a pilot study. Arch Dermatol Res 2015;307:705–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawada C, Yoshida T, Yoshida H, Matsuoka R, et al. Ingested hyaluronan moisturizes dry skin. Nutr J 2014;13:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pérez-Sánchez A, Barrajón-Catalán E, Herranz-López M, Micol V. Nutraceuticals for skin care: a comprehensive review of human clinical studies. Nutrients 2018;10:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vollmer DL, West VA, Lephart ED. Enhancing skin health: by oral administration of natural compounds and minerals with implications to the dermal microbiome. Int J Mol Sci 2018;19:3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guaitolini E, Cavezzi A, Cocchi S, Colucci R, et al. Randomized, placebo-controlled study of a nutraceutical based on hyaluronic acid, L-carnosine, and methylsulfonylmethane in facial skin aesthetics and well-being. J Clin Aesthet Dermatol 2019;12:40–5. [PMC free article] [PubMed] [Google Scholar]
- 80.Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg 2013;39:443–8. [DOI] [PubMed] [Google Scholar]
- 81.Bonaparte JP, Ellis D. Skin biomechanical changes after injection of onabotulinum toxin A: prospective assessment of elasticity and pliability. Otolaryngol Head Neck Surg 2014;150:949–55. [DOI] [PubMed] [Google Scholar]
- 82.Sapra P, Demay S, Sapra S, Khanna J, et al. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin A formulas to reduce signs of facial aging. J Clin Aesthet Dermatol 2017;10:34–44. [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J. Clinical effects on skin texture and hydration of the face using microbotox and microhyaluronicacid. Plast Reconstr Surg Glob Open 2018;6:e1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bukhari SN, Roswandi NL, Waqas M, Habib H, et al. Hyaluronic acid, a promising skin rejuvenating biomedicine: a review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int J Biol Macromol 2018;120:1682–95. [DOI] [PubMed] [Google Scholar]
- 85.Cavallini M, Papagni M, Ryder TJ, Patalano M. Skin quality improvement with VYC-12, a new injectable hyaluronic acid: objective results using digital analysis. Dermatol Surg 2019;45:1598–604. [DOI] [PubMed] [Google Scholar]
- 86.Niforos F, Ogilvie P, Cavallini M, Leys C, et al. VYC-12 injectable gel is safe and effective for improvement of facial skin topography: a prospective study. Clin Cosmet Investig Dermatol 2019;12:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Marrakchi S, Maibach HI. Biophysical parameters of skin: map of human face, regional, and age-related differences. Contact Derm 2007;57:28–34. [DOI] [PubMed] [Google Scholar]
- 88.Hamer MA, Jacobs LC, Lall JS, Wollstein A, et al. Validation of image analysis techniques to measure skin aging features from facial photographs. Skin Res Technol 2015;21:392–402. [DOI] [PubMed] [Google Scholar]
- 89.Jain R, Huang P, Ferraz RM. A new tool to improve delivery of patient-engaged care and satisfaction in facial treatments: the Aesthetic Global Ranking Scale. J Cosmet Dermatol 2017;16:132–43. [DOI] [PubMed] [Google Scholar]
- 90.Charrow A, Xia FD, Joyce C, Mostaghimi A. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol 2017;153:193–8. [DOI] [PubMed] [Google Scholar]
- 91.Makino ET, Kadoya K, Sigler ML, Hino PD, et al. Development and clinical assessment of a comprehensive product for pigmentation control in multiple ethnic populations. J Drugs Dermatol 2016;15:1562–70. [PubMed] [Google Scholar]
- 92.Chien AL, Qi J, Grandhi R, Kim N, et al. Effect of age, gender, and sun exposure on ethnic skin photoaging: evidence gathered using a new photonumeric scale. J Natl Med Assoc 2018;110:176–81. [DOI] [PubMed] [Google Scholar]
- 93.Ebede T, Papier A. Disparities in dermatology educational resources. J Am Acad Dermatol 2006;55:687–90. [DOI] [PubMed] [Google Scholar]
- 94.Nijhawan RI, Jacob SE, Woolery-Lloyd H. Skin of color education in dermatology residency programs: does residency training reflect the changing demographics of the United States? J Am Acad Dermatol 2008;59:615–8. [DOI] [PubMed] [Google Scholar]
- 95.Zhang J, Hou W, Feng S, Chen X, et al. Classification of facial wrinkles among Chinese women. J Biomed Res 2017;31:108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tian B. Wrinkle severity grading scale: a southeast asia study. OALib 2018;05:1–5. [Google Scholar]
- 97.Graham JA, Jouhar AJ. The importance of cosmetics in the psychology of appearance. Int J Dermatol 1983;22:153–6. [DOI] [PubMed] [Google Scholar]
- 98.Serra M, Bohnert K, Narda M, Granger C, et al. Brightening and improvement of facial skin quality in healthy female subjects with moderate hyperpigmentation or dark spots and moderate facial aging. J Drugs Dermatol 2018;17:1310–5. [PubMed] [Google Scholar]
- 99.Bonaparte JP, Ellis D. Alterations in the elasticity, pliability, and viscoelastic properties of facial skin after injection of onabotulinum toxin A. JAMA Facial Plast Surg 2015;17:256–63. [DOI] [PubMed] [Google Scholar]
- 100.Herndon JH, Makino ET, Jiang LI, Stephens TJ, et al. Long-term multi-product facial regimen in subjects with moderate-to-severe photodamage and hyperpigmentation. J Clin Aesthet Dermatol 2015;8:16–21. [PMC free article] [PubMed] [Google Scholar]
- 101.Pandya AG, Hynan LS, Bhore R, Riley FC, et al. Reliability assessment and validation of the Melasma Area and Severity Index (MASI) and a new modified MASI scoring method. J Am Acad Dermatol 2011;64:78–83. [DOI] [PubMed] [Google Scholar]
- 102.Vanaman Wilson MJ, Jones IT, Bolton J, Larsen L, et al. A randomized, investigator-blinded comparison of two topical regimens in Fitzpatrick skin types III-VI with moderate to severe facial hyperpigmentation. J Drugs Dermatol 2017;16:1127–32. [PubMed] [Google Scholar]
- 103.Balkrishnan R, McMichael AJ, Camacho FT, Saltzberg F, et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol 2003;149:572–7. [DOI] [PubMed] [Google Scholar]
- 104.Downie J, Schneider K, Goberdhan L, Makino ET, et al. Combination of in-office chemical peels with a topical comprehensive pigmentation control product in skin of color subjects with facial hyperpigmentation. J Drugs Dermatol 2017;16:301–6. [PubMed] [Google Scholar]
- 105.Geddes ER, Stout AB, Friedman PM. Retrospective analysis of the treatment of melasma lesions exhibiting increased vascularity with the 595-nm pulsed dye laser combined with the 1927-nm fractional low-powered diode laser. Lasers Surg Med 2017;49:20–6. [DOI] [PubMed] [Google Scholar]
- 106.Foolad N, Prakash N, Shi VY, Kamangar F, et al. The use of facial modeling and analysis to objectively quantify facial redness. J Cosmet Dermatol 2016;15:43–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Logger JG, de Vries FM, van Erp PE, de Jong EM, et al. Noninvasive objective skin measurement methods for rosacea assessment: a systematic review. Br J Dermatol 2019;182:55–66. [DOI] [PubMed] [Google Scholar]
- 108.Kim EH, Kim YC, Lee ES, Kang HY. The vascular characteristics of melasma. J Dermatol Sci 2007;46:111–6. [DOI] [PubMed] [Google Scholar]
- 109.Lee HI, Lim YY, Kim BJ, Kim MN, et al. Clinicopathologic efficacy of copper bromide plus/yellow laser (578 nm with 511 nm) for treatment of melasma in Asian patients. Dermatol Surg 2010;36:885–93. [DOI] [PubMed] [Google Scholar]
- 110.Tan J, Liu H, Leyden JJ, Leoni MJ. Reliability of clinician erythema assessment grading scale. J Am Acad Dermatol 2014;71:760–3. [DOI] [PubMed] [Google Scholar]
- 111.Draelos ZD, Fuller BB. Efficacy of 1% 4-ethoxybenzaldehyde in reducing facial erythema. Dermatol Surg 2005;31:881–5. [DOI] [PubMed] [Google Scholar]
- 112.Makino ET, Yano S, Chen T, Mehta R. Efficacy of a comprehensive serum in Japanese subjects with moderate to severe facial hyperpigmentation. J Drugs Dermatol 2017;16:36–40. [Google Scholar]
- 113.Petitjean A, Sainthillier JM, Mac-Mary S, Muret P, et al. Skin radiance: how to quantify? Validation of an optical method. Skin Res Technol 2007;13:2–8. [DOI] [PubMed] [Google Scholar]
- 114.Musnier C, Piquemal P, Beau P, Pittet JC. Visual evaluation in vivo of 'complexion radiance' using the C.L.B.T. sensory methodology. Skin Res Technol 2004;10:50–6. [DOI] [PubMed] [Google Scholar]
- 115.Arbuckle R, Clark M, Harness J, Bonner N, et al. Item reduction and psychometric validation of the oily skin self assessment scale (OSSAS) and the oily skin impact scale (OSIS). Value Health 2009;12:828–37. [DOI] [PubMed] [Google Scholar]
- 116.Werschler WP, Trookman NS, Rizer RL, Ho ET, et al. Enhanced efficacy of a facial hydrating serum in subjects with normal or self-perceived dry skin. J Clin Aesthet Dermatol 2011;4:51–5. [PMC free article] [PubMed] [Google Scholar]
- 117.Bloemen MC, van Gerven MS, van der Wal MB, Verhaegen PD, et al. An objective device for measuring surface roughness of skin and scars. J Am Acad Dermatol 2011;64:706–15. [DOI] [PubMed] [Google Scholar]
- 118.Fabi SG, Zaleski-Larsen L, Bolton J, Mehta RC, et al. Optimizing facial rejuvenation with a combination of a novel topical serum and injectable procedure to increase patient outcomes and satisfaction. J Clin Aesthet Dermatol 2017;10:14–8. [PMC free article] [PubMed] [Google Scholar]
- 119.Jiang LI, Stephens TJ, Goodman R. SWIRL, a clinically validated, objective, and quantitative method for facial wrinkle assessment. Skin Res Technol 2013;19:492–8. [DOI] [PubMed] [Google Scholar]
- 120.Carruthers J, Donofrio L, Hardas B, Murphy DK, et al. Development and validation of a photonumeric scale for evaluation of facial fine lines. Dermatol Surg 2016;42(Suppl 1):S227–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kane MA, Blitzer A, Brandt FS, Glogau RG, et al. Development and validation of a new clinically-meaningful rating scale for measuring lateral canthal line severity. Aesthet Surg J 2012;32:275–85. [DOI] [PubMed] [Google Scholar]
- 122.Shoshani D, Markovitz E, Monstrey SJ, Narins DJ. The modified Fitzpatrick Wrinkle Scale: a clinical validated measurement tool for nasolabial wrinkle severity assessment. Dermatol Surg 2008;34(Suppl 1):S85–91. [DOI] [PubMed] [Google Scholar]
- 123.Ning Y, Qing Z, Qing W, Li L. Evaluating photographic scales of facial pores and diagnostic agreement of tests using latent class models. J Cosmet Laser Ther 2017;19:64–7. [DOI] [PubMed] [Google Scholar]
- 124.Boyce ST, Supp AP, Wickett RR, Hoath SB, et al. Assessment with the dermal torque meter of skin pliability after treatment of burns with cultured skin substitutes. J Burn Care Rehabil 2000;21:55–63. [DOI] [PubMed] [Google Scholar]
- 125.Leal Silva HG. Facial laxity rating scale validation study. Dermatol Surg 2016;42:1370–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


