ABSTRACT
Enterotoxigenic Escherichia coli (ETEC) and Shiga toxin-producing E. coli (STEC) strains are the causative agents of severe foodborne diseases in both humans and animals. In this study, porcine pathogenic E. coli strains (n = 277) as well as porcine commensal strains (n = 188) were tested for their susceptibilities to 34 bacteriocin monoproducers to identify the most suitable bacteriocin types inhibiting porcine pathogens. Under in vitro conditions, the set of pathogenic E. coli strains was found to be significantly more susceptible to the majority of tested bacteriocins than commensal E. coli. Based on the production of bacteriocins with specific activity against pathogens, three potentially probiotic commensal E. coli strains of human origin were selected. These strains were found to be able to outcompete ETEC strains expressing F4 or F18 fimbriae in liquid culture and also decreased the severity and duration of diarrhea in piglets during experimental ETEC infection as well as pathogen numbers on the last day of in vivo experimentation. While the extents of the probiotic effect were different for each strain, the cocktail of all three strains showed the most pronounced beneficial effects, suggesting synergy between the tested E. coli strains.
IMPORTANCE Increasing levels of antibiotic resistance among bacteria also increase the need for alternatives to conventional antibiotic treatment. Pathogenic Escherichia coli represents a major diarrheic infectious agent of piglets in their postweaning period; however, available measures to control these infections are limited. This study describes three novel E. coli strains producing antimicrobial compounds (bacteriocins) that actively inhibit a majority of toxigenic E. coli strains. The beneficial effect of three potentially probiotic E. coli strains was demonstrated under both in vitro and in vivo conditions. The novel probiotic candidates may be used as prophylaxis during piglets’ postweaning period to overcome common infections caused by E. coli.
KEYWORDS: probiotic, Escherichia, E. coli, bacteriocin, pig, ETEC, STEC
INTRODUCTION
Pathogenic Escherichia coli strains, including enterotoxigenic E. coli (ETEC) and Shiga-toxigenic E. coli (STEC) strains, are important pathogens in postweaning piglets causing significant decreases in weight gain and increases in mortality (1–4). Porcine pathogenic ETEC strains often harbor specific colonization factors, including fimbrial adhesins F4 (K88) and F18 (3). When attached, ETEC produces enterotoxins (heat-labile toxin [LT] and the heat-stable toxins [STa and/or STb]), which cause diarrhea as a result of induced electrolyte imbalance (5, 6). STEC strains produce Shiga toxins (Stx1 and/or Stx2), and infection in piglets (edema disease), associated with Stx2e production, manifests as edema in various tissues and neurological signs such as ataxia, paralysis, and incoordination, with mortality rates of up to 90% (7, 8). Moreover, ETEC strains are the most frequent bacterial cause of traveler’s and infant diarrhea, resulting in considerable morbidity and mortality in developing countries (9), while STEC strains are the causative agents of severe human foodborne diseases (10, 11).
The extensive use of antibiotics results in the selection of antibiotic-resistant bacteria that can potentially be transmitted to humans (12). While the use of antibiotics in animal husbandry is under surveillance and limited with regulations in Europe, unregulated usage is still quite frequent in some states (13, 14). Therefore, there is a need for novel antimicrobial strategies. In this regard, probiotic bacteria producing antimicrobial proteins or peptides (bacteriocins) are of interest (for reviews, see references 15–17).
Probiotics are viable microorganisms that, when ingested in sufficient amounts, exert beneficial effects on the host (18). Probiotics can directly compete with pathogens for an ecological niche and can also inhibit their growth by the production of antimicrobial compounds such as bacteriocins. Furthermore, probiotics can modulate the host’s immune response, help in maintaining intestinal barrier integrity, and inactivate enterotoxins (19). The beneficial effect of the human probiotic E. coli Nissle 1917 in freshly weaned piglets has been demonstrated (20). Moreover, the positive effect of the application of probiotic E. coli together with prebiotics (raw potato starch) has been experimentally shown (21).
Bacteriocins are natural antimicrobials produced by certain bacterial strains. E. coli has been shown to produce two types of bacteriocin molecules, including colicins (>10 kDa) and microcins (<10 kDa) (15, 22, 23). The ability to produce bacteriocins (bacteriocinogeny) is a common feature, and more than half of E. coli strains isolated from human fecal microbiota produce at least one bacteriocin type (15, 24). Among pathogenic E. coli strains isolated from pigs, bacteriocinogeny was found in 60% of tested isolates (25). The use of bacteriocinogenic E. coli strains with in vitro activity against ETEC from piglets (26) was found to be an effective approach also proven by experiments under in vivo conditions in piglets (21, 27, 28). However, the selection of probiotic strains based on the production of effective bacteriocins against specific pathogens has not been tested yet.
In this study, we have selected three human commensal E. coli strains based on their bacteriocin production determined by an extensive in vitro study and activity against toxigenic E. coli. Here, the effect of these potentially probiotic strains against pathogens is demonstrated under both in vitro and in vivo conditions.
RESULTS
Distribution of toxins and serogroups among porcine pathogenic E. coli strains.
Porcine pathogenic E. coli strains were isolated from diseased piglets. Out of 277 isolates, 166 were determined to be ETEC producing one or more enterotoxins, 82 were characterized as STEC as they harbored only gene for Stx2, and 29 strains were classified as STEC/ETEC as they harbored determinants for both Shiga toxin and enterotoxins. No pathogenic strains that encoded Stx1 were found. Among ETEC strains, six different combinations of toxin production were found. Among the strains of the STEC/ETEC pathotype, five different combinations were observed. For a detailed distribution of toxin determinants, see Table S1 in the supplemental material. In addition, the distribution of serogroups among pathogenic strains was determined according to methods described previously by Salajka et al. (29) (for results, see Table S2).
Susceptibility of porcine pathogenic E. coli to bacteriocin types.
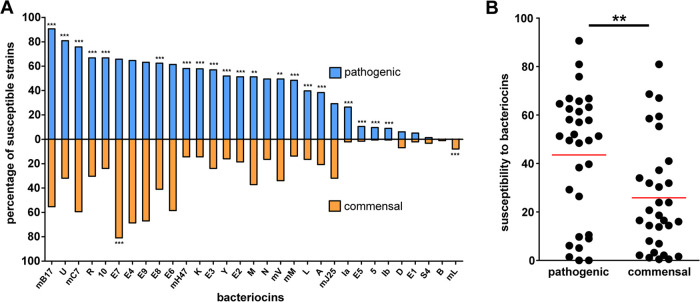
All 277 pathogenic isolates were tested for their susceptibilities to 34 bacteriocin monoproducers. Among microcins, the broadest antimicrobial activity was found for microcin B17 (which inhibited 90.6% of pathogenic isolates), followed by microcins C7 and H47, which inhibited 75.8 and 58.1% of all isolates, respectively. For colicins, the broadest antimicrobial activity was found for colicins U, R, 10, E7, E4, E9, E8, E6, E3, and K, which inhibited 57 to 80.9% of pathogenic isolates. Complete lists of bacteriocin spectra are shown in Fig. 1A and Table S3. Moreover, strains of the STEC and ETEC pathotypes differed in their susceptibilities to most of the tested bacteriocins. In general, STEC tended to be the more susceptible pathotype than ETEC (Table S4).
FIG 1.
Susceptibilities of pathogenic and commensal E. coli to bacteriocins. (A) Comparison of the percentages of susceptible porcine pathogenic and commensal strains to 34 individual bacteriocin types. Fisher’s exact test was used for statistical comparisons (**, P < 0.01; ***, P < 0.001). (B) Overall susceptibilities of porcine pathogenic and commensal E. coli strains to bacteriocin types. Each dot represents a single bacteriocin type inhibiting a certain percentage from the set of either pathogenic or commensal E. coli strains. Unpaired two-tailed Student’s t test was used for statistical comparisons (**, P < 0.01; red bars, means). Bacteriocins FY, JS, and Z were omitted from analyses as they did not inhibit any tested E. coli strain.
Comparison of susceptibilities of porcine pathogenic E. coli and porcine commensal E. coli.
To determine bacteriocin inhibition of commensal porcine microbiota, the set of 188 nontoxigenic E. coli strains isolated from healthy piglets was tested. Interestingly, the overall susceptibility to bacteriocins was found to be significantly higher in pathogenic E. coli than in commensal E. coli strains (P < 0.01) (Fig. 1B). Specifically, the antimicrobial activities of 22 bacteriocin types (colicins A, E2, E3, E5, E8, Ia, Ib, K, L, M, N, R, U, Y, 5, and 10 and microcins B17, C7, H47, M, and V) were found to be significantly higher against the set of pathogenic strains. Inversely, the activities of 2 bacteriocins, colicin E7 and microcin L, were significantly lower against pathogenic isolates (Fig. 1A).
Selection of three probiotic candidates.
Based on the results of the inhibition spectra of the different bacteriocin types against pathogenic and commensal E. coli strains, a large set of previously characterized commensal E. coli strains of human origin with known bacteriocinogeny (24, 25) was searched for a suitable combination of bacteriocin production with special emphasis on multiproducers of effective bacteriocins. Selection was also performed with regard to strain phylogroup and a limited number of encoded virulence factors. From this set, the two most suitable candidates were selected (B771 and B1172) and reanalyzed for the presence of bacteriocin and virulence determinants and phylogenetic group (Table 1). The third candidate (582) was selected from the laboratory stock.
TABLE 1.
Characteristics of potentially probiotic and pathogenic strains selected for further testing
| Strain | Phylogenetic group | Bacteriocinogeny | Virulence factors |
|---|---|---|---|
| Probiotic candidates | |||
| 582 | A | E1, B, M, mV | fimA, pap |
| B771 | A | Ia, Y | fimA, pap, aer |
| B1172 | B2 | E1, K, mB17 | fimA, pap, aer |
| Pathogenic strains | |||
| 1739 | A | Ib | F18, STa, LT, α-hly |
| 12919 | A | F4, STb, LT | |
Inhibition activity of selected probiotic candidates in liquid culture.
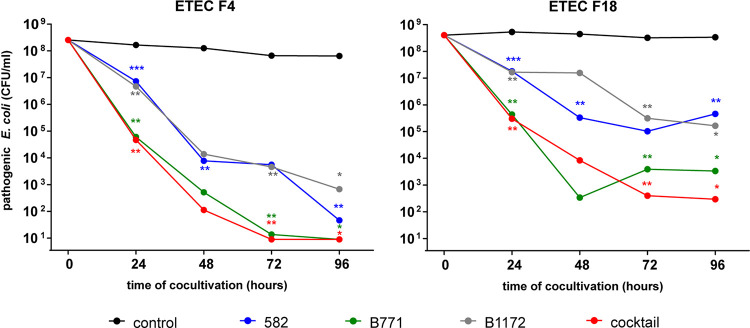
The inhibitory activities of the selected probiotic candidates against pathogenic E. coli isolates were tested by cocultivation of potentially probiotic strains with two different ETEC strains isolated from piglets with acute diarrhea, ETEC 12919 and ETEC 1739, encoding fimbriae F4 and F18 (Table 1), respectively, reflecting the fact that E. coli F4 and F18 are most commonly found to cause postweaning diarrhea in piglets (30). After 24 h of cocultivation, every probiotic candidate decreased pathogen numbers by at least 1 order of magnitude (Fig. 2). By the end of cocultivation, ETEC 12919 (F4) was almost eliminated by every single probiotic candidate. In the case of ETEC 1739 (F18), which seemed to be more resistant to the probiotic effect, numbers decreased by at least 2 orders of magnitude by the end of the experiment (∼99% of pathogens were killed). Among the tested potentially probiotic strains, the best activity was found for strain B771 and the cocktail of all three potentially probiotic strains (Fig. 2).
FIG 2.
In vitro activity of potentially probiotic strains against two pathogenic E. coli strains. Each probiotic candidate and a cocktail of all probiotic candidates together were cocultivated with two pathogenic E. coli (ETEC 12919 [F4] and ETEC 1739 [F18]) strains, respectively. Pathogen numbers were quantified every 24 h for 4 days and plotted (means). The control curve (black) represents the average number of pathogens cultivated without the addition of any probiotic candidate. Colored curves represent average numbers of pathogenic E. coli bacteria cocultivated with one of three probiotic candidates (582, B771, and B1172) or a mixture of them (cocktail). Data represent average numbers obtained from six independent experiments. Two-tailed Student’s t test was used for statistical comparison of the control sample with others (*, P < 0.05; **, P < 0.01; ***, P < 0.001). The detection limit of the method was 9 CFU/ml.
Experimental infection of piglets and effect of probiotic candidates.
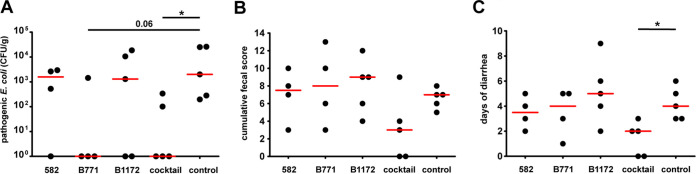
A group of 25 weaned piglets was randomly divided into 5 subgroups with 5 piglets in every group. All piglets were inoculated (day 2) with 106 CFU of a mixture of two ETEC strains, 1739 (F18) and 12919 (F4). For their characteristics, see Table 1 (the application of E. coli F4 and F18 in experimental piglets was reviewed in reference 31). Four groups of piglets were also inoculated with a dose of a single probiotic candidate (582, B771, or B1172) or a combination of all three candidates (cocktail) on the day prior to experimental infection (day 1) (109 CFU per dose) (see Materials and Methods) and then every 24 h. One group served as a control and therefore remained untreated with probiotic candidates. The treatment of piglets with the cocktail of probiotic candidates resulted in a significant reduction of pathogen counts by the last day of experimentation in comparison to the untreated control (P < 0.05) (Fig. 3A). Moreover, in this group, the cumulative fecal score showed a decreasing trend in comparison to the controls, but the results were not found to be significantly different (Fig. 3B). The average number of days that piglets in groups suffered from any type of diarrhea is shown in Fig. 3C. The piglets in a group treated with the cocktail of probiotic candidates showed a significant decrease of the period of diarrhea in comparison to the controls (P < 0.05). Moreover, only the group treated with the cocktail of probiotic candidates had any piglets that did not suffer from any type of diarrhea during the entire experiment (n = 2). According to the microbiota analysis of piglets, by the end of the experiment, no significant differences in bacterial diversity between treated groups and untreated groups were found (Fig. S1). However, probiotic treatment with the cocktail of probiotic candidates resulted in improved clinical conditions of piglets (Fig. 3), specifically, lower numbers of pathogens shed by the end of the experiment (P < 0.05), a trend toward decreased fecal scores, and shorter periods of pathogen shedding (P < 0.05).
FIG 3.
Probiotic activity of bacteriocinogenic E. coli during experimental infection in piglets. Piglets were randomly divided into 5 groups (n = 5 for each group) and inoculated with the probiotic E. coli candidate and pathogenic E. coli (see Materials and Methods). The probiotic dose was administered 24 h before pathogen challenge and then on a daily basis. Piglet fecal samples were collected daily for 14 days. Two piglets died during the experiment (in the groups treated with probiotic candidates 582 and B771) and therefore were omitted from further analyses. (A) CFU of pathogens on the last day of the experiment (day 14) for each experimental piglet. The detection limit of the method was 9 CFU/ml. (B) Cumulative fecal score (0, formed nondiarrheic feces; 1, mild diarrhea with nonformed feces; 2, severe diarrhea; 3, watery diarrhea; 4, diarrhea with blood) for the duration of the experiment for every piglet. (C) Number of days that piglets suffered from any type of diarrhea (i.e., fecal score of 1 to 4). A two-tailed Mann-Whitney U test was used for statistical comparisons (red bars, medians) (numbers above black lines represent nearly significant P values [*, P < 0.05]; every single dot represents one experimental piglet).
DISCUSSION
This study was designed to test the probiotic potential of bacteriocinogenic E. coli strains against ETEC and STEC strains causing enteric infections in pigs. Although pigs are not considered to be an important reservoir of zoonotic E. coli due to the requirement for species-specific binding mediated by the fimbrial adhesins (32), in some cases, pigs can serve as a possible reservoir of STEC that is potentially transmissible to humans. Pigs were found to shed STEC serotypes that were found to be associated with human illnesses (33), and several outbreaks were linked to the consumption of pork (4).
The porcine pathogens tested in this study were sampled from more than 50 farms in different districts of the Czech Republic over a period covering almost 2 decades (1996 to 2016). It is therefore expected that the isolated porcine pathogens are representative ETEC and STEC strains in the Czech Republic and perhaps also in other countries in central Europe. Similarly, the set of control strains comprised representative commensal E. coli strains isolated from 188 individual healthy piglets from 10 different farms in the Czech Republic.
Interestingly, the bacteriocin activity against pathogens was found to be broader than the activity against commensal strains. The broadest activity was found for microcin B17 and colicin U, which inhibited 90.6 and 80.9% of all tested pathogens, respectively. Several studies have already shown bacteriocin inhibitory effects on ETEC strains, including the activities of colicin E1 and colicin N (34) and also the activities of imperfectly determined colicin types S4/N and B/D (26), which did not show good activity during in vitro testing in this study. Although the ultimate reason for the increased susceptibility of pathogenic strains to bacteriocins is as yet unclear, it could reflect the fact that receptors for bacteriocins are often virulence factors or other factors contributing to bacterial colonization of the host (15, 35–38). The selected targeting of virulence factors or even their domains important for bacterial virulence has already been demonstrated for colicins U and Y (38), potentially limiting the emergence of fully pathogenic but bacteriocin-resistant strains. Only colicin E7 and microcin L were found to have broader activity against commensals than pathogenic strains, which makes their production unwanted for probiotic strains. Despite this fact, colicin E7 was tested and considered a viable candidate in other studies (39–41).
In general, great differences in the percentages of inhibited strains for different bacteriocin types were found, indicating that there are substantial differences in the frequencies of functional bacteriocin receptors and/or translocation proteins able to mediate bacteriocin activity. In addition to bacteriocin binding and translocation, susceptibility to a given bacteriocin is also influenced by the presence of producers of the same bacteriocin type also harboring immunity to the bacteriocin produced (using the production of either an immunity protein, an efflux pump, or both). Accordingly, the highest susceptibility of pathogenic strains was found for the least frequent bacteriocin types, e.g., for microcin B17, which is rarely produced by tested pathogenic strains (0.75%) (25).
The three human commensal E. coli strains that were chosen for this study were selected due to their bacteriocin-producing spectra targeting inhibition toward the pathogenic strains. Moreover, bacteriocin types with relatively limited effects on commensal strains were considered. All three potentially probiotic strains were previously isolated from human fecal samples and were previously characterized (24, 62, 63). The best inhibition activity, both in vitro and in vivo, was observed in strain B771, producing colicins Y and Ia, and in the cocktail of all three strains producing 8 different bacteriocin types, including the highly efficient microcins B17 and V and colicins K and Y (a close homolog of colicin U) (38, 42–45). While strains 582 and B771 belong to phylogenetic group A, which is the most abundant phylogroup among pig isolates (25, 46), this phylogroup is less frequent among humans (24, 47). In contrast, strain B1172 belonged to phylogroup B2, which is the most abundant phylogroup among human E. coli strains (24, 47), supporting its possible use in human applications. In general, the phylogroup classification (with respect to the particular host) increases the probability that probiotic strains will be able to colonize the same niche as the pathogen (46, 48). Consistent with this, the B1172 strain appeared to be the least active probiotic candidate during in vivo testing in pigs (Fig. 3), while its activity during in vitro testing was comparable to those of the other probiotic candidates tested (Fig. 2). In contrast to the other two strains, the B1172 strain promises higher activity in humans. All three probiotic candidates do not harbor any gene for the tested toxins (STa, STb, LT, Shiga toxins, and hemolysins) but harbor genes for type 1 and type P fimbriae, and strains B771 and B1172 also harbor a gene involved in aerobactin synthesis. While all these virulence factors may provide an advantage during gut colonization and are quite common among human commensal strains (47), the absence of other tested virulence factors likely ensures a low pathogenicity of these probiotic candidates.
The selected probiotic candidates were tested under in vivo conditions in weaned piglets to clearly demonstrate their probiotic effect. The probiotic activity is quite complex and includes not only bacteriocin production and activity but also attachment to enterocytes and other factors that have an impact on E. coli fitness and gut colonization capacity (49, 50). Similar to our experimental design, Schroeder et al. (20) tested if pretreatment with the probiotic E. coli Nissle 1917 has an effect on diarrhea in piglets caused by ETEC Abbotstown. As in our study, pretreatment with probiotic E. coli was able to decrease clinical signs of diarrhea. Thus, pretreatment and treatment of piglets with probiotic E. coli strains in their postweaning period could be an effective method to prevent pathogenic E. coli from causing diarrhea through enterotoxin synthesis. Furthermore, Cutler et al. (51) showed that the addition of colicin E1 to the diet of piglets decreases the incidence and severity of experimental postweaning diarrhea caused by ETEC. In the work of Setia et al. (26), the authors selected two probiotic candidates inhibiting an important veterinary pathogen, E. coli F4. Two E. coli strains (UM-2 and UM-7) produced bacteriocins and utilized starch and inulin (present in pigs’ feed). The combined application of the probiotic candidates and prebiotics (potato starch) resulted in better growth performance of piglets, reduced diarrhea, and also increased microbial diversity (21). For experimental infection of piglets by pathogenic E. coli, ETEC strains were chosen since infection by this pathotype (postweaning diarrhea) occurs more often, experimental infection is easily inducible, and clinical symptoms can be easily measured in comparison to the edema disease caused by STEC (52). However, we assume that the chosen probiotic candidates would have beneficial effects even during infection by STEC since these strains were, in general, more susceptible than ETEC strains under in vitro conditions (see Table S4 in the supplemental material).
The application of potentially probiotic strains did not result in significant microbiota changes, although there were detectable differences between probiotic-treated and probiotic-untreated pigs (Fig. S1). The application of the cocktail resulted in the reduction of some taxa in the treated (cocktail) group (Bacteroidales RF16 group, Streptococcus, and Terrisporobacter), while the abundance of others was increased (Lachnospiraceae Eubacterium hallii group and Mogibacterium). Similar to our findings, Wang et al. (53) also detected decreased abundances of Terrisporobacter and increased abundances of Lachnospiraceae in piglets treated with a Lactobacillus plantarum probiotic. The lower abundance of Terrisporobacter may be considered beneficial given that members of the genus Terrisporobacter have been associated with infection in humans (54). The genus Mogibacterium was previously considered a potential biomarker of swine dysentery (55); however, it was also positively correlated with the average daily weight gain of piglets (56). Unlike in other studies (21, 57–59), the microbial diversity in our study was not significantly affected by the administration of a cocktail of probiotic candidates.
Most importantly, the cocktail of probiotic candidates showed the best performance compared to individual probiotic candidates, suggesting an unexpected synergy between the chosen strains. Only the cocktail containing all three probiotic candidates was found to significantly decrease the period of diarrhea in experimental piglets from 4 to 2 days (Fig. 3C) and to also lower the fecal score. The use of several different probiotic strains in a single probiotic preparation thus could result in an increased beneficial effect on the host, likely as a result of complementary antibacterial action and/or a decreased risk of antibiotic resistance to multiple bacteriocins (15). Similarly, Khafipour et al. (28) found that fecal scores were slightly lower (although nonsignificantly) in piglets treated with a cocktail of two potentially probiotic E. coli strains (UM-2 and UM-7), while the quantity of ETEC was found to be significantly smaller in piglets treated with a cocktail than in an untreated control. Moreover, Budič et al. (40) found a bacteriocin cocktail to be effective in overcoming bacteriocin resistance to a single bacteriocin.
Taken together, three potentially probiotic E. coli strains of human origin were shown to exert probiotic effects on pathogenic ETEC of porcine origin under both in vitro and in vivo conditions. Following the administration of these probiotic E. coli candidates, piglet diarrhea caused by ETEC was milder, and the shedding of the pathogen ended faster than in the untreated controls. While the extents of the probiotic effect were different for each strain, the cocktail of all three strains showed the most pronounced beneficial effects, suggesting synergy between the tested probiotic E. coli candidates. This study, based on extensive in vitro testing, promises the selection of suitable probiotic candidates with potential activity against vast spectra of pathogenic E. coli strains.
MATERIALS AND METHODS
Bacterial strains and cultivation conditions.
A set of 277 toxigenic E. coli strains isolated from diseased piglets (i.e., diarrheic piglets) from more than 50 farms in the Czech Republic came from the strain collection of the National Reference Laboratory for E. coli (Veterinary Research Institute, Brno, Czech Republic). Strains were collected over 10 years between 1996 and 2016 and were previously characterized (25). Two pathogenic strains with chloramphenicol resistance were selected from the laboratory strain collection for in vitro and in vivo experiments based on detected virulence determinants. ETEC strain 12919 of serotype O149 was hemolytic and encoded type F4 fimbriae and LT and STb enterotoxins. ETEC strain 1739 was hemolytic and of serotype O149, harboring type F18 fimbriae and producing enterotoxins LT and STa. Both strains were reanalyzed with respect to bacteriocin production, phylogenetic group identification, and the presence of virulence determinants typical for E. coli, as described in detail previously (25, 60).
A set of 188 commensal E. coli strains was isolated in 2018 from 188 individual healthy piglets from 10 different farms in the Czech Republic. Fecal samples were collected from the rectum of individual healthy piglets using sterile swabs. Endo agar (Hi-Media, Mumbai, India) was used for the selection of an E. coli-like colony from every swab. Colonies were subsequently determined to be E. coli using COLItest and ENTEROtest 16 (Erba Lachema, Brno, Czech Republic). All strains tested negative for the presence of determinants for enterotoxins (STa, STb, and LT) (61) and Shiga toxins (stx1 and stx2) (25) using PCR.
Probiotic E. coli candidates B771 and B1172 were selected from the set of bacteriocinogenic E. coli strains of human origin (n = 695) deposited in our laboratory stock and described previously (24, 25). Both strains were reanalyzed with respect to bacteriocin production, phylogenetic group identification, and the presence of virulence determinants typical for E. coli, as described in detail previously (25). Besides this well-characterized set of human commensals producing bacteriocins, E. coli 582 (previously strain 18) (62, 63) of human origin was selected from our laboratory stock based on the previously indicated multiproduction of bacteriocins. This strain was subsequently analyzed for the presence of bacteriocin determinants, phylogenetic group, and the presence of virulence determinants according to methods described previously by Micenková et al. (24) and, based on these results, was used as a third probiotic candidate.
A set of bacteriocin monoproducers was used for testing E. coli susceptibility to each bacteriocin. The following set of producers was described previously (24, 26) and was used with a few modifications: E. coli BZB2101/pColA-CA31 (A. P. Pugsley), BZB2102 (516)/pColB (A. P. Pugsley), BZB2103/pColD-CA23 (A. P. Pugsley), B1876/pColE1 (D. Šmajs), 189BM/pColE2-P9 (B. A. D. Stocker), 185M4/pColE3-CA38 (P. Fredericq), BZB2107/pColE4 (A. P. Pugsley), BZB2108/pColE5-099 (A. P. Pugsley), BZB2150/pColE6-CT14 (A. P. Pugsley), 245BM/pColE7 (V. Horák), W3110/pColE8 (J. R. James), and W3110/pColE9 (J. R. James); Yersinia frederiksenii Y27601/pColFY (J. Bosák); E. coli BZB2279/pColIa-CA53 (A. P. Pugsley) and B1056/ColIb (D. Šmajs); Shigella sonnei (colicinotype 7)/pColJs (J. Šmarda); E. coli 361/79/pColK (J. Šmarda and P. Fredericq); Serratia marcescens JF246 and 29/80/pColL (J. Šmarda) (64); E. coli PAP1/pColM-BZBNC22 (A. P. Pugsley), BZB2123/pColN-284 (A. P. Pugsley), ROAR029/pColR (O. Rendueles), and K-12/pColS4 (D. Šmajs); Shigella boydii M592 (serovar 8)/pColU (V. Horák); E. coli K339/pColY (D. Friedman); E. coli B1356/pColZ (D. Šmajs), pCol5 and pCol10 (H. Pilsl), and BZB2283 (527) (mccB17) (A. P. Pugsley); and E. coli TOP10F′/pDS601 (mccC7) (D. Šmajs), B642 (mccH47) (D. Šmajs), HKII BP52C (757) (mccJ25) (D. Šmajs), B2201 (mccL) (D. Šmajs), B2472 (mccM) (D. Šmajs), and 1.7a (525) (mccV) (J. Šmarda and P. Fredericq).
Bacterial strains were cultivated using tryptone-yeast (TY) broth and agar. For the selection of hemolytic pathogenic strains, blood agar was used. TY broth was prepared by mixing 8 g/liter tryptone (Hi-Media), 5 g/liter yeast extract (Hi-Media), and 5 g/liter sodium chloride (Penta, Prague, Czech Republic) in water. The same composition was used for TY agar additionally supplemented with agar powder (1.2% and 0.66%; Hi-Media). Blood agar plates were provided by the University Hospital Brno. For the selection of pathogenic strains, media with the addition of chloramphenicol (25 mg/liter) (Sigma-Aldrich, Germany) were used.
PCR detection of virulence factors in pathogenic E. coli.
The detection of variants of enterotoxin (STa and STb) was performed using colony PCR. A bacterial colony resuspended in 200 μl of sterile water was used as a template for multiplex PCR. Primers were STa1 (5′-TTTCTGTATTATCTTTCCCC-3′), STa2 (5′-ATTACAACAAAGTTCACAGC-3′), STb1 (5′-TCTTCTTGCATCTATGTTCG-3′), and STb2 (5′-TCTCTAACCCCTAAAAAACC-3′) (amplicon lengths of 168 nucleotides [nt] and 138 nt, respectively) and were previously reported by Alexa et al. (65). PCR thermal cycling conditions were as follows: an initial cycle of 5 min at 94°C was followed by 30 cycles with alternating temperatures of 92°C, 48°C, and 72°C (45 s each) and a final extension step for 10 min at 72°C. Determinants for thermolabile enterotoxin and Shiga toxins were previously determined by Bosák et al. (25).
For two ETEC strains used in in vivo and in vitro testing (12919 and 1739), the fimbrial type was determined by colony PCR. Primers were F4-F (5′-TGAATGACCTGACCAATGGTGGAACC-3′), F4-R (5′-GCGTTTACTCTTTGAATCTGTCCGAG-3′), F18-F (5′-TGGCACTGTAGGAGATACCATTCAGC-3′), and F18-R (5′-ACTTACAGTGCTATTCGACGCCTTAA-3′) (amplicon lengths of 478 nt and 268 nt, respectively) and were previously reported by Zajacova et al. (61).
Susceptibilities of pathogenic and commensal strains to bacteriocins.
In order to investigate the susceptibilities of E. coli strains to individual bacteriocins, monoproducers of bacteriocins were stabbed into TY agar (1.2%) and cultivated for 24 h at 37°C as described previously (24, 66). Next, these bacteriocin monoproducers were killed by chloroform vapors (except for those producing microcins H47 and M, which are chloroform sensitive [67]) and overlaid with 3.5 ml of TY agar (0.66%) supplemented with 100 μl of a culture of the pathogenic/commensal strain cultivated in TY broth overnight. Petri dishes were then cultivated overnight at 37°C. The formation of a zone of inhibited growth around a monoproducer stab of at least 1.5 mm was considered indicative of susceptibility to a particular bacteriocin type.
Cocultivation of probiotic candidates with pathogenic strains in vitro.
Potentially probiotic strains were cultivated in 5 ml of TY broth overnight at 37°C with shaking at 200 rpm. Pathogenic strains (ETEC 12919 and 1739) were cultivated in the same way. After cultivation, 100 μl of potentially probiotic (or, in the case of the cocktail, 33 μl of each probiotic candidate) and 100 μl of pathogenic strain cultures were transferred into 5 ml sterile TY broth and cocultivated for 24 h at 37°C at 200 rpm. A 24-h culture was used since higher levels of production of several colicin (68, 69) and microcin (70) types were previously described in stationary phase. As a negative control, 100 μl of the pathogen without any probiotic candidate addition was cultivated. Every 24 h, 100 μl of the cocultivation culture was transferred into 5 ml of new sterile TY broth and cultivated. The culture samples were serially diluted 10-fold (100 to 10−8), spread on TY agar plates (1.2%) with chloramphenicol, and cultivated at 37°C. The next day, chloramphenicol-resistant colonies, representing pathogens, were counted, and numbers of pathogens (CFU per milliliter) were determined for each. This process was repeated for 4 days.
Analysis of probiotic activity under in vivo conditions.
Animal experiments and handling of animals were performed by licensed staff at an accredited facility of the Veterinary Research Institute. Twenty-five piglets (Sus scrofa domesticus Improved White) were obtained from the Bioproduct Knapovec a.s. farm (Ústí nad Orlicí, Czech Republic), where piglets are raised for the meat industry. Piglets were weaned on their 28th day of life and subsequently transported to the Veterinary Research Institute, where they were randomly distributed into 5 groups (n = 5). Each group was kept in an individual pen in a separate room, without the possibility of transmission of fecal bacteria between pens. Piglets had access to water and feed ad libitum. Before experimental infection, rectal swabs were taken for the exclusion of the presence of ETEC infection using PCR. As chloramphenicol resistance was chosen as a marker for the selection of experimental pathogens, the swabs were also used for the exclusion of the presence of chloramphenicol-resistant hemolytic bacteria by spreading swabs on blood agar with chloramphenicol.
For the preparation of inocula for piglets, probiotic E. coli candidates (582, B771, and B1172) were cultivated in 200 ml of TY broth for 16 h at 37°C. The optical density (OD) value was measured, a volume containing 109 CFU was centrifuged (3,000 relative centrifugal force [RCF] for 20 min at 4°C), the supernatant was removed, and the pellet was resuspended in 1 ml of sterile phosphate-buffered saline (PBS) (Hi-Media). Next, 333 μl of 40% sterile glycerol (Sigma-Aldrich, Germany) was added, and bacterial strains were frozen at −80°C until use. Before inoculation, strains were thawed and centrifuged (3,000 RCF for 20 min at 4°C), the supernatant was removed, and the pellet was resuspended in 5 ml of sterile PBS. In the case of the cocktail inoculum, equal concentrations of the three probiotic candidates were mixed to obtain a final concentration of 109 CFU. For the preparation of pathogenic inocula, pathogenic strains (ETEC 12919 and 1739) were freshly cultivated according to methods described previously by Salajka et al. (29). After cultivation, both pathogenic strains were mixed into a final volume of 4 ml to a final concentration of 106 CFU/ml, and this suspension was used for every single piglet inoculation.
The in vivo experiment was performed as follows. Twenty-five piglets were randomly divided into five groups with five piglets in each group. During the whole experiment (i.e., 14 days), four groups of piglets were inoculated with a dose of potentially probiotic bacteria daily (three groups with one of the three probiotic candidates and the fourth group with the mixture of the three probiotic candidates [cocktail]) by the oral application of 5 ml of the probiotic suspension (109 CFU/ml) to each piglet. Piglets in the fifth group represent controls of untreated ETEC infection. One hour after the second dose of potentially probiotic bacteria, all piglets were challenged with a mixture of two pathogenic E. coli strains (106 CFU/ml; strains ETEC 12919 and ETEC 1739), including the control group. Fecal samples were collected daily from the recta, and the fecal score was evaluated by a veterinarian (0, formed nondiarrheic feces; 1, mild diarrhea with nonformed feces [pasty]; 2, severe diarrhea [mushy]; 3, watery diarrhea; 4, diarrhea with blood) (71). On the last day of the experiment, piglets were euthanized. Piglets were also weighed before and after the experiment, but no significant changes between treated groups and untreated controls were observed.
Piglet feces, collected daily, were weighed and resuspended in 2 ml of sterile PBS. Tubes with fecal samples were briefly centrifuged to remove large particles (500 RCF for 1 min), and the supernatant was collected. The supernatant was diluted by serial dilutions (100 to 10−8) in PBS and inoculated on blood agar with chloramphenicol. Plates were cultivated aerobically overnight at 37°C. Chloramphenicol-resistant colonies with hemolysis, representing experimental infection, were counted, and the CFU of each pathogen per gram of fecal sample were determined for each fecal sample.
Microbiota analysis.
On the final day of the in vivo experiment, fecal samples were collected from each rectum using a sterile swab. Swabs were then stored at −20°C until analysis. DNA isolation was performed using a DNeasy PowerLyzer PowerSoil kit (Qiagen, Germany) according to the manufacturer’s protocol. Isolated DNA was used as a template in PCRs targeting the hypervariable V4 region of the bacterial 16S rRNA gene according to the 16S metagenomic sequencing library preparation protocol (Illumina, San Diego, CA). Sequencing was performed using MiSeq reagent kits v2 on a MiSeq 2000 sequencer according to the manufacturer’s instructions (Illumina, USA).
Sequence preprocessing included quality trimming using the Trimmomatic sequence tool (72), subsequent joining by the fastq-join utility (73), and demultiplexing. In the final step, joined reads were subjected to operational taxonomic unit (OTU) picking with a 97% sequence similarity threshold. OTU picking was performed using the uclust tool (74). OTU picking was followed by taxonomy assignment using the usearch-based (74) method of the QIIME 1.9.1 (75) toolset against the Silva v123 16S rRNA database (76).
The alpha diversity of metagenomic samples was calculated from OTU tables with abundances of individual taxonomic groups using Chao, Shannon, and Simpson diversity metrics. Beta diversity was calculated using UniFrac distance metrics. Mann-Whitney U tests and t tests were performed using the wilcox.test and pairwise.t.test functions in the R (77) stats package (78). The analysis of similarity (ANOSIM) permutation test was carried out using the vegan R package (79). Data were visualized using the R package ggplot2 (80).
Statistical analyses.
Prism 5 software (GraphPad) was used for statistical analyses. Fisher’s exact test was used for statistical comparisons of susceptibilities of pathogens and commensals. Susceptibility to bacteriocins and bacterial growth during in vitro experiments were analyzed using unpaired two-tailed Student’s t test. The nonparametric Mann-Whitney U test was used to analyze bacterial CFU in feces, average fecal scores, and duration of diarrhea. P values of less than 0.05 were considered statistically significant and are denoted with asterisks in the figures (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Ethical approval.
The experimental protocol was approved by the Branch Commission for Animal Welfare of the Ministry of Agriculture of the Czech Republic (permission no. MZe 1876) in accordance with act no. 246/1992 Coll. on the protection of animals against torture, as subsequently amended, and with decree 419/2012 Coll. on the protection, breeding, and use of experimental animals (https://en.svscr.cz/wp-content/files/publications/ib1304a.pdf).
ACKNOWLEDGMENTS
This work was supported by funds from the Faculty of Medicine MU to junior researcher J.B. (ROZV/23/LF13/2019 and ROZV/28/LF/2020) and from the Ministry of Education, Youth, and Sports (LM2018121, CZ.02.1.01/0.0/0.0/18_046/0015975, and CZ.02.1.01/0.0/0.0/16_013/0001761). I.K. and Z.T. were supported by the project RVO0518 from the Ministry of Agriculture of the Czech Republic.
Footnotes
Supplemental material is available online only.
Contributor Information
David Šmajs, Email: dsmajs@med.muni.cz.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Rhouma M, Fairbrother JM, Beaudry F, Letellier A. 2017. Post weaning diarrhea in pigs: risk factors and non-colistin-based control strategies. Acta Vet Scand 59:31. 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luppi A, Gibellini M, Gin T, Vangroenweghe F, Vandenbroucke V, Bauerfeind R, Bonilauri P, Labarque G, Hidalgo Á. 2016. Prevalence of virulence factors in enterotoxigenic Escherichia coli isolated from pigs with post-weaning diarrhoea in Europe. Porcine Health Manag 2:20. 10.1186/s40813-016-0039-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fairbrother JM, Nadeau É, Gyles CL. 2005. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev 6:17–39. 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 4.Tseng M, Fratamico PM, Manning SD, Funk JA. 2014. Shiga toxin-producing Escherichia coli in swine: the public health perspective. Anim Health Res Rev 15:63–75. 10.1017/S1466252313000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luppi A. 2017. Swine enteric colibacillosis: diagnosis, therapy and antimicrobial resistance. Porcine Health Manag 3:16. 10.1186/s40813-017-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun Y, Kim SW. 2017. Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim Nutr 3:322–330. 10.1016/j.aninu.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matise I, Sirinarumitr T, Bosworth BT, Moon HW. 2000. Vascular ultrastructure and DNA fragmentation in swine infected with Shiga toxin-producing Escherichia coli. Vet Pathol 37:318–327. 10.1354/vp.37-4-318. [DOI] [PubMed] [Google Scholar]
- 8.Casanova NA, Redondo LM, Dailoff GC, Arenas D, Fernández Miyakawa ME. 2018. Overview of the role of Shiga toxins in porcine edema disease pathogenesis. Toxicon 148:149–154. 10.1016/j.toxicon.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 9.Bourgeois AL, Wierzba TF, Walker RI. 2016. Status of vaccine research and development for enterotoxigenic Escherichia coli. Vaccine 34:2880–2886. 10.1016/j.vaccine.2016.02.076. [DOI] [PubMed] [Google Scholar]
- 10.Majowicz SE, Scallan E, Jones-Bitton A, Sargeant JM, Stapleton J, Angulo FJ, Yeung DH, Kirk MD. 2014. Global incidence of human Shiga toxin-producing Escherichia coli infections and deaths: a systematic review and knowledge synthesis. Foodborne Pathog Dis 11:447–455. 10.1089/fpd.2013.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Newell DG, La Ragione RM. 2018. Enterohaemorrhagic and other Shiga toxin-producing Escherichia coli (STEC): where are we now regarding diagnostics and control strategies? Transbound Emerg Dis 65:49–71. 10.1111/tbed.12789. [DOI] [PubMed] [Google Scholar]
- 12.Marshall BM, Levy SB. 2011. Food animals and antimicrobials: impacts on human health. Clin Microbiol Rev 24:718–733. 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maron DF, Smith TJS, Nachman KE. 2013. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Global Health 9:48. 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EMA Committee for Medicinal Products for Veterinary Use, EFSA Panel on Biological Hazards, Murphy D, Ricci A, Auce Z, Beechinor JG, Bergendahl H, Breathnach R, Bureš J, Duarte Da Silva JP, Hederová J, Hekman P, Ibrahim C, Kozhuharov E, Kulcsár G, Lander Persson E, Lenhardsson JM, Mačiulskis P, Malemis I, Markus‐Cizelj L, Michaelidou-Patsia A, Nevalainen M, Pasquali P, Rouby J-C, Schefferlie J, Schlumbohm W, Schmit M, Spiteri S, Srčič S, Taban L, Tiirats T, Urbain B, Vestergaard E, Wachnik-Święcicka A, Weeks J, Zemann B, Allende A, Bolton D, Chemaly M, Fernandez Escamez PS, Girones R, Herman L, Koutsoumanis K, Lindqvist R, Nørrung B, Robertson L, Ru G, Sanaa M, Simmons M, Skandamis P, Snary E, Speybroeck N, et al. 2017. EMA and EFSA joint scientific opinion on measures to reduce the need to use antimicrobial agents in animal husbandry in the European Union, and the resulting impacts on food safety (RONAFA). EFSA J 15:e04666. 10.2903/j.efsa.2017.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosák J, Hrala M, Micenková L, Šmajs D. 2021. Non-antibiotic antibacterial peptides and proteins of Escherichia coli: efficacy and potency of bacteriocins. Expert Rev Anti Infect Ther 19:309–322. 10.1080/14787210.2020.1816824. [DOI] [PubMed] [Google Scholar]
- 16.Gillor O, Kirkup BC, Riley MA. 2004. Colicins and microcins: the next generation antimicrobials. Adv Appl Microbiol 54:129–146. 10.1016/S0065-2164(04)54005-4. [DOI] [PubMed] [Google Scholar]
- 17.Meade E, Slattery MA, Garvey M. 2020. Bacteriocins, potent antimicrobial peptides and the fight against multi drug resistant species: resistance is futile? Antibiotics (Basel) 9:32. 10.3390/antibiotics9010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quigley EMM. 2019. Prebiotics and probiotics in digestive health. Clin Gastroenterol Hepatol 17:333–344. 10.1016/j.cgh.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Collado M, Isolauri E, Salminen S, Sanz Y. 2009. The impact of probiotic on gut health. Curr Drug Metab 10:68–78. 10.2174/138920009787048437. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder B, Duncker S, Barth S, Bauerfeind R, Gruber AD, Deppenmeier S, Breves G. 2006. Preventive effects of the probiotic Escherichia coli strain Nissle 1917 on acute secretory diarrhea in a pig model of intestinal infection. Dig Dis Sci 51:724–731. 10.1007/s10620-006-3198-8. [DOI] [PubMed] [Google Scholar]
- 21.Krause DO, Bhandari SK, House JD, Nyachoti CM. 2010. Response of nursery pigs to a synbiotic preparation of starch and an anti-Escherichia coli K88 probiotic. Appl Environ Microbiol 76:8192–8200. 10.1128/AEM.01427-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, Postle K, Riley M, Slatin S, Cavard D. 2007. Colicin biology. Microbiol Mol Biol Rev 71:158–229. 10.1128/MMBR.00036-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S. 2007. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep 24:708–734. 10.1039/b516237h. [DOI] [PubMed] [Google Scholar]
- 24.Micenková L, Bosák J, Štaudová B, Kohoutová D, Čejková D, Woznicová V, Vrba M, Ševčíková A, Bureš J, Šmajs D. 2016. Microcin determinants are associated with B2 phylogroup of human fecal Escherichia coli isolates. Microbiologyopen 5:490–498. 10.1002/mbo3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosák J, Hrala M, Pirková V, Micenková L, Čížek A, Smola J, Kučerová D, Vacková Z, Budinská E, Koláčková I, Šmajs D. 2019. Porcine pathogenic Escherichia coli strains differ from human fecal strains in occurrence of bacteriocin types. Vet Microbiol 232:121–127. 10.1016/j.vetmic.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Setia A, Bhandari SK, House JD, Nyachoti CM, Krause DO. 2009. Development and in vitro evaluation of an Escherichia coli probiotic able to inhibit the growth of pathogenic Escherichia coli K88. J Anim Sci 87:2005–2012. 10.2527/jas.2008-1400. [DOI] [PubMed] [Google Scholar]
- 27.Bhandari SK, Opapeju FO, Krause DO, Nyachoti CM. 2010. Dietary protein level and probiotic supplementation effects on piglet response to Escherichia coli K88 challenge: performance and gut microbial population. Livest Sci 133:185–188. 10.1016/j.livsci.2010.06.060. [DOI] [Google Scholar]
- 28.Khafipour E, Munyaka PM, Nyachoti CM, Krause DO, Rodriguez-Lecompte JC. 2014. Effect of crowding stress and Escherichia coli K88+ challenge in nursery pigs supplemented with anti-Escherichia coli K88+ probiotics. J Anim Sci 92:2017–2029. 10.2527/jas.2013-7043. [DOI] [PubMed] [Google Scholar]
- 29.Salajka E, Salajkova Z, Alexa P, Hornich M. 1992. Colonization factor different from K88, K99, F41 and 987P in enterotoxigenic Escherichia coli strains isolated from postweaning diarrhoea in pigs. Vet Microbiol 32:163–175. 10.1016/0378-1135(92)90103-z. [DOI] [PubMed] [Google Scholar]
- 30.Dubreuil JD, Isaacson RE, Schifferli DM. 8 September 2016, posting date. Animal enterotoxigenic Escherichia coli. EcoSal Plus 2016 10.1128/ecosalplus.ESP-0006-2016. [DOI] [PMC free article] [PubMed]
- 31.Luise D, Lauridsen C, Bosi P, Trevisi P. 2019. Methodology and application of Escherichia coli F4 and F18 encoding infection models in post-weaning pigs. J Anim Sci Biotechnol 10:53. 10.1186/s40104-019-0352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wasteson Y. 2001. Zoonotic Escherichia coli. Acta Vet Scand Suppl 95:79–84. [PubMed] [Google Scholar]
- 33.Kaufmann M, Zweifel C, Blanco M, Blanco JE, Blanco J, Beutin L, Stephan R. 2006. Escherichia coli O157 and non-O157 Shiga toxin-producing Escherichia coli in fecal samples of finished pigs at slaughter in Switzerland. J Food Prot 69:260–266. 10.4315/0362-028x-69.2.260. [DOI] [PubMed] [Google Scholar]
- 34.Stahl CH, Callaway TR, Lincoln LM, Lonergan SM, Genovese KJ. 2004. Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob Agents Chemother 48:3119–3121. 10.1128/AAC.48.8.3119-3121.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilsl H, Šmajs D, Braun V. 1999. Characterization of colicin S4 and its receptor, OmpW, a minor protein of the Escherichia coli outer membrane. J Bacteriol 181:3578–3581. 10.1128/JB.181.11.3578-3581.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Šmajs D, Weinstock GM. 2001. The iron- and temperature-regulated cjrBC genes of Shigella and enteroinvasive Escherichia coli strains code for colicin Js uptake. J Bacteriol 183:3958–3966. 10.1128/JB.183.13.3958-3966.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Šmajs D, Weinstock GM. 2001. Genetic organization of plasmid ColJs, encoding colicin Js activity, immunity, and release genes. J Bacteriol 183:3949–3957. 10.1128/JB.183.13.3949-3957.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosák J, Micenková L, Doležalová M, Šmajs D. 2016. Colicins U and Y inhibit growth of Escherichia coli strains via recognition of conserved OmpA extracellular loop 1. Int J Med Microbiol 306:486–494. 10.1016/j.ijmm.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 39.Schamberger GP, Phillips RL, Jacobs JL, Diez-Gonzalez F. 2004. Reduction of Escherichia coli O157:H7 populations in cattle by addition of colicin E7-producing E. coli to feed. Appl Environ Microbiol 70:6053–6060. 10.1128/AEM.70.10.6053-6060.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Budič M, Rijavec M, Petkovšek Ž, Žgur-Bertok D. 2011. Escherichia coli bacteriocins: antimicrobial efficacy and prevalence among isolates from patients with bacteraemia. PLoS One 6:e28769. 10.1371/journal.pone.0028769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazurek-Popczyk J, Pisarska J, Bok E, Baldy-Chudzik K. 2020. Antibacterial activity of bacteriocinogenic commensal Escherichia coli against zoonotic strains resistant and sensitive to antibiotics. Antibiotics (Basel) 9:411–413. 10.3390/antibiotics9070411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Šmajs D, Pilsl H, Braun V. 1997. Colicin U, a novel colicin produced by Shigella boydii. J Bacteriol 179:4919–4928. 10.1128/jb.179.15.4919-4928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Riley MA, Cadavid L, Collett MS, Neely MN, Adams MD, Phillips CM, Neel JV, Friedman D. 2000. The newly characterized colicin Y provides evidence of positive selection in pore-former colicin diversification. Microbiology 146:1671–1677. 10.1099/00221287-146-7-1671. [DOI] [PubMed] [Google Scholar]
- 44.Šmajs D, Matějková P, Weinstock GM. 2006. Recognition of pore-forming colicin Y by its cognate immunity protein. FEMS Microbiol Lett 258:108–113. 10.1111/j.1574-6968.2006.00201.x. [DOI] [PubMed] [Google Scholar]
- 45.Šmajs D, Doležalová M, Macek P, Žídek L. 2008. Inactivation of colicin Y by intramembrane helix-helix interaction with its immunity protein. FEBS J 275:5325–5331. 10.1111/j.1742-4658.2008.06662.x. [DOI] [PubMed] [Google Scholar]
- 46.Carlos C, Pires MM, Stoppe NC, Hachich EM, Sato MIZ, Gomes TAT, Amaral LA, Ottoboni LMM. 2010. Escherichia coli phylogenetic group determination and its application in the identification of the major animal source of fecal contamination. BMC Microbiol 10:161. 10.1186/1471-2180-10-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micenková L, Bosák J, Vrba M, Ševčíková A, Šmajs D. 2016. Human extraintestinal pathogenic Escherichia coli strains differ in prevalence of virulence factors, phylogroups, and bacteriocin determinants. BMC Microbiol 16:218. 10.1186/s12866-016-0835-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gordon DM, Cowling A. 2003. The distribution and genetic structure of Escherichia coli in Australian vertebrates: host and geographic effects. Microbiology 149:3575–3586. 10.1099/mic.0.26486-0. [DOI] [PubMed] [Google Scholar]
- 49.Scaldaferri F, Gerardi V, Mangiola F, Lopetuso LR, Pizzoferrato M, Petito V, Papa A, Stojanovic J, Poscia A, Cammarota G, Gasbarrini A. 2016. Role and mechanisms of action of Escherichia coli Nissle 1917 in the maintenance of remission in ulcerative colitis patients: an update. World J Gastroenterol 22:5505–5511. 10.3748/wjg.v22.i24.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cordonnier C, Thévenot J, Etienne-Mesmin L, Alric M, Livrelli V, Blanquet-Diot S. 2017. Probiotic and enterohemorrhagic Escherichia coli: an effective strategy against a deadly enemy? Crit Rev Microbiol 43:116–132. 10.1080/1040841X.2016.1185602. [DOI] [PubMed] [Google Scholar]
- 51.Cutler SA, Lonergan SM, Cornick N, Johnson AK, Stahl CH. 2007. Dietary inclusion of colicin E1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob Agents Chemother 51:3830–3835. 10.1128/AAC.00360-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato T, Hamabata T, Takita E, Matsui T, Sawada K, Imaoka T, Nakanishi N, Nakayama K, Tsukahara T. 2017. Improved porcine model for Shiga toxin-producing Escherichia coli infection by deprivation of colostrum feeding in newborn piglets. Anim Sci J 88:826–831. 10.1111/asj.12769. [DOI] [PubMed] [Google Scholar]
- 53.Wang J, Ji H, Wang S, Liu H, Zhang W, Zhang D, Wang Y. 2018. Probiotic Lactobacillus plantarum promotes intestinal barrier function by strengthening the epithelium and modulating gut microbiota. Front Microbiol 9:1953. 10.3389/fmicb.2018.01953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cheng MP, Domingo MC, Lévesque S, Yansouni CP. 2016. A case report of a deep surgical site infection with Terrisporobacter glycolicus/T. mayombei and review of the literature. BMC Infect Dis 16:529. 10.1186/s12879-016-1865-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burrough ER, Arruda BL, Plummer PJ. 2017. Comparison of the luminal and mucosa-associated microbiota in the colon of pigs with and without swine dysentery. Front Vet Sci 4:139. 10.3389/fvets.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiros TG, Derakhshani H, Pinloche E, D’Inca R, Marshall J, Auclair E, Khafipour E, Van Kessel A. 2018. Effect of live yeast Saccharomyces cerevisiae (Actisaf Sc 47) supplementation on the performance and hindgut microbiota composition of weanling pigs. Sci Rep 8:5315. 10.1038/s41598-018-23373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Šmajs D, Bureš J, Šmarda J, Chaloupková E, Květina J, Förstl M, Kohoutová D, Kuneš M, Rejchrt S, Lesná J, Kopáčová M. 2012. Experimental administration of the probiotic Escherichia coli strain Nissle 1917 results in decreased diversity of E. coli strains in pigs. Curr Microbiol 64:205–210. 10.1007/s00284-011-0051-x. [DOI] [PubMed] [Google Scholar]
- 58.Suzzi G, Tofalo R, Golí N, Veljoví K, Diní M, Lukí J, Mihajloví S, Tolinački M, Tolinački T, Živkoví M, Begoví J, Mrvaljeví I, Terzí C-Vidojeví A. 2017. Promotion of early gut colonization by probiotic intervention on microbiota diversity in pregnant sows. Front Microbiol 8:2028. 10.3389/fmicb.2017.02028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin D, Chang SY, Bogere P, Won KH, Choi JY, Choi YJ, Lee HK, Hur J, Park BY, Kim Y, Heo J. 2019. Beneficial roles of probiotics on the modulation of gut microbiota and immune response in pigs. PLoS One 14:e0220843. 10.1371/journal.pone.0220843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Clermont O, Bonacorsi S, Bingen E. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl Environ Microbiol 66:4555–4558. 10.1128/aem.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zajacova ZS, Konstantinova L, Alexa P. 2012. Detection of virulence factors of Escherichia coli focused on prevalence of EAST1 toxin in stool of diarrheic and non-diarrheic piglets and presence of adhesion involving virulence factors in astA positive strains. Vet Microbiol 154:369–375. 10.1016/j.vetmic.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 62.Šmarda J. 1963. Lysogenie a colicinogenie bakterií Escherichia coli. PhD thesis. Masaryk University, Brno, Czech Republic. [Google Scholar]
- 63.Šmarda J, Obdržálek V. 1966. Colicine Q. Zentralbl Bakteriol Orig 200:493–497. [PubMed] [Google Scholar]
- 64.Šmarda J, Šmajs D. 1998. Colicins—exocellular lethal proteins of Escherichia coli. Folia Microbiol 43:563–582. 10.1007/BF02816372. [DOI] [PubMed] [Google Scholar]
- 65.Alexa P, Rychlík I, Nejezchleb A, Hamřík J. 1997. Identification of enterotoxin-producing strains of Escherichia coli by PCR and biological methods. Vet Med (Praha) 42:97–100. [PubMed] [Google Scholar]
- 66.Bosák J, Micenková L, Vrba M, Ševčíková A, Dědičová D, Garzetti D, Šmajs D. 2013. Unique activity spectrum of colicin FY: all 110 characterized Yersinia enterocolitica isolates were colicin FY susceptible. PLoS One 8:e81829. 10.1371/journal.pone.0081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patzer SI, Baquero MR, Bravo D, Moreno F, Hantke K. 2003. The colicin G, H and X determinants encode microcins M and H47, which might utilize the catecholate siderophore receptors FepA, Cir, Fiu and IroN. Microbiology 149:2557–2570. 10.1099/mic.0.26396-0. [DOI] [PubMed] [Google Scholar]
- 68.Eraso JM, Chidambaram M, Weinstock GM. 1996. Increased production of colicin E1 in stationary phase. J Bacteriol 178:1928–1935. 10.1128/jb.178.7.1928-1935.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kuhar I, Žgur-Bertok D. 1999. Transcription regulation of the colicin K cka gene reveals induction of colicin synthesis by differential responses to environmental signals. J Bacteriol 181:7373–7380. 10.1128/JB.181.23.7373-7380.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moreno F, Gónzalez-Pastor JE, Baquero MR, Bravo D. 2002. The regulation of microcin B, C and J operons. Biochimie 84:521–529. 10.1016/S0300-9084(02)01452-9. [DOI] [PubMed] [Google Scholar]
- 71.Trckova M, Lorencova A, Hazova K, Sramkova Zajacova Z. 2016. Prophylaxis of post-weaning diarrhoea in piglets by zinc oxide and sodium humate. Vet Med (Praha) 60:351–360. 10.17221/8382-VETMED. [DOI] [Google Scholar]
- 72.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aronesty E. 2011. Command-line tools for processing biological sequencing data. https://expressionanalysis.github.io/ea-utils/.
- 74.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 75.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pe˜a AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and Web-based tools. Nucleic Acids Res 41:D590–D596. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gentleman R. 2008. R programming for bioinformatics. CRC Press, Boca Raton, FL. [Google Scholar]
- 78.R Core Team. 2019. R: the R project for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.r-project.org/. [Google Scholar]
- 79.Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Szoecs E, Wagner H. 2017. Vegan: community ecology package. R package version 2.4-3. https://cran.r-project.org/web/packages/vegan/index.html.
- 80.Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, New York, NY. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S4, Fig. S1. Download AEM03121-20_Supp_1_seq4.pdf, PDF file, 0.4 MB (359.7KB, pdf)