ABSTRACT
Coenzyme A (CoA) is an essential cofactor present in all domains of life and is involved in numerous metabolic pathways, including fatty acid metabolism, pyruvate oxidation through the tricarboxylic acid (TCA) cycle, and the production of secondary metabolites. This characteristic makes CoA a commercially valuable compound in the pharmaceutical, cosmetic, and clinical industries. However, CoA is difficult to accumulate in living cells at a high level, since it is consumed in multiple metabolic pathways, hampering its manufacturing by typical cell cultivation and extraction approaches. The feedback inhibition by CoA to a biosynthetic enzyme, pantothenate kinase (PanK), is also a serious obstacle for the high-titer production of CoA. To overcome this challenge, in vitro production of CoA, in which the CoA biosynthetic pathway was reconstructed outside cells using recombinant thermophilic enzymes, was performed. The in vitro pathway was designed to be insensitive to the feedback inhibition of CoA using CoA-insensitive type III PanK from the thermophilic bacterium Thermus thermophilus. Furthermore, a statistical approach using design of experiments (DOE) was employed to rationally determine the enzyme loading ratio to maximize the CoA production rate. Consequently, 0.94 mM CoA could be produced from 2 mM d-pantetheine through the designed pathway. We hypothesized that the insufficient conversion yield is attributed to the high Km value of T. thermophilus PanK toward ATP. Based on these observations, possible CoA regulation mechanisms in T. thermophilus and approaches to improve the feasibility of CoA production through the in vitro pathway have been investigated.
IMPORTANCE The biosynthesis of coenzyme A (CoA) in bacteria and eukaryotes is regulated by feedback inhibition targeting type I and type II pantothenate kinase (PanK). Type III PanK is found only in bacteria and is generally insensitive to CoA. Previously, type III PanK from the hyperthermophilic bacterium Thermotoga maritima was shown to defy this typical characteristic and instead shows inhibition toward CoA. In the present study, phylogenetic analysis combined with functional analysis of type III PanK from thermophiles revealed that the CoA-sensitive behavior of type III PanK from T. maritima is uncommon. We cloned type III PanKs from Thermus thermophilus and Geobacillus sp. strain 30 and showed that neither enzyme’s activities were inhibited by CoA. Furthermore, we utilized type III PanK for a one-pot cascade reaction to produce CoA.
KEYWORDS: coenzyme A, in vitro metabolic engineering, pantothenate kinase, thermophiles
INTRODUCTION
Coenzyme A (CoA) is an essential cofactor that is ubiquitously distributed in all domains of life, including eukaryotes, bacteria, and archaea. CoA is involved in numerous metabolic pathways such as fatty acid metabolism, pyruvate oxidation through the tricarboxylic acid (TCA) cycle, and the production of secondary metabolites. In most bacteria and eukaryotes, CoA is synthesized from d-pantothenate via five-step conversion by pantothenate kinase (PanK), phosphopantothenate-cysteine ligase (CoaB), phosphopantothenoylcysteine decarboxylase (CoaC), pantetheine-phosphate adenylyltransferase (PPAT), and dephospho-CoA (dPCoA) kinase (DPCK) (Fig. 1). Similar to many metabolites, CoA biosynthesis is controlled via feedback inhibition. The main target of this inhibition in bacteria and eukaryotes is usually PanK, which is inhibited by CoA, acetyl-CoA, and other CoA derivatives (1–4). To date, PanK enzymes have been classified into three types based on their primary structures. Type I and type III are found in a wide range of bacteria, and type II PanK is found mostly in eukaryotes. Type I and type II are typically inhibited by CoA and its derivatives, with a few exceptions. In contrast, type III PanK (PanK-III) is not inhibited by CoA, and this has been demonstrated experimentally for enzymes from Helicobacter pylori, Bacillus subtilis, and Pseudomonas aeruginosa (5, 6).
FIG 1.
Typical coenzyme A biosynthesis pathway. CoA biosynthesis in bacteria and eukaryotes is usually regulated by feedback inhibition to type I and type II PanKs. Type III PanK is found only in bacteria and is not a target for feedback inhibition by CoA and its derivatives.
The lack of sensitivity to CoA makes PanK-III a compelling candidate for biotechnology applications. Previously, our group demonstrated the feasibility of using thermophilic enzymes for in vitro metabolic engineering in which multiple enzymes are assembled in a cell-free environment to construct an in vitro synthetic pathway for the biocatalytic manufacture of commercially important metabolites (7, 8). In vitro metabolic engineering can be beneficial for CoA manufacturing as it introduces only the necessary enzymes into a reaction cocktail for CoA production, minimizing side reactions and allowing high-yield production of CoA from its precursors. More importantly, in comparison with previous CoA production methods (9–11), the use of thermophilic enzymes allows easy purification through simple heat treatment of crude lysate from a recombinant mesophile (e.g., Escherichia coli) overexpressing these enzymes, reducing time-consuming procedures for enzyme preparation (12, 13).
When we started this project, PanK-III from thermophilic bacteria had rarely been explored. The only biochemically characterized PanK-III from thermophiles was from the hyperthermophilic bacterium Thermotoga maritima (TmPanK) (14). Interestingly, despite being PanK-III, TmPanK was demonstrated to be sensitive to feedback inhibition by CoA. This is the first report of CoA inhibition of PanK-III, and it motivated us to explore an “ordinal (CoA-insensitive)” PanK-III from thermophilic sources and apply it to the in vitro production of CoA.
In this study, we performed a phylogenetic analysis of PanK-III of a thermophilic origin. Among the enzymes assessed, PanK-III from Thermus thermophilus (TtPanK) and PanK-III from Geobacillus sp. strain 30 (GbPanK) (39) were selected and assayed to demonstrate their insensitivity to CoA and acetyl-CoA. TtPanK was then utilized in tandem with other CoA biosynthesis enzymes for in vitro CoA production. To this end, we showed that the absence of feedback inhibition allowed higher-titer CoA production through the in vitro pathway.
RESULTS
Exploration of thermophilic PanK-III enzymes.
A BLAST search was performed on the genome sequences of thermophilic and hyperthermophilic bacteria obtained from the TEMPURA database (15) using the amino acid sequence of TmPanK as a query. BLAST identified hundreds of hits with various sequence similarities to TmPanK, and we constructed a phylogenetic tree showing three main clusters of PanK-III shared between thermophiles and hyperthermophiles (Fig. 2). The clusters were approximately separated by taxa, and some could be further grouped based on class, order, or family. Cluster A included only bacteria in the phylum Firmicutes, mainly from the Clostridia and Bacilli classes. It should be noted that this cluster did not include all Firmicutes from the list of bacteria. Cluster B consisted of multiple phyla, the majority of which were in the Thermotogae class. As TmPanK is included in this cluster, other PanK-IIIs in the cluster are suspected to show similar characteristics. Cluster C was the most diverse, in which some microbes were clustered based on their environmental conditions, such as sulfur bacteria.
FIG 2.
Unrooted phylogenetic tree of PanK-III in thermophilic and hyperthermophilic bacteria. The tree was generated by Clustal Omega using sequences acquired from BLASTp of PanK-III from T. maritima in a thermophilic database. The bar indicates the number of substitutions per 100 sites of amino acid residues. Abbreviations: R. marinus, Rhodothermus marinus; M. ruber, Meiothermus ruber; D. thermophilum, Dictyoglomus thermophilum; T. autotrophica, Thermodesulfitimonas autotrophica.
Among the selections, PanK-III from T. thermophilus HB8 (TtPanK) belonging to the Thermaceae family in cluster C was selected because of its high-growth-temperature characteristics and because it is a model organism for thermophiles. This protein (33.6% identical to TmPanK) was encoded by the gene TTHA1374. The TtPanK gene was cloned into the pET19b vector, which contains a histidine tag at the N terminus. TtPanK was expressed in E. coli, and the recombinant protein was heat treated, followed by His tag purification.
Enzymatic properties of TtPanK.
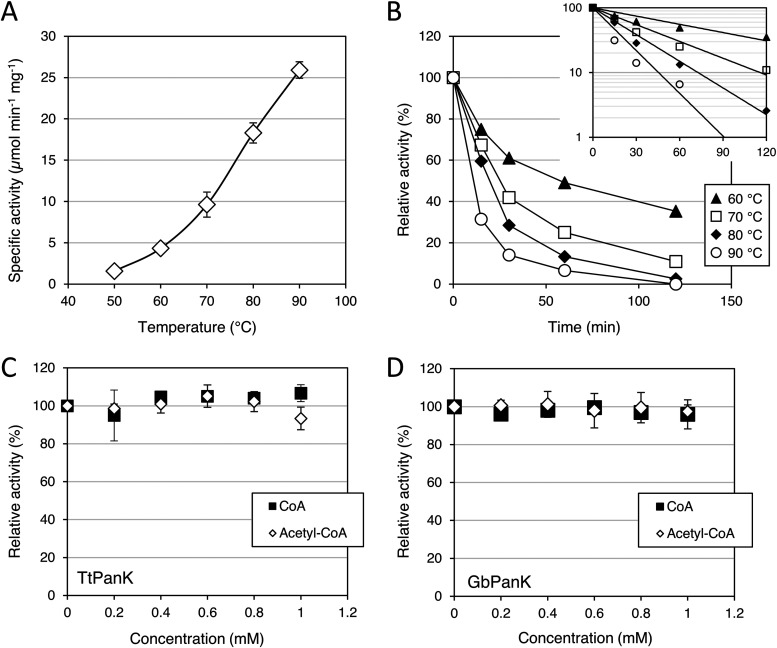
The optimum growth temperature of T. thermophilus is between 65°C and 72°C (16). Therefore, TtPanK is expected to exhibit thermophilic behavior. TtPanK activity was assayed by measuring the production of ADP released by the phosphorylation of d-pantothenate at the designated temperature after 30 min. First, the effects of temperature on TtPanK activity were examined at temperatures of between 50°C and 90°C. The activity of TtPanK continued to increase with increasing temperature (Fig. 3A). The thermostability of TtPanK was examined by incubating the protein at 60°C, 70°C, 80°C, and 90°C for various periods of time. The protein retained its activity well at 60°C, which was slightly lower than the optimum growth temperature. The half-lives of TtPanK were calculated to be 71 min, 35 min, 22 min, and 14 min at 60°C, 70°C, 80°C, and 90°C, respectively (Fig. 3B). The optimum pH of TtPanK was between pH 6 and 8; the enzyme activity decreased to 40% at pH 9 or above (data not shown).
FIG 3.
Enzymatic properties of PanK-III from thermophiles. (A) Effects of temperature on TtPanK activity. Data are means ± standard deviations (SD) (n = 3). (B) Thermostability of TtPanK. A logarithmic plot is shown at the top right. The 100% relative activity corresponds to 9.6 μmol min−1 mg−1. Data are means ± SD (n = 3). (C and D) Effect of CoA and acetyl-CoA on TtPanK (C) and GbPanK (D). GbPanK was assayed at 60°C due to lower thermostability. The 100% relative activity corresponds to 9.6 and 7.3 μmol min−1 mg−1 for TtPanK and GbPanK, respectively. Data are means ± SD (n = 3).
Following general enzyme characteristic assays, the sensitivity of TtPanK to CoA was assayed at various concentrations of CoA and acetyl-CoA in the reaction mixture from 0 to 1 mM. As expected from PanK-III, TtPanK activity was not inhibited by CoA and acetyl-CoA (Fig. 3C). In contrast, the activity of TmPanK almost completely halted when CoA reached 0.4 mM (14). To further validate which of the PanK-IIIs were outliers, PanK-III from Geobacillus sp. 30 (GbPanK) belonging to cluster A in the phylogenetic tree was cloned and assayed. GbPanK activity was not inhibited by CoA or acetyl-CoA; this was similar to the results for TtPanK (Fig. 3D).
Construction of the in vitro CoA biosynthetic pathway.
With the discovery of a CoA-insensitive thermophilic PanK-III, TtPanK, we constructed an in vitro pathway for CoA production using thermophilic enzymes. PanK is known to phosphorylate d-pantothenate as well as its cystaminated derivative, d-pantetheine, as an alternate substrate in CoA biosynthesis (17, 18). The phosphorylation of d-pantetheine by PanK allows us to bypass the enzyme CoaBC and directly produces d-4′-phosphopantetheine. d-Pantoate, a starting compound for CoA production in archaea (19), was also tested to check the substrate specificity of TtPanK. The activity of TtPanK toward d-pantetheine and d-pantoate was assayed and confirmed (see Fig. S1 in the supplemental material). TtPanK preferably used d-pantothenate over d-pantetheine at an approximately 300-fold decrease in the specific activity. Nevertheless, this trade-off in specific activity is worthwhile, taking the cost of CTP and the preparation of CoaB and CoaC into consideration. The other two enzymes, PPAT and DPCK, were both taken from T. thermophilus (TtPPAT and TtDPCK, respectively), as the recombinant production of both enzymes in E. coli has been reported (20, 21).
All the enzymes used in this experiment were expressed in E. coli Rosetta2(DE3)/pLysS with a knockdown mutation in adenylate kinase (ADK). Previously, our group observed an unexpected depletion of ATP accompanied by AMP accumulation during in vitro bioconversion with thermophilic enzymes, probably due to thermally stable host-borne enzymes (7). The following analysis suggested that ADK from E. coli, which catalyzes the reversible transphosphorylation between ATP and AMP (ATP + AMP ⇔ 2 ADP), is the cause of this observation. Previously, similar contamination of host-borne protein in a heat treatment approach to enzyme purification from Salmonella enterica serovar Typhimurium was eliminated by the deletion of the contaminating gene (22). Accordingly, we attempted to knock out the adk gene using the λ-Red recombination technique (23). However, this trial was not successful, indicating the importance of ADK in E. coli viability. Instead, a site-specific mutation (the alteration of the 87th proline to serine, which has been reported to reduce the thermal stability of ADK [24]) was introduced into the ADK gene in the E. coli genome using the CRISPR-Cas9 system (K. Honda and K. Okano, unpublished data).
The activity of TtDPCK was confirmed experimentally by observing the conversion of dephospho-CoA to CoA. TtPPAT activity could not be measured because of the absence of a commercially available substrate (d-4′-phosphopantetheine). Nevertheless, TtPPAT showed high expression levels even after the heat treatment process, suggesting that the protein was properly folded into an active form (Fig. S2). The production of both dephospho-CoA and CoA was detected using the three heat-purified enzymes and d-pantetheine as the substrates.
Determination of the enzyme loading ratio.
In our previous work, we determined the optimum loading ratio of enzymes (i.e., the optimum distribution of the total amount of enzymes) to maximize the flux through an in vitro pathway using a kinetic modeling approach (7). However, in the present study, this approach could not be used because a pathway intermediate, d-4′-phosphopantetheine, was not commercially available. Hence, we employed a statistical design of experiments (DOE) approach (25) to rationally determine the appropriate enzyme loading ratio based on the enzyme concentration instead of kinetic parameters (see the supplemental material for detailed methods and results). DOE allows us to evaluate the impact of each input factor in the system on the response and make educated guesses to increase, decrease, or exclude the factors. In this context, using enzyme concentrations as input factors and metabolite concentrations as the response, the individual enzyme concentration was increased from a fixed baseline, based on statistical significance, to the response value in a stepwise manner until it reached the peak, a process usually referred to as response surface methods. The three enzymes were initialized to 0.3 μg wet cells μl−1 as the baseline concentration, and after parameter optimization, the concentrations of TtPanK, TtPPAT, and TtDPCK were 0.75, 0.605, and 1.07 μg wet cells μl−1, respectively (the enzyme amount is normalized using the wet weight of E. coli cells used for the preparation of the indicated amounts of the enzyme) (see “Enzyme preparation” in Materials and Methods, below). The band densitometry observed by SDS-PAGE analysis of heat-treated crude extracts of E. coli producing this enzyme showed that the concentrations of TtPanK, TtPPAT, and TtDPCK used in the cascade reaction were 0.86, 1.40, and 3.75 ng recombinant protein μl−1, respectively.
In vitro production of CoA.
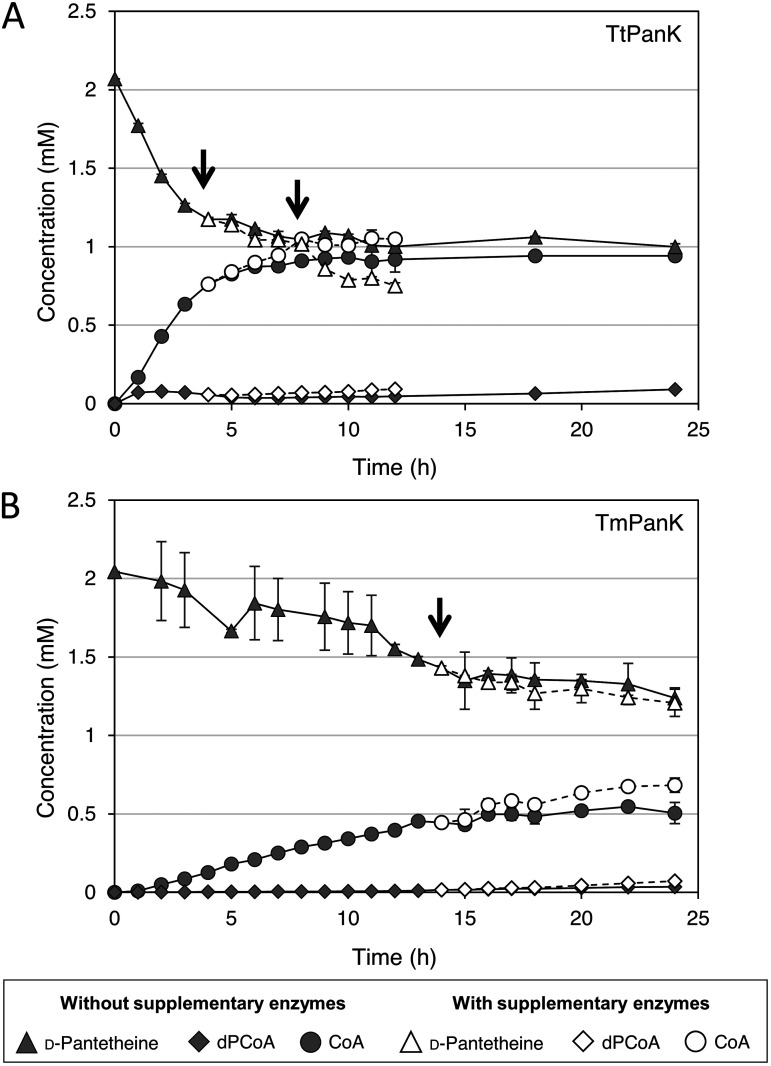
Using the acquired enzyme loading ratio, we performed in vitro production of CoA in a batch reaction with 2 mM d-pantetheine and 6 mM ATP as the substrates at 70°C. The reaction mixture was intermittently sampled for 24 h, and concentrations of the substrates and products were quantified. The CoA concentration increased at a high rate for the first 4 h of the reaction and started to stagnate afterward (Fig. 4A). A maximum titer of 0.93 mM was achieved after 10 h of incubation, with a conversion yield of 46.7% (mol/mol) (Fig. 4A, black fill), after which the CoA concentration remained constant. As the reaction was attempted at a high temperature, thermal inactivation of enzymes could be the cause of the stop in CoA production. To confirm this possibility, fresh enzyme solutions containing the three enzymes were added to the reaction mixture after 4 h of incubation and further added at 8 h of incubation. The supplementary addition of fresh enzymes had a moderately positive impact on the CoA titer, increasing the maximum titer to 1.05 mM (Fig. 4A, white fill). However, other factors that interfered with further increases in the CoA titer seemed to be present. We suspected that the high Km of TtPanK for ATP (10.7 mM) and the apparent inhibition of TtPanK by ADP were two of the factors contributing to the halt in CoA production (Fig. S3). This was supported by the low ATP and increased ADP concentrations in the reaction mixture (Fig. S4).
FIG 4.
CoA production through the in vitro synthetic pathway. Shown are the metabolite profiles of d-pantetheine, dephospho-CoA (dPCoA), and CoA in a cascade reaction in reaction mixtures containing TtPanK (A) and TmPanK (B). The same amounts of TtPPAT and TtDPCK were added for both reactions. Times of enzyme addition are marked by arrows (4 h and 8 h for TtPanK and 14 h for TmPanK), and the metabolite profile is shown with white fill. Assays were carried out at 70°C in a 100-μl assay mixture consisting of 2 mM d-pantetheine, 6 mM ATP, 10 mM MgCl2, 60 mM KCl, 60 mM NH4Cl, and a designated amount of the heat-purified enzyme solution in 50 mM Tris-HCl (pH 7.5). Data are means ± SD (n = 3).
Furthermore, we evaluated the effect of PanK feedback inhibition by CoA in an in vitro CoA production system. A CoA production assay was performed by replacing TtPanK with the same enzyme units of TmPanK (4.6 × 105 U ml−1; 1 U of the enzyme is defined as the amount catalyzing the phosphorylation of 1 μmol d-pantetheine per min under standard assay conditions) under the same assay conditions (Fig. S5). A maximum CoA titer of 0.55 mM was achieved at 22 h of incubation, with a conversion yield of 27.5% (mol/mol) (Fig. 4B, black fill). To exclude the effect of enzyme inactivation, supplementary enzymes were added to the mixture after 14 h of incubation. The CoA production rate was recovered by the addition of fresh enzymes, and a maximum titer of 0.68 mM was achieved at 22 to 24 h (Fig. 4B, white fill). However, the consumption rate of d-pantetheine in the reaction with or without the supplementary enzyme was not significantly different. This observation indicated that the increase in CoA production was mainly caused by the addition of TtPPAT and TtDPCK, and TmPanK hardly worked even after its additional supplementation, probably due to the inhibitory effect of accumulated CoA.
DISCUSSION
In this study, we identified CoA-insensitive PanK-III from thermophiles and demonstrated its application in the in vitro production of CoA. Phylogenetic analysis of PanK-III from thermophiles revealed that the previously described CoA-sensitive PanK-III from T. maritima can be clustered in a distinct group of PanK-IIIs. Functional analysis of PanK-IIIs from T. thermophilus and Geobacillus sp. 30, both of which belong to a different cluster than TmPanK, exhibited typical CoA-insensitive PanK-III behavior. Hence, based on phylogenetic and functional analyses, our results suggest that only select groups of thermophiles, mainly in the Thermotogae class, exhibit CoA-sensitive PanK-III. This raises the questions of how T. thermophilus regulates CoA concentrations, as only a single PanK-III gene is present in the genome sequence, and why there is a disparity in PanK-III characteristics among thermophiles.
Since the discovery of PanK-III, the regulation of CoA levels in bacteria harboring PanK-III remains unclear. The unusually high Km for ATP is a shared characteristic of PanK-IIIs, as demonstrated in T. thermophilus (Km = 10.7 mM) and other bacteria (H. pylori, Km = 9.59 mM; B. subtilis, Km = 3.05 mM; P. aeruginosa, Km = 7.17 mM; T. maritima, Km = 6.04 mM). The high-Km characteristic of PanK-III opens up the possibility of another phosphate donor being used as the main cofactor. To address this issue, PanK-III from H. pylori was tested against multitudes of phosphate donors, and it exhibited significantly less activity using GTP and CTP than using ATP (5). Some thermophilic enzymes are known to preferably use PPi as a phosphate donor instead of ATP (26, 27). We tested the plausibility of this for TtPanK by monitoring orthophosphate formation accompanied by PPi-dependent enzymatic phosphorylation using a malachite green assay (28); however, no significant accumulation of orthophosphate was detected (data not shown). It seems, therefore, that ATP is indeed the main phosphate donor for PanK-III.
If the high Km of PanK-III is the main regulator of CoA in these organisms, considering CoA metabolic function in the TCA cycle and fatty acid metabolism, there could be a correlation between cell growth and the intracellular concentrations of ATP and CoA. Previous studies in E. coli showed that the ATP concentration in vivo does not vary with the growth rate (29) and that, on average, 1.54 mM ATP is present (30). On the other hand, independent studies have shown that the concentrations of CoA and its thioesters in multiple bacteria are influenced by the type and availability of the carbon source, aerobic or anaerobic cultivation, and other cultivation conditions (31, 32). From these examples, there seems to be no correlation between ATP and CoA concentrations in the cell. However, all of these studies were performed using bacteria encoding type I PanK, where feedback inhibition toward CoA has been thoroughly established (2). Investigating the correlation between CoA and ATP concentrations in the cell would elucidate this matter. Subsequently, CoA regulation in these bacteria may be as simple as feedback inhibition of other CoA biosynthetic enzymes upstream and/or downstream of PanK. For example, the upstream enzyme ketopantoate reductase is the target of feedback inhibition in archaea (33).
Furthermore, we demonstrated in vitro CoA production using thermophilic enzymes. Although the yield was lower than expected, it was still comparable to those of other established methods for CoA production (summarized in reference 34). Compared to a similar in vitro method for CoA production with mesophilic enzymes (11), our approach is performed in a one-step one-pot reaction, and the use of thermophilic enzymes allows us to skip time-consuming enzyme purification procedures. We have also employed DOE to determine the enzyme loading ratio with a positive increase in CoA production. DOE is a powerful tool for this purpose as it allows us to skip the tedious process of individual enzyme characterization that has to be done for kinetic modeling. Enzymes with no commercially available substrate (i.e., TtPPAT) and those with difficult assay conditions can be included in the cascade reaction mixtures in rational amounts. In addition, DOE is not limited to enzyme concentrations, and other factors such as temperature, cofactor concentrations, and buffer pH can also be taken into consideration.
Although the use of PanK-III without feedback inhibition had a positive impact on CoA production, the high Km of TtPanK for ATP was rather troublesome. Screening of PanK-III of other origins or protein engineering of TtPanK to improve its affinity for ATP may increase the feasibility of this system. Alternatively, the integration of an ATP generation/recycling system to maintain ATP and ADP concentrations should push the reaction to its completion. The use of single enzymes such as polyphosphate kinase (35, 36) and acetate kinase (37), or an ATP regeneration cascade through a carbon rearrangement module (38), is a possible strategy to address this challenge.
MATERIALS AND METHODS
Gene cloning and construction of expression vectors.
The oligonucleotide primers used in this study are summarized in Table 1. The gene coding for TtPanK (TTHA1374) was amplified by PCR from the genomic DNA of Thermus thermophilus HB8 using the primer pair TtPanK-F and -R. The amplicon was digested with NdeI and BamHI and introduced into the corresponding sites of pET19b (Merck Japan, Tokyo, Japan). The gene encoding PanK-III from Geobacillus sp. 30 (39) was amplified from the bacterial genome with the primer set GbPanK-F and -R and introduced at the NdeI and XhoI restriction sites of pET19b. Similarly, genes encoding DPCK (TTHA0926) were PCR amplified from the T. thermophilus HB8 genome and inserted into the pET21a expression vector. The expression vector for TTHA0929, encoding PPAT of T. thermophilus, was obtained from the Riken T. thermophilus HB8 expression library (40).
TABLE 1.
Oligonucleotide primers used in this study
| Primer | Sequence (5′→3′) | Description |
|---|---|---|
| TtPanK-F | AGTCTACATATGCTCCTCGCGGTGGACATCa | Gene cloning for type III PanK from the Thermus thermophilus genome |
| TtPanK-R | ACGGGATCCTCACCCTCGCCCCAGGTGb | |
| GbPanK-F | AGTCTACATATGATTTTTGTGTTAGATGTTa | Gene cloning for type III PanK from the Geobacillus sp. 30 genome |
| GbPanK-R | ACGGTCCTCGAGTCATTGTTTTTTATCTACATTc | |
| TmPanK-F | AAAGGATCCCATATGTACCTCCTCGTGGACGTGGGTAAa | Gene cloning for type III PanK from the Thermotoga maritima genome |
| TmPanK-R | AAAGAATTCTCAATCTCCGAAGCAGAAATGd | |
| TtDPCK-F | TACGTCATATGGGCCACGAGGCGAAGCACCCCATTa | Gene cloning for DPCK from the Thermus thermophilus genome |
| TtDPCK-R | AGCTAGAATTCTCAGCCTCGCCCTCCTTTGGCCCCCCCGGTGd | |
Underlining indicates the recognition site of NdeI.
Underlining indicates the recognition site of BamHI.
Underlining indicates the recognition site of XhoI.
Underlining indicates the recognition site of EcoRI.
Enzyme preparation.
E. coli Rosetta2(DE3)/pLysS adk-P87S cells (Honda and Okano, unpublished) transformed with a pET-derived plasmid were cultivated in Luria-Bertani (LB) medium supplemented with 100 μg ml−1 ampicillin and 30 μg ml−1 chloramphenicol (Fujifilm Wako Pure Chemical Corporation, Osaka, Japan). A 500-ml Erlenmeyer flask containing 50 ml of medium was inoculated with 500 μl of a culture of the recombinant strain grown overnight. Cells were cultivated at 30°C with orbital shaking at 180 rpm using an MMS-310 multishaker (Eyela, Tokyo, Japan). After cultivation for 4 h, isopropyl thiogalactoside (IPTG) at a final concentration of 0.2 mM was added to induce gene expression, and the cells were further cultivated for 18 h. Cells were harvested by centrifugation at 8,000 × g at 4°C for 5 min, washed once with 50 mM Tris-HCl (pH 7.5), and resuspended in the same buffer at a concentration of 200 mg wet cells ml−1. Cells were then disrupted using a UD 201 ultrasonicator (Tomy, Tokyo, Japan) at output 3, duty 75, and 10 flashes 5 times while being cooled during the whole process. The crude lysate was incubated at 70°C for 30 min in a water bath and centrifuged at 15,000 × g at 4°C for 10 min to remove cell debris and heat-denatured proteins. The resulting supernatants were used as enzyme solutions unless stated otherwise.
Recombinant PanK was further purified by affinity chromatography using a HisTrap HP column (1 ml; GE Healthcare Life Science) with a single-step elution method according to the manufacturer’s instructions. The binding buffer consisted of 0.5 M NaCl and 30 mM imidazole in 50 mM Tris-HCl (pH 7.5), and the elution buffer consisted of 0.5 M NaCl and 0.5 M imidazole in 50 mM Tris-HCl (pH 7.5). Acquired elution solutions were analyzed by SDS-PAGE to determine protein purity. Amicon Ultra-15 centrifugal filter units (Merck, NJ, USA) were used for buffer exchange with 50 mM Tris-HCl (pH 7.5) before the enzymatic assay.
Protein concentrations were measured by the Bradford assay using a Bio-Rad assay system (Bio-Rad, CA, USA). Alternatively, the protein concentration of heat-purified samples was determined by band densitometry after separation on SDS-PAGE gels stained with Coomassie brilliant blue R-250 (CBB). The band intensity of the protein of interest was quantified with GelAnalyzer (version 19.1; www.gelanalyzer.com), and protein concentrations were estimated using bovine serum albumin (Nacalai Tesque, Kyoto, Japan) of a known concentration as a standard. The apparent protein concentrations of TtPanK, TtPPAT, and TtDPCK were 1.1, 2.3, and 3.5 μg recombinant protein mg wet cells−1.
Phylogenetic analysis.
BLAST analysis was conducted using the Genome Benchwork (version 3.5.0) (NCBI) database. The amino acid sequence of TmPanK (GenBank accession number WP_004080709.1) was used as a query against bacteria with optimum growth temperatures ≥45°C obtained from the TEMPURA database (15). The acquired BLAST result was subjected to multiple-sequence alignment using Clustal Omega (41), and an unrooted phylogenetic tree was constructed using the neighbor-joining method.
PanK enzymatic assay.
PanK activity was assayed by determining ATP consumption and ADP formation in the reaction using reverse-phase high-performance liquid chromatography (HPLC) analysis. The standard assay was performed at 70°C for 30 min in a mixture (100 μl) consisting of 5 mM calcium d-pantothenate (Tokyo Chemical Industry, Tokyo, Japan), 5 mM ATP (Oriental Yeast, Tokyo, Japan), 10 mM MgCl2, 60 mM NH4Cl, 60 mM KCl, and a designated amount of the enzyme in 50 mM Tris-HCl buffer (pH 7.5). As an alternative substrate, d-pantetheine was prepared by reducing d-pantethine (Merck) with an equivalent molar amount of 1,4-dithiothreitol (Fujifilm Wako). d-Pantoate was prepared by hydrolyzing d-pantolactone (Tokyo Chemical Industry) in an equimolar concentration of an NaOH solution. Enzyme solutions were preincubated at 70°C for 2 min before adding the substrates. The reaction was stopped by adding 20 μl of 1 M HCl to the mixture. Samples were centrifuged at 20,400 × g at 4°C for 5 min, and the supernatants were subjected to HPLC analysis. The analysis was performed using a 250-by-4.6 Cosmosil 5C18-AR-II column (Nacalai Tesque) with a binary gradient of 20 mM tert-butylamine (pH 6.8; adjusted by H3PO4) and 20 mM tert-butylamine (pH 6.8) (plus 10% [vol/vol] methanol) as solvents A and B, respectively, at a flow rate of 1 ml min−1. The sample injection volume was set to 15 μl, and the UV-visible (UV-vis) detector was set at a wavelength of 254 nm. The initial condition (0% solvent B) was increased linearly to 100% solvent B over 10 min. After a 2-min hold at 100% solvent B, there was a linear decrease to 0% solvent B over 15 s, followed by a 5-min hold at the initial condition (0% solvent B).
Statistical analysis.
Basic statistical analysis was performed using R statistical software (version 4.0.2) with R built-in packages and the “tidyverse” package (42). Enzyme kinetic parameters were calculated by fitting experimental results by nonlinear regression to specified Michaelis-Menten models with the “nls” function. The design of experiments for the determination of the enzyme loading ratio was analyzed by the “qualityTools” package (version 1.55) (43).
In vitro CoA production.
The in vitro CoA production assay was performed at 70°C in a mixture (100 μl) consisting of 2 mM d-pantetheine, 6 mM ATP, 10 mM MgCl2, 60 mM KCl, 60 mM NH4Cl, and designated amounts of heat-treated enzyme solutions in 50 mM Tris-HCl buffer (pH 7.5). The reaction was stopped by adding 10 vol% of 200 mM Tris(2-carboxyethyl)phosphine followed by 10 vol% of 1 M HCl to the mixture to denature the enzymes. The samples were centrifuged at 20,400 × g at 4°C for 5 min, and the supernatant was subjected to HPLC analysis. The HPLC method has been previously reported (11). HPLC allowed the detection of d-pantetheine, dephospho-CoA, and CoA. The method for measurement of adenosine phosphate levels was identical to that used in the PanK assay mentioned above.
ACKNOWLEDGMENTS
This work was partly supported by a research grant from the Institute for Fermentation, Osaka (IFO), Japan. This work was also supported in part by the research funding program of Novozymes, Japan.
Footnotes
Supplemental material is available online only.
Contributor Information
Kohsuke Honda, Email: honda@icb.osaka-u.ac.jp.
Robert M. Kelly, North Carolina State University
REFERENCES
- 1.Leonardi R, Zhang YM, Rock CO, Jackowski S. 2005. Coenzyme A: back in action. Prog Lipid Res 44:125–153. 10.1016/j.plipres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 2.Vallari DS, Jackowski S, Rock CO. 1987. Regulation of pantothenate kinase by coenzyme A and its thioesters. J Biol Chem 262:2468–2471. 10.1016/S0021-9258(18)61527-3. [DOI] [PubMed] [Google Scholar]
- 3.Zhang YM, Rock CO, Jackowski S. 2006. Biochemical properties of human pantothenate kinase 2 isoforms and mutations linked to pantothenate kinase-associated neurodegeneration. J Biol Chem 281:107–114. 10.1074/jbc.M508825200. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YM, Rock CO, Jackowski S. 2005. Feedback regulation of murine pantothenate kinase 3 by coenzyme A and coenzyme A thioesters. J Biol Chem 280:32594–32601. 10.1074/jbc.M506275200. [DOI] [PubMed] [Google Scholar]
- 5.Brand LA, Strauss E. 2005. Characterization of a new pantothenate kinase isoform from Helicobacter pylori. J Biol Chem 280:20185–20188. 10.1074/jbc.C500044200. [DOI] [PubMed] [Google Scholar]
- 6.Hong BS, Yun MK, Zhang YM, Chohnan S, Rock CO, White SW, Jackowski S, Park HW, Leonardi R. 2006. Prokaryotic type II and type III pantothenate kinases: the same monomer fold creates dimers with distinct catalytic properties. Structure 14:1251–1261. 10.1016/j.str.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Hanatani Y, Imura M, Taniguchi H, Okano K, Toya Y, Iwakiri R, Honda K. 2019. In vitro production of cysteine from glucose. Appl Microbiol Biotechnol 103:8009–8019. 10.1007/s00253-019-10061-4. [DOI] [PubMed] [Google Scholar]
- 8.Honda K, Kimura K, Ninh PH, Taniguchi H, Okano K, Ohtake H. 2017. In vitro bioconversion of chitin to pyruvate with thermophilic enzymes. J Biosci Bioeng 124:296–301. 10.1016/j.jbiosc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu S, Esumi A, Komaki R, Yamada H. 1984. Production of coenzyme A by a mutant of Brevibacterium ammoniagenes resistant to oxypantetheine. Appl Environ Microbiol 48:1118–1122. 10.1128/AEM.48.6.1118-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu S, Komaki R, Tani Y, Yamada H. 1983. A high yield method for the preparative synthesis of coenzyme A by combination of chemical and enzymic reactions. FEBS Lett 151:303–306. 10.1016/0014-5793(83)80091-X. [DOI] [PubMed] [Google Scholar]
- 11.Mouterde LMM, Stewart JD. 2016. An efficient chemoenzymatic synthesis of coenzyme A and its disulfide. Org Process Res Dev 20:954–959. 10.1021/acs.oprd.6b00059. [DOI] [Google Scholar]
- 12.Ninh PH, Honda K, Sakai T, Okano K, Ohtake H. 2015. Assembly and multiple gene expression of thermophilic enzymes in Escherichia coli for in vitro metabolic engineering. Biotechnol Bioeng 112:189–196. 10.1002/bit.25338. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Honda K, Sakai T, Okano K, Omasa T, Hirota R, Kuroda A, Ohtake H. 2012. Synthetic metabolic engineering—a novel, simple technology for designing a chimeric metabolic pathway. Microb Cell Fact 11:120. 10.1186/1475-2859-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimosaka T, Tomita H, Atomi H. 2016. Regulation of coenzyme A biosynthesis in the hyperthermophilic bacterium Thermotoga maritima. J Bacteriol 198:1993–2000. 10.1128/JB.00077-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato Y, Okano K, Kimura H, Honda K. 2020. TEMPURA: database of growth TEMPeratures of usual and RAre prokaryotes. Microbes Environ 35:ME20074. 10.1264/jsme2.ME20074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oshima T, Imahori K. 1974. Description of Thermus thermophilus (Yoshida and Oshima) comb. nov., a nonsporulating thermophilic bacterium from a Japanese thermal spa. Int J Syst Evol Microbiol 24:102–112. [Google Scholar]
- 17.Balibar CJ, Hollis-Symynkywicz MF, Tao J. 2011. Pantethine rescues phosphopantothenoylcysteine synthetase and phosphopantothenoylcysteine decarboxylase deficiency in Escherichia coli but not in Pseudomonas aeruginosa. J Bacteriol 193:3304–3312. 10.1128/JB.00334-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rana A, Seinen E, Siudeja K, Muntendam R, Srinivasan B, van der Want JJ, Hayflick S, Reijngoud DJ, Kayser O, Sibon OCM. 2010. Pantethine rescues a Drosophila model for pantothenate kinase-associated neurodegeneration. Proc Natl Acad Sci U S A 107:6988–6993. 10.1073/pnas.0912105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimosaka T, Makarova KS, Koonin EV, Atomi H. 2019. Identification of dephospho-coenzyme A (dephospho-CoA) kinase in Thermococcus kodakarensis and elucidation of the entire CoA biosynthesis pathway in archaea. mBio 10:e01146-19. 10.1128/mBio.01146-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seto A, Murayama K, Toyama M, Ebihara A, Nakagawa N, Kuramitsu S, Shirouzu M, Yokoyama S. 2005. ATP-induced structural change of dephosphocoenzyme A kinase from Thermus thermophilus HB8. Proteins 58:235–242. 10.1002/prot.20276. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi H, Inagaki E, Fujimoto Y, Kuroishi C, Nodake Y, Nakamura Y, Arisaka F, Yutani K, Kuramitsu S, Yokoyama S, Yamamoto M, Miyano M, Tahirov TH. 2004. Structure and implications for the thermal stability of phosphopantetheine adenylyltransferase from Thermus thermophilus. Acta Crystallogr D Biol Crystallogr 60:97–104. 10.1107/s0907444903025319. [DOI] [PubMed] [Google Scholar]
- 22.Parsons SM, Koshland DE, Jr.. 1974. A rapid isolation of phosphoribosyladenosine triphosphate synthetase and comparison to native enzyme. J Biol Chem 249:4104–4109. 10.1016/S0021-9258(19)42489-7. [DOI] [PubMed] [Google Scholar]
- 23.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haase GHW, Brune M, Reinstein J, Pai EF, Pingoud A, Wittinghofer A. 1989. Adenylate kinases from thermosensitive Escherichia coli strains. J Mol Biol 207:151–162. 10.1016/0022-2836(89)90446-4. [DOI] [PubMed] [Google Scholar]
- 25.Gray CT. 1988. Introduction to quality engineering: designing quality into products and processes, Taguchi G. 1986. Asian productivity organization. Qual Reliab Eng Int 4:198. 10.1002/qre.4680040216. [DOI] [Google Scholar]
- 26.Liu B, Hong Y, Wu L, Li Z, Ni J, Sheng D, Shen Y. 2007. A unique highly thermostable 2-phosphoglycerate forming glycerate kinase from the hyperthermophilic archaeon Pyrococcus horikoshii: gene cloning, expression and characterization. Extremophiles 11:733–739. 10.1007/s00792-007-0079-9. [DOI] [PubMed] [Google Scholar]
- 27.Siebers B, Hensel R. 2001. Pyrophosphate-dependent phosphofructokinase from Thermoproteus tenax. Methods Enzymol 331:54–62. 10.1016/s0076-6879(01)31046-7. [DOI] [PubMed] [Google Scholar]
- 28.Baykov AA, Evtushenko OA, Avaeva SM. 1988. A malachite green procedure for orthophosphate determination and its use in alkaline phosphatase-based enzyme immunoassay. Anal Biochem 171:266–270. 10.1016/0003-2697(88)90484-8. [DOI] [PubMed] [Google Scholar]
- 29.Schneider DA, Gourse RL. 2004. Relationship between growth rate and ATP concentration in Escherichia coli: a bioassay for available cellular ATP. J Biol Chem 279:8262–8268. 10.1074/jbc.M311996200. [DOI] [PubMed] [Google Scholar]
- 30.Yaginuma H, Kawai S, Tabata KV, Tomiyama K, Kakizuka A, Komatsuzaki T, Noji H, Imamura H. 2014. Diversity in ATP concentrations in a single bacterial cell population revealed by quantitative single-cell imaging. Sci Rep 4:6522. 10.1038/srep06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chohnan S, Izawa H, Nishihara H, Takamura Y. 1998. Changes in size of intracellular pools of coenzyme A and its thioesters in Escherichia coli K-12 cells to various carbon sources and stresses. Biosci Biotechnol Biochem 62:1122–1128. 10.1271/bbb.62.1122. [DOI] [PubMed] [Google Scholar]
- 32.Chohnan S, Furukawa H, Fujio T, Nishihara H, Takamura Y. 1997. Changes in the size and composition of intracellular pools of nonesterified coenzyme A and coenzyme A thioesters in aerobic and facultatively anaerobic bacteria. Appl Environ Microbiol 63:553–560. 10.1128/AEM.63.2.553-560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomita H, Imanaka T, Atomi H. 2013. Identification and characterization of an archaeal ketopantoate reductase and its involvement in regulation of coenzyme A biosynthesis. Mol Microbiol 90:307–321. 10.1111/mmi.12363. [DOI] [PubMed] [Google Scholar]
- 34.Mouterde LMM, Stewart JD. 2019. Isolation and synthesis of one of the most central cofactors in metabolism: coenzyme A. Org Process Res Dev 23:19–30. 10.1021/acs.oprd.8b00348. [DOI] [Google Scholar]
- 35.Iwamoto S, Motomura K, Shinoda Y, Urata M, Kato J, Takiguchi N, Ohtake H, Hirota R, Kuroda A. 2007. Use of an Escherichia coli recombinant producing thermostable polyphosphate kinase as an ATP regenerator to produce fructose 1,6-diphosphate. Appl Environ Microbiol 73:5676–5678. 10.1128/AEM.00278-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Restiawaty E, Iwasa Y, Maya S, Honda K, Omasa T, Hirota R, Kuroda A, Ohtake H. 2011. Feasibility of thermophilic adenosine triphosphate-regeneration system using Thermus thermophilus polyphosphate kinase. Process Biochem 46:1747–1752. 10.1016/j.procbio.2011.05.021. [DOI] [Google Scholar]
- 37.Yan B, Ding Q, Ou L, Zou Z. 2014. Production of glucose-6-phosphate by glucokinase coupled with an ATP regeneration system. World J Microbiol Biotechnol 30:1123–1128. 10.1007/s11274-013-1534-7. [DOI] [PubMed] [Google Scholar]
- 38.Wei X, Xie L, Job Zhang Y-HP, You C. 2018. Stoichiometric regeneration of ATP by a NAD(P)/CoA-free and phosphate-balanced in vitro synthetic enzymatic biosystem. ChemCatChem 10:5597–5601. 10.1002/cctc.201801562. [DOI] [Google Scholar]
- 39.Tsuji N, Honda K, Wada M, Okano K, Ohtake H. 2014. Isolation and characterization of a thermotolerant ene reductase from Geobacillus sp. 30 and its heterologous expression in Rhodococcus opacus. Appl Microbiol Biotechnol 98:5925–5935. 10.1007/s00253-014-5668-9. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama S, Hirota H, Kigawa T, Yabuki T, Shirouzu M, Terada T, Ito Y, Matsuo Y, Kuroda Y, Nishimura Y, Kyogoku Y, Miki K, Masui R, Kuramitsu S. 2000. Structural genomics projects in Japan. Nat Struct Biol 7:943–945. 10.1038/80712. [DOI] [PubMed] [Google Scholar]
- 41.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. 2011. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wickham H, Averick M, Bryan J, Chang W, McGowan LD, François R, Grolemund G, Hayes A, Henry L, Hester J, Kuhn M, Pedersen TL, Miller E, Bache SM, Müller K, Ooms J, Robinson D, Seidel DP, Spinu V, Takahashi K, Vaughan D, Wilke C, Woo K, Yutani H. 2019. Welcome to the Tidyverse. J Open Source Softw 4:1686. 10.21105/joss.01686. [DOI] [Google Scholar]
- 43.Roth T. 2016. qualityTools: statistics in quality science. http://www.r-qualitytools.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 to S5, Supplemental Information for design of experiment approach (Fig. SI to SIII, Tables SI to SV). Download AEM00541-21_Supp_1_seq3.pdf, PDF file, 0.4 MB (416.3KB, pdf)