Abstract
Simple Summary
The analysis of circulating tumor cells (CTCs) as a “real-time liquid biopsy” in epithelial tumors for personalized medicine has received tremendous attention over the past years, with important clinical implications. In metastatic colorectal cancer (mCRC), the CellSearch® system has already demonstrated its prognostic value and interest in monitoring treatment response, but the number of recovered CTCs remains low. In this article, we evaluate the early prognostic and predictive value of viable CTCs in patients with mCRC treated with FOLFIRI–bevacizumab with an alternative approach, the functional EPISPOT assay. This study shows that viable CTCs can be detected in patients with mCRC before and during FOLFIRI–bevacizumab treatment and that CTC detection at D28 and the D0–D28 CTC kinetics evaluated with the EPISPOT assay are associated with response to treatment.
Abstract
Background: Circulating tumor cells (CTCs) allow the real-time monitoring of tumor course and treatment response. This prospective multicenter study evaluates and compares the early predictive value of CTC enumeration with EPISPOT, a functional assay that detects only viable CTCs, and with the CellSearch® system in patients with metastatic colorectal cancer (mCRC). Methods: Treatment-naive patients with mCRC and measurable disease (RECIST criteria 1.1) received FOLFIRI–bevacizumab until progression or unacceptable toxicity. CTCs in peripheral blood were enumerated at D0, D14, D28, D42, and D56 (EPISPOT assay) and at D0 and D28 (CellSearch® system). Progression-free survival (PFS) and overall survival (OS) were assessed with the Kaplan–Meier method and log-rank test. Results: With the EPISPOT assay, at least 1 viable CTC was detected in 21% (D0), 15% (D14), 12% (D28), 10% (D42), and 12% (D56) of 155 patients. PFS and OS were shorter in patients who remained positive, with viable CTCs between D0 and D28 compared with the other patients (PFS = 7.36 vs. 9.43 months, p = 0.0161 and OS = 25.99 vs. 13.83 months, p = 0.0178). The prognostic and predictive values of ≥3 CTCs (CellSearch® system) were confirmed. Conclusions: CTC detection at D28 and the D0–D28 CTC dynamics evaluated with the EPISPOT assay were associated with outcomes and may predict response to treatment.
Keywords: circulating tumor cells, colorectal cancer, EPISPOT assay, CellSearch® system, predictive value
1. Introduction
In western countries, colorectal cancer (CRC) is one of the most frequently diagnosed cancers and a leading cause of cancer death. In Europe, an estimated 499,700 new cases occurred in 2018, and 242,500 patients died of CRC in the same year [1].
CRC’s high mortality rate is due to the development of distant unresectable metastases in more than 50% of patients at some point during the disease course [2]. In this setting, the current guidelines recommend the use of cytotoxic chemotherapy regimens that combine fluoropyrimidine with oxaliplatin or irinotecan and a targeted agent (bevacizumab or cetuximab/panitumumab) as first-line standard-of-care therapy, [3,4,5]. Although the RAS oncogene’s mutational status is an unquestionable marker to select patients who are unlikely to benefit from EGFR antibody therapy [6,7], robust biomarkers for predicting outcome and early treatment response are still lacking, especially for bevacizumab-based regimens [8].
The “liquid biopsy” has been introduced for the analysis of circulating tumor cells (CTCs) in the blood of patients with solid cancers, and many clinical trials have focused on this new approach for precision medicine over the past decade [9]. Specifically, the most aggressive tumor cells are actively released by the tumor and/or metastases in body fluids [10]. They can be isolated from peripheral blood and were the first “liquid biopsy” component investigated as a biomarker in many cancer types [9]. In metastatic CRC, the CTC prognostic value has been clinically validated using the FDA-cleared CellSearch® system (www.cellsearchctc.com, accessed on 21 May 2021). Briefly, three large prospective studies demonstrated that patients with ≥3 CTCs before chemotherapy have shorter progression-free (PFS) and overall survival (OS) [10,11,12]. They also found that the CTC number remains a strong prognostic factor after a few treatment cycles and might also help monitor the treatment response. In these studies, most patients received the fluoropyrimidine–oxaliplatin combination and bevacizumab as first-line treatment. With the CellSearch® system, CTC capture is based on immunoselection using antibodies against the epithelial cell surface adhesion molecule (EpCAM) [13]. However, CTCs are phenotypically heterogeneous, and some may not express epithelial markers anymore or weakly, especially if they have undergone an epithelial-to-mesenchymal transition [14,15]. Consequently, these subpopulations might not be detected by the CellSearch® system, underlining the need to develop alternative approaches to improve CTC enrichment.
In this context, we developed a functional assay called the Epithelial ImmunoSPOT assay (EPISPOT) that selects viable CTCs based on the detection of specific secreted tumor-associated proteins. Therefore, EPISPOT enumerates only viable CTCs, irrespective of EpCAM expression, because this innovative technology is always combined with depletion of leukocytes [16]. Using cytokeratin-19 (CK19) as the released protein to detect CTCs in the bloodstream, we have already validated the prognostic value of functional CTCs in a prospective study with more than 250 patients with metastatic breast cancer. We found that functional CTCs are correlated with OS and could be used in combination with the CTCs detected by the CellSearch® system to refine the prognostic stratification of these patients [17]. Moreover, in non-metastatic CRC, the CK19-EPISPOT assay detected more CTCs than the CellSearch® system in peripheral and mesenteric blood samples from patients with treatment-naïve tumors [18].
Therefore, we carried out a prospective study, called COLOSPOT, on patients with untreated metastatic CRC, about to receive FOLFIRI (folinic acid, fluorouracil, and irinotecan) and bevacizumab as first-line therapy, to further investigate the clinical utility of viable CTCs detected with the CK19-EPISPOT assay. The objectives were to assess the prognostic and early predictive values of viable CTC enumeration and their dynamics during treatment using the CK19-EPISPOT assay and to compare the CTC detection of the CK19-EPISPOT assay and the CellSearch® system (the gold standard).
2. Materials and Methods
2.1. Study Design
We carried out a multicenter prospective study named “COLOSPOT” (ClinicalTrials.gov: NCT01596790) in 11 medical centers in France. The human investigations were performed after approval by the human investigation committee Sud Méditerranée III (Ref: 2011.11.01). Patients with untreated metastatic colorectal adenocarcinoma, with measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, who started first-line systemic therapy with FOLFIRI–bevacizumab were eligible. Other inclusion criteria were: patients older than 18 years and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) score of 0 to 2. Chemotherapy was continued until disease progression, unacceptable toxicity, or patient/investigator’s decision. Tumor response was assessed every 8 weeks during the first year of treatment and every 3 months thereafter until disease progression or for a maximum period of 2 years. Tumor response was evaluated using contrast-enhanced chest–abdomen–pelvis computed tomography images and the RECIST 1.1 criteria. All patients gave their written informed consent before inclusion.
For CTC enumeration, peripheral blood samples were drawn just before and during therapy, as follows: for the EPISPOT assay, 15 mL of blood was collected in EDTA tubes at baseline (D0) and at day 14 (D14), day 28 (D28), day 42 (D42) and day 56 (D56) after treatment initiation. For the CellSearch system, 10 mL of peripheral blood was collected in CellSave tubes (Silicon Biosystems-Menarini) at D0 and D28, based on the data previously reported by Cohen et al., showing that the conversion of baseline unfavorable (≥3 CTCs/7.5 mL of blood) to favorable (<3 CTCs/7.5 mL of blood) CTC profiles at 3–5 weeks is associated with significantly longer PFS and OS [10]. All blood samples were sent to LCCRH–Montpellier, where all the CTC detection experiments were processed.
2.2. CTC Isolation and Enumeration
All CK19-EPISPOT assays were performed at LCCRH–Montpellier. The detailed procedure of the EPISPOT assay has been previously described [16]. Briefly, within 24 h after blood collection, leukocytes were depleted with RosetteSep CTC enrichment cocktails (#15167) from Stemcell Technologies. Then, the enriched fraction was frozen in liquid nitrogen (90% fetal calf serum + 10% DMSO) and unfrozen when all samples from the same patient were obtained. The idea was to run a single CK19-EPISPOT experiment per patient, avoiding inter-assay variation during the follow-up. Enriched cells were cultured in 96-well plates (MAIPN4550, Milipore, Darmstadt, Germany), precoated with an anti-CK19 antibody (Ks19.1, Progen, Heidelberg, Germany), to capture CK19-releasing CTCs. After 48 h, wells were washed to remove cells, and CK19 molecules captured by the coating antibody were detected with a second anti-CK19 antibody (Ks19.2, Progen) conjugated to the AlexaFluor 555 fluorochrome. Single fluorescent CK19 immunospots were counted under a fluorescent microscope equipped with a camera and computer-assisted analysis (KS ELISPOT, Carl Zeiss Vision, Oberkochen, Germany). Results were expressed as the number of cells per 15 mL of blood.
All CellSearch® analyses were performed within 96 h after blood collection using the CellSearch® CTC kit (7900001, Silicon Biosystem, Menarini, Bologna, Italy), according to the manufacturer’s instructions. This method enriches CTCs via positive selection with magnetic beads coated with anti-EpCAM antibodies, followed by immunofluorescence-based detection. CTCs are Pan-CK(+), DAPI(+), and CD45(−). Results are expressed as the number of cells per 7.5 mL of blood.
2.3. Statistical Analyses
Data were summarized with medians and ranges for continuous variables and frequency for categorical variables. Fischer’s exact test was used to study the correlation between CTC detection and clinical–pathological characteristics. Concordance between technologies was assessed at D0 and D28 by calculating the intraclass correlation coefficient.
PFS and OS were analyzed with the Kaplan–Meier method. Survival curves were compared with the non-parametric log-rank test (p ≤ 0.05 was considered significant). PFS was defined as the elapsed time from blood collection to disease progression or death from any cause. Patients who began a second-line treatment without disease progression were censored at the date of treatment switch. OS was defined as the elapsed time from blood collection to death from any cause.
Univariate and multivariate Cox proportional hazards regression models were used to obtain the unadjusted and fully adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
Statistical analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Clinical and Tumor Characteristics
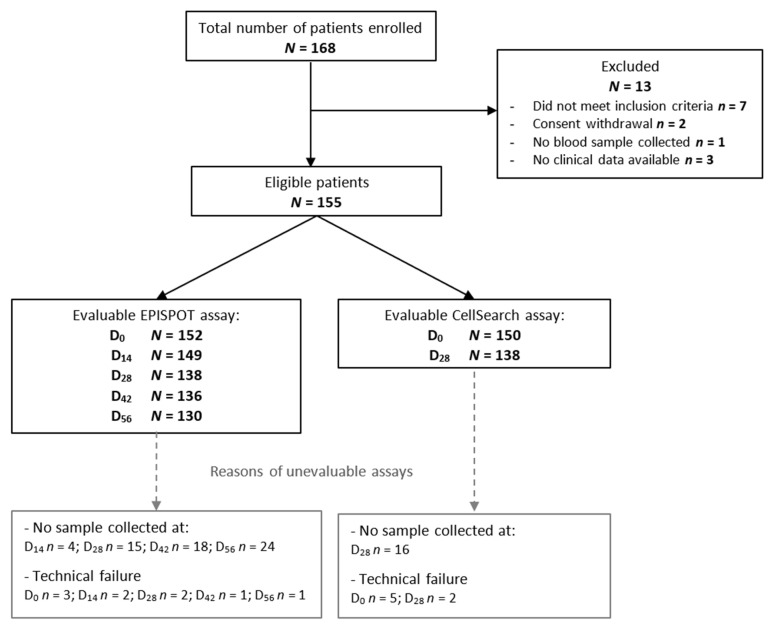
Between April 2012 and September 2016, 168 patients were enrolled in the study, among whom 155 met the inclusion and exclusion criteria and were assessable. The number of patients included at each stage of the analysis and the reasons for exclusion are summarized in the study flowchart (Figure 1).
Figure 1.
Study flowchart showing the number of included patients and the number of patients in whom CTCs could be assessed in peripheral blood samples at different time points before (D0) and during treatment (EPISPOT: D14, D28, D42, D56; CellSearch®: D28). Abbreviations: N, number; D, day.
The patient and tumor characteristics are summarized in Table S1. At the time of the final analysis (July 2019), the median follow-up was 24.5 months (range, 0.99–75.04 months), and the median PFS and OS were 9.4 (95% CI, 8.1–10.2 months) and 26.2 months (95% CI, 21.3–29.8 months), respectively.
3.2. CTC Prevalence at Different Time Points and Correlation with Baseline Characteristics
Table S2 summarizes the results obtained with the CK19-EPISPOT and CellSearch® assays at different time points. With the EPISPOT assay, 32/152 (21%) patients had ≥1 CTC/sample and 18/152 had ≥2 CTCs/sample (11.8%) at D0. During treatment, the number of patients with at least 1 CTC decreased to 15.4% at D14, 12.3% at D28, 9.6% at D42, and 11.5% at D56. According to the CellSearch® assay, 59/150 (39.3%) and 13/138 (9.4%) patients had ≥3 CTCs/sample at D0 and D28, respectively.
The concordance between methods was low, as indicated by the Cohen Κ coefficient of 0.23 (p = 0.002) and 0.34 (p ≤ 0.0001) at D0 and D28, respectively.
Only CTCs detected with the CellSearch® system at D0 (≥3) correlated significantly with some biological and clinical characteristics. Baseline performance status was worse and more patients had synchronous metastases, liver involvement, and abnormal CEA levels in the group with ≥3 CTCs/sample than in the group with <3 CTCs/sample at D0 (Table 1).
Table 1.
Patient characteristics and correlation with CTC number. CTCs were detected with two methods: CK19-EPISPOT and CellSearch®.
| Parameters | EPISPOT (n = 152) | CellSearch® (n = 150) | ||||
|---|---|---|---|---|---|---|
| ≥1 | <1 | p-Value (Fisher) | ≥3 | <3 | p-Value (Fisher) | |
| Age | ||||||
| <70 years | 21 (66%) | 77 (64%) | 1 | 42 (71%) | 55 (61%) | 0.22 |
| ≥70 years | 11 (34%) | 43 (36%) | 17 (29%) | 36 (39%) | ||
| Sex | ||||||
| Men | 23 (72%) | 73 (61% | 0.31 | 35 (59%) | 58 (64%) | 0.61 |
| Women | 9 (28%) | 47 (39%) | 24 (41%) | 33 (36%) | ||
| Baseline ECOG PS score | ||||||
| 0 | 15 (47%) | 67 (57%) | 0.32 | 22 (39%) | 59 (66%) | 0.0021 |
| 1–2 | 17 (53%) | 50 (43%) | 35 (61%) | 31 (34%) | ||
| CRC localization | ||||||
| Right | 11 (37%) | 39 (32%) | 0.67 | 21 (37%) | 29 (32%) | 0.59 |
| Left | 19 (63%) | 81 (68%) | 36 (63%) | 62 (68%) | ||
| Metastases | ||||||
| Synchronous | 23 (74%) | 77 (65%) | 0.40 | 48 (83%) | 50 (57%) | 0.0012 |
| Metachronous | 8 (26%) | 41 (35%) | 10 (17%) | 38 (43%) | ||
| Nb of organs with metastases | ||||||
| 1 | 14 (45%) | 47 (39%) | 0.55 | 21 (36%) | 39 (43%) | 0.49 |
| >1 | 17 (55%) | 73 (61%) | 37 (64%) | 51 (57%) | ||
| Liver metastases | ||||||
| Yes | 26 (84%) | 97 (81%) | 0.80 | 54 (93%) | 66 (73%) | 0.0025 |
| No | 5 (16%) | 23 (19%) | 4 (7%) | 24 (27%) | ||
| RAS status | ||||||
| Wild type | 10 (38%) | 30 (31%) | 0.49 | 13 (29%) | 26 (34%) | 0.69 |
| Mutant | 16 (62%) | 66 (69%) | 32 (71%) | 50 (66%) | ||
| B-RAF status | ||||||
| Wild type | 28 (97%) | 92 (92%) | 0.68 | 46(92%) | 74(95%) | 0.71 |
| Mutant | 1 (3%) | 8 (8%) | 4(8%) | 4(5%) | ||
| CEA value | ||||||
| Normal | 8 (25%) | 36 (31%) | 0.66 | 7 (12%) | 37 (42%) | 0.0001 |
| >normal | 24 (75%) | 81 (69%) | 51 (88%) | 52 (58%) | ||
Abbreviations: M, men; W, women; CRC, colorectal cancer; PS, performance status.
3.3. CTC Presence Correlates with PFS and OS in Patients with Metastatic CRC
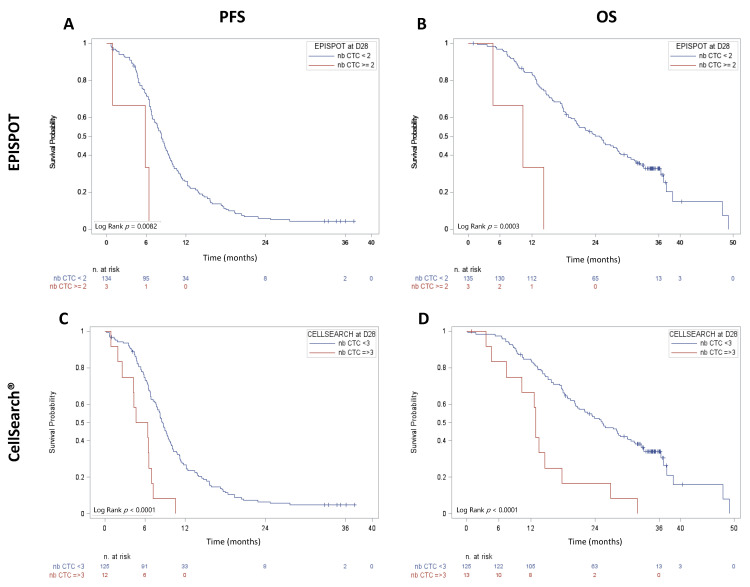
Considering the CTC data obtained with the CK19-EPISPOT assay, the number of viable CTCs at D28, but not at D0, was significant correlated with PFS and OS (Figure 2A,B). PFS and OS were shorter in patients with ≥2 CTCs than in patients without or with only 1 CTC (median PFS = 5.82 months, 95% CI (0.92–6.37 months) vs. 8.28 months, 95% CI (7.20–9.17 months); p = 0.0082 and median OS = 10.28 months, 95% CI (4.63–14.26 months) vs. 24.84 months, 95% CI (20.11–28.45 months); p = 0.0003). Similar results were obtained for CTCs at D42 and OS. No prognostic correlation was observed using 1 CTC as cut-off, regardless of the sampling time (Table S3).
Figure 2.
PFS and OS in patients with metastatic CRC at D28. CTCs were enumerated after the first two chemotherapy cycles (D28) with the (A,B) CK19-EPISPOT (≥2 vs. <2) and (C,D) CellSearch® (≥3 vs. <3) assays.
With the CellSearch® system, at D0, OS was shorter in patients with ≥3 CTCs than in those with <3 CTCs (median OS = 19.1 months, 95% CI (15.57–21.59 months) vs. 37.3 months, 95% CI (26.81–44.58 months); p < 0.0001). Conversely, PFS was not significantly different (data not shown). At D28, ≥3 CTCs was associated with shorter PFS and OS compared with <3 CTCs (median PFS = 5.50 months, 95% CI (1.90–6.93 months) vs. 8.64 months, 95% CI (7.67–9.56 months); p < 0.0001 and median OS = 12.91 months, 95% CI (4.63–17.77 months) vs. 25.27 months, 95% CI (20.40–30.10 months); p < 0.0001 respectively) (Figure 2C,D).
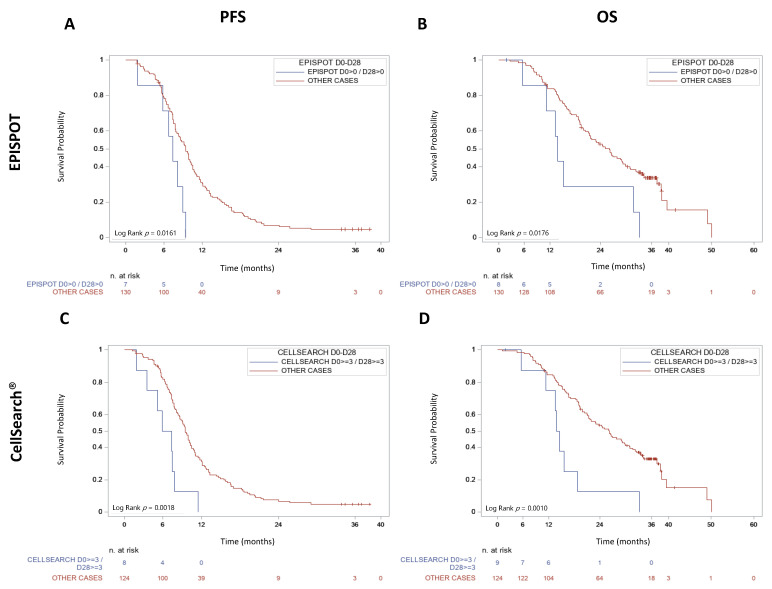
3.4. CTC Kinetics between D0 and D28 Correlates with PFS and OS
To study the CTC kinetics between D0 and D28, patients were divided in two groups: (1) CTC-positive at D0 and D28, and (2) CTC-negative at D0 and D28 or CTC-positive only at D0 or D28. PFS and OS were significant shorter in patients in the first group, with both the CK19-EPISPOT method (median PFS = 7.36 months, 95% CI (1.84–8.97 months) vs. 9.43 months, 95% CI (8.08–10.25 months); p = 0.0161 and median OS = 13.83 months, 95% CI (5.55–31.63 months) vs. 25.99 months, 95% CI (20.99–29.17 months); p = 0.0176) and the CellSearch® method (median PFS = 6.6 months, 95% CI (1.84–7.85 months) vs. 9.46 months, 95% CI (8.54–10.31 months); p = 0.0018 and median OS = 14.13 months, 95% CI (5.55–18.69 months) vs. 26.18 months, 95% CI (21.29–29.83 months); p = 0.0010) (Figure 3).
Figure 3.
PFS and OS in metastatic CRCs according to the D0–D28 CTC kinetics. Patients were divided into two groups in the function of CTC enumeration at D0 and D28, using the (A,B) CK19-EPISPOT and (C,D) CellSearch® assays.
Univariate analysis confirmed that the early CTC dynamics (both assays), ECOG PS at D0, and BRAF mutational status were predictors of PFS and OS. A primary tumor localized to the right colon also significantly correlated with worse OS (Table 2).
Table 2.
Univariate Cox regression analysis for PFS and OS prediction.
| Parameters | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age: ≥70 vs. <70 years | 1.04 | 0.74–1.46 | 0.84 | 1.08 | 0.72–1.62 | 0.71 |
| Sex: W vs. M | 0.84 | 0.6–1.19 | 0.32 | 1.28 | 0.86–1.89 | 0.22 |
| ECOG PS: 1–2 vs. 0 | 1.46 | 1.05–2.05 | 0.0259 | 2.66 | 1.77–3.99 | <0.0001 |
| Right vs. left colon | 1.07 | 0.75–1.51 | 0.72 | 1.54 | 1.03–2.31 | 0.04 |
| Synchronous vs. metachronous mets | 0.78 | 0.55–1.11 | 0.17 | 1.24 | 0.81–1.88 | 0.32 |
| N of organs with mets: >1 vs. 1 | 1.21 | 0.86–1.69 | 0.27 | 1.34 | 0.9–2 | 0.15 |
| Liver mets vs. no-liver mets | 0.9 | 0.59–1.37 | 0.62 | 1.53 | 0.87–2.69 | 0.14 |
| CEA: >nal vs. nal | 1.04 | 0.71–1.5 | 0.85 | 1.46 | 0.92–2.32 | 0.11 |
| RAS: MT vs. WT | 0.76 | 0.51–1.12 | 0.16 | 0.71 | 0.45–1.12 | 0.14 |
| B-RAF: MT vs. WT | 3.27 | 1.61–6.64 | 0.001 | 7.39 | 3.36–16.25 | <0.0001 |
| D0-D28 CTC kinetics (EPISPOT): Positive at both time points (≥1) vs. other cases | 2.52 | 1.15–5.52 | 0.0204 | 2.48 | 1.14–5.37 | 0.0219 |
| D0-D28 CTC kinetics (CellSearch®): Positive at both time points (≥3) vs. other cases | 3.02 | 1.45–6.3 | 0.0031 | 3.22 | 1.54–6.74 | 0.0019 |
Abbreviations: M, men; W, women; HR, hazard ratio; vs., versus; PS, performance status; nal, normal; mets, metastases; CEA, carcinoembryonic antigen; MT, mutated; WT, wild type; D, day; PFS, progression-free survival; OS, overall survival; CI, confidence interval.
In multivariate analysis, D0–D28 CTC kinetics according to the CK19-EPISPOT assay (HR 2.445, 95% CI (1.04–5.78), p = 0.0414) and the CellSearch® system (HR 2.461, 95% CI (1.06–5.74), p = 0.037) remained significant predictors of PFS but not of OS (Table 3).
Table 3.
Multivariate Cox regression analysis for PFS and OS prediction.
| Parameters | PFS | OS | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| ECOG PS: 1–2 vs. 0 | 2.48 | 1.51–4.07 | 0.0003 | |||
| B-RAF: MT vs. WT | 3.046 | 1.43–6.5 | 0.0043 | 5.34 | 2.23–12.79 | 0.0002 |
| D0–D28 CTC kinetics (EPISPOT): Positive at both time points (≥1) vs. other cases | 2.445 | 1.04–5.78 | 0.0414 | |||
| D0–D28 CTC kinetics (CellSearch®): Positive at both time points (≥3) vs. other cases | 2.461 | 1.06–5.74 | 0.037 | |||
Abbreviations: HR, hazard ratio; vs., versus; PS, performance status; MT, mutated; WT, wild type; D, day; PFS, progression-free survival; OS, overall survival; CI, confidence interval.
4. Discussion
More than a decade ago, it was shown that CTC enumeration is a prognostic factor in metastatic breast, prostate, and colorectal cancer [10,19,20]. In this field of expertise, it was then important to show the clinical validity of CTCs with meta-analyses of thousands of cancer patients [21] and, especially, to demonstrate their clinical utility for introducing them in daily clinical practice [22]. CTC clinical validity and utility have been reported for metastatic breast cancer; conversely, in CRCs, many key questions are still unanswered.
To determine whether viable CTCs are clinically relevant in patients with metastatic CRC as an early criterion of response to FOLFIRI–bevacizumab treatment, we performed a prospective multicenter study in which peripheral blood samples were tested before and during treatment, with two different CTC detection technologies: (i) the EPISPOT assay to detect viable CTCs, and (ii) the FDA-cleared CellSearch® system. We then determined whether the subpopulation of viable CTCs detected with the EPISPOT assay is clinically relevant for the prognosis and as an early biomarker to predict clinical outcomes after treatment initiation. We assessed the CTC count at different time points and different CTC cut-offs for the EPISPOT assay because this system is still under study. Conversely, on the basis of the work by Cohen et al., with the CellSearch® system, we only tested CTCs at D0 and D28 and considered only the cut-off of ≥3 CTCs [10].
The studied population is representative because their OS (26.2 months) and PFS (9.4 months) are consistent with previously reported data on unselected patients with metastatic CRC treated with FOLFIRI and bevacizumab [23]. The detection of viable CTCs could be assessed in most patients during their routine follow-up at 11 centers in France, demonstrating the feasibility of this technique in clinical practice. During treatment, we found significant correlations between survival and the presence of viable CTCs (threshold: ≥2 CTCs) at D28 (PFS and OS) and D42 (only OS). Moreover, the D0–D28 CTC kinetics predicted both PFS and OS and was an independent factor of PFS by multivariate analysis. This finding confirms the clinical interest of the CTC kinetic previously assessed with ISET technology [24] or other assays [25] for early detection of poor outcomes in patients with metastatic CRC under treatment. During the last decade, the EPISPOT assay’s prognostic value has already been demonstrated in advanced breast, prostate, and head and neck cancer as well as in melanoma and non-metastatic CRC [18,26,27,28]. The prognostic value of the early kinetics of viable CTCs has already been reported in recurrent and metastatic head and neck squamous cell carcinoma [28].
According to the CellSearch® system, 40% of patients had ≥3 CTCs, in line with previous studies (24–52% of untreated patients with metastatic CRC) [10,11,12,29,30]. We then confirmed that the CellSearch® system, using the conventional cut-off of 3 CTCs, provides prognostic information before and early after initiation of the first line of treatment. PFS and OS were significantly shorter in patients who became or remained positive (≥3 CTCs) after 4 weeks of chemotherapy (D28), demonstrating that they did not benefit from therapy.
Considering the detection of viable CTCs (EPISPOT), the number of positive patients was lower at baseline compared with the CellSearch® system, and it decreased during treatment. Thus, the low number of patients with unfavorable CTC evolution according to the EPISPOT assay is a limitation of our study. As already shown in previous studies [17,26,27,28], the concordance between EPISPOT and CellSearch® technologies for CTC detection was low at baseline and during treatment. This could be explained by the fact that the EPISPOT assay detects only CK19-releasing viable CTCs and not the others (e.g., apoptotic CTCs). Moreover, the enrichment and detection steps are different. The CellSearch® system uses positive selection based on EpCAMs to enrich CTCs, whereas the EPISPOT assay is combined with negative selection by leukocyte depletion. In the CellSearch® system, detection is based on Pan CK, DAPI, and CD45 staining of fixed CTCs. Conversely, the EPISPOT assay detects only CK19-releasing CTCs in culture. Despite this low agreement, the dynamic CTC count, which changes with both methods, remained significantly correlated with PFS in multivariate analysis, suggesting that these assays are complementary for predicting clinical outcomes during treatment. Interestingly, CTC positivity (≥3 cells) by CellSearch® is correlated with surrogate markers of tumor burden ([30,31] and the present study), but not the presence of viable CTCs. This might suggest that their predictive value is not directly linked to the tumor mass changes but more to the identification of an aggressive chemotherapy-resistant subpopulation of tumor cells that are certainly at the origin of cancer progression.
Currently, we are developing a new version of the EPISPOT assay, named EPIDROP (EPIspot in a DROP), that combines EPISPOT and CellSearch® strategies and might represent an ideal liquid biopsy tool. Indeed, with this new technology, we can detect the total amount of CTCs by immunostaining, as done by the CellSearch® system, and also the subset of viable CTCs on the basis of their ability to secrete, shed, or release some proteins. EPIDROP might also allow the use of a larger panel of CTC biomarkers, such as VEGF monitoring during bevacizumab therapy. This innovative technology should open new avenues to detect CTCs that are relevant as prognostic and early predictive information in metastatic CRC with high specificity and sensitivity.
5. Conclusions
The CK19-EPISPOT assay detects viable CTCs in metastatic CRC. This prospective study shows that real-time liquid biopsy for CTC analysis could be clinically relevant in this setting, particularly to monitor the early response to FOLFIRI–bevacizumab.
Acknowledgments
The authors express their gratitude to the patients who participated in this trial. They thank all the participating physicians, the supporting staff, especially Julie Duval and Anne Cadène, and Elisabetta Andermarcher for assistance with her comments and proofreading, which greatly improved the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13122966/s1, Table S1: Patient and tumor characteristics (n = 155), Table S2: CTC detection at each time point, Table S3: Prognostic value of CTCs according to the CK19-EPISPOT assay.
Author Contributions
Conceptualization, C.A.-P. and T.M.; Methodology, J.-P.D.; Validation, L.C., C.A.-P. and T.M.; Formal analysis, L.C. and F.P.; Data curation, L.C., T.M. and F.P.; Resources, H.S., B.L., C.d.l.F., E.T., E.F., S.O., R.G., L.M., M.F., M.Y., E.A. and T.M.; Writing—original draft preparation, L.C., C.A.-P. and T.M.; Writing—review and editing, L.C., E.A., H.S., B.L., C.d.l.F., E.T., E.F., S.O., R.G., L.M., M.F., M.Y., C.A.-P. and T.M.; Supervision, C.A.-P. and T.M.; Funding acquisition, C.A.-P. and T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Cancer Institute (INCa) “Recherche Translationnelle-Projet Libre 2011” and the General Direction for Caregiving (DGOS) for patient recruitment and analyses in the COLOSPOT study (NCT01596790). We also received financial support from F Hoffmann-La Roche Ltd., Basel, Switzerland. The LCCRH was also supported by a SIRIC Montpellier Cancer Grant (INCa_Inserm_DGOS_12553) for staff salary.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Ethics Committee Sud Méditerranée III (Ref: 2011.11.01).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in the main article and its supplementary material.
Conflicts of Interest
C.A.P. has received an honorarium from Menarini. T.M. discloses research funding from ROCHE and AMGEN; an honorarium from AMGEN, SANOFI, BMS, SANDOZ, and AAA; and travel, accommodations, and expenses paid by AMGEN. The other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Dyba T., Randi G., Bettio M., Gavin A., Visser O., Bray F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer. 2018;103:356–387. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fedewa S.A., Ahnen D.J., Meester R.G.S., Barzi A., Jemal A. Colorectal cancer statistics, 2017. CA Cancer J. Clin. 2017;67:177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 3.Benson A.B., Venook A.P., Al-Hawary M.M., Arain M.A., Chen Y.J., Ciombor K.K., Cohen S., Cooper H.S., Deming D., Farkas L., et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:329–359. doi: 10.6004/jnccn.2021.0012. [DOI] [PubMed] [Google Scholar]
- 4.Van Cutsem E., Cervantes A., Adam R., Sobrero A., van Krieken J., Aderka D., Aguilar E.A., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 5.Phelip J.M., Tougeron D., Léonard D., Benhaim L., Desolneux G., Dupré A., Michel P., Penna C., Tournigand C., Louvet C., et al. Metastatic colorectal cancer (mCRC): French intergroup clinical practice guidelines for diagnosis, treatments and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR) Dig. Liver Dis. 2019;51:1357–1363. doi: 10.1016/j.dld.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 6.Loupakis F., Ruzzo A., Cremolini C., Vincenzi B., Salvatore L., Santini D., Masi G., Stasi I., Canestrari E., Rulli E., et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br. J. Cancer. 2009;101:715–721. doi: 10.1038/sj.bjc.6605177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douillard J.-Y., Oliner K.S., Siena S., Tabernero J., Burkes R., Barugel M., Humblet Y., Bodoky G., Cunningham D., Jassem J., et al. Panitumumab–FOLFOX4 Treatment and RAS Mutations in Colorectal Cancer. N. Engl. J. Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 8.Cidon E.U., Alonso P., Masters B. Markers of Response to Antiangiogenic Therapies in Colorectal Cancer: Where are We Now and What should be Next? Clin. Med. Insights Oncol. 2016;10:CMO.S34542. doi: 10.4137/CMO.S34542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pantel K., Alix-Panabières C. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Cohen S.J., Punt C.J.A., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C., et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 11.Tol J., Koopman M., Miller M.C., Tibbe A., Cats A., Creemers G.J.M., Vos A.H., Nagtegaal I., Terstappen L.W.M.M., Punt C.J.A. Circulating tumour cells early predict progression-free and overall survival in advanced colorectal cancer patients treated with chemotherapy and targeted agents. Ann. Oncol. 2010;21:1006–1012. doi: 10.1093/annonc/mdp463. [DOI] [PubMed] [Google Scholar]
- 12.Sastre J., Maestro M.L., Gómez-España A., Rivera F., Valladares M., Massuti B., Benavides M., Gallen M., Marcuello E., Abad A., et al. Circulating Tumor Cell Count Is a Prognostic Factor in Metastatic Colorectal Cancer Patients Receiving First-Line Chemotherapy Plus Bevacizumab: A Spanish Cooperative Group for the Treatment of Digestive Tumors Study. Oncologist. 2012;17:947–955. doi: 10.1634/theoncologist.2012-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riethdorf S., Fritsche H., Müller V., Rau T., Schindlbeck C., Rack B., Janni W., Coith C., Beck K., Jänicke F., et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clin. Cancer Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 14.Mikolajczyk S.D., Millar L.S., Tsinberg P., Coutts S.M., Zomorrodi M., Pham T., Bischoff F.Z., Pircher T.J. Detection of EpCAM-Negative and Cytokeratin-Negative Circulating Tumor Cells in Peripheral Blood. J. Oncol. 2011;2011:252361. doi: 10.1155/2011/252361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serrano M.J., Ortega F.G., Cubero M.J.A., Nadal R., Sánchez F.G.O., Salido M., Rodríguez M., García-Puche J.L., Delgado-Rodriguez M., Solé F., et al. EMT and EGFR in CTCs cytokeratin negative non-metastatic breast cancer. Oncotarget. 2014;5:7486–7497. doi: 10.18632/oncotarget.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soler A., Cayrefourcq L., Mazel M., Alix-Panabières C. EpCAM-Independent Enrichment and Detection of Viable Circulating Tumor Cells Using the EPISPOT Assay. In: Magbanua M.J.M., Park J.W., editors. Circulating Tumor Cells. Volume 1634. Springer; New York, NY, USA: 2017. pp. 263–276. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez J.-M., Fehm T., Orsini M., Cayrefourcq L., Maudelonde T., Pantel K., Alix-Panabières C. Prognostic Relevance of Viable Circulating Tumor Cells Detected by EPISPOT in Metastatic Breast Cancer Patients. Clin. Chem. 2014;60:214–221. doi: 10.1373/clinchem.2013.215079. [DOI] [PubMed] [Google Scholar]
- 18.DeNeve E., Riethdorf S., Ramos J., Nocca D., Coffy A., Daurès J.-P., Maudelonde T., Fabre J.-M., Pantel K., Alix-Panabières C. Capture of Viable Circulating Tumor Cells in the Liver of Colorectal Cancer Patients. Clin. Chem. 2013;59:1384–1392. doi: 10.1373/clinchem.2013.202846. [DOI] [PubMed] [Google Scholar]
- 19.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W., et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 20.De Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K., Raghavan D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 21.Bidard F.-C., Peeters D.J., Fehm T., Nolé F., Gisbert-Criado R., Mavroudis D., Grisanti S., Generali D., Garcia-Saenz J.A., Stebbing J., et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: A pooled analysis of individual patient data. Lancet Oncol. 2014;15:406–414. doi: 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 22.Bidard F.-C., Jacot W., Kiavue N., Dureau S., Kadi A., Brain E., Bachelot T., Bourgeois H., Gonçalves A., Ladoire S., et al. Efficacy of Circulating Tumor Cell Count–Driven vs Clinician-Driven First-line Therapy Choice in Hormone Receptor–Positive, ERBB2-Negative Metastatic Breast Cancer. JAMA Oncol. 2021;7:34–41. doi: 10.1001/jamaoncol.2020.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cremolini C., Loupakis F., Antoniotti C., Lupi C., Sensi E., Lonardi S., Mezi S., Tomasello G., Ronzoni M., Zaniboni A., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall sur-vival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 24.E Silva V.S., Chinen L., Abdallah E.A., Damascena A., Paludo J., Chojniak R., Dettino A., Mello C.A.L., Alves V.S., Fanelli M.F. Early detection of poor outcome in patients with metastatic colorectal cancer: Tumor kinetics evaluated by circulating tumor cells. OncoTargets Ther. 2016;9:7503–7513. doi: 10.2147/OTT.S115268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C., Chen F., Wang S., Xiong B. Circulating Tumor Cells in Gastrointestinal Cancers: Current Status and Future Perspectives. Front. Oncol. 2019;9:1427. doi: 10.3389/fonc.2019.01427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuske A., Gorges T.M., Tennstedt P., Tiebel A.-K., Pompe R.S., Preißer F., Prues S., Mazel M., Markou A., Lianidou E., et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. Sci. Rep. 2016;6:39736. doi: 10.1038/srep39736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cayrefourcq L., De Roeck A., Garcia C., Stoebner P.-E., Fichel F., Garima F., Perriard F., Daures J.-P., Meunier L., Alix-Panabières C. S100-EPISPOT: A New Tool to Detect Viable Circulating Melanoma Cells. Cells. 2019;8:755. doi: 10.3390/cells8070755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garrel R., Mazel M., Perriard F., Vinches M., Cayrefourcq L., Guigay J., Digue L., Aubry K., Alfonsi M., Delord J.-P., et al. Circulating Tumor Cells as a Prognostic Factor in Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma: The CIRCUTEC Prospective Study. Clin. Chem. 2019;65:1267–1275. doi: 10.1373/clinchem.2019.305904. [DOI] [PubMed] [Google Scholar]
- 29.Krebs M., Renehan A., Backen A., Gollins S., Chau I., Hasan J., Valle J.W., Morris K., Beech J., Ashcroft L., et al. Circulating Tumor Cell Enumeration in a Phase II Trial of a Four-Drug Regimen in Advanced Colorectal Cancer. Clin. Color. Cancer. 2015;14:115–122.e2. doi: 10.1016/j.clcc.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 30.Sastre J., de la Orden V., Martínez A., Bando I., Balbín M., Bellosillo B., Palanca S., Gomez M.I.P., Mediero B., Llovet P., et al. Association Between Baseline Circulating Tumor Cells, Molecular Tumor Profiling, and Clinical Characteristics in a Large Cohort of Chemo-naïve Metastatic Colorectal Cancer Patients Prospectively Collected. Clin. Color. Cancer. 2020;19:e110–e116. doi: 10.1016/j.clcc.2020.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Kaifi J.T., Kunkel M., Dicker D.T., Joude J., E Allen J., Das A., Zhu J., Yang Z., E Sarwani N., Li G., et al. Circulating tumor cell levels are elevated in colorectal cancer patients with high tumor burden in the liver. Cancer Biol. Ther. 2015;16:690–698. doi: 10.1080/15384047.2015.1026508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the main article and its supplementary material.