Abstract
Objective:
To study the prospective association between N-terminal pro-Brain natriuretic peptide (NT-proBNP), and changes in weight and obesity risk in a community-based population.
Methods:
We analyzed data from 9,681 participants from the Atherosclerosis Risk in Communities Study, at two time points 6-years apart. Among people without obesity at baseline, we used multivariable logistic regression models to examine the association between baseline levels of NT-proBNP and incident obesity. We used multivariable linear regression model to examine the association between changes in NT-proBNP (visit 2 serum and visit 4 plasma samples) and changes in weight.
Results:
The prevalence of obesity increased from 28% to 35% in the 6-year follow up period. Compared with individuals in the highest NT-proBNP quartile, those in the lowest were more likely to have obesity at baseline (OR 1.25; 95%CI 1.08-1.45) and, among people who were not obese at baseline, more likely to develop obesity at follow-up (OR 1.35; 95%CI 1.07-1.69). Changes in NT-proBNP were inversely associated with weight change.
Conclusions:
In this prospective study, lower levels of NT-proBNP were associated with higher risk of obesity, and changes in NT-proBNP were inversely associated with changes in weight. This suggests that NPs or their pathways may be potential targets in the treatment of obesity.
Keywords: Obesity, Weight, NT-proBNP, Metabolic diseases
Introduction
Natriuretic peptides (NPs) are biochemical markers of cardiac function, which correlate with the severity of heart failure (HF) and also strongly predict future HF development (1). The main stimulus for NP release is distension of cardiac myocytes, which can occur in situations of increased ventricular wall stress caused by processes such as hypertension and volume overload (2). These hormones have well established protective cardiovascular effects. Neprilysin inhibitors (Entresto) are widely used in the treatment of congestive HF, promoting vasodilation, natriuresis, and decreased renin and aldosterone secretion (3). The action of NPs, however, is not restricted to the heart-kidney axis. They also may play a role in metabolic pathways and the development of insulin resistance. In adipose tissue, NPs are known to stimulate lipolysis, mitochondrial biogenesis and browning of adipocytes (4).
Cross-sectional studies have demonstrated that diabetes and obesity are inversely associated with lower levels of BNP and NT-proBNP. Wang et al (5) found that, among 3389 participants of the Framingham Heart Study offspring cohort without HF, body mass index (BMI) was inversely associated with NP levels. Compared to those with a normal BMI, men and women with obesity had 40% and 38% lower plasma BNP levels, respectively. In the setting of HF, NT-proBNP is widely used to aid diagnosis, stratify cardiovascular risk and monitor therapy effect. This inverse association of BMI with NP levels is also present in those with HF, and BMI-specific cutoff-points for HF diagnosis and prediction have been proposed in the literature (6-8). Possible explanations for the low levels of NPs in obesity are impaired synthesis and release from the cardiomyocytes or, less likely, increased clearance by NP receptor-C (9-12).
A growing number of studies suggest that NPs are not only associated with but may also protect against metabolic diseases. Transgenic mice overexpressing BNP were protected against diet-induced obesity and insulin resistance, despite being fed a high fat diet (13). Preclinical studies have also shown that BNP treatment in obese mice improves glucose tolerance and insulin sensitivity (14,15). Similar observations have been seen in human studies. In a subsample of 3,019 participants from the Jackson Heart Study, a cohort of African-American adults from Mississippi, BNP levels had a U-shape association with the incidence of metabolic syndrome (16). The Atherosclerosis Risk in Communities Study (ARIC) also demonstrated that NT-proBNP levels were inversely associated with diabetes risk, even after multivariable adjustments (17). Likewise, the Women’s Health Study (18), the Cardiovascular Health Study (19) and the Multi-Ethnic Study of Atherosclerosis (20) have additionally provided support for an inverse association between NPs levels and incident diabetes. However, longitudinal data regarding the direct association of changes in NPs with weight change and risk of obesity, independently of changes in other cardiometabolic risk markers, are limited.
We hypothesized that lower baseline levels and decreases in NT-proBNP over time would be independently associated with increases in weight and a higher risk of obesity in the community-based population of the ARIC Study.
Methods
Study population
The ARIC Study is an ongoing prospective cohort of 15,792 middle-aged adults designed to investigate the etiology of atherosclerosis and its clinical outcomes. Participants were recruited from four communities in the United States (Forsyth County, North Carolina; Jackson, Mississippi; suburbs of Minneapolis, Minnesota; and Washington County, Maryland). Participants were enrolled from 1987 to 1989 and underwent clinical examinations approximately every three years thereafter for 3 more visits (1990-1992, 1993-1995, 1996-1998). Subsequent visits also occurred in 2011-2013, 2016-2017 and 2018-2019. In this analysis, we used data on NT-proBNP available from visits 2 (1990-92) and 4 (1996-98). More information about data collection is published elsewhere (21).
A total of 11,449 participants attended both visits. We excluded participants with race/ethnicity other than black or white (n=31) and black individuals from Minneapolis and Washington County centers (n=38); those with missing data for variables of interest (n=745); and those missing NT-proBNP levels (n=954). The final sample size was composed of 9,681 adults. For the analyses examining the incidence of obesity, individuals with obesity at baseline were excluded (n=2,741).
The ARIC study has been approved by the Institutional Review Boards (IRB) at all participating institutions: University of North Carolina at Chapel Hill IRB, Wake Forest University IRB, Johns Hopkins University IRB, University of Minnesota IRB and University of Mississippi Medical Center IRB. Written informed consent was obtained from all study participants. All methods were carried out in accordance with the relevant guidelines and regulations for human subject research, in accordance with the Declaration of Helsinki (22).
Measurements
All covariates were assessed at baseline following standard protocols. BMI was calculated from measured weight and height at both visits and obesity was defined as a BMI ≥ 30 kg/m2. Diabetes was defined as fasting (≥8 hours) blood glucose ≥126 mg/dL, non-fasting blood glucose ≥ 200 mg/dL, self-reported physician diagnosed diabetes or “sugar in blood”, or use of medication for diabetes in the past two weeks. Hypertension was defined as mean systolic blood pressure (BP) ≥ 140 mmHg, mean diastolic BP ≥ 90 mmHg, or use of medication for high blood pressure in the past 2 weeks. Atherosclerotic cardiovascular disease (ASCVD) was defined as history of coronary heart disease and/or stroke.
Assays of NT-proBNP levels were conducted in serum (visit 2) and plasma (visit 4) samples that had been stored at −70°C. Measurements were made using a sandwich immunoassay method on a Roche Elecsys 2010 Analyzer at visit 2, and an ECLIA immunoassay on an automated Cobas e411 analyzer at visit 4. The lower detection limit for both assays was 5 pg/mL, and participants with unmeasurable levels were assigned a value of 2.5 pg/mL. NT-proBNP, glucose, total cholesterol, HDL- and LDL-cholesterol, triglycerides and hs-CRP were re-calibrated based on published equations to minimize any systematic differences across study visits (23).
Statistical Analyses
We compared the characteristics of the study participants by quartiles of NT-proBNP at baseline. P-values for linear trends across NT-proBNP quartiles were obtained by assigning the median NT-proBNP value in each quartile and modeling this ordinal variable continuously. We used multivariable logistic regression models to estimate the association of NT-proBNP quartiles with prevalence of obesity at baseline. Among those who did not have obesity at baseline, we examined the risk of developing obesity at 6 years according to baseline quartiles of NT-proBNP. Two adjustment models were used: Model 1 included age, sex, race-center, smoking status, eGFR, hypertension, diabetes, heart failure and ASCVD (coronary heart disease or stroke); Model 2 included the variables in model 1 plus total cholesterol, HDL-cholesterol and use of lipid lowering medication. We analyzed the associations of baseline deciles of NT-proBNP with 6-year change in weight. The reference category for change in NT-proBNP was the one with values crossing zero. We also used linear regression to evaluate weight change as a linear spline which was regressed on log-transformed change in NT-proBNP (knots located at deciles of change in log-NT-proBNP). We conducted sensitivity analyses excluding patients with HF prior to visit 4. We also conducted a sensitivity analysis using BMI (kg/m2) as a continuous dependent variable in a linear regression model. All statistical analyses were performed using Stata v15.1 software, and a p value < 0.05 was considered statistically significant.
Results
Baseline data
Mean age at baseline (1990-1992) was 57 years, 56% were women and 78% were white. The prevalence of obesity was 28%. NT-proBNP levels were almost 12% lower in participants with obesity compared to those without obesity at baseline (77.0 ± 135.3 pg/mL in people with obesity and 87.1 ± 439.5 pg/mL in people without obesity). The prevalence of coronary heart disease generally increased with increasing quartiles of NT-proBNP levels at baseline, while the prevalence of diabetes, levels of BMI, glucose, triglycerides, total cholesterol, and eGFR decreased (Table 1, P for trend <0.0001). Individuals in the lowest quartile of NT-proBNP levels at baseline were less likely to have hypertension, HF and coronary heart disease compared to those in the highest quartile (Table 1).
Table 1.
Characteristics of participants according to NT-proBNP quartiles at baseline (visit 2, 1990-1992), the Atherosclerosis Risk in Communities (ARIC), n=9681
| Q1 (≤ 27.17 pg/mL) |
Q2 (27.19 – 50.79 pg/mL) |
Q3 (50.81 – 90.58 pg/mL) |
Q4 (≥ 90.63 pg/mL) |
P-value for trend |
|
|---|---|---|---|---|---|
| N | 2,422 | 2,419 | 2,421 | 2,419 | |
| Age, years | 55.0 (5.3) | 56.3 (5.4) | 57.2 (5.7) | 58.3 (5.7) | <0.0001 |
| Female (%) | 838 (34.6%) | 1270 (52.5%) | 1611 (66.5%) | 1727 (71.4%) | <0.0001 |
| Race (%) | |||||
| White | 1644 (67.9%) | 1908 (78.9%) | 2004 (82.8%) | 2029 (83.9%) | <0.0001 |
| Black | 778 (32.1%) | 511 (21.1%) | 417 (17.2%) | 390 (16.1%) | <0.0001 |
| Current smokers (%) | 474 (19.6%) | 431 (17.8%) | 466 (19.2%) | 492 (20.3%) | 0.164 |
| Hypertension (%) | 727 (30.0%) | 709 (29.3%) | 755 (31.2%) | 1003 (41.5%) | <0.0001 |
| Diabetes (%) | 393 (16.2%) | 320 (13.2%) | 274 (11.3%) | 258 (10.7%) | <0.0001 |
| History of coronary heart disease or stroke (%) | 62 (2.6%) | 87 (3.6%) | 124 (5.1%) | 289 (11.9%) | |
| Prevalent heart failure (%) | 74 (3.1%) | 88 (3.6%) | 72 (3.0%) | 140 (5.8%) | <0.0001 |
| Use of medication for hypertension, diabetes or lipid lowering (%) | 779 (32.2%) | 762 (31.5%) | 784 (32.4%) | 1061 (43.9%) | <0.0001 |
| Weight, kg | 84.1 ± 15.3 | 80.6 ± 16.0 | 76.7 ± 16.8 | 75.6 ± 17.1 | <0.0001 |
| BMI, kg/m2 | 28.7 ± 4.9 | 28.2 ± 5.0 | 27.5 ± 5.5 | 27.3 ± 5.5 | <0.0001 |
| Obesity [BMI≥30 kg/m2] (%) | 781 (32.2%) | 716 (29.6%) | 622 (25.7%) | 622 (25.7%) | <0.0001 |
| Waist circumference, cm | 100.3 ± 12.4 | 98.8 ± 13.3 | 96.1 ± 14.6 | 95.4 ± 15.2 | <0.0001 |
| Glucose, mg/dL | 102.1 (94.4, 112.6) | 99.2 (92.5, 107.8) | 97.3 (90.6, 104.9) | 96.3 (90.6, 104.9) | <0.0001 |
| Glycated hemoglobin, %-point | 5.5 (5.2, 5.9) | 5.4 (5.2, 5.8) | 5.4 (5.2, 5.7) | 5.4 (5.2, 5.7) | <0.0001 |
| Triglycerides, mg/dL | 126.1 (90.5, 178.8) | 117.9 (85.5, 166.7) | 112.9 (82.4, 160.6) | 111.8 (82.4, 156.5) | <0.0001 |
| HDL-cholesterol, mg/dL | 20.1 (17.9, 23.0) | 20.9 (17.9, 23.8) | 21.6 (18.7, 26.0) | 22.3 (18.7, 26.7) | <0.0001 |
| Total cholesterol, mg/dL | 204.7 (180.8, 231.5) | 203.7 (179.8, 228.5) | 202.7 (180.8, 227.5) | 201.7 (178.8, 227.5) | 0.005 |
| CRP, mg/L | 2.0 (1.0, 4.0) | 2.1 (1.0, 4.4) | 2.3 (1.1, 4.9) | 2.6 (1.2, 5.7) | <0.0001 |
| eGFR, mL/min/1.73 m2 | 99.1 (91.3, 107.6) | 97.7 (89.5, 105.3) | 96.9 (89.0, 104.8) | 95.6 (86.1, 103.3) | <0.0001 |
Data is presented as number (%), mean ± standard deviation or median (interquartile range). BMI=Body mass index; CRP=C-Reactive Protein; eGFR=Estimated glomerular filtration rate; HDL=High-density lipoprotein
Outcomes
Obesity
In cross-sectional analyses, individuals in the lowest quartile of NT-proBNP (≤ 27.2 pg/mL) were more likely to have obesity at baseline than those in the highest quartile (OR 1.37, 95%CI 1.18-1.58; Model 1; Table 2). After additional adjustment for lipids (Model 2), the association was slightly attenuated, but remained significant and strong (OR 1.25, 95%CI 1.08-1.45). Results were similar in a sensitivity analysis using BMI (kg/m2) as a continuous dependent variable in a linear regression model (Table S1).
Table 2.
Odds ratios (95% confidence intervals) of prevalent obesity (BMI≥30 kg/m2) at baseline according to quartiles of NT-proBNP at baseline, N=9,681
| NT-proBNP categories (pg/mL) | ||||
|---|---|---|---|---|
| Q1 (≤ 27.17) |
Q2 (27.19 – 50.79) |
Q3 (50.81 – 90.58) |
Q4 (≥ 90.63) |
|
| Model 1 | 1.37 (1.18-1.58)* | 1.26 (1.10-1.45)* | 1.05 (0.91—1.21) | 1 (reference) |
| Model 2 | 1.25 (1.08-1.45)* | 1.17 (1.02-1.35)* | 1.03 (0.89 – 1.18) | 1 (reference) |
Model 1: Adjusted for age, sex, race-center, smoking status, eGFR, hypertension, diabetes, heart failure, ASCVD (coronary heart disease or stroke). Model 2: Model 1 plus total cholesterol, HDL-cholesterol and lipid lowering medication.
p<0.05
ASCVD= atherosclerotic cardiovascular disease; eGFR=Estimated glomerular filtration rate; HDL=High-density lipoprotein
During the 6-year follow up period, the overall prevalence of obesity increased from 28% to 35%. The risk of developing obesity during the 6-year period was highest among individuals in the lowest quartile of NT-proBNP in both models (Table 3).
Table 3.
Odds ratios (95% confidence intervals) of incident obesity (BMI≥30 kg/m2) at the 6-year follow up visit (visit 4, 1996-1998) among those participants who were non-obese at baseline (visit 2, 1990-1992), N=6,940
| NT-proBNP categories (pg/mL) | |||||
|---|---|---|---|---|---|
| Q1 (≤ 27.17) |
Q2 (27.19 – 50.79) |
Q3 (50.81 – 90.58) |
Q4 (≥ 90.63) |
P-value for trend |
|
| Model 1 | 1.51 (1.21-1.89)* | 1.49 (1.21-1.83)* | 1.09 (0.89-1.35) | 1 (reference) | <0.0001 |
| Model 2 | 1.35 (1.07-1.69)* | 1.37 (1.11-1.69)* | 1.06 (0.85-1.30) | 1 (reference) | 0.003 |
Model 1: Adjusted for age, sex, race-center, smoking status, eGFR, hypertension, diabetes, heart failure, ASCVD (coronary heart disease or stroke). Model 2: Model 1 plus total cholesterol, HDL-cholesterol and lipid lowering medication.
p<0.05
ASCVD= atherosclerotic cardiovascular disease; eGFR=Estimated glomerular filtration rate; HDL=High-density lipoprotein
Change in weight
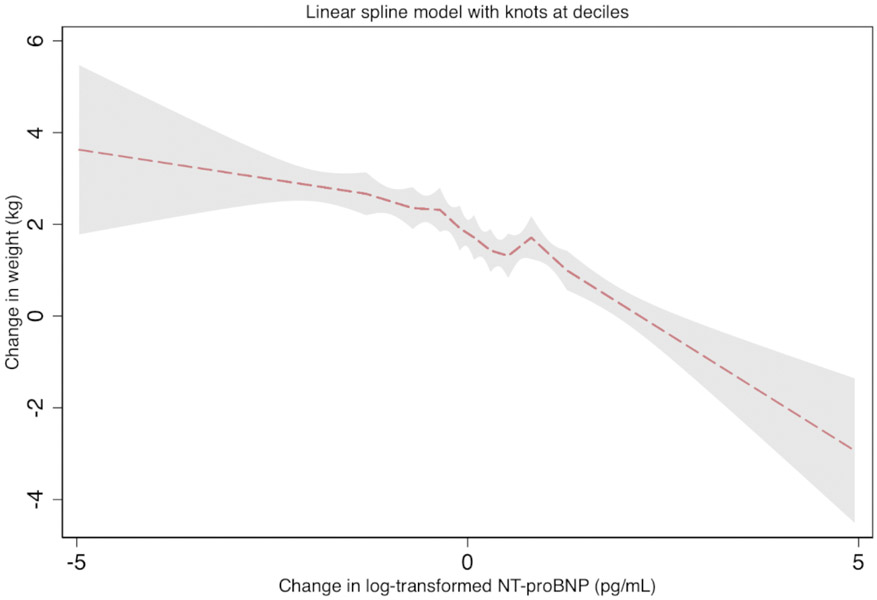
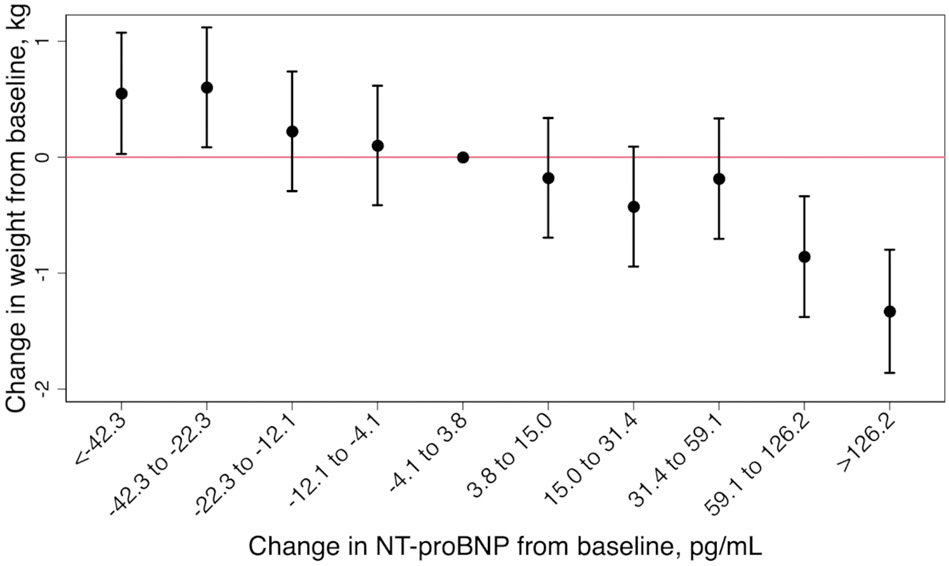
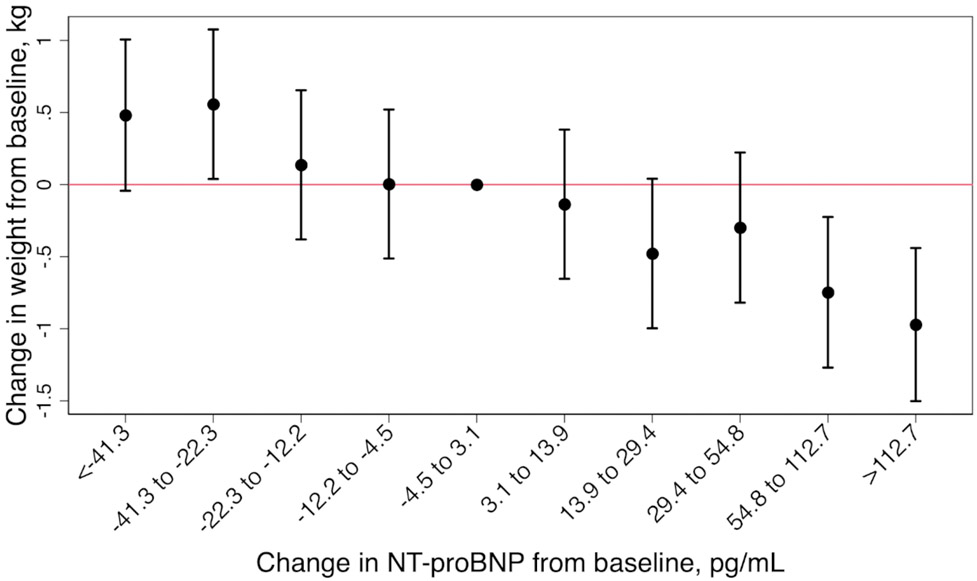
We found an inverse association between change in weight and change in NT-proBNP level (Figure 1). Participants who had an increase in NT-proBNP between the two visits greater than or equal to the 90th percentile of change in NT-proBNP (≥126.2 pg/mL), decreased their weight by an average of ~1.3 kg (mean weight change −1.33; 95%CI −1.86 to −0.80). Statistically significant decreases in weight were observed when NT-proBNP levels increased by more than 59.1 pg/mL in the 6-year follow up (Figure 2A). Decreases in NT-proBNP levels of more than 22.3 pg/mL over the same period were associated with statistically significant increases in weight (decrease in NT-proBNP −22.3 to −42.3, mean weight change 0.55 kg, 95% CI (0.03, 1.07); decrease in NT-proBNP <−42.3, mean weight change 0.60 kg, 95% CI (0.09, 1.12)). Results were similar in a sensitivity analysis excluding participants with HF (Figure 2B).
Figure 1. Adjusted six-year change in log-transformed NT-proBNP with change in weight (β coefficient, 95%CI), the Atherosclerosis Risk in Communities (ARIC) Study (1990-1992 to 1996-1998).
The association between weight change as a linear spline with log-transformed change in NT-proBNP. The dashed line is the β coefficient and the shaded region is the 95% confidence interval. Knots are located at deciles of change in log NT-proBNP. This model is adjusted for age, sex, race-center, smoking status, estimated glomerular filtration rate, hypertension, diabetes, heart failure, atherosclerotic cardiovascular disease (coronary heart disease or stroke), total cholesterol, high-density lipoprotein-cholesterol and lipid lowering medication. We found an inverse association between change in weight and change in NT-proBNP level.
Figure 2. Change in weight by deciles of change in NT-proBNP over the 6-year follow up period (A) and after exclusion of participants with heart failure occurring at or prior to visit 4 (B), the Atherosclerosis Risk in Communities (ARIC) Study (1990-1992 to 1996-1998).
(A) and (B) are adjusted for age, sex, race-center, smoking status, estimated glomerular filtration rate, hypertension, diabetes, heart failure, atherosclerotic cardiovascular disease (coronary heart disease or stroke), total cholesterol, high-density lipoprotein-cholesterol and lipid lowering medication. Statistically significant decreases in weight were observed when NT-proBNP levels increased by more than 59.1 pg/mL (A) and 54.8 pg/mL (B) in the 6-year follow up. Decreases in NT-proBNP levels of more than 22.3 pg/mL over the same period were associated with statistically significant increases in weight (A).
Discussion
In this prospective cohort of 9,681 adults, we found that lower NT-proBNP levels at baseline were associated with an increased risk of obesity, and changes in NT-proBNP levels over a 6-year follow-up period were inversely associated with changes in weight. This relationship was not driven by incident HF, which is known to directly affect NT-proBNP levels. Consistent with other cross-sectional studies, individuals with obesity also had lower levels of NT-proBNP compared to individuals without obesity. To our knowledge, this is the first study to show the prospective association of baseline levels and changes in NT-proBNP with the incidence of obesity and changes in weight over time.
NPs and obesity
Several mechanisms have been proposed to explain the inverse relation between NPs and obesity. In the adipose tissue, BNP has a lipolytic effect via stimulation of GC-A and GC-B. Activation of these receptors induce a rise in cyclic guanosine monophosphate (cGMP) levels and subsequent activation of cGMP-dependent protein kinase 1 (cGK1), which in turn modify the lipid droplet surface to facilitate lipolysis (3). The existence of this pathway is supported by in vitro studies on isolated human fat cells (13,24-26). Notably, the impact of NPs on adipose tissue may be altered in individuals with obesity or insulin resistance, with attenuation or loss of the protective lipolytic effect of NPs (27,28).
NPs and hormones
Natriuretic peptides are also linked with modulated expression of numerous hormones, and may indirectly influence food intake. In a randomized clinical trial with 10 healthy men, acute intravenous infusion of BNP decreased circulating ghrelin concentrations, an important hormone that stimulates appetite (29). Participants reported decreased hunger and increased feeling of satiety, demonstrating an induced anorexic effect of BNP infusion. However, this study only evaluated the acute effect of infusion with BNP, and changes in other variables affected by NPs (for example, insulin) could be responsible for the decrease in ghrelin. In contrast, adiponectin, which is responsible for the regulation of various metabolic pathways, has a positive association with NT-proBNP levels. Higher levels of adiponectin are associated with decreased BMI and lower risk of metabolic syndrome and diabetes. Fully understanding the link between NPs and the endocrine system is essential to interpret the interplay of these peptides with obesity.
Directionality of the relationship between NPs and adiposity
Low levels of NPs are also proposed to be a consequence of obesity (5). Several mechanisms have been suggested, such as reduced synthesis and release from the heart. Substances released by the adipose tissue could be involved in suppressing production of NPs by the myocytes. Higher glycosylation of proBNP, seen in individuals with obesity, leads to impaired conversion of this molecule and, consequently, to lower plasma concentrations of BNP and NT-proBNP. Individuals with obesity also appear to have increased expression of neprilysin, an endopeptidase that degrades NPs. Taking into account these investigations along with our findings, it is possible to assume that the relationship between NPs and adiposity is bidirectional, with each factor influencing the levels of the other. Previous work has demonstrated inverse associations of NT-proBNP with the development of metabolic syndrome (18,30) and diabetes (31). While this study does not directly address metabolic syndrome and diabetes, it is likely that an increase in weight, and thus lower levels of NPs, contribute to a greater likelihood of these metabolic abnormalities.
Study limitations
Our study has some limitations. We had only one measure of NT-proBNP at each visit, and this peptide is known to vary within individuals over short periods of time, similar to other neuro-hormones (32). We did not have measures of BNP; however, NT-proBNP and BNP are secreted in equimolar amounts after cleavage of proBNP, and previous studies have shown that their levels are closely correlated (33). Nevertheless, the biological variation of NT-proBNP is much less than that of BNP (34). We also did not have information on lean and fat body mass, which could be useful since some studies suggest that the percentage of fat body mass may be more closely associated with BNP levels than BMI (35). It is also possible that differences in other factors which were not evaluated in this analysis could influence the associations of NPs with weight change. Due to the observational nature of the study, we cannot exclude the possibility of residual confounding.
Study strengths
Nonetheless, our study has important strengths. We used data from the ARIC study, a prospective cohort that follows a biracial community-based sample since 1987 using standardized and rigorous data collection methods. Our large sample and availability of serial measurements of multiple cardiometabolic markers allowed us to investigate, over a 6-year follow up period, the relationship between natriuretic peptides and weight. We were able to show the prospective association of baseline levels and changes in NT-proBNP with incident obesity controlling for other risk factors.
Conclusion
In conclusion, we found inverse associations of baseline and longitudinal NT-proBNP levels with weight change and incidence of obesity. Our results suggest that NPs or their pathways may be potential targets in the treatment of obesity. However, the molecular networks involving the natriuretic system are complex and additional research, especially interventional studies, is needed to address the clinical relevance of our findings.
Supplementary Material
Cross-sectional studies have demonstrated that obesity is associated with lower levels of NT-proBNP. Longitudinal data evaluating the association between natriuretic peptides (NPs) and weight change are scarce.
Lower levels of NT-proBNP were associated with higher risk of obesity, and changes in NT-proBNP were inversely associated with changes in weight.
NPs or their pathways may be potential targets in the treatment of obesity. Nevertheless, interventional studies are needed to examine the therapeutic potential of NPs.
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding: The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, HHSN268201700004I). Reagents for the NT-proBNP assays were donated by the Roche Diagnostics Corporation. This research was supported by NIH/NIDDK R01DK089174 to Dr. Selvin and NIH/NHLBI grant R01 HL134320 to Drs. Ballantyne and Selvin. Dr. Selvin was also supported by NIH/NHLBI grant K24 HL152440. Ms. Sbaraini was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior Brasil (CAPES) - Finance Code 001. Dr. Tang is supported by NIH/NIDDK F30DK120160. Dr. Schaan is supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Nakagawa Y, Nishikimi T, Kuwahara K. Atrial and brain natriuretic peptides: Hormones secreted from the heart. Peptides 2019;111:18–25. [DOI] [PubMed] [Google Scholar]
- 2.Gruden G, Landi A, Bruno G. Natriuretic Peptides, Heart, and Adipose Tissue: New Findings and Future Developments for Diabetes Research. Diabetes Care 2014;37:2899. [DOI] [PubMed] [Google Scholar]
- 3.Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. American Journal of Physiology-Heart and Circulatory Physiology 2013;304:H358–H368. [DOI] [PubMed] [Google Scholar]
- 4.Collins S A heart–adipose tissue connection in the regulation of energy metabolism. Nature Reviews Endocrinology 2014;10:157–163. [DOI] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D et al. Impact of Obesity on Plasma Natriuretic Peptide Levels. Circulation 2004;109:594–600. [DOI] [PubMed] [Google Scholar]
- 6.Daniels LB, Clopton P, Bhalla V et al. How obesity affects the cut-points for B-type natriuretic peptide in the diagnosis of acute heart failure: Results from the Breathing Not Properly Multinational Study. American Heart Journal 2006;151:999–1005. [DOI] [PubMed] [Google Scholar]
- 7.Christenson RH, Azzazy HME, Duh S-H, Maynard S, Seliger SL, deFilippi CR. Impact of Increased Body Mass Index on Accuracy of B-Type Natriuretic Peptide (BNP) and N-Terminal proBNP for Diagnosis of Decompensated Heart Failure and Prediction of All-Cause Mortality. Clinical Chemistry 2010;56:633–641. [DOI] [PubMed] [Google Scholar]
- 8.Ndumele CE, Matsushita K, Sang Y et al. N-Terminal Pro-Brain Natriuretic Peptide and Heart Failure Risk Among Individuals With and Without Obesity: The Atherosclerosis Risk in Communities (ARIC) Study. Circulation 2016;133:631–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayes-Genis A, DeFilippi C, Januzzi JL Jr. Understanding Amino-Terminal Pro-B-Type Natriuretic Peptide in Obesity. American Journal of Cardiology 2008;101:S89–S94. [DOI] [PubMed] [Google Scholar]
- 10.Clerico A, Fontana M, Vittorini S, Emdin M. The search for a pathophysiological link between gender, cardiac endocrine function, body mass regulation and cardiac mortality: Proposal for a working hypothesis. Clinica Chimica Acta 2009;405:1–7. [DOI] [PubMed] [Google Scholar]
- 11.Morabito D, Vallotton MB, Lang U. Obesity Is Associated With Impaired Ventricular Protein Kinase C-MAP Kinase Signaling and Altered ANP mRNA Expression in the Heart of Adult Zucker Rats. Journal of Investigative Medicine 2001;49:310. [DOI] [PubMed] [Google Scholar]
- 12.Das Sandeep R, Drazner Mark H, Dries Daniel L et al. Impact of Body Mass and Body Composition on Circulating Levels of Natriuretic Peptides. Circulation 2005;112:2163–2168. [DOI] [PubMed] [Google Scholar]
- 13.Miyashita K, Itoh H, Tsujimoto H et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes 2009;58:2880–2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plante E, Menaouar A, Danalache BA, Broderick TL, Jankowski M, Gutkowska J. Treatment with brain natriuretic peptide prevents the development of cardiac dysfunction in obese diabetic db/db mice. Diabetologia 2014;57:1257–67. [DOI] [PubMed] [Google Scholar]
- 15.Coue M, Badin PM, Vila IK et al. Defective Natriuretic Peptide Receptor Signaling in Skeletal Muscle Links Obesity to Type 2 Diabetes. Diabetes 2015;64:4033–45. [DOI] [PubMed] [Google Scholar]
- 16.Musani SK, Vasan RS, Bidulescu A et al. Aldosterone, C-reactive protein, and plasma B-type natriuretic peptide are associated with the development of metabolic syndrome and longitudinal changes in metabolic syndrome components: findings from the Jackson Heart Study. Diabetes care 2013;36:3084–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lazo M, Young JH, Brancati FL et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes 2013;62:3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett BM, Cook NR, Chasman DI et al. Prospective evaluation of B-type natriuretic peptide concentrations and the risk of type 2 diabetes in women. Clinical chemistry 2013;59:557–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brutsaert EF, Biggs ML, Delaney JA et al. Longitudinal assessment of N-terminal pro-B-type natriuretic peptide and risk of diabetes in older adults: The cardiovascular health study. Metabolism 2016;65:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez OA, Duprez DA, Bahrami H et al. Changes in N-terminal pro-B-type natriuretic peptide and incidence of diabetes: The Multi-Ethnic Study of Atherosclerosis (MESA). Diabetes Metab 2015;41:378–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 1989;129:687–702. [PubMed] [Google Scholar]
- 22.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. 1989/April/01 ed: Am J Epidemiol, 1989:687–702. [PubMed] [Google Scholar]
- 23.Parrinello CM, Grams ME, Couper D et al. Recalibration of blood analytes over 25 years in the atherosclerosis risk in communities study: impact of recalibration on chronic kidney disease prevalence and incidence. Clin Chem 2015;61:938–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SengenES C, Berlan M, De Glisezinski I, Lafontan MAX, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. The FASEB Journal 2000;14:1345–1351. [PubMed] [Google Scholar]
- 25.Bordicchia M, Liu D, Amri E-Z et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birkenfeld AL, Boschmann M, Moro C et al. Lipid Mobilization with Physiological Atrial Natriuretic Peptide Concentrations in Humans. The Journal of Clinical Endocrinology & Metabolism 2005;90:3622–3628. [DOI] [PubMed] [Google Scholar]
- 27.Santhekadur PK, Kumar DP, Seneshaw M, Mirshahi F, Sanyal AJ. The multifaceted role of natriuretic peptides in metabolic syndrome. Biomed Pharmacother 2017;92:826–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moro C Targeting cardiac natriuretic peptides in the therapy of diabetes and obesity. Expert Opinion on Therapeutic Targets 2016;20:1445–1452. [DOI] [PubMed] [Google Scholar]
- 29.Vila G, Grimm G, Resl M et al. B-type natriuretic peptide modulates ghrelin, hunger, and satiety in healthy men. Diabetes 2012;61:2592–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruno G, Barutta F, Landi A et al. Levels of N-terminal pro brain natriuretic peptide are enhanced in people with the uncomplicated metabolic syndrome: a case-cohort analysis of the population-based Casale Monferrato study. Diabetes/Metabolism Research and Reviews 2015;31:360–367. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson M, Jujic A, Hedblad B et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab 2012;97:638–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Apple FS, Panteghini M, Ravkilde J et al. Quality Specifications for B-Type Natriuretic Peptide Assays. Clinical Chemistry 2020;51:486–493. [DOI] [PubMed] [Google Scholar]
- 33.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet 2003;362:316–22. [DOI] [PubMed] [Google Scholar]
- 34.Wu AH, Smith A, Wieczorek S et al. Biological variation for N-terminal pro- and B-type natriuretic peptides and implications for therapeutic monitoring of patients with congestive heart failure. Am J Cardiol 2003;92:628–31. [DOI] [PubMed] [Google Scholar]
- 35.Huang F-Y, Wang H, Huang B-T et al. The influence of body composition on the N-terminal pro-B-type natriuretic peptide level and its prognostic performance in patients with acute coronary syndrome: a cohort study. Cardiovasc Diabetol 2016;15:58–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.