Abstract
Objective: To study the impact of age, gender, and presence of diabetes (any type) on the risk of early deaths (180-day mortality) in patients starting long-term hemodialysis (HD) therapy.
Design: Systematic review of the literature.
Setting: Out-patient (non-hospitalized), community-based HD therapy world-wide.
Participants: Patients with advanced chronic kidney disease (CKD) starting long-term HD treatment for end-stage renal disease (ESRD).
Methods: Medline and EMBASE were searched for studies published between 1/1/1985 and 12/31/2017. Observational studies involving adult subjects commencing HD were included. Data extracted included population characteristics and settings. In addition, patient or treatment related factors studied with reference to their relationship with the risk of early mortality were documented. The Quality in Prognosis Studies tool was used to assess risk of bias in individual studies. Findings were summarized, and a narrative account was drawn.
Results: Included were 26 studies (combined population 1,098,769; representing 287,085 person-years of observation for early mortality). There were 17 cohort and 9 case-control studies. Risk of bias was low in 13 and high in a further 13 studies. Patients who died in the early period were older than those who survived. Mortality rates increased with advancing age. Female gender was associated with slightly increased early mortality rates in larger and higher quality studies. The available data showed conflicting results in relation to the association of diabetes and risk of early mortality.
Conclusions: This systematic review evaluated the impact of key demographic and co-morbid factors on risk of early mortality in patients starting maintenance HD. The information could help in delivering more tailored prognostic information and planning of future interventions.
Keywords: Dialysis, End-stage renal disease, Mortality, Early-mortality, Elderly
Chronic kidney disease (CKD) represents an ever-increasing public health problem world-wide. Its prevalence is increasing,1,2 as are the numbers of patients who require regular dialysis treatment.3 The onset of hemodialysis (HD) therapy in patients with advanced CKD heralds a period of abrupt changes; patients experience severe disruptions in their lifestyles from frequent hospital visits and are exposed to multiple medical procedures.4,5 HD treatment itself imposes additional physiological demands on patients, as it is associated with impairment of myocardial function6 and accelerated loss of residual renal function.7 Combined with worsening indices of nutrition and inflammation with advancing CKD,8-10 an increasing burden of comorbid illnesses11 and a decline in functional status in the elderly,12 the risk of decompensation is high in the early days of HD therapy.13 Mortality rates are highest in the first few weeks of treatment before stabilizing to lower levels.14-16
As research progresses towards delivering interventions to reduce early mortality in new HD starters, eg, by better preparing patients for dialysis through pre-dialysis educational programs,17 pro-actively anticipating and managing common problems faced by dialysis starters,18 and starting HD incrementally rather than abruptly;19 there is a growing need to coalesce the global literature on risk factors for early mortality by subjecting it to a rigorous review process. An authoritative understanding of the impact of key risk factors on early mortality rates can help in counselling patients who are about to make the life-changing transition in to dialysis-dependency.20-22 It can also help ensure that new interventions are targeted towards those at highest risk to maximize their impact.
A systematic review of the literature was conducted to summarize the impact of key risk factors for early mortality after initiation of maintenance HD. In this paper, we report our findings in relation to three such factors: age, gender, and a background of diabetes, and their association with risk of early mortality in patients starting long-term HD treatment.
Materials and Methods
Search Strategy
Medline and EMBASE databases were searched using keywords and subject headings encompassing three separate themes: (a) hemodialysis [h*modialysis or ‘renal replacement’ or subject headings ‘dialysis’ or ‘renal replacement therapy’]; (b) mortality [mortality or death* or survival* or outcome*]; and (c) early or initial [initia$ or early or (first adj2 day$) or (first adj2 week$) or (first adj2 month$) or (soon adj1 after) or (short adj1 term)]. The results were combined using the operator AND, ie, A AND B AND C. Hence, the search strategy aimed to retrieve records of studies related to early mortality in patients starting hemodialysis. Searches were restricted to publications between January 1, 1985 and December 31, 2017 in English language. Publications before 1985 were deemed unlikely to represent current practices and the changed patient demographics in the modern era. Reference lists of relevant studies were also screened for additional publications.
Eligibility Criteria
Observational studies involving subjects starting HD for end stage renal disease (ESRD), evaluating any baseline factor for its relationship with post-HD mortality, and reporting mortality as one of the outcome measures were eligible for inclusion. Early mortality was defined as all-cause mortality with in the first 6 months of starting HD. Studies primarily focusing on those receiving other forms of renal replacement therapy (RRT) (ie, peritoneal dialysis and transplantation) were excluded.
Data Items
Basic study characteristics including the study objectives, settings, selection and number of participants, proportion of patients stating HD (as opposed to other forms of RRT), duration of follow-up, and measurement of outcomes (ie, definition and ascertainment of early mortality) were extracted. Baseline patient characteristics and risk factors studied (eg, age, gender), number of subjects with and without the risk factors, the number of deaths or mortality rates in each group (if available) were documented. The summary risk estimates were extracted (if available), which included odds ratios, relative risks, or hazard ratios (with confidence intervals and P values). If studies adjusted their risk estimates for confounding factors, the adjusted estimates were also recorded. A note was made of the statistical method used to compare means or proportions.
Risk of Bias in Individual Studies
Risk of bias within studies were assessed using the Quality in Prognosis Studies (QUIPS) instrument.23 This tool prompts users to assess the risk of bias in six domains: (1) study participation, (2) study attrition, (3) prognostic factor measurement, (4) outcome measurement, (5) study confounding, and (6) statistical analysis and reporting. In the QUIPS instrument, summated scores for each study are not recommended.24 Instead, an overall judgement of the risk of bias is suggested based on the relative impact of biases in the six main domains. We attributed a lower risk of bias to studies that carefully recruited their subjects, ie, an unselected cohort of adult patients starting maintenance HD for ESRD with a complete follow-up record of all study participants, attention to ascertainment of the outcome measure (all-cause mortality), and non-selective reporting of the outcome. Based on these principles, studies were classified as low or high risk of bias:
Low risk: low risk rating in all domains
High risk: any high or moderate risk domains
Synthesis of Results
The included studies are summarized in Table 1 along with key study and participant characteristics. A narrative account was constructed for each risk factor and presented in the free text. As much as possible, attempt has been made to quantify the risks, but due to the heterogeneity found within the studies in terms of their base population, selection methods, evaluation of risk factors, presentation of data, and follow-up, a meta-analysis was not conducted. In some cases, limited pooling of data from different studies (restricted to only those studies where raw data could be extracted) have also been presented; this has been done to show a comparison of early deaths (numbers or rates) in each risk category. Crude early mortality rates were calculated for these studies from raw data and are presented along with their 95% confidence intervals (CI). In the narrative synthesis, more emphasis has been placed in reporting the findings of higher quality studies. Findings linked with lower quality evidence have been highlighted
Table 1.
List of included studies with key study and participant characteristics.
| Study | Settings | Objective | N | Ave. age (yrs) | % Male | HD as first RRT modality | Definition of EM (days) |
|---|---|---|---|---|---|---|---|
| Foley et al, 201436 | US; Nationwide. Data from United States Renal Data System | To enumerate weekly mortality rates during the first year of RRT | 498,566 | 51% >65 yrs | 56% | 94% | 90 |
| Chan et al, 201135 | US; Dialysis centers operated by Fresenius Medical Care North America with 1500 dialysis facilities | To describe the characteristics, mortality and hospitalization risks for a cohort of patients starting chronic dialysis | 303,289 | 63 | 54% | 94% | 90 |
| Robinson et al, 201440 | Participating dialysis units in 11 countries across western Europe, US, Japan, Australia and NZ | To evaluate mortality patterns over the course of HD treatment in 11 countries participating in the DOPPS study | 86,886 | 63 | 59% | 100% | 120 |
| Tsakiris et al, 199945 | Dialysis centers across 28 European countries affiliated with ERA-EDTA | To define the incidence and factors related to deaths within 90 days of starting RRT | 78,534 | 54 | 59% | 82% | 90 |
| Yazawa et al, 201649 | Japan; National ESRD registry | To study the impact of functional status on early deaths | 33,281 | 69 | 65% | 100% | 90 |
| Ivory et al 201746 | Australia and New Zealand; population-wide data from ANZDATA registry | To develop a risk prediction tool for 6-month mortality | 23,658 | 61 | 60% | NS | 180 |
| Lukowsky et al, 201231 | US; Multicenter; centers affiliated with a large private dialysis provider | To test if patterns and risk factors associated with early mortality differ from those in later dialysis therapy periods | 18,707 | 63 | 55% | 100% | 90 |
| Soucie et al, 199641 | US; three states: Georgia, North Carolina and South Carolina | To identify factors associated with mortality at the onset of dialysis | 15,245 | 57 | 49% | 80% | 90 |
| Zhao et al, 201750 | China; Beijing regional ESRD registry | To study risk factors for early mortality | 11,955 | 58 | 56% | 100% | 120 |
| Roca-Tey et al, 201648 | Spain (Catalonia); regional ESRD registry | To evaluate the impact on vascular access type of mortality | 9,956 | NS | NS | 100% | 120 |
| McQuillan et al, 201242 | Canada; Multicenter, 35 dialysis sites covering a population base of 7.6 million people | To determine the incidence and risk factors for 90-day mortality | 4,807 | 66 | 60% | 100% | 90 |
| Bradbury et al, 200743 | US; multicenter. Randomly chosen dialysis facilities in DOPPS 1 and DOPPS 2 studies | To examine the magnitude of associations between various patient characteristics and mortality | 4,802 | 26% >75 yrs | 56% | 100% | 120 |
| Couchoud et al, 200933 | France; population based. Patients starting dialysis in 16 French regions (covering 79% of French population) | To develop and validate a clinical score to assess risk of mortality within 6 months of starting RRT in elderly patients with ESRD | 2,500 | 81 | 60% | NS | 180 |
| Chua et al, 201439 | Singapore; Single-center | To examine factors associated with early death. To enable formulation of an effective risk prediction scoring system | 983 | 60 | 52% | 72% | 90 |
| Wolf et al, 200734 | US; multicenter, 569 dialysis facilities in 37 states | To test if decreased levels of untreated 25D and 1,25D are associated with increased early mortality | 825 | 63 | 53% | 100% | 90 |
| Barrett et al, 199738 | Canada; multicenter. 11 dialysis centers affiliated with Universities across Canada. | To predict factors for early death in patients starting dialysis | 822 | 58 | 59% | 62% | 180 |
| Serafinceanu et al, 201426 | Romania; Single center in Bucharest | To identify demographic and clinical risk factors associated with early mortality in diabetic patients | 788 | 56 | 58% | 65% | 90 |
| Metcalf et al, 200027 | Scotland; nationwide study | To determine the major influences on death within the first 90 days of RRT | 532 | 65 | 60% | 77% | 90 |
| Kessler et al, 200329 | France; Multicenter. All 13 Nephrology units in the metropolitan region of Lorraine | To study the impact of nephrology referral on outcomes after the start of HD | 502 | 63 | 59% | 80% | 90 |
| Khan et al, 199532 | UK; Aberdeen, single center | To study the influence of various factors on deaths during 90 days of starting RRT | 459 | 65 | 50% | NS | 90 |
| De Lima et al, 199844 | Brazil; single center in Sao Paulo | To determine the relationship between baseline characteristics and prognosis of dialysis patients | 395 | NS | NS | NS | 90 |
| Biesenbach et al, 200728 | Austria; single center | To evaluate differences in risk of early death between diabetics and non-diabetics | 334 | NS | NS | NS | 90 |
| Foley et al, 199430 | Canada; Newfoundland, single tertiary care center | To identify and quantify the accuracy of predictors of death within 6 months of starting maintenance dialysis | 325 | 35% >70 yrs | 65% | NS | 180 |
| Fabian et al, 201651 | South Africa, multi-center | To evaluate the impact of ‘Healthy Start’ intervention on mortality | 269 | 54 | 64% | 78% | 90 |
| Arai et al, 201437 | Japan; single-center, Yokosuka Kyosai Hospital | To investigate the 6-month mortality of Japanese patients aged > 75 years who recently started dialysis | 202 | 80 | 60% | 97% | 180 |
| Rubio et al, 201747 | Spain (Malaga); multi-center | To study the impact of clinical parameters of mortality | 147 | 68 | 71% | 100% | 180 |
US: United States; UK: United Kingdom; NZ: New Zealand; HD: hemodialysis; EM: early mortality; NS: not specified; ESRD: end-stage renal disease; RRT: renal replacement therapies; DOPPS: Dialysis Outcomes and Practice Patterns Study; N: number of participants; Ave: Average.
Protocol and Registration
A four-member academic advisory panel at Hull York Medical School regularly reviewed the methods and conduct of the systematic review throughout its course. It was registered with the International Prospective Register of Systematic Reviews (PROSPERO)25 on April 2, 2016 (registration number: CRD-42016037016).
Results
Search Results
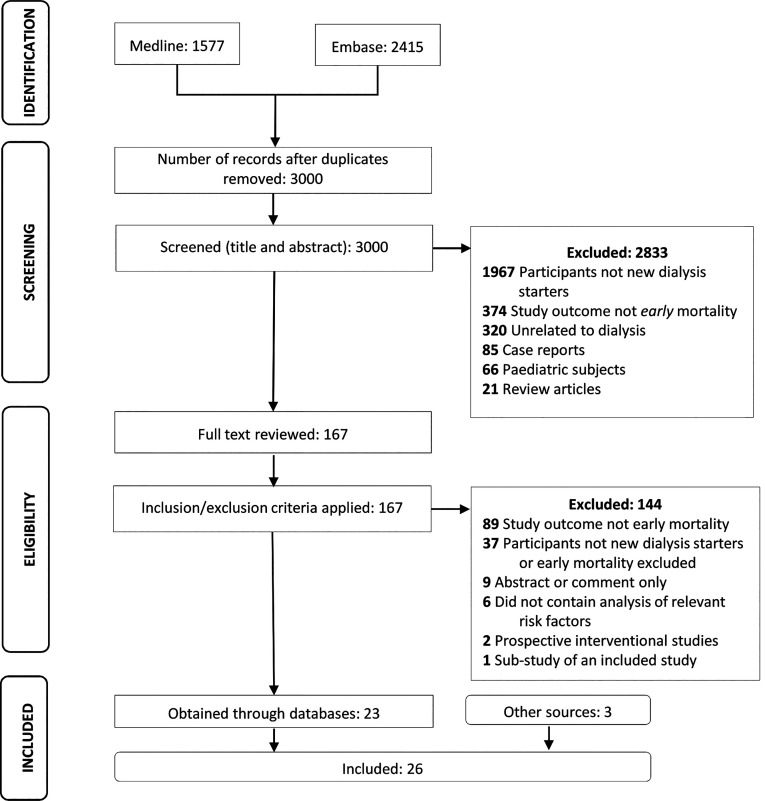
After removing duplicates, a total of 3,000 citations were obtained from database searches (Figure 1). Screening of titles and abstracts resulted in the exclusion of 2,833 citations. Full texts of the remaining 167 citations were reviewed; 26 studies met the eligibility criteria and were included.
Figure 1.
Prisma flow chart showing study selection process.
Description of Included Studies
The included studies26-51 are listed in Table 1. These collectively represented a population of 1,098,769 new dialysis starters and 287,085 person-years of observation for early mortality. There were 17 cohort27,29-31,33,35-38,40-43,45,48-50 and 9 case-control26,28,32,34,39,44,46,47,51 studies. Six studies were based in the United States (combined number of participants: 841,434);31,34-36,41,43 three in Canada (5,954 participants);30,38,42 two each in United Kingdom,27,32 France,29,33 Spain,47,48 and Japan37,49 (47,579 participants); and one each in Austria,28 Brazil,44 China,50 Romania,26 Singapore,39 and South Africa51 (14,724 participants). In addition, there were three multinational studies involving the Australia-New Zealand region,46 European countries,45 and a group of 11 industrialized nations40 (189,078 participants).
Numbers of participants in the included studies ranged between 14747 and 498,566;36 median duration of follow-up for early mortality: 90-days (range 90–180 days); and average age of participants: 54 years45,51 to 81 years.33 The proportion of men ranged between 49%41 and 65%;30 this figure was not reported in some studies.28,44,48 The proportion of patients receiving HD as first RRT modality ranged between 62%38 and 100%,31,34,40,42,43,48-50,52-54 although this information was not reported in several studies.28,30,32,44,46
Risk of Bias
Of the studies included, 13 were at low risk of bias27,29,31,32,34,36,38,40,41,43,46,49 and 13 at high risk.26,28,30,33,37,39,42,44,45,47,48,50,51 The summary of risk of bias assessment is presented in Supplementary Table S1 (available online).
The Impact of Age on Early Mortality
In total, 25 studies 26,28-51 with a combined population of 1,098,237 people examined age as a risk factor. The presentation of data differed in these studies. Eleven studies26,28,31,32,34,35,39,44,46,47,51 compared mean ages in those who died early vs. the survivors (case-control design). Patients who died early were older in all instances. In one study (n=1,000), the mean ages (± standard deviation [SD]) of patients who died in the first 90 days (n=250) and of those who survived this period (n=750) were 71 (±13) and 61 (±16) years, respectively; t-test P < 0.01.34 Some studies also adjusted for potential confounding factors,31,35,39,44 producing similar outcomes; ie, patients who died early were older. Lukowsky et al,31 reported the mean age of patients who died in the first 3 months vs. those who survived > 2 years were 72 and 60 years, respectively. For every 10 year increase in age, the hazard ratio of mortality increased by 1.50 (95% CI 1.43-1.56).
Ten studies30,33,40-43,45,48-50 presented their findings as number of early deaths within various age brackets (longitudinal cohort design). All these studies demonstrated higher early mortality rates in older subjects. Soucie et al41 (n=15,245) showed that in age groups < 45, 45–64, 65–74, and > 75 years, percentage of deaths in the first 90-days of starting HD were 2.3%, 4.6%, 9.5%, and 11.6%, respectively; P value < 0.001. Similarly, Robinson et al40 stratified their cohort (n=86,899) into age groups of < 45, 45–54, 55–64, 65–74, and > 75 years; crude mortality rates were 6.0, 11.4, 17.4, 28.2, and 45.6 per 100 person-years, respectively, in these groups. This equated to adjust hazard ratios for early mortality of 1.08, 1.24, 1.55, 1.53, and 1.59, respectively, in the groups when compared to those who survived to between 121- and 365-days post-HD, after accounting for confounding factors. Every 5-year increase in age was associated with 1.22 (95% CI 1.21 – 1.23, P value < 0.001) times increased risk of early mortality (adjusted for sex, race, and diabetes).
Four studies29,36-38 reported summary statistics only (ie, odds or hazard ratios for early deaths) in pre-defined age brackets, again confirming higher risk of early mortality with increasing age. In Kessler et al,29 the odds (and 95% CI) of 90-day mortality were reported at 2.9 (0.7-12.4), 6.0 (1.7-21.0), 4.4 (1.3-15.3), and 11.2 (3.0-41.4) in age groups 50–60, 60–70, 70–80, and > 80 years when compared to those aged < 50. Studies that recruited only elderly patients did not demonstrate any association between increasing age and early mortality.33,37
The Impact of Gender on Early Mortality
There were 21 studies26,28-31,33-37,39-48,50 with a combined population of 1,063,406 participants, that examined gender and its association with risk of early mortality. This included 14 cohort29-31,33,35-37,40-43,45,48,50 and 7 case-control26,28,34,39,44,46,47 studies. The number of participants ranged between 14747 and 498,566.36 Risk of bias was rated as low and high in 9 studies29,31,34-36,40,41,43,46 and 12 studies,26,28,30,33,37,39,42,44,45,47,48,50 respectively. The proportion of males in these studies averaged 55.8% and ranged between 49.4%41 to 65.0%.30
Risk of early mortality was higher in women in three studies26,36,40 and higher in men in two studies;41,42 whereas, the remaining 16 studies showed no overall increased risk in either gender. The three studies26,36,40 showing increased risk in women were larger (combined population of 586,240), with the risk of bias in these studies rated as low in two36,40 and high one study.26 In contrast, the two studies41,42 showing increased risk in men were smaller (combined population of 20,052), with risk of bias being low and high (one study in each category).
The largest study by Foley et al,36 a retrospective cohort study involving 498,566 patients (56.1% male), showed that after adjusting for multiple potential confounding factors, the risk of early mortality in women was higher compared to men, hazard ratio 1.06 (95% CI 1.04-1.09). The overall risk of bias in this study was low. Of the six studies with low risk of bias,29,31,35,36,39,43 one36 showed increased risk of early mortality in women, and none showed increased in men.
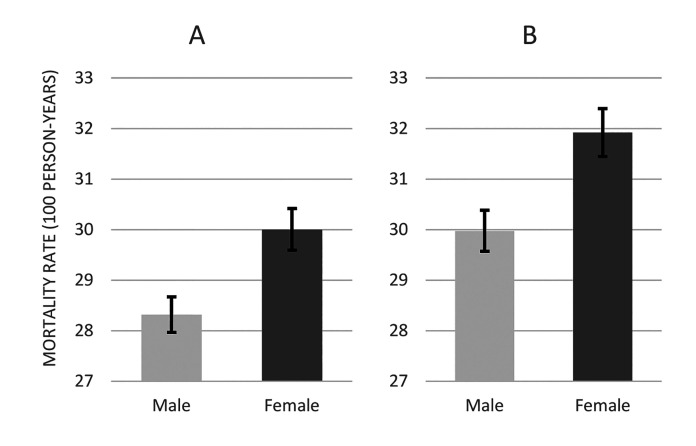
Of the 21 studies examining gender as a risk factor, 1426,31,33-35,39-43,45,46,48,50 contained data amenable to extraction and further analysis. Data from these studies were pooled together to construct a cohort of 563,110 patients, which included 315,224 males and 247,886 females. The number of early deaths in each group was 24,696 (7.8%) and 20,256 (8.2%). This equated to crude early mortality rates of 28.3 (95% CI 28.0-28.7) and 30.0 (95% CI 29.6-30.4) per 100 person-years in men and women, respectively (Figure 2A). When analyses were restricted to high-quality studies only (ie, low risk of bias), 453,587 remained in the cohort overall: 250,447 men and 203,140 women. The number of patients who died in the early period in these two groups were 20,906 (8.3%) and 17,708 (8.7%), respectively. This equated to crude early mortality rates of 30.0 (95% CI 29.7-30.4) and 31.9 (95% CI 31.4-32.4) per 100 person-years in men and women, respectively (Figure 2B). Hence, the risk of early mortality was marginally higher in women compared to men.
Figure 2.
Early mortality rates in male and female participants when data from 14 studies (see text) were pooled together (A) for all 14 studies (B) for studies with low risk of bias.
The Impact of Diabetes on Early Mortality
A total of 17 studies27-29,32-34,36,39-41,43-48,50 were found in this category, combined population 736,279. Number of subjects ranged between 14747 and 498,566;36 median 1,000. The proportion of patients with diabetes as the cause of ESRD ranged between 7.1%32 and 64.6%.39 Number of studies with high and low risk of bias were eight28,33,39,44,45,47,48,50 and nine,27,29,32,34,36,40,41,43,46 respectively.
Eight studies27,28,32,34,39,44,46,47 compared the proportion of subjects with diabetes in those who died early vs the survivors (case-control design). Overall, subjects with diabetes were not at increased risk of early mortality. In the largest study in this group, Wolf et al34 compared characteristics of 250 patients who died early to 750 matched controls. The proportion of subjects with diabetes as the cause of ESRD were identical; 43% in both groups. Khan et al32 compared 42 patients who died within 90 days of starting HD with 42 controls; the proportion of patients with diabetes in both groups were identical (3/42 in each group). In contrast, two small studies with high risk of bias28,44 did report an association between early mortality and a background of diabetic nephropathy. De Lima et al44 compared those who died early to a group of subjects who survived more than 10 years of HD treatment. The proportion of subjects with diabetes were 35% and 0%, respectively. The results were not adjusted for any potential confounders.
Nine studies29,33,36,40,41,43,45,48,50 compared mortality rates in patients with and without history of diabetes (cohort design). Overall, results were mixed, with some showing diabetes to confer an increased risk of early mortality whilst others demonstrating a protective effect. Robinson et al40 included 86,886 new dialysis starters. Within the first 120 days of starting HD, 8.1% of patients with diabetic nephropathy died; in comparison, this rate was 9.2% in those with other renal etiologies. Hence, diabetes was said to be protective. Another large study, however, appeared to show an opposite effect. In the study by Tsakiris et al45 (n=78,534), percentage of early mortality was 5.0% in patients with background of diabetes and 2.6% in those without. In the remaining six studies in this category, three29,33,41 showed increased risk, two36,43 no overall effect, and one27 showed a protective effect of diabetes.
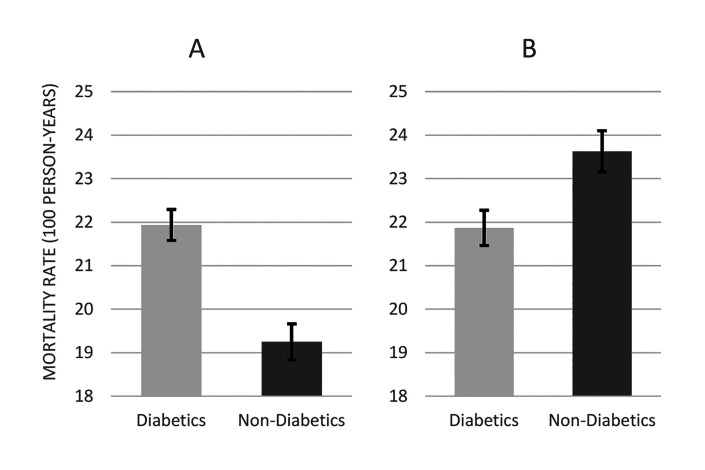
In total, nine studies27,33,39-41,43,45,46,48 with a combined population of 223,096 participants contained data that enabled the construction of two cohorts of patients: one with diabetes (n=59,893) and another without diabetes (n=163,203). The number patients who died in the early period were 4,514 (7.5%) and 10,354 (6.3%) in the two groups, respectively. This equated to crude early mortality rates of 21.9 (95% CI 21.3-22.6) and 19.2 (95% CI 18.9-19.6) per 100 person-years in patients with diabetes and those without diabetes, respectively (Figure 3A). When analyses were restricted to high-quality studies only (ie, low risk of bias), 131,085 remained in the cohort overall: 45,119 patients with diabetes and 85,966 without diabetes. The number of patients who died in the early period in these two groups were 3,406 (7.5%) and 7,118 (8.3%), respectively. This equated to crude early mortality rates of 21.9 (95% CI 21.1-22.6) and 23.6 (95% CI 23.1-24.2) per 100 person-years in diabetics and non-diabetics, respectively (Figure 3B). Hence, although initial analyses not accounting for quality suggested higher mortality in diabetic patients, this effect was not found after restricting analyses to high quality studies.
Figure 3.
Early mortality rates in patients with and without diabetes when data from 9 studies (see text) were pooled together (A) for all 9 studies (B) for studies with low risk of bias.
Discussion
This systematic review of the literature examined the impact of key risk factors on early mortality after commencement of maintenance HD. The results confirm previous findings that increasing age is associated with increased risk of death soon after starting maintenance HD. We have also shown a slightly increased risk of early mortality in women. The current data do not allow for firm conclusions to be drawn on the association of diabetes with increased risk of early mortality.
Experience from pre-dialysis care shows that many patients with CKD who are about to start dialysis have limited understanding of the outcomes of dialysis treatment.55 Providing tailored information to this patient group may be particularly difficult given the paucity of available resources to inform any discussions about the early days of dialysis, a particularly risky period in the care of patients with advanced CKD. This systematic review, therefore, goes some way in addressing this knowledge gap and aims to help patients make informed choices about their future care. It is hoped that the findings of this systematic review will stimulate further efforts to design interventions for reduction in the risk of early mortality.
The data reported in this paper formed part of a wider systematic review of literature examining risk factors and interventions related to reducing the risk of early mortality in the incident HD population. It was not possible to include findings related to all the risk factors examined due to space limitations. The reporting here prioritizes the most commonly studied or the most important modifiable risk factors for early mortality.
Studies included in this systematic review were quite diverse in terms of their design, settings (originating 12 different countries), baseline differences in participant characteristics, duration of observation for early mortality (90, 120 or 180 days), types of comparator groups used, and the reporting of outcomes. Consequently, a quantitative synthesis of all the available evidence was not possible. We were able to draw a limited comparison of crude mortality rates in isolated risk groups (depending on the reporting of raw data). This was done mainly to support the narrative account; these comparisons were not adjusted for potential confounding factors due to lack of sufficient data. Therefore, the results are to be interpreted with caution.
Overall, 13 studies were judged to be at high risk of bias. Selection bias was the largest contributor to the high overall risk of bias. Only a minority of studies set out specifically to investigate risk factors for early mortality in unselected incident dialysis population.27,30,31,35,38-43 In most cases, the studies’ primary focus was not necessarily to investigate risk factors for early mortality, but rather on addressing other objectives such as quantification of weekly mortality rates in new dialysis starters,36 comparison of baseline characteristics between patients who died early and those who survived long-term on dialysis,44 mortality risk prediction in the elderly,33 risk associations in diabetic subjects,26,28 and the study of specific and limited risk factors for early mortality (eg, mobility in the elderly,37 vitamin D status in those not previously treated with vitamin D supplements,34 and basic patient demographics).32,45 This had implications on the selection of study participants, measurement of risk exposure, analytical methods used, and reporting of outcomes. The reporting of these studies was generally in keeping with their respective pre-defined objectives; however, for the purposes of this systematic review, there were significant omissions in the design and reporting of these studies that led to them being judged as at high risk of bias. The majority of studies reporting early mortality rates in new HD starters, and those examining the effects of risk factors and interventions on early mortality, in this patient population, were of low quality.
Elderly patients are disproportionately affected by early mortality. Introducing HD incrementally, rather than abruptly starting HD at full doses, has been proposed as a way of making the start easier for this patient group.56 This could attenuate the disruptive effects of regular HD regimen on patients’ life-styles and preserve residual renal function,57,58 which could improve survival and quality of life. Female gender was also associated with a slightly increased risk of early mortality. Interestingly, this observation seems to corroborate findings that the most commonly used measure of dialysis effectiveness (the ‘Kt/Vurea’) under-estimates true dialysis requirements in women.59,60 It raises the possibility that women may be under-dialyzed if put on the same treatment program as men. Further research is needed before this can be proven definitively.
Conclusions
This systematic review of literature summarizes the impact of age, gender and diabetes on risk of early mortality after commencement of maintenance HD. Results show that older age and possibly female gender are associated with higher risks of early mortality whereas the literature currently does not support the notion of increased risks in those with diabetes. Future interventions61 should aim to target these high-risk groups as this is likely to lead to highest impact in terms of reducing early mortality rates.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Rhian Gabe (Hull York Medical School), Professor Trevor Sheldon (Hull York Medical School) and Professor Una Macleod (Hull York Medical School) for their helpful comments during this study.
References
- 1.Jha V, Modi GK.. Getting to know the enemy better—the global burden of chronic kidney disease. Kidney Int. 2018;94(3):462-464. [DOI] [PubMed] [Google Scholar]
- 2.Luyckx VA, Tonelli M, Stanifer JW.. The global burden of kidney disease and the sustainable development goals. Bull World Health Organ. 2018;96(6):414-422D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas B, Wulf S, Bikbov B, et al. Maintenance dialysis throughout the world in years 1990 and 2010. J Am Soc Nephrol. 2015;26(11):2621-2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McIntyre CW, Rosansky SJ.. Starting dialysis is dangerous: how do we balance the risk? Kidney Int. 2012;82(4):382-387. [DOI] [PubMed] [Google Scholar]
- 5.Broers NJ, Cuijpers AC, van der Sande FM, Leunissen KM, Kooman JP.. The first year on haemodialysis: a critical transition. Clin Kidney J. 2015;8(3):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McIntyre CW, Burton JO, Selby NM, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3(1):19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bargman JM, Golper TA.. The importance of residual renal function for patients on dialysis. Nephrol Dial Transplant. 2005;20(4):671-673. [DOI] [PubMed] [Google Scholar]
- 8.Ikizler TA, Greene JH, Wingard RL, Parker RA, Hakim RM.. Spontaneous dietary protein intake during progression of chronic renal failure. J Am Soc Nephrol. 1995;6(5):1386-1391. [DOI] [PubMed] [Google Scholar]
- 9.Ledebo I, Kessler M, van Biesen W, et al. Initiation of dialysis—opinions from an international survey: Report on the Dialysis Opinion Symposium at the ERA‐EDTA Congress, 18 September 2000, Nice. Nephrol Dial Transplant. 2001;16(6):1132-1138. [DOI] [PubMed] [Google Scholar]
- 10.Broers NJH, Cuijpers ACM, van der Sande FM, Leunissen KML, Kooman JP.. The first year on haemodialysis: a critical transition. Clin Kidney J. 2015;8(3):271-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikizler TA. A patient with CKD and poor nutritional status. Clin J Am Soc Nephrol. 2013;8(12):2174-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE.. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins AJ, Foley RN, Gilbertson DT, Chen SC.. The state of chronic kidney disease, ESRD, and morbidity and mortality in the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Supplement 1):S5-S11. [DOI] [PubMed] [Google Scholar]
- 14.Noordzij M, Jager KJ.. Increased mortality early after dialysis initiation: a universal phenomenon. Kidney Int. 2014;85(1):12-14. [DOI] [PubMed] [Google Scholar]
- 15.Cohen LM, Ruthazer R, Moss AH, Germain MJ.. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol. 2010;5(1):72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hazara AM, Bhandari S.. Early mortality rates after commencement of maintenance hemodialysis: a systematic review and meta-analysis. Ther Apher Dial. 2019. October 1. doi: 10.1111/1744-9987.13437. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Lacson E Jr, Wang W, DeVries C, et al. Effects of a nationwide predialysis educational program on modality choice, vascular access, and patient outcomes. Am J Kidney Dis. 2011;58(2):235-242. [DOI] [PubMed] [Google Scholar]
- 18.Wingard RL, Chan KE, Lazarus JM, Hakim RM.. The “right” of passage: surviving the first year of dialysis. Clin J Am Soc Nephrol. 2009;4(Supplement 1):S114-S120. [DOI] [PubMed] [Google Scholar]
- 19.Hazara AM, Bhandari S.. Can incremental haemodialysis reduce early mortality rates in patients starting maintenance haemodialysis? Curr Opin Nephrol Hypertens. 2019;28(6):641647. [DOI] [PubMed] [Google Scholar]
- 20.Morton RL, Tong A, Howard K, Snelling P, Webster AC.. The views of patients and carers in treatment decision making for chronic kidney disease: systematic review and thematic synthesis of qualitative studies. BMJ. 2010;340:c112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer SC, de Berardis G, Craig JC, et al. Patient satisfaction with in-center haemodialysis care: an international survey. BMJ Open. 2014;4(5):e005020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(2):195-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayden JA, Côté P, Bombardier C.. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144(6):427-437. [DOI] [PubMed] [Google Scholar]
- 24.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C.. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280-286. [DOI] [PubMed] [Google Scholar]
- 25.National Institute for Health Research. NIHR. PROSPERO . International prospective register of systematic reviews. Centre for Reviews and Dissemination, University of York, York, UK. Available at: www.crd.york.ac.uk/prospero. Accessed 24 November 2016. [Google Scholar]
- 26.Serafinceanu C, Neculaescu C, Cimponeriu D, Timar R, Covic AC.. Impact of gender and dialysis modality on early mortality risk in diabetic ESRD patients: data from a large single center cohort. Int Urol Nephrol. 2014;46(3):607-614. [DOI] [PubMed] [Google Scholar]
- 27.Metcalfe W, Khan IH, Prescott GJ, Simpson K, Macleod AM.. Can we improve early mortality in patients receiving renal replacement therapy? Kidney Int. 2000;57(6):2539-2545. [DOI] [PubMed] [Google Scholar]
- 28.Biesenbach G, Loipl J, Schmekal B, Janko O.. Different risk factors and causes for early death after initiating dialysis in diabetic and non-diabetic patients. Renal Failure 2007;29(1):49-53. [Google Scholar]
- 29.Kessler M, Frimat L, Panescu V, Briançon S.. Impact of nephrology referral on early and midterm outcomes in ESRD: EPidémiologie de l’Insuffisance REnale chronique terminale en Lorraine (EPIREL): results of a 2-year, prospective, community-based study. Am J Kidney Dis. 2003;42(3):474-485. [DOI] [PubMed] [Google Scholar]
- 30.Foley RN, Parfrey PS, Hefferton D, Singh I, Simms A, Barrett BJ.. Advance prediction of early death in patients starting maintenance dialysis. Am J Kidney Dis. 1994;23(6):836-845. [DOI] [PubMed] [Google Scholar]
- 31.Lukowsky LR, Kheifets L, Arah OA, Nissenson AR, Kalantar-Zadeh K.. Patterns and predictors of early mortality in incident hemodialysis patients: new insights. Am J Nephrol. 2012;35(6):548-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khan IH, Catto GRD, Edward N, MacLeod AM.. Death during the first 90 days of dialysis: A case control study. Am J Kidney Dis. 1995;25(2):276-280. [DOI] [PubMed] [Google Scholar]
- 33.Couchoud C, Labeeuw M, Moranne O, et al. ; French Renal Epidemiology and Information Network (REIN) registry . A clinical score to predict 6-month prognosis in elderly patients starting dialysis for end-stage renal disease. Nephrol Dial Transplant. 2009;24(5):1553-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf M, Shah A, Gutierrez O, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004-1013. [DOI] [PubMed] [Google Scholar]
- 35.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM.. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642-2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Foley RN, Chen SC, Solid CA, Gilbertson DT, Collins AJ.. Early mortality in patients starting dialysis appears to go unregistered. Kidney Int. 2014;86(2):392-398. [DOI] [PubMed] [Google Scholar]
- 37.Arai Y, Kanda E, Kikuchi H, et al. Decreased mobility after starting dialysis is an independent risk factor for short-term mortality after initiation of dialysis. Nephrology (Carlton). 2014;19(4):227-233. [DOI] [PubMed] [Google Scholar]
- 38.Barrett BJ, Parfrey PS, Morgan J, et al. Prediction of early death in end-stage renal disease patients starting dialysis. Am J Kidney Dis. 1997;29(2):214-222. [DOI] [PubMed] [Google Scholar]
- 39.Chua HR, Lau T, Luo N, et al. Predicting first-year mortality in incident dialysis patients with end-stage renal disease - the UREA5 study. Blood Purif. 2014;37(2):85-92. [DOI] [PubMed] [Google Scholar]
- 40.Robinson BM, Zhang J, Morgenstern H, et al. Worldwide, mortality risk is high soon after initiation of hemodialysis. Kidney Int. 2014;85(1):158-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soucie JM, McClellan WM.. Early death in dialysis patients: risk factors and impact on incidence and mortality rates. J Am Soc Nephrol. 1996;7(10):2169-2175. [DOI] [PubMed] [Google Scholar]
- 42.McQuillan R, Trpeski L, Fenton S, Lok CE.. Modifiable risk factors for early mortality on hemodialysis. Int J Nephrol. 2012;2012:1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradbury BD, Fissell RB, Albert JM, et al. Predictors of early mortality among incident US hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol. 2007;2(1):89-99. [DOI] [PubMed] [Google Scholar]
- 44.Galvão De Lima JJ, Américo da Fonseca J, Godoy AD.. Baseline variables associated with early death and extended survival on dialysis. Ren Fail. 1998;20(4):581-587. [DOI] [PubMed] [Google Scholar]
- 45.Tsakiris D, Jones EHP, Briggs JD, et al. Deaths within 90 days from starting renal replacement therapy in the ERA– EDTA Registry between 1990 and 1992. Nephrol Dial Transplant. 1999;14(10):2343-2350. [DOI] [PubMed] [Google Scholar]
- 46.Ivory SE, Polkinghorne KR, Khandakar Y, et al. Predicting 6-month mortality risk of patients commencing dialysis treatment for end-stage kidney disease. Nephrol Dial Transplant. 2017;32(9):1558-1565. [DOI] [PubMed] [Google Scholar]
- 47.Rebollo Rubio A, Morales Asencio JM, Pons Raventos ME.. Biomarkers associated with mortality in patients undergoing dialysis. J Ren Care. 2017;43(3):163-174. [DOI] [PubMed] [Google Scholar]
- 48.Roca-Tey R, Arcos E, Comas J, Cao H, Tort J; Catalan Renal Registry Committee . Starting hemodialysis with catheter and mortality risk: persistent association in a competing risk analysis. J Vasc Access. 2016;17(1):20-28. [DOI] [PubMed] [Google Scholar]
- 49.Yazawa M, Kido R, Ohira S, et al. Early Mortality Was Highly and Strongly Associated with Functional Status in Incident Japanese Hemodialysis Patients: A Cohort Study of the Large National Dialysis Registry. PLoS One. 2016;11(6):e0156951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao X, Wang M, Zuo L.. Early mortality risk in incident Chinese hemodialysis patients: a retrospective cohort study. Ren Fail. 2017;39(1):526-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fabian J, Van Jaarsveld K, Maher HA, Gaylard P.. Early survival on maintenance dialysis therapy in South Africa: evaluation of a pre-dialysis education programme. Clin Exp Nephrol. 2016;20(1):118-125. [DOI] [PubMed] [Google Scholar]
- 52.Eghan BA, Amoako-Atta K, Kankam CA, Nsiah-Asare A.. Survival pattern of hemodialysis patients in Kumasi, Ghana: A summary of forty patients initiated on hemodialysis at a new hemodialysis unit. Hemodial Int. 2009;13(4):467-471. [DOI] [PubMed] [Google Scholar]
- 53.Otero-López MS, Martínez-Ocaña JC, Betancourt-Castellanos L, Rodríguez-Salazar E, García-García M.. Two prognostic scores for early mortality and their clinical applicability in elderly patients on haemodialysis: poor predictive success in individual patients. Nefrologia. 2012;32(2):213-220. [DOI] [PubMed] [Google Scholar]
- 54.Abderrahim E, Zouaghi K, Kheder A, et al. Impact of initial blood pressure on the mortality of diabetics undergoing renal replacement therapy. Transplant Proc. 2004;36(6):1820-1823. [DOI] [PubMed] [Google Scholar]
- 55.Finkelstein FO, Story K, Firanek C, et al. Perceived knowledge among patients cared for by nephrologists about chronic kidney disease and end-stage renal disease therapies. Kidney Int. 2008;74(9):1178-1184. [DOI] [PubMed] [Google Scholar]
- 56.Marshall MR. Observations of twice a week hemodialysis. Kidney Int. 2016;90(5):936-938. [DOI] [PubMed] [Google Scholar]
- 57.Wong J, Vilar E, Davenport A, Farrington K.. Incremental haemodialysis. Nephrol Dial Transplant. 2015;30(10):1639-1648. [DOI] [PubMed] [Google Scholar]
- 58.Golper TA, Mehrotra R.. The intact nephron hypothesis in reverse: an argument to support incremental dialysis. Nephrol Dial Transplant. 2015;30(10):1602-1604. [DOI] [PubMed] [Google Scholar]
- 59.Spalding EM, Chandna SM, Davenport A, Farrington K.. Kt/V underestimates the hemodialysis dose in women and small men. Kidney Int. 2008;74(3):348-355. [DOI] [PubMed] [Google Scholar]
- 60.Davenport A. Is hemodialysis patient survival dependent upon small solute clearance (Kt/V)?: If so how can Kt/V be adjusted to prevent under dialysis in vulnerable groups? Semin Dial. 2017;30(2):86-92. [DOI] [PubMed] [Google Scholar]
- 61.Hazara AM, Allgar V, Twiddy M, Bhandari S.. A mixed-method feasibility study of a novel transitional regime of incremental haemodialysis: study design and protocol. Clin Exp Nephrol. 2021. June 8; doi: 10.1007/s10157-021-02072-1. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.