Abstract
We conducted a prospective population-based cohort study to assess risk factors for infection, hospitalization, and death from SARS-CoV-2. The study comprised the people covered by the Health Service of Navarre, Spain. Sociodemographic variables and chronic conditions were obtained from electronic healthcare databases. Confirmed infections, hospitalizations, and deaths from SARS-CoV-2 were obtained from the enhanced epidemiological surveillance during the second SARS-CoV-2 epidemic surge (July–December 2020), in which diagnostic tests were widely available. Among 643,757 people, 5497 confirmed infections, 323 hospitalizations, 38 intensive care unit admissions, and 72 deaths from SARS-CoV-2 per 100,000 inhabitants were observed. A higher incidence of confirmed infection was associated with people aged 15–29 years, nursing home residents, healthcare workers, people born in Latin America or Africa, as well as in those diagnosed with diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD), chronic kidney disease, dementia, severe obesity, hypertension and functional dependence. The risk of hospitalization in the population was associated with males, higher age, nursing home residents, Latin American or African origin, and those diagnosed with immunodeficiency, diabetes, cardiovascular disease, COPD, asthma, kidney disease, cerebrovascular disease, cirrhosis, dementia, severe obesity, hypertension and functional dependence. The risk of death was associated with males, higher age, nursing home residents, Latin American origin, low income level, immunodeficiency, diabetes, cardiovascular disease, COPD, kidney disease, dementia, and functional dependence. This study supports the prioritization of the older population, nursing home residents, and people with chronic conditions and functional dependence for SARS-CoV-2 prevention and vaccination, and highlights the need for additional preventive support for immigrants.
Keywords: SARS-CoV-2 infection, COVID-19, cohort study, COVID-19 hospitalization, COVID-19 severity, mortality, risk factor, epidemiology, inequality, Spain
1. Introduction
SARS-CoV-2 has produced more than one epidemic surge of COVID-19 during 2020 in many countries [1]. Although COVID-19 is a mild condition in most individuals, it can be life threatening for others [2]. Knowing the risk factors for infection, hospitalization and death from COVID-19 in the population may be useful for addressing clinical management, preventive measures, and vaccination programs [3]. Many studies have reported the association of sociodemographic characteristics and pre-existing conditions with severe disease and mortality from COVID-19 in clinical series or epidemiological surveillance [4,5,6,7]. Other studies have compared the characteristics of positive and negative testers [8,9]. However, studies describing risk factors for COVID-19 outcomes in the general population are scarce [10,11,12], although they are necessary to assess the risk affecting individuals in the population.
Increased odds of sociodemographic characteristics and pre-existing conditions in patients with severe COVID-19 have been reported in the first epidemic surge [13,14,15,16,17]. The low sensitivity in detecting very early cases and the limited availability of diagnostic tests in the first epidemic surge could lead to a non-representative view of the COVID-19 outcomes in the population. Between July and December 2020, there was a second epidemic surge of SARS-CoV-2 in Europe [1]. The analysis of this surge may provide a less biased view given the improvement in diagnosing cases regardless of severity and that incidence had not yet been affected by vaccination.
The current study aimed to evaluate sociodemographic characteristics, chronic conditions and health-related variables as independent risk factors for confirmed infection, hospitalization, intensive care unit admission, and death from SARS-CoV-2 in the second epidemic surge. As the World Health Organization has proposed priority groups for vaccination that include nursing home residents, functional dependents, older age groups and individuals with certain chronic conditions [3], we also aimed to evaluate these prioritizations in the study population.
2. Materials and Methods
2.1. Study Design and Setting
A prospective population-based cohort study was performed in Navarre, Spain, where the Health Service provides universal healthcare, free at the point of service. During the second SARS-CoV-2 epidemic surge, the wide availability of tests allowed the testing not only of all symptomatic patients and of close contacts of cases regardless of symptoms, but also the screening of population groups in specific circumstances.
The cohort included people covered by the Navarre Health Service at least from July 2019, as well as children born in Navarre after this date, so we ensured that basic medical records were available for each person. The period for prospective detection of SARS-CoV-2 infections was defined from July to December 2020. Hospitalizations and deaths from SARS-CoV-2 infections were considered in a follow-up period of 30 days after infection diagnosis. People who had been confirmed for SARS-CoV-2 infection before July 2020 were removed from the cohort.
2.2. Variables
The outcomes of interest were SARS-CoV-2 confirmed infection, hospitalization, intensive care unit admission and death.
Confirmed cases were defined as patients who tested positive for SARS-CoV-2 by commercial tests based on reverse transcription quantitative real-time polymerase chain reaction or antigen test in a respiratory tract sample. The antigen test was used in symptomatic patients within 5 days of the COVID-19 symptom onset [18].
COVID-19 hospitalized cases included those admitted for 24 h or more and those who died in the emergency room before admission. Deaths were obtained from electronic medical records and the mortality registry. As part of the epidemiological surveillance, medical doctors reviewed hospital admissions and deaths to identify those related to COVID-19, and only those were considered for the present study.
Sociodemographic characteristics, chronic conditions and other health-related variables at baseline were obtained from the electronic medical records. This source of information has demonstrated high sensitivity and specificity to detect chronic medical conditions [19].
Sociodemographic variables included sex, age group (0–14, 15–29, 30–49, 50–59, 60–69, 70–79 and ≥80 years old), nursing home residence, healthcare work, place of birth (Spain, Europe, Latin America, North Africa, sub-Saharan Africa, and others), place of residence (<5000, 5000–50,000, and >50,000 inhabitants), and annual taxable income level in four categories.
Major chronic conditions considered were: immunodeficiency (primary immunodeficiency, HIV infection or transplant recipient), diabetes, cardiovascular disease, chronic obstructive pulmonary disease (COPD), asthma, chronic kidney disease, cerebrovascular disease, liver cirrhosis, dementia, hematological malignancy, non-hematological cancer, severe obesity (body mass index ≥ 40 kg/m2), and hypertension. The lack of registered diagnosis of chronic disease was considered as not having that condition.
From the electronic medical records, we also obtained the history of hospitalization in the prior 12 months, the smoking status (non-smoker, former smoker, current smoker, and unknown), and the functional dependence (Barthel’s index <40) [20].
2.3. Statistical Analysis
The database was anonymized before the analysis. The cumulative incidence of SARS-CoV-2 confirmed infection, hospitalization, intensive care unit admission, and death per 100,000 inhabitants was calculated for each category of the analyzed variables. Poisson regression models were used to assess the independent effect of each variable for the analyzed outcomes. For every variable, the sex- and age-adjusted relative risk (RR) and the fully adjusted RR with their 95% confidence intervals (CI) were calculated. p-values < 0.05 were considered statistically significant.
The population was categorized in hierarchical categories for COVID-19 vaccination priority in the following order: nursing home residents, functional dependents, and age groups starting from the oldest and split into two categories according to the presence or not of any major chronic condition. The proportion and the risk of each COVID-19 outcome were calculated in each category.
2.4. Ethical Aspects
This study was approved by the Ethical Committee for Clinical Research of Navarre, which waived the requirement of obtaining informed consent (approval code: PI2020/45).
3. Results
3.1. Cumulative Incidence by Population Characteristics
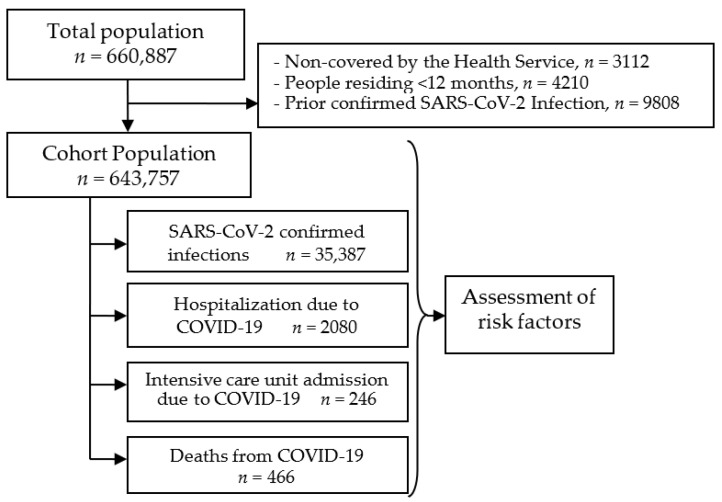
The cohort included 643,757 people: 35,387 of them were confirmed for SARS-CoV-2 infection in the study period, 2080 were hospitalized, 246 were admitted to the intensive care unit, and 466 died from COVID-19 (Figure 1). These figures supposed cumulative incidences of 5497, 323, 38, and 72 per 100,000 inhabitants, respectively. The infections confirmed in the study period were 72% of all SARS-CoV-2 infections confirmed during the first 12 months of the pandemic.
Figure 1.
Scheme of the study.
The cumulative incidence of SARS-CoV-2 infection was high in all population groups, ranging from 3.6% in people aged 70–79 years to 13.8% in nursing home residents, followed by people born in Latin America (11.2%) or North Africa (7.6%), people with dementia (7.4%) and functional dependence (7.4%), and people aged 15–29 years (7.6%) (Table 1).
Table 1.
Association between potential predictive factors and confirmed SARS-CoV-2 infection in the general population cohort.
| Infections | Sex- and Age-Adjusted Analysis | Fully Adjusted Analysis * | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Cases per 100,000 | RR | 95% CI | p Value | RR | 95% CI | p Value | |
| Total | 35,387 | 5497 | ||||||
| Sex | ||||||||
| Female | 18,215 | 5609 | 1 | 1 | ||||
| Male | 17,172 | 5383 | 0.95 | 0.93–0.97 | <0.001 | 0.98 | 0.96–1.00 | 0.078 |
| Age, years | ||||||||
| 0–14 | 5625 | 5457 | 0.99 | 0.95–1.02 | 0.441 | 1.01 | 0.97–1.05 | 0.526 |
| 15–29 | 7640 | 7611 | 1.37 | 1.33–1.42 | <0.001 | 1.28 | 1.24–1.33 | <0.001 |
| 30–49 | 10,248 | 5544 | 1.00 | 0.97–1.03 | 0.976 | 0.96 | 0.93–0.99 | 0.017 |
| 50–59 | 5187 | 5541 | 1 | 1 | ||||
| 60–69 | 2986 | 4204 | 0.76 | 0.72–0.79 | <0.001 | 0.75 | 0.72–0.79 | <0.001 |
| 70–79 | 1899 | 3557 | 0.64 | 0.61–0.68 | <0.001 | 0.59 | 0.56–0.62 | <0.001 |
| 80+ | 1802 | 4818 | 0.86 | 0.82–0.91 | <0.001 | 0.64 | 0.59–0.68 | <0.001 |
| Nursing home resident | 681 | 13,830 | 3.28 | 3.02–3.55 | <0.001 | 3.24 | 2.98–3.53 | <0.001 |
| Healthcare worker | 692 | 6290 | 1.11 | 1.03–1.20 | 0.005 | 1.23 | 1.14–1.33 | <0.001 |
| Place of birth | ||||||||
| Spain | 26,779 | 4959 | 1 | 1 | ||||
| Europe | 1049 | 4114 | 0.80 | 0.75–0.85 | <0.001 | 0.81 | 0.76–0.86 | <0.001 |
| Latin America | 5738 | 11,175 | 2.11 | 2.04–2.17 | <0.001 | 2.08 | 2.01–2.14 | <0.001 |
| North Africa | 1213 | 7586 | 1.45 | 1.36–1.53 | <0.001 | 1.44 | 1.36–1.53 | <0.001 |
| Sub-Saharan Africa | 459 | 6387 | 1.23 | 1.13–1.35 | <0.001 | 1.21 | 1.10–1.32 | <0.001 |
| Other | 149 | 3951 | 0.75 | 0.64–0.88 | 0.001 | 0.75 | 0.64–0.88 | 0.001 |
| Place of residence | ||||||||
| >50,000 inhabitants | 11,249 | 5548 | 1.06 | 1.03–1.09 | <0.001 | 1.01 | 0.99–1.04 | 0.355 |
| 5000–50,000 inhabitants | 12,711 | 5708 | 1.07 | 1.05–1.10 | <0.001 | 1.04 | 1.02–1.07 | 0.001 |
| <5000 inhabitants | 11,427 | 5234 | 1 | 1 | ||||
| Income level | ||||||||
| Very low | 1734 | 6201 | 1.16 | 1.10–1.22 | <0.001 | 1.00 | 0.95–1.05 | 0.929 |
| Low | 20,437 | 5760 | 1.10 | 1.08–1.13 | <0.001 | 0.99 | 0.97–1.02 | 0.521 |
| Middle | 12,983 | 5064 | 1 | 1 | ||||
| High | 233 | 5080 | 0.98 | 0.86–1.11 | 0.747 | 0.99 | 0.87–1.13 | 0.922 |
| Smoking status | ||||||||
| Never smoker | 3191 | 3884 | 1 | 1 | ||||
| Current smoker | 6119 | 5788 | 0.63 | 0.60–0.66 | <0.001 | 0.67 | 0.64–0.70 | <0.001 |
| Former smoker | 1235 | 5136 | 0.98 | 0.92–1.04 | 0.445 | 1.01 | 0.95–1.07 | 0.785 |
| Unknown | 24,842 | 5752 | 0.88 | 0.85–0.90 | <0.001 | 0.87 | 0.85–0.90 | <0.001 |
| Hospitalization in prior year | 1917 | 5718 | 1.13 | 1.08–1.18 | <0.001 | 1.09 | 1.04–1.14 | 0.001 |
| Immunodeficiency | 267 | 5501 | 1.04 | 0.93–1.18 | 0.487 | 1.00 | 0.89–1.13 | 0.984 |
| Diabetes | 1893 | 4992 | 1.14 | 1.08–1.19 | <0.001 | 1.06 | 1.01–1.11 | 0.024 |
| Cardiovascular disease | 2736 | 5216 | 1.07 | 1.03–1.12 | 0.001 | 1.08 | 1.03–1.12 | <0.001 |
| COPD | 1404 | 5074 | 1.04 | 0.99–1.10 | 0.112 | 1.10 | 1.04–1.16 | 0.001 |
| Asthma | 2330 | 5535 | 0.97 | 0.93–1.01 | 0.162 | 1.00 | 0.96–1.04 | 0.969 |
| Chronic kidney disease | 989 | 5130 | 1.16 | 1.08–1.24 | <0.001 | 1.11 | 1.04–1.19 | 0.002 |
| Cerebrovascular disease | 470 | 4868 | 1.10 | 1.00–1.21 | 0.048 | 0.99 | 0.90–1.09 | 0.841 |
| Liver cirrhosis | 632 | 5244 | 1.11 | 1.03–1.21 | 0.008 | 1.06 | 0.98–1.15 | 0.127 |
| Dementia | 369 | 7420 | 1.72 | 1.54–1.92 | <0.001 | 1.25 | 1.11–1.40 | <0.001 |
| Hematological malignancy | 110 | 4073 | 0.85 | 0.70–1.02 | 0.087 | 0.87 | 0.72–1.05 | 0.139 |
| Non-hematological cancer | 1695 | 4363 | 0.96 | 0.91–1.01 | 0.090 | 0.98 | 0.93–1.03 | 0.454 |
| Severe obesity | 527 | 6295 | 1.24 | 1.13–1.35 | <0.001 | 1.18 | 1.08–1.29 | <0.001 |
| Hypertension | 4543 | 4666 | 1.07 | 1.03–1.12 | <0.001 | 1.05 | 1.01–1.09 | 0.013 |
| Functional dependence | 339 | 7399 | 1.65 | 1.48–1.85 | <0.001 | 1.22 | 1.08–1.38 | 0.001 |
COPD, chronic obstructive pulmonary diseases; RR, relative risk; CI, confidence interval, * Adjusted for all the variables in the table.
The cumulative incidence of hospitalization, intensive care unit admission and death by COVID-19 showed important differences among population groups. The highest risk of hospitalization was observed in nursing home residents (3.3%), followed by people with functional dependence (2.5%), dementia (2.2%), or aged 80 years and older (1.5%). The highest risk of intensive care unit admission was observed in people with severe obesity (191 per 100,000), liver cirrhosis (133 per 100,000), and aged 70–79 years (127 per 100,000). The highest risk of mortality from COVID-19 was found in nursing home residents (2.3%), functional dependents (2.1%), and persons with dementia (1.7%) or aged 80 years and over (0.9%).
3.2. Predictive Factors for Infection, Hospitalization and Severe Outcomes
The fully adjusted RR of SARS-CoV-2 confirmed infection in the population was significantly higher in people aged 15–29 years, nursing home residents, healthcare workers, people born in Latin America, North Africa or sub-Saharan Africa, people residing in municipalities of 5000–50,000 inhabitants, as well as in those diagnosed with diabetes, cardiovascular disease, COPD, chronic kidney disease, dementia, severe obesity, hypertension and functional dependence (Table 1).
Hospitalization with COVID-19 in the population was independently associated with males, higher age, nursing home residents, people born in Latin America, North Africa or sub-Saharan Africa, those with very low income level, residence in municipalities >5000 inhabitants and hospitalization in the prior 12 months, as well as with people diagnosed with immunodeficiency, diabetes, cardiovascular disease, COPD, asthma, chronic kidney disease, cerebrovascular disease, liver cirrhosis, dementia, severe obesity, hypertension and functional dependence (Table 2).
Table 2.
Association between potential predictive factors and COVID-19 hospitalization in the general population cohort.
| Hospitalizations | Sex- and Age-Adjusted Analysis | Fully Adjusted Analysis * | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Cases per 100,000 | RR | 95% CI | p Value | RR | 95% CI | p Value | |
| Total | 2080 | 323 | ||||||
| Sex | ||||||||
| Female | 1000 | 308 | 1 | 1 | ||||
| Male | 1080 | 339 | 1.27 | 1.16–1.38 | <0.001 | 1.32 | 1.21–1.45 | <0.001 |
| Age, years | ||||||||
| 0–14 | 24 | 23 | 0.06 | 0.04–0.09 | <0.001 | 0.07 | 0.04–0.10 | <0.001 |
| 15–29 | 48 | 48 | 0.12 | 0.09–0.17 | <0.001 | 0.11 | 0.08–0.15 | <0.001 |
| 30–49 | 368 | 199 | 0.51 | 0.44–0.59 | <0.001 | 0.48 | 0.41–0.55 | <0.001 |
| 50–59 | 365 | 390 | 1 | 1 | ||||
| 60–69 | 353 | 497 | 1.28 | 1.10–1.48 | 0.001 | 1.25 | 1.07–1.45 | 0.004 |
| 70–79 | 365 | 684 | 1.77 | 1.53–2.05 | <0.001 | 1.49 | 1.27–1.75 | <0.001 |
| 80+ | 557 | 1489 | 3.95 | 3.46–4.51 | <0.001 | 2.42 | 2.04–2.87 | <0.001 |
| Nursing home resident | 162 | 3290 | 3.56 | 3.00–4.22 | <0.001 | 3.23 | 2.69–3.88 | <0.001 |
| Healthcare worker | 22 | 200 | 0.76 | 0.50–1.16 | 0.199 | 0.98 | 0.64–1.51 | 0.936 |
| Place of birth | ||||||||
| Spain | 1640 | 304 | 1 | 1 | ||||
| Europe | 62 | 243 | 1.30 | 1.01–1.69 | 0.043 | 1.27 | 0.98–1.64 | 0.075 |
| Latin America | 296 | 576 | 3.70 | 3.24–4.23 | <0.001 | 3.47 | 3.02–3.99 | <0.001 |
| North Africa | 53 | 331 | 2.22 | 1.68–2.94 | <0.001 | 2.17 | 1.63–2.89 | <0.001 |
| Sub-Saharan Africa | 21 | 292 | 1.86 | 1.20–2.87 | 0.005 | 1.63 | 1.05–2.54 | 0.029 |
| Other | 8 | 212 | 1.30 | 0.65–2.60 | 0.463 | 1.28 | 0.64–2.57 | 0.489 |
| Place of residence | ||||||||
| >50,000 inhabitants | 723 | 357 | 1.17 | 1.05–1.30 | 0.004 | 1.14 | 1.02–1.27 | 0.019 |
| 5000–50,000 inhabitants | 695 | 312 | 1.20 | 1.08–1.34 | 0.001 | 1.16 | 1.04–1.29 | 0.007 |
| <5000 inhabitants | 662 | 303 | 1 | 1 | ||||
| Income level | ||||||||
| Very low | 102 | 365 | 2.04 | 1.66–2.52 | <0.001 | 1.27 | 1.02–1.58 | 0.034 |
| Low | 1288 | 363 | 1.28 | 1.16–1.41 | <0.001 | 1.05 | 0.95–1.16 | 0.372 |
| Middle | 677 | 264 | 1 | 1 | ||||
| High | 13 | 283 | 1.08 | 0.62–1.86 | 0.796 | 1.11 | 0.64–1.92 | 0.715 |
| Smoking status | ||||||||
| Never smoker | 191 | 233 | 1 | 1 | ||||
| Current smoker | 611 | 578 | 0.54 | 0.45–0.64 | <0.001 | 0.54 | 0.46–0.65 | <0.001 |
| Former smoker | 181 | 753 | 1.05 | 0.89–1.24 | 0.580 | 1.02 | 0.86–1.21 | 0.798 |
| Unknown | 1097 | 254 | 0.86 | 0.77–0.96 | 0.006 | 0.84 | 0.76–0.94 | 0.002 |
| Hospitalization in prior year | 243 | 725 | 1.52 | 1.33–1.74 | <0.001 | 1.28 | 1.11–1.47 | 0.001 |
| Immunodeficiency | 36 | 742 | 2.04 | 1.47–2.84 | <0.001 | 1.67 | 1.20–2.32 | 0.003 |
| Diabetes | 408 | 1076 | 1.61 | 1.43–1.80 | <0.001 | 1.33 | 1.18–1.49 | <0.001 |
| Cardiovascular disease | 411 | 784 | 1.33 | 1.19–1.50 | <0.001 | 1.18 | 1.05–1.33 | 0.007 |
| COPD | 195 | 705 | 1.29 | 1.11–1.50 | 0.001 | 1.30 | 1.11–1.51 | 0.001 |
| Asthma | 147 | 349 | 1.29 | 1.09–1.53 | 0.003 | 1.27 | 1.07–1.50 | 0.006 |
| Chronic kidney disease | 275 | 1426 | 1.65 | 1.43–1.89 | <0.001 | 1.41 | 1.23–1.63 | <0.001 |
| Cerebrovascular disease | 135 | 1398 | 1.58 | 1.32–1.89 | <0.001 | 1.27 | 1.06–1.52 | 0.011 |
| Liver cirrhosis | 105 | 871 | 1.66 | 1.36–2.02 | <0.001 | 1.42 | 1.17–1.74 | 0.001 |
| Dementia | 108 | 2172 | 1.89 | 1.54–2.32 | <0.001 | 1.28 | 1.02–1.59 | 0.032 |
| Hematological malignancy | 24 | 889 | 1.40 | 0.94–2.10 | 0.099 | 1.38 | 0.92–2.06 | 0.119 |
| Non-hematological cancer | 255 | 656 | 0.97 | 0.85–1.11 | 0.651 | 0.96 | 0.84–1.11 | 0.605 |
| Severe obesity | 79 | 944 | 2.20 | 1.75–2.75 | <0.001 | 1.79 | 1.42–2.25 | <0.001 |
| Hypertension | 840 | 863 | 1.27 | 1.15–1.41 | <0.001 | 1.11 | 1.01–1.25 | 0.040 |
| Functional dependence | 116 | 2532 | 2.28 | 1.87–2.79 | <0.001 | 1.54 | 1.24–1.91 | <0.001 |
COPD, chronic obstructive pulmonary diseases; RR, relative risk; CI, confidence interval; *Adjusted for all the variables in the table.
The fully adjusted RR of intensive care unit admission for COVID-19 in the population was statistically significantly higher in males, older age up to 70–79 years, people born in Latin America or North Africa, people residing in municipalities of 5000–50,000 inhabitants, and those diagnosed with asthma, severe obesity and hypertension (Table 3).
Table 3.
Association between potential predictive factors and intensive care unit admission for COVID-19 in the general population cohort.
| Intensive Care Unit Admissions | Sex- and Age-Adjusted Analysis | Fully Adjusted Analysis * | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Cases per 100,000 | RR | 95% CI | p Value | RR | 95% CI | p Value | |
| Total | 246 | 38 | ||||||
| Sex | ||||||||
| Female | 92 | 28 | 1 | 1 | ||||
| Male | 154 | 48 | 1.79 | 1.38–2.31 | <0.001 | 2.02 | 1.53–2.66 | <0.001 |
| Age, years | ||||||||
| 0–14 | 1 | 1 | 0.02 | 0.00–0.11 | <0.001 | 0.02 | 0–0.14 | <0.001 |
| 15–29 | 2 | 2 | 0.03 | 0.01–0.13 | <0.001 | 0.03 | 0.01–0.11 | <0.001 |
| 30–49 | 30 | 16 | 0.26 | 0.17–0.40 | <0.001 | 0.23 | 0.15–0.37 | <0.001 |
| 50–59 | 59 | 63 | 1 | 1 | ||||
| 60–69 | 72 | 101 | 1.62 | 1.15–2.29 | 0.006 | 1.73 | 1.21–2.46 | 0.003 |
| 70–79 | 68 | 127 | 2.07 | 1.46–2.93 | <0.001 | 2.21 | 1.49–3.29 | <0.001 |
| 80+ | 14 | 37 | 0.64 | 0.36–1.15 | 0.139 | 0.72 | 0.37–1.38 | 0.320 |
| Nursing home resident | 4 | 81 | 1.47 | 0.54–4.01 | 0.455 | 2.07 | 0.75–5.74 | 0.161 |
| Healthcare worker | 4 | 36 | 1.10 | 0.41–2.99 | 0.850 | 1.55 | 0.56–4.23 | 0.397 |
| Place of birth | ||||||||
| Spain | 175 | 32 | 1 | 1 | ||||
| Europe | 7 | 27 | 1.31 | 0.61–2.80 | 0.491 | 1.24 | 0.57–2.67 | 0.588 |
| Latin America | 55 | 107 | 6.73 | 4.88–9.30 | <0.001 | 6.15 | 4.34–8.72 | <0.001 |
| North Africa | 7 | 44 | 2.84 | 1.32–6.10 | 0.008 | 2.88 | 1.30–6.36 | 0.009 |
| Sub-Saharan Africa | 2 | 28 | 1.67 | 0.41–6.80 | 0.472 | 1.38 | 0.34–5.70 | 0.654 |
| Other | 0 | 0 | NE | NE | ||||
| Place of residence | ||||||||
| >50,000 inhabitants | 84 | 41 | 1.55 | 1.11–2.17 | 0.010 | 1.39 | 0.99–1.95 | 0.061 |
| 5000–50,000 inhabitants | 104 | 47 | 1.97 | 1.43–2.71 | <0.001 | 1.80 | 1.30–2.50 | <0.001 |
| <5000 inhabitants | 58 | 27 | 1 | 1 | ||||
| Income level | ||||||||
| Very low | 17 | 61 | 2.79 | 1.66–4.70 | <0.001 | 1.49 | 0.85–2.61 | 0.162 |
| Low | 131 | 37 | 1.21 | 0.93–1.59 | 0.158 | 0.96 | 0.72–1.27 | 0.755 |
| Middle | 96 | 37 | 1 | 1 | ||||
| High | 2 | 44 | 1.08 | 0.27–4.38 | 0.917 | 1.15 | 0.28–4.67 | 0.846 |
| Smoking status | ||||||||
| Never smoker | 30 | 37 | 1 | 1 | ||||
| Current smoker | 67 | 63 | 0.50 | 0.32–0.77 | 0.002 | 0.57 | 0.36–0.89 | 0.014 |
| Former smoker | 27 | 112 | 0.97 | 0.62–1.53 | 0.895 | 1.01 | 0.64–1.60 | 0.961 |
| Unknown | 122 | 28 | 0.72 | 0.52–0.98 | 0.037 | 0.77 | 0.56–1.05 | 0.099 |
| Hospitalization in prior year | 16 | 48 | 0.89 | 0.54–1.49 | 0.662 | 0.84 | 0.50–1.41 | 0.516 |
| Immunodeficiency | 5 | 103 | 1.93 | 0.80–4.69 | 0.145 | 1.66 | 0.68–4.06 | 0.267 |
| Diabetes | 46 | 121 | 1.56 | 1.11–2.17 | 0.009 | 1.21 | 0.86–1.72 | 0.276 |
| Cardiovascular disease | 33 | 63 | 1.00 | 0.69–1.47 | 0.988 | 0.90 | 0.61–1.33 | 0.595 |
| COPD | 22 | 80 | 1.14 | 0.73–1.78 | 0.559 | 1.22 | 0.78–1.92 | 0.386 |
| Asthma | 23 | 55 | 1.94 | 1.26–2.99 | 0.003 | 1.84 | 1.19–2.83 | 0.006 |
| Chronic kidney disease | 22 | 114 | 1.70 | 1.07–2.68 | 0.025 | 1.49 | 0.94–2.39 | 0.093 |
| Cerebrovascular disease | 7 | 73 | 0.89 | 0.42–1.91 | 0.774 | 0.85 | 0.40–1.83 | 0.679 |
| Liver cirrhosis | 16 | 133 | 1.72 | 1.03–2.86 | 0.037 | 1.43 | 0.85–2.39 | 0.173 |
| Dementia | 0 | 0 | NE | NE | ||||
| Hematological malignancy | 1 | 37 | 0.52 | 0.07–3.72 | 0.516 | 0.55 | 0.08–3.91 | 0.548 |
| Non-hematological cancer | 29 | 75 | 0.87 | 0.59–1.30 | 0.506 | 0.92 | 0.61–1.37 | 0.673 |
| Severe obesity | 16 | 191 | 3.69 | 2.22–6.13 | <0.001 | 3.05 | 1.81–5.14 | <0.001 |
| Hypertension | 100 | 103 | 1.53 | 1.15–2.03 | 0.003 | 1.36 | 1.01–1.83 | 0.041 |
| Functional dependence | 1 | 22 | 0.42 | 0.06–3.05 | 0.392 | 0.52 | 0.07–3.81 | 0.520 |
COPD, chronic obstructive pulmonary diseases; NE, no events; RR, relative risk; CI, confidence interval; *Adjusted for all the variables in the table.
An increased risk of death from COVID-19 in the population was independently observed in males, higher ages, nursing home residents, people born in Latin America, those with very low and low incomes, and those hospitalized in the prior 12 months, as well as in people with immunodeficiency, diabetes, cardiovascular disease, COPD, chronic kidney disease, dementia and functional dependence (Table 4).
Table 4.
Association between potential predictive factors and death from COVID-19 in the general population cohort.
| Deaths | Sex- and Age-Adjusted Analysis | Fully Adjusted Analysis * | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Cases per 100,000 | RR | 95% CI | p Value | RR | 95% CI | p Value | |
| Total | 466 | 72 | ||||||
| Sex | ||||||||
| Female | 240 | 74 | 1 | 1 | ||||
| Male | 226 | 71 | 1.42 | 1.19–1.71 | <0.001 | 1.61 | 1.31–1.97 | <0.001 |
| Age, years | ||||||||
| 0–29 | 0 | 0 | NE | NE | ||||
| 30-49 | 2 | 1 | 0.06 | 0.01-0.28 | <0.001 | 0.06 | 0.01-0.27 | <0.001 |
| 50-59 | 16 | 17 | 1 | 1 | ||||
| 60–69 | 32 | 45 | 2.65 | 1.45–4.83 | 0.002 | 2.44 | 1.33–4.48 | 0.004 |
| 70–79 | 72 | 135 | 8.00 | 4.65–13.75 | <0.001 | 5.88 | 3.34–10.34 | <0.001 |
| 80+ | 344 | 920 | 56.53 | 34.22–93.37 | <0.001 | 24.43 | 14.12–42.29 | <0.001 |
| Nursing home resident | 112 | 2275 | 5.30 | 4.25–6.62 | <0.001 | 4.19 | 3.28–5.36 | <0.001 |
| Healthcare worker | 0 | 0 | NE | NE | ||||
| Place of birth | ||||||||
| Spain | 444 | 82 | 1 | 1 | ||||
| Europe | 1 | 4 | 0.25 | 0.04–1.80 | 0.169 | 0.23 | 0.03–1.67 | 0.148 |
| Latin America | 16 | 31 | 2.64 | 1.59–4.40 | <0.001 | 2.57 | 1.52–4.36 | 0.001 |
| North Africa | 3 | 19 | 1.96 | 0.63–6.14 | 0.247 | 2.03 | 0.64–6.43 | 0.230 |
| Sub-Saharan Africa | 2 | 28 | 3.96 | 0.97–16.09 | 0.055 | 3.41 | 0.82–14.08 | 0.090 |
| Other | 0 | 0 | NE | NE | ||||
| Place of residence | ||||||||
| >50,000 inhabitants | 147 | 72 | 0.87 | 0.70–1.08 | 0.203 | 1.00 | 0.80–1.25 | 0.988 |
| 5000–50,000 inhabitants | 135 | 61 | 1.06 | 0.85–1.32 | 0.611 | 1.07 | 0.86–1.34 | 0.537 |
| <5000 inhabitants | 184 | 84 | 1 | 1 | ||||
| Income level | ||||||||
| Very low | 18 | 64 | 3.52 | 2.12–5.86 | <0.001 | 1.95 | 1.15–3.32 | 0.013 |
| Low | 352 | 99 | 1.66 | 1.31–2.10 | <0.001 | 1.35 | 1.06–1.72 | 0.016 |
| Middle | 95 | 37 | 1 | 1 | ||||
| High | 1 | 22 | 0.65 | 0.09–4.68 | 0.671 | 0.67 | 0.09–4.79 | 0.687 |
| Smoking status | ||||||||
| Never smoker | 29 | 35 | 1 | 1 | ||||
| Current smoker | 200 | 189 | 0.77 | 0.51–1.15 | 0.202 | 0.67 | 0.44–1.01 | 0.058 |
| Former smoker | 47 | 195 | 1.08 | 0.78–1.51 | 0.643 | 1.03 | 0.74–1.44 | 0.852 |
| Unknown | 190 | 44 | 0.97 | 0.79–1.18 | 0.741 | 0.80 | 0.65–0.99 | 0.039 |
| Hospitalization in prior year | 88 | 262 | 1.72 | 1.36–2.17 | <0.001 | 1.30 | 1.02–1.65 | 0.034 |
| Immunodeficiency | 8 | 165 | 2.74 | 1.36–5.52 | 0.005 | 2.22 | 1.10–4.48 | 0.027 |
| Diabetes | 143 | 377 | 1.58 | 1.29–1.92 | <0.001 | 1.29 | 1.05–1.58 | 0.014 |
| Cardiovascular disease | 172 | 328 | 1.52 | 1.25–1.84 | <0.001 | 1.33 | 1.09–1.63 | 0.004 |
| COPD | 69 | 249 | 1.58 | 1.22–2.05 | 0.001 | 1.47 | 1.12–1.91 | 0.005 |
| Asthma | 28 | 67 | 1.05 | 0.72–1.54 | 0.796 | 1.03 | 0.70–1.51 | 0.886 |
| Chronic kidney disease | 134 | 695 | 1.73 | 1.41–1.13 | <0.001 | 1.48 | 1.20–1.83 | <0.001 |
| Cerebrovascular disease | 56 | 580 | 1.45 | 1.09–1.92 | 0.010 | 1.04 | 0.78–1.38 | 0.803 |
| Liver cirrhosis | 22 | 183 | 1.52 | 0.99–2.34 | 0.056 | 1.37 | 0.89–2.11 | 0.156 |
| Dementia | 83 | 1669 | 2.89 | 2.26–3.69 | <0.001 | 1.56 | 1.19–2.04 | 0.002 |
| Hematological malignancy | 9 | 333 | 1.54 | 0.80–2.98 | 0.201 | 1.59 | 0.82–3.09 | 0.167 |
| Non-hematological cancer | 64 | 165 | 0.72 | 0.55–0.93 | 0.014 | 0.72 | 0.55–0.94 | 0.014 |
| Severe obesity | 10 | 119 | 1.24 | 0.66–2.33 | 0.497 | 0.88 | 0.47–1.66 | 0.701 |
| Hypertension | 314 | 322 | 1.36 | 1.11–1.66 | 0.003 | 1.23 | 1.00–1.51 | 0.055 |
| Functional dependence | 95 | 2073 | 3.77 | 2.98–4.76 | <0.001 | 2.24 | 1.72–2.90 | <0.001 |
COPD, chronic obstructive pulmonary diseases; NE, no events; RR, relative risk; CI, confidence interval; * Adjusted for all the variables in the table.
Current smokers, but not former smokers, had a significantly lower risk of SARS-CoV-2 confirmed infection, hospitalization, and intensive care unit admission for COVID-19.
3.3. Assessing Priority Groups for Vaccination
Regardless of other variables, nursing home residents and functional dependents presented the highest risks of COVID-19 hospitalization and death. Outside of these groups, aging was associated with an increased risk of hospitalization and death. In every age group, people with major chronic conditions had a higher risk of hospitalization and death. For some age groups, the presence of major chronic conditions increased the risk more than being 10 years older (Table 5). The vaccination of nursing home residents, people with functional dependence and people aged 80 years and over will cover the population groups in which 79% of deaths by COVID-19 occurred, but only those that give rise to 31% of hospitalizations and 8% of intensive care unit admissions. Extending vaccination to all people aged 50 years and over will cover the population in which 79% of hospitalizations, 87% of intensive care unit admissions and 99% of deaths from COVID-19 occurred (Table 5).
Table 5.
Hospitalization, intensive care unit admission and deaths from COVID-19 in hierarchical categories for COVID-19 vaccination priority in the general population cohort (n = 643,757). Figures presented are the number, proportion (%) of all events and events per 100,000 inhabitants.
| COVID-19 Hospitalization | Intensive Care Unit Admission by COVID-19 | Death from COVID-19 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Categories | n | % | Events per 100,000 | n | % | Events per 100,000 | n | % | Events per 100,000 |
| Nursing home resident | 162 | 7.8 | 3290 | 4 | 1.6 | 81 | 112 | 24.0 | 2275 |
| Functional dependent | 86 | 4.1 | 2288 | 1 | 0.4 | 27 | 55 | 11.8 | 1463 |
| ≥80 years | |||||||||
| Chronic conditions | 323 | 15.5 | 1411 | 11 | 4.5 | 48 | 171 | 36.7 | 747 |
| No chronic conditions | 69 | 3.3 | 789 | 3 | 1.2 | 34 | 30 | 6.4 | 343 |
| 70–79 years | |||||||||
| Chronic conditions | 232 | 11.2 | 741 | 46 | 18.7 | 147 | 46 | 9.9 | 147 |
| No chronic conditions | 93 | 4.5 | 449 | 21 | 8.5 | 101 | 11 | 2.4 | 53 |
| 60–69 years | |||||||||
| Chronic conditions | 184 | 8.8 | 583 | 39 | 15.9 | 123 | 21 | 4.5 | 66 |
| No chronic conditions | 152 | 7.3 | 391 | 31 | 12.6 | 80 | 7 | 1.5 | 18 |
| 50–59 years | |||||||||
| Chronic conditions | 144 | 6.9 | 517 | 27 | 11.0 | 97 | 7 | 1.5 | 25 |
| No chronic conditions | 204 | 9.8 | 312 | 31 | 12.6 | 47 | 4 | 0.9 | 6 |
| 0–49 years | |||||||||
| Chronic conditions | 106 | 5.1 | 162 | 17 | 6.9 | 26 | 1 | 0.2 | 2 |
| No chronic conditions | 325 | 15.6 | 101 | 15 | 6.1 | 5 | 1 | 0.2 | 0.3 |
| Total | 2080 | 100.0 | 323 | 246 | 100.0 | 38 | 466 | 100.0 | 72 |
COPD, chronic obstructive pulmonary diseases; NE, no events; RR, relative risk; CI, confidence interval.
4. Discussion
The present population-based cohort study shows important differences in the incidence of COVID-19 hospitalizations and severe outcomes according to the characteristics of the individuals that lead to defining high-risk groups. Many of these findings are consistent with the increased risk of severe outcomes among COVID-19 cases that have been associated with specific conditions [13,14,15,16,17]. We also provide population-based information on possible differences in the risk of infection due to susceptibility or increased exposure to SARS-CoV-2 infection. Therefore, we show a complete perspective to assess the priority groups for healthcare and preventive interventions in the population.
Since the first pandemic surge, protocols were implemented to prevent cases in nursing homes [21]; however, people residing in these facilities still presented a three-fold higher risk of infection than other people with similar characteristics did in the second surge, demonstrating the exceptional difficulties for preventing transmission in these places. The excess risk in nursing home residents was similar for SARS-CoV-2 infection and severe outcomes, suggesting that the excess risk for greater severity was due to the increased risk of infection, but not due to late or worse medical care.
Age was a very important risk factor for the outcomes evaluated. The highest risk for SARS-CoV-2 infection was observed in the group aged 15–29 years that had been less affected in the first surge due to the early closure of educational centers [7]. The risk of hospitalization for COVID-19 increased progressively with age, admission to intensive care units increased up to the age group of 70–79 years, and the risk of death rose exponentially with age. Although males did not show a higher incidence of confirmed infection [22], consistent with the literature, they presented a higher risk of hospitalization and severe outcomes, indicating their worse prognosis for this infection [17,23]. Healthcare workers presented an excess of confirmed infection but did not present excess hospitalization or severe outcomes, suggesting timely and effective medical care.
Compared to natives, people born in Latin America and Africa showed a higher risk of confirmed infection, hospitalization and severe outcomes. Possible explanations of these findings are their frequent work as caregivers or in other socially exposed activities, greater number of cohabitants, greater use of public transport, and possibly, worse access to health promotion, preventive measures and early diagnosis. A higher susceptibility related to ethnicity has also been suggested [24], but this variable was not available in the present study. Regardless of the explanation, specific interventions are urgently needed to reduce this excess risk.
Residents in municipalities of more than 5000 inhabitants presented an increased risk of SARS-CoV-2 infection that was probably related to increased social interaction. This excess risk was also observed for hospitalization admission by COVID-19. Very low- and low-income levels were risk factors for SARS-CoV-2 confirmed infection, hospitalization and mortality in the analysis only adjusted for sex and age. The association with COVID-19 mortality remained in the fully adjusted analysis, suggesting a possible delay in access to medical care.
Current smokers showed a lower risk of diagnosed SARS-CoV-2 infection and hospitalization, but they did not have a lower risk of COVID-19 mortality. These results should be considered carefully due to the high proportion of missing values in smoking status. Nevertheless, similar findings have been found in other studies [8,10,25]. These results offer a different perspective from studies reporting that smoking is associated with increased severity in COVID-19 patients [14,26]. More studies are needed to clarify the effect of tobacco on SARS-CoV-2 transmission [24,27].
The higher risk of SARS-CoV-2 infection associated with some chronic conditions, such as diabetes, cardiovascular disease, COPD, chronic kidney disease, dementia, severe obesity, hypertension and functional dependence, is especially concerning because chronic conditions also increase the risk of severe illness in the case of SARS-CoV-2 infection [7]. These conditions may increase the susceptibility to infection, and chronic patients could be exposed to infection from caregivers or in visits to healthcare centers.
Our results are consistent with many other studies showing the increased risk of severe COVID-19 outcomes among patients with major chronic conditions [13,14,15,16,17,28]. Almost all major chronic conditions were independent risk factors for COVID-19 hospitalization; asthma, severe obesity and hypertension were also related to intensive care unit admission; and several major chronic conditions were risk factors for COVID-19 mortality. However, the increased risk associated with major chronic comorbidities was not greater than the risk associated with increasing one or two decades of age.
Hypertension was independently associated with SARS-CoV-2 infection, hospitalization and intensive care unit admission, as has been reported in other studies [29], but this is in contrast with results from the same region in the first epidemic surge when hypertension was not an independent risk factor in the analysis adjusted for hypertension-related comorbidities [30].
The main strengths of our study are that we evaluated four COVID-19 outcomes using a prospective population-based cohort design and that only laboratory-confirmed cases were considered in a period with high availability of tests. Information was obtained from administrative and clinical records before the beginning of the follow-up to prevent information bias.
Some limitations should also be mentioned. Comorbidity severity and treatments, clinical manifestations of COVID-19, and the treatment received at the hospital were not available. A positive antigen test was considered confirmatory in patients with symptoms since the specificity of this test has been proved high in these cases [31]. Predictors for severe COVID-19 outcomes may be different in other places and other epidemic surges, especially after the introduction of the SARS-CoV-2 vaccine. Temporary residents and non-resident immigrants were not included in this study. Although they are a small proportion of the population, this exclusion may have affected the results.
5. Conclusions
These results support the prioritization of preventive interventions and COVID-19 vaccination programs in nursing home residents, people with functional dependence, older populations, and those with chronic conditions because they have a higher risk of severe outcomes than the rest of the population. Healthcare workers were at a higher risk of infection, but not for severe outcomes. Since people born in Latin America and Africa were at higher risk of infection and severe outcomes, they may need specific preventive interventions, better access to healthcare, and priority in vaccination programs.
Acknowledgments
The members of the Working Group for the Study of COVID-19 in Navarra are: Carlos Ibero Esparza, Mercedes Herranz, Irati Arregui, Carmen Martín, Ana Miqueleiz, Ana Navascués, Isabel Polo, Camino Trobajo-Sanmartín, Carmen Ezpeleta (Complejo Hospitalario de Navarra, Pamplona, España); Ingrid Esteve, Igberto Tordoya, Delia Quílez (Hospital Reina Sofía de Tudela); Francisco Lameiro, Ana Isabel Álvaro (Hospital García Orcoyen de Estella); Esther Albéniz, Fernando Elía, Javier Gorricho (Servicio Navarro de Salud-Osasunbidea, Pamplona, Spain); Eva Ardanaz, Nieves Ascunce, Maite Arriazu, Fernando Baigorria, Aurelio Barricarte, Cristina Burgui, Itziar Casado, Enrique de la Cruz, Jorge Díaz, María Ederra, Nerea Egüés, Manuel García Cenoz, Nerea Iriarte, Iván Martínez-Baz, Conchi Moreno-Iribas, Marian Nuín, Carmen Sayón, Juana Vidán, Jesús Castilla and Marcela Guevara (Instituto de Salud Pública y Laboral de Navarra—IdiSNA—CIBERESP, Pamplona, Spain).
Author Contributions
Conceptualization, J.C. and M.G.; methodology, J.C., M.G. I.M.-B., I.C. and C.B.; validation, A.M., F.B., C.I.-E., A.N., C.T.-S. and C.E.; formal analysis, J.C. and M.G.; investigation, F.B., C.I.-E., I.M.-B. and I.C.; resources, C.E.; data curation, A.M., F.B., C.I.-E., A.N., C.T.-S., I.C., C.B. and C.E.; writing—original draft preparation, J.C. and M.G.; writing—review and editing, C.T.-S., I.M.-B. and I.C.; supervision, J.C. and C.E.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Horizon 2020 program of the European Commission, project I-MOVE-COVID-19, grant agreement number 101003673; Heath Department of the Navarre Government (Pyto 2018/43), and by the Carlos III Institute of Health with the European Regional Development Fund, grant numbers COV20/00542 and PI20/01323.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Ethics Committee for Clinical Research of Navarre (approval code: PI2020/45).
Informed Consent Statement
Patient consent was waived by the Ethics Committee for Clinical Research of Navarre.
Data Availability Statement
Availability of individual-level data needs authorization of the Department of Health of the Navarra Government.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.European Centre for Disease Prevention and Control COVID-19 Situation Update for the EU/EEA, as of 20 January 2021. [(accessed on 23 January 2021)]; Available online: https://www.ecdc.europa.eu/en/cases-2019-ncov-eueea.
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) WHO SAGE Roadmap for Prioritizing Uses of Covid-19 Vaccines in the Context of Limited Supply. Version 1.1. Geneva. 13 November 2020. [(accessed on 16 April 2021)]; Available online: https://cdn.who.int/media/docs/default-source/immunization/sage/covid/sage-prioritization-roadmap-covid19-vaccines_31a59ccd-1fbf-4a36-a12f-73344134e49d.pdf?sfvrsn=bf227443_36&download=true.
- 4.Karagiannidis C., Mostert C., Hentschker C., Voshaar T., Malzahn J., Schillinger G., Klauber J., Janssens U., Marx G., Weber-Carstens S., et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: An observational study. Lancet Respir. Med. 2020;8:853–862. doi: 10.1016/S2213-2600(20)30316-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piroth L., Cottenet J., Mariet A.S., Bonniaud P., Blot M., Tubert-Bitter P., Quantin C. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir Med. 2021;9:251–259. doi: 10.1016/S2213-2600(20)30527-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Working Group for the Surveillance and Control of COVID-19 in Spain. Members of the Working Group for the Surveillance and Control of COVID-19 in Spain The first wave of the COVID-19 pandemic in Spain: Characterisation of cases and risk factors for severe outcomes, as at 27 April 2020. Euro. Surveill. 2020;25:2001431. doi: 10.2807/1560-7917.ES.2020.25.50.2001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., Andrews N., Byford R., Dabrera G., Elliot A., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect. Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reilev M., Kristensen K.B., Pottegård A., Lund L.C., Hallas J., Ernst M.T., Christiansen C.F., Sørensen H.T., Johansen N.B., Brun N.C., et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: A nationwide cohort. Int. J. Epidemiol. 2020;49:1468–1481. doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Williamson E.J., Walker A.J., Bhaskaran K., Bacon S., Bates C., Morton C.E., Curtis H.J., Mehrkar A., Evans D., Inglesby P., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clift A.K., Coupland C.A.C., Keogh R.H., Diaz-Ordaz K., Williamson E., Harrison E.M., Hayward A., Hemingway H., Horby P., Mehta N., et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: National derivation and validation cohort study. BMJ. 2020;371:m3731. doi: 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergman J., Ballin M., Nordström A., Nordström P. Risk factors for COVID-19 diagnosis, hospitalization, and subsequent all-cause mortality in Sweden: A nationwide study. Eur. J. Epidemiol. 2021;36:287–298. doi: 10.1007/s10654-021-00732-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang J., Zheng Y., Gou X., Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q., Ji R., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J., Li Q., Jiang C., Zhou Y., Liu S., et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis. J. Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim L., Garg S., O’Halloran A., Whitaker M., Pham H., Anderson E.J., Armistead I., Bennett N.M., Billing L., Como-Sabetti K., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. Coronavirus Disease 2019 (COVID-19)-associated Hospitalization Surveillance Network (COVID-NET) Clin. Infect. Dis. 2020;16:ciaa1012. doi: 10.1093/cid/ciaa1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., Aaron J.G., Claassen J., Rabbani L.E., Hastie J., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pijls B.G., Jolani S., Atherley A., Derckx R.T., Dijkstra J.I.R., Franssen G.H.L., Hendriks S., Richters A., Venemans-Jellema A., Zalpuri S., et al. Demographic risk factors for COVID-19 infection, severity, ICU admission and death: A meta-analysis of 59 studies. BMJ Open. 2020;11:e044640. doi: 10.1136/bmjopen-2020-044640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control Case Definition for Coronavirus Disease 2019 (COVID-19), as of 29 May 2020. [(accessed on 16 April 2021)]; Available online: https://www.ecdc.europa.eu/en/covid-19/surveillance/case-definition.
- 19.Moreno-Iribas C., Sayon-Orea C., Delfrade J., Ardanaz E., Gorricho J., Burgui R., Nuin M., Guevara M. Validity of type 2 diabetes diagnosis in a population-based electronic health record database. BMC Med. Inform. Decis. Mak. 2017;17:34. doi: 10.1186/s12911-017-0439-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hobart J.C., Thompson A.J. The five item Barthel index. J. Neurol. Neurosurg. Psychiatry. 2001;71:225–230. doi: 10.1136/jnnp.71.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grupos de trabajo COVID-19 de la Comisión Delegada y del Comité Consultivo del Consejo Territorial de Servicios Sociales y del Sistema para la Autonomía y Atención a la Dependencia Ministerio de Derechos Sociales y Agenda 2030. Informe del Grupo de Trabajo COVID 19 y Residencias. Vers. final (24/11/2020) [(accessed on 16 April 2021)]; Available online: https://www.mscbs.gob.es/ssi/imserso/docs/GTCOVID_19_RESIDENCIAS.pdf.
- 22.Pollán M., Pérez-Gómez B., Pastor-Barriuso R., Oteo J., Hernán M.A., Pérez-Olmeda M., Sanmartín J.L., Fernández-García A., Cruz I., Fernández de Larrea N., et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet. 2020;396:535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pastor-Barriuso R., Pérez-Gómez B., Hernán M.A., Pérez-Olmeda M., Yotti R., Oteo-Iglesias J., Sanmartín J.L., León-Gómez I., Fernández-García A., Fernández-Navarro P., et al. Infection fatality risk for SARS-CoV-2 in community dwelling population of Spain: Nationwide seroepidemiological study. BMJ. 2020;371:m4509. doi: 10.1136/bmj.m4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killerby M.E., Link-Gelles R., Haight S.C., Schrodt C.A., England L., Gomes D.J., Shamout M., Pettrone K., O’Laughlin K., Kimball A., et al. Characteristics associated with hospitalization among patients with COVID-19—Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G., Henry B.M. Active smoking is not associated with severity of coronavirus disease 2019 (COVID-19) Eur. J. Intern. Med. 2020;75:107–108. doi: 10.1016/j.ejim.2020.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy R.K., Charles W.N., Sklavounos A., Dutt A., Seed P.T., Khajuria A. The effect of smoking on COVID-19 severity: A systematic review and meta-analysis. J. Med. Virol. 2021;93:1045–1056. doi: 10.1002/jmv.26389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organization Smoking and COVID-19. Scientific Brief. 30 June 2020. [(accessed on 16 April 2021)]; Available online: https://escholarship.org/content/qt22m8z3sq/qt22m8z3sq.pdf.
- 28.Fresán U., Guevara M., Elía F., Albéniz E., Burgui C., Castilla J., Working Group for the Study of COVID-19 in Navarra Independent role of severe obesity as a risk factor for COVID-19 hospitalization: A Spanish population-based cohort study. Obesity. 2021;29:29–37. doi: 10.1002/oby.23029. [DOI] [PubMed] [Google Scholar]
- 29.Wang B., Li R., Lu Z., Huang Y. Does comorbidity increase the risk of patients with COVID-19: Evidence from meta-analysis. Aging. 2020;12:6049–6057. doi: 10.18632/aging.103000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fresán U., Guevara M., Trobajo-Sanmartín C., Burgui C., Ezpeleta C., Castilla J. Hypertension and related comorbidities as potential risk factors for COVID-19 hospitalization and severity: A prospective population-based cohort study. J. Clin. Med. 2021;10:1194. doi: 10.3390/jcm10061194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merino P., Guinea J., Muñoz-Gallego I., González-Donapetry P., Galán J.C., Antona N., Cilla G., Hernáez-Crespo S., Díaz-de Tuesta J.L., Gual-de Torrella A., et al. Multicenter evaluation of the Panbio™ COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021;27:758–761. doi: 10.1016/j.cmi.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Availability of individual-level data needs authorization of the Department of Health of the Navarra Government.