Abstract
Objective
To evaluate the quantitative changes of respiratory functions for critically ill COVID-19 patients with mechanical ventilation, computational fluid dynamics (CFD) analysis was performed based on patient-specific three-dimensional airway geometry.
Methods
37 cases of critically ill patients with COVID-19 admitted to the ICU of Huangshi Traditional Chinese Medicine Hospital from February 1st to March 20th, 2020 were retrospectively analyzed. 5 patients whose clinical data met the specific criteria were finally cataloged into death group (2 patients) and survival group (3 patients). The patient-specific three-dimensional airways were reconstructed from the central airways down to the 4th-5th bifurcation of the tracheobronchial tree. The volume changes of bronchi were calculated during the disease progression according to the comparison of two CT scans. Additionally, the changes of air flow resistance were analyzed using numerical simulation of CFD.
Results
Pearson correlation analysis demonstrated that there was negative correlation between the change of volume (ΔV) and the change of resistance (ΔR) for all COVID-19 patients (r=-0.7025). For total airway volume, an average decrease of -11.41±15.71% was observed in death group compared to an average increase of 1.86±10.80% in survival group (p=0.0232). For air flow through airways in lower lobe, the resistance increases for death group by 10.97±77.66% and decreases for survival group by -45.49±42.04% (p=0.0246).
Conclusion
The variation of flow resistance in the airway could be used as a non-invasive functional evaluation for the prognosis and outcome of critically ill patients with COVID-19. The ‘virtual’ pulmonary function test by integrating follow-up CT scans with patient-derived CFD analysis could be a potentially powerful way in improving the efficiency of treatment for critically ill patients with COVID-19.
Keywords: Computational fluid dynamics, COVID-19, Flow resistance
1. Introduction
Track back to December 2019, an unexpected outbreak of a highly contagious new coronavirus pneumonia (COVID-19) has rapidly swept around the globe [1], [2], [3], [4], [5], [6]. By November 2020, the outbreak had grown to infect more than 57 million people with more than 1.3 million deaths in more than 200 countries/territories [7]. It is estimated that approximately 13.8% of infections developed into severe disease and 6.1% were critically ill [8]. The mortality rate of critically ill patients is reported to be over 37% [9]. Unfortunately, there is no evidence of any effective treatment for COVID-19 to date. Clinical control in critically ill cases is mainly based on supportive care including invasive mechanical ventilation, which can significantly improve the severe respiratory distress of these patients [10,11]. However, the quantitative and effective evaluation of functional changes in patients with mechanical ventilation is hardly be assessed since the regular pulmonary function testing is not applicable to these critically ill patients.
Computational fluid dynamics (CFD) is a technique that allows the simulation of flow patterns through three-dimensional models using a computational grid [12]. Based on the three-dimensional reconstruction of patient's CT images, CFD can be applied in the field of respiratory modeling where the flows inside the virtual airways are quantitatively analyzed and the influences of different morphology of airway on the flow pattern are investigated [13], [14], [15], [16]. In previous studies, CFD analysis has demonstrated special advantages compared with traditional assessment in management of COPD patients [17,18], calculation of drug distribution in airways [19], [20], [21] and therapeutic effect evaluation of asthmatics [22,23]. In this study, we hypothesize that the patient-specific CFD airway analysis shows airway resistance variations that correlate with the development of the disease, and subsequently provides a useful approach for evaluation of respiratory functions and further prognosis of critically ill COVID-19 patients. To this end, retrospective investigation is taken where the morphological and CFD analysis for patient-specific airways result in a novel non-invasive functional assessment.
2. Methods
2.1. Patient recruitment
In this study, a total of 37 cases of critically ill patients with COVID-19 admitted to the ICU of Huangshi Traditional Chinese Medicine Hospital from February 1st to March 20th, 2020 were retrospectively analyzed. On admission, the nucleic acid test results of all these patients were positive, and their CT suggested pneumonia. According to the diagnostic criteria of Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment (7th edition) issued by the National Health Council (China) [24], all cases included in this study were critically ill patients, and all of them met at least one of the following criteria: 1) respiratory failure occurred, and required mechanical ventilation; 2) shock occurred; 3) combined with other organ failure, and admission to the ICU. This study was approved by the Ethics Committee of Huangshi Traditional Chinese Medicine Hospital (HSZYPJ-2020-018-01), and all patients provided written informed consent.
This study collected all clinical data and CT data of critical patients with COVID-19 during their hospitalization. The specific criteria for inclusion are as follows: (1) all patients included in this study have their CT data reflecting the dynamic changes from the initial stage to the improvement of the condition or death; 2) the interval between CT scans was about 10 days, and at least 2 times of the CT scans were available for analysis; 3) the CT data were in DICOM format, with the layer thickness less than 1.5 mm; The exclusion criteria of this study include: 1) with no CT examination; 2) with no CT data at the initial stage; 3) their CT layer thickness didn't meet the criterion of the analysis; 4) the interval between CT scans were too long that the CT data could not reflect the entire dynamic process. Based on the aforementioned criteria, 5 critical patients with COVID-19 were finally cataloged into two groups, i.e., death group (Patients #1 & #2) and survival group (Patients #3, #4 & #5), for the further morphological and CFD analysis on individual airways. The patient demographics are summarized in Table 1 and the schematic representation for inclusion is shown in Fig. 1.
Table 1.
Patient demographics.
| Patient No. | Gender | Age | Height (cm) | Weight (kg) | Outcome | Duration of invasive | Interval between two |
|---|---|---|---|---|---|---|---|
| Patient No. | Gender | (years old) | Height (cm) | Weight (kg) | Outcome | ventilation (days) | CT scans for analysis (days) |
| 1 | male | 71 | 165 | 65 | dead | 42 | 18 |
| 2 | male | 69 | 164 | 72 | dead | 36 | 10 |
| 3 | male | 32 | 181 | 92.5 | survived | 16 | 17 |
| 4 | male | 48 | 174 | 72 | survived | 13 | 27 |
| 5 | male | 48 | 175 | 80 | survived | 46 | 33 |
Fig. 1.
Schematic representation of patient populations included in the study.
2.2. Reconstruction of three-dimensional airway
As a prerequisite for the assessment of changes in airway volumes and CFD-determined resistance, three-dimensional reconstruction of patient-specific airway geometries was carried out based on the CT images. All CT scans were taken with a multi-slice scanner with 64 receptors, and all images had a pixel size of approximately 0.7 mm. The DICOM (Digital Imaging and Communications in Medicine) images obtained by CT scans were then imported to a commercially developed software Mimics (Materialise, Leuven, Belgium) to implement the semi-automatic segmentation and three-dimensional reconstruction. Limited by the resolution of the CT scans, the constructed airway models started from the central airways down to the 4th-5th bifurcation of the tracheobronchial tree, where the airway diameter is around 1-2 mm. The same procedure was applied to the pre and post CT images for constructing three-dimensional model. Subsequently, the airways of pre and post models were made topology corresponding and the endings were pruned to the same length, which is necessary for a comparison of airway volume and resistance changes. The airway models were subdivided in accordance with Ikeda [25], and the volume was measured before and after the treatment.
2.3. CFD calculation
Homogeneous, incompressible, and Newtonian flow was assumed in the models. Since the influence of viscous effects could be neglected in the regions with highest resistance, i.e., the 4th-7th bifurcation, the air in the airway was considered as a laminar flow. Air was modeled to have a density of 1.225 kg/m3. The airway wall was assumed to be rigid without displacement. The governing equations were Navier-Stokes equations in three-dimension, which are time-averaged equations of motion for fluid flow. Therefore, the flow can be described by
| (1) |
| (2) |
In these equations, t represents time, ρf is the fluid density, is the velocity vector, p is the fluid pressure, and the viscous stress tensor is given by .
To solve the flow numerically, meshing procedure was performed by ANSYS software to generate a computational grid with average mesh size of 3 × 106 tetrahedral cells. Mesh independent analysis was implemented to ensure enough grids were constructed to capture variations of CFD parameters. After that, transient CFD simulation was processed by Fluent (ANSYS, 19.0) and the pressure-based coupled solver based on SIMPLE algorithms was used to the solve the equations. The respiratory cycle was set as a period of 5.1 s, which represents the breath frequency of about 12 times per minute for a normal adult. Besides, the tidal volume is set as 500 ml [26] based on physical facts. Building time defined function, the time-dependent velocity curve was specified as the inlet boundary condition as shown in Fig. 2 . At the outlets, the static pressure was uniformly defined to be zero pascal using an iterative process to reflect the internal flow distribution. The time step size was set to 0.051 s. The convergence criterion was satisfied when the residual of continuity was less than 10−4.
Fig. 2.
Inlet velocity curve.
CFD allows the calculation of flow properties, including pressure, velocity, and mass flow rate inside the entire flow domain, which enables the determination of airway resistance. The resistance of certain branches Ri was related with the pressure drop over the branches Δp and the mass flow rate through the branches F,
| (3) |
where Ri denotes to resistances of separate bronchial section [27].
2.4. Statistical analysis
The statistical software was SPSS 20.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as median± SEM unless stated otherwise. The student's t-test was used for comparison of changes of volumes and resistance between death and survival groups. p<0.05 was considered statistically significant. The Pearson correlation coefficient was calculated to investigate the correlation between the changes of volume and the changes of resistance.
3.Results
3.1. Air flow characteristics during disease development
To investigate the changes in the air flow characteristics during the disease progression, the CFD analysis were performed on all patients based on 3D airway models reconstructed by their CT images at two time points. A movie of change of velocity magnitude at mid-plane for Patient #2 during one respiratory cycle was provided in Supplementary Material. The cross-section profiles of velocity magnitude and pressure for two cases (Patient #2 from the death group and Patient #5 from the survival group) at mid-plane at 0.85 s are presented in Fig. 3 . In the main bronchi, a slight increase of 3.18% and 1.23% in average flow velocity was observed for the death and survival cases respectively. However, the drop of pressure showed a decrease of -4.07% for the survival case. This indicates that the resistance in the main bronchi decreased for the survival case and that the improved respiratory functions can be expected. On the contrast, it is worth noting that the drop of pressure for the death case increases to 2 times during the disease progression, suggesting that the respiratory function was getting worsen for this patient.
Fig. 3.
Left: The cross-section profiles of velocity magnitude (m/s) and pressure (pascal) of Patient #2 (the death group) at the first CT scan (top) and the second scan (bottom). Right: The cross-section profiles of velocity magnitude (m/s) and pressure (pascal) of Patient #5 (the survival group) at the first CT scan (top) and the second scan (bottom).
3.2. Negative correlation between ΔV and ΔR
The change of volume (ΔV) and the change of resistance (ΔR) of each bronchial section were averaged in the survival group and the death group, respectively. Table 2 provides a summary of ΔV and ΔR in the main bronchi and several bronchial sections for all patients. The opposite trend between ΔV and ΔR was found in most bronchial sections as well as the main bronchi. A statistical analysis (Pearson correlation analysis) between the results yielded significant negative correlation between ΔV and ΔR for all these COVID-19 patients (Fig. 4 ; r=-0.7025, p=0.002).
Table 2.
Changes of volume and resistance in the main bronchi and several bronchial sections for all patients. ΔV represents the changes in airway volume and ΔR represents the changes in airway resistance.
| Patient | Change | B1R | B2R | B3R | B4R | B12L | B6R | B9L | MAIN |
|---|---|---|---|---|---|---|---|---|---|
| 1 | ΔV | -0.1366 | 0.0522 | 0.0815 | -0.3112 | -0.0061 | -0.0050 | -0.2248 | -0.0333 |
| ΔR | 0.3319 | -0.6382 | -0.0288 | 0.6856 | 0.1124 | -0.4688 | 0.9184 | -0.3380 | |
| 2 | ΔV | -0.1425 | 0.2392 | -0.2175 | -0.4661 | -0.2220 | -0.3846 | -0.4813 | -0.3834 |
| ΔR | 0.3395 | -0.4625 | 0.4642 | 0.8594 | -0.4730 | 0.8304 | -0.8411 | 0.2269 | |
| 3 | ΔV | 0.3687 | 0.3706 | 0.1961 | 0.2460 | 0.6603 | 0.1428 | 0.4010 | 0.0658 |
| ΔR | -0.4296 | -0.5551 | -0.1970 | -0.2323 | -0.5928 | -0.2111 | -0.9995 | -0.4517 | |
| 4 | ΔV | 0.0045 | -0.3772 | -0.0472 | -0.0969 | -0.5554 | 0.1347 | -0.1596 | -0.1399 |
| ΔR | 0.0497 | 0.6585 | 0.5556 | 0.1766 | 0.2882 | -0.5944 | -0.2595 | 0.0059 | |
| 5 | ΔV | 0.0998 | 0.2552 | 0.1395 | 0.0704 | -0.4420 | -0.1962 | -0.8123 | 0.1168 |
| ΔR | -0.8508 | -0.9665 | -0.9741 | -0.6569 | -0.9981 | -0.8875 | 0.2230 | -0.4874 |
Fig. 4.
The correlation between ∆V and ∆R.
3.3. Differences between the survival group and the death group
Fig. 5 presents the changes in bronchial volume for all patients. In the survival group and the death group, the mean of the volume changes and resistance changes were measured. Table 3 provides an overview of the quantitative comparison between the death group and the survival group. The volume changes in total bronchial (ΔVtotal) showed a mean decrease of -11.41±15.71% for death group and an increase of 1.86±10.80% for survival group (p=0.0232). An average decrease of -20.84±17.51% was observed in main airway volume (ΔVmain) for death group compared to an average increase of 1.42±11.10% for survival group (p=0.2705). For airway volume in the upper lung lobe (ΔVupper), an average decrease of -15.89±19.72% was observed for death group compared to an average increase of 5.95±31.76% for survival group (p=0.4929). Besides, for airway volume in the lower lung lobe (ΔVlower), an average decrease of -27.40±18.03% was observed for death group compared to a more slightly decrease of -8.16±38.37% for survival group (p=0.4186).
Fig. 5.
Volume changes in the death group (Patients #1 & #2) and the survival group (Patients #3, #4 & #5).
Table 3.
Comparison of ΔV and ΔR between the death group and the survival group.
| Death group | Survival group | p-value | |
|---|---|---|---|
| ΔVtotal | -11.41± 15.71% | 1.86± 10.80% | 0.0232* |
| ΔRtotal | 9.49± 36.36% | -34.95± 10.94% | 0.4886 |
| ΔVmain | -20.84± 17.51% | 1.42± 11.10% | 0.2705 |
| ΔRmain | -5.55± 28.25% | -31.10± 22.46% | 0.4485 |
| ΔVupper | -15.89± 19.72% | 5.95± 31.76% | 0.4929 |
| ΔRupper | 11.91± 48.65% | -31.50± 53.81% | 0.5854 |
| ΔVlower | -27.40± 18.03% | -8.16± 38.37% | 0.4186 |
| ΔRlower | 10.97± 77.66% | -45.49± 42.04% | 0.0246* |
Note. Data are presented as mean± SD. *p < 0.05 was considered to be statistically significant.
Total = Total bronchial; Main = Main airway; Upper = Upper lung lobe; Lower = lower lung lobe.
The CFD-based airway resistance calculations indicated the total resistance (ΔRtotal) increases for death group by 9.49±36.36% and decreases for survival group by -34.95±10.94% (p=0.4886). Note the distal resistance in the lower lung (ΔRlower) lobe showed an average increase of 10.97±77.66% for death group and an average decrease of -45.49±42.04% for survival group (p=0.0246). Statistical results suggested that both the volume changes in total bronchial (ΔVtotal) and the flow resistance change in the lower lung lobe (ΔRlower) showed significant difference (p<0.05) between death and survival group.
4. Discussion
To the best of our knowledge, this is the first study to investigate the air flow changes in the airway for critically ill patients with COVID-19 by using CFD analysis. The analysis of retrospective cases demonstrated that the patients’ prognosis was significantly related to the changes of airway volume and resistance. Since there are no registered drugs to treat COVID-19 disease currently, management is based mainly on supportive care including mechanical ventilation especially in severe cases. However, the changes of respiratory function after ventilation can hardly be detected quantitatively for critical ill patients. To this end, the current study presented a novel non-invasive analysis by comparing morphological changes and performing CFD calculation on patient-derived 3D airway models. We believe the integration with computational techniques such as CFD analysis could play a valuable role in improving the efficiency of treatment for critically ill patients with COVID-19.
Among all the patients included in this study, the age of the two cases in the death group was significantly higher than that of the survival group, which is consistent with previous studies reported that age is a warning factor with strong predictive value in COVID-19 disease [28,29]. Fig. 6 shows the chest CT images during the disease progression for Patient #2 in the death group. Several typical changes caused by COVID-19 virus infection in the lung lesion can be observed from the two CT scans (with an interval of 10 days), including (a) typical ground glass opacity (GGO) in the upper and lower lung lobes at the early stage of the disease (Fig. 6 A&C) and (b) pulmonary fibrosis and its induced tractive pulmonary bullae at the middle and late stages (Fig. 6 B&D). In addition, the autopsy results from the lung of critically ill patients with COVID-19 have demonstrated that the virus destroyed the anatomical structure of the alveoli, resulting in the formation of hyaline membranes in the alveolar cavity, thickening of the alveolar walls, the formation of a large number of microthrombi, and the pulmonary fibrosis with varying degrees [30], [31], [32]. All these anatomical and pathological features during the disease progression contribute to the changes in bronchial volume and resistance in the death cases. To be specific, the volumes of most bronchial sections were decreasing, and consequently an increase of the airflow resistance was observed in the death group (Tables 2 & 3).
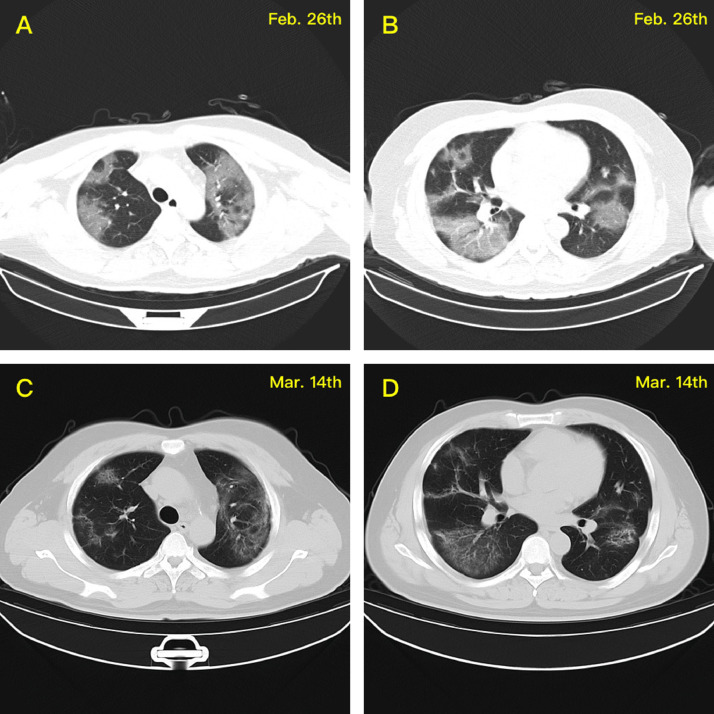
Fig. 6.
CT scans for Patient #2 indicate typical changes caused by COVID-19 virus infection in the lung lesion, including typical ground glass opacity (GGO) in the upper and lower lung lobes at the early stage of the disease (A&C) and pulmonary fibrosis and its induced tractive pulmonary bullae at the middle and late stages (B&D).
In contrast to the death group, a general increase of airway volume and a reduction of flow resistance were found for the three cases in the survival group (Table 2). This CFD result was demonstrated to be consistent with the prognosis of these patients. For example, the CT images of Patient #3 in survival group (Fig. 7 ) indicated that the exudation from the lung was obviously absorbed after effective treatment. The disease progression of Patient #5 was much more complicate. This patient was transferred to the ICU with mechanical ventilation seven days after symptoms developed. After eight days, this patient received non-invasive ventilation. However, four days later, he received mechanical ventilation again for another nine days due to the worsened condition. Therefore, his lung showed obvious uneven lesions with fibrosis and exudation caused by the relapse of illness. Correspondingly, the CFD analysis for Patient #5 (Table 2) showed that the volumes of several bronchi (B6R, B9L and B12L) even decrease during the progression, which may produce bad effect on the prognosis and recovery of this patient.
Fig. 7.
CT scans for Patient #3 indicate that the exudation from the lung was obviously absorbed after effective treatment.
It is noteworthy that both the reduced volume of the lower lobe bronchi (B6R and B9L) and the significantly increased resistance in the airway could be observed for the patients who maintained mechanical ventilation for a long time and eventually died. In addition, the difference of flow resistance between the death group and the survival group showed significant at the lower lung lobe (Table 3). This can be attributed to the trapped airway due to the deposition of inflammatory secretion in response to gravity after a long period of time lying supine [33,34]. To this end, prone ventilation may be suggested for critically ill COVID-19 patients who require long duration of mechanical ventilation, in order to improve their lung ventilation or even their prognosis. The CFD analysis for airway flow regarding to prone ventilation need further studies in the future work.
STUDY LIMITATIONS. First, one of the major limitations of the study is its small sample size. CFD calculations were applied on the limited retrospective cases, therefore the findings of this study may or may not be generalizable to other COVID-19 patients. Prospective studies should be performed to confirm the additive value of CFD parameters in respiratory function assessment for critically ill patients with mechanical ventilation. Second, CFD analysis based on individual CT images are limited by the resolution of CT scans, which only allows a reconstruction of 3D airway models up to the region of airway diameters around 1-2 mm. However, evidence has demonstrated that the main part of the airway resistance is caused by the region between the 4th and 7th bifurcation. In addition, CT scans is the most commonly used imaging modality for COVID-19 patients to date. Finally, the role of drug therapy was not included, which can be important in a study to investigate the improvement of CFD parameters caused by drug therapy. Combined CFD and drug therapy for COVID-19 patients or acute respiratory distress syndrome (ARDS) patients would be of huge interest.
5. Conclusions
This retrospective study demonstrates the application of patient-derived CFD analysis in non-invasive assessment of the respiratory function for clinically ill COVID-19 patients with mechanical ventilation. It is found that the change of airway resistance not only correlated well with the volume changes of the bronchial, but also showed significantly increasing in the lower lobe for the death group. The ‘virtual’ pulmonary function test by integrating follow-up CT scans with patient-derived CFD analysis provides the quantitative evaluation of respiratory functional improvement, suggesting that CFD analysis may have a role in improving the efficiency of treatment and predicting the prognosis and outcome for clinically ill patients with COVID-19.
Footnotes
Fund for this project: This research is supported by the National Nature Science Foundation of China (Grant numbers 61821002, 12072074, 11772093, 11972117, 11972118), Jiangsu science and technology development project (BE2017745 Diagnosis of early lung cancer using endobronchial optical coherence imaging technology), the Fundamental Research Funds for the Central Universities, the National Demonstration Center for Experimental Biomedical Engineering Education, and the Funds for Young Zhishan Scholars (Southeast University, Nanjing, China).
Conflict of interest: All other authors declared no potential conflict of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cmpb.2021.106257.
Appendix. Supplementary materials
References
- 1.Guan W.-j., Ni Z.-y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N., Zhang D., Wang W., et al. New Engl. J. Med. 2020. A novel coronavirus from patients with pneumonia in China, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai Y., Yao L., Wei T., et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323(14):1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan L.T., Nguyen T.V., Luong Q.C., et al. Importation and human-to-human transmission of a novel coronavirus in Vietnam. N. Engl. J. Med. 2020;382(9):872–874. doi: 10.1056/NEJMc2001272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giovanetti M., Benvenuto D., Angeletti S., Ciccozzi M. The first two cases of 2019-nCoV in Italy: Where they come from? J. Med. Virol. 2020;92(5):518–521. doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet North Am. Ed. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO Coronavirus Disease (COVID-19) Dashboard. https://covid19.who.int/table.
- 8.Lima CMAdO Information about the new coronavirus disease (COVID-19) Radiol. Bras. 2020;53(2):V–VI. doi: 10.1590/0100-3984.2020.53.2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quah P., Li A., Phua J. Mortality rates of patients with COVID-19 in the intensive care unit: a systematic review of the emerging literature. Crit. Care. 2020;24:1–4. doi: 10.1186/s13054-020-03006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. Statpearls [internet] StatPearls Publishing; 2020. Features, evaluation and treatment coronavirus (COVID-19) [PubMed] [Google Scholar]
- 11.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata AA. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson J.D., Wendt J. Vol. 206. Springer; 1995. (Computational Fluid Dynamics). [Google Scholar]
- 13.Leong S., Chen X., Lee H., Wang D. A review of the implications of computational fluid dynamic studies on nasal airflow and physiology. Rhinology. 2010;48(2):139. doi: 10.4193/Rhin09.133. [DOI] [PubMed] [Google Scholar]
- 14.Mylavarapu G., Murugappan S., Mihaescu M., Kalra M., Khosla S., Gutmark E. Validation of computational fluid dynamics methodology used for human upper airway flow simulations. J. Biomech. 2009;42(10):1553–1559. doi: 10.1016/j.jbiomech.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Van Ertbruggen C., Hirsch C., Paiva M. Anatomically based three-dimensional model of airways to simulate flow and particle transport using computational fluid dynamics. J. Appl. Physiol. 2005;98(3):970–980. doi: 10.1152/japplphysiol.00795.2004. [DOI] [PubMed] [Google Scholar]
- 16.Walters D.K., Luke W.H. Computational fluid dynamics simulations of particle deposition in large-scale, multigenerational lung models. J. Biomech. Eng. 2011;133(1) doi: 10.1115/1.4002936. [DOI] [PubMed] [Google Scholar]
- 17.De Backer L.A., Vos W., De Backer J., Van Holsbeke C., Vinchurkar S., De Backer W. The acute effect of budesonide/formoterol in COPD: a multi-slice computed tomography and lung function study. Eur. Respir. J. 2012;40(2):298–305. doi: 10.1183/09031936.00072511. [DOI] [PubMed] [Google Scholar]
- 18.Burrowes K.S., Doel T., Brightling C. Computational modeling of the obstructive lung diseases asthma and COPD. J. Transl. Med. 2014;12(2):1–8. doi: 10.1186/1479-5876-12-S2-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soni B., Aliabadi S. Large-scale CFD simulations of airflow and particle deposition in lung airway. Comput. Fluids. 2013;88:804–812. [Google Scholar]
- 20.Nowak N., Kakade P.P., Annapragada A.V. Computational fluid dynamics simulation of airflow and aerosol deposition in human lungs. Ann. Biomed. Eng. 2003;31(4):374–390. doi: 10.1114/1.1560632. [DOI] [PubMed] [Google Scholar]
- 21.Pourmehran O., Gorji T.B., Gorji-Bandpy M. Magnetic drug targeting through a realistic model of human tracheobronchial airways using computational fluid and particle dynamics. Biomech. Model. Mechanobiol. 2016;15(5):1355–1374. doi: 10.1007/s10237-016-0768-3. [DOI] [PubMed] [Google Scholar]
- 22.De Backer J., Vos W., Devolder A., et al. Computational fluid dynamics can detect changes in airway resistance in asthmatics after acute bronchodilation. J. Biomech. 2008;41(1):106–113. doi: 10.1016/j.jbiomech.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 23.Vinchurkar S., De Backer L., Vos W., Van Holsbeke C., De Backer J., De Backer W. A case series on lung deposition analysis of inhaled medication using functional imaging based computational fluid dynamics in asthmatic patients: effect of upper airway morphology and comparison with in vivo data. Inhal. Toxicol. 2012;24(2):81–88. doi: 10.3109/08958378.2011.644351. [DOI] [PubMed] [Google Scholar]
- 24.Commission CNH. 7th edition. 2020. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment. [Google Scholar]
- 25.Netter F.H. 1980. The CIBA collection of medical illustrations, vol. 7. [Google Scholar]
- 26.Hrasˇka V., Photiadis J., Haun C., et al. Pulmonary artery sling with tracheal stenosis. Multimed. Man. Cardiothorac. Surg. 2009;2009(123) doi: 10.1510/mmcts.2008.003343. [DOI] [PubMed] [Google Scholar]
- 27.De Backer L., Vos W., Salgado R., et al. Functional imaging using computer methods to compare the effect of salbutamol and ipratropium bromide in patient-specific airway models of COPD. Int. J. Chronic Obstruct. Pulm. Dis. 2011;6:637. doi: 10.2147/COPD.S21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies N.G., Klepac P., Liu Y., et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. MedRxiv. 2020 doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 29.Klopfenstein T., Toko L., Royer P.-Y., Lepiller Q., Gendrin V., Zayet S. Features of anosmia in COVID-19. Méd. Mal. Infect. 2020 doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mason R.J. Eur. Respir. Soc. 2020. Pathogenesis of COVID-19 from a cell biology perspective. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C., Xie J., Zhao L., et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID-19 patients. EBio Med. 2020;57 doi: 10.1016/j.ebiom.2020.102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao X., Li T., He Z., et al. A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua bing li xue za zhi= Chin. J. Pathol. 2020;49 doi: 10.3760/cma.j.cn112151-20200312-00193. E009-E009. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z., Zhong M., Jiang L., et al. Effects of the lower airway secretions on airway opening pressures and suction pressures in critically Ill COVID-19 patients: a computational simulation. Ann. Biomed. Eng. 2020:1–11. doi: 10.1007/s10439-020-02648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moutsopoulos H.M. Anti-inflammatory therapy may ameliorate the clinical picture of COVID-19. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.