ABSTRACT
Caldicellulosiruptor species are hyperthermophilic, Gram-positive anaerobes and the most thermophilic cellulolytic bacteria so far described. They have been engineered to convert switchgrass to ethanol without pretreatment and represent a promising platform for the production of fuels, chemicals, and materials from plant biomass. Xylooligomers, such as xylobiose and xylotriose, that result from the breakdown of plant biomass more strongly inhibit cellulase activity than do glucose or cellobiose. High concentrations of xylobiose and xylotriose are present in C. bescii fermentations after 90 h of incubation, and removal or breakdown of these types of xylooligomers is crucial to achieving high conversion of plant biomass to product. In previous studies, the addition of exogenous β-d-xylosidase substantially improved the performance of glucanases and xylanases in vitro. β-d-Xylosidases are, in fact, essential enzymes in commercial preparations for efficient deconstruction of plant biomass. In addition, the combination of xylanase and β-d-xylosidase is known to exhibit synergistic action on xylan degradation. In spite of its ability to grow efficiently on xylan substrates, no extracellular β-d-xylosidase was identified in the C. bescii genome. Here, we report that the coexpression of a thermal stable β-d-xylosidase from Thermotoga maritima and a xylanase from Acidothermus cellulolyticus in a C. bescii strain containing the A. cellulolyticus E1 endoglucanase significantly increased the activity of the exoproteome as well as growth on xylan substrates. The combination of these enzymes also resulted in increased growth on crystalline cellulose in the presence of exogenous xylan.
IMPORTANCE Caldicellulosiruptor species are bacteria that grow at extremely high temperature, more than 75°C, and are the most thermophilic bacteria so far described that are capable of growth on plant biomass. This native ability allows the use of unpretreated biomass as a growth substrate, eliminating the prohibitive cost of preprocessing/pretreatment of the biomass. They only grow under strictly anaerobic conditions, and the combination of high temperature and the lack of oxygen reduces the cost of fermentation and contamination by other microbes. They have been genetically engineered to convert switchgrass to ethanol without pretreatment and represent a promising platform for the production of fuels, chemicals, and materials from plant biomass. In this study, we introduced genes from other cellulolytic bacteria and identified a combination of enzymes that improves growth on plant biomass. An important feature of this study is that it measures growth, validating predictions made from adding enzyme mixtures to biomass.
KEYWORDS: Caldicellulosiruptor, biomass deconstruction, consolidated bioprocessing, xylanase, β-d-xylosidase
INTRODUCTION
Members of the genus Caldicellulosiruptor are hyperthermophilic anaerobes and the most thermophilic cellulolytic bacteria so far described. Unlike most cellulolytic species of the Clostridium genus that rely on complex protein structures, called cellulosomes, Caldicellulosiruptor species secrete primarily free multifunctional enzymes into the exoproteome. One such cellulase, CelA, is the most abundant enzyme secreted by C. bescii (1, 2) and has been shown to outperform mixtures of commercially available exo- and endoglucanases in vitro (3). Moreover, C. bescii uses both xylans and glucans simultaneously and has the ability to grow well on xylan (1, 4). While most strain engineering of C. bescii has focused on improving cellulolytic activity (5–7), improving hemicellulolytic activity is essential to make consolidated bioprocessing by C. bescii an industrially relevant process (8). Hemicelluloses, particularly in the form of xylobiose and xylotriose, strongly inhibit cellulase activity, even more so than glucose or cellobiose (9). A previous study showed that high concentrations of xylobiose and, to a lesser extent, xylotriose accumulate in C. bescii fermentations after 90 h of incubation (1). Complete removal or breakdown of xylooligomers is crucial to achieve higher conversion of plant biomass, and, in fact, β-d-xylosidases are essential in commercial enzyme preparations for efficient deconstruction of plant biomass, as the activity of cellulases and hemicellulases in these mixtures has been shown to be inhibited by xylobiose. Previous studies also showed that the addition of exogenous β-d-xylosidase substantially improved the performance of glucanases and xylanases in vitro (8, 10, 11). In spite of its ability to grow efficiently on xylan substrates, no extracellular β-d-xylosidase was identified in the C. bescii genome (4, 5). To investigate whether the addition of a secreted β-d-xylosidase would improve growth, xylan utilization, and cellulose utilization, even in the presence of xylan, by C. bescii, we cloned and expressed a thermal stable β-d-xylosidase from Thermotoga maritima (Tm_0076; GenBank accession number AAD35170) in C. bescii using the CelA signal sequence for protein export. This gene was chosen because the enzyme was reported to be maximally active at 90°C (12). We then examined the effect of coexpression of this β-d-xylosidase with a Family 10 xylanase (Acel_0180) from A. cellulolyticus on the activity of the exoproteome in a strain containing the A. cellulolyticus E1 endoglucanase, previously shown to act synergistically to improve the cellulolytic activity of the C. bescii exoproteome (6). The combination of xylanase and β-d-xylosidase is known to act synergistically on xylan degradation (10). We selected the xylanase based on a previous study showing that introduction of this enzyme, including the tandem carbohydrate binding modules (CBM2 and CBM3) located at the C terminus, significantly improved the ability of C. bescii to utilize xylan (13). The combination of these enzymes increased the overall activity of the C. bescii exoproteome and improved growth on xylan as well as growth on crystalline cellulose, even in the presence of exogenous xylan in the growth medium.
RESULTS AND DISCUSSION
Heterologous expression and secretion of a β-d-xylosidase from T. maritima in a C. bescii strain containing the A. cellulolyticus endoglucanase E1.
To construct an expression vector for Tm_0076 in C. bescii (see Fig. S1 in the supplemental material), the gene was amplified from T. maritima genomic DNA (gDNA) and cloned into a shuttle vector, pSKW28, under the transcriptional control of the C. bescii S-layer promoter (14, 15). The native Tm_0076 signal peptide was replaced with the CelA signal sequence, as CelA is the most abundant extracellular enzyme produced by C. bescii (1, 2), and previous work showed that it can be used to drive secretion of other heterologous proteins, including the secretion of cellobiose phosphorylase from T. maritima in C. bescii (7). The plasmid was introduced into a C. bescii strain, JWCB52, that contains the E1 protein from A. cellulolyticus, a thermostable endo-1,4-β-glucanase (GH5) with a Family 2 carbohydrate-binding module, inserted into the C. bescii chromosome at the chromosomal integration site one (CIS1) (15). Strain JWCB52 also contains a deletion of pyrF, and transformants were selected for uracil prototrophy to generate JWCB95, which was grown at 65°C to accommodate the expression of the C. thermocellum pyrF gene used for complementation and plasmid selection. To verify the presence of the plasmid, primers (DC460 and DC228) were used to amplify the portion of the plasmid containing the open reading frame of the targeted proteins with annealing to regions of the plasmid outside the gene to avoid amplification of sequences residing on the chromosome (Fig. S2A). Total DNA from strain JWCB95 was also back transformed into E. coli, and two different restriction endonuclease digests performed on plasmid DNA purified from three independent back transformants resulted in digestion patterns identical to those of the original plasmid (Fig. S2B). These results indicated that the plasmid was successfully transformed into C. bescii and structurally stable during transformation and replication in C. bescii and back transformation into E. coli.
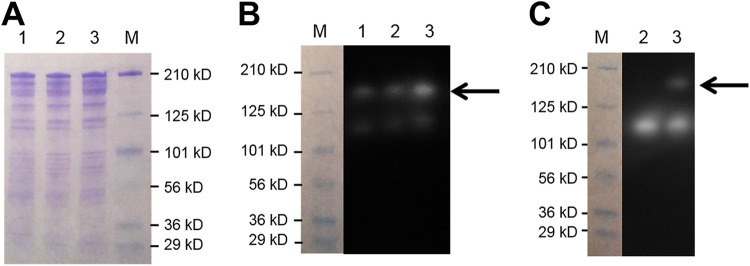
For detection of β-d-xylosidase activity, the extracellular protein (ECP) fraction from JWCB95 (Fig. 1) culture supernatants was first separated in an SDS-PAGE gel (Fig. 2A) and then renatured in the same gel, followed by infusion with 4-methylumbelliferyl β-d-xylopyranoside (MUX). As shown in Fig. 2B, two protein bands exhibiting β-d-xylosidase activity were detected in the parent and E1 expression strain. The C. bescii genome contains only two genes annotated as potential extracellular xylosidases, Cbes_2371 (34 kDa) and Cbes_0182 (152 kDa), both containing GH43s, known to exhibit xylosidases (16, 17). Protein bands consistent with molecular masses of ∼160 and ∼110 kDa were detected, and these species most likely represent the intact Cbes_0182 and functional truncations. Note that we have also observed similar functional truncations of cellulases in the C. bescii exoproteome. The predicted molecular mass of a T. maritima β-d-xylosidase dimer (Tm_0076) would be ∼174 kDa, which is close to the molecular weight of Cbes_0182, making it difficult to separate the two. However, comparison of the band intensities from the zymogram showed that the activity corresponding to this molecular weight in JWCB95 was more than twice that in JWCB82 (the parental strain) or JWCB73 (the E1 expression strain).
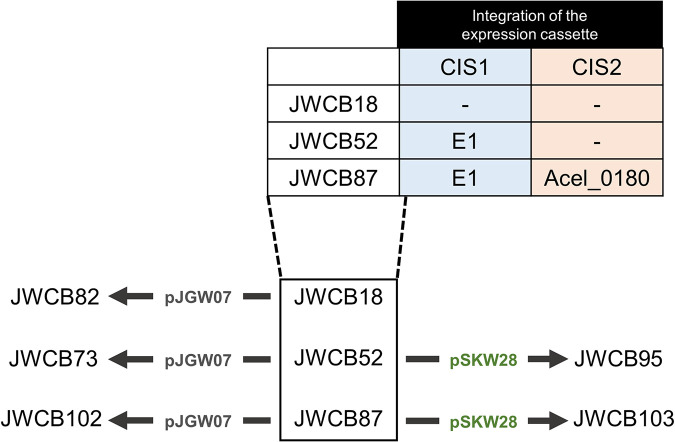
FIG 1.
Strain construction flow chart. The A. cellulolyticus E1 and Acel_0180 genes were inserted into the JWCB18 chromosome at chromosomal integration sites one (CIS1) and CIS2, respectively. The plasmids with (pSKW28) and without (pJGW07) the TM_0076 gene from T. maritima then were introduced into the JWCB18, JWCB52, and JWCB87 strains.
FIG 2.
Confirmation of β-d-xylosidase expression and activity in C. bescii. (A) SDS-PAGE analysis of concentrated extracellular proteins (10 μg). (B) Zymogram analysis of concentrated extracellular proteins (15 μg) using 0.3 mM MUX as a substrate for detecting protein bands with β-d-xylosidase activity. (C) Zymogram analysis of concentrated extracellular proteins (15 μg) using 5 mM MUG as a substrate for detecting protein bands with β-d-glucosidase activity. M, prestained SDS-PAGE standards, broad range (Bio-Rad Laboratories); 1, JWCB82 (parental strain); 2, JWCB73 (E1-expressing strain); 3, JWCB95 (E1 + Tm_0076-expressing strain).
To confirm the expression of Tm_0076 and remove the background from native C. bescii CAZymes, we performed zymogram analysis using 4-methylumbelliferyl β-d-glucopyranoside (MUG), a substrate specific for β-d-glucosidase activity, as Tm_0076 (GH3) should exhibit activity on this substrate but not Cbes_0182 (GH43).
A clearing zone corresponding to a protein of a molecular mass of 160 kDa was obtained from the JWCB95 strain but was not present in the JWCB73 strain (Fig. 2C). We conclude from these analyses that the T. maritima β-d-xylosidase was expressed, secreted, and functional in C. bescii. We believe that this band corresponds to a dimer of Tm_0076 (∼174 kDa). The maintenance of multimers of thermostable proteins in SDS-PAGE gels is well documented. For example, the β-d-glucosidase from Pyrococcus furiosus (BGLPf) appears to form a dimer that is stable in the presence of sodium dodecyl sulfate, and this dimer migrated in reducing SDS-PAGE even after incubation at 95°C (18).
Heterologous expression and secretion of a xylanase from A. cellulolyticus in a C. bescii strain containing the A. cellulolyticus endoglucanase E1.
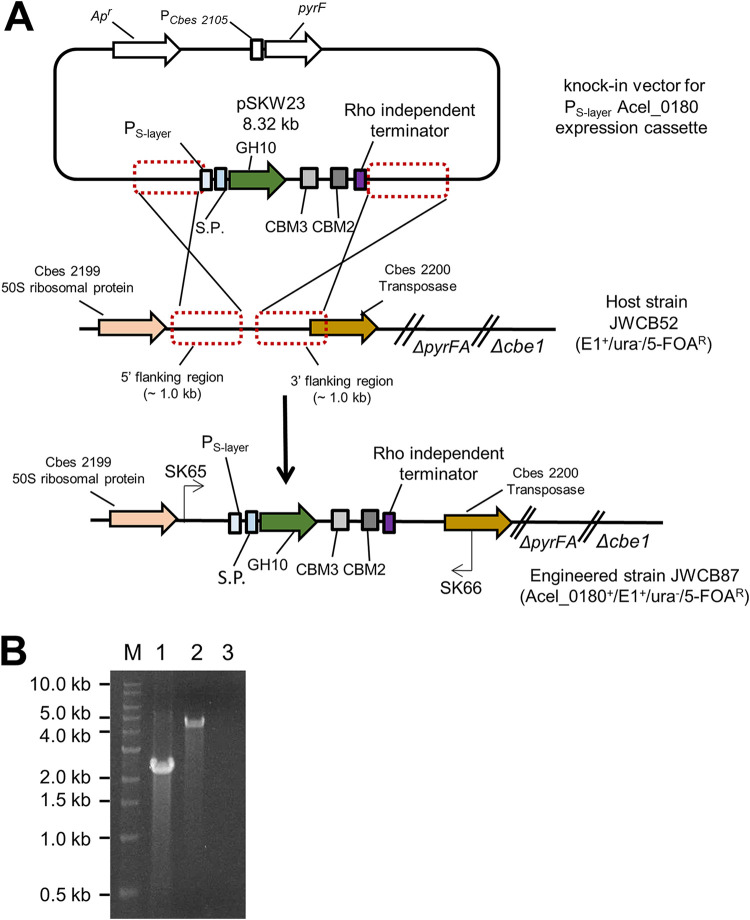
To generate a strain containing the A. cellulolyticus xylanase (Acel_0180) in the C. bescii strain containing E1, the xylanase expression cassette was amplified by PCR from a replicating shuttle vector, pSKW10 (13), and cloned into an integrational expression vector, pSKW23 (Fig. 3A). The PS-layer-Acel_0180 cassette was flanked by two 1-kb DNA regions of homology from the intergenic region between Cbes2199 and Cbes2200 (CIS2), previously determined to be available without affecting growth or resulting in a detectable phenotype (19). JWCB52 contained the A. cellulolyticus E1 gene inserted into the intergenic region between Cbes0863 and Cbes0864, designated CIS1 (5). Uracil prototrophic transformants were serially passaged as described previously (14) to allow segregation of merodiploids containing a mixture of the integrated Acel_0180 expression cassette and the parental strain (JWCB52) genomes. This resulted in strain JWCB87 [ΔpyrFA ldh::ISCbe4 Δcbe1 CIS1::PS-layerAcel0614(E1) CIS2::PS-layerAcel0180 (xylanase)] (Table 1). Verification of insertion of the xylanase gene into the JWCB87 chromosome was performed using PCR amplification with primers SK65 and SK66 (Fig. 3B) and sequencing the PCR products. The parental strain, JWCB52, produced the expected wild-type 2.2-kb band, whereas amplification of JWCB87 produced 4.4 kb, indicating an insertion of the xylanase expression cassettes within the targeted region (Fig. 3B). Expression and secretion of the A. cellulolyticus xylanase in C. bescii was confirmed using zymogram analysis (Fig. S3).
FIG 3.
Chromosomal integration of the Acel_0180 xylanase gene into the C. bescii genome. (A) A depiction of the chromosomal location and integration event of the Acel_0180 expression cassette. SP, signal peptide; GH10, a Family 10 glycoside hydrolase; CBM3, a Family 3 carbohydrate-binding module; CBM2, a Family 2 carbohydrate-binding module. (B) Agarose gel showing PCR products amplified using primers SK65 and SK66 annealing to regions outside the site of integration in the parent strain, JWCB52 (ΔpyrFA+ E1), 2.2 kb (lane 1); the newly constructed strain JWCB87 (ΔpyrFA+ E1 + Acel_0180), 4.4 kb (lane 2); no template PCR control (lane 3); and NEB 1-kb DNA ladder (lane M).
TABLE 1.
Strains and plasmids used in this study
| Name | Description | Reference or source |
|---|---|---|
| E. coli | ||
| JW532 | DH5α containing pSKW23 (Aprr) | This study |
| JW536 | DH5α containing pSKW28 (Aprr) | This study |
| C. bescii | ||
| JWCB18 | ΔpyrFA ldh::ISCbe4 Δcbe1(ura/5-FOAr) | 25 |
| JWCB52 | ΔpyrFA ldh::ISCbe4 Δcbe1 CIS1::PS-layerAcel0614(E1) (ura/5-FOAr) | 5 |
| JWCB87 | ΔpyrFA ldh::ISCbe4 Δcbe1 CIS1::PS-layerAcel0614(E1) CIS2::PS-layerAcel0180 (ura/5-FOAr) | This study |
| JWCB73 | JWCB52 containing pJGW07 (ura+/5-FOAs) | 13 |
| JWCB82 | JWCB18 containing pJGW07 (ura+/5-FOAs) | This study |
| JWCB95 | JWCB52 containing pSKW28 (ura+/5-FOAs) | This study |
| JWCB102 | JWCB87 containing pJGW07 (ura+/5-FOAs) | This study |
| JWCB103 | JWCB87 containing pSKW28 (ura+/5-FOAs) | This study |
| Plasmids | ||
| pJGW07 | E. coli/C. bescii shuttle vector containing the C. thermocellum pyrF gene (Aprr) | 27 |
| pSKW10 | Source of the Acel_0180 expression cassette | 13 |
| pSKW22 | Integrational vector for C. bescii CIS2 (Aprr) | 19 |
| pSKW23 | Integrational vector containing Acel_0180 expression cassette (PS-layerAcel0180) (Aprr) | This study |
| pSKW28 | Expression vector containing PS-layerTm0076 (Aprr) | This study |
Coexpression of the T. maritima β-d-xylosidase and the A. cellulolyticus xylanase in the C. bescii strain containing E1 was synergistic for xylan degradation.
To first test β-d-xylosidase activity in this strain, cells were grown at 65°C, and the extracellular protein fraction from JWCB82 (the parental strain), JWCB73 (containing E1), JWCB95 (containing E1 and the T. maritima β-d-xylosidase), JWCB102 (containing E1 and the A. cellulolyticus xylanase), and JWCB103 (containing E1, the β-d-xylosidase, and the xylanase) were compared. Expression and secretion of the Acel_0180 xylanase and Tm_0076 β-d-xylosidase were confirmed by zymogram analysis (Fig. S3). The extracellular protein fraction was then assayed for β-d-xylosidase activity at 65°C and 75°C on p-nitrophenyl β-d-xylopyranoside (pNP-X), a substrate specific for β-d-xylosidase activity. The stability and activity of native C. bescii xylan-degrading enzymes are known to decrease significantly at temperatures higher than 85°C (13). While the optimal temperature for growth of T. maritima is 80°C (20), the optimal temperature for activity of the β-d-xylosidase was reported to be 90°C (12). For the strains expressing β-d-xylosidase there was, as expected, an increase in β-d-xylosidase activity. Culture supernatants from JWCB95 showed a 70% (P = 0.019) increase over the parental strain at 65°C and a 46% (P = 0.009) higher β-d-xylosidase activity at 75°C. Culture supernatants from JWCB103 showed a 41% (P = 0.003) increase at 65°C and a 27% (P = 0.035) increase at 75°C (Fig. 4A).
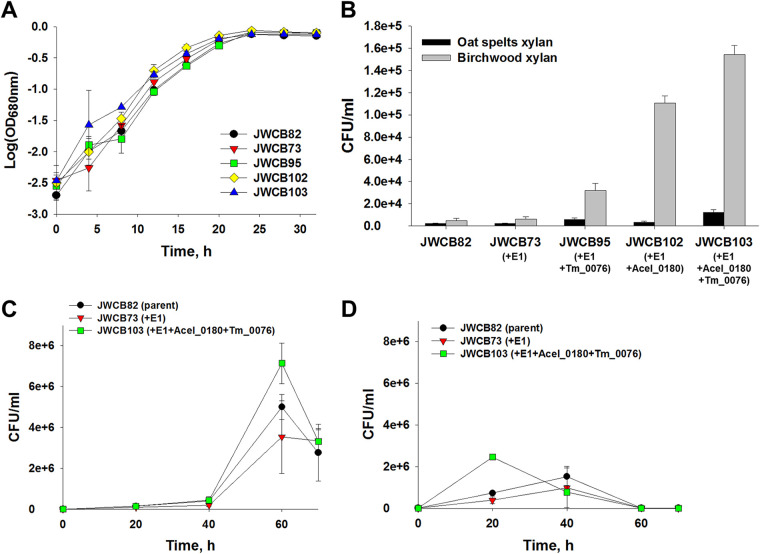
FIG 4.
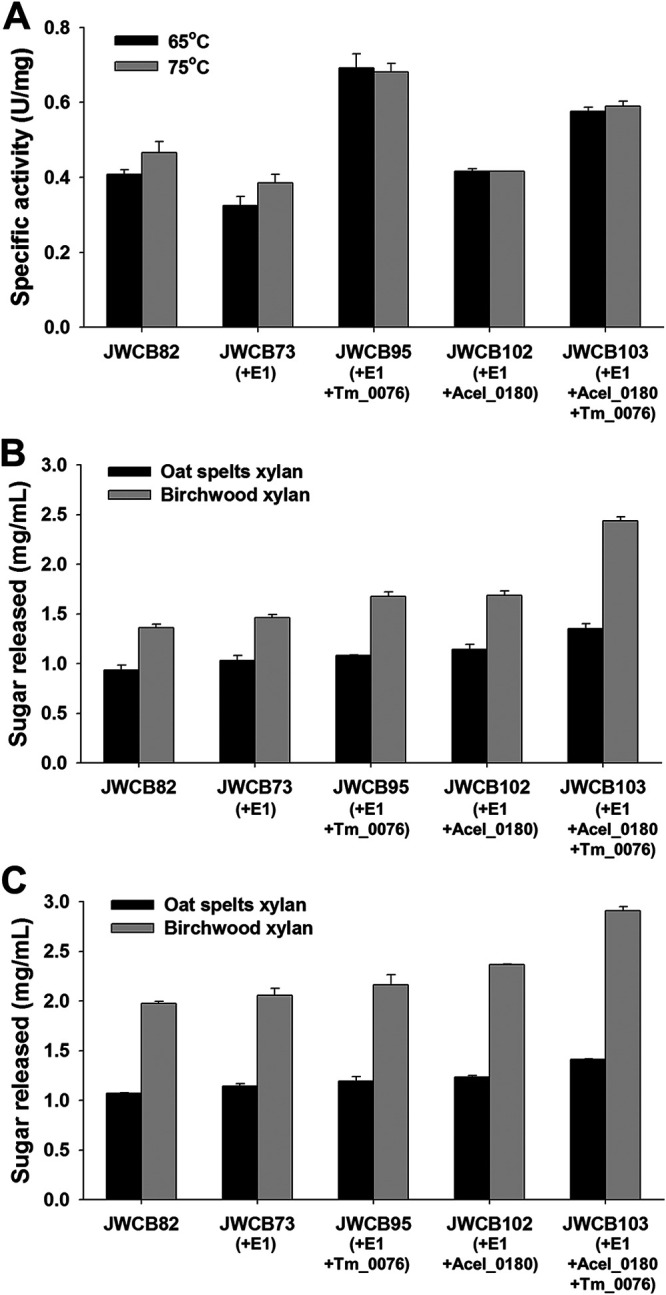
Effects of expression of Tm_0076 β-d-xylosidase and Acel_0180 xylanase on the activity of the C. bescii exoproteome. (A) The enzyme was incubated at 65°C or 75°C for 10 min in reaction buffer containing 5 mM pNP-X, 20 mM MES buffer (pH 5.5), 1 mM DTT, 1 mM CaCl2, and 1 mM MgCl2. (B and C) Relative enzymatic activity of the extracellular fraction of C. bescii strains on oat spelt and birchwood xylans. Activity of extracellular protein (25 μg/ml concentrated protein) on oat spelt and birchwood xylans was measured after 12 h of incubation at 65°C (B) or 75°C (C). JWCB82, the parent strain used in these experiments; JWCB73, the E1 expression strain; JWCB95, the E1 expression strain containing Tm_0076; JWCB102, the E1 and Acel_0180 expression strain; JWCB103, the E1 and Acel_0180 expression strain containing Tm_0076. Results are means from triplicate experiments, and error bars indicate standard deviations.
Xylobiose is a known inhibitor of xylanase activity, and studies have shown that the exogenous addition of β-d-xylosidase markedly improved the performance of some xylanases (10, 11). To examine whether expression of the β-d-xylosidase enhanced the xylan-degrading activity of the exoproteome, enzyme assays were performed at 65°C using oat spelt and birchwood xylans as substrates using the same three strains. Oat spelt xylan is a complex arabinoxylan, branched with arabinose residues. Birchwood xylan is a simpler, primarily unsubstituted xylose polymer with traces of uronic acids as side groups (more than 90% β-1,4-linked xylose residues) (21, 22). As previously reported (13), the exoproteome from a xylanase-expressing strain (JWCB102) showed 23% (P = 0.026) higher activity on oat spelt xylan and 24% (P = 0.008) higher activity on birchwood xylan than the parental strain, JWCB82 (Fig. 4B). Increased activity of the concentrated culture supernatants from the β-d-xylosidase-expressing strain was observed for both oat spelt and birchwood xylans compared to the parental strain, indicating that increasing the extracellular β-d-xylosidase does, in fact, increase xylan hydrolysis. In JWCB102, the β-d-xylosidase most likely aids the native C. bescii xylanases, but an even greater synergy is observed in JWCB103, which also expresses the A. cellulolyticus xylanase. The activity of the exoproteome from JWCB95 (the β-d-xylosidase expression strain without the A. cellulolyticus xylanase) on oat spelt and birchwood xylans increased 16% (P = 0.009) and 23% (P < 0.001), and the activity from JWCB103 (the β-d-xylosidase and A. cellulolyticus xylanase coexpression strain) increased 45% (P < 0.001) and 79% (P < 0.001), respectively (Fig. 4B). Total xylanase activity increased at 75°C (Fig. 4C), but the pattern was the same (Fig. 4C). These results suggest that the T. maritima β-d-xylosidase acts synergistically with both the native and A. cellulolyticus xylanases to deconstruct xylans more efficiently.
Coexpression of the T. maritima β-d-xylosidase and the A. cellulolyticus xylanase in the C. bescii strain containing E1 resulted in an increase in the ability of C. bescii to grow on xylan substrates.
To examine the effect of the expression of the heterologous β-d-xylosidase and xylanase on the growth of the C. bescii strain containing E1, growth was first measured on the soluble substrate, cellobiose, as the sole carbon source, a disaccharide that does not require the activity of either a xylanase or cellulase. As shown in Fig. 5A, growth of the JWCB82 (the parental strain), JWCB73 (the E1 expression strain), JWCB95 (the E1 expression strain containing the A. cellulolyticus xylanase), JWCB102 (the E1 expression strain containing the T. maritima β-d-xylosidase), and JWCB103 (the E1 expression strain containing both the A. cellulolyticus xylanase and T. maritima β-d-xylosidase) strains was virtually identical. While doubling time of JWCB102 was slightly shorter than that of JWCB82, it was not significant (P = 0.81). This result indicates that expression of these heterologous enzymes had no obvious effect on growth in general.
FIG 5.
Growth of C. bescii strains on cellobiose (A), xylan substrates (B), Avicel (C), or Avicel plus oat spelt xylan (D). (A) Growth as measured by OD at 680 nm. (B) Viable cell numbers after 36 h of cultivation on xylan substrates. (C and D) Growth of recombinant strains on Avicel without (C) and with (D) the addition of xylan. JWCB82, the parent strain used in these experiments; JWCB73, the E1 expression strain; JWCB95, the E1 expression strain containing Tm_0076; JWCB102, the E1 and Acel_0180 expression strain; JWCB103, the E1 and Acel_0180 expression strain containing Tm_0076. Results are the means from duplicate experiments, and error bars indicate standard deviations.
As previously reported (13) and as shown in Fig. 5B, growth of JWCB102 (the E1-expressing strain containing the A. cellulolyticus xylanase) on birchwood xylan was 23.0-fold (P = 0.008) higher than that of the JWCB82 parental strain. JWCB95, containing E1 and the Tm_0076 β-d-xylosidase, resulted in a dramatic increase in the ability of this strain to grow on xylan, a 2.7 (P = 0.076)- and 6.7 (P = 0.039)-fold increase on oat spelt and birchwood xylans, respectively, over that of the JWCB82 parental strain (Fig. 5B). Growth of JWCB103 (the E1 expression strain containing both the A. cellulolyticus xylanase and T. maritima β-d-xylosidase) was even more dramatic, a 5.7 (P = 0.052)- and 32.1 (P = 0.009)-fold increase on oat spelt and birchwood xylans, respectively, over that of the JWCB82 parental strain. We suggest that in both JWCB102 and JWCB103, Acel_0180 helps release more xylobiose or xylotriose in the medium and further reduces the degree of polymerization of the deconstructed xylans, rendering them readily transported and used as a carbon source. This is apparently more pronounced in the case of the simpler substrate (birchwood xylans) than the more complex branched substrate (oat spelt xylans). On oat spelt xylan, the activity of Acel_0180 as well as other xylanases is likely limited by increased branching that reduces accessibility. In addition, the released xylans, most likely branched, may not be as readily transported or utilized by the microorganism. In JWCB103, the increased growth is most likely due to decreased inhibition by xylobiose or xylotriose, resulting from the action of the β-d-xylosidase on xylanases including Acel_0180, leading to increased overall solubilization. This is also demonstrated by the difference in growth between JWCB95 and JWCB73, where the additional β-d-xylosidase is augmenting the native xylanases in the C. bescii exoproteome, leading to higher growth.
The combination of the β-d-xylosidase and xylanase substantially improves the activity of the exoproteome on cellulose, even in the presence of exogenous xylan.
To test whether the Tm_0076 β-d-xylosidase and Acel_0180 xylanase do, in fact, aid in cellulose utilization by relieving inhibition of xylobiose or xylotriose accumulating in the media, growth was measured on Avicel with and without the addition of xylan. The strains JWCB073, JWCB082, and JWCB103 were grown on both 5 g/liter Avicel alone and 5 g/liter Avicel plus 2.5 g/liter oat spelt xylan at 65°C. Oats spelt xylan was chosen because it is a poor substrate for growth (Fig. 5B), it should be inhibitory because it contains branched arabinose residues, and its deconstruction is less affected by the presence of Tm_0076 β-d-xylosidase and Acel_0180 (Fig. 4B). On Avicel alone, the combination of E1, the Tm_0076 β-d-xylosidase, and the Acel_0180 xylanase (JWCB103) resulted in significantly better growth than the parent strain (JWCB82) or the E1-containing strain (JWCB73), a 43% increase compared to the parent strain JWCB082 at 60 h (Fig. 5C). These results indicate that the combination of the β-d-xylosidase and xylanase allows C. bescii to more readily utilize the negligible (∼5%) xylan content in Avicel. Additionally, this increase could also be due to the fact that the GH3 in Tm_0076 possesses β-d-glucosidase activity (12) or that the fibronectin-like domain aids in the deconstruction of Avicel, as shown for other biomass-degrading enzymes (23). Growth of JWCB103 on Avicel in the presence of exogenous oat spelt xylan resulted in significant differences in both the timing and overall amount of growth. Perhaps the most striking difference was the almost total elimination of a lag phase for JWCB103 and a reduced lag time for JWCB73 and JWCB82 (Fig. 5D) compared to growth on Avicel alone. The overall growth was also less than that on Avicel alone. This might be explained by the fact that C. bescii can utilize the easily accessible xylan, a preferred carbon source, in oat spelt xylan, resulting in increased biomass and the production of complex biomass-degrading enzymes earlier in the fermentation. We also believe that part of this increase is due to the xylan content in Avicel but is not sufficient to explain that level of increased growth. While this is true for both JWCB73 and JWCB82, the inhibition of oat spelt xylan during fermentation, resulting in less biomass production, is more clear.
Conclusions.
The ability of C. bescii to deconstruct nonpretreated plant biomass, its ability to grow anaerobically at high temperature, and its ability to use both C5 and C6 sugars simultaneously make it of special interest for use in consolidated bioprocessing to produce fuels, chemicals, and materials from this sustainable substrate. C. bescii also represents a model for understanding the fundamentals of plant cell wall deconstruction given its unusual cellulolytic activity. In previous studies, the supplementation of its secretome with heterologous CAZyme cassettes led to significant increases in cellulolytic activity and growth on complex substrates. In this study, we examined CAZyme cassettes with specific predicted synergy, a β-d-xylosidase thought to relieve substrate inhibition, and a xylanase, while also likely to be susceptible to inhibition by xylooligomers (in this case xylobiose and xylotriose), might augment the native C. bescii xylanases. Taken together, the data presented support those predictions. Significant increases in the enzymatic activity of the exoproteome as well as dramatic effects on growth were observed, suggesting synergistic interactions between CAZymes in vivo. We further suggest that this kind of study will facilitate the optimization and the synergy within and with these heterologous CAZyme cassettes to further improve thermophilic consolidated bioprocessing in other microbes. Finally, we suggest that the results shown in this study represent an important step toward addressing the inhibition of xylooligomers in consolidated bioprocessing at industrially relevant (high) solids loadings.
MATERIALS AND METHODS
Strains, media, and culture conditions.
E. coli and C. bescii strains used in this study are listed in Table 1. C. bescii strains were grown anaerobically at 65°C on solid or in liquid low-osmolarity defined (LOD) medium (24), as described previously, with 5 g/liter maltose or cellobiose as the sole carbon source for routine growth and transformation experiments (25). For growth of uracil auxotrophs, the medium contained 40 μM uracil. E. coli DH5α was used as the host for plasmid DNA construction and preparation using standard techniques. E. coli cells were cultured in LB broth containing apramycin (50 μg/ml). Plasmid DNA was isolated using a Qiagen miniprep kit (Qiagen, Valencia, CA, USA). Chromosomal DNA from C. bescii strains was extracted using the Quick-gDNA mINIpREP (Zymo, Irving, CA), as described previously (26).
Construction of a shuttle vector for β-d-xylosidase expression.
Q5 high-fidelity DNA polymerase (New England BioLabs, Ipswich, MA, USA) was used for all PCRs. Restriction enzymes (New England BioLabs, Ipwich, MA, USA) and the fast-link DNA ligase kit (Epicentre Biotechnologies, Madison, WI, USA) were used for plasmid constructions according to the manufacturer’s instructions. To construct pSKW28, a 2.3-kb DNA fragment containing the coding sequence of Tm_0076 was amplified using primers SK74 (with an XmaI site) and SK75 (with an AvrII site) using T. maritima MSB8 gDNA as the template. In addition, an 8.1-kb DNA fragment containing the C. bescii replication origin from BAS2, an apramycin resistance gene cassette (Aprr), a C. thermocellum pyrF expression cassette, the regulatory region of Cbes2303 (S-layer protein), the signal CelA signal sequence, a C-terminal 6× histidine tag, and a Rho-independent transcription terminator was amplified with primers SK21 (with XmaI site) and DC700 (with AvrII site) using pSKW10 (13) as the template. These two linear DNA fragments were digested with XmaI and AvrII and ligated to construct pSKW28 (see Fig. S1B in the supplemental material). Primers used are listed in Table 2.
TABLE 2.
List of primers used in this studya
| Name | Sequence (5′→3′) | Restriction enzyme | Description |
|---|---|---|---|
| SK74 | CCGCCCGGGATGGAACTGTACAGGGATCCTTC | XmaI | To construct pSKW23 |
| SK75 | AGACCTAGGCTCCTCGCAGGCTTCCGT | AvrII | |
| SK21 | CCGCCCGGGAAACGAACCAGCCCTAACCTCT | XmaI | To construct pSKW23 |
| DC700 | AGACCTAGGCATCACCATCACCATCACTAATAAT | AvrII | |
| DC460 | AGAGAGCGATCGACAGTTTGATTACAGTTTAGTCAGAGCT | PvuI | To construct pSKW28 |
| DC461 | AGAAGAAGGCGGCCGCTTGGTTCCTTAAATCTAAGAGGTATGA | NotI | |
| SK61 | AGAGAGCGATCGAGTGTTTTAAAAAGTGGCTAAAGATTAGAAGC | PvuI | To construct pSKW28 |
| SK62 | AGAAGAAGGCGGCCGCAGGTAAGTCTAAACTATTTAGCTGGTTGAG | NotI | |
| DC460 | AGAGAGCGATCGACAGTTTGATTACAGTTTAGTCAGAGCT | PvuI | To confirm transformants containing pSKW28 |
| DC228 | ATCATCCCCTTTTGCTGATG | ||
| SK65 | ATTAACTTGCTCAAAAACCTTGGCA | To verify the targeted insertion of the Acel_0180 expression cassette | |
| SK66 | TTGCAGCAGTGAGAAAACCTATG |
The italicized sequences indicate the recognition sites of the corresponding restriction enzymes.
Construction of a vector for insertion of the Family 10 xylanase (Acel_0180) from Acidothermus cellulolyticus into the C. bescii chromosome.
To construct pSKW23, the 2.3-kb Acel_0180 expression cassette, containing the regulatory region of Cbes2303 (S-layer protein), the CelA signal sequence, a C-terminal 6× histidine tag, and a Rho-independent transcription terminator, was amplified by PCR with primers DC460 (with PvuI site) and DC461 (with NotI site) using pSKW10 as the template. The 6.0-kb DNA fragment containing the 5′ flanking region (1.0 kb) and the 3′ flanking region (1.0 kb) of the targeted insertion site (an intergenic region between Cbes2199 and Cbes2200) in the C. bescii genome was amplified with primers SK61 (with PvuI site) and SK62 (with NotI site) using pSKW22 (19) as the template. These two linear DNA fragments were digested with PvuI and NotI and ligated to construct pSKW23 (Fig. 3A). Primers used are listed in Table 2.
Transformation, screening, purification, and sequence verification of engineered C. bescii strains.
Constructed plasmids were introduced into E. coli DH5α by electroporation in a 1-mm-gap cuvette at 1.8 kV, and transformants were selected for apramycin resistance. All plasmids were sequenced by automatic sequencing (Genewiz, South Plainfield, NJ, USA). Electrotransformation of C. bescii cells was performed as previously described (27). After an electropulse with plasmid DNA (∼0.5 μg), cultures were recovered in low-osmolarity complex (LOC) medium (24) at 75°C and transferred into liquid LOD medium (24) without uracil to select uracil prototrophy. For selection of shuttle vectors, cultures were plated on solid LOD medium to obtain isolated colonies, and total DNA was extracted for PCR confirmation. Taq polymerase (Sigma, St. Louis, MO, USA) was used for PCRs. PCR amplification was done with primers (DC460 and DC228) outside the gene cassette on the plasmid to confirm the presence of gene insertion. To insert Acel_0180 into the C. bescii chromosome, recovery cultures were transferred into liquid LOD medium (24) without uracil to select uracil prototrophic transformants, and transformants were inoculated into nonselective liquid defined medium with 40 μM uracil and incubated overnight at 65°C to allow loop-out of the plasmid. The cultures were then plated onto 5-fluorootic acid (5-FOA) (8 mM) containing solid medium, and transformants containing the insertion were purified by two additional passages under selection on solid medium and screened a second time by PCR. The insertion of the Acel_0180 expression cassette in the targeted region was confirmed by PCR amplification using primers (SK65 and SK66) outside the homologous regions used to construct the insertion, generating JWCB87, and the PCR product was sequenced. Primers used are listed in Table 2.
Preparation of extracellular protein and zymogram analysis.
To collect the extracellular protein (ECP) fraction, C. bescii cells were grown in 2 liters of LOD medium with 40 mM morpholinepropanesulfonic acid (MOPS) in closed bottles at 65°C with shaking at 150 rpm to an optical density at 680 nm (OD680) of 0.25 to 0.3. Culture broth was centrifuged (6,000 × g at 4°C for 15 min) and filtered (glass fiber, 0.7 μm) to separate cells. The 2 liters of ECP was loaded onto a hollow fiber cartridge with a 3-kDa molecular mass cut off (GE Healthcare, Buckinghamshire, UK) and eluted with 50 ml buffer 20 mM morpholineethanesulfonic acid (MES)/2 mM β-mercaptoethanol (pH 5.5). The 50 ml EP was concentrated (∼25 times) with a Vivaspin column (10-kDa molecular mass cut off; Sartorius, Göttingen, Germany). Protein concentrations were determined using the Bio-Rad protein assay kit with bovine serum albumin (BSA) as the standard. ECP samples (10 μg) were electrophoresed in 4 to 20% gradient Mini-Protean TGX gels (Bio-Rad), and protein bands were visualized by staining with Coomassie brilliant blue G-250. For detection of in-gel β-d-xylosidase activity, ECP samples (15 μg) were electrophoresed in 4 to 20% gradient Mini-Protean TGX gels (Bio-Rad). After soaking the gel for 1 h in 2.5% (vol/vol) Triton X-100 solution to remove the SDS, the zymogram gel was incubated at 75°C for 20 min in reaction buffer containing 0.3 mM 4-methylumbelliferyl β-d-xylopyranoside, 20 mM MES (pH 5.5), 1 mM dithiothreitol (DTT), 1 mM CaCl2, and 1 mM MgCl2. The presence of fluorescent reaction product was visualized under UV light using a gel document system. Detection of in-gel β-d-glucosidase activity was performed similarly to that of β-d-xylosidase activity, but the substrate was 5 mM 4-methylumbelliferyl β-d-glucopyranoside. For the zymogram analysis of xylanase, ECP samples (15 μg) were electrophoresed in 12% polyacrylamide gel with a 5% stacking gel containing 0.1% birchwood xylan. After removing SDS and incubating the gel in the reaction buffer as described above, the gel was submerged in 0.1% (wt/vol) Congo red solution for 30 min and destained with 1 M NaCl until pale-red hydrolysis zones appeared. The reaction was stopped by dipping the gel into a 5% acetic acid solution. Quantification of band intensity was carried out using densitometry software (Total Lab 1.01; Nonlinear Dynamics Ltd.).
Enzyme activity assays.
The reaction mixture for β-d-xylosidase activity contained 750 μl distilled water, 100 μl of 200 mM MES buffer (pH 5.5), 10 μl of 100 mM DTT, 10 μl of 100 mM CaCl2, 10 μl of 100 mM MgCl2, and 20 μl of the crude enzyme solution and was preheated at 75°C for 10 min. The absorbance change at 65°C and 75°C and 405-nm wavelength was monitored using a Jenway Genova spectrophotometer after adding 100 μl of 50 mM p-nitrophenyl β-d-xylopyranoside (pNP-X; Sigma, USA). One unit of β-d-xylosidase activity was defined as the amount of enzyme needed to release 1 μmol p-NP (p-nitrophenol) from pNP-X per min. Specific enzyme activity (U/mg protein) was estimated by dividing the enzyme activity by the total protein concentration. Protein concentrations were determined using the Bio-Rad protein assay kit. Enzyme activity on xylan substrates was measured using 10 g/liter of either oat spelt or birchwood xylan (Sigma, USA) in MES reaction buffer (pH 5.5) as previously described (28). Cells were grown in a 2-liter volume of LOD medium with 40 mM MOPS and 5 g/liter maltose as the carbon source. A concentration of 25 μg/ml extracellular protein fraction was added to each reaction mixture and incubated at 65°C and 75°C for 12 h. Reducing sugars in the supernatant were measured using dinitrosalicylic acid (DNS). Samples and standards (xylose) were mixed 1:1 with DNS reaction solution, boiled for 2 min, and measured at the OD575. Activity was reported as milligram per milliliter of sugar released.
Growth of recombinant strains on cellobiose and xylan.
To measure growth on cellobiose, cells were subcultured twice in LOD medium with 5 g/liter maltose as the sole carbon source and used to inoculate media with cellobiose (1% total volume for all experiments) as the sole carbon source to a final concentration of 5 g/liter in 50 ml LOD medium with 40 mM MOPS and then incubated at 65°C with shaking at 150 rpm. Cell growth on cellobiose was measured by OD680 using a Jenway Genova spectrophotometer. To measure growth on oat spelt and birchwood xylans, both the subculture and the initial culture were performed in LOD medium with 5 g/liter oat spelt and birchwood xylans. Numbers of CFU were measured by plating cells on LOC medium.
Growth of recombinant strains on Avicel with and without the addition of xylan.
Frozen cells were revived and then subcultured twice in LOD medium with 5 g/liter maltose as the sole carbon source and with 40 mM MOPS. The second subculture was grown to mid-log phase and used to inoculate 50 ml of LOD medium supplemented with 40 mM MOPS with either 5 g/liter of Avicel or 5 g/liter Avicel plus 2.5 g/liter oat spelt xylan. A 0.2% (vol/vol) inoculum was used, and cultures were incubated at 65°C with shaking at 150 rpm. Growth was measured as number of CFU of serially diluted samples and plating on LOC medium with 5 g/liter maltose. Plates were incubated anaerobically at 65°C for 4 days.
ACKNOWLEDGMENTS
Funding was provided by the BioEnergy Science Center (BESC) and the Center for Bioenergy Innovation (CBI), from the U.S. Department of Energy Bioenergy Research Centers, supported by the Office of Biological and Environmental Research in the DOE Office of Science. This work was authored in part by Alliance for Sustainable Energy, LLC, the Manager and Operator of the National Renewable Energy Laboratory for the U.S. Department of Energy (DOE) under contract no. DE-AC36-08GO28308.
The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government.
Footnotes
Supplemental material is available online only.
Contributor Information
Janet Westpheling, Email: janwest@uga.edu.
Rebecca E. Parales, University of California, Davis
REFERENCES
- 1.Yang SJ, Kataeva I, Hamilton-Brehm SD, Engle NL, Tschaplinski TJ, Doeppke C, Davis M, Westpheling J, Adams MW. 2009. Efficient degradation of lignocellulosic plant biomass, without pretreatment, by the thermophilic anaerobe “Anaerocellum thermophilum” DSM 6725. Appl Environ Microbiol 75:4762–4769. 10.1128/AEM.00236-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lochner A, Giannone RJ, Rodriguez M, Jr, Shah MB, Mielenz JR, Keller M, Antranikian G, Graham DE, Hettich RL. 2011. Use of label-free quantitative proteomics to distinguish the secreted cellulolytic systems of Caldicellulosiruptor bescii and Caldicellulosiruptor obsidiansis. Appl Environ Microbiol 77:4042–4054. 10.1128/AEM.02811-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunecky R, Alahuhta M, Xu Q, Donohoe BS, Crowley MF, Kataeva IA, Yang SJ, Resch MG, Adams MWW, Lunin VV, Himmel ME, Bomble YJ. 2013. Revealing nature's cellulase diversity: the digestion mechanism of Caldicellulosiruptor bescii CelA. Science 342:1513–1516. 10.1126/science.1244273. [DOI] [PubMed] [Google Scholar]
- 4.Dam P, Kataeva I, Yang SJ, Zhou FF, Yin YB, Chou WC, Poole FL, Westpheling J, Hettich R, Giannone R, Lewis DL, Kelly R, Gilbert HJ, Henrissat B, Xu Y, Adams MWW. 2011. Insights into plant biomass conversion from the genome of the anaerobic thermophilic bacterium Caldicellulosiruptor bescii DSM 6725. Nucleic Acids Res 39:3240–3254. 10.1093/nar/gkq1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung D, Young J, Cha M, Brunecky R, Bomble YJ, Himmel ME, Westpheling J. 2015. Expression of the Acidothermus cellulolyticus E1 endoglucanase in Caldicellulosiruptor bescii enhances its ability to deconstruct crystalline cellulose. Biotechnol Biofuels 8:113. 10.1186/s13068-015-0296-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim SK, Chung D, Himmel ME, Bomble YJ, Westpheling J. 2017. In vivo synergistic activity of a CAZyme cassette from Acidothermus cellulolyticus significantly improves the cellulolytic activity of the C. bescii exoproteome. Biotechnol Bioeng 114:2474–2480. 10.1002/bit.26366. [DOI] [PubMed] [Google Scholar]
- 7.Kim SK, Himmel ME, Bomble YJ, Westpheling J. 2017. Expression of a cellobiose phosphorylase from Thermotoga maritima in Caldicellulosiruptor bescii improves the phosphorolytic pathway and results in a dramatic increase in cellulolytic activity. Appl Environ Microbiol 84:e02348-17. 10.1128/AEM.02348-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qing Q, Wyman CE. 2011. Supplementation with xylanase and beta-xylosidase to reduce xylo-oligomer and xylan inhibition of enzymatic hydrolysis of cellulose and pretreated corn stover. Biotechnol Biofuels 4:18. 10.1186/1754-6834-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qing Q, Yang B, Wyman CE. 2010. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour Technol 101:9624–9630. 10.1016/j.biortech.2010.06.137. [DOI] [PubMed] [Google Scholar]
- 10.Huang D, Liu J, Qi YF, Yang KX, Xu YY, Feng L. 2017. Synergistic hydrolysis of xylan using novel xylanases, β-xylosidases, and an α-L-arabinofuranosidase from Geobacillus thermodenitrificans NG80-2. Appl Microbiol Biotechnol 101:6023–6037. 10.1007/s00253-017-8341-2. [DOI] [PubMed] [Google Scholar]
- 11.Yang XZ, Shi PJ, Huang HQ, Luo HY, Wang YR, Zhang W, Yao B. 2014. Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem 148:381–387. 10.1016/j.foodchem.2013.10.062. [DOI] [PubMed] [Google Scholar]
- 12.Xue YM, Shao WL. 2004. Expression and characterization of a thermostable β-xylosidase from the hyperthermophile, Thermotoga maritima. Biotechnol Lett 26:1511–1515. 10.1023/B:BILE.0000044454.70768.81. [DOI] [PubMed] [Google Scholar]
- 13.Kim SK, Chung D, Himmel ME, Bomble YJ, Westpheling J. 2016. Heterologous expression of family 10 xylanases from Acidothermus cellulolyticus enhances the exoproteome of Caldicellulosiruptor bescii and growth on xylan substrates. Biotechnol Biofuels 9:176. 10.1186/s13068-016-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung D, Cha M, Guss AM, Westpheling J. 2014. Direct conversion of plant biomass to ethanol by engineered Caldicellulosiruptor bescii. Proc Natl Acad Sci U S A 111:8931–8936. 10.1073/pnas.1402210111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung D, Young J, Bomble YJ, Vander Wall TA, Groom J, Himmel ME, Westpheling J. 2015. Homologous expression of the Caldicellulosiruptor bescii CelA reveals that the extracellular protein is glycosylated. PLoS One 10:e0119508. 10.1371/journal.pone.0119508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan DB, Stoller JR, Lee CC, Chan VJ, Wagschal K. 2017. Biochemical characterization of a GH43 β-xylosidase from Bacteroides ovatus. Appl Biochem Biotechnol 182:250–260. 10.1007/s12010-016-2324-0. [DOI] [PubMed] [Google Scholar]
- 17.Barker IJ, Petersen L, Reilly PJ. 2010. Mechanism of xylobiose hydrolysis by GH43 β-xylosidase. J Phys Chem B 114:15389–15393. 10.1021/jp107886e. [DOI] [PubMed] [Google Scholar]
- 18.Kado Y, Inoue T, Ishikawa K. 2011. Structure of hyperthermophilic β-glucosidase from Pyrococcus furiosus. Acta Crystallogr Sect F Struct Biol Cryst Commun 67:1473–1479. 10.1107/S1744309111035238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SK, Chung D, Himmel ME, Bomble YJ, Westpheling J. 2019. Heterologous co-expression of two β-glucanases and a cellobiose phosphorylase resulted in a significant increase in the cellulolytic activity of the Caldicellulosiruptor bescii exoproteome. J Ind Microbiol Biotechnol 46:687–695. 10.1007/s10295-019-02150-0. [DOI] [PubMed] [Google Scholar]
- 20.Huber R, Langworthy TA, Koenig H, Thomm M, Woese CR, Sleytr UB, Stetter KO. 1986. Thermotoga maritima sp. nov. represents a new genus of unique extremely thermophilic eubacteria growing up to 90°C. Arch Microbiol 144:324–333. 10.1007/BF00409880. [DOI] [Google Scholar]
- 21.Liab K, Azadi P, Collins R, Tolan J, Kim JS, Eriksson KEL. 2000. Relationships between activities of xylanases and xylan structures. Enzyme Microb Technol 27:89–94. 10.1016/S0141-0229(00)00190-3. [DOI] [PubMed] [Google Scholar]
- 22.Puls J, Schroder N, Stein A, Janzon R, Saake B. 2005. Xylans from oat spelts and birch kraft pulp. Macromol Symp 232:85–92. 10.1002/masy.200551410. [DOI] [Google Scholar]
- 23.Kataeva IA, Seidel RD, Shah A, West LT, Li XL, Ljungdahl LG. 2002. The fibronectin type 3-like repeat from the Clostridium thermocellum cellobiohydrolase CbhA promotes hydrolysis of cellulose by modifying its surface. Appl Environ Microbiol 68:4292–4300. 10.1128/aem.68.9.4292-4300.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farkas J, Chung D, Cha M, Copeland J, Grayeski P, Westpheling J. 2013. Improved growth media and culture techniques for genetic analysis and assessment of biomass utilization by Caldicellulosiruptor bescii. J Ind Microbiol Biotechnol 40:41–49. 10.1007/s10295-012-1202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung D, Farkas J, Westpheling J. 2013. Overcoming restriction as a barrier to DNA transformation in Caldicellulosiruptor species results in efficient marker replacement. Biotechnol Biofuels 6:82. 10.1186/1754-6834-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung D, Huddleston JR, Farkas J, Westpheling J. 2011. Identification and characterization of CbeI, a novel thermostable restriction enzyme from Caldicellulosiruptor bescii DSM 6725 and a member of a new subfamily of HaeIII-like enzymes. J Ind Microbiol Biotechnol 38:1867–1877. 10.1007/s10295-011-0976-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groom J, Chung D, Young J, Westpheling J. 2014. Heterologous complementation of a pyrF deletion in Caldicellulosiruptor hydrothermalis generates a new host for the analysis of biomass deconstruction. Biotechnol Biofuels 7:132. 10.1186/s13068-014-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanafusa-Shinkai S, Wakayama J, Tsukamoto K, Hayashi N, Miyazaki Y, Ohmori H, Tajima K, Yokoyama H. 2013. Degradation of microcrystalline cellulose and non-pretreated plant biomass by a cell-free extracellular cellulase/hemicellulase system from the extreme thermophilic bacterium Caldicellulosiruptor bescii. J Biosci Bioeng 115:64–70. 10.1016/j.jbiosc.2012.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1 to S3. Download AEM00524-21_Supp_1_seq11.pdf, PDF file, 0.3 MB (260.3KB, pdf)