ABSTRACT
The present study investigated the impact of on-farm anaerobic digestion on the abundance of enteric bacteria, antibiotic resistance-associated gene targets, and the horizontal transfer potential of extended-spectrum β-lactamase (ESBL) genes. Samples of raw and digested manure were obtained from six commercial dairy farms in Ontario, Canada. Digestion significantly abated populations of viable coliforms in all six farms. Conjugative transfer of plasmids carrying β-lactamase genes from manure bacteria enriched overnight with buffered peptone containing 4 mg/liter cefotaxime into a β-lactam-sensitive green fluorescent protein (GFP)-labeled Escherichia coli recipient strain was evaluated in patch matings. Digestion significantly decreased the frequency of the horizontal transfer of ESBL genes. Twenty-five transconjugants were sequenced, revealing six distinct plasmids, ranging in size from 40 to 180 kb. A variety of ESBL genes were identified: blaCTX-M-1, blaCTX-M-14, blaCTX-M-15, blaCTX-M-27, blaCTX-M-55, and blaPER-1. blaCTX-M-15 was the most prevalent ESBL gene detected on plasmids harbored by transconjugants. Various mobile genetic elements were found located proximal to resistance genes. Ten gene targets, including sul1, str(A), str(B), erm(B), erm(F), intI1, aadA, incW, blaPSE, and blaOXA-20, were quantified by quantitative PCR on a subset of 18 raw and 18 digested samples. Most targets were significantly more abundant in raw manure; however, erm(B) and erm(F) targets were more abundant in digested samples. Overall, on-farm digestion of dairy manure abated coliform bacteria, a number of antibiotic resistance-associated gene targets, and the potential for in vitro conjugation of plasmids conferring resistance to extended-spectrum β-lactams and other classes of antibiotics into E. coli CV601.
IMPORTANCE Using livestock manure for fertilization can entrain antibiotic-resistant bacteria into soil. Manure on some dairy farms is anaerobically digested before being land applied. Recommending the widespread implementation of the practice should be founded on understanding the impact of this treatment on various endpoints of human health concern. Although lab-scale anaerobic treatments have shown potential for reducing the abundance of antibiotic resistance genes, there are very few data from commercial farms. Anaerobic digestion of manure on six dairy farms efficiently abated coliform bacteria, E. coli, and a majority of antibiotic resistance-associated gene targets. In addition, the conjugation potential of plasmids carrying ESBL genes into introduced E. coli strain CV601 was reduced. Overall, anaerobic digestion abated coliform bacteria, the genes that they carry, and the potential for ESBL-carrying plasmid transfer.
KEYWORDS: antibiotic resistance, dairy manure, anaerobic digestion, extended-spectrum β-lactamase genes, plasmid conjugation
INTRODUCTION
Manures from poultry, cattle, and swine farms are a valued source of nutrients and organic matter when used as fertilizers in crop production (1). However, fecal material can contain viral, bacterial, or parasitic pathogens that can threaten human health, and thus these amendments must be managed judiciously, particularly when used to grow crops destined for direct human consumption (2). Manures from animals that receive antibiotics are enriched in antibiotic-resistant bacteria and may contain excreted antibiotic residues (3, 4). There is a concern that contamination of agricultural soil with antibiotic-resistant bacteria and antibiotic residues will increase the reservoir of antibiotic resistance in crop production systems that are amended with manures (5, 6). Presumably, human exposure to this additional burden of resistant bacteria through consumption of contaminated crops represents a health risk (7).
Pretreatment of manures prior to land application to reduce the burden of antibiotic-resistant bacteria and destroy antibiotic residues should reduce this potential health risk (8). Treatment options commonly practiced on commercial farms consist of aerobic composting or anaerobic digestion at either mesophilic or thermophilic temperatures (9). Anaerobic digestion offers the advantage of generating biogas (methane, hydrogen, and carbon dioxide) that can be exploited for energy production, and thus this approach is widely practiced in many jurisdictions (10–13). The technology employed for anaerobic digestion ranges from the rudimentary to the quite sophisticated and thus has the advantage of being usable across a range of income settings (14).
The potential abatement of antibiotic-resistant bacteria in manure by anaerobic digestion and how this varies with process parameters are as yet not completely understood (15, 16). Process parameters, including temperature, pH, organic loading rate, and hydraulic retention time, are important factors for the efficient removal of pathogens, veterinary antibiotics, and antibiotic resistance genes while contributing to increased biogas production (15, 16). There have been several studies on the effect of temperature on the abundance of antibiotic resistance genes with findings that are often conflicting or inconsistent, indicating that other factors must be important (17, 18).
Antibiotic resistance and associated risk factors in bovine/dairy manure have been studied previously (19–21). Bovine manure not only improved the phosphorus and organic matter in soil because of its ideal C/N ratio (22/1) for soil microorganisms but also had lower antibiotic resistance gene diversity and abundance compared to those of other animal manures (22, 23). Dairy farm manure was previously found to contain extended-spectrum β-lactamase (ESBL)-/AmpC-encoding genes, including blaCTX-M-1, -2, -14, -15, -32, and -55 and blaTEM-52 (19, 21, 24, 25). Other antibiotic resistance genes detected in dairy manure confer resistance to tetracycline [tet(C), tet(G), tet(M), tet(Q), tet(X), tet(W)], sulfonamides (sul1, sul2), macrolides [mef(A), erm(B), erm(Q)], and aminoglycosides and fluoroquinolones [aac(6′)-Ib-cr] (20, 26). Mobile genetic elements such as plasmids, integrons, or transposons are normally associated with antibiotic resistance genes to facilitate the gene transfer among bacterial communities (26). Network analysis revealed the strong cooccurrence of mobile genetic elements (intI1, intI2, and ISCR1) and six antibiotic resistance genes (27).
In previous work with manure sourced from a commercial dairy farm, anaerobic digestion abated viable enteric bacteria but did not reduce the abundance of antibiotic resistance genes quantified by quantitative PCR (qPCR) (28). In the present study, manures prior to and after anaerobic digestion were sampled from six commercial dairy farms in Ontario, Canada, on a monthly basis over approximately 1 year. A detailed description of manure types as well as manure sampling methods can be found in the supplemental material (Text S1 and Fig. S2). Various enteric bacteria were quantified by viable plate count, and the abundance of selected gene targets associated with antibiotic resistance was quantified by qPCR. The potential for in vitro conjugal transfer of plasmids conferring resistance to β-lactam antibiotics from manure communities into an introduced GFP-tagged Escherichia coli recipient (CV601) was determined. Overall, results from the present study indicate that under commercial conditions, anaerobic digestion efficiently removed coliform bacteria and a majority of resistance genes. However, it had little effect on Gram-positive bacteria and increased the abundance of the gene targets ermB and ermF.
RESULTS
Impact of anaerobic digestion on the abundance of viable enteric bacteria.
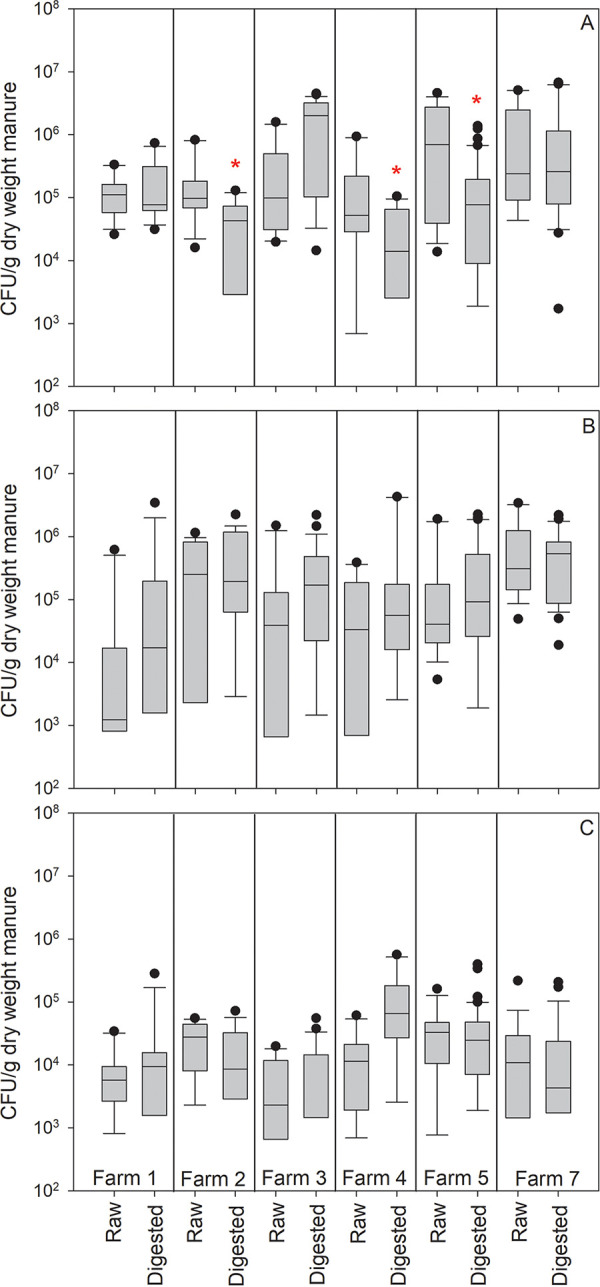
A variety of bacteria in raw and in digested manure were enumerated by viable plate count. Total coliforms and E. coli colonies enumerated on Chromocult agar medium were consistently 1.1 log10 to 2.2 log10-fold lower in digested samples than in raw samples across all six participating farms (Fig. 1). In contrast, there was little impact of digestion on the abundance of Gram-positive bacteria, including Enterococcus spp., Staphylococcus spp., and presumptive Clostridium perfringens (Fig. 2).
FIG 1.
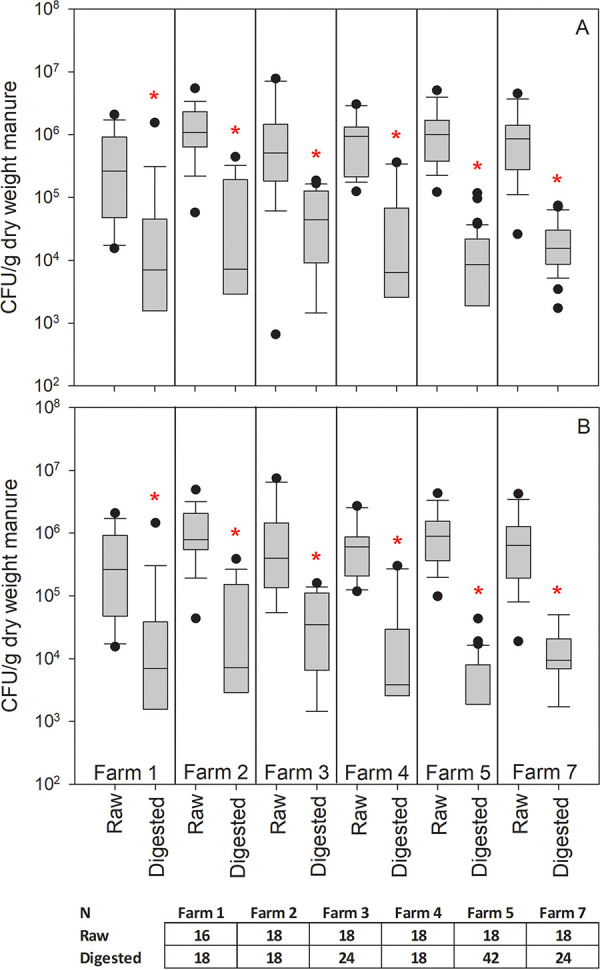
A comparison of coliform bacterial counts in raw and digested manure across the six participating farms: (A) total coliforms and (B) E. coli. Boxes represent the 25th to 75th percentile, whiskers indicate the 10th and 90th percentiles, and dots represent outliers outside the 10th and 90th percentiles. Asterisks indicate a statistically significant difference between treatments (Mann-Whitney U test, P < 0.05). N represents the number of samples collected for each treatment at each farm location.
FIG 2.
A comparison of selected Gram-positive viable counts in raw and digested manure across the six participating farms: (A) Enterococcus spp., (B) Staphylococcus spp., and (C) Clostridium perfringens. Box plot description and sample numbers per farm are indicated in Fig. 1. Asterisks indicate a statistically significant difference (Mann-Whitney U test, P < 0.05) between raw manure and digested manure.
In vitro conjugation frequency from raw and digested manure into E. coli CV601.
Transconjugants were identified as ESBL or non-ESBL based on the outcome of the ESBL confirmation test. Manure samples that yielded ESBL transconjugants were identified as carrying the ESBL phenotype, samples that yielded non-ESBL transconjugants were considered non-ESBL phenotype, and samples that yielded cefotaxime-resistant transconjugants of either or both phenotypes (ESBL, non-ESBL, or both) were considered extended-spectrum cephalosporinase (ESC) phenotype. Raw manure samples more frequently yielded transconjugants with the ESC, ESBL, and non-ESBL phenotypes than did digested manure samples (Table 1). However, raw samples only had significantly greater odds of yielding transconjugants with the ESC and ESBL phenotypes compared to those of digested samples (Table 2). The mean (log10) and median transformed conjugation frequency were higher for raw samples than for digested samples (Table 1). The log10 transformed conjugation frequency was significantly greater for raw samples than for digested samples, and the log10 transformed conjugation frequency was significantly higher in samples collected in the fall and winter than in samples collected in the spring (Table 3). Based on the variance components in all the models, it appears that farm-level effects contribute to a substantial proportion of the variance in these outcomes (Tables 2 and 3). The assumption of homoscedasticity was met for the standardized residuals in the multilevel linear model and the best linear unbiased predictions (BLUPs) of all models. The normality assumption for these residuals and BLUPs was either met or only symmetrical or mildly skewed. No outliers were identified in any of the multilevel models fitted.
TABLE 1.
Prevalence of samples that have ESC, ESBL, and non-ESBL phenotypes and mean (log10) and median enhanced conjugation frequency estimated for all samples and when stratified by processing stage and seasona
| Stratification parameters | % of samples with phenotype (95% CI) |
Mean log10 conjugation frequency (95% CI) | Conjugation frequency (interquartile range) | ||
|---|---|---|---|---|---|
| ESCb | ESBLc | Non-ESBLd | |||
| Processing stage | |||||
| Digested (n = 63) | 57.1 (44.0, 69.5) | 44.4 (31.9, 57.5) | 19.0 (10.2, 30.9) | −7.6 (−8.3, −7.0) | 0 (0, 2 × 10−6) |
| Raw (n = 63) | 82.5 (70.9, 90.9) | 60.3 (47.2, 72.4) | 33.3 (22.0, 46.3) | −6.8 (−7.5, −6.1) | 8.0 × 10−8 (0, 1.9 × 10−5) |
| Season | |||||
| Spring (n = 50) | 56.0 (41.3, 70.0) | 36.0 (22.9, 50.8) | 24.0 (13.1, 38.2) | −7.8 (−8.5, −7.1) | 0 (0, 7.5 × 10−7) |
| Summer (n = 30) | 83.3 (65.3, 94.4) | 66.7 (47.2, 82.7) | 30.0 (14.7, 49.4) | −6.9 (−7.9, −6.0) | 5.4 × 10−8 (0, 1.2 × 10−5) |
| Fall (n = 12) | 75.0 (42.8, 94.5) | 58.3 (27.7, 84.8) | 33.3 (9.9, 65.1) | −7.1 (−8.6, −5.6) | 1.2 × 10−7 (0, 1.7 × 10−5) |
| Winter (n = 34) | 76.5 (58.8, 89.3) | 61.8 (43.6, 77.8) | 23.5 (10.7, 41.2) | −6.7 (−7.6, −5.7) | 6.9 × 10−7 (0, 1.9 × 10−5) |
| Overall (n = 126) | 69.8 (61.0, 77.7) | 52.4 (43.3, 61.3) | 26.2 (18.8, 34.8) | −7.2 (−7.7, −6.8) | 2.3 × 10−9 (0, 8.0 × 10−6) |
Phenotypes of transconjugants were determined by ESBL confirmation assay and binned into either ESBL or non-ESBL phenotypes.
ESC, samples yielding transconjugants of any phenotypes (ESBL, non-ESBL, or both phenotypes). CI, confidence interval.
ESBL, samples yielding ESBL phenotype transconjugants.
Non-ESBL, samples yielding non-ESBL phenotype transconjugants.
TABLE 2.
The results of multilevel logistic regression models examining the associations between processing stage and season on the odds of samples having transconjugants that have ESC, ESBL, and non-ESBL phenotypesa
| Stratification parameters | ESCb |
ESBLc |
Non-ESBLd |
||||||
|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | OR | 95% CI | P value | |
| Processing stage | |||||||||
| Digested | Referent | Referent | Referent | ||||||
| Raw | 9.05 | 2.12, 38.69 | 0.003 | 3.53 | 1.16, 10.73 | 0.026 | 2.52 | 0.97, 6.54 | 0.057 |
| Variance components | Variance (VPCe) | 95% CI | Variance (VPCe) | 95% CI | Variance (VPCe) | 95% CI | |||
| Farm | 1.38 (15.3%) | 0.10, 18.24 | 3.80 (34.0%) | 0.56, 26.03 | 1.40 (28.9%) | 0.23, 8.44 | |||
| Farm on date of sampling | 4.33 (48.1%) | 0.91, 20.58 | 4.08 (36.5%) | 1.01, 16.53 | 0.16 (3.3%) | 5.25 × 10−6, 4.98 × 103 | |||
Phenotypes of transconjugants were determined by ESBL confirmation assay and binned into either ESBL or non-ESBL phenotypes. OR, odds ratio.
ESC, samples yielding transconjugants of any phenotypes: ESBL, non-ESBL, or both.
ESBL, samples yielding ESBL phenotype transconjugants.
Non-ESBL, samples yielding non-ESBL phenotype transconjugants.
Variance partition coefficients (VPC) estimated using the latent variable technique.
TABLE 3.
The results of a multilevel linear regression model examining the associations between processing stage and season on the log10 conjugation frequency
| Stratification parameters | βa | 95% CI | P value |
|---|---|---|---|
| Digestion stage | |||
| Digested | Referent | ||
| Raw | 0.82 | 0.23, 1.41 | 0.006 |
| Season | |||
| Spring | Referent | ||
| Summer | 0.92 | −0.09, 1.93 | 0.075 |
| Fall | 1.59 | 0.15, 3.03 | 0.030 |
| Winter | 1.13 | 0.16, 2.11 | 0.022 |
| Variance components | Variance (VPCb) | 95% CI | |
| Farm | 2.69 (40.8%) | 0.77, 9.34 | |
| Farm on date of sampling | 1.07 (16.2%) | 0.40, 2.82 | |
| Sample | 2.83 (42.9%) | 2.00, 4.01 |
Model coefficients.
Variance partition coefficients.
Genotypic and phenotypic characterization of recovered plasmids.
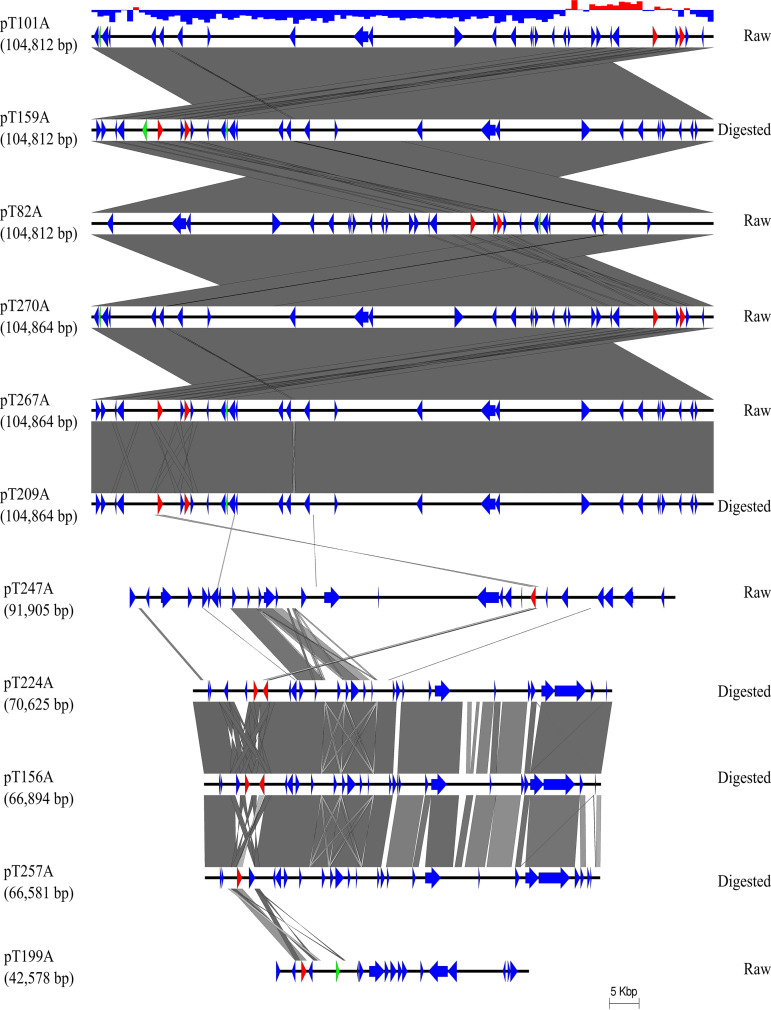
A total of 459 plasmids were isolated, digested with EcoRI, and binned into 10 distinct restriction enzyme (RE) profiles. Both raw and digested manures from a farm sampling appeared to share similar RE profile clusters where they both had the most prevalent plasmid (pT267A) (Table S1). Other less frequent plasmid RE profiles were detected in either raw manures (pT145A, pT247A, pT277A, pT308A) or digested manures (pT156A, pT224A, pT295A, pT476A).
Based on preliminary RE profiles, a subset of 25 transconjugants with the ESBL phenotype was selected and subjected to whole-genome sequencing on the Illumina MiSeq short-read sequencing platform. Eleven of these transconjugants were further sequenced on the MinION long-read sequencing platform. Hybrid assembly was used to completely close plasmid sequences. Key characteristics of the plasmids are presented in Table 4. Plasmid incompatibility groups detected by Mob-suite included IncI1, IncN, IncFIIA, IncFII, IncX1, and IncC (Table 4). The most common plasmid (∼100 kb pT267A) carrying numerous antibiotic resistance genes was found across all participating farms in both raw and digested manure. Snippy analysis showed that there were between one and seven single nucleotide polymorphisms (SNPs) when Illumina assemblies of transconjugants, likely carrying this common plasmid, were mapped against a reference genome with a closed plasmid sequence (Table S2).
TABLE 4.
Plasmid characteristics and plasmid-borne resistance genes in 25 transconjugants using Miseq/MinION sequencing platforms
| Plasmid ID | Resistance genes on the plasmidb,c | Farm/sample type | Inc groupd,e | Plasmid size (bp) | Predicted mobility | Predicted resistance phenotypef |
|---|---|---|---|---|---|---|
| pT82Aa | aac (6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 4/raw | Inc− | 104,812 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT101Aa | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 4/raw | Inc− | 104,812 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT159Aa | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 1/digested | Inc− | 104,812 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT209Aa | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 7/digested | Inc− | 104,864 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT267Aa | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 5/raw | Inc− | 104,864 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT270Aa | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 5/raw | Inc− | 104,864 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT221A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 7/digested | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT286A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 2/raw | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT496A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 5/raw | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT304A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 3/raw | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT306A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 3/raw | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT409A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 7/digested | Inc− | ∼96,702 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT478A | aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, blaCTX-M-15, blaOXA-1, catB4, dfrA1, floR, lnu(G), sul2 | Farm 4/raw | Inc− | ∼96,650 | Conjugative | Cip Gen Str Kan Amp Axo Tmp Chl Lin Sxz |
| pT145A | blaCTX-M-15 | Farm 1/raw | IncI1 | ∼85,051 | Conjugative | Amp Axo |
| pT277A | blaCTX-M-15 | Farm 1/raw | IncI1 | ∼83, 223 | Conjugative | Amp Axo |
| pT308A | blaCTX-M-14 | Farm 3/raw | IncI1 | ∼94,724 | Conjugative | Amp Axo |
| pT247Aa | blaCTX-M-14 | Farm 2/raw | IncI1 | 91,905 | Conjugative | Amp Axo |
| pT199Aa | blaCTX-M-1 | Farm 7/raw | IncN | 42,578 | Conjugative | Amp Axo |
| pT257Aa | blaCTX-M-27 | Farm 2/digested | IncFIIA, IncFII | 66,581 | Conjugative | Amp Axo |
| pT455A | blaCTX-M-27 | Farm 2/digested | IncN | ∼41,970 | Conjugative | Amp Axo |
| pT295Aa | aph(3′')-Ib, aph(6)-Id, blaCTX-M-27, blaTEM-1B, dfrA14, sul2, tet(A) | Farm 2/digested | IncN, IncX1 | 77,311 | Conjugative | Str Kan Amp Axo Tmp Sxz Tet |
| pT476A | blaCTX-M-55, blaTEM-1B | Farm 7/digested | IncX1 | ∼40,307 | Conjugative | Amp Axo |
| pT224Aa | blaCTX-M-55, blaTEM-206, fosA3 | Farm 7/digested | IncFIIA, IncFII | 70,625 | Conjugative | Amp Axo Fos |
| pT156Aa | blaCTX-M-55, blaTEM-206 | Farm 1/digested | IncFIIA, IncFII | 61,659 | Conjugative | Amp Axo |
| pT413Aa | aadA2, blaPER-1, sul1g, tet(E), tet(X), mph(E), msr(E), aph(3′)-Ia, tet(C) | Farm 5/raw | IncC | 187,012 | Conjugative | Str Amp Axo Ery Azi |
Samples were sequenced on both Miseq and MinION platforms, followed by hybrid assembly. These plasmids had complete closed sequences of precise sizes.
Genes in bold were identified as ESBL genes.
Resistance genes were identified by starAMR tool.
Inc group, plasmid size, and predicted mobility were determined by whole-genome sequencing and the MOB-suite tool.
Inc−, plasmid with no detectable Inc type.
Cip, ciprofloxacin; Gen, gentamicin; Str, streptomycin; Amp, ampicillin; Axo, ceftriaxone; Tmp, trimethoprim; Chl, chloramphenicol; Lin, lincomycin; Sxz, sulfisoxazole; Ery, erythromycin; Azi, azithromycin; Tet, tetracycline; Fos, fosfomycin.
This plasmid had two copies of the sul1 gene.
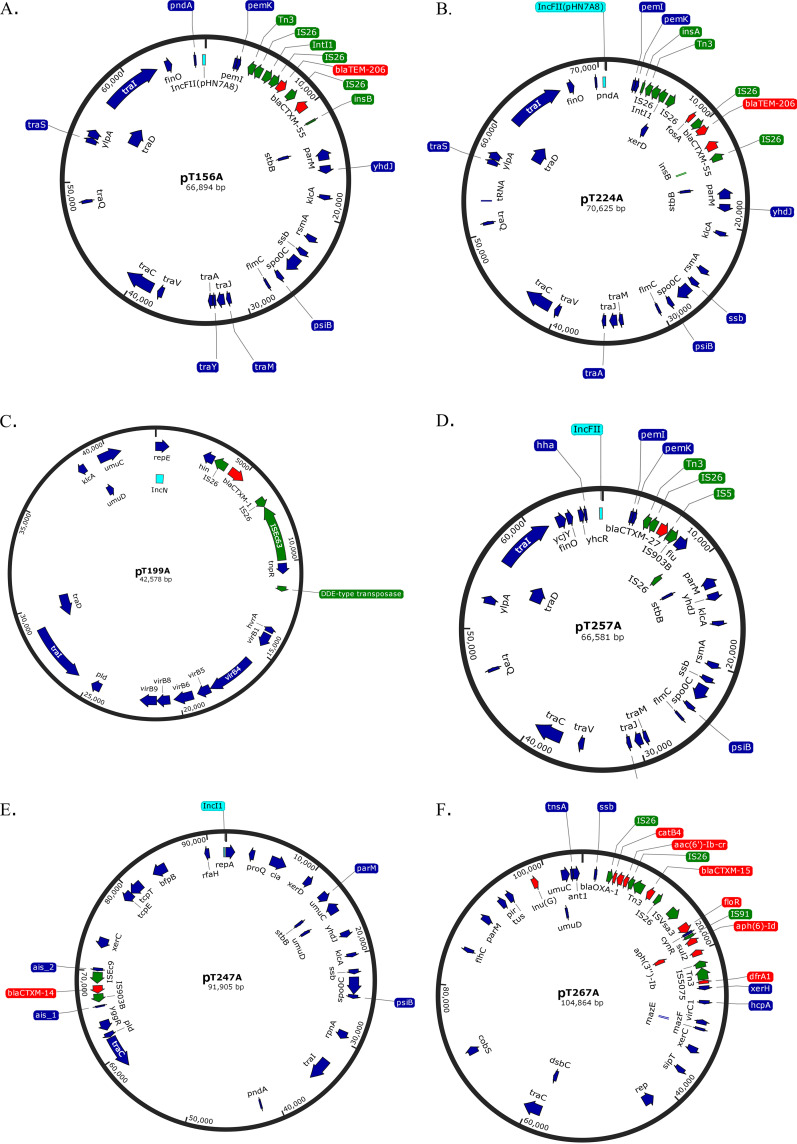
Eleven complete closed plasmids were aligned and their sequence identity was compared (Fig. 3). On this basis, six likely identical plasmids which were captured in six individual transconjugants were identified and designated pT82A, pT101A, pT159A, pT270A, pT267A, and pT209A. Three plasmids, pT156A, pT224A, and pT257A, shared a majority of their sequence in common, while pT199A and pT247A were more unique in their sequences. For the most prevalent plasmid (pT267A) which was present in both raw and digested manure samples, the average GC content of areas containing resistance genes and mobile genetic elements was about 54%, whereas the rest of DNA plasmid had an average GC content of 37.5%. Maps of six distinct complete plasmids were constructed (Fig. 4). A variety of mobile genetic elements were found in areas surrounding resistance genes including Tn3, Tn7, IS26, intI1, insA, insB, ISEc63, ISEc9, IS903B, IS5, IS91, IS5075, and ISVsa3. Five of the six plasmids carried IS26.
FIG 3.
Comparison of 11 closed plasmid sequences obtained from hybrid assembly on short- and long-read sequencing platforms. Coding sequences annotated by PROKKA tool are represented by colored arrows. Red arrows are β-lactamase genes, green arrows are mobile genetic elements, and blue arrows are other functional genes. The top graph showed GC content of the first plasmid sequence: red, GC content greater than 50%; blue, GC content lower than 50%.
FIG 4.
Plasmid maps of six distinct plasmids harboring ESBL genes which were captured in E. coli CV601 strain. (A) pT156A, (B) pT224A, (C) pT199A, (D) pT257A, (E) pT247A, (F) pT267A. Red and turquoise arrows are resistance genes and incompatibility plasmid sequence, respectively, detected by starAMR tool. Green arrows are mobile genetic elements detected by RAST and BLAST tools. Dark blue arrows are other functional genes which were annotated by PROKKA tool. Figures were created using SnapGene.
Putative β-lactamases and other antibiotic resistance genes carried by the 25 sequenced plasmids were identified using the StarAMR tool (Table 4). Six ESBL genes were detected: blaCTX-M-15, blaCTX-M-55, blaCTX-M-1, blaCTX-M-14, blaCTX-M-27, and blaPER-1. The most common ESBL gene was blaCTX-M-15, which was carried by 13 of the 25 plasmids. Other genes were associated with resistance to macrolides [mph(E), msr(E)], tetracyclines [tet(A), tet(C), tet(E), tet(X)], lincomycin [lnu(G)], florfenicol (floR), sulfonamides (sul2), chloramphenicol (catB4), trimethoprim (dfrA1), and aminoglycosides (aac(6′)-Ib-cr). AMR genes detected with the ABRicate tool were similar to those detected by starAMR (Table S3), except that ABRicate also reported genes with lower coverage than 50% and a different blaTEM variant. ABRicate identified the gene as blaTEM-141, while starAMR identified it as the blaTEM-206 gene. Both variants had the same coverage of 86.64% with our gene sequence.
Antibiotic susceptibility of the 25 transconjugants was determined phenotypically using the Sensititre Gram-negative susceptibility panels. They were all confirmed to possess ESBL phenotypes. Most of the antibiotic resistance phenotypes were consistent with their corresponding genotypes based on Clinical and Laboratory Standards Institute (CLSI) standards (99) except for gentamicin (Gen) and ciprofloxacin (Cip) (Table 5). In these cases, the plasmids carried the resistance gene aac(6′)-Ib-cr but did not confer phenotypic resistance. MICs in most transconjugants (i.e., T82A, T101A, T159A, and T209A) carrying this plasmid-mediated gene were slightly elevated (MICCip = 0.06 to 0.12 mg/liter, MICGen = 2 mg/liter) compared to that in the host E. coli CV601 alone (MICCip = 0.03 mg/liter, MICGen = 1 mg/liter).
TABLE 5.
Antibiotic resistance phenotypes of 25 transconjugants using Gram-negative susceptibility panels (CMV4AGNF and ESB1F) on the Sensititre automated systema,b
| Antibiotic(s) | Phenotype for transconjugant |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T156A | T247A | T277A | T224A | T413A | T308A | T257A | T455A | T199A | T295A | T304A | T306A | T476A | T409A | T478A | T496A | T082A | T270A | T145A | T159A | T101A | T267A | T286A | T221A | T209A | |
| Amoxicillin/clavulanic acid | S | S | S | S | S | S | S | S | I | I | I | I | I | I | I | I | I | R | S | R | R | R | R | R | R |
| Ampicillin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Azithromycin | S | S | S | S | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Cefazolin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefepime | S | S | S | S | S | S | S | S | R | S | R | S | S | R | R | R | R | R | S | R | R | R | R | R | R |
| Cefotaxime | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cefoxitin | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | I | S | S | S | S | S | S | S |
| Cefpodoxime | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Ceftazidime | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Ceftriaxone | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Cephalothin | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R | R |
| Chloramphenicol | S | S | S | S | S | S | S | S | S | S | R | R | S | R | R | R | R | R | S | R | R | R | R | R | R |
| Ciprofloxacin | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Gentamicin | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Imipenem | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Meropenem | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Nalidixic acid | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Piperacillin/tazobactam | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S | I | S | S | S | S | S | S | S |
| Streptomycinc | S | S | S | S | R | S | S | S | S | S | R | R | S | R | R | R | R | R | S | R | R | R | R | R | R |
| Sulfisoxazolec | S | S | S | S | NS | S | S | S | S | NS | NS | NS | S | NS | NS | NS | NS | NS | S | NS | NS | NS | NS | NS | NS |
| Tetracycline | S | S | S | S | R | S | S | S | S | R | S | S | S | S | S | S | S | S | S | S | S | S | S | S | S |
| Trimethoprim/sulfamethoxazole | S | S | S | S | S | S | S | S | S | R | R | R | S | R | R | R | R | R | S | R | R | R | R | R | R |
The results were interpreted by the Sensititre automated system unless otherwise stated.
Streptomycin and sulfisoxazole results were interpreted according to the breakpoint guideline provided by U.S. Food and Drug Administration (FDA) (https://www.fda.gov/media/108180/download).
R, resistant (bolded for clarity); I, intermediate; S, susceptible; NS, not susceptible. NS only applies to sulfisoxazole where MICs were limited by the range of tested panels; however, the values were larger than the susceptible breakpoint in the guideline provided by U.S. Food and Drug Administration (FDA) (https://www.fda.gov/media/108180/download).
Abundance of selected gene targets associated with antibiotic resistance or horizontal gene transfer.
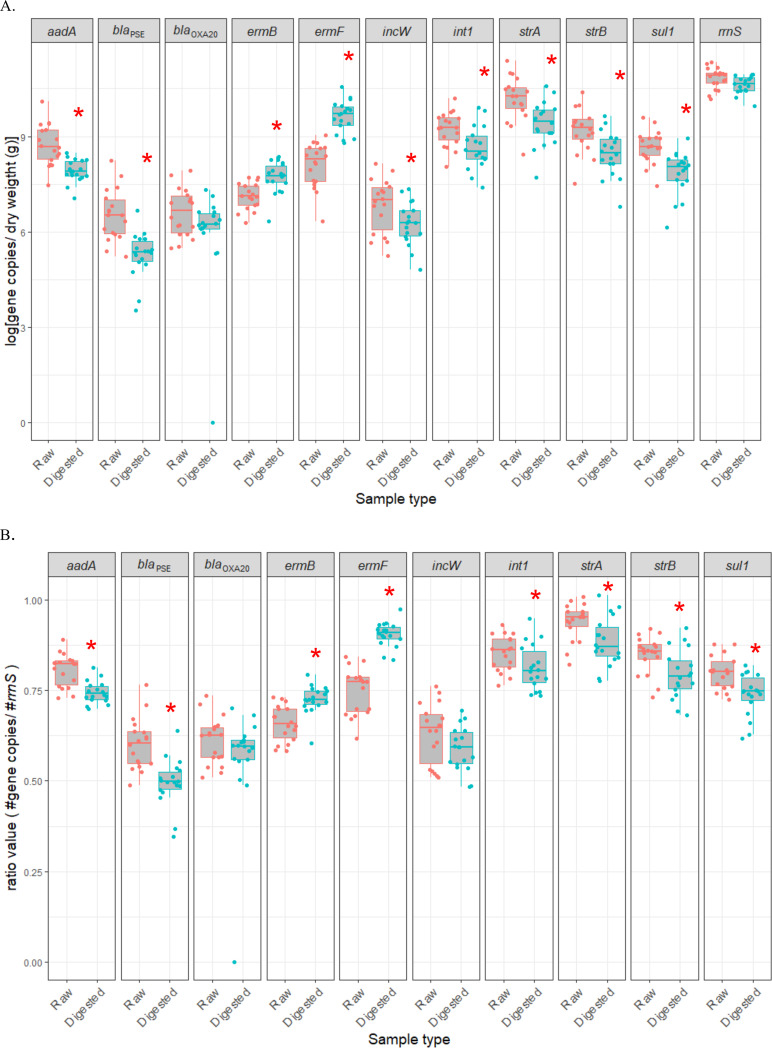
Most gene targets, including aadA, int1, blaPSE, str(A), str(B), and sul1, were less abundant in digested manure than in raw manure (Fig. 5). In contrast, both ermB and ermF gene targets increased in abundance with digestion, and the blaOXA20 target did not change in abundance (Fig. 5). The abundance of total bacteria based on the 16S rRNA gene (rrnS) did not significantly decrease in digested samples (Fig. 5a). The impact of digestion on the abundance of all gene targets except incW was the same whether expressed as the absolute or the relative abundance (Fig. 5a and b). The incW plasmid incompatibility group gene target was significantly reduced in absolute abundance with digestion, whereas it was not reduced in relative abundance (Fig. 5a).
FIG 5.
A comparison of antibiotic resistance-associated gene targets in raw and digested samples across six farms. (A) The log of [gene copy number per matter dry weight (g)]. (B) The ratio of the gene copy number to the number of total bacteria. Asterisks indicate a statistically significant difference (P < 0.05).
DISCUSSION
As revealed by viable plate count, anaerobic digestion significantly reduced the abundance of viable Gram-negative bacteria but not that of Gram-positive bacteria. These results are consistent with a previous study in which most of Gram-negative bacteria significantly decreased with digestion but Clostridium perfringens and Enterococcus spp. did not (29). In another study, E. coli and total coliform counts decreased below the limit of detection within 4 to 7 days of dry mesophilic anaerobic codigestion of food waste and pig manure, whereas it took longer to remove Enterococcus spp. (12 days) (30). It is likely due to the fact that these bacteria are more thermotolerant (Enterococcus spp., Staphylococcus spp.) or that they are spore-forming (Clostridium perfringens) (9, 31). In our study, digesters at most farms were operated in the mesophilic temperature range of 38.5 to 40°C.
With E. coli CV601 as the recipient, bacteria enriched from raw manure as the donor yielded transconjugants more frequently than did bacteria enriched from digested manure. Presumably, this was due to the significant reduction in the abundance of potential donors, as evidenced by viable plate counts. Manure was preenriched with buffered peptone containing cefotaxime prior to inoculation with the E. coli GFP-tagged CV601 recipient. In the absence of this preenrichment step, we were unsuccessful in obtaining any transconjugants in digested samples. In other studies, biparental/triparental mating was used to capture mobilizing plasmids from granules of an anaerobic wastewater treatment plant, cow manure, swine manure slurries, and fresh water; however, none of these studies investigated the fate of these plasmids after anaerobic digestion (32–34). In our study, capturing plasmid-mediated ESBL genes in either preenriched raw or preenriched digested manures was still achievable without the addition of helper plasmids such as pBBR1MCS-derivative plasmids. Although there was a significant decrease in cefotaxime-resistant transconjugants found in digested manures compared to that found in raw manures, both raw and digested samples appeared to share similar RE profile clusters. The prevalent plasmid, pT267A (∼100 kb), was present abundantly in both raw and digested manures from all farms participating in this study. We also identified other less frequent plasmids that were only found in either raw or digested manures.
The variance components from our multilevel models also provided us with additional insights concerning our data. Interestingly, farm-level effects appeared to contribute to a substantial proportion of the variance in the outcomes examined, although we should be cautious in this interpretation due to the lack of precision in our estimates (i.e., wide confidence intervals for the variance components). Farm-level factors, which could not be statistically analyzed due to the relatively small number of farms included in our study, that may explain this farm-level variance include retention time, manure input and output flows, pH, and other differences in digestion processes. Future studies, using a larger number of farms, would allow researchers to measure the effect of these manure digestion processing factors.
A variety of ESBL genes were identified in 25 sequenced transconjugants, including blaCTX-M-1, blaCTX-M-14, blaCTX-M-15, blaCTX-M-27, blaCTX-M-55, and blaPER-1. The blaCTX-M-15 gene was the most prevalent ESBL gene detected. This is not surprising because blaCTX-M variants are commonly found in both clinical and community settings (35–38). First discovered in the 1980s, they became the most commonly encountered ESBL genes and have become more frequently found than blaTEM and blaSHV (38). The CTX-M lineage was divided into at least five phylogroups: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25 (38). In our study, two phylogroups were identified: CTX-M-1 (blaCTX-M-1, blaCTX-M-15, blaCTX-M-55) and CTX-M-9 (blaCTX-M-14, blaCTX-M-27). The blaCTX-M-15 gene was the most prevalent gene among them in our study; this agrees with previous reports regarding the global spread of this gene (39–45). The non-CTX-M ESBL blaPER-1 gene, originally from Pseudomonas aeruginosa, was also found in other species, including Salmonella enterica serovar Typhimurium, Proteus mirabilis, and Providencia stuartii (41, 46–48).
Other non-ESBL β-lactamase genes which colocated with one blaCTX-M- gene were also detected, including blaTEM-1b, blaTEM-206, and blaOXA-1. The blaTEM-1b gene, colocated with either blaCTX-M-27 or blaCTXM-55, was considered one of the parental penicillinase genes along with blaTEM-1a and blaTEM-2 genes. Their derivatives, resulting from various combinations of eight amino acid substitutions, including five amino acids which expanded the enzymatic substrate specificity, were classified as TEM-type ESBL genes (49). More interestingly, there was a rare case where an E. coli isolate carrying this blaTEM-1b gene expressed ESBL phenotype. Lagace-Wiens et al. postulated that the strain either hyperproduced non-ESBL β-lactamase or got permeability reduced (50). There is very little available information concerning blaTEM-206, which was classified as a non-ESBL gene in one study (51). Two different blaTEM variants were reported by two different tools (blaTEM-141 by ABRicate versus blaTEM-206 by starAMR) because our truncated sequence, which lacked 115 bp of the front sequence, shared the same coverage (87%) with both of them (Fig. S1). Therefore, this gene might not be functional at all in our case. The blaOXA-1, another non-ESBL gene in E. coli, was found to cooccur with blaCTX-M-15. This cooccurrence was also observed in several previous studies (39, 42, 52). Also, there was an association between the OXA-1 enzyme and reduced susceptibility to penicillin/β-lactamase inhibitor combinations among ESBL-producing E. coli (53).
ESBL-producing E. coli isolates are more likely to be multidrug resistant, gentamicin resistant, and fluoroquinolone resistant than are AmpC-producing E. coli isolates (37, 54). In the present study, the most prevalent ESBL gene (blaCTX-M-15) was found to colocate with multiple other resistance genes: aac(6′)-Ib-cr, aph(3′')-Ib, aph(6)-Id, catB4, dfrA1, floR, lnu(G), and sul2. Although some plasmids harbored a determinant for gentamicin and ciprofloxacin resistance [aac(6′)-Ib-cr], their phenotypic MIC values did not meet the CLSI standard to be interpreted as “resistant.” Nevertheless, MICs in some transconjugants were slightly elevated compared to that in the host alone. The aac(6′)-Ib-cr gene, a derivative of aminoglycoside acetyltransferase aac(6′)-Ib, was first reported in 2003 and has widely disseminated ever since (44, 45, 55). This gene is interesting because it was shown to confer resistance to two distinctly different antibiotic classes (aminoglycoside and fluoroquinolone) (55). Other resistance genes were also detected along with ESBL genes, including fosA3 that confers resistance to fosfomycin: mph(E) and msr(E) for macrolide resistance and tet(A), tet(C), tet(E), and tet(X) for tetracycline resistance.
Resistance genes found in manures are likely related to the use of drugs that were approved for veterinary medication (prescription drug list; PDL) such as oxytetracycline, penicillin, florfenicol, trimethoprim-sulfadoxine, and ceftiofur (15, 56). These antibiotics were used by the participating farms; a full list of drugs (antibiotics included) is found in Table S5. Penicillins, penicillin combinations, cephalosporins, tetracyclines, trimethoprim-sulfonamide combinations, and lincosamides are the most commonly used antibiotic classes in dairy farms in Canada for dry cow therapy and clinical mastitis treatment (57). Penicillin G procaine (Pen G), ampicillin (Polyflex), and ceftiofur (Excede) might contribute to the dissemination and maintenance of β-lactamase, especially ESBL genes. Other drugs such as florfenicol, oxytetracycline, trimethoprim, and lincomycin could promote ESBL plasmids that were also resistant to other drugs as shown in this study.
Mobile genetic elements such as plasmids, transposons, and insertion sequences have been known to largely contribute to the spread of antibiotic resistance genes (58, 59). Plasmids found in our study fell within the size range of 40 to 180 kb. The most prevalent plasmid had a low GC content backbone which is similar to previous observations (60, 61). Only areas containing resistance genes appeared to have a higher GC content, suggesting that these genes were recruited from foreign DNA in this plasmid. In addition, a variety of insertion sequences and integrases were found near resistance genes. Among them, IS26 was detected in a majority of our sequenced plasmids. IS26, first discovered almost 4 decades ago, is a major contributor to the evolution and/or the dissemination of antibiotic resistance genes (62–65). IS26 was found to cooccur with several β-lactamase genes, such as blaS2A and blaTEM-1 (62, 66). Two mechanisms were presented to explain IS26 movement: cointegrate forming and cut-and-paste mechanisms (64). The end product is normally an array of two or more directly oriented IS26s (64). In another study, IS26 was shown to use replicative transposition to reorganize clinically isolated plasmids (67). Only one out of four possible recombination outcomes resulted in two inversely oriented IS26s after replicative transposition provided that the donor insertion sequence (IS) and target sites were present in the same replicon (intramolecular transposition) and DNA rearrangement occurred in the trans pathway (58, 67). In our study, a sophisticated structure of multiple disoriented IS26s along with the presence of other mobile genetic elements were seen in several plasmids, suggesting multiple gene exchange events.
The majority of gene targets significantly decreased in abundance after digestion except for blaOXA20, erm(B), and erm(F). The abundance of erm(B) and erm(F) significantly increased in digested samples. The abundance of erm(B) and erm(F) was also seen to increase in municipal wastewater treatment plants (68). This might be due to the shift of certain bacterial populations, for example from nutrient removal functional bacteria (i.e., Nitrospira, Dechloromonas, Dokdonella, Comamonas, Thauera, and Zoogloea) to fermentative bacteria (i.e., Smithella, Petrimonas, Saccharicrinis, Syntrophomonas, and Ercella) (68). Lab-scale mesophilic anaerobic digestion also amplified both erm(B) and erm(F) genes, while thermophilic digestion provided more effective reduction of erm(B) and erm(F) (69). Both erm(B) and erm(F) are carried by various Gram-positive bacteria of animal origin, including Streptococcus spp., Staphylococcus spp., and Peptostreptococcus spp. (70).
The present study showed that on-farm anaerobic digestion reduced the horizontal transfer potential of plasmids carrying ESC genes including ESBL genes into E. coli CV601, consistent with the abatement of viable coliform bacteria. Anaerobic digestion abated some but not all antibiotic resistance gene targets. This inconsistent response is presumably due to shifts in the population carrying these genes according to those that perish during digestion and those that survive or proliferate. Future studies on the shift of bacterial populations during digestion using amplicon/shotgun sequencing will help unravel the dynamics of populations and the genes that they carry.
MATERIALS AND METHODS
Participating farms, anaerobic digestion process, and manure sampling method.
Seven farms were recruited, but in the present study, farm six was not sampled since this farm composted manure rather than processing the manure through a digestion system. The six participating farms in this project were located within 500 km of London, Ontario, Canada. The herd size for each farm is indicated in Fig. S2. Cow breeds at theses farms were Holsteins.
All farm digestion systems run on a continuous flow methodology, through mesophilic/thermophilic digesters (Fig. S2). Two digesters typically run in a range of 38.5 to 40°C with a retention time of roughly 48 days in farms 1, 2, 3, and 4. For farms 1 and 3, the output of the secondary digester continued going through screw presses to separate solids from liquids. Farm 5 had three digesters operated at 38 to 39°C (digesters 1 and 2) and 51 to 52°C (digester 3) with a total retention time of 100 to 110 days depending on feeding rates. Farm 7 had only a primary digester operated at mesophilic temperatures (38 to 39°C). After this digester, the material went through two screw presses to remove more moisture (dewatered) and were fed into two rotating kiln dryers running at 150°C. Digesters had a volume of about 1,000 m3 each, except for the digester on farm 7 with a size of 3,000 m3.
Samples were collected on a monthly basis from the six farms during an approximately 1-year period, except for 1 month when samples were collected on a biweekly basis. A brief description of the manure sampling approach from raw manure to final digestate, including sampling points, can be found in Text S1. The drug list in Table S5 was obtained from the dairy farms’ record keeping book because dairy farms were required to record drug usage on the farm as part of their animal care program.
Sample preparation and enumeration of total enteric bacteria.
A total of 106 raw and 138 digested manure samples of multiple digestion stages as indicated in Fig. S2 were used for bacterial enumeration. Manure samples were prepared for bacterial enumeration as 10-fold serial dilutions (10−1 through 10−4) by aseptically adding 5 g of manure into 45 ml of sterile sodium metaphosphate buffer (2 g/liter, pH 7.0). Subsequent serial dilutions were prepared by aseptically transferring 1 ml of the previous dilution to 9 ml of sterile metaphosphate buffer and vortexed for 30 s.
Total coliforms and Escherichia coli were enumerated by direct plating of 100 μl of each serial dilution onto Chromocult agar (Millipore-Sigma, Toronto, ON). Plates were incubated at 37°C for 18 to 20 h. Enterococcus spp. were enumerated by direct plating as above onto m-Enterococcus agar (Thermo Fisher Scientific, Toronto, ON). Plates were incubated at 37°C for 48 h. Staphylococcus spp. were enumerated by direct plating onto Mannitol salt agar (MSA, VWR International, Mississauga, ON) and Chromagar Staphylococcus agar (Dalynn Biologicals, Calgary, AB) and incubated for 48 h at 37°C. Clostridium perfringens was enumerated by direct plating of serial dilutions onto mCP agar (Accumedia, VWR International, Mississauga, ON) and incubated at 44.5°C for 24 h in anaerobic boxes under anaerobic conditions (Anaeropak system, VWR International).
Conjugation method with enriched manure samples.
Conjugation experiments used 174 enriched samples obtained from the six dairy farms as donors. Sixty-three samples were raw and 111 were digested. Manure was enriched by adding 1 g (if solid, raw manure) or 1 ml (if slurry, digested manure) into 9 ml buffered peptone water (Difco) supplemented with cefotaxime (4 mg/liter) overnight under static condition at 30°C before being used in conjugation experiments.
Overnight enriched manure was centrifuged at 300 × g for 5 min to remove particulates, and the supernatant was decanted into a clean sterile conical tube. Subsequently, the supernatant was centrifuged at 8000 × g, 15 min to collect bacteria. The pellet was resuspended in 2 ml 1/10× LB, centrifuged at 3,100 × g in 5 min, washed once with 2 ml 1/10× LB, and centrifuged again. Finally, the pellet was resuspended in 200 μl saline.
The β-lactam-sensitive GFP-labeled E. coli CV601 (gfp+kanRrifR) was used as the recipient strain in conjugation experiments (71–73). The strain carries some chromosomal resistance genes [aph(3′)-III, mdf(A)], and its antimicrobial resistance phenotype can be found in Table S4. The strain was inoculated into LB supplemented with rifampin (50 mg/liter) and cultured overnight at 37°C on a rotary shaker set at 200 rpm. The next day, cells were harvested by centrifugation at 3,100 × g for 5 min, washed twice with 1/10× LB, then resuspended in 100 μl saline and mixed with the above enriched manure in a ratio of 1:1, and spotted onto LB plates supplemented with cycloheximide (100 mg/liter) plates. Manure aliquots and the recipient strain E. coli CV601 were also spotted individually on LB plus cycloheximide (100 mg/liter) plates as negative controls.
After overnight incubation at 30°C, mating spots were washed and resuspended in saline, and different dilutions were plated on Chromocult medium containing rifampin (50 mg/liter), kanamycin (50 mg/liter), and cefotaxime (4 mg/liter) to select transconjugants. Negative controls were also plated onto the same selective medium. Transconjugant green-florescent phenotypes were also confirmed using a handheld UV light.
Conjugation frequency was calculated by taking the ratio of the number of colonies counted on transconjugant-selective plates (Chromocult agar supplemented rifampin [50 mg/liter], kanamycin [50 mg/liter], and cefotaxime [4 mg/liter]) over the number of colonies on recipient-selective plates (Chromocult agar supplemented rifampin [50 mg/liter], kanamycin [50 mg/liter]) which supported the growth of both transconjugants and plasmid-free recipients.
ESBL disc diffusion confirmation test.
The ESBL confirmation disc set was used to confirm ESBL phenotypes. This set is a combination of four individual discs of cefotaxime/cefotaxime plus clavulanic acid/ceftazidime/ceftazidime plus clavulanic acid, (BD BBL, Thermo Fisher Scientific, Ottawa, ON). Potential ESBL transconjugants were restreaked on transconjugant-selective plates to obtain pure colonies and confirm their cefotaxime-resistant phenotypes. Following this, they were mixed in 10 ml saline solution and swabbed on Mueller-Hinton agar (Oxoid) plates before the four diffusion discs were applied. Plates were then incubated at 37°C overnight. The results were interpreted by comparing the diameters around the discs following the Clinical and Laboratory Standards Institute (CLSI) guidelines (99).
Based on the results, transconjugants were determined to possess either ESBL or non-ESBL phenotype. Samples where phenotypic ESBL transconjugants were obtained were recorded as ESBL phenotypes. Likewise, samples where non-ESBL phenotype transconjugants were obtained were recorded as non-ESBL phenotypes. Samples that had transconjugants of any phenotype (ESBL, non-ESBL, or both) were recorded as extended-spectrum cephalosporinase (ESC). The numbers were used for statistical analysis as described further below.
Susceptibility tests.
MICs of various antimicrobial agents were determined using the Sensititre automated system (Trek Diagnostic Systems, Cleveland, OH, USA) using the Gram-negative (CMV4AGNF and ESB1F) susceptibility panels. The MIC data were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) breakpoints (99) and the breakpoint guideline provided by the U.S. Food and Drug Administration (https://www.fda.gov/media/108180/download).
Plasmid miniprep, enzyme digestion, and whole-genome DNA extraction.
Plasmids were isolated from 459 transconjugants obtained from the conjugation experiment using either the Qiaprep Spin Miniprep kit (Qiagen, Toronto, ON) or the Plasmid Mini AX kit (A&A Biotechnology, Gdynia, Poland). They were then digested with EcoRI and run electrophoretically on 0.8% agarose gel at 110 V for 50 min.
For whole-genome DNA extraction, strains were inoculated in LB supplemented with cefotaxime (4 mg/liter) and incubated overnight at 37°C. On the next day, 1 ml cell culture was collected and used for genome DNA extraction using Lucigen MasterPure Complete DNA and RNA with additional RNase step (Lucigen, Mandel Scientific, Guelph, ON, Canada) following the manufacturer’s manual.
Illumina/MinION sequencing protocol and bioinformatics tools.
Illumina paired-end sequencing was performed on the MiSeq platform (Illumina, Inc., San Diego, CA, USA) using the 600-cycle sequencing kit with libraries prepared using Nextera XT at the National Microbiology Laboratory (Guelph, ON, Canada) to a target of 60-fold coverage.
Oxford Nanopore MinION (Oxford Nanopore Technologies, New York, NY) sequencing was performed according to the default manufacturer protocol for rapid barcoding. Samples were prepared using the SQK-RBK004 rapid barcoding kit and subsequently run on a FLO-MIN106 R9.4 flow cell. Each multiplexed run produced between 4,719 and 111,488 reads per sample, with the mean read length ranging between 3,485 and 11,880 bp. Albacore v. 2.1.3 (Oxford Nanopore Technologies) was used to perform demultiplexing, base-calling, and quality filtering of the raw reads.
Illumina only and hybrid de novo assemblies of transconjugants’ whole genomes were produced using the Unicycler pipeline v. 0.4.4 (74). MOB-suite v. 2.0.0 was used to characterize the plasmid content of the de novo assemblies (75). MOB-recon was used to reconstruct the individual plasmids in the draft de novo assemblies, and plasmid typing was performed on each plasmid using MOB-typer using the default parameters for both. PROKKA (Galaxy version 1.13+galaxy1) and RAST (https://rast.nmpdr.org) were used to annotate genes on plasmids (76–80). Mobile genetic elements that were detected by RAST were further specified by blasting sequences against the NCBI nonredundant database (81). Snippy tool (Galaxy version 4.4.3+galaxy0) was used to determine SNPs between a reference genome with closed plasmid sequence and Illumina assemblies of transconjugants carrying potentially identical plasmids (82). The assemblies were also used as input to StarAMR (Galaxy version 0.7.1+galaxy1) and ABRicate (Galaxy version 0.8) to detect antibiotic resistance genes based on the resfinder resistance gene database (83–88). Plasmid maps were constructed using ApE v. 2.0.47 (https://jorgensen.biology.utah.edu/wayned/ape/) and SnapGene v. 5.1.3.1. Easyfig v. 2.2.2 was used to create multialignment figure (89).
Extraction of DNA from manure samples for quantitative PCR.
A subset of 18 raw samples and 18 digested samples were randomly chosen for further investigation using quantitative PCR (qPCR). A total of 250 mg of solid manure was used for DNA extraction using the DNeasy PowerSoil kit (Qiagen, Canada) following the manufacturer’s instructions. The final elution volume was 100 μl. If manure samples were liquid, 1 ml of each sample was centrifuged at 15,871 × g for 5 min in a tabletop centrifuge, and the pellet was treated with the Qiagen DNeasy PowerSoil kit following the manufacturer’s instructions. The final elution volume was 100 μl. DNA concentrations and quality were determined using a NanoDrop ND-1000 microspectrophotometer (NanoDrop Technologies, Wilmington, DE).
Detection and quantification of antibiotic resistance-associated gene targets.
The abundance of 10 selected gene targets associated with antibiotic resistance or horizontal gene transfer was determined by qPCR using a Bio-Rad CFX96 real-time PCR instrument with Bio-Rad CFX Manager software, version 3.0, as described previously (90, 91). The primers and hydrolysis probes used in the present study are listed in Table 6. All primers and hydrolysis probes were synthesized by Sigma (Sigma-Aldrich, Toronto, ON). The reaction was performed with the Brilliant II QPCR Master mix (Agilent, Toronto, ON, Canada) for TaqMan PCR and the Brilliant II SYBR green Low ROX qPCR Master mix (Agilent) for SYBR green PCR. A total of 2 μL of template DNA (corresponding to 0.1 to 10 ng DNA) was added and PCR grade water (Sigma-Aldrich, Toronto, ON) was used to reach a final volume of 25 μl. The PCRs were run in Hard-Shell Thin-Wall 96-Well Skirted PCR plates with clear bottom (Bio-Rad Laboratories, Canada). An optically clear and adhesive seal, the Microseal ‘B’ seal (Bio-Rad Laboratories, Canada) was placed on a plate before the PCR run. Each sample reaction included the no template control reaction and was run with the following cycle conditions: 1 cycle at 95°C for 10 min followed by 40 cycles at 95°C for 15 s and the required annealing temperature as specified in Table 6 for extension time of 35 s. For the SYBR green assay, a melting curve step was added in order to check the purity of the PCR product. This step consisted of a ramp temperature increase from 65 to 95°C, with an increment of 0.5°C and holding for 5 s for each step. The identities of the quantified gene targets were ensured on the basis of hybridization when using TaqMan chemistry or melting behavior when using SYBR green.
TABLE 6.
Primers and probes used for quantitative PCR in detection of antibiotic resistance genes
| Primer or probe | Sequence (5′-3′)a,b | Concn (nM) | Annealing temp (oC); extension time (s) | Amplicon size (bp) | Target | Reference |
|---|---|---|---|---|---|---|
| Universal bacteria | ||||||
| BACT1369F | CGGTGAATACGTTCYCGG | 300 | 59; 40 | 123 | rrnS gene | 94 |
| PROK1492R | GGWTACCTTGTTACGACTT | |||||
| TM1389F | HEX-CTTGTACACACCGCCCGTC-BHQ1 | 300 | ||||
| int1 | ||||||
| Int1-F2 | TCGTGCGTCGCCATCACA | 400 | 62; 60 | 67 | Integrase class 1 | 95 |
| Int1-R2 | GCTTGTTCTACGGCACGTTTGA | |||||
| sul1 | ||||||
| sul1-F | GACTGCAGGCTGGTGGTTAT | 200 | 64; 60 | 105 | Sulfamethazine resistance gene 1 | 91 |
| sul1-R | GAAGAACCGCACAATCTCGT | |||||
| str(A) | ||||||
| strA-F | TATGGTTGTTTGCCATGGTG | 400 | 62; 60 | 126 | Streptomycin phosphotransferase A | 96 |
| strA-R | TTCTCTTCGGCGTTAGCAAT | |||||
| str(B) | ||||||
| strB-F | ATCGCTTTGCAGCTTTGTTT | 300 | 61; 30 | 143 | Streptomycin phosphotransferase B | 96 |
| strB-R | ATGATGCAGATCGCCATGTA | |||||
| strB-P | HEX-ATGCCTCGGAACTGCGT-BHQ1 | 200 | ||||
| erm(B) | ||||||
| ermB-F | AAAACTTACCCGCCATACCA | 400 | 65; 60 | 139 | Erythromycin resistance gene locus B | 97 |
| ermB-R | TTTGGCGTGTTTCATTGCTT | |||||
| erm(F) | ||||||
| ermF-F | TCGTTTTACGGGTCAGCACTT | 300 | 61; 30 | 182 | Erythromycin resistance gene locus F | 97 |
| ermF-R | CAACCAAAGCTGTGTCGTTT | |||||
| aad(A) | ||||||
| aadA-F | CAGCGCAATGACATTCTTGC | 200 | 63; 30 | 294 | Aminoglycoside adenylyltransferase | 96 |
| aadA-R | GTCGGCAGCGACA(C/T)CCTCG | |||||
| aadA-P | HEX-TGGTAGGTCCAGCGGCGGAG-BHQ1 | 300 | ||||
| blaOXA-20 | ||||||
| blaOXA20-F | TGATGATTGTCGAAGCCAAA | 400 | 60; 60 | 101 | Oxacillinase gene blaOXA20 (group II) | 98 |
| blaOXA20-R | GCCTGTAGGCCACTCTACCC | |||||
| blaPSE | ||||||
| blaPSE-F | ACCGTATTGAGCCTGATTTA | 400 | 59; 30 | 101 | Beta-lactamase PSE gene | 98 |
| blaPSE-R | GCCGGCAATACTGAACGTAG | 28 | ||||
| blaPSE-P | HEX-TCTTGGATGGTGAACAATCAAG -BHQ1 | 300 | ||||
| IncW repA | ||||||
| IncW-F | GGCCATCGTATCAACGAGAT | 300 | 61; 30 | 153 | Plasmid incompatibility group W | 91 |
| IncW-R | ATTGGTGCGCTCAAAGTAGC | |||||
| IncW-P | HEX-AGCTGGCTTAGTCGGCTACA-BHQ1 | 200 |
HEX, 2′,4′,5′,7′-tetrachloro-6-carboxy-4,7-dichlorofluorescein succinimidyl ester.
BHQ-1, black hole quencher-1.
The quantity of each experimental sample was calculated using a standard curve as described in a previous study (91). The DNA fragment was cloned in the pSC-A-amp/kan plasmid using the StrataClone PCR cloning kit (Agilent) and transformed into E. coli. Plasmids were extracted using the Qiagen plasmid midi kit (Qiagen, Mississauga, ON). The plasmids were then linearized by NotI enzyme (NewEngland Bio Labs, Mississauga, ON) and purified with the Qiagen QIAquick PCR purification kit. Plasmid copy numbers were calculated using the measured DNA concentration from NanoDrop ND-1000 microspectrophotometer (NanoDrop Technologies, Wilmington, DE). The standard curve consisted of 10-fold serial dilution (from 106 down to 1 copy per μl) of a known target plasmid in triplicate and was included in each plate for PCR. The limit of quantification (LOQ) was set at the lowest dilution giving three positive results in the linearity range when tested with negative soil samples. If the gene target was detected at a copy number between one and four copies per reaction, it was considered to be detected below the LOQ.
Statistical analysis.
Bacteriological enumeration data for the raw and digested samples were evaluated for normality using the Shapiro-Wilk test. Bacteriological data did not show a normal distribution and subsequently were analyzed using the Mann-Whitney U test to determine significance in treatment effect with a P value cutoff of <0.05. Bacteriological enumeration data were plotted as box plots in SigmaPlot (v. 13.0, Systat Software, San Jose, CA).
Antibiotic resistance-associated gene targets in raw and digested samples were evaluated for normality using the Shapiro-Wilk test. Gene targets that did not show a normal distribution [erm(F), blaOXA-20] subsequently were analyzed using the analysis of variance (ANOVA) on ranks test to determine significance in treatment effect with a P value cutoff of <0.05. Other targets that met normality criteria [sul1, str(A), str(B), erm(B), intI1, aadA, incW, blaPSE] were analyzed using the one-way ANOVA test with a significant P value cutoff of <0.05. The statistical analysis was done using SigmaPlot (v. 13.0, Systat Software, San Jose, CA). The data were then plotted as box plots using RStudio (v. 1.2.1335, RStudio Inc., Boston, MA).
The conjugation results from 63 raw samples and 63 digested samples were used in multilevel logistic and linear regression models. To evaluate the effect of anaerobic digestion on conjugation, these digested samples were taken from the digestate holding pit for farm 7 and from the secondary digesters for other farms (Fig. S2). The prevalence estimates of samples with transconjugants that have ESC, ESBL, and non-ESBL phenotypes were reported with their 95% exact confidence intervals. The mean (log10) and median transformed conjugation frequencies were reported with their 95% confidence intervals and interquartile ranges, respectively. All estimates were reported for all samples and when stratified by processing stage and season.
Multilevel logistic regression models were fitted to examine the associations between manure processing stage (i.e., raw versus digested) and season (i.e., spring [March, April, May], summer [June, July, August], fall [September, October, November], and winter [December, January, February]) and the odds of a sample having the following characteristics: presence of transconjugants resistant to ESC, presence of transconjugants with an ESBL phenotype (ESBL), and presence of transconjugants with a non-ESBL phenotype. A multilevel linear regression model was fitted to examine the associations between the same independent variables and the log10 of the conjugation frequency. The conjugation frequency was log transformed to meet model assumptions concerning the normality and homoscedasticity of residuals. Where the conjugation frequency was 0, half the lowest value recorded was used for analyses since the log10 of zero is mathematically undefined. For all the multilevel models, random intercepts were included for the samples collected on the same date on a specific farm and for farm. Processing stage was forced into all models, and season was included if it was a statistically significant independent variable or acted as a confounding variable (92). Season was considered a confounding variable if its removal from the model resulted in a 20% or greater change in the model coefficient for processing stage. Interaction effects between processing stage and season were not examined due to small sample sizes for interaction terms. Variance partition coefficients were estimated for each model with the latent variable technique used for the multilevel logistic regression models (93). The standardized residuals (multilevel linear model) and the best linear unbiased predictions (BLUPs for all models) were examined using normal quantile plots and scatterplots of the BLUPs against the predicted outcomes to determine if they met the assumptions of normality and homoscedasticity, respectively. The significance level for all analyses was 5% (i.e., α = 0.05). All multilevel models were fitted using the “melogit” (multilevel logistic regression) and “mixed” (multilevel linear regression) commands using Stata 15 statistical software (StataCorp, College Station, TX).
Data availability.
Complete nucleotide sequences of 11 closed plasmids were deposited in GenBank under accession numbers MW298652 to MW298662. Whole-genome sequences can be accessed on the NCBI server under BioProject ID PRJNA681611.
ACKNOWLEDGMENTS
We thank our farm cooperators, without whom we would not have been able to undertake this work. Research funding was provided by the Canadian Institute for Health Research (CIHR) in support of the JPIAMR ARMIS project and by Agriculture and Agri-Food Canada.
Footnotes
Supplemental material is available online only.
Contributor Information
Edward Topp, Email: ed.topp@canada.ca.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Larney FJ, Hao X, Topp E. 2011. Manure management, p 247–263. In Hatfield JL, Sauer TJ (ed), Soil management: building a sustainable base for agriculture. American Society for Agronomy, Madison WI. [Google Scholar]
- 2.World Health Organization. 2003. Codex Alimentarius—code of hygienic practice for fresh fruits and vegetables. CAC/RCP 53–2003. World Health Organization, Rome, Italy. http://www.fao.org/ag/agn/CDfruits_en/others/docs/alinorm03a.pdf. [Google Scholar]
- 3.Kumar K, C Gupta S, Chander Y, Singh AK. 2005. Antibiotic use in agriculture and its impact on the terrestrial environment. Adv Agron 87:1–54. doi: 10.1016/S0065-2113(05)87001-4. [DOI] [Google Scholar]
- 4.Kemper N. 2008. Veterinary antibiotics in the aquatic and terrestrial environment. Ecol Indic 8:1–13. doi: 10.1016/j.ecolind.2007.06.002. [DOI] [Google Scholar]
- 5.Heuer H, Schmitt H, Smalla K. 2011. Antibiotic resistance gene spread due to manure application on agricultural fields. Curr Opin Microbiol 14:236–243. doi: 10.1016/j.mib.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 6.Ghirardini A, Grillini V, Verlicchi P. 2020. A review of the occurrence of selected micropollutants and microorganisms in different raw and treated manure—environmental risk due to antibiotics after application to soil. Sci Total Environ 707:136118. doi: 10.1016/j.scitotenv.2019.136118. [DOI] [PubMed] [Google Scholar]
- 7.Ashbolt NJ, Amezquita A, Backhaus T, Borriello P, Brandt KK, Collignon P, Coors A, Finley R, Gaze WH, Heberer T, Lawrence JR, Larsson DG, McEwen SA, Ryan JJ, Schonfeld J, Silley P, Snape JR, Van den Eede C, Topp E. 2013. Human health risk assessment (HHRA) for environmental development and transfer of antibiotic resistance. Environ Health Perspect 121:993–1001. doi: 10.1289/ehp.1206316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruden A, Larsson DG, Amezquita A, Collignon P, Brandt KK, Graham DW, Lazorchak JM, Suzuki S, Silley P, Snape JR, Topp E, Zhang T, Zhu YG. 2013. Management options for reducing the release of antibiotics and antibiotic resistance genes to the environment. Environ Health Perspect 121:878–885. doi: 10.1289/ehp.1206446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Effenberger M, Bachmaier J, Garces G, Gronauer A, Wilderer PA, Lebuhn M. 2006. Mesophilic-thermophilic-mesophilic anaerobic digestion of liquid dairy cattle manure. Water Sci Technol 53:253–261. doi: 10.2166/wst.2006.256. [DOI] [PubMed] [Google Scholar]
- 10.Hills DJ, Roberts DW. 1981. Anaerobic digestion of dairy manure and field crop residues. Agricultural Wastes 3:179–189. doi: 10.1016/0141-4607(81)90026-3. [DOI] [Google Scholar]
- 11.Li J, Wei L, Duan Q, Hu G, Zhang G. 2014. Semi-continuous anaerobic co-digestion of dairy manure with three crop residues for biogas production. Bioresour Technol 156:307–313. doi: 10.1016/j.biortech.2014.01.064. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Li Y, Zhang D, Li G, Lu J, Li S. 2016. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour Technol 217:50–55. doi: 10.1016/j.biortech.2016.01.111. [DOI] [PubMed] [Google Scholar]
- 13.Cuetos MJ, Fernández C, Gómez X, Morán A. 2011. Anaerobic co-digestion of swine manure with energy crop residues. Biotechnol Bioproc E 16:1044–1052. doi: 10.1007/s12257-011-0117-4. [DOI] [Google Scholar]
- 14.Marchaim U. 1992. Chapter seven: anaerobic processes, plant design and control, p 61–87. In Biogas processes for sustainable development. Food and Agriculture Organization of the United Nations, Rome, Italy. [Google Scholar]
- 15.Gurmessa B, Pedretti EF, Cocco S, Cardelli V, Corti G. 2020. Manure anaerobic digestion effects and the role of pre- and post-treatments on veterinary antibiotics and antibiotic resistance genes removal efficiency. Sci Total Environ 721:137532. doi: 10.1016/j.scitotenv.2020.137532. [DOI] [PubMed] [Google Scholar]
- 16.Nag R, Auer A, Markey BK, Whyte P, Nolan S, O’Flaherty V, Russell L, Bolton D, Fenton O, Richards K, Cummins E. 2019. Anaerobic digestion of agricultural manure and biomass—critical indicators of risk and knowledge gaps. Sci Total Environ 690:460–479. doi: 10.1016/j.scitotenv.2019.06.512. [DOI] [PubMed] [Google Scholar]
- 17.Huang X, Zheng J, Tian S, Liu C, Liu L, Wei L, Fan H, Zhang T, Wang L, Zhu G, Xu K. 2019. Higher temperatures do not always achieve better antibiotic resistance gene removal in anaerobic digestion of swine manure. Appl Environ Microbiol 85:e02878-18. doi: 10.1128/AEM.02878-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zou Y, Xiao Y, Wang H, Fang T, Dong P. 2020. New insight into fates of sulfonamide and tetracycline resistance genes and resistant bacteria during anaerobic digestion of manure at thermophilic and mesophilic temperatures. J Hazard Mater 384:121433. doi: 10.1016/j.jhazmat.2019.121433. [DOI] [PubMed] [Google Scholar]
- 19.Gonggrijp MA, Santman-Berends I, Heuvelink AE, Buter GJ, van Schaik G, Hage JJ, Lam T. 2016. Prevalence and risk factors for extended-spectrum beta-lactamase- and AmpC-producing Escherichia coli in dairy farms. J Dairy Sci 99:9001–9013. doi: 10.3168/jds.2016-11134. [DOI] [PubMed] [Google Scholar]
- 20.Li MM, Ray P, Knowlton KF, Pruden A, Xia K, Teets C, Du P. 2020. Fate of pirlimycin and antibiotic resistance genes in dairy manure slurries in response to temperature and pH adjustment. Sci Total Environ 710:136310. doi: 10.1016/j.scitotenv.2019.136310. [DOI] [PubMed] [Google Scholar]
- 21.Wepking C, Avera B, Badgley B, Barrett JE, Franklin J, Knowlton KF, Ray PP, Smitherman C, Strickland MS. 2017. Exposure to dairy manure leads to greater antibiotic resistance and increased mass-specific respiration in soil microbial communities. Proc R Soc B 284:20162233. doi: 10.1098/rspb.2016.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almeida RF, Queiroz IDS, Mikhael JER, Oliveira RC, Borges EN. 2019. Enriched animal manure as a source of phosphorus in sustainable agriculture. Int J Recycl Org Waste Agricult 8:203–210. doi: 10.1007/s40093-019-00291-x. [DOI] [Google Scholar]
- 23.Qian X, Gu J, Sun W, Wang XJ, Su JQ, Stedfeld R. 2018. Diversity, abundance, and persistence of antibiotic resistance genes in various types of animal manure following industrial composting. J Hazard Mater 344:716–722. doi: 10.1016/j.jhazmat.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 24.Ibrahim DR, Dodd CE, Stekel DJ, Ramsden SJ, Hobman JL. 2016. Multidrug resistant, extended spectrum beta-lactamase (ESBL)-producing Escherichia coli isolated from a dairy farm. FEMS Microbiol Ecol 92:fiw013. doi: 10.1093/femsec/fiw013. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki M, Miyake M, Maseda H, Qi G, Pan Z, Ihara I, Umetsu K. 2019. Thermophilic anaerobic digestion is an effective treatment for reducing cefazolin-resistant bacteria and ESBL-producers in dairy manure. J Mater Cycles Waste Manag 21:293–299. doi: 10.1007/s10163-018-0789-3. [DOI] [Google Scholar]
- 26.Qian X, Sun W, Gu J, Wang XJ, Zhang YJ, Duan ML, Li HC, Zhang RR. 2016. Reducing antibiotic resistance genes, integrons, and pathogens in dairy manure by continuous thermophilic composting. Bioresour Technol 220:425–432. doi: 10.1016/j.biortech.2016.08.101. [DOI] [PubMed] [Google Scholar]
- 27.Sun W, Gu J, Wang X, Qian X, Peng H. 2019. Solid-state anaerobic digestion facilitates the removal of antibiotic resistance genes and mobile genetic elements from cattle manure. Bioresour Technol 274:287–295. doi: 10.1016/j.biortech.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Tien YC, Li B, Zhang T, Scott A, Murray R, Sabourin L, Marti R, Topp E. 2017. Impact of dairy manure pre-application treatment on manure composition, soil dynamics of antibiotic resistance genes, and abundance of antibiotic-resistance genes on vegetables at harvest. Sci Total Environ 581–582:32–39. doi: 10.1016/j.scitotenv.2016.12.138. [DOI] [PubMed] [Google Scholar]
- 29.Masse D, Gilbert Y, Topp E. 2011. Pathogen removal in farm-scale psychrophilic anaerobic digesters processing swine manure. Bioresour Technol 102:641–646. doi: 10.1016/j.biortech.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 30.Jiang Y, Dennehy C, Lawlor PG, Hu Z, Zhan X, Gardiner GE. 2018. Inactivation of enteric indicator bacteria and system stability during dry co-digestion of food waste and pig manure. Sci Total Environ 612:293–302. doi: 10.1016/j.scitotenv.2017.08.214. [DOI] [PubMed] [Google Scholar]
- 31.Onyango LA, Alreshidi MM. 2018. Adaptive metabolism in staphylococci: survival and persistence in environmental and clinical settings. J Pathog 2018:1092632. doi: 10.1155/2018/1092632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown CJ, Sen D, Yano H, Bauer ML, Rogers LM, Van der Auwera GA, Top EM. 2013. Diverse broad-host-range plasmids from freshwater carry few accessory genes. Appl Environ Microbiol 79:7684–7695. doi: 10.1128/AEM.02252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yanagiya K, Maejima Y, Nakata H, Tokuda M, Moriuchi R, Dohra H, Inoue K, Ohkuma M, Kimbara K, Shintani M. 2018. Novel self-transmissible and broad-host-range plasmids exogenously captured from anaerobic granules or cow manure. Front Microbiol 9:2602. doi: 10.3389/fmicb.2018.02602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smalla K, Heuer H, Götz A, Niemeyer D, Krögerrecklenfort E, Tietze E. 2000. Exogenous isolation of antibiotic resistance plasmids from piggery manure slurries reveals a high prevalence and diversity of IncQ-like plasmids. Appl Environ Microbiol 66:4854–4862. doi: 10.1128/AEM.66.11.4854-4862.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gao L, Hu J, Zhang X, Wei L, Li S, Miao Z, Chai T. 2015. Application of swine manure on agricultural fields contributes to extended-spectrum beta-lactamase-producing Escherichia coli spread in Tai'an, China. Front Microbiol 6:313. doi: 10.3389/fmicb.2015.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brolund A, Sandegren L. 2016. Characterization of ESBL disseminating plasmids. Infect Dis (Lond) 48:18–25. doi: 10.3109/23744235.2015.1062536. [DOI] [PubMed] [Google Scholar]
- 37.Canton R, Coque TM. 2006. The CTX-M beta-lactamase pandemic. Curr Opin Microbiol 9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 38.Canton R, Gonzalez-Alba JM, Galan JC. 2012. CTX-M enzymes: origin and diffusion. Front Microbiol 3:110. doi: 10.3389/fmicb.2012.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyd DA, Tyler S, Christianson S, McGeer A, Muller MP, Willey BM, Bryce E, Gardam M, Nordmann P, Mulvey MR. 2004. Complete nucleotide sequence of a 92-kilobase plasmid harboring the CTX-M-15 extended-spectrum beta-lactamase involved in an outbreak in long-term-care facilities in Toronto, Canada. AAC 48:3758–3764. doi: 10.1128/AAC.48.10.3758-3764.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brigante G, Luzzaro F, Perilli M, Lombardi G, Coli A, Rossolini GM, Amicosante G, Toniolo A. 2005. Evolution of CTX-M-type beta-lactamases in isolates of Escherichia coli infecting hospital and community patients. Int J Antimicrob Agents 25:157–162. doi: 10.1016/j.ijantimicag.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Lartigue MF, Fortineau N, Nordmann P. 2005. Spread of novel expanded-spectrum β-lactamases in Enterobacteriaceae in a university hospital in the Paris area, France. Clin Microbiol Infect 11:588–591. doi: 10.1111/j.1469-0691.2005.01172.x. [DOI] [PubMed] [Google Scholar]
- 42.Lavollay M, Mamlouk K, Frank T, Akpabie A, Burghoffer B, Ben Redjeb S, Bercion R, Gautier V, Arlet G. 2006. Clonal dissemination of a CTX-M-15 beta-lactamase-producing Escherichia coli strain in the Paris area, Tunis, and Bangui. Antimicrob Agents Chemother 50:2433–2438. doi: 10.1128/AAC.00150-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carvalho I, Tejedor-Junco MT, Gonzalez-Martin M, Corbera JA, Silva V, Igrejas G, Torres C, Poeta P. 2020. Escherichia coli producing extended-spectrum beta-lactamases (ESBL) from domestic camels in the canary islands: a one health approach. Animals 10:1295. doi: 10.3390/ani10081295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braun SD, Ahmed MFE, El-Adawy H, Hotzel H, Engelmann I, Weiß D, Monecke S, Ehricht R. 2016. Surveillance of extended-spectrum beta-lactamase-producing Escherichia coli in dairy cattle farms in the Nile Delta, Egypt. Front Microbiol 7:1020. doi: 10.3389/fmicb.2016.01020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dandachi I, Fayad E, El-Bazzal B, Daoud Z, Rolain JM. 2019. Prevalence of extended-spectrum beta-lactamase-producing gram-negative bacilli and emergence of mcr-1 colistin resistance gene in Lebanese swine farms. Microb Drug Resist 25:233–240. doi: 10.1089/mdr.2018.0110. [DOI] [PubMed] [Google Scholar]
- 46.Pagani L, Migliavacca R, Pallecchi L, Matti C, Giacobone E, Amicosante G, Romero E, Rossolini GM. 2002. Emerging extended-spectrum beta-lactamases in Proteus mirabilis. J Clin Microbiol 40:1549–1552. doi: 10.1128/jcm.40.4.1549-1552.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vahaboglu H, Hal LM, Mulazimoglu L, Dodanli S, Yildirim I, Livermore DM. 1995. Resistance to extended-spectrum cephalosporins, caused by PER-I β-lactamase, in Salmonella typhimurium from Istanbul, Turkey. J Med Microbiol 43:294–299. doi: 10.1099/00222615-43-4-294. [DOI] [PubMed] [Google Scholar]
- 48.Danel F, Hall LM, Gur D, Akalin HE, Livermore DM. 1995. Transferable production of PER-1 β-lactamase in Pseudomonas aeruginosa. J Antimicrob Chemother 35:281–294. doi: 10.1093/jac/35.2.281. [DOI] [PubMed] [Google Scholar]
- 49.Goussard S, Courvalin P. 1991. Sequence of the genes blaT-1B and blaT-2. Gene 102:71–73. doi: 10.1016/0378-1119(91)90540-R. [DOI] [PubMed] [Google Scholar]
- 50.Lagace-Wiens PR, Tailor F, Simner P, DeCorby M, Karlowsky JA, Walkty A, Hoban DJ, Zhanel GG. 2011. Activity of NXL104 in combination with beta-lactams against genetically characterized Escherichia coli and Klebsiella pneumoniae isolates producing class A extended-spectrum beta-lactamases and class C beta-lactamases. Antimicrob Agents Chemother 55:2434–2437. doi: 10.1128/AAC.01722-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strauß LM, Dahms C, Becker K, Kramer A, Kaase M, Mellmann A. 2015. Development and evaluation of a novel universal beta-lactamase gene subtyping assay for blaSHV, blaTEM and blaCTX-M using clinical and livestock-associated Escherichia coli. J Antimicrob Chemother 70:710–715. doi: 10.1093/jac/dku450. [DOI] [PubMed] [Google Scholar]
- 52.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob Agents Chemother 54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Livermore DM, Day M, Cleary P, Hopkins KL, Toleman MA, Wareham DW, Wiuff C, Doumith M, Woodford N. 2019. OXA-1 beta-lactamase and non-susceptibility to penicillin/beta-lactamase inhibitor combinations among ESBL-producing Escherichia coli. J Antimicrob Chemother 74:326–333. doi: 10.1093/jac/dky453. [DOI] [PubMed] [Google Scholar]
- 54.Baudry PJ, Nichol K, DeCorby M, Mataseje L, Mulvey MR, Hoban DJ, Zhanel GG. 2008. Comparison of antimicrobial resistance profiles among extended-spectrum-beta-lactamase-producing and acquired AmpC beta-lactamase-producing Escherichia coli isolates from Canadian intensive care units. Antimicrob Agents Chemother 52:1846–1849. doi: 10.1128/AAC.01176-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Robicsek A, Strahilevitz J, Jacoby GA, Macielag M, Abbanat D, Park CH, Bush K, Hooper DC. 2006. Fluoroquinolone-modifying enzyme: a new adaptation of a common aminoglycoside acetyltransferase. Nat Med 12:83–88. doi: 10.1038/nm1347. [DOI] [PubMed] [Google Scholar]
- 56.Wallace JS, Garner E, Pruden A, Aga DS. 2018. Occurrence and transformation of veterinary antibiotics and antibiotic resistance genes in dairy manure treated by advanced anaerobic digestion and conventional treatment methods. Environ Pollut 236:764–772. doi: 10.1016/j.envpol.2018.02.024. [DOI] [PubMed] [Google Scholar]
- 57.Saini V, McClure JT, Leger D, Dufour S, Sheldon AG, Scholl DT, Barkema HW. 2012. Antimicrobial use on Canadian dairy farms. J Dairy Sci 95:1209–1221. doi: 10.3168/jds.2011-4527. [DOI] [PubMed] [Google Scholar]
- 58.Partridge SR, Kwong SM, Firth N, Jensen SO. 2018. Mobile genetic elements associated with antimicrobial resistance. Clin Microbiol Rev 31:e00088-17. doi: 10.1128/CMR.00088-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang T, Zhang XX, Ye L. 2011. Plasmid metagenome reveals high levels of antibiotic resistance genes and mobile genetic elements in activated sludge. PLoS One 6:e26041. doi: 10.1371/journal.pone.0026041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kyselková M, Chrudimský T, Husník F, Chroňáková A, Heuer H, Smalla K, Elhottová D. 2016. Characterization of tet(Y)-carrying LowGC plasmids exogenously captured from cow manure at a conventional dairy farm. FEMS Microbiol Ecol 92:fiw075. doi: 10.1093/femsec/fiw075. [DOI] [PubMed] [Google Scholar]
- 61.Heuer H, Kopmann C, Binh CT, Top EM, Smalla K. 2009. Spreading antibiotic resistance through spread manure: characteristics of a novel plasmid type with low %G+C content. Environ Microbiol 11:937–949. doi: 10.1111/j.1462-2920.2008.01819.x. [DOI] [PubMed] [Google Scholar]
- 62.Lee KY, Hopkins JD, Syvanen M. 1990. Direct involvement of IS26 in an antibiotic resistance operon. J Bacteriol 172:3229–3236. doi: 10.1128/jb.172.6.3229-3236.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mollet B, Iida S, Shepherd J, Arber W. 1983. Nucleotide sequence of IS26, a new prokaryotic mobile genetic element. Nucleic Acids Res 11:6319–6330. doi: 10.1093/nar/11.18.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harmer CJ, Moran RA, Hall RM. 2014. Movement of IS26-associated antibiotic resistance genes occurs via a translocatable unit that includes a single IS26 and preferentially inserts adjacent to another IS26. mBio 5:e01801-14. doi: 10.1128/mBio.01801-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harmer CJ, Hall RM. 2016. IS26-mediated formation of transposons carrying antibiotic resistance genes. mSphere 1:e00038-16. doi: 10.1128/mSphere.00038-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Martin C, Gomez-Lus R, Ortiz JM, Garcia-Lobo JM. 1987. Structure and mobilization of an ampicillin and gentamicin. Antimicrob Agents Chemother 31:1266–1270. doi: 10.1128/aac.31.8.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He S, Hickman AB, Varani AM, Siguier P, Chandler M, Dekker JP, Dyda F. 2015. Insertion sequence IS26 reorganizes plasmids in clinically isolated multidrug-resistant bacteria by replicative transposition. mBio 6:e00762-15. doi: 10.1128/mBio.00762-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tong J, Fang P, Zhang J, Wei Y, Su Y, Zhang Y. 2019. Microbial community evolution and fate of antibiotic resistance genes during sludge treatment in two full-scale anaerobic digestion plants with thermal hydrolysis pretreatment. Bioresour Technol 288:121575. doi: 10.1016/j.biortech.2019.121575. [DOI] [PubMed] [Google Scholar]
- 69.Ma Y, Wilson CA, Novak JT, Riffat R, Aynur S, Murthy S, Pruden A. 2011. Effect of various sludge digestion conditions on sulfonamide, macrolide, and tetracycline resistance genes and class I integrons. Environ Sci Technol 45:7855–7861. doi: 10.1021/es200827t. [DOI] [PubMed] [Google Scholar]
- 70.Chung WO, Werckenthin C, Schwarz S, Roberts MC. 1999. Host range of the ermF rRNA methylase gene in bacteria of human and animal origin. J Antimicrob Chemother 43:5–14. doi: 10.1093/jac/43.1.5. [DOI] [PubMed] [Google Scholar]
- 71.Tschape H, Tietze E, Koch C. 1981. Characterization of conjugative R plasmids belonging to the new incompatibility group IncU. J Gen Microbiol 127:155–160. doi: 10.1099/00221287-127-1-155. [DOI] [PubMed] [Google Scholar]
- 72.Jutkina J, Rutgersson C, Flach CF, Joakim Larsson DG. 2016. An assay for determining minimal concentrations of antibiotics that drive horizontal transfer of resistance. Sci Total Environ 548–549:131–138. doi: 10.1016/j.scitotenv.2016.01.044. [DOI] [PubMed] [Google Scholar]
- 73.Heuer H, Krögerrecklenfort E, Wellington EMH, Egan S, van Elsas JD, van Overbeek L, Collard J-M, Guillaume G, Karagouni AD, Nikolakopoulou TL, Smalla K. 2002. Gentamicin resistance genes in environmental bacteria: prevalence and transfer. FEMS Microbiol Ecol 42:289–302. doi: 10.1111/j.1574-6941.2002.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 74.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Robertson J, Nash JHE. 2018. MOB-suite: software tools for clustering, reconstruction and typing of plasmids from draft assemblies. Microb Genom 4:e000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cuccuru G, Orsini M, Pinna A, Sbardellati A, Soranzo N, Travaglione A, Uva P, Zanetti G, Fotia G. 2014. Orione, a web-based framework for NGS analysis in microbiology. Bioinformatics 30:1928–1929. doi: 10.1093/bioinformatics/btu135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA, 3rd, Stevens R, Vonstein V, Wattam AR, Xia F. 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. doi: 10.1038/srep08365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res 42:D206–D214. doi: 10.1093/nar/gkt1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 81.Johnson M, Zaretskaya I, Raytselis Y, Merezhuk Y, McGinnis S, Madden TL. 2008. NCBI BLAST: a better web interface. Nucleic Acids Res 36:W5–W9. doi: 10.1093/nar/gkn201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seemann T. 2015. Snippy: fast bacterial variant calling from NGS reads. GitHub, https://github.com/tseemann/snippy.
- 83.Carattoli A, Zankari E, Garcia-Fernandez A, Voldby Larsen M, Lund O, Villa L, Moller Aarestrup F, Hasman H. 2014. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58:3895–3903. doi: 10.1128/AAC.02412-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Feldgarden M, Brover V, Haft DH, Prasad AB, Slotta DJ, Tolstoy I, Tyson GH, Zhao S, Hsu CH, McDermott PF, Tadesse DA, Morales C, Simmons M, Tillman G, Wasilenko J, Folster JP, Klimke W. 2019. Validating the AMRFinder tool and resistance gene database by using antimicrobial resistance genotype-phenotype correlations in a collection of isolates. Antimicrob Agents Chemother 63:e00483–19. doi: 10.1128/AAC.00483-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seemann T. 2016. ABRicate: mass screening of contigs for antibiotic resistance genes. GitHub, https://github.com/tseemann/abricate.
- 87.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petkau A. 2018. Staramr, GitHub. https://github.com/phac-nml/staramr.
- 89.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rahube TO, Marti R, Scott A, Tien YC, Murray R, Sabourin L, Zhang Y, Duenk P, Lapen DR, Topp E. 2014. Impact of fertilizing with raw or anaerobically digested sewage sludge on the abundance of antibiotic-resistant coliforms, antibiotic resistance genes, and pathogenic bacteria in soil and on vegetables at harvest. Appl Environ Microbiol 80:6898–6907. doi: 10.1128/AEM.02389-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marti R, Tien YC, Murray R, Scott A, Sabourin L, Topp E. 2014. Safely coupling livestock and crop production systems: how rapidly do antibiotic resistance genes dissipate in soil following a commercial application of swine or dairy manure? Appl Environ Microbiol 80:3258–3265. doi: 10.1128/AEM.00231-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dohoo IR, Martin SW, Stryhn HE. 2009. Veterinary epidemiologic research (2nd Edition). University of Prince Edward Island, Charlottetown, Prince Edward Island, Canada. [Google Scholar]
- 93.Pearl DL. 2014. Making the most of clustered data in laboratory animal research using multi-level models. Ilar J 55:486–492. doi: 10.1093/ilar/ilu034. [DOI] [PubMed] [Google Scholar]
- 94.Suzuki MT, Taylor LT, DeLong EF. 2000. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 59-nuclease assays. Appl Environ Microbiol 66:4605–4614. doi: 10.1128/AEM.66.11.4605-4614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gaze WH, Zhang L, Abdouslam NA, Hawkey PM, Calvo-Bado L, Royle J, Brown H, Davis S, Kay P, Boxall AB, Wellington EM. 2011. Impacts of anthropogenic activity on the ecology of class 1 integrons and integron-associated genes in the environment. ISME J 5:1253–1261. doi: 10.1038/ismej.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Walsh F, Ingenfeld A, Zampicolli M, Hilber-Bodmer M, Frey JE, Duffy B. 2011. Real-time PCR methods for quantitative monitoring of streptomycin and tetracycline resistance genes in agricultural ecosystems. J Microbiol Methods 86:150–155. doi: 10.1016/j.mimet.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 97.Knapp CW, Zhang W, Sturm BS, Graham DW. 2010. Differential fate of erythromycin and beta-lactam resistance genes from swine lagoon waste under different aquatic conditions. Environ Pollut 158:1506–1512. doi: 10.1016/j.envpol.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 98.Bert F, Branger C, Lambert-Zechovsky N. 2002. Identification of PSE and OXA beta-lactamase genes in Pseudomonas aeruginosa using PCR-restriction fragment length polymorphism. J Antimicrob Chemother 50:11–18. doi: 10.1093/jac/dkf069. [DOI] [PubMed] [Google Scholar]
- 99.CLSI . 2015. Performance standards for antimicrobial susceptibility testing, 25th ed. CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Text S1, Tables S1 to S5, Fig. S1 and S2. Download AEM02980-20_Supp_1_seq4.pdf, PDF file, 0.7 MB (687.9KB, pdf)
Data Availability Statement
Complete nucleotide sequences of 11 closed plasmids were deposited in GenBank under accession numbers MW298652 to MW298662. Whole-genome sequences can be accessed on the NCBI server under BioProject ID PRJNA681611.