Abstract
Objective:
Dietary supplements and alternative therapies are commercialized as a panacea for obesity/weight gain as a result of the minimal regulatory requirements in demonstrating efficacy. These products may indirectly undermine the value of guideline-driven obesity treatments. We systematically reviewed the literature of purported dietary supplements and alternative therapies for weight loss.
Methods:
A systematic review evaluated the efficacy of dietary supplements and alternative therapies for weight loss aged ≥18 years. We searched Medline (Pubmed), Cochrane, Web of Science, CINAHL, EMBASE (Ovid), and PsychINFO (EBSCO). Risk of bias and results were summarized qualitatively.
Results:
Of 20,504 citations, we reviewed 1,743 full-text articles, of which 315 were randomized controlled trials evaluating the efficacy of 14 purported dietary supplements, therapies or a combination thereof. Risk of bias and sufficiency of data varied widely. Few studies (n=52 [16.5%]) were classified low risk and sufficient to support efficacy. Of these, only 16 (31%) noted significant pre/post inter-group differences in weight (range: 0.3,4.93 kg).
Conclusions:
Dietary supplements and alternative therapies for weight loss have a limited, high-quality evidence-base of efficacy. Practitioners and patients should be aware of the scientific evidence of claims before recommending use.
Keywords: Dietary supplements, obesity, weight loss, treatment, regulatory, herbal supplements, nutraceuticals, complementary therapies
INTRODUCTION
Obesity rates in the United States (USA) continue to rise(1) which may lead to obesity-related complications such as cardiometabolic dysfunction, malignancy, disability and premature mortality (2–4). Obesity prevention strategies and treatments continue to evolve, and include intensive lifestyle interventions, public health programs, Food and Drug Administration (FDA)-approved pharmacotherapies, endoscopic and surgical bariatric therapies (5), which potentially promote 5-35% weight-loss (6–9), Such programs can be delivered in primary care, community- or research-based settings (10–13). Patients often struggle to lose or maintain weight (14) either due to lack of efficacy of existing FDA-approved therapies or due to a lack of access to healthcare professionals providing obesity treatments. This prompts a search for easily available non-prescription dietary supplements or alternative therapies to satisfy consumer and clinician’s desire for effective, low-risk, and low-cost options for achieving weight loss (15–19).
Of adults attempting weight-loss, 33.9% report using dietary supplements; these estimates are higher in younger adults, women, and in lower socioeconomic groups (20,21). The number of marketed weight-loss supplements exceed 40% of the 776 dietary supplements identified in a FDA-drug database (22). They are also marketed by popular press, influencers, and celebrities (23,24). Hence, healthcare professionals are faced with questions about their effectiveness for weight gain (25,26), and similar to consumers, have major misconceptions about such purported treatments, including beliefs that they are thoroughly evaluated for safety/efficacy by the FDA in advance of marketing (20,27–30). Healthcare professionals have incomplete information about ‘clinically proven’ claims touted by promoters of these therapies. These assumptions lead consumers to believe that ‘natural’ or ‘clinically proven’ non-prescription products are safer than FDA-approved medications, despite, in some cases, post-marketing recalls (20,31).
Passage of the Dietary Supplement Health and Education Act (DSHEA) in 1994 led to the deregulation of the dietary supplement industry. The National Institute of Health’s Office of Dietary Supplements was established thereafter, whose mission is to strengthen the knowledge and understanding of dietary supplements by evaluating scientific information, stimulating and supporting research and educating the public (32). Studies funded by this Institute require a product integrity profile including a collaborative approach for commercial-based product documentation. While this institute’s work has importantly advanced the science, members of The Obesity Society believed it was important to evaluate and perform a qualitative synthesis of non-FDA therapies to provide scientific evidence to guide members. This paper differs from previous societal reviews by focusing specifically on weight loss as an outcome (33,34). We conducted a systematic review of randomized controlled trials (RCTs) of dietary supplements and alternative therapies, with a goal of closing the knowledge gap among clinicians, enhancing the evidence supporting their efficacy, and to counsel their patients (35,36).
METHODS
We conducted a systematic literature review using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (37) (Table S1).
Study Protocol
Initial searches were conducted by two research librarians (HBB, PJB) on January 30th, 2018. The search strategy (Table S2) included subject headings and key words to capture the concepts of weight loss and alternative therapies. Syntax was adjusted to conform to each of the following electronic databases searched on January 30th, 2018: Medline (PubMed) [1946-current]; Cochrane Library (Wiley) [various]; Web of Science [1900-Current]; and CINAHL (EBSCO) [1981-Current]. EMBASE (Ovid) [1974-Current] was searched on February 5th, 2018. No other limits were applied, but we separated animal from human studies during screening. The authors solicited additional potential treatments from their own clinical experiences and asked leading experts within the Clinical Committee of The Obesity Society for their input. Our focus was on peer-reviewed RCTs; we omitted gray literature, websites, abstract submissions, clinical trial registries or conference proceedings. Reference lists from systematic reviews were evaluated; articles were manually reviewed for additional studies.
Selection Criteria
Selection criteria was guided by the Patients, Intervention, Controls, Outcomes, and Study Type framework. Inclusion criteria included: English language articles, overweight or obesity (BMI ≥25kg/m2), and studies focusing on intentional weight loss. We limited our review to adults aged ≥18 years, as the Committee believed that the use of dietary supplements or alternative therapies would be low in children and has been published (38). Following full-text review and identification of studies, the team modified the protocol to include RCTs rather than observational trials. We excluded non-randomized controlled trials including cohort trials, case reports/series, systematic reviews or meta-analyses, commentaries, editorials, cross-sectional studies, animal studies, bariatric surgery, or studies focusing on FDA-approved medications.
Data Extraction
All citations were combined using Endnote X8 (Thomson Reuters). A total of 20,504 citations were identified using a priori criteria (Figure 1) after which duplicates were removed, (n=6,476) leaving 14,028 citations. Each set of supplements/therapies was assigned to a set of reviewers, each conducting a test review of title/abstracts of 200 citations for which concordance rates exceeded 75%. At that point, each reviewer set evaluated title/abstracts of each grouping using the predefined inclusion/exclusion criteria. Discrepancies were adjudicated by a senior member of the Clinical Committee and reconciled before full-text review. Full-text manuscripts (n=1,743) were obtained with the assistance of the reference librarians, and a second-level screening that applied exclusion criteria in a hierarchical manner was conducted.
Figure 1:
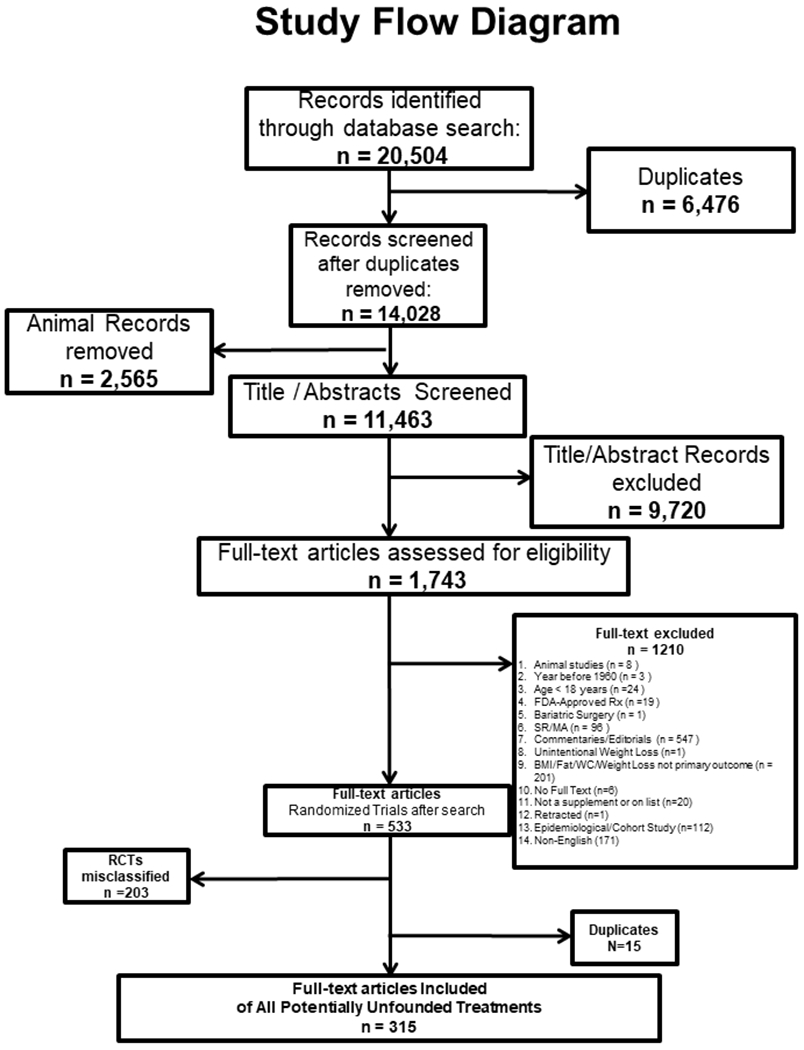
Participant Flow Diagram for Systematic Review of Randomized Controlled Trials
Quality Review
Each included study was assessed using the Cochrane Collaboration’s Risk-of-Bias Tool(39). The following elements were evaluated: sequence generation, allocation concealment, and blinding (participants, healthcare providers, data collectors, outcome assessors). The review deliberately excluded incomplete outcome data, selective outcome reporting or other sources of bias as the volume of articles and heterogeneity of results would prevent an accurate aggregate summary of the results. Each full-text set was evaluated in duplicate, with disagreements adjudicated by a third reviewer. Categories were designated as a high/low/unclear level of bias representing low/high/unclear study quality. The proportion of low bias was tabulated for each product based on the reviewer’s interpretation (Table 1). Articles fulfilling low risk of bias within each category were identified and summarized.
Table 1:
Cochrane Risk of Bias Among Studies with >5 Randomized Controlled Trials
| Dietary Supplement or Alternative Therapy | # of studies | # Overall Low Bias | Sequence Generation | Allocation Concealment | Blinding |
|||
|---|---|---|---|---|---|---|---|---|
| Participants | Healthcare Providers | Data Collectors | Outcome Assessors | |||||
| Acupuncture | 45 | 2% | 42% | 18% | 27% | 7% | 7% | 4% |
| Calcium & Vitamin D | 22 | 23% | 68% | 45% | 64% | 68% | 68% | 55% |
| Chitosan | 9 | 11% | 67% | 44% | 44% | 22% | 11% | 11% |
| Chocolate/Cocoa | 6 | 11% | 33% | 50% | 33% | 50% | 50% | 33% |
| Chromium | 6 | 50% | 83% | 67% | 67% | 83% | 83% | 50% |
| Ephedra or Caffeine | 31 | 32% | 45% | 65% | 97% | 87% | 74% | 74% |
| Garcinia and/or Hydroxycitrate | 15 | 27% | 47% | 27% | 67% | 73% | 60% | 47% |
| Green Tea | 38 | 42% | 76% | 63% | 79% | 79% | 76% | 71% |
| Guar Gum | 5 | 0% | 40% | 0% | 40% | 20% | 0% | 0% |
| Conjugated Linoleic Acid | 31 | 16% | 84% | 71% | 90% | 32% | 39% | 23% |
| Mind-Body | 22 | 9% | 45% | 32% | 18% | 14% | 32% | 23% |
| Phaseolus | 7 | 0% | 14% | 29% | 86% | 71% | 0% | 0% |
| Phenylpropylamine | 5 | 20% | 40% | 60% | 180% | 40% | 60% | 60% |
| Pyruvate | 7 | 0% | 29% | 71% | 29% | 57% | 0% | 20% |
Criteria are based on the summary for each dietary supplement and alternative therapy of included randomized controlled trials. Percentages reflect the proportion of studies fulfilling “low risk of bias” for the given category according to the Cochrane Collaboration tool criteria (see Appendices). The proportion fulfilling Low is determined by the number of “Low” responses divided by the overall number of studies
Study-Level Outcomes:
A priori, we decided to present only dietary supplements or alternative therapies consisting of five or more RCTs. Study-level data are presented in aggregate in Table 2 and 3 and detailed data in Appendices 3–17. Key data included: study year, journal, country, funding source, participant characteristics, description of the arms, number of subjects per arm, and study duration. Mean (± standard deviation) or age-range if not reported, the count (proportion) of females, count (proportion) of non-Caucasian race, mean body mass index, and waist circumference or waist-hip ratio if not designated, were abstracted. The writing group proposed presenting all study outcomes; however, due to the heterogeneity and complexity of the results and analytical methods used, the authors presents the key outcome of weight (baseline, follow-up, weight change), the intra/inter-group p-value differences, and if weight was not listed, other relevant body composition measures. Qualitative summary of the region, industry funding, range of participants and study duration are in Table 2. Table 3 outlines age range, whether authors indicated significance among arms, if reported, and the number of studies with overall low risk of bias that were significant.
Table 2:
Summary Characteristics of Studies
| Dietary Supplement or Alternative Therapy | # RCTs | NA | SA | Geographic Region | Africa | ANZ | Industry Fundeda | # participants | Study Duration | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Europe | Asia | N (%) | NR | Low | High | Low | High | |||||||
| Acupuncture | 45 | 1 (2%) | --- | 4 (9%) | 38 (84% | --- | 2 (4) | 1 (2%) | 18 (40%) | 5 | 105 | 3 weeks | 24 weeks | |
| Calcium & Vitamin D | 22 | 11 (50%) | 2 (9%) | 4 (18%) | 5 (23) | --- | --- | 6 (27%) | 4 (18%) | 6 | 170 | 2 weeks | 2 years | |
| Chitosan | 9 | 3 (33%) | --- | 2 (22%) | 3 (33%) | --- | 1 (11%) | 3 (33%) | 4 (44%) | 10 | 125 | 1 month | 24 weeks | |
| Chocolate/Cocoa | 6 | 3 (50%) | --- | 2 (33%) | --- | --- | 1 (16%) | 2 (33%) | 4 (67%) | 4 | 15 | 21 days | 18 weeks | |
| Chromium | 6 | 5 (83%) | --- | --- | --- | --- | 1 (17%) | 5 (83%) | 1 (17%) | 6 | 66 | 40 days | 24 weeksb | |
| Ephedra or Caffeine | 31 | 14 (45%) | --- | 11 (35%) | 5 (16%) | --- | 1 (3%) | 19 (61%) | 2 (7%) | 4 | 84 | 1 week | 9 months | |
| Garcinia | 15 | 3 (20%) | 2 (13%) | 2 (13%) | 8 (53%) | --- | --- | 4 (27%) | 9 (60%) | 10 | 69 | 2 weeks | 16 weeks | |
| Green Tea | 38 | 8 (21%) | --- | 10 (26%) | 19 (50%) | --- | 1 (3%) | 12 (32%) | 9 (24%) | 5 | 123 | 6 weeks | 12 months | |
| Guar Gum | 5 | --- | 1 (20%) | 3 (60%) | 1 (20%) | --- | --- | 1 (20%) | 2 (40%) | 9 | 48 | 6 weeks | 6 months | |
| Conjugated Linoleic Acid | 31 | 12 (39%) | 2 (7%) | 14 (45%) | 3 (10%) | --- | --- | 15 (48%) | 7 (23%) | 7 | 173 | 3 weeks | 24 months | |
| Mind-Body | 22 | 12 (55%) | --- | 8 (36%) | 2 (9%) | --- | --- | 0 (0%) | 15 (68%) | 5 | 100 | 4 weeks | 18 months | |
| Phaseolus | 7 | --- | --- | 5 (71%) | 2 (29%) | --- | --- | 2 (29%) | 3 (43%) | 12 | 62 | 30 days | 24 weeks | |
| Phenylpropylamine | 5 | 5 (100%) | --- | --- | --- | --- | --- | 3 (60%) | 2 (40%) | 7 | 51 | 6 weeks | 12 weeks | |
| Pyruvate | 7 | 6 (86%) | --- | 1 (14% | --- | --- | --- | 1 (14%) | 3 (43%) | 6 | 18 | 21 days | 6 weeks | |
Number of participants reflect the lowest/highest number of participants in each arm of the study.
Abbreviations: ANZ – Australia/New Zealand; NA – North America; NR – not reported; RCT – randomized controlled trial; SA – South America
Included as industry funded even if part of the study received an element of funding. Not reported indicates funding was not reported in the study
One study is listed as not-reported
Table 3:
Summary Characteristics of Studies
| Dietary Supplement or Alternative Therapy | Age (years) | Reporting of Weighta | Weight Change in Low Bias Studies | ||||
|---|---|---|---|---|---|---|---|
| # RCTs | Low | High | NR p-value | Noted Significantd | # Low Biase | Significantf | |
| Acupuncture | 45 | 18 | 64 | 18 (40%) | 18 (40%) | 2 (4.4%) | -- |
| Calcium & Vitamin D | 22 | 20.1 | 62.4 | 8 (36%) | 3 (13.6) | 5 (22.7%) | --- |
| Chitosan | 9 | 23.1 | 54.5 | 3 (33%) | 5 (56%) | 1 (11%) | 1 (11%) |
| Chocolate/Cocoa | 6 | 20 | 60 | 4 (67%) | 1 (17%) | 1 (11%) | --- |
| Chromium | 6 | 25 | 75 | 1 (17%) | 3 (50%) | 3 (50%) | 1 (16%) |
| Ephedra or Caffeine | 31 | 22 | 50.1 | 11 (35%) | 14 (45%) | 10 (32%) | 5 (16%) |
| Garcinia and/or Hydroxycitrate | 15 | 21 | 60 | 3 (20%) | 5 (33%) | 4 (27%) | 2 (13%) |
| Green Tea | 38 | 19 | 81.4 | 13 (34%) | 8 (21%) | 16 (42%) | 2 (5.3%) |
| Guar Gum | 5 | 51.6 | 63.6 | 3 (60%) | 1 (20%) | --- | --- |
| Conjugated Linoleic Acid | 31 | 18 | 65 | 8 (26%) | 4 (13%) | 5 (16%) | 1 (3.2%) |
| Mind-Body | 22 | 20 | 65 | 4 (18%) | 6 (27%) | 2 (9.1%) | --- |
| Phaseolus | 7 | 22 | 66 | 3 (43%)c | 3 (43%) | --- | --- |
| Phenylpropylamine | 5 | 18 | 65 | 1 (20%) | 3 (60%) | 1 (20%) | --- |
| Pyruvate | 7 | 22.5 | 48.7b | 2 (29%) | 4 (57%) | --- | --- |
Abbreviation: NR – not reported; RCT – randomized controlled trial
reporting the number of studies that compared pre/post intervention between arms.
two studies did not report age or age range
One study had two substudies – one of which did not report an overall p-value
significance as noted/outlined by the study authors
Number of RCTs that fulfilled criteria for low risk of bias across all categories of Cochrane Collaboration tool
Proportion of low risk of bias studies that demonstrated statistically significant changes pre/post intervention between groups.
RESULTS
Of the 20,504 total citations, we reviewed 1,743 full-text articles and included 315 in our full-text review (Figure 1). The most common reason for full-text exclusion was study type (i.e., commentary, editorial, non-randomized study). Each of the dietary supplements or alternative therapies is presented, recognizing that some studies consisted of multiple therapies and could be categorized in varied categories. Fifty-two studies were classified as high quality (e.g., low risk of bias), of which 16 demonstrated significant pre/post weight changes compared to placebo (range, 0.3,4.9 kg).
Acupuncture
Acupuncture is a traditional Chinese practice that involves placing small needles, with or without electrical stimulation, at various points in the body in order to adjust physiological functions and address numerous ailments (40). Studies examining the mechanism by which acupuncture impacts weight loss have demonstrated changes in central nervous neurotransmitters regulating hunger and satiety as well as improving insulin resistance, lipid metabolism and appetite reduction (41,42). Of the 45 RCTs conducted between 1981 and 2016, only a small fraction (n=2, 4%) was identified to have a low degree of bias (43,44). The majority (n=38) were from Asia followed by Europe (n=4). Eighteen (40%) did not report their funding source and the remaining were funded by government sources. Types of acupuncture were varied, including auricular, wrist, electrical, abdominal and laser. Study arms consisted of 5 to 105 participants, with a follow-up range of 3-24 weeks. Median age of participants was in their 30’s, none were above age 65 years, and the majority of studies were conducted in females. There were 18 studies that did not report between-group pre/post changes. Of studies that did, 18 of 27 (67%) reported significant differences between arms; all reported changes <5% and <5kg between groups. The two studies demonstrating low risk of bias either did not report the differences between the arms (43) or were not significant (44) (see Tables S3A–D).
Calcium-Vitamin D Supplementation
Calcium is a mineral necessary for heart, muscle, nerve, and blood clotting function. Vitamin D aids absorption of calcium in the body through the formation of hormone calcitriol. Joint supplementation of calcium and vitamin D is proposed to regulate adipocyte lipid metabolism and triglyceride storage, improving metabolic health and reducing body weight (45,46). Only five of the 22 RCTs (23%) conducted between 2007 and 2018 were classified as having an overall low risk of bias (47–51). Half of the studies (n=11) were conducted in North America, and of the 22, 6 (27%) were industry-sponsored, and four did not report their funding sources. The number of participants ranged from 6-170 per arm, and the range of the study duration was 2 weeks to 2 years. Participant age ranged from 20.1 to 62.4 years. Studies were generally conducted in females. Of the 22 studies, eight did not report changes between arms over time; three studies (9%) demonstrated significant findings of decreased weight over time. Of the 5 articles with low bias (23%) (47–51), none demonstrated significant differences in weight change between groups (see Tables S4A–D).
Chitosan
Chitosan is a linear polysaccharide that is extracted with alkaline substances from the hard exterior of crustaceans, such as shellfish. Chitosan is used medically to decrease bleeding for wound dressings and has been proposed, due to its ability to bind to lipids and decrease their absorption in the gastrointestinal tract, to lower cholesterol and body weight (52,53). Nine studies (conducted between 1996 and 2016) were identified, of which only one was classified as having low risk of bias (54). Studies were conducted in North America (n=2), Southeast Asia and Europe (n=2 each). Three were industry sponsored, and the number of participants ranged from 10-125 per arm, with a range for follow-up of 1 month to 24 weeks. The median age was in the 30s (range, 23.1-54.5), no participants were above age 65, and most studies were conducted in females. Five studies demonstrated statistically significant differences between arms, and three did not report any such differences. The article with low risk of bias demonstrated a statistically significant pre/post between group change in weight of 2.3 kg over 6 weeks (54) (see Tables S5A–D).
Chocolate/Cocoa
The cocoa bean is a dried and fully fermented seed of the theobroma cacao tree. These seeds can be ground and roasted, forming chocolate. Cocoa contains significant amounts of bioactive compounds, including antioxidant polyphenols and methylxanthines (caffeine and theobromine), which might promote weight loss by browning white adipocytes and improving lipid catabolic metabolism insulin resistance, endothelial function and oxidative stress (55–57). Only one of the six trials (conducted between 2008 and 2015) was classified as having a low risk of bias (58). Trials were conducted in the USA, Europe, Australia and Mexico. Four trials did not report funding; the remaining were industry funded. Counts per arm ranged from 4 to 15, with trial duration ranging from 21 days to 18 weeks. Participant age ranged from 20 to 60 years. One study demonstrated significant differences between arms at follow-up; the remaining were non-significant or did not report these values. The study with a low risk of bias (58) did not show any significant changes in weight (see Tables S6A–D).
Chromium
Chromium is a non-essential biometal, present in many foods (mostly attributed to food processed in stainless steel equipment). It is proposed to play a role in glucose, lipid, and amino acid metabolism by its potentiating effects on insulin action. It may directly enhance serotonin activity and/or impacting potential downstream effects on dopaminergic signaling on central insulin receptors. By altering these neurotransmitters, chromium is thought to affect several pathways involved in the central control of food intake, satiety and energy hemostasis (59–63). Of the six RCTs (between 1996 and 2016), three had a low risk of bias (n=3). Five studies (83%) were conducted in the USA; all were industry sponsored. The number of participants ranged from 6 to 66 per arm, and study duration ranged from 40 days to 24 weeks. One study included only females; three did not report sex of participants. Of the six trials, one did not report differences between groups, and of those that reported results, only two reported a significant weight-loss. Of the 3 studies with low bias (64–66) only one demonstrated a significant reduction in weight (−1.4 ± 2.9 kg) with 400 µg of chromium over 72 days (65) (see Tables S7A–D).
Ephedra/Caffeine
The plant ephedra sinica’s main proposed active ingredient for weight loss is ephedrine which is often taken is conjugation with caffeine. Its proposed mechanism of action for weight loss is that it enhances and speeds up metabolism promoting a fat burning metabolic state (67). Thirty-one RCTs published between 1975 and 2017 were identified, among which 10 studies had a low degree of bias (31%). Fourteen (45%) were conducted in the USA while 11 (35%) were conducted in Europe. Funding sources were not revealed in two studies, with industry sponsoring 19 (61%) studies. Study duration ranged from 1 week to 9 months, the number in each arm ranged from 4 to 84 participants, and most were conducted in women. None were aged >50 years (range, 22 to 50.1 years). Fourteen (45%) studies noted pre/post significant weight changes between arms over time; yet, 11 studies did not report such outcomes. Of the 10 studies showing a low risk of bias (68–77), five reported statistically significant decreases in weight ranging from 0.3 to 4.9 kg; results on inter-group pre/post weight changes were otherwise not reported (68,69,71,72,75) (see Tables S8A–D).
Garcinia and/or Hydroxycitrate
Garcinia is a plant native to India and Southeast Asia, whose rind contains hydroxycitric acid (HCA) which is purported to affect appetite by preventing fat storage blocking ATP-citralyase. ATP-citralyase is an enzyme in the step to cellular fatty acid synthesis and storage hence may inhibit lipogenesis (78). Fifteen RCTs were identified between 1998 and 2016. Only four of the 15 studies (27%) showed low bias scores for methodologic and reporting quality. Of these 15 studies, eight were conducted in Asia, three in the USA, two in Brazil and one each in several other countries. Funding sources were not revealed for 9 studies; the remaining were funded by a combination of local government and industry sponsorship. Duration of the studies lasted from 2 weeks to 16 weeks. The number of participants in each arm ranged from 10 to 69 subjects, with participant age ranging from 21 to 60 years. Three studies did not assess differences in weight between arms pre/post, and five studies (33%) reported significant decrease in weight. Of the trials noting low risk of bias, two studies reported significant weight loss (ranging from −1.3 kg to −3.6 kg) (79,80) whereas, the other two were non-significant (81,82) (see Tables S9A–D).
Green Tea
Green tea is a form of the Camellia sinensis plant that is made from steaming and pan-frying the leaves, without fermentation. Green tea contains caffeine, which is proposed to contribute to appetite suppression and stimulate thermogenesis (83,84). Antioxidants, including the catechin epigallocatechin gallate inhibit the breakdown of norepinephrine leading to increased calorie burning (67,85). Thirty-eight RCTs published between 2005 and 2017 were identified; of these, 16 (42%) were categorized as having an overall low degree of bias. Of these 38, the majority were conducted in Asia (50%). Funding sources were not revealed in 9 (24%) studies and the remaining were funded by both industry and governments. The duration of the studies was between 6 weeks and 12 months. The number of participants in each arm ranged from 5 to 123 subjects. Sex composition was variable (female (30-100%). Only 8 studies (21%) demonstrated significant different values between groups over time; 13 (34%) did not report statistical measures for weight change. Sixteen studies noted a low risk of bias (86–101), 5 (31%) included only women and 7 (44%) studies were < 3 months in duration. Only two of the 16 studies demonstrated statistically significant improvements in weight ranging from 1.5 to 1.7kg (96,99), both lasting less than 8 weeks (see Tables S10A–D).
Guar Gum
Guar gum is a dietary fiber derived from seeds of the Cyamopsis tetragonolobus plant, and is an ingredient in some food products, used as an emulsifier and thickener in baked goods. It is purported to promote weight loss by acting as a bulking agent in the gut, delaying gastric emptying and increasing satiety (102,103). Five RCTs were identified between 1980 and 2013 with none showing a low degree of bias for overall methodological quality. Of these five, two were conducted in Italy; investigators in Brazil, Saudi Arabia, and Finland contributed to one study each. Funding sources were not revealed for two studies; the remaining were funded by a combination of local government and industry sponsorship. The duration of the studies lasted between 6 weeks and 6 months. The number of participants in each arm ranged from 9 to 48. Sex composition was variable (female, 38 to 72%). Mean age of most participants was between 51.6 and 63.6 years. Three studies did not report pre/post changes between groups; one of the remaining studies demonstrated a significant difference in weight (see Tables S11A–D).
Conjugated Linoleic Acid (CLA)
Conjugated Linoleic Acid (CLA) is a naturally occurring omega 6 fatty acid derivative of linoleic acid that is typically found in dairy and meat products. Studies based on the animal model have shown positive benefits including diabetes management, improved immune system, reduction of atherosclerosis biomarkers, and body composition changes- reduced body fat mass and increased lean body mass (104). In humans, dietary CLA may improve insulin sensitivity and lipid metabolism specifically in reducing plasma triglyceride levels and low-density cholesterol (105). Thirty-one RCTs were identified between 2001 and 2016 with five (16%) showing low degree of bias. Of these 31, eight were conducted in USA, four in Canada, and 14 in Europe. Funding source was not revealed in 7 studies; the remaining were funded by a combination of government and industry. The study duration was between from 3 weeks and 24 months. Participants in each arm ranged from 7 to 173. Sex composition was variable (female 10-100%), with no women in 6 studies. Mean age of most of the participants was between 18 and 65 years. There were 8 studies that did not report between group data over time. Of the five high quality studies (e.g., low risk of bias) (106–110), only one demonstrated a statistically significant weight loss in their two intervention (vs. placebo) groups ranging from 1.5 to 3.0 kg (106) (see Tables S12A–D).
Mind-Body
Mind-body interventions that were evaluated for weight loss efficacy included behavioral therapies (e.g., mindfulness, stress management), hypnosis, meditation, and massage. In general, these strategies are designed to target maladaptive food behaviors such as cravings and hedonic eating (111). Of the 22 RCTs conducted between 1980 and 2017, only 2 (9%) were classified as having low risk of bias (112,113). Studies were primarily conducted in North America (n=12), Europe (n=8) and Asia (n=2). Funding was not reported in the majority of the studies (n=15, 68%); of the 6 reported, all were governmental/institutional. The number of participants ranged within each cohort between 5 and 100. Length of follow-up ranged from 4 weeks to 18 months. Percent women ranged from 10-100%, with 7 including only women. Only 6 (27%) studies reported significant findings for greater weight loss compared to control. Two studies with low risk of bias studies did not report pre/post intra-group differences in weight change (see Tables S13A–D).
Phaseolus
Phaseolus vulgaris is extracted from the white kidney bean and thought to inhibit the activity of alpha amylase thereby interfering with absorption of carbohydrates (114). Seven RCTs identified between 2007 and 2014. None of these studies fulfilled low risk of bias across each category. Of these seven, five were conducted in Europe (71%) and the remaining in Asia. Funding sources were not revealed for three studies (43%); the remaining were funded by industry and local universities. Study duration ranged from 30 days to 24 weeks and the number of participants in each arm ranged from 12 to 62 subjects. Mean age ranged from 22 to 66 years. The number of women in each arm ranged from 38-74%. Three studies reported significant weight loss compared to placebo, while three did not report such statistical changes (see Table S14A–D).
Phenylpropylamine
Phenylpropylamine (PPA) is similar in structure to amphetamine and ephedrine and may act as an appetite suppressant to induce weight loss, believing to act via the alpha-1 adrenergic receptor (115). There were five RCTs (published between 1982 and 1999). Only one trial was categorized as having a low degree of bias for all categories. All studies were published in the USA, three were industry funded (two not reported), and the number of participants ranged from 7 to 51. Study duration ranged from 6 to 12 weeks. The ages of participants ranged from 18 to 65 years. Number of women ranged from 56-100% with one study not reporting sex composition. Only one study did not report the differences at follow-up between both arms. Three studies demonstrated significant differences between arms, and the remaining did not report on significance. The one high quality (low risk of bias study) did not demonstrate significant changes in weight between groups at follow-up (116) (see Tables S15A–D).
Pyruvate
Pyruvate is a product of glycolysis, the first step in the breakdown of glucose to produce energy, and a key intermediate of several metabolic pathways in the cell. Pyruvate has been proposed to aid with lipid metabolism through reversible conversion to phosphoenolpyruvate and to increase glucose uptake by skeletal muscles, providing a potential for weight loss (117). There were seven RCTs published between 1992 and 2009, of which none were categorized as having an overall low degree of bias. All but one was published in the USA, one was funded by industry, and two did not report their funding. Study duration ranged from 21 days to 6 weeks, with number per arm ranging from 6 to 18 participants. The age of participants ranged from 22.5 to 48.7 years (two studies did not report age). The number of women ranged from 0–100%. Two studies did not report differences between arms while four studies were considered by the authors statistically significant (see Tables S16A–D).
Other Miscellaneous
Supporting Tables S17A–D represents 39 miscellaneous categories consisting of 66 RCTs. Due to the heterogeneity and number of compounds, no summaries are provided for these. Bias assessment, study and baseline characteristics, and outcome measures are presented in the supplementary table. Sixteen studies (24%) were categorized as high quality, of which six demonstrated significant weight loss ranging from 0.7 to 3 kg (see Tables S17A–D).
DISCUSSION
This review provides healthcare professionals with evidence-based data on the quality and efficacy of dietary supplements and alternative therapies purported to cause weight loss. Despite their high usage, our data suggest a dearth of high-quality RCTs evaluating weight loss outcomes. Published studies are of small sample sizes, short study follow-up, poor study design, and often lead to inconsistent conclusions and results reporting. Our findings underscore the need for well-designed and powered trials to minimize bias and provide definitive clinical efficacy.
This evaluation specifically focuses on the impact of supplements on weight and weight-related measures in adult patients with overweight and obesity. Society-based statements are dated (33,34) as were two high-quality reviews (25,118) published over 15 years ago. Yet, these authors limited their scope to double-blind randomized trials, did not use PRISMA, and limited their analysis to specific compounds. A separate review of reviews excluded combination supplements (119); however, it only included nine reviews of individual supplements and that too was over nine years old. While a number of reviews are available on individual compounds, few in fact evaluate a spectrum of supplements as ours.
Trials were published from different geographical regions of the world, with some dietary supplements or alternative therapies favoring certain regions over others. Despite this variation, it would be important to embrace cultural treatments using vigorous scientific inquiry. This includes increasing the sample size and study duration, but also evaluating both homogeneous and heterogeneous ethnic and age-appropriate cohorts. Our evaluation suggests the importance of conducting appropriate statistical analyses for their design and oversight. Other studies assessed changes within but not across each arm. Others suggested statistically significant changes when there were none, or, in our assessment, incorrect evaluation methods were applied. In those that did use appropriate methods or were of high quality, few were statistically significant, with findings not clinically significant. The methodological inconsistencies observed provide considerable opportunities for future research endeavors.
High-quality evidence is needed prior to embracing products within clinical practice. By following PRISMA, we applied the Cochrane Bias Review to our articles and observed that the majority of articles were classified as having low quality. Much is due to the challenges in study design. Many of the included articles had small sample sizes, failed to control for key confounding variables, and had different doses and formulations of each compound making the interpretation rather difficult. While key weight-loss studies such as the Diabetes Prevention Program (120) or the Look-AHEAD (121) lasted a year or more, very few of the included trials in this review lasted a year or longer. Longer-term trials are needed to ascertain their impact on weight-loss. Our evaluation highlights the importance of the efforts put forth by the Office of Dietary Supplements to advance the science of nutritional supplementation and a need for high-quality research.
This review provides contemporaneous evaluation that can provide a scientific basis for practicing clinicians. Each supplement is listed in the dietary supplement label database (https://dsld.od.nih.gov/dsld/). The use of research librarians enhanced the validity of our process and approach.(122) Using the major medical databases rather than complementary databases, our team felt that this approach consisted of higher integrity journal articles (123), despite the publication and location bias that may exist in other databases (124–126). A substantial amount of the integrated care literature in PubMed is not discoverable in its MeSH-indexed subset, signifying that a search relying on MeSH terms only will miss non-indexed but highly relevant content. We also recommend the addition of Embase to a PubMed search if the emerging topics often to be found in conference abstracts are of interest.
There are a number of limitations. Studies were heterogeneous and participants may not necessarily be representative of the typical patient presenting for weight loss. While our initial intentions were to include observational studies, the volume of studies, their design and outcomes, and the importance of limiting to RCTs, made this impossible. Further, the extensive heterogeneity of patient populations, interventions, outcomes and lack of statistical comparisons prevent us from conducting a formal meta-analysis. Many of the compounds consisted of different doses, formulations or were combined with other supplements. We acknowledge these limitations and attempted to group as best as we could. The majority of full-text studies had intra-group comparisons, rather than inter-group comparisons over time. While the Cochrane tool attempts to standardize quality assessments, this may be prone to subjectivity and/or interpretation of the articles. We attempted to mitigate this through trained evaluators and adjudicators.
Implications/Next Steps
These findings are critically important to understanding the science behind dietary supplements and alternative therapies purported for weight-loss. The importance of an academic partnership with industry to conduct well-designed, randomized, double-blinded controlled trials of sufficient duration to demonstrate efficacy of each category would be helpful. Studies should be free of potential commercial bias; our findings suggest this may not currently be the case. Longer duration is needed to understand the beneficial outcomes of weight-loss dietary supplements to provide higher quality recommendations. The process outlined by the Office of Dietary Supplements in promoting research endeavors in this field reduces bias, enhances product integrity through quality control measures, can add to the scientific base. All studies should be registered in clinicaltrials.gov. It is unknown whether adding compounds to existing lifestyle interventions could enhance efficacy, and it would be helpful to explore those strategies.
CONCLUSIONS
Despite a number of citations evaluating dietary supplements and alternative therapies for weight-loss, this review does not support strong, high-quality evidence of efficacy for any of products. There is considerable heterogeneity in trial design, bias, efficacy, and duration, suggesting a need to develop trials accounting for methodological flaws.
Supplementary Material
What is already known about this subject?
Dietary supplements and alternative therapies for weight loss are widely available and used for the treatment of obesity
Deregulation of the dietary supplement industry permits marketing of such products without stringent regulatory approval
Use of dietary supplements and alternative therapies among adults with obesity is high despite their limited evidence base
What are the new findings in your manuscript?
There is weak evidence for the efficacy of dietary supplements and alternative therapies
The number of randomized controlled trials varies depending on the therapy examined
Design of existing trials is hampered by a significant risk of bias because of methodological inconsistencies
How might your results change the direction of research or focus of clinical practice?
A critical need to strengthen the industry-academic relationship to design high quality trials in this sphere
Emphasis on clearer outcomes, enhanced methodological rigor and study duration are needed
The interaction between existing lifestyle interventions and existing supplements should be evaluated in future trials
ACKNOWLEDGEMENTS
The authors would like to specifically thank Nayan Agarwal, Allegra Bermudez, Tyler Gooding, Nichole Jannah, Ridhima Kapoor, Melanie Peterson, and Tiffany Driesse for their assistance. We additionally would like to thank other members of The Obesity Society’s Clinical and Executive Committees for their input.
FINANCIAL DISCLOSURE
Dr. Batsis’ research reported in this publication was supported in part by the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Apolzan has received funding from a food company to investigate topics from this manuscript. Dr. Golden reports consulting with Novo Nordisk and Unjury. Dr. Heymsfield reports personal fees from Medifast. Dr. Kidambi is Medical Director for TOPS Center for Metabolic Health at the Medical College of Wisconsin, which is supported by TOPS Inc. Dr. Rubino reports consulting and speaking for Novo Nordisk, and Astra Zeneca. Dr. Saunders has a relationship with Intellihealth Inc. There was no funding from The Obesity Society for the development of the work from this manuscript.
ABBREVIATIONS
- DSHEA
Dietary Supplement Health and Education Act
- FDA
Food and Drug Administration
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized Controlled Trial
Footnotes
There are no conflicts of interest pertaining to this manuscript
ETHICS APPROVAL: n/a
REFERENCES
- 1.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007-2008 to 2015-2016. Jama 2018;319: 1723–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. Jama 2005;293: 1868–1874. [DOI] [PubMed] [Google Scholar]
- 3.Walter S, Kunst A, Mackenbach J, Hofman A, Tiemeier H. Mortality and disability: the effect of overweight and obesity. International journal of obesity (2005) 2009;33: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 4.Alley DE, Chang VW. The changing relationship of obesity and disability, 1988-2004. Jama 2007;298: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 5.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS Guideline for the Management of Overweight and Obesity in Adults. Circulation 2014;129: S102–S138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saunders KH, Umashanker D, Igel LI, Kumar RB, Aronne LJ. Obesity Pharmacotherapy. The Medical clinics of North America 2018;102: 135–148. [DOI] [PubMed] [Google Scholar]
- 7.Barenbaum SR, Saunders KH, Igel LI, Shukla AP, Aronne LJ. Obesity: When to consider surgery. The Journal of family practice 2018;67: 614; 616,; 618,; 620. [PubMed] [Google Scholar]
- 8.Li Z, Maglione M, Tu W, Mojica W, Arterburn D, Shugarman LR, et al. Meta-analysis: pharmacologic treatment of obesity. Annals of internal medicine 2005;142: 532–546. [DOI] [PubMed] [Google Scholar]
- 9.Wolfe BM, Kvach E, Eckel RH. Treatment of Obesity: Weight Loss and Bariatric Surgery. Circulation research 2016;118: 1844–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCrabb S, Lane C, Hall A, Milat A, Bauman A, Sutherland R, et al. Scaling-up evidence-based obesity interventions: A systematic review assessing intervention adaptations and effectiveness and quantifying the scale-up penalty. Obes Rev 2019. [DOI] [PubMed] [Google Scholar]
- 11.LeBlanc ES, Patnode CD, Webber EM, Redmond N, Rushkin M, O’Connor EA. Behavioral and Pharmacotherapy Weight Loss Interventions to Prevent Obesity-Related Morbidity and Mortality in Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018;320: 1172–1191. [DOI] [PubMed] [Google Scholar]
- 12.Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. Jama 2014;312: 1779–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemmens VE, Oenema A, Klepp KI, Henriksen HB, Brug J. A systematic review of the evidence regarding efficacy of obesity prevention interventions among adults. Obes Rev 2008;9: 446–455. [DOI] [PubMed] [Google Scholar]
- 14.Loveman E, Frampton GK, Shepherd J, Picot J, Cooper K, Bryant J, et al. The clinical effectiveness and cost-effectiveness of long-term weight management schemes for adults: a systematic review. Health Technol Assess 2011;15: 1–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varkevisser RDM, van Stralen MM, Kroeze W, Ket JCF, Steenhuis IHM. Determinants of weight loss maintenance: a systematic review. Obes Rev 2019;20: 171–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robertson C, Avenell A, Boachie C, Stewart F, Archibald D, Douglas F, et al. Should weight loss and maintenance programmes be designed differently for men? A systematic review of long-term randomised controlled trials presenting data for men and women: The ROMEO project. Obesity research & clinical practice 2016;10: 70–84. [DOI] [PubMed] [Google Scholar]
- 17.MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, et al. NIH working group report: Innovative research to improve maintenance of weight loss. Obesity (Silver Spring, Md) 2015;23: 7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas JG, Bond DS, Phelan S, Hill JO, Wing RR. Weight-loss maintenance for 10 years in the National Weight Control Registry. Am J Prev Med 2014;46: 17–23. [DOI] [PubMed] [Google Scholar]
- 19.Dombrowski SU, Knittle K, Avenell A, Araujo-Soares V, Sniehotta FF. Long term maintenance of weight loss with non-surgical interventions in obese adults: systematic review and meta-analyses of randomised controlled trials. Bmj 2014;348: g2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pillitteri JL, Shiffman S, Rohay JM, Harkins AM, Burton SL, Wadden TA. Use of dietary supplements for weight loss in the United States: results of a national survey. Obesity (Silver Spring) 2008;16: 790–796. [DOI] [PubMed] [Google Scholar]
- 21.Blanck HM, Khan LK, Serdula MK. Use of nonprescription weight loss products: results from a multistate survey. JAMA 2001;286: 930–935. [DOI] [PubMed] [Google Scholar]
- 22.Tucker J, Fischer T, Upjohn L, Mazzera D, Kumar M. Unapproved Pharmaceutical Ingredients Included in Dietary Supplements Associated With US Food and Drug Administration Warnings. JAMA network open 2018;1: e183337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WY, Linn CT, Fu CS, Sukoco BM. The role of endorsers, framing, and rewards on the effectiveness of dietary supplement advertisements. J Health Commun 2012;17: 54–75. [DOI] [PubMed] [Google Scholar]
- 24.Hoffman SJ, Tan C. Biological, psychological and social processes that explain celebrities' influence on patients' health-related behaviors. Arch Public Health 2015;73: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pittler MH, Ernst E. Dietary supplements for body-weight reduction: a systematic review. Am J Clin Nutr 2004;79: 529–536. [DOI] [PubMed] [Google Scholar]
- 26.Pittler MH, Schmidt K, Ernst E. Adverse events of herbal food supplements for body weight reduction: systematic review. Obes Rev 2005;6: 93–111. [DOI] [PubMed] [Google Scholar]
- 27.Blendon RJ, DesRoches CM, Benson JM, Brodie M, Altman DE. Americans’ views on the use and regulation of dietary supplements. Archives of internal medicine 2001;161: 805–810. [DOI] [PubMed] [Google Scholar]
- 28.Marinac JS, Buchinger CL, Godfrey LA, Wooten JM, Sun C, Willsie SK. Herbal products and dietary supplements: a survey of use, attitudes, and knowledge among older adults. J Am Osteopath Assoc 2007;107: 13–20; quiz 21–13. [PubMed] [Google Scholar]
- 29.He Z, Barrett LA, Rizvi R, Payrovnaziri SN, Zhang R. Exploring the Discrepancies in Actual and Perceived Benefits of Dietary Supplements Among Obese Patients. Stud Health Technol Inform 2019;264: 1474–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Durante KM, Whitmore B, Jones CA, Campbell NR. Use of vitamins, minerals and herbs: a survey of patients attending family practice clinics. Clin Invest Med 2001;24: 242–249. [PubMed] [Google Scholar]
- 31.Cohen PA, Maller G, DeSouza R, Neal-Kababick J. Presence of banned drugs in dietary supplements following FDA recalls. Jama 2014;312: 1691–1693. [DOI] [PubMed] [Google Scholar]
- 32.Mission, Origin, and Mandate: National Institutes of Health: Office of Dietary Supplements Bethesda, MD: National Institutes of Health; 2019. [cited 2019 November 24]. [Google Scholar]
- 33.Mechanick JI, Brett EM, Chausmer AB, Dickey RA, Wallach S. American Association of Clinical Endocrinologists medical guidelines for the clinical use of dietary supplements and nutraceuticals. Endocr Pract 2003;9: 417–470. [DOI] [PubMed] [Google Scholar]
- 34.Vogel JH, Bolling SF, Costello RB, Guarneri EM, Krucoff MW, Longhurst JC, et al. Integrating complementary medicine into cardiovascular medicine. A report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents (Writing Committee to Develop an Expert Consensus Document on Complementary and Integrative Medicine). Journal of the American College of Cardiology 2005;46: 184–221. [DOI] [PubMed] [Google Scholar]
- 35.Nichter M, Thompson JJ. For my wellness, not just my illness: North Americans’ use of dietary supplements. Cult Med Psychiatry 2006;30: 175–222. [DOI] [PubMed] [Google Scholar]
- 36.Wu W-Y, Linn CT, Fu C-S, Sukoco BM. The Role of Endorsers, Framing, and Rewards on the Effectiveness of Dietary Supplement Advertisements. Journal of Health Communication 2012;17: 54–75. [DOI] [PubMed] [Google Scholar]
- 37.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Bmj 2009;339: b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hunt K, Ernst E. The evidence-base for complementary medicine in children: a critical overview of systematic reviews. Arch Dis Child 2011;96: 769–776. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj 2011;343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steyer TE, Ables A. Complementary and Alternative Therapies for Weight Loss. Primary Care: Clinics in Office Practice 2009;36: 395–406. [DOI] [PubMed] [Google Scholar]
- 41.Kim SY, Shin IS, Park YJ. Effect of acupuncture and intervention types on weight loss: a systematic review and meta-analysis. Obesity Reviews 2018;19: 1585–1596. [DOI] [PubMed] [Google Scholar]
- 42.Belivani M, Dimitroula C, Katsiki N, Apostolopoulou M, Cummings M, Hatzitolios AI. Acupuncture in the treatment of obesity: a narrative review of the literature. Acupuncture in medicine : journal of the British Medical Acupuncture Society 2013;31: 88–97. [DOI] [PubMed] [Google Scholar]
- 43.Ching HY, Wu SL, Chen WC, Hsieh CL. Effects of auricular acupressure on body weight parameters in patients with chronic schizophrenia. Evidence-based complementary and alternative medicine : eCAM 2012;2012: 151737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fogarty SS L; Harris D; Zaslawski C; Mathai ML; McAinch AJ A randomised cross-over pilot study investigating the use of acupuncture to promote weight loss and mental health in overweight and obese individuals participating in a weight loss program. Eating and weight disorders : EWD 2015;20: 379–387. [DOI] [PubMed] [Google Scholar]
- 45.Calcium and Vitamin D: Important at Every Age: NIH Osteoporosis and Related Bone Disease National Resource Center: National Institute of Health; 2018. [cited 2020 November 25]. Available from: https://www.bones.nih.gov/health-info/bone/bone-health/nutrition/calcium-and-vitamin-d-important-every-age. .
- 46.Zhu W, Cai D, Wang Y, Lin N, Hu Q, Qi Y, et al. Calcium plus vitamin D3 supplementation facilitated fat loss in overweight and obese college students with very-low calcium consumption: a randomized controlled trial. Nutrition journal 2013;12: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mason C, Xiao L, Imayama I, Duggan C, Wang CY, Korde L, et al. Vitamin D3 supplementation during weight loss: a double-blind randomized controlled trial. The American journal of clinical nutrition 2014;99: 1015–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenblum JL, Castro VM, Moore CE, Kaplan LM. Calcium and vitamin D supplementation is associated with decreased abdominal visceral adipose tissue in overweight and obese adults. The American journal of clinical nutrition 2012;95: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salehpour A, Hosseinpanah F, Shidfar F, Vafa M, Razaghi M, Dehghani S, et al. A 12-week double-blind randomized clinical trial of vitamin D(3) supplementation on body fat mass in healthy overweight and obese women. Nutrition journal 2012;11: 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shapses SA, Heshka S, Heymsfield SB. Effect of calcium supplementation on weight and fat loss in women. The Journal of clinical endocrinology and metabolism 2004;89: 632–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yanovski JA, Parikh SJ, Yanoff LB, Denkinger BI, Calis KA, Reynolds JC, et al. Effects of calcium supplementation on body weight and adiposity in overweight and obese adults: a randomized trial. Annals of internal medicine 2009;150: 821–829, w145–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gades MD, Stern JS. Chitosan supplementation does not affect fat absorption in healthy males fed a high-fat diet, a pilot study. International Journal of Obesity 2002;26: 119–122. [DOI] [PubMed] [Google Scholar]
- 53.Patrulea V, Ostafe V, Borchard G, Jordan O. Chitosan as a starting material for wound healing applications. European Journal of Pharmaceutics and Biopharmaceutics 2015;97: 417–426. [DOI] [PubMed] [Google Scholar]
- 54.Woodgate DE, Conquer JA. Effects of a stimulant-free dietary supplement on body weight and fat loss in obese adults: a six-week exploratory study. Current therapeutic research, clinical and experimental 2003;64: 248–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal 2011;15: 2779–2811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Barisic V, Kopjar M, Jozinovic A, Flanjak I, Ackar D, Milicevic B, et al. The Chemistry behind Chocolate Production. Molecules 2019;24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jang MH, Kang NH, Mukherjee S, Yun JW. Theobromine, a Methylxanthine in Cocoa Bean, Stimulates Thermogenesis by Inducing White Fat Browning and Activating Brown Adipocytes. Biotechnology and Bioprocess Engineering 2018;23: 617–626. [Google Scholar]
- 58.Njike VY, Faridi Z, Shuval K, Dutta S, Kay CD, West SG, et al. Effects of sugar-sweetened and sugar-free cocoa on endothelial function in overweight adults. International Journal of Cardiology 2011;149: 83–88. [DOI] [PubMed] [Google Scholar]
- 59.Davis CM, Vincent JB. Chromium oligopeptide activates insulin receptor tyrosine kinase activity. Biochemistry 1997;36: 4382–4385. [DOI] [PubMed] [Google Scholar]
- 60.Attenburrow MJ, Odontiadis J, Murray BJ, Cowen PJ, Franklin M. Chromium treatment decreases the sensitivity of 5-HT2A receptors. Psychopharmacology (Berl) 2002;159: 432–436. [DOI] [PubMed] [Google Scholar]
- 61.Brownley KA, Boettiger CA, Young L, Cefalu WT. Dietary chromium supplementation for targeted treatment of diabetes patients with comorbid depression and binge eating. Med Hypotheses 2015;85: 45–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hua Y, Clark S, Ren J, Sreejayan N. Molecular mechanisms of chromium in alleviating insulin resistance. The Journal of nutritional biochemistry 2012;23: 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vincent JB, Lukaski HC. Chromium. Adv Nutr 2018;9: 505–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen TJH, Blum K, Kaats G, Braverman ER, Eisenberg A, Sherman M, et al. Chromium Picolinate (CrP) a putative anti-obesity nutrient induces changes in body composition as a function of the Taq1 dopamine D2 receptor polymorphisms in a randomized double-blind placebo controlled study. Gene Ther Mol Biol 2007;11B: 161–170. [Google Scholar]
- 65.Diaz MLW BA; Li Y; Anderson RA; Campbell WW Chromium picolinate and conjugated linoleic acid do not synergistically influence diet- and exercise-induced changes in body composition and health indexes in overweight women. The Journal of nutritional biochemistry 2008;19: 61–68. [DOI] [PubMed] [Google Scholar]
- 66.Yazaki Y, Faridi Z, Ma Y, Ali A, Northrup V, Njike VY, et al. A pilot study of chromium picolinate for weight loss. Journal of alternative and complementary medicine (New York, NY) 2010;16: 291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diepvens K, Westerterp KR, Westerterp-Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 2007;292: R77–R85. [DOI] [PubMed] [Google Scholar]
- 68.Boozer CN, Daly PA, Homel P, Solomon JL, Blanchard D, Nasser JA, et al. Herbal ephedra/caffeine for weight loss: a 6-month randomized safety and efficacy trial. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2002;26: 593–604. [DOI] [PubMed] [Google Scholar]
- 69.Boozer CN, Nasser JA, Heymsfield SB, Wang V, Chen G, Solomon JL. An herbal supplement containing Ma Huang-Guarana for weight loss: a randomized, double-blind trial. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2001;25: 316–324. [DOI] [PubMed] [Google Scholar]
- 70.Buemann B, Marckmann P, Christensen NJ, Astrup A. The effect of ephedrine plus caffeine on plasma lipids and lipoproteins during a 4.2 MJ/day diet. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 1994;18: 329–332. [PubMed] [Google Scholar]
- 71.Chong PW, Beah ZM, Grube B, Riede L. IQP-GC-101 reduces body weight and body fat mass: a randomized, double-blind, placebo-controlled study. Phytotherapy research : PTR 2014;28: 1520–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greenway FdJ-L L; Martin C; Roberts A; Grundy I; Parker C Dietary herbal supplements with phenylephrine for weight loss. Journal of medicinal food 2006;9: 572–578. [DOI] [PubMed] [Google Scholar]
- 73.Greenway FLDJ L; Blanchard D; Frisard M; Smith SR Effect of a dietary herbal supplement containing caffeine and ephedra on weight, metabolic rate, and body composition. Obes Res 2004;12: 1152–1157. [DOI] [PubMed] [Google Scholar]
- 74.Griboff SIB R A double blind clinical evaluation of a phenylpropanolamine-caffeine-vitamine combination and a placebo in the treatment of exogenous obesity. Current therapeutic research, clinical and experimental 1975;17: 535–543. [PubMed] [Google Scholar]
- 75.Hackman RMH PJ; Schwartz HJ; Rutledge JC; Watnik MR; Noceti EM; Stohs SJ; Stern JS; Keen CL Multinutrient supplement containing ephedra and caffeine causes weight loss and improves metabolic risk factors in obese women: a randomized controlled trial. International journal of obesity (2005) 2006;30: 1545–1556. [DOI] [PubMed] [Google Scholar]
- 76.Kim HJP JM; Kim JA; Ko BP Effect of herbal Ephedra sinica and Evodia rutaecarpa on body composition and resting metabolic rate: a randomized, double-blind clinical trial in Korean premenopausal women. Journal of acupuncture and meridian studies 2008;1: 128–138. [DOI] [PubMed] [Google Scholar]
- 77.Roure R, Oddos T, Rossi A, Vial F, Bertin C. Evaluation of the efficacy of a topical cosmetic slimming product combining tetrahydroxypropyl ethylenediamine, caffeine, carnitine, forskolin and retinol, In vitro, ex vivo and in vivo studies. International journal of cosmetic science 2011;33: 519–526. [DOI] [PubMed] [Google Scholar]
- 78.Vasques CA, Schneider R, Klein-Júnior LC, Falavigna A, Piazza I, Rossetto S. Hypolipemic effect of Garcinia cambogia in obese women. Phytotherapy research : PTR 2014;28: 887–891. [DOI] [PubMed] [Google Scholar]
- 79.Toromanyan E, Aslanyan G, Amroyan E, Gabrielyan E, Panossian A. Efficacy of Slim339 in reducing body weight of overweight and obese human subjects. Phytotherapy research : PTR 2007;21: 1177–1181. [DOI] [PubMed] [Google Scholar]
- 80.Mattes RD, Bormann L. Effects of (-)-hydroxycitric acid on appetitive variables. Physiology & behavior 2000;71: 87–94. [DOI] [PubMed] [Google Scholar]
- 81.Hayamizu KI Y; Kaneko I; Shen M; Okuhara Y; Shigematsu N; Tomi H; Furuse M; Yoshino G; Shimasaki H Effects of Garcinia cambogia (Hydroxycitric Acid) on Visceral Fat Accumulation: A Double-Blind, Randomized, Placebo-Controlled Trial. Current Therapeutic Research - Clinical and Experimental 2003;64: 551–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heymsfield SBA DB; Vasselli JR; Pietrobelli A; Greenfield D; Nunez C; Heymsfield SB; Allison DB; Vasselli JR; Pietrobelli A; Greenfield D; Nunez C Garcinia cambogia (hydroxycitric acid) as a potential antiobesity agent: a randomized controlled trial. JAMA: Journal of the American Medical Association 1998;280: 1596–1600. [DOI] [PubMed] [Google Scholar]
- 83.Schubert MM, Irwin C, Seay RF, Clarke HE, Allegro D, Desbrow B. Caffeine, coffee, and appetite control: a review. International journal of food sciences and nutrition 2017;68: 901–912. [DOI] [PubMed] [Google Scholar]
- 84.Harpaz E, Tamir S, Weinstein A, Weinstein Y. The effect of caffeine on energy balance. J Basic Clin Physiol Pharmacol 2017;28: 1–10. [DOI] [PubMed] [Google Scholar]
- 85.Carrasco-Pozo C, Cires MJ, Gotteland M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. Journal of medicinal food 2019;22: 753–770. [DOI] [PubMed] [Google Scholar]
- 86.Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutrition research (New York, NY) 2012;32: 421–427. [DOI] [PubMed] [Google Scholar]
- 87.Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin-3-gallate on insulin resistance and associated metabolic risk factors: randomized controlled trial. The British journal of nutrition 2009;101: 886–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome--a randomized placebo-controlled trial. Journal of the Society for Gynecologic Investigation 2006;13: 63–68. [DOI] [PubMed] [Google Scholar]
- 89.Chen IJ, Liu CY, Chiu JP, Hsu CH. Therapeutic effect of high-dose green tea extract on weight reduction: A randomized, double-blind, placebo-controlled clinical trial. Clinical nutrition (Edinburgh, Scotland) 2016;35: 592–599. [DOI] [PubMed] [Google Scholar]
- 90.Dostal AMA A; Espejo L; Kurzer MS Long-Term Supplementation of Green Tea Extract Does Not Modify Adiposity or Bone Mineral Density in a Randomized Trial of Overweight and Obese Postmenopausal Women. The Journal of nutrition 2016;146: 256–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dostal AMS H; Espejo L; Arikawa AY; Stendell-Hollis NR; Kurzer MS Green Tea Extract and Catechol-O-Methyltransferase Genotype Modify Fasting Serum Insulin and Plasma Adiponectin Concentrations in a Randomized Controlled Trial of Overweight and Obese Postmenopausal Women. The Journal of nutrition 2016;146: 38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hsu CHL YL; Lin SC; Tsai TH; Huang CJ; Chou P Does supplementation with green tea extract improve insulin resistance in obese type 2 diabetics? A randomized, double-blind, and placebo-controlled clinical trial. Alternative medicine review : a journal of clinical therapeutic 2011;16: 157–163. [PubMed] [Google Scholar]
- 93.Hsu CHT TH; Kao YH; Hwang KC; Tseng TY; Chou P Effect of green tea extract on obese women: a randomized, double-blind, placebo-controlled clinical trial. Clinical nutrition (Edinburgh, Scotland) 2008;27: 363–370. [DOI] [PubMed] [Google Scholar]
- 94.Kim HJK J; Storni C; Song HJ; Cho YG Effect of green mate in overweight volunteers: A randomized placebo-controlled human study. J Funct Food 2012;4: 287–293. [Google Scholar]
- 95.Kobayashi MK T; Ukawa Y; Sagesaka YM; Fukuhara I Green tea beverages enriched with catechins with a galloyl moiety reduce body fat in moderately obese adults: a randomized double-blind placebo-controlled trial. Food & function 2016;7: 498–507. [DOI] [PubMed] [Google Scholar]
- 96.Ling P, Li DL, Lei MR, Zhang LQ, Zhou LX. Preparation-containing node of Lotus Rhizome, green tea and Panax notoginseng for obese adults. Chinese Journal of Clinical Rehabilitation 2005;9: 231–233. [Google Scholar]
- 97.Mangine GT, Gonzalez AM, Wells AJ, McCormack WP, Fragala MS, Stout JR, et al. The effect of a dietary supplement (N-oleyl-phosphatidyl-ethanolamine and epigallocatechin gallate) on dietary compliance and body fat loss in adults who are overweight: a double-blind, randomized control trial. Lipids in health and disease 2012;11: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Miyazaki R, Kotani K, Ayabe M, Tsuzaki K, Shimada J, Sakane N, et al. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: A randomized controlled trial. Geriatrics and Gerontology International 2013;13: 622–629. [DOI] [PubMed] [Google Scholar]
- 99.Montelius C, Erlandsson D, Vitija E, Stenblom EL, Egecioglu E, Erlanson-Albertsson C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite 2014;81: 295–304. [DOI] [PubMed] [Google Scholar]
- 100.Pilaczynska-Szczesniak L, Rzymski P, Pischel I. Influence of combined botanical extract preparation on body composition - Results from double blind randomized clinical trial. Archives of Medical Science 2006;2: 171–178. [Google Scholar]
- 101.Rondanelli M, Opizzi A, Solerte SB, Trotti R, Klersy C, Cazzola R. Administration of a dietary supplement ( N-oleyl-phosphatidylethanolamine and epigallocatechin-3-gallate formula) enhances compliance with diet in healthy overweight subjects: a randomized controlled trial. The British journal of nutrition 2009;101: 457–464. [DOI] [PubMed] [Google Scholar]
- 102.Pittler MH, Ernst E. Guar gum for body weight reduction: meta-analysis of randomized trials. Am J Med 2001;110: 724–730. [DOI] [PubMed] [Google Scholar]
- 103.Ríos-Hoyo A, Gutiérrez-Salmeán G. New Dietary Supplements for Obesity: What We Currently Know. Current Obesity Reports 2016;5: 262–270. [DOI] [PubMed] [Google Scholar]
- 104.Gaullier JMH J; Hoivik HO; Hoye K; Syvertsen C; Nurminiemi M; Hassfeld C; Einerhand A; O’Shea M; Gudmundsen O Six months supplementation with conjugated linoleic acid induces regional-specific fat mass decreases in overweight and obese. The British journal of nutrition 2007;97: 550–560. [DOI] [PubMed] [Google Scholar]
- 105.Lee KN, Kritchevsky D, Pariza MW. Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 1994;108: 19–25. [DOI] [PubMed] [Google Scholar]
- 106.Falcone PHT CY; Carson LR; Joy JM; Mosman MM; Vogel RM; McCann TR; Crona KP; Griffin JD; Kim MP; Moon JR Subcutaneous and segmental fat loss with and without supportive supplements in conjunction with a low-calorie high protein diet in healthy women. PloS one 2015;10: e0123854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madry E, Chudzicka-Strugala I, Grabanska-Martynska K, Malikowska K, Grebowiec P, Lisowska A, et al. Twelve weeks CLA supplementation decreases the hip circumference in overweight and obese women. A double-blind, randomized, placebo-controlled trial. Acta scientiarum polonorum Technologia alimentaria 2016;15: 107–113. [DOI] [PubMed] [Google Scholar]
- 108.Rao AV, Andrews K, Logan A. A double-blind, randomized-controlled trial of a nutritional supplement (abs+) containing conjugated linoleic acid (CLA) and epigallocatechin-gallate (EGCG) in human weight loss. Journal of Herbs, Spices and Medicinal Plants 2006;12: 67–76. [Google Scholar]
- 109.Riserus U, Arner P, Brismar K, Vessby B. Treatment with dietary trans10cis12 conjugated linoleic acid causes isomer-specific insulin resistance in obese men with the metabolic syndrome. Diabetes care 2002;25: 1516–1521. [DOI] [PubMed] [Google Scholar]
- 110.Riserus U, Berglund L, Vessby B. Conjugated linoleic acid (CLA) reduced abdominal adipose tissue in obese middle-aged men with signs of the metabolic syndrome: a randomised controlled trial. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity 2001;25: 1129–1135. [DOI] [PubMed] [Google Scholar]
- 111.Butryn ML, Webb V, Wadden TA. Behavioral treatment of obesity. Psychiatr Clin North Am 2011;34: 841–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ito HY O; Kira Y; Tanaka T; Matsuoka R The effects of auricular acupuncture on weight reduction and feeding-related cytokines: a pilot study. BMJ open gastroenterology 2015;2: e000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tapper K, Shaw C, Ilsley J, Hill AJ, Bond FW, Moore L. Exploratory randomised controlled trial of a mindfulness-based weight loss intervention for women. Appetite 2009;52: 396–404. [DOI] [PubMed] [Google Scholar]
- 114.Obiro WC, Zhang T, Jiang B. The nutraceutical role of the Phaseolus vulgaris alpha-amylase inhibitor. The British journal of nutrition 2008;100: 1–12. [DOI] [PubMed] [Google Scholar]
- 115.Greenway F, Herber D, Raum W, Herber D, Morales S. Double-blind, randomized, placebo-controlled clinical trials with non-prescription medications for the treatment of obesity. Obes Res 1999;7: 370–378. [DOI] [PubMed] [Google Scholar]
- 116.Greenway F, Herber D, Raum W, Herber D, Morales S. Double-blind, randomized, placebo-controlled clinical trials with non-prescription medications for the treatment of obesity. Obesity research, 1999, pp 370–378. [DOI] [PubMed] [Google Scholar]
- 117.Miller ATJ, Thomas BM. Pyruvate Metabolism in Obesity. The American journal of clinical nutrition 1956;4: 619–624. [DOI] [PubMed] [Google Scholar]
- 118.Pittler MH, Ernst E. Complementary therapies for reducing body weight: a systematic review. Int J Obes (Lond) 2005;29: 1030–1038. [DOI] [PubMed] [Google Scholar]
- 119.Onakpoya IJ, Wider B, Pittler MH, Ernst E. Food supplements for body weight reduction: a systematic review of systematic reviews. Obesity (Silver Spring, Md) 2011;19: 239–244. [DOI] [PubMed] [Google Scholar]
- 120.Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes care 2002;25: 2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Look ARG. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring, Md) 2014;22: 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Meert D, Torabi N, Costella J. Impact of librarians on reporting of the literature searching component of pediatric systematic reviews. Journal of the Medical Library Association : JMLA 2016;104: 267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tober M PubMed, ScienceDirect, Scopus or Google Scholar – Which is the best search engine for an effective literature research in laser medicine? Medical Laser Application 2011;26: 139–144. [Google Scholar]
- 124.Dickersin K The existence of publication bias and risk factors for its occurrence. JAMA 1990;263: 1385–1389. [PubMed] [Google Scholar]
- 125.Pittler MH, Abbot NC, Harkness EF, Ernst E. Location bias in controlled clinical trials of complementary/alternative therapies. J Clin Epidemiol 2000;53: 485–489. [DOI] [PubMed] [Google Scholar]
- 126.Sood A, Knudsen K, Sood R, Wahner-Roedler DL, Barnes SA, Bardia A, et al. Publication bias for CAM trials in the highest impact factor medicine journals is partly due to geographical bias. Journal of clinical epidemiology 2007;60: 1123–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


