Abstract
Adipose-derived stem cells (ASCs) secrete many cytokines, proteins, growth factors, and extracellular vesicles with beneficial outcomes that can be used in regenerative medicine. It has great potential, and the development of new treatment strategies using the ASCs secretome is of global interest. Besides cytokines, proteins, and growth factors, the therapeutic effect of secretome is hidden in non-coding RNAs such as miR-21, miR-24, and miR-26 carried via exosomes secreted by adequate cells. The whole secretome, including ASC-derived exosomes (ASC-exos) has been proven in many studies to have immunomodulatory, proangiogenic, neurotrophic, and epithelization activity and can potentially be used for neurodegenerative, cardiovascular, respiratory, inflammatory, and autoimmune diseases as well as wound healing treatment. Due to limitations in the use of stem cells in cell-based therapy, its secretome with emphasis on exosomes seems to be a reasonable and safer alternative with increased effectiveness and fewer side effects. Moreover, the great advantage of cell-free therapy is the possibility of biobanking the ASCs secretome. In this review, we focus on the current state of knowledge on the use of the ASCs secretome in stem cell-free therapy.
Keywords: adipose-derived stem cells, extracellular vesicles, secretome, stem cell therapy
1. Introduction
Mesenchymal stem cells (MSCs) have a great potential for use in medicine and stem cell therapy. The therapeutic potential of MSCs is mainly based on their ability to self-renew and differentiate towards tissue-specific cells, thus tissue regeneration [1,2]. To standardize the concept and the possibility of comparing the results of research in possible medical application, in 2006, the International Society for Cellular Therapy (ISCT) published three minimum criteria for defining MSC cells. According to ISCT, these cells must be plastic-adherent, capable of differentiating into osteoblasts, adipocytes and chondroblasts, and phenotypically be ≥95% CD105, CD90, and CD73 positive and negative for CD45, CD34, CD14 or CD11b, CD79alpha or CD19 and HLA-DR [3]. MSCs can be hypothetically obtained from almost all human tissues, e.g., the marrow spaces of long bones, cord blood, dental pulp, placenta, and synovial fluids, first discovered and described by Friedenstein’s group (1976), Muluen’s group (1978), Shi’s group (2003), and Atala’s group (2006), respectively [4,5,6,7]. However, due to many limitations in isolation and abundance, the main sources today are bone marrow (BM) and adipose tissue (AT) [2,8]. BM-MSCs were the first discovered and described at the end of the twentieth century, and naturally, BM became the first and most important source of MSCs [8]. However, subsequent researchers provided evidence that multipotent stem cells can be successfully isolated from other tissues and that higher densities of MSCs can be found in AT than BM. At the same time, the invasiveness of donor sampling is reduced [9].
Adipose-derived stem cells are mesenchymal stem cells harvested from AT capable of self-renewal and multipotent differentiation towards adipocytes, osteoblasts, chondrocytes, myocytes, neurocytes, and other types of cells [10,11,12,13,14]. ASCs isolated from AT meet the criteria established by the ISCT and are considered a promising tool for use in regenerative medicine, including the treatment of degenerative, inflammatory, and autoimmune diseases [13]. ASCs have already been shown to have the potential for use in multiple sclerosis [14], rheumatoid arthritis, osteoarthritis [13,15,16,17,18], fistulae [18,19], diabetes mellitus [20,21], dyslipidemia, and cardiovascular diseases [22,23] treatment as well as skin aging and wound healing [24,25,26]. The great advantage of MSCs isolated from AT is that they can be easily obtained by liposuction from autologous subcutaneous adipose tissue with high cellular activity without any ethical concerns comparing to embryonic stem cells, the obtainment of which is associated with the destruction of the embryo. This makes ASCs a suitable choice for use in cell-based therapies, apart from those that may be questionable and controversial [10].
ASCs may also be considered a therapeutic tool due to their autocrine and paracrine factors and by increasing the recruitment of endogenous precursors. They secrete many significant proteins, including growth factors (GF) and cytokines [27,28,29], as well as extracellular vesicles (EV) and RNAs [30,31,32] to support cell regeneration, proliferation, differentiation, and migration [33]. Attempts have been made to optimize the conditions of stem cell-based therapy with naive ASCs by manipulating the route of administration and dose of cells and the timing [34,35,36] of homing to the site of inflammation, however, without fully satisfactory results [37,38]. The Sacerdote group has been studying the use of ASCs for years [34]. In 2013, they showed that 1 × 106 human ASCs administered intravenously can reduce pain in a mouse model with neuropathic pain. However, they suggested validation of ASCs dose and the time of treatment to improve this effect [35]. In a clinical study by Alvaro-Gracia et al., after three months of treatment of the rheumatoid arthritis patients (three groups: 1, 2, and 4 million cells/kg by three intravenous administrations on days 1, 8, and 15), clinical benefits were not sustained but many side effects were observed [36]. Therefore, a new strategy for ASCs based therapy is needed, and ASCs secretome can be a promising tool for medical purposes as a safe therapeutic agent for cell-free-based therapy with easy storage for long-term use [39,40].
This review provides information on the current state of knowledge about secretome of adipose-derived stem cells, with particular focus on their exosomes. The study used all available sources such as PubMed, Google Scholar, and Clinical Trials (European and World) databases. The authors collected data and summarized information from in vitro, in vivo, and clinical trials using adipose-derived stem cells secretome in the context of use in stem cell-free therapy. The literature study was based on keywords such as adipose-derived stem cells, secretome, exosomes, growth factors, cytokines, cell-free, therapy that were used alone or in combination with diabetes mellitus, cardiovascular, wound healing, neurodegenerative, skeletal, respiratory, metabolic, regeneration, disease, and skin aging.
2. Adipose-Derived Stem Cells Characteristics
2.1. Nomenclature
The International Fat Applied Technology Society has proposed and adapted the term adipose-derived stem cells (ASCs) to standardize it to avoid confusion in nomenclature. In the older literature, adipose-derived stem cells are referred to by various terms and abbreviations: adipose-derived adult stem (ADAS) cells; adipose mesenchymal stem cells (AdMSCs); adipose-derived adult stromal cells; adipose-derived stromal cells (ADSCs); adipose stromal cells (ASCs); adipose-derived stromal/stem cells (ASCs); vascular stromal/stem cells; preadipocytes; processed lipoaspirate (PLA) cells; pericytes and lipoblasts and that inconsistent nomenclature in the literature has led to confusion [41,42,43,44]. In line with International Fat Applied Technology Society recommendations, we use the term adipose-derived stem cells in this study.
2.2. Sources
Adipose-derived stem cells can be harvested from adipose tissue also containing adipocytes, preadipocytes, pericytes, and immune cells such as eosinophils, macrophages, and innate lymphoid cells (ILCs), T cells, and B cells, fibroblasts, and endothelial cells [45,46]. Before International Fat Applied Technology in 2004 defined the nomenclature and adapted the term ASCs with a specific phenotype, referring only to mesenchymal stem cells isolated from the vascular stromal fraction, the terms preadipocytes and pericytes had previously been used confusingly [41,42,43]. Adipose tissue can be divided into white (WAT) and brown (BAT) adipose tissue as a site for energy storage and expenditure, respectively. Adipose-derived stem cells from WAT are characterized by a different expression of cell surface markers than BAT, e.g., ASCs from BAT express noticeably more TMEM-26, SSEA-4, CD106, CD105, HLA-A,B,C, and CD-137 but less CD86 and LIN, than WAT [47]. Subcutaneous AT is most commonly used for ASCs isolation due to its easy and non-invasive obtaining procedure from the abdomen, thigh, and arm. [48,49]. Moreover, those obtained from thighs show the best viability [50]. However, it can also be obtained from the craniofacial, pericardial, perirenal, omentum, intestine, bone marrow, buttocks, pulmonary (WAT regions) cervical, paravertebral, supraclavicular, axillary, suprarenal (BAT regions) regions [43,49,51]. The type of harvested area and the type of adipose tissue affects proliferation, endocrine function, gene expression, surface antigens, and the differentiation potentials of adipose-derived stem cells [50,52]. Nepali et al. have already shown that abdominal ASCs exhibit better chondrogenic but poorer osteogenic potential than orbital ASCs [53].
Moreover, the origin of the adipose-derived stem cells is critical, and the age, weight, and disease state of the donor may affect the condition and properties of isolated ASCs. It has been shown that the older donor of ASCs the lower proliferation and differentiation potential and the lower growth factors secretion ability [9,36,54]. Furthermore, ASCs obtained from obese donors are characterized by decreased expression of phenotypic mesenchymal stem cells markers, an excessive immune response, and thus a lower self-renewal, differentiation potential [55,56], and a higher capacity for migration, invasion, and phagocytosis [57]. Studies have also shown that type 1 and type 2 diabetes mellitus, hypercholesterolemia, hypertension, and smoking have negative effects on the pluripotency and self-renewal of isolated ASCs [56,58]. On the other hand, it has been shown that gender and chemotherapy have not shown much effect on ASCs [59]. However, it is necessary to use exosomes isolated from healthy individuals to avoid a reduction in the therapeutic potential and potential side effects caused by bioactive exosomes, the content of which depends on the state of secretory cells, therefore their pathological effects should be assessed [60].
2.3. Phenotype
Adipose-derived stem cells are a heterogenic population and no unique surface markers have yet been described. However, they express markers characteristic of mesenchymal stem cells established by ISCT and the International Federation for Adipose Therapeutics and Science (IFATS) and are described by CD73(+), CD90(+), CD105(+), and CD36(+) but also CD31(−), CD45(−), CD11b(−), CD106(−). The expression of CD36 and lack of expression of CD106 distinguish ASCs from BM-MSCs [61]. Moreover, ASCs express β-1 the integrin (CD29) that participates in angiogenesis and CD44 hyaluronate and osteopontin receptor, which is crucial in extracellular matrix development and pathological processes such as neoplasia [62,63]. Although, they also show the characteristics of neural phenotypic profile through the expression of NEUROD1, SOX3, and PAX6 and markers determining core circuitry self-renewal: SOX2, NANOG, OCT4 [64]. There are inconsistent data, confounding if ASCs isolated from stromal vascular fraction may express CD34 [65,66] and CD106 [67] or not [68]. It is recognized that cultured MSCs do not express CD34 contrary to freshly isolated cells. Some studies have shown that CD34 is present at the beginning of culture in freshly isolated ASCs but after the first and subsequent passages (2–4) disappears [69] or remains at a low level [70].
ASCs markers also depend on adipose tissue origin, and it has been proven that orbital-ASCs express less HLA-DR, CD31, and CD44 than abdominal-ASCs [53]. It has also been shown that the ASCs phenotype changes progressively with passages in vitro. Stromal-cell associated markers such as CD13, CD29, CD44, CD63, CD73, CD90, CD166 were present after subsequent passages but with increased levels [68,70]. Zhu et al. have also shown that ASCs, retained their phenotype after 25 passages, as well as multilineage differentiation, and proliferation capacity but decreased compared to the first passage [71]. Furthermore, Atat et al. confirmed that the expansion of the passaged ASCs did not influence their differentiation capacity [72]. Interestingly, Griffin et al. showed that the phenotype and differentiation potential are not changed by comparing ASCs from healthy and systematic sclerosis donors but only the proliferation, invasion, and migration capacity of these cells, which may suggest that the diseases does not alter the self-renewal potency of ASCs [73]. Similarly, Mieczkowska et al. showed that ASCs from oncological surgery patients exhibit comparable phenotypes to those from healthy donors [70].
3. Adipose-Derived Stem Cells Secretome
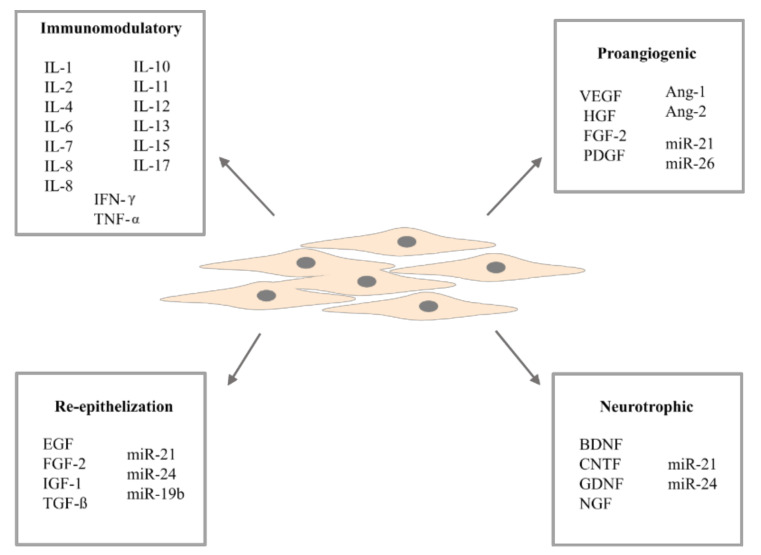
Adipose-derived stem cells produce many molecules responsible for cell signaling, such as cytokines [29,74], growth factors [28,75], morphogens [76], chemokines [27,77], and extracellular vesicles [78,79,80], improving various cellular mechanisms. Interestingly, compared to BM-MSCs—the second most frequently considered MSCs for stem cell therapy—ASCs secrete more growth factors, and this feature increases their regenerative capacity [2,81]. It has also been shown, in vivo, that ASCs are better in the bioactive factors secretion such as nerve growth factor (NGF), hepatocyte growth factor (HGF), monocyte chemotactic protein 1 (MCP-1), colony stimulator of granulocyte-macrophage factor (GM-CSF), granulocyte colony stimulating-factor (CSF), interleukin 1 receptor antagonist (IL-1RA), interleukin (IL)-6 and IL-8 versus bone marrow (BM)-MSCs [27]. This implies a better differentiation, migration, proliferation, and autocrine activity compared to BM-MSCs; moreover, an excellent ASCs paracrine potential has been revealed. Additionally, ASCs undergo senescence later than BM-MSCs [82,83]. Due to several limitations of cell therapy, in terms of increasing the effectiveness of ASCs and in view of safety and costs, the dose and frequency of cell application cannot be increased indefinitely. One strategy for enhancing ASCs therapy is to use their secretome with particular emphasis on extracellular vesicles. This approach promises higher efficiency than naive ASCs without having to use ASCs alone [37] (Figure 1).
Figure 1.
Different types of secretory activity of adipose-derived stem cells.
3.1. Cytokines and Growth Factors
Adipose-derived stem cells are characterized by high trophic activity and secretion of a large amount of proteins, growth factors, and pro- and anti-inflammatory cytokines exerting benefits towards cells’ regenerative capacity. They are considered to be highly immunomodulating cells, exceeding the suppressive effect of BM-MSCs by secreting more anti-inflammatory IL-6 and transforming growth factor-β1 (TGF-β1) [84]. However, under different conditions, IL-6 can have anti- and pro-inflammatory properties similar to IL-2 [77]. It has been shown that relevant levels of IL-2 affect ASCs function by transcriptional dysregulation [85] and also that IL-6 enhances ALP activity and promotes osterix expression, and thus osteogenesis [86,87]. Moreover, it has been shown that orbital ASCs secrete higher concentrations of IL-6, IL-8, eotaxin, fractalkine, and IL-10, but lower concentrations of basic fibroblast growth factor (FGF-2) and vascular endothelial growth factor (VEGF) than abdominal ASCs [53]. In addition, high seeding density, long-term culturing, and passaging in vitro reduce the concentration of IL-6 and VEGF [72,88].
In addition to bifunctional IL-2 and IL-6, adipose-derived stem cells secrete other cytokines with a well-defined pro-inflammatory (IL-7, IL-8, IL-9, IL-11, IL-12, IL-15, IL-17, interferon-gamma IFN-γ, IL-1β, and tumor necrosis factor-alpha TNF-α) or anti-inflammatory (IL-1Ra, IL-4, IL-10, and IL-13) properties [29,44,89]. It has been shown that TNF-α and IL-1β secretion by macrophages mediates the inhibitory effect on ASCs adipogenesis, and also that combination of TNF-α or IL-1β with IFN-γ can enhance the immunomodulatory properties of ASCs mainly dependent on indoleamine 2, 3-dioxidase (IDO) or inducible nitric oxide synthase (iNOS) [74]. IFN-γ triggers ASCs to elicit immunosuppressive factors [90]. In addition, the signaling protein (PGE2) secreted by ASCs has been found to have an immunosuppressive effect [91,92]. It has been shown that IL-4 and IL-17 have an inhibitory effect on adipogenic differentiation by promoting lipolysis and suppressing proadipogenic factors gene expression, respectively [93,94].
In addition to cytokines, ASCs secrete many growth factors that influence cellular processes that promote regeneration. The proangiogenic and antiapoptotic properties of ASCs are provided by trophic factors secreted by these cells such as VEGF, FGF-2, TGF-β, HGF, and GM-CSF [95]. HGF and VEGF also induce neurogenic responses [33]. Interestingly, the secretion of HGF involved in vasculogenesis and angiogenesis [96] is significantly increased after ASCs stimulation with FGF-2 or epidermal growth factor (EGF) [29]. However, platelet-derived growth factor (PDGF) secreted by ASCs plays an essential role in angiogenesis [97] and it has been shown that cell stimulation with PDGF enhances the release of extracellular vesicles and thus proangiogenic properties [98]. Adipose-derived stem cells also secrete insulin-like growth factor (IGF) promoting, proliferation, self-renewal, and differentiation of cells [99] but its level decrease with donor age [100]. Nevertheless, IGF-1, EGF, FGF-2, and TGFα are essential wound healing factors, enhancing these processes and cell migration [101].
3.2. Extracellular Vesicles
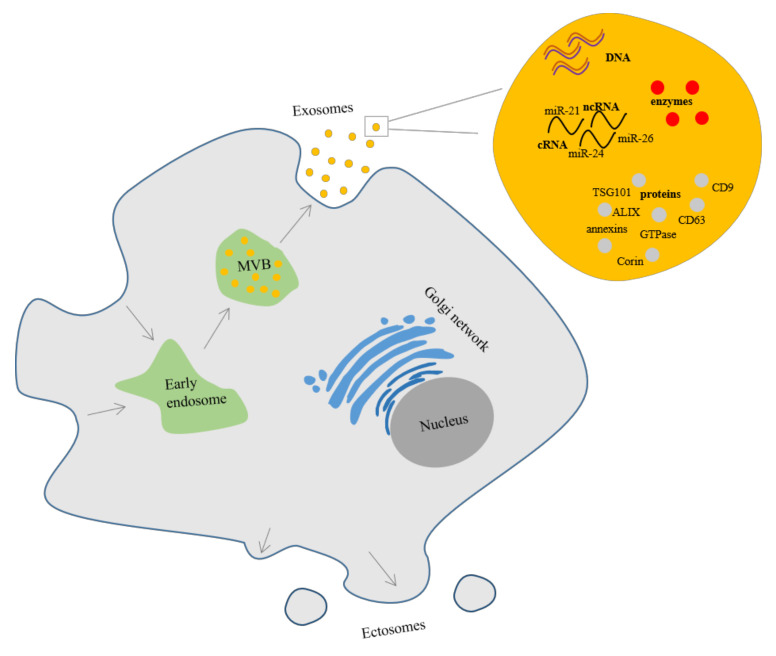
Extracellular vesicles (EV) are secreted by cells into the extracellular matrix and carry biomarkers that influence numerous cellular processes. These self-contained lipid bilayer vesicles are composed of lipids, proteins, and nucleic acids [102], but cannot replicate because they do not contain a functional nucleus [103]. EV can be formed by outward budding of the cell membrane or from inward endosome. Exosomes are formed from exosome precursors called intraluminal vesicles (ILVs) in the endocytic cisternae membrane. ILVs accumulation leads to the formation of multivesicular bodies (MVBs), which over time exocytically fuse with the plasma membrane and can be released into the extracellular space. Whereas, ectosomes are quickly generated in the plasma membrane (Figure 2) [104]. The term nanovesicles can also be found in the literature. Nanovesicles are membrane-bounded structures of various origins, e.g., exosomes, so basically exosomes are nanovesicles. Nanovesicles can be artificially generated by sequential cell membrane penetration and can be used in cell-based therapy [105].
Figure 2.
Exosomes and ectosomes secretion in adipose-derived stem cell.
EVs have attracted a lot of attention over the last decade and there has been a growing number of scientific papers on them. There is no consensus yet on the description of specific markers for EVs subtypes. Due to this, the International Society for Extracellular Vesicle published in 2018 an updated minimum information statement on extracellular vesicles (MISEV2018) referring to meaningful changes in nomenclature. MISEV2018 recommends to describe subtypes of EVs in published researches by (a) physical characteristics such as density (low, middle, high, with each range defined) or size (“small EVs” (sEVs): <100 nm or <200 nm and “medium/large EVs” (m/lEVs): >200 nm (large and/or medium)) (b) biochemical composition (CD63+/CD81+-EVs, Annexin A5-stained EVs, etc.); or (c) descriptions of cell conditions or origins (podocyte EVs, hypoxic EVs, large oncosomes, apoptotic bodies). However, using subtype terms based on subcellular origin is allowed: endosome-origin “exosomes” and plasma membrane-derived “ectosomes” (microparticles/microvesicles), while a demonstration of origin is shown [106].
3.2.1. Extracellular Vesicles Composition
Adipose-derived stem cells secrete exosomes (Figure 2) with a diameter of 30–100 nm [30] or according to other sources up to 150 nm [107,108]. Exosomes contain cytoskeleton, heat shock, and transmembrane proteins, enzymes, lipids, and DNA [102]. Moreover, they carry coding (mRNA) and non-coding RNA (e.g., rRNA, miRNA) in various subtypes but with a strong emphasis on short non-coding RNA [109,110]. Non-coding RNAs are RNA molecules that cannot be translated into a protein and the functions of many have not been understood yet, however, carried by exosomes play a role in cellular mediated communication [110]. Interestingly, Xing et al. identified 1185 proteins in ASC-derived exosomes (ASC-exos) and showed that exosomes are crucial not only for cell-to-cell communication but also for metabolic and cellular processes and the regulation of biological processes. They also showed that ASC-exos are characterized by exosomal markers such as CD9, CD63, as well as tumor susceptibility gene (TSG) 101 [111]. In turn, Ni et al. identified 1466 proteins in ASCs-exos and some of them are associated with the proliferation and regeneration pathways (e.g., PI3K-Akt or WNT) such as LAMC1, LAMA4, LAMB2, LAMB1, MEK1, MEK2, IKBKA, IKKA CHUK, RELN, VTN, WNT2, PEDF, and SERPINF1 [112]. Interestingly, González-Cubero et al. showed that exosomes derived from ASC-conditioned medium were characterized by a strong enhancement of CD3, CD45, CD56, HLA-ABC, and HLA-DRDPDQ expression [113].
3.2.2. Control of the Secretion of Extracellular Vesicles
The specific molecule content makes exosomes (due to their specific biological properties) modulators with different effects on the target recipient cells. ASC-exos provide benefits in therapeutic use due to their stability in the human body [30] as opposed to whole ASCs which die after 48 h of systemic infusion [38]. Moreover, the use of exosomes is safer, because of the reduced risk of tumorigenicity. They can also be readily produced in large quantities with well-established protocols in under laboratory conditions, and their components are less prone to cellular degradation [30]. Importantly, exosomes can affect not only neighboring cells by transporting bioactive cargo, but also cells and host tissues over a greater distance [105].
Despite the benefits of exosome use, the regulation of their content and reproducibility is important to provide successful clinical applications. The function and secretion quantity of exosomes depends on their donor. It has been shown that ASCs from obese donors and those from omental depots secrete more exosomes than lean donors and subcutaneous depots, respectively, which is reflected in the disease state [114]. However, exosome secretion can be enhanced with in vitro molecule modulators. Wang et al. showed that MSCs treated with N-methyldopamine and norepinephrine for 24 h produce three times more exosomes without altering their features [115]. The exosomal content is not random and is controlled by posttranslational modifications and specific endogenous target sequences at the stage of exosome formation, most importantly, miRNAs are the most preferred exosome loaded RNAs [116]. Nevertheless, due to the improvement in the exosome efficiency in therapy, their function can also be boosted by the engineering content modifications through genetic and chemical methods and may be used as an ideal drug delivery agent [109,117].
4. Therapeutic Potential of Adipose-Derived Stem Cells Secretome
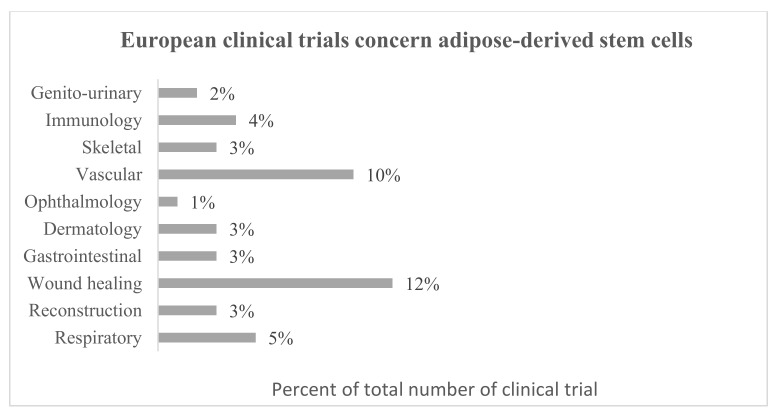
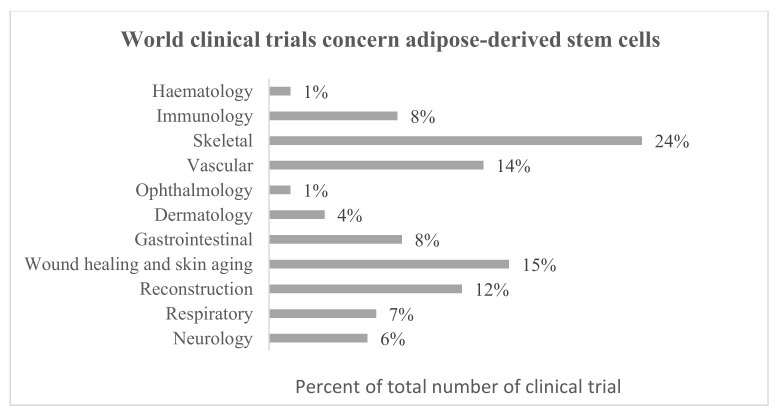
Adipose-derived stem cells are getting more and more interest due to their use in regenerative medicine. Currently, 1259 MSC-containing investigational medicinal products, including 406 ASCs are registered in the Clinical Trials database [118] and also 138 and 46 in the European Clinical Trials database [119], respectively. Regarding European clinical trials database, the greatest number of ASC studies concern wound healing (fistulae, skin burns, and ulcers) and vascular applications (myocardial infarction, ischemia, and heart failure). ASCs are also studied for respiratory (respiratory distress, bacterial and COVID-19 pneumonia), immunomodulatory (HIV infection, amyotrophic lateral, and systemic sclerosis), skeletal (spinal cord injury, osteoarthritis, atrophic pseudarthrosis), dermatology (scars, cutis laxa, lichens, epidermal necrolysis), reconstructive (breasts reconstructions), gastrointestinal (hyposalivation and xerostomia), genito-urinary (urinary incontinence, erectile dysfunction), and ophthalmology (dry eye disease) applications (Figure 3). On the other hand, regarding the world Clinical Trial database, most research is focused on skeletal applications but also wound healing with the extension of research on skin aging. In this database are also registered clinical trials concerning haematological (e.g., anemia) and neurological (e.g., Parkinson’s disease) applications (Figure 4). Many clinical and laboratory research show the enormous potential of using ASCs in cell therapy (Table 1 and Table 2).
Figure 3.
Summary of European clinical trials concern adipose-derived stem cells based on all clinical trials available in the database (years 2007–2021) [119].
Figure 4.
Summary of World clinical trials concern adipose-derived stem cells based on all clinical trials available in the database (years 2007–2021) [118].
Table 1.
Clinical trials of adipose-derived stem cells exosomes for cell-free based therapies. N/A—Not available [111].
| NCT no. | Title | Status | Condition/ Disease |
Administration | Intervention/ Treatment |
Results |
|---|---|---|---|---|---|---|
| NCT04313647 | A tolerance clinical study on aerosol inhalation of mesenchymal stem cells exosomes in healthy volunteers | Recruiting | Healthy volunteers | Aerosol inhalation | 2 × 108, 4 × 108, 8 × 108, 16 × 108, 20 × 108 nano vesicles/3 mL to be administrated at once to different participants sets | N/A |
| NCT04388982 | Open-label, single-center, phase I/Ⅱ clinical trial to evaluate the safety and the efficacy of exosomes derived from allogenic adipose mesenchymal stem cells in patients with mild to moderate dementia due to Alzheimer’s disease | Recruiting | Dementia due to Alzheimer’s disease | Nasal drip | 5 µg, 10 µg, 20 µg exosomes/1 mL exosomes, twice a week for 12 weeks | N/A |
| NCT04544215 | A clinical study of allogeneic human adipose-derived mesenchymal progenitor cell exosomes (haMPC-Exos) nebulizer for the treatment of carbapenem-resistant gram-negative bacilli-induced pulmonary infection | Recruiting | Drug resistant pulmonary infection | Aerosol inhalation | 8 × 108, 16 × 108 nano vesicles/3 mL per day for 7 days | N/A |
| NCT04276987 | A pilot clinical study on aerosol inhalation of the exosomes derived from allogenic adipose mesenchymal stem cells in the treatment of severe patients with novel coronavirus pneumonia | Completed | Coronavirus pneumonia | Aerosol inhalation | 2 × 108 nano vesicles/3 mL per day for 5 days | N/A |
Table 2.
Adipose-derived stem cells’ secretome investigations for cell-free therapy.
| Paracrine Factors | Model | Therapeutic Effect | References |
|---|---|---|---|
| Neurodegenerative Diseases | |||
| BDNF, GDNF | Mouse Parkinson disease model | Protection of dopaminergic neurons. | [128] |
| Exosomes | Mice Alzheimer disease Model | Reduction of amyloid beta (Aβ) levels and the Aβ42/40. Increasing cell survival and decrease of neuronal death in the hippocampus and the cerebral cortex. Decrease deposition of Aβ plaques in Alzheimer Disease by exosomal neprilysin. |
[134,135] |
| Exosomes | Mice-derived neural cells from Huntington’s disease mouse model | Reduction of accumulation of mHtt aggregates, mitochondrial dysfunction and cell apoptosis by p-CREB-PGC1α pathway activation in Huntington’s disease. | [136] |
| Exosomes | Mice-derived neuronal cells from Amyotrophic lateral sclerosis disease mouse model | Reduction of cytosolic superoxide dismutase 1 and restore of the abnormal reduction of mitochondrial proteins. | [137] |
| Exosomes | Mouse progressive Multiple Sclerosis model | Brain atrophy reduction and remyelination promotion. Reduction of Th1 and Th17 levels. |
[138] |
| Exosomes | Brain injured male Sprague-Dawley rats | Reduction of inflammatory indicator (Ly6G+/CD11b/c+) and immune (CD3+/CD4+/CD3+/CD8+) cells. Reduction of early and late apoptotic cells. |
[31] |
| Exosomes (containing or depleted of MALAT1) |
Rats following a mild controlled cortical impact | Modulation of inflammation-related after traumatic brain injury by MALAT1. | [139] |
| Cardiovascular diseases | |||
| Exosomes | Endothelial cells | Promotion of vascularization by overexpressing miR-21. | [30] |
| VEGF | Human microvascular endothelial cells and mouse Hindlimb Ischemia model |
Reduction of endothelial cells apoptosis. Perfusion improvement in ischemic hindlimbs. |
[95] |
| IGF-1, BDNF | Cerebellar granule neurons | Blocking postischemic p38 activation. Protection of neural cells. |
[140] |
| Exosomes | Rat Acute Ischemic Stroke model | Increase of neural regeneration. Reduction of brain infarct zone, brain swelling, and shrinkage. |
[144] |
| Exosomes released by PDGF-stimulated adipose-derived stem cells | Acute Hindlimb Ischemia mouse model | Muscle protection from Acute Ischemia. | [145] |
| Engineered modified exosomes encoding VEGF | Nude mouse fat transplantation model | Improvement of neo-angiogenesis and vascularization by promoting cell proliferation and vascular maturity. | [143] |
| Exosomes | Vascular endothelial cells and nude mice transplantation model |
Promoting exogenous angiogenesis. Increase the proliferation, migration, tube formation, and VEGF secretion. Improving the survival of fat grafts. |
[142] |
| Metabolic diseases | |||
| PDGF | Diabetic patients adipose-derived stem cells and SCID Wound mice model |
Increased proliferation, migration, and homing to sites of inflammation. | [151] |
| Exosomes | Diabetic rat model | Restored erectile function due to corin content in exosomes. | [154] |
| Exosomes | Mouse podocyte MPC5 cells and spontaneous diabetes mice |
Reducing high glucose-induced increase of cell death. Reduction of urine protein, serum creatinine, blood urea nitrogen, and podocyte apoptosis. |
[156] |
| Exosomes (miR-26a-5p) |
Mouse podocyte MPC5 cells and spontaneous diabetes mice |
Protection of cells from injury. Protection against diabetic nephropathy. |
[157] |
| Exosomes | Peritoneal macrophages and obesity mouse model |
Improving obesity-related inflammation and metabolism. | [60] |
| Exosomes | Sepsis-induced acute kidney injury mouse model | Renal Protective effect in acute kidney injury. | [158] |
| Respiratory diseases | |||
| HGF | Pulmonary emphysema rat model | Increased alveolar and vascular repair. | [161] |
| Artifical Nanovesicles | MLE-12 epithelial cells and elastase-induced emphysema mice |
Proliferation increase. Inhibition of emphysema primarily by an FGF2-dependent pathway. |
[162] |
| Exosomes | Histone-induced acute lung injury mice | Improvement of survival. Inhibition histone-mediated lung hemorrhage edema, Reduction of vascular hyper-permeability. |
[80] |
| Skeletal tissue regeneration | |||
| Exosomes | Adipose-derived stem cells | Induction of osteogenic differentiation. | [166] |
| Exosomes | Synovial fibroblasts and periosteal cells | Chondrogenesis promotion. Increased Collagen type II and β-catenin expression. Increased proliferation (miR-145) and chondrogenic potential (miR-221). |
[167] |
| Conditioned Media | Osteoarthritic mice model | Rapid and lasting pain relief. No effect on tissue regeneration. |
[153] |
| Exosomes | Chondrocytes from osteoarthritic patients | Reduction of MMP-1, MMP-3, MMP-13 and ADAMTS-5. Increased collagen II expression. |
[169] |
| Exosomes | Chondrocytes from osteoarthritic patients | Reduction of MMP-3 expression. Increased collagen II expression. |
[168] |
| Whole secretome/isolated exosomes | Mouse myoblast cell line C2C12 and C57BL/6 mice |
Increased cell proliferation, skeletal muscle differentiation and migration. Enhancing of regeneration of skeletal muscle in acute damage. |
[165] |
| Exosomes | Massive rotator cuff tear rat model | Prevention of atrophy, fatty infiltration, inflammation, and vascularization of muscles. | [170] |
| Wound healing and skin aging | |||
| Platelet-rich plasma and conditioned Media | Fibroblasts and keratinocytes isolated from skin sample | Increased cell proliferation and migration. | [172] |
| Conditioned Media | Human epidermal keratinocyte neonatal cells | Acceleration of keratinocyte differentiation via miR-24 upregulation. | [173] |
| Exosomes | Human keratinocyte cell line HaCaT | Increased migration and proliferation by exosomal miR-21. Enhancing MMP-9 expression. Promotion of wound healing. |
[176] |
| Exosomes | Human keratinocyte cell line HaCaT | TGF-β pathway regulation by miR-19b. Promotion of wound healing. |
[79] |
| Exosomes | Human keratinocyte cell line HaCaT | Promotion of wound healing via Wnt/β-catenin pathway. Increased proliferation and migration. Apoptosis inhibition. |
[78] |
| Conditioned media | Human keratinocyte cell line HaCaTs and normal human dermal fibroblasts |
Anti-photoaging activity. Reduced IL-6 secretion. |
[183] |
| Engineered modified exosomes miR-21-5p | Human keratinocyte cell line HaCaT and fullthickness skin defects diabetic rat model |
Promotion of wound healing via Wnt/β-catenin pathway. Improved wound healing by re-epithelialization, collagen remodeling, angiogenesis, and vessel maturation. |
[178] |
| Conditioned Media | Ischemia/reperfusion flap mice model | Increased cell proliferation and the number of hair follicles. Prevention from flap necrosis after skin flap transplantation. |
[174] |
| Exosomes | Human umbilical vein endothelial cell | Reduction of inflammation and apoptosis. Enhancing skin flap recovery. |
[175] |
| Exosomes | Human embryonic kidney 293 cells | Prolonged the survival of vascularized composite allografts after transplantation. Downregulation of CD4 + T and Th1 cells. Upregulation Tr1 and Treg cell. |
[32] |
| Conditioned media | Human dermal fibroblasts | Reduced cellular senescence of skin cells. Improved collagen I, collagen III, elastin, and TIMP-1 expression. |
[182] |
| Exosomes | Atopic dermatitis mouse model | Decreased level of IgE and eosinophiles in blood and CD86+ and CD206+ cells in skin lesion. Reduction of (IL)-4, IL-23, IL-31. |
[179] |
| Exosomes | Chronic allergic dermatitis mouse model | Promotion of epidermal barrier repair. Reduction of IL-5, IL-13, TNF-α, IFN-γ, IL-17, and TSLP. |
[180] |
Two main perspectives are crucial in stem-cell-based therapy. First, the ability of cells, which are the progeny of implanted cells, to rebuild tissues by cell differentiation and proliferation, and second immune cells, due to their trophic activity and stimulation of endogenous regenerative factors [120]. The ASCs secretome can be obtained during culturing in vitro from the culture medium, and then conditioned medium after removal of cell debris can be used directly or fractioned as concentrated formula [121]. Below, we summarize the current stage of knowledge of ASCs and their use in neurodegenerative, inflammatory, cardiovascular, respiratory, and metabolic diseases as well as skin aging and wound healing with particular emphasis on cell-free-based therapy.
4.1. Neurodegenerative Diseases
Neurodegenerative diseases such as Alzheimer’s (AD), Huntington’s (HD), Parkinson’s (PD), Machado–Joseph (Spinocerebellar ataxia type 3, SCA3) diseases, and multiple sclerosis (MS) are characterized by progressive deterioration and/or death of nerve cells that disable brain function [122]. Adipose-derived stem cells secrete angiogenic and antiapoptotic factors such as VEGF, IGF [123,124], TGF-β [125], and HGF involved in neurogenic responses and tissue regeneration [33], as well as neurotrophic factors such as brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), glial cell line-derived neurotrophic factor (GDNF) [126,127,128], and NGF [129] are required for neurons survival and growth. Choi et al. reported that ASCs therapy in a mouse PD model restored the activity of dopaminergic neurons and mitochondrial complex I, improving the behavioral performance of mice after three weeks [130]. Recently, Park and Chang have also shown in a mouse PD model that BDNF and GDNF expressed by ASCs have an effect on the protection of dopaminergic neurons and could be used in the treatment of PD [128]. Trophic factors from ASCs can also be used in the treatment of SCA3 due to protective function of neurons by reducing the production of reactive oxygen species [131]. Furthermore, it has been proven that miR-21 from ASCs stimulates neuronal differentiation [132] as well as miR-24 [133].
In addition, ASC-exos show a key role in neuroprotection and neuroregeneration and have a great potential to be used in neurodegenerative disorders. It has been shown that treatment with ASC-exos can reduce amyloid beta (Aβ) levels and the Aβ42/40 ratio causing neuronal mitochondrial dysfunction in the transgenic mice-derived Alzheimer’s disease, thereby increasing cell survival and reducing neuronal death in the hippocampus and cerebral cortex [134]. ASC-exos carry enzymatically active neprilysin (NEP) that reduces the deposition of Aβ plaques in AD [135]. In turn, Lee et al. showed in in vitro mouse-derived neural cells that ASC-exos can reduce mHtt aggregate accumulation, mitochondrial dysfunction, and cell apoptosis by activating the p-CREB-PGC1α pathway and can also be used in HD treatment [136]. It has also been shown by the same authors that ASC-exos can be used in amyotrophic lateral sclerosis (ALS). Neuronal cells from the G93A ALS mouse model after treatment with ASC-exos showed a reduced level of cytosolic superoxide dismutase 1 (SOD1), which restored the abnormal reduction of mitochondrial proteins [137]. The potential for the use of ASCs extracellular vesicles in MS has also been demonstrated by Laso-Garcia et al. They have shown in a mouse model of progressive multiple sclerosis that administration of EV diminishes brain atrophy and promotes remyelination as well as reduces Th1 and Th17 levels [138]. ASC-derived exosomes used in brain-injured rats have been shown to reduce the inflammatory index (Ly6G+/CD11b/c+), as well as expression of immune cells (CD3+/CD4+/CD3+/CD8+ cells) and apoptotic cells in the circulatory system. Thereby, ASC-exos might also have the potential to prevent brain damage and neurological complications (e.g., sepsis-associated encephalopathy) that are affected by sepsis syndrome [31]. In addition, the long noncoding RNA MALAT1 from ASC-exos modulates the immune response after traumatic brain injury in vivo [139]. One Clinical Trial is currently approved to investigate the impact of ASC-exos in Alzheimer’s disease treatment (NCT04388982). The participants will be treated with exosomes from allogenic adipose-derived stem cells to cure mild to moderate dementia caused by Alzheimer’s disease at three different doses to verify their safety and efficacy. This non-randomized trial now is currently in recruitment and it is estimated to be completed in April 2022.
4.2. Cardiovascular Diseases
Adipose-derived stem cells are of great interest in angiogenic therapy as they are good and abundant source of proangiogenic proteins such as FGF-2, HGF, VEGF, PDGF, Ang-1, Ang-2 [33,58]. They secrete a higher concentration of Ang, LIF, and TGF-β1 factors than BM-MSCs and equal levels of VEGF-A and HGF [81]. In 2009, Wei et al. showed that concentrated ASC conditioned media can block postischemic p38 activation with concomitant neuroprotective function of IGF-1 and BDNF, thus protecting neurons in vitro and in vivo and preventing loss of hippocampal and cortical volume [140]. Similarly, Rehman et al. showed that conditioned media after ASC culture under hypoxia, administrated to mice with hind limbs showed a fivefold increased level of VEGF, and a marked improvement in perfusion [95]. The neovascularization ability and directing ASCs to the fate of vascular cells by VEGF is well known. It has been shown that after ischemia within the myocardial infarction its level increases [141]. Recently, Zhu et al. showed that extracellular vesicles from ASCs promote VEGF secretion by vascular endothelial cells in vitro and in vivo contributing angiogenesis via the let-7/argonaute 1 (AGO1)/VEGF signaling pathway [142]. In addition, Yu et al. showed that mRNA-engineered modified ASCs encoding VEGF promotes and improves vascularization and neo-angiogenesis by improving cell proliferation and vascular maturity [143]. ASC-exos have the potential to be used after ischemic stroke. Chen et al. showed that they can enhance neural regeneration and significantly reduce the infarct after ischemic stroke in a rat model. The administrated dose of ASC-exos, after the third day of acute ischemic stroke reduced cerebral edema and contraction [144]. It has been shown that ASC-exos overexpress miR-21 thus promoting vascularization of endothelial cells [30]. Interestingly, an increased level of PDGF changed the RNA and protein content in extracellular vesicles by modifying the expression of anti-inflammatory and immunomodulatory factors. These changes enhance the production of IL-10 and TGF-β1 and stimulate the formation of T-cells in order to protect the muscle from acute ischemia in vivo [145].
4.3. Metabolic Diseases
Cardiovascular diseases, as well as metabolic diseases (such as diabetes mellitus), may be the effect of obesity. Adipose-derived stem cells have the potential to contribute to the pathophysiology of obesity and metabolic disorders due to their bioactive secretome. Due to their immunomodulatory properties, they can regulate the metabolic inflammation during obesity, and therefore can be used in the treatment of metabolic diseases [146,147]. Moreover, ASCs isolated from WAT are desired to be used in cell therapy for metabolism disorders. They can be easily differentiated into brown adipocytes and this conversion is dependent on PPARγ agonist and differentiated cells express a higher level of uncoupling protein 1 (UCP1) important for adaptive thermogenesis. It has been already shown that ASCs-implanted animals demonstrated less weight gain than animals in the control group [146,148,149]. Recently, Ikemoto et al. showed that ASCs isolated from diabetic and non-diabetic patients exhibit the same phenotype and can be differentiated into insulin-producing cells (IPCs) characterized by the autonomous insulin secretion. They showed in a diabetic mellitus 1 mouse model that IPCs from diabetic 1 patient ASCs can affect normoglycemia through prolonged insulin secretion. Moreover, the programmed cell death ligand-1 (PDL-1) secreted by these cells can be considered as circumvention of immunity [20]. ASCs also have the potential to be used in type 2 diabetes mellitus. It has been shown that ASCs treatment improves insulin sensitivity and results in the lower number of β-cells death [21].
A similar observation was made by Cao et al. where ASC-treated mice had better glucose tolerance, reduced triglyceride, and high-density lipoprotein level as well as reduced expression of IL-6, but also increased InsR and PPARγ expression [150]. The specific properties of ASCs are related to their secretome. Zhao et al. showed that exosomes transferred to macrophages induce the anti-inflammatory M2 macrophages phenotype by STAT3 and transactivation of arginase 1 and IL-10 increasing expression of uncoupling protein 1. These results suggest that exosomes can be used in clinical treatment due to improved obesity-related inflammation and metabolism [60]. Furthermore, it has been shown that PDGF can reverse the changes induced by diabetes, such as reduced proliferation and inhibition of migration, as well as targeting sites of inflammation and impaired function of diabetic ASCs [151].
Neuropathic pain, a prevalent compilation of diabetic mellitus and it has been shown that ASCs [34] and ASC conditioned media can be used in advanced peripheral painful neuropathy [152]. Brini et al. showed that diabetic mice after ASC conditioned media (from 2 × 106 ASC culture) treatment restored the balance of pro-inflammatory cytokines and prevented the loss of skin innervation. Most importantly reversed allodynia and hyperalgesia effect lasted up to 12 weeks [151]. Interestingly, the same group of Sacerdote in 2021 published a paper in which conditioned media (from 2 × 106 ASCs) after injection into the tail vein in osteoarthritic mice showed rapid and lasting pain relief but did not affect tissue regeneration [153].
Exosomes have also been shown to positively affect chronic complications of diabetes mellitus such as erectile dysfunction, cognitive decline, diabetic nephropathy, or wounds. Wang et al. used exosomes isolated from ASCs in a rat model and showed that corin (transmembrane serine proteinase, converting pro-atrial natriuretic peptide (ANP) and pro-brain natriuretic peptide (BNP) to active forms) present in these exosomes has a beneficial therapeutic influence on erectile dysfunction treatment [154]. ASCs also have neuroprotective properties regardless of cause and have been shown to be of use in the prevention of cognitive impairment in diabetic mellitus patients by peripheral reduction of advanced glycation end products [155]. In turn, Jin et al. showed that ASC-exos attenuate diabetic nephropathy and reduce amount of serum creatinine, urea nitrogen, and urine protein, as well as podocyte apoptosis in a mouse model. These effects were caused by enhanced miR-486 expression and thus inhibition of the Smad1/mTOR podocyte signaling pathway [156]. On the other hand, Duan et al. showed that miR-26a-5p from ASC-exos also protects against diabetic nephropathy by targeting toll-like receptor 4 (TLR4), silencing the NF-κB pathway, and downregulating VEGF-A secretion [157]. Furthermore, Gao et al. suggest that ASC-exos renal protective effect may be through the SIRT1 signaling pathway [158]. The use of exosomes in wound healing from diabetes mellitus and other causes is described in the chapter on wound healing and skin aging.
4.4. Respiratory Diseases
Acute respiratory distress syndrome is often observed in critically ill patients (~10% of all patients in intensive care units worldwide), and is most commonly associated with sepsis, pneumonia (bacterial and viral), severe trauma or aspiration of gastric, and/or oral and oesophageal contents [159]. Paracrine factors such as IFN-γ, IL-1β, IL-6, IL-10, TGF-β1, TGF-6, VEGF, HGF, PGE2, Ang-1, and IDO secreted by ASCs can attenuate lung injury [160]. Shigemura et al. showed that HGF from ASCs can improve gas exchange thus treat pulmonary emphysema [161]. In turn, Kim et al. showed that ASCs nanovesicles can also inhibit pulmonary emphysema by FGF-2 dependent pathway and can be used in cell-free therapy in a lower dose than naturally delivered ASC-exos demonstrating high treatment efficiency [162]. It has been also shown that ASCs have the potential for use in acute lung injury (ALI)—severe pulmonary inflammation—treatment by increased secretion of IL-6 in anti-inflammatory modulation [163]. Recently, ASC-exos have been shown to alleviate histone-induced ALI by activating PI3K/Akt pathway. Exosomal miR-126 has been shown to be a key factor in increasing Akt phosphorylation in the mouse ALI model [80].
The national clinical database has registered one study of ASC-exos in drug resistance gram-negative bacilli pulmonary infection (NCT04544215). This randomized study with 60 participants will use exosome aerosol inhalation in two doses (8 × 108 and 16 × 108 for seven days) and placebo groups. The results are not yet available, the current state of this study is recruitment, and the end of the study is estimated for July 2023.
Adipose-derived stem cells are also of great interest for treatment of COVID-19 respiratory distress. It has been shown that 13 patients with COVID-19 pneumonia treated with ASCs, exhibited clinical and biological improvement and a reduction in secreted inflammatory indicators such as C-reactive protein, IL-6, ferritin, LDH, and d-dimer [164]. ASC-exos are also being tested. A clinical trial (NCT04276987) with 24 critically ill patients is testing the use of conventional treatment and ASC-exos by inhaling an aerosol five times (2 × 108 nanovesicles at day 1, 2, 3, 4, 5) but the results are not yet published.
4.5. Skeletal Tissue Regeneration
Skeletal tissue clinical trials are registered in the highest number of all clinical trials using ASCs (Figure 4). Most of these studies focus on osteoarthritis. The ASCs secretome also has the potential to regenerate skeletal tissue. Mitchel et al. showed that ASCs secretome increases tissue regeneration and homeostasis of the mouse myoblast cell line by promoting skeletal muscle proliferation, differentiation, and migration. This may be due to the presence of soluble proteins and exosomal immunomodulatory miR-21 [165]. On the other hand, ASC-exos has been shown to induce osteogenic differentiation [166] and also suppress inflammation by upregulating miR-145 and miR-221, and therefore can be helpful in the treatment of osteoarthritis [167]. It has already been shown in the case of chondrocytes isolated from osteoarthritic patients that treatment with ASCs extracellular vesicles reduces MMP-1, MMP-3, MMP-13, and ADAMTS-5 and increases collagen II expression [168,169]. ASC-exos also have the potential to regenerate muscles. Wang et al. showed in a rat model that exosomes can prevent muscle degradation in torn rotator cuffs by atrophy, fat infiltration, inflammation, and prevention of vascularization. However, they pointed out that they had used a population of young, although mature rats, while torn rotator cuffs are common in the elderly population and that additional studies with older animals are required [170].
4.6. Wound Healing and Skin Aging
The skin as the first physical barrier against physical and biological damage and its self-repairing ability, protect against dehydration and thermal, chemical, and physical stress. Repairing physical damage is a dynamic process involving overlapping stages such as homeostasis, inflammation, proliferation, re-epithelization, and fibrosis [171]. ASCs secrete four major growth factors that promote re-epithelization: EGF, FGF-2, IGF-1, and TGF-β. These factors induce the necessary biological effects for tissue repair including cell migration, proliferation, and differentiation but also promotion of angiogenesis and extracellular production and inflammation resolution [101]. It has already been shown that co-treatment with administration of ASCs and conditioned ASCs media increases the proliferation of dermal keratinocytes and fibroblast in the skin [172] but also the maturation of fibroblasts associated with miR-24 upregulation [173]. Moreover, Pu et al. showed that ASCs conditioned media prevent flap necrosis after skin flap transplantation by increasing proliferation and secretion of IL-6 [174]. Similarly, Bai et al. showed that ASC-exos enhances skin flap recovery by reducing inflammation and apoptosis [175]. ASC-exos prolong the survival of vascularized composite allografts after transplantation by downregulating CD4+ T and Th1 cells and upregulating Tr1 and Treg cells [32]. It shows that the use of ASCs conditioned media as well as exosomes may be a promising approach in reconstructive and plastic surgery.
Tissue repairing requires the mitigation of inflammatory insults. Besides secreted immunomodulatory factors by ASCs, it is possible to govern inflammatory pathways by extracellular vesicles assisted by transfer at the site of injury with RNA derived from adipose-derived stem cells [105]. It has been shown that ASC-exos express miR-21, which increases migration and proliferation of HaCaT cells by enhancing the matrix metalloproteinase 9 (MMP-9) expression via the PI3K/AKT pathway, thereby increasing wound healing [176]. Furthermore, miR-19b from ASC-exos promotes wound healing by regulating the TGF-β pathway through targeting chemokine C-C motif ligand 1 (CCL1) by modulating the CCL1/TGF-β signaling axis [75]. Interestingly, TGF-β secreted by ASCs has been shown to cross react with growth differentiation factor 11 (GDF11) to reverse keratinocytes aging and trigger skin rejuvenation [177]. Improved re-epithelialization, collagen remodeling, angiogenesis, and vessel maturation and thus wound healing were also demonstrated in diabetic mice treated with engineered ASC-exos containing miR-21-5p. In Lv et al.’s study, keratinocytes proliferation and migration and accelerated wound healing were performed via the Wnt/β-catenin signaling pathway in vitro [178], confirming the results obtained by Ma et al. a year earlier, where ASC-exos was shown to improve wound healing also via the Wnt/β-catenin pathway [78]. ASC-exos can also be used as a potential factor in alleviating atopic dermatitis. It has been found that an in vivo model of atopic dermatitis after ASC-exos injection exhibited reduced clinical score, decreased level of serum IgE and blood eosinophil counts, as well as CD86+ and CD206+ cells in skin lesions, as well as diminished the infiltration of mast cells. Moreover, it has been shown that levels of inflammatory cytokines such as IL-4, IL-23, and IL-31 were reduced [179]. Recently, Shin et al. showed that not only the levels of these cytokines are reduced after ASC-exos treatment in atopic dermatitis, but also IL-5, IL-13, TNF-α, IFN-γ, IL-17, and TSLP. They also showed that after ASC-exos treatment, cells restored expression of genes responsible for lipid metabolism, the cell cycle, and the inflammatory response, as well as improving the skin barrier [180].
Furthermore, adipose-derived stem cells and their secretome can be used in skin rejuvenation and wrinkle reduction, mostly by stimulating collagen synthesis and regulating the proliferation and migration of dermal fibroblasts [181]. Guo et al. showed the protective function of ASCs conditioned media on dermal fibroblasts and keratinocytes against UVB-induced photoaging. They showed that reduced skin cellular senescence was observed in the group stimulated with conditioned media after UVB irradiation. Moreover, treatment with ASCs conditioned media improved collagen I, collagen III, elastin, and TIMP-1 expression. However, they also showed that inhibitory effect of ASCs conditioned media on human dermal fibroblasts senescence decreased with fibroblasts passages [182]. In turn, Li et al. showed that ASCs conditioned media may downregulate mitogen-activated protein kinases (MAPKs), activator protein 1 (AP-1), and nuclear factor kappa B (NF-κB) UVB-induced signaling pathways and reduce IL-6 secretion [183].
5. Conclusions
Adipose-derived stem cells secretome is considered as a potential agent for treatment of various diseases, e.g., multiple sclerosis, rheumatoid arthritis, osteoarthritis, fistulae, diabetes mellitus, and cardiovascular diseases, but also in skin aging and wound healing. ASCs properties are effected by the specific content of its secretome: cytokines, proteins, growth factors, and exosomes with several types of RNAs, characterized by extensive bioactivity including immunomodulatory, antiapoptotic, angiogenic, vasculogenic, neurogenic, and epithelial activity. The application of bioactive factors without administering whole cells is a safer alternative treatment and may be more effective. Nowadays, many in vitro and in vivo studies from the last four years confirm effectiveness of ASCs secretome therapy, mostly with ASCs derived exosomes. Several clinical trials are currently being evaluated for safety and effectiveness of ASC-derived exosomes therapy, but the results are not yet available. Nevertheless, further research in vivo and in vitro are needed to understand the specific role of ASCs secretome in repairing of damaged and diseased tissues.
Author Contributions
A.T. designed and wrote the manuscript and created all figures and tables. A.B.-Z. conceived of the project, coordinated and revised the manuscript critically for important intellectual content, and gave final approval of this version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
This research has not been supported from external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing interest(s).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Varderidou-Minasian S., Lorenowicz M.J. Mesenchymal stromal/stem cell-derived extracellular vesicles in tissue repair: Challenges and opportunities. Theranostics. 2020;10:5979–5997. doi: 10.7150/thno.40122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samsonraj R.M., Raghunath M., Nurcombe V., Hui J.H., Van Wijnen A.J., Cool S.M. Concise Review: Multifaceted Characterization of Human Mesenchymal Stem Cells for Use in Regenerative Medicine. Stem Cells Transl. Med. 2017;6:2173–2185. doi: 10.1002/sctm.17-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominici M., Le Blanc K., Mueller I., Slaper-Cortenbach I., Marini F.C., Krause D.S., Deans R.J., Keating A., Prockop D.J., Horwitz E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 4.Friedenstein A.J., Gorskaja J.F., Kulagina N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976;4:267–274. [PubMed] [Google Scholar]
- 5.Prindull G., Prindull B., Meulen N. Haematopoietic stem cells (CFUc) in human cord blood. Acta Paediatr. 1978;67:413–416. doi: 10.1111/j.1651-2227.1978.tb16347.x. [DOI] [PubMed] [Google Scholar]
- 6.Miura M., Gronthos S., Zhao M., Lu B., Fisher L.W., Robey P.G., Shi S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA. 2003;100:5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delo D.M., De Coppi P., Bartsch G., Jr., Atala A. Amniotic fluid and placental stem cells. Methods Enzymol. 2006;419:426–438. doi: 10.1016/S0076-6879(06)19017-5. [DOI] [PubMed] [Google Scholar]
- 8.Berebichez-Fridman R., Montero-Olvera P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018;18:264–277. doi: 10.18295/squmj.2018.18.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamed-Ahmed S., Fristad I., Lie S.A., Suliman S., Mustafa K., Vindenes H., Idris S.B. Adipose-derived and bone marrow mesenchymal stem cells: A donor-matched comparison. Stem Cell Res. Ther. 2018;9:168. doi: 10.1186/s13287-018-0914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai R., Wang Z., Samanipour R., Koo K.-I., Kim K. Adipose-Derived Stem Cells for Tissue Engineering and Regenerative Medicine Applications. Stem Cells Int. 2016;2016:6737345. doi: 10.1155/2016/6737345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang T., He D., Kleiner G., Kuluz J.T. Neuron-like Differentiation of Adipose-Derived Stem Cells from Infant Piglets in Vitro. J. Spinal Cord Med. 2007;30:S35–S40. doi: 10.1080/10790268.2007.11753967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu C.-H., Tong Y.-W., Yeh W.-L., Lei K.F., Chen A.C.-Y. Self-Renewal and Differentiation of Adipose-Derived Stem Cells (ADSCs) Stimulated by Multi-Axial Tensile Strain in a Pneumatic Microdevice. Micromachines. 2018;9:607. doi: 10.3390/mi9110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parate D., Kadir N.D., Celik C., Lee E.H., Hui J.H.P., Franco-Obregón A., Yang Z. Pulsed electromagnetic fields potentiate the paracrine function of mesenchymal stem cells for cartilage regeneration. Stem Cell Res. Ther. 2020;11:46. doi: 10.1186/s13287-020-1566-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández O., Izquierdo G., Fernández V., Leyva L., Reyes V., Guerrero M., León A., Arnaiz C., Navarro G., Páramo M.D., et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE. 2018;13:e0195891. doi: 10.1371/journal.pone.0195891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi E.W., Shin I.S., Song J.W., Lee M., Yun T.W., Yang J., Choi K.-S., Kim S.-J. Effects of Transplantation of CTLA4Ig-Overexpressing Adipose Tissue-Derived Mesenchymal Stem Cells in Mice with Sustained Severe Rheumatoid Arthritis. Cell Transplant. 2016;25:243–259. doi: 10.3727/096368915X688470. [DOI] [PubMed] [Google Scholar]
- 16.Ueyama H., Okano T., Orita K., Mamoto K., Sobajima S., Iwaguro H., Nakamura H. Local transplantation of adipose-derived stem cells has a significant therapeutic effect in a mouse model of rheumatoid arthritis. Sci. Rep. 2020;10:3076. doi: 10.1038/s41598-020-60041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonomoto K., Yamaoka K., Kaneko H., Yamagata K., Sakata K., Zhang X., Kondo M., Zenke Y., Sabanai K., Nakayamada S., et al. Spontaneous Differentiation of Human Mesenchymal Stem Cells on Poly-Lactic-Co-Glycolic Acid Nano-Fiber Scaffold. PLoS ONE. 2016;11:e0153231. doi: 10.1371/journal.pone.0153231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Álvarez P.D.-A., Garcia-Arranz M., Georgiev-Hristov T., Olmo D.G. A new bronchoscopic treatment of tracheomediastinal fistula using autologous adipose-derived stem cells. Thorax. 2008;63:374–376. doi: 10.1136/thx.2007.083857. [DOI] [PubMed] [Google Scholar]
- 19.Olmo D.G., Herreros D., De-La-Quintana P., Guadalajara H., Trébol J., Georgiev-Hristov T., Garcia-Arranz M. Adipose-Derived Stem Cells in Crohn’s Rectovaginal Fistula. Case Rep. Med. 2010;2010:961758. doi: 10.1155/2010/961758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikemoto T., Tokuda K., Wada Y., Gao L., Miyazaki K., Yamada S., Saito Y., Imura S., Morine Y., Shimada M. Adipose Tissue from Type 1 Diabetes Mellitus Patients Can Be Used to Generate Insulin-Producing Cells. Pancreas. 2020;49:1225–1231. doi: 10.1097/MPA.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 21.Wang M., Song L., Strange C., Dong X., Wang H. Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes. Mol. Ther. 2018;26:1921–1930. doi: 10.1016/j.ymthe.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu G.-Y., Liu J., Wang Y.-L., Liu Y., Shao Y., Han Y., Qin Y.-R., Xiao F.-J., Li P.-F., Zhao L.-J., et al. Adipose-Derived Mesenchymal Stem Cells Ameliorate Lipid Metabolic Disturbance in Mice. Stem Cells Transl. Med. 2016;5:1162–1170. doi: 10.5966/sctm.2015-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perin E.C., Sanz-Ruiz R., Sanchez P.L., Lasso J., Pérez-Cano R., Alonso-Farto J.C., Pérez-David E., Santos M.E.F., Serruys P.W., Duckers H.J., et al. Adipose-derived regenerative cells in patients with ischemic cardiomyopathy: The PRECISE Trial. Am. Heart J. 2014;168:88–95.e2. doi: 10.1016/j.ahj.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.-W., Scutaru T.T., Ghetu N., Carasevici E., Lupascu C.D., Ferariu D., Pieptu D., Coman C.-G., Danciu M. The Effects of Adipose-Derived Stem Cell-Differentiated Adipocytes on Skin Burn Wound Healing in Rats. J. Burn Care Res. 2017;38:1–10. doi: 10.1097/BCR.0000000000000466. [DOI] [PubMed] [Google Scholar]
- 25.Gaur M., Dobke M., Lunyak V.V. Mesenchymal Stem Cells from Adipose Tissue in Clinical Applications for Dermatological Indications and Skin Aging. Int. J. Mol. Sci. 2017;18:208. doi: 10.3390/ijms18010208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heo J.S., Kim S., Yang C.E., Choi Y., Song S.Y., Kim H.O. Human Adipose Mesenchymal Stem Cell-Derived Exosomes: A Key Player in Wound Healing. Tissue Eng. Regen. Med. 2021 doi: 10.1007/s13770-020-00316-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banaś A., Teratani T., Yamamoto Y., Tokuhara M., Takeshita F., Osaki M., Kawamata M., Kato T., Okochi H., Ochiya T. IFATS Collection: In Vivo Therapeutic Potential of Human Adipose Tissue Mesenchymal Stem Cells after Transplantation into Mice with Liver Injury. Stem Cells. 2008;26:2705–2712. doi: 10.1634/stemcells.2008-0034. [DOI] [PubMed] [Google Scholar]
- 28.Trzyna A., Pikuła B., Ludwin A., Kocan B., Banaś-Ząbczyk A. The influence of an electromagnetic field on adipose-derived stem/stromal cells’ growth factor secretion: Modulation of FGF-2 production by in vitro exposure. Arch. Biol. Sci. 2020;72:339–347. doi: 10.2298/ABS200321028T. [DOI] [Google Scholar]
- 29.Kilroy G.E., Foster S.J., Wu X., Ruiz J., Sherwood S., Heifetz A., Ludlow J.W., Stricker D.M., Potiny S., Green P., et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J. Cell. Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 30.An Y., Zhao J., Nie F., Qin Z., Xue H., Wang G., Li D. Exosomes from Adipose-Derived Stem Cells (ADSCs) Overexpressing miR-21 Promote Vascularization of Endothelial Cells. Sci. Rep. 2019;9:12861. doi: 10.1038/s41598-019-49339-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang C.L., Chen H.H., Chen K.H., Chiang J.Y., Li Y.C., Lin H.S., Sung P.H., Yip H.K. Adipose-derived mesenchymal stem cell-derived exosomes markedly protected the brain against sepsis syndrome induced injury in rat. Am. J. Transl. Res. 2019;11:3955–3971. [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Xue S., Zhang S., Cheng K., Ye Q. Exosomes from donor-derived adipose mesenchymal stem cells prolong the survival of vascularized composite allografts. J. Cell. Physiol. 2021;236:5895–5905. doi: 10.1002/jcp.30274. [DOI] [PubMed] [Google Scholar]
- 33.Dubey N.K., Mishra V.K., Dubey R., Deng Y.-H., Tsai F.-C., Deng W.-P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018;19:2200. doi: 10.3390/ijms19082200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Franchi S., Castelli M., Amodeo G., Niada S., Ferrari D., Vescovi A.L., Brini A.T., Panerai A.E., Sacerdote P. Adult Stem Cell as New Advanced Therapy for Experimental Neuropathic Pain Treatment. BioMed Res. Int. 2014;2014:470983. doi: 10.1155/2014/470983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sacerdote P., Niada S., Franchi S., Arrigoni E., Rossi A., Yenagi V., de Girolamo L., Panerai A.E., Brini A.T. Systemic Administration of Human Adipose-Derived Stem Cells Reverts Nociceptive Hypersensitivity in an Experimental Model of Neuropathy. Stem Cells Dev. 2013;22:1252–1263. doi: 10.1089/scd.2012.0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Álvaro-Gracia J.M., Jover J.A., García-Vicuña R., Carreño L., Alonso A., Marsal S., Blanco F., Martínez-Taboada V.M., Taylor P., Martín-Martín C., et al. Intravenous administration of expanded allogeneic adipose-derived mesenchymal stem cells in refractory rheumatoid arthritis (Cx611): Results of a multicentre, dose escalation, randomised, single-blind, placebo-controlled phase Ib/IIa clinical trial. Ann. Rheum. Dis. 2017;76:196–202. doi: 10.1136/annrheumdis-2015-208918. [DOI] [PubMed] [Google Scholar]
- 37.Seo Y., Shin T.-H., Kim H.-S. Current Strategies to Enhance Adipose Stem Cell Function: An Update. Int. J. Mol. Sci. 2019;20:3827. doi: 10.3390/ijms20153827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ankrum J., Ong J.F., Karp J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Y., Zeng Q., Han Q., Xia W. Effect of pH, temperature and freezing-thawing on quantity changes and cellular uptake of exosomes. Protein Cell. 2019;10:295–299. doi: 10.1007/s13238-018-0529-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar A., Xu Y., Yang E., Du Y. Stemness and Regenerative Potential of Corneal Stromal Stem Cells and Their Secretome after Long-Term Storage: Implications for Ocular Regeneration. Investig. Opthalmol. Vis. Sci. 2018;59:3728–3738. doi: 10.1167/iovs.18-23824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baer P.C. Adipose-derived mesenchymal stromal/stem cells: An update on their phenotype in vivo and in vitro. World J. Stem Cells. 2014;6:256–265. doi: 10.4252/wjsc.v6.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barry F.P., Murphy J. Mesenchymal stem cells: Clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 2004;36:568–584. doi: 10.1016/j.biocel.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Bunnell B.A., Flaat M., Gagliardi C., Patel B., Ripoll C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods. 2008;45:115–120. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kocan B., Maziarz A., Tabarkiewicz J., Ochiya T., Banaś-Ząbczyk A. Trophic Activity and Phenotype of Adipose Tissue-Derived Mesenchymal Stem Cells as a Background of Their Regenerative Potential. Stem Cells Int. 2017;2017:1653254. doi: 10.1155/2017/1653254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zuk P.A., Zhu M., Mizuno H., Huang J., Futrell J.W., Katz A.J., Benhaim P., Lorenz H.P., Hedrick M.H. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 46.Wang Q., Wu H. T Cells in Adipose Tissue: Critical Players in Immunometabolism. Front. Immunol. 2018;9:2509. doi: 10.3389/fimmu.2018.02509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silva F.J., Holt D.J., Vargas V., Yockman J., Boudina S., Atkinson D., Grainger D.W., Revelo M.P., Sherman W., Bull D.A., et al. Metabolically Active Human Brown Adipose Tissue Derived Stem Cells. Stem Cells. 2014;32:572–581. doi: 10.1002/stem.1595. [DOI] [PubMed] [Google Scholar]
- 48.Si Z., Wang X., Sun C., Kang Y., Xu J., Wang X., Hui Y. Adipose-derived stem cells: Sources, potency, and implications for regenerative therapies. Biomed. Pharmacother. 2019;114:108765. doi: 10.1016/j.biopha.2019.108765. [DOI] [PubMed] [Google Scholar]
- 49.Gesta S., Tseng Y.-H., Kahn C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Tsekouras A., Mantas D., Tsilimigras I.D., Moris D., Kontos M., Zografos C.G. Comparison of the Viability and Yield of Adipose-Derived Stem Cells (ASCs) from Different Donor Areas. In Vivo. 2017;31:1229–1234. doi: 10.21873/invivo.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feijóo-Bandín S., Rodríguez-Penas D., García-Rúa V., Mosquera-Leal A., González-Juanatey J.R., Lago F. Adipokines at the Cardiovascular System: Role in Health and Disease. SM J. Endocrinol. Metab. 2016;2:1009. [Google Scholar]
- 52.Prunet-Marcassus B., Cousin B., Caton D., André M., Penicaud L., Casteilla L. From heterogeneity to plasticity in adipose tissues: Site-specific differences. Exp. Cell Res. 2006;312:727–736. doi: 10.1016/j.yexcr.2005.11.021. [DOI] [PubMed] [Google Scholar]
- 53.Nepali S., Park M., Lew H., Kim O. Comparative Analysis of Human Adipose-Derived Mesenchymal Stem Cells from Orbital and Abdominal Fat. Stem Cells Int. 2018;2018:3932615. doi: 10.1155/2018/3932615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schipper B.M., Marra K.G., Zhang W., Donnenberg A.D., Rubin J.P. Regional Anatomic and Age Effects on Cell Function of Human Adipose-Derived Stem Cells. Ann. Plast. Surg. 2008;60:538–544. doi: 10.1097/SAP.0b013e3181723bbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harrison M.A.A., Wise R.M., Benjamin B.P., Hochreiner E.M., Mohiuddin O.A., Bunnell B.A. Adipose-Derived Stem Cells from Obese Donors Polarize Macrophages and Microglia toward a Pro-Inflammatory Phenotype. Cells. 2020;10:26. doi: 10.3390/cells10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oñate B., Vilahur G., Camino-López S., Díez-Caballero A., Ballesta-López C., Ybarra J., Moscatiello F., Herrero J., Badimon L. Stem cells isolated from adipose tissue of obese patients show changes in their transcriptomic profile that indicate loss in stemcellness and increased commitment to an adipocyte-like phenotype. BMC Genom. 2013;14:625. doi: 10.1186/1471-2164-14-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Badimon L., Cubedo J. Adipose tissue depots and inflammation: Effects on plasticity and resident mesenchymal stem cell function. Cardiovasc. Res. 2017;113:1064–1073. doi: 10.1093/cvr/cvx096. [DOI] [PubMed] [Google Scholar]
- 58.Badimon L., Oñate B., Vilahur G. Adipose-derived Mesenchymal Stem Cells and Their Reparative Potential in Ischemic Heart Disease. Revista Española Cardiología. 2015;68:599–611. doi: 10.1016/j.recesp.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 59.Varghese J., Griffin M., Mosahebi A., Butler P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017;8:45. doi: 10.1186/s13287-017-0483-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao H., Shang Q., Pan Z., Bai Y., Lining Z., Zhang H., Zhang Q., Guo C., Zhang L., Wang Q. Exosomes from Adipose-Derived Stem Cells Attenuate Adipose Inflammation and Obesity through Polarizing M2 Macrophages and Beiging in White Adipose Tissue. Diabetes. 2018;67:235–247. doi: 10.2337/db17-0356. [DOI] [PubMed] [Google Scholar]
- 61.Bourin P., Bunnell B.A., Casteilla L., Dominici M., Katz A.J., March K.L., Redl H., Rubin J.P., Yoshimura K., Gimble J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Di Maggio N., Martella E., Frismantiene A., Resink T.J., Schreiner S., Lucarelli E., Jaquiery C., Schaefer D.J., Martin I., Scherberich A. Extracellular matrix and α5β1 integrin signaling control the maintenance of bone formation capacity by human adipose-derived stromal cells. Sci. Rep. 2017;7:44398. doi: 10.1038/srep44398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Morath I., Hartmann T.N., Orian-Rousseau V. CD44: More than a mere stem cell marker. Int. J. Biochem. Cell Biol. 2016;81:166–173. doi: 10.1016/j.biocel.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 64.Gentile P., Piccinno M.S., Calabrese C. Characteristics and Potentiality of Human Adipose-Derived Stem Cells (hASCs) Obtained from Enzymatic Digestion of Fat Graft. Cells. 2019;8:282. doi: 10.3390/cells8030282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mitchell J.B., McIntosh K., Zvonic S., Garrett S., Floyd E., Kloster A., Di Halvorsen Y., Storms R.W., Goh B., Kilroy G., et al. Immunophenotype of Human Adipose-Derived Cells: Temporal Changes in Stromal-Associated and Stem Cell-Associated Markers. Stem Cells. 2006;24:376–385. doi: 10.1634/stemcells.2005-0234. [DOI] [PubMed] [Google Scholar]
- 66.Lin C.-S., Ning H., Lin G., Lue T.F. Is CD34 truly a negative marker for mesenchymal stromal cells? Cytotherapy. 2012;14:1159–1163. doi: 10.3109/14653249.2012.729817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gronthos S., Franklin D.M., Leddy H.A., Robey P.G., Storms R.W., Gimble J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001;189:54–63. doi: 10.1002/jcp.1138. [DOI] [PubMed] [Google Scholar]
- 68.Yang Z.X., Han Z.-B., Ji Y.R., Wang Y.W., Liang L., Chi Y., Yang S.G., Li L.N., Luo W.F., Li J.P., et al. CD106 Identifies a Subpopulation of Mesenchymal Stem Cells with Unique Immunomodulatory Properties. PLoS ONE. 2013;8:e59354. doi: 10.1371/journal.pone.0059354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suga H., Matsumoto D., Eto H., Inoue K., Aoi N., Kato H., Araki J., Yoshimura K. Functional Implications of CD34 Expression in Human Adipose-Derived Stem/Progenitor Cells. Stem Cells Dev. 2009;18:1201–1210. doi: 10.1089/scd.2009.0003. [DOI] [PubMed] [Google Scholar]
- 70.Mieczkowska A., Schumacher A., Filipowicz N., Wardowska A., Zieliński M., Madanecki P., Nowicka E., Langa P., Deptuła M., Zieliński J., et al. Immunophenotyping and transcriptional profiling of in vitro cultured human adipose tissue derived stem cells. Sci. Rep. 2018;8:11339. doi: 10.1038/s41598-018-29477-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhu Y., Liu T., Song K., Fan X., Ma X., Cui Z. Adipose-derived stem cell: A better stem cell than BMSC. Cell Biochem. Funct. 2008;26:664–675. doi: 10.1002/cbf.1488. [DOI] [PubMed] [Google Scholar]
- 72.El Atat O., Antonios D., Hilal G., Hokayem N., Abou-Ghoch J., Hashim H., Serhal R., Hebbo C., Moussa M., Alaaeddine N. An Evaluation of the Stemness, Paracrine, and Tumorigenic Characteristics of Highly Expanded, Minimally Passaged Adipose-Derived Stem Cells. PLoS ONE. 2016;11:e0162332. doi: 10.1371/journal.pone.0162332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Griffin M., Ryan C.M., Pathan O., Abraham D., Denton C.P., Butler P.E.M. Characteristics of human adipose derived stem cells in scleroderma in comparison to sex and age matched normal controls: Implications for regenerative medicine. Stem Cell Res. Ther. 2017;8:23. doi: 10.1186/s13287-016-0444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma H., Li Y.-N., Song L., Liu R., Li X., Shang Q., Wang Y., Shao C., Shi Y. Macrophages inhibit adipogenic differentiation of adipose tissue derived mesenchymal stem/stromal cells by producing pro-inflammatory cytokines. Cell Biosci. 2020;10:88. doi: 10.1186/s13578-020-00450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noverina R., Widowati W., Ayuningtyas W., Kurniawan D., Afifah E., Laksmitawati D.R., Rinendyaputri R., Rilianawati R., Faried A., Bachtiar I., et al. Growth factors profile in conditioned medium human adipose tissue-derived mesenchymal stem cells (CM-hATMSCs) Clin. Nutr. Exp. 2019;24:34–44. doi: 10.1016/j.yclnex.2019.01.002. [DOI] [Google Scholar]
- 76.Tratwal J., Mathiasen A.B., Juhl M., Brorsen S.K., Kastrup J., Ekblond A. Influence of vascular endothelial growth factor stimulation and serum deprivation on gene activation patterns of human adipose tissue-derived stromal cells. Stem Cell Res. Ther. 2015;6:62. doi: 10.1186/s13287-015-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Blaber S.P., Webster R.A., Hill C.J., Breen E.J., Kuah D., Vesey G., Herbert B.R. Analysis of in vitro secretion profiles from adipose-derived cell populations. J. Transl. Med. 2012;10:172. doi: 10.1186/1479-5876-10-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ma T., Fu B., Yang X., Xiao Y., Pan M. Adipose mesenchymal stem cell-derived exosomes promote cell proliferation, migration, and inhibit cell apoptosis via Wnt/β-catenin signaling in cutaneous wound healing. J. Cell. Biochem. 2019;120:10847–10854. doi: 10.1002/jcb.28376. [DOI] [PubMed] [Google Scholar]
- 79.Cao G., Chen B., Zhang X., Chen H. Human Adipose-Derived Mesenchymal Stem Cells-Derived Exosomal microRNA-19b Promotes the Healing of Skin Wounds through Modulation of the CCL1/TGF-β Signaling Axis. Clin. Cosmet. Investig. Dermatol. 2020;13:957–971. doi: 10.2147/CCID.S274370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mizuta Y., Akahoshi T., Guo J., Zhang S., Narahara S., Kawano T., Murata M., Tokuda K., Eto M., Hashizume M., et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res. Ther. 2020;11:508. doi: 10.1186/s13287-020-02015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Adolfsson E., Helenius G., Friberg Ö., Samano N., Frøbert O., Johansson K. Bone marrow- and adipose tissue-derived mesenchymal stem cells from donors with coronary artery disease; growth, yield, gene expression and the effect of oxygen concentration. Scand. J. Clin. Lab. Investig. 2020;80:318–326. doi: 10.1080/00365513.2020.1741023. [DOI] [PubMed] [Google Scholar]
- 82.Ding D.-C., Chou H.-L., Hung W.-T., Liu H.-W., Chu T.-Y. Human adipose-derived stem cells cultured in keratinocyte serum free medium: Donor’s age does not affect the proliferation and differentiation capacities. J. Biomed. Sci. 2013;20:59. doi: 10.1186/1423-0127-20-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wu H., Li J.-Z., Xie B.-D., Tian H., Fang S.-H., Jiang S.-L., Kang K. Lower Senescence of Adipose-Derived Stem Cells than Donor-Matched Bone Marrow Stem Cells for Surgical Ventricular Restoration. Stem Cells Dev. 2018;27:612–623. doi: 10.1089/scd.2017.0271. [DOI] [PubMed] [Google Scholar]
- 84.Ceccarelli S., Pontecorvi P., Anastasiadou E., Napoli C., Marchese C. Immunomodulatory Effect of Adipose-Derived Stem Cells: The Cutting Edge of Clinical Application. Front. Cell Dev. Biol. 2020;8:236. doi: 10.3389/fcell.2020.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Niu P., Smagul A., Wang L., Sadvakas A., Sha Y., Pérez L.M., Nussupbekova A., Amirbekov A., Akanov A.A., Gálvez B.G., et al. Transcriptional profiling of interleukin-2-primed human adipose derived mesenchymal stem cells revealed dramatic changes in stem cells response imposed by replicative senescence. Oncotarget. 2015;6:17938–17957. doi: 10.18632/oncotarget.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huh J.-E., Lee S.Y. IL-6 is produced by adipose-derived stromal cells and promotes osteogenesis. Biochim. Biophys. Acta. 2013;1833:2608–2616. doi: 10.1016/j.bbamcr.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 87.Bastidas-Coral A.P., Bakker A.D., Zandieh-Doulabi B., Kleverlaan C.J., Bravenboer N., Forouzanfar T., Klein-Nulend J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016;2016:1318256. doi: 10.1155/2016/1318256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu Y., Hoogduijn M.J., Baan C.C., Korevaar S.S., De Kuiper R., Yan L., Wang L., Van Besouw N.M. Adipose Tissue-Derived Mesenchymal Stem Cells Have a Heterogenic Cytokine Secretion Profile. Stem Cells Int. 2017;2017:4960831. doi: 10.1155/2017/4960831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Prichard H.L., Reichert W., Klitzman B. IFATS collection: Adipose-derived stromal cells improve the foreign body response. Stem Cells. 2008;26:2691–2695. doi: 10.1634/stemcells.2008-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ghannam S., Bouffi C., Djouad F., Jorgensen C., Noël D. Immunosuppression by mesenchymal stem cells: Mechanisms and clinical applications. Stem Cell Res. Ther. 2010;1:2. doi: 10.1186/scrt2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cui L., Yin S., Liu W., Li N., Zhang W., Cao Y. Expanded Adipose-Derived Stem Cells Suppress Mixed Lymphocyte Reaction by Secretion of Prostaglandin E2. Tissue Eng. 2007;13:1185–1195. doi: 10.1089/ten.2006.0315. [DOI] [PubMed] [Google Scholar]
- 92.Kang J.W., Kang K.-S., Koo H.C., Park J.R., Choi E.W., Park Y.H. Soluble Factors-Mediated Immunomodulatory Effects of Canine Adipose Tissue-Derived Mesenchymal Stem Cells. Stem Cells Dev. 2008;17:681–694. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 93.Tsao C.-H., Shiau M.-Y., Chuang P.-H., Chang Y.-H., Hwang J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014;55:385–397. doi: 10.1194/jlr.M041392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zúñiga L.A., Shen W.-J., Joyce-Shaikh B., Pyatnova E.A., Richards A.G., Thom C., Andrade S.M., Cua D.J., Kraemer F.B., Butcher E.C. IL-17 Regulates Adipogenesis, Glucose Homeostasis, and Obesity. J. Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rehman J., Traktuev D., Li J., Merfeld-Clauss S., Temm-Grove C.J., Bovenkerk J.E., Pell C.L., Johnstone B.H., Considine R.V., March K.L. Secretion of Angiogenic and Antiapoptotic Factors by Human Adipose Stromal Cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 96.Ieda Y., Fujita J., Ieda M., Yagi T., Kawada H., Ando K., Fukuda K. G-CSF and HGF: Combination of vasculogenesis and angiogenesis synergistically improves recovery in murine hind limb ischemia. J. Mol. Cell. Cardiol. 2007;42:540–548. doi: 10.1016/j.yjmcc.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 97.Ball S.G., Shuttleworth C.A., Kielty C.M. Mesenchymal stem cells and neovascularization: Role of platelet-derived growth factor receptors. J. Cell. Mol. Med. 2007;11:1012–1030. doi: 10.1111/j.1582-4934.2007.00120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lopatina T., Bruno S., Tetta C., Kalinina N., Porta M., Camussi G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal. 2014;12:26. doi: 10.1186/1478-811X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Youssef A., Aboalola D., Han V.K.M. The Roles of Insulin-Like Growth Factors in Mesenchymal Stem Cell Niche. Stem Cells Int. 2017;2017:9453108. doi: 10.1155/2017/9453108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang C., Li X., Dang H., Liu P., Zhang B.O., Xu F. Insulin-like growth factor 2 regulates the proliferation and differentiation of rat adipose-derived stromal cells via IGF-1R and IR. Cytotherapy. 2019;21:619–630. doi: 10.1016/j.jcyt.2018.11.010. [DOI] [PubMed] [Google Scholar]
- 101.Chicharro D., Carrillo J.M., Rubio M., Cugat R., Cuervo B., Guil S., Forteza J., Moreno V., Vilar J.M., Sopena J. Combined plasma rich in growth factors and adipose-derived mesenchymal stem cells promotes the cutaneous wound healing in rabbits. BMC Vet. Res. 2018;14:288. doi: 10.1186/s12917-018-1577-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Doyle L.M., Wang M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells. 2019;8:727. doi: 10.3390/cells8070727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Welch J.L., Stapleton J.T., Okeoma C.M. Vehicles of intercellular communication: Exosomes and HIV-1. J. Gen. Virol. 2019;100:350–366. doi: 10.1099/jgv.0.001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Meldolesi J. Exosomes and Ectosomes in Intercellular Communication. Curr. Biol. 2018;28:R435–R444. doi: 10.1016/j.cub.2018.01.059. [DOI] [PubMed] [Google Scholar]
- 105.Fatima F., Ekstrom K., Nazarenko I., Maugeri M., Valadi H., Hill A.F., Camussi G., Nawaz M. Non-coding RNAs in Mesenchymal Stem Cell-Derived Extracellular Vesicles: Deciphering Regulatory Roles in Stem Cell Potency, Inflammatory Resolve, and Tissue Regeneration. Front. Genet. 2017;8:161. doi: 10.3389/fgene.2017.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang W., Bai X., Zhao B., Li Y., Zhang Y., Li Z., Wang X., Luo L., Han F., Zhang J., et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018;370:333–342. doi: 10.1016/j.yexcr.2018.06.035. [DOI] [PubMed] [Google Scholar]
- 108.Cha H., Hong S., Park J.H., Park H.H. Stem Cell-Derived Exosomes and Nanovesicles: Promotion of Cell Proliferation, Migration, and Anti-Senescence for Treatment of Wound Damage and Skin Ageing. Pharmaceutics. 2020;12:1135. doi: 10.3390/pharmaceutics12121135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiong M., Zhang Q., Hu W., Zhao C., Lv W., Yi Y., Wu Y., Wu M. Exosomes from Adipose-Derived Stem Cells: The Emerging Roles and Applications in Tissue Regeneration of Plastic and Cosmetic Surgery. Front. Cell Dev. Biol. 2020;8:574223. doi: 10.3389/fcell.2020.574223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.O’Brien K., Breyne K., Ughetto S., Laurent L.C., Breakefield X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xing X., Han S., Cheng G., Ni Y., Li Z., Li Z. Proteomic Analysis of Exosomes from Adipose-Derived Mesenchymal Stem Cells: A Novel Therapeutic Strategy for Tissue Injury. BioMed Res. Int. 2020;2020:6094562. doi: 10.1155/2020/6094562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ni J., Li H., Zhou Y., Gu B., Xu Y., Fu Q., Peng X., Cao N., Fu Q., Jin M., et al. Therapeutic Potential of Human Adipose-Derived Stem Cell Exosomes in Stress Urinary Incontinence—An In Vitro and In Vivo Study. Cell. Physiol. Biochem. 2018;48:1710–1722. doi: 10.1159/000492298. [DOI] [PubMed] [Google Scholar]
- 113.González-Cubero E., González-Fernández M.L., Gutiérrez-Velasco L., Navarro-Ramírez E., Villar-Suárez V. Isolation and characterization of exosomes from adipose tissue-derived mesenchymal stem cells. J. Anat. 2021;238:1203–1217. doi: 10.1111/joa.13365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Patel R.S., Carter G., El Bassit G., Patel A.A., Cooper D.R., Murr M., Patel N.A. Adipose-derived stem cells from lean and obese humans show depot specific differences in their stem cell markers, exosome contents and senescence: Role of protein kinase C delta (PKCδ) in adipose stem cell niche. Stem Cell Investig. 2016;3:2. doi: 10.3978/j.issn.2306-9759.2016.01.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang J., Bonacquisti E.E., Brown A.D., Nguyen J. Boosting the Biogenesis and Secretion of Mesenchymal Stem Cell-Derived Exosomes. Cells. 2020;9:660. doi: 10.3390/cells9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Guduric-Fuchs J., O’Connor A., Camp B., O’Neill C.L., Medina R.J., Simpson D.A. Selective extracellular vesicle-mediated export of an overlapping set of microRNAs from multiple cell types. BMC Genom. 2012;13:357. doi: 10.1186/1471-2164-13-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Liang Y., Duan L., Lu J., Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–3195. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.ClinicalTrials.gov. [(accessed on 8 April 2021)]; Available online: www.clinicaltrials.gov.
- 119.European Union Clinical Trials Register. [(accessed on 8 April 2021)]; Available online: www.clinicaltrialsregister.eu.
- 120.Ruetze M., Richter W. Adipose-derived stromal cells for osteoarticular repair: Trophic function versus stem cell activity. Expert Rev. Mol. Med. 2014;16:e9. doi: 10.1017/erm.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Phelps J., Sanati-Nezhad A., Ungrin M., Duncan N.A., Sen A. Bioprocessing of Mesenchymal Stem Cells and Their Derivatives: Toward Cell-Free Therapeutics. Stem Cells Int. 2018;2018:9415367. doi: 10.1155/2018/9415367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Araldi R.P., D’Amelio F., Vigerelli H., De Melo T.C., Kerkis I. Stem Cell-Derived Exosomes as Therapeutic Approach for Neurodegenerative Disorders: From Biology to Biotechnology. Cells. 2020;9:2663. doi: 10.3390/cells9122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sadat S., Gehmert S., Song Y.-H., Yen Y., Bai X., Gaiser S., Klein H., Alt E. The cardioprotective effect of mesenchymal stem cells is mediated by IGF-I and VEGF. Biochem. Biophys. Res. Commun. 2007;363:674–679. doi: 10.1016/j.bbrc.2007.09.058. [DOI] [PubMed] [Google Scholar]
- 124.Nakagami H., Maeda K., Morishita R., Iguchi S., Nishikawa T., Takami Y., Kikuchi Y., Saito Y., Tamai K., Ogihara T., et al. Novel Autologous Cell Therapy in Ischemic Limb Disease through Growth Factor Secretion by Cultured Adipose Tissue-Derived Stromal Cells. Arterioscler. Thromb. Vasc. Biol. 2005;25:2542–2547. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 125.Kandasamy M., Lehner B., Kraus S., Sander P.R., Marschallinger J., Rivera F.J., Trümbach D., Ueberham U., Reitsamer H.A., Strauss O., et al. TGF-β signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med. 2014;18:1444–1459. doi: 10.1111/jcmm.12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Razavi S., Razavi M.R., Esfahani H.Z., Kazemi M., Mostafavi F.S. Comparing brain-derived neurotrophic factor and ciliary neurotrophic factor secretion of induced neurotrophic factor secreting cells from human adipose and bone marrow-derived stem cells. Dev. Growth Differ. 2013;55:648–655. doi: 10.1111/dgd.12072. [DOI] [PubMed] [Google Scholar]
- 127.Wei X., Zhao L., Zhong J., Gu H., Feng D., Johnstone B., March K., Farlow M., Du Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci. Lett. 2009;462:76–79. doi: 10.1016/j.neulet.2009.06.054. [DOI] [PubMed] [Google Scholar]
- 128.Park H., Chang K.-A. Therapeutic Potential of Repeated Intravenous Transplantation of Human Adipose-Derived Stem Cells in Subchronic MPTP-Induced Parkinson’s Disease Mouse Model. Int. J. Mol. Sci. 2020;21:8129. doi: 10.3390/ijms21218129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kalbermatten D.F., Schaakxs D., Kingham P.J., Wiberg M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011;344:251–260. doi: 10.1007/s00441-011-1142-5. [DOI] [PubMed] [Google Scholar]
- 130.Choi H.S., Kim H.J., Oh J.-H., Park H.-G., Ra J.C., Chang K.-A., Suh Y.-H. Therapeutic potentials of human adipose-derived stem cells on the mouse model of Parkinson’s disease. Neurobiol. Aging. 2015;36:2885–2892. doi: 10.1016/j.neurobiolaging.2015.06.022. [DOI] [PubMed] [Google Scholar]
- 131.Chen Y.-S., Hong Z.-X., Lin S.-Z., Harn H.-J. Identifying Therapeutic Targets for Spinocerebellar Ataxia Type 3/Machado–Joseph Disease through Integration of Pathological Biomarkers and Therapeutic Strategies. Int. J. Mol. Sci. 2020;21:3063. doi: 10.3390/ijms21093063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Riordan N.H., Ichim T.E., Min W.-P., Wang H., Solano F., Lara F., Alfaro M., Rodriguez J.P., Harman R.J., Patel A.N., et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Li C., Fei K., Tian F., Gao C., Song Y. Adipose-derived mesenchymal stem cells attenuate ischemic brain injuries in rats by modulating miR-21-3p/MAT2B signaling transduction. Croat. Med. J. 2019;60:439–448. doi: 10.3325/cmj.2019.60.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lee M., Ban J.-J., Yang S., Im W., Kim M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87–93. doi: 10.1016/j.brainres.2018.03.034. [DOI] [PubMed] [Google Scholar]
- 135.Katsuda T., Tsuchiya R., Kosaka N., Yoshioka Y., Takagaki K., Oki K., Takeshita F., Sakai Y., Kuroda M., Ochiya T. Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci. Rep. 2013;3:1197. doi: 10.1038/srep01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lee M., Liu T., Im W., Kim M. Exosomes from adipose-derived stem cells ameliorate phenotype of Huntington’s disease in vitro model. Eur. J. Neurosci. 2016;44:2114–2119. doi: 10.1111/ejn.13275. [DOI] [PubMed] [Google Scholar]
- 137.Lee M., Ban J.-J., Kim K.Y., Jeon G.S., Im W., Sung J.-J., Kim M. Adipose-derived stem cell exosomes alleviate pathology of amyotrophic lateral sclerosis in vitro. Biochem. Biophys. Res. Commun. 2016;479:434–439. doi: 10.1016/j.bbrc.2016.09.069. [DOI] [PubMed] [Google Scholar]
- 138.Laso-García F., Ramos-Cejudo J., Carrillo-Salinas F.J., Ortega L.O., Feliú A., Frutos M.G.-D., Mecha M., Díez-Tejedor E., Guaza C., Gutiérrez-Fernández M. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS ONE. 2018;13:e0202590. doi: 10.1371/journal.pone.0202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Patel N.A., Moss L.D., Lee J.-Y., Tajiri N., Acosta S., Hudson C., Parag S., Cooper D.R., Borlongan C.V., Bickford P.C. Long noncoding RNA MALAT1 in exosomes drives regenerative function and modulates inflammation-linked networks following traumatic brain injury. J. Neuroinflamm. 2018;15:204. doi: 10.1186/s12974-018-1240-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei X., Du Z., Zhao L., Feng D., Wei G., He Y., Tan J., Lee W.-H., Hampel H., Dodel R., et al. IFATS Collection: The Conditioned Media of Adipose Stromal Cells Protect against Hypoxia-Ischemia-Induced Brain Damage in Neonatal Rats. Stem Cells. 2009;27:478–488. doi: 10.1634/stemcells.2008-0333. [DOI] [PubMed] [Google Scholar]
- 141.Kranz A., Rau C., Kochs M., Waltenberger J. Elevation of Vascular Endothelial Growth Factor-A Serum Levels Following Acute Myocardial Infarction. Evidence for its Origin and Functional Significance. J. Mol. Cell. Cardiol. 2000;32:65–72. doi: 10.1006/jmcc.1999.1062. [DOI] [PubMed] [Google Scholar]
- 142.Zhu Y., Zhang J., Hu X., Wang Z., Wu S., Yi Y. Extracellular vesicles derived from human adipose-derived stem cells promote the exogenous angiogenesis of fat grafts via the let-7/AGO1/VEGF signalling pathway. Sci. Rep. 2020;10:5313. doi: 10.1038/s41598-020-62140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu F., Witman N., Yan D., Zhang S., Zhou M., Yan Y., Yao Q., Ding F., Yan B., Wang H., et al. Human adipose-derived stem cells enriched with VEGF-modified mRNA promote angiogenesis and long-term graft survival in a fat graft transplantation model. Stem Cell Res. Ther. 2020;11:490. doi: 10.1186/s13287-020-02008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chen K.-H., Chen C.-H., Wallace C.G., Yuen C.-M., Kao G.-S., Chen Y.-L., Shao P.-L., Chen Y.-L., Chai H.-T., Lin K.-C., et al. Intravenous administration of xenogenic adipose-derived mesenchymal stem cells (ADMSC) and ADMSC-derived exosomes markedly reduced brain infarct volume and preserved neurological function in rat after acute ischemic stroke. Oncotarget. 2016;7:74537–74556. doi: 10.18632/oncotarget.12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lopatina T., Favaro E., Grange C., Cedrino M., Ranghino A., Occhipinti S., Fallo S., Buffolo F., Gaykalova D.A., Zanone M.M., et al. PDGF enhances the protective effect of adipose stem cell-derived extracellular vesicles in a model of acute hindlimb ischemia. Sci. Rep. 2018;8:17458. doi: 10.1038/s41598-018-36143-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lu K.-Y., Dass K.T.P., Lin S.-Z., Harn H.-J., Liu S.-P. The application of stem cell therapy and brown adipose tissue transplantation in metabolic disorders. Cytotherapy. 2020;22:521–528. doi: 10.1016/j.jcyt.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 147.Silva K.R., Baptista L.S. Adipose-derived stromal/stem cells from different adipose depots in obesity development. World J. Stem Cells. 2019;11:147–166. doi: 10.4252/wjsc.v11.i3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang J.P., Anderson A.E., McCartney A., Ory X., Ma G., Pappalardo E., Bader J., Elisseeff J.H. Metabolically Active Three-Dimensional Brown Adipose Tissue Engineered from White Adipose-Derived Stem Cells. Tissue Eng. 2017;23:253–262. doi: 10.1089/ten.tea.2016.0399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pisani D.F., Djedaini M., Beranger G.E., Elabd C., Scheideler M., Ailhaud G.P., Amri E.-Z. Differentiation of human adipose-derived stem cells into “brite” (brown-in-white) adipocytes. Front. Endocrinol. 2011;2:87. doi: 10.3389/fendo.2011.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cao M., Pan Q., Dong H., Yuan X., Li Y., Sun Z., Dong X., Wang H. Adipose-derived mesenchymal stem cells improve glucose homeostasis in high-fat diet-induced obese mice. Stem Cell Res. Ther. 2015;6:208. doi: 10.1186/s13287-015-0201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Capilla-González V., López-Beas J., Escacena N., Aguilera Y., de la Cuesta A., Ruiz-Salmerón R., Martín F., Hmadcha A., Soria B. PDGF Restores the Defective Phenotype of Adipose-Derived Mesenchymal Stromal Cells from Diabetic Patients. Mol. Ther. 2018;26:2696–2709. doi: 10.1016/j.ymthe.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brini A.T., Amodeo G., Ferreira L.M., Milani A., Niada S., Moschetti G., Franchi S., Borsani E., Rodella L.F., Panerai A.E., et al. Therapeutic effect of human adipose-derived stem cells and their secretome in experimental diabetic pain. Sci. Rep. 2017;7:9904. doi: 10.1038/s41598-017-09487-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Amodeo G., Niada S., Moschetti G., Franchi S., Savadori P., Brini A.T., Sacerdote P. Secretome of human adipose-derived mesenchymal stem cell relieves pain and neuroinflammation independently of the route of administration in experimental osteoarthritis. Brain Behav. Immun. 2021;94:29–40. doi: 10.1016/j.bbi.2021.03.011. [DOI] [PubMed] [Google Scholar]
- 154.Wang J., Mi Y., Wu S., You X., Huang Y., Zhu J., Zhu L. Exosomes from adipose-derived stem cells protect against high glucose-induced erectile dysfunction by delivery of corin in a streptozotocin-induced diabetic rat model. Regen. Ther. 2020;14:227–233. doi: 10.1016/j.reth.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wartchow K.M., Rodrigues L., Lissner L.J., Federhen B.C., Selistre N.G., Moreira A., Gonçalves C.-A., Sesterheim P. Insulin-producing cells from mesenchymal stromal cells: Protection against cognitive impairment in diabetic rats depends upon implant site. Life Sci. 2020;251:117587. doi: 10.1016/j.lfs.2020.117587. [DOI] [PubMed] [Google Scholar]
- 156.Jin J., Shi Y., Gong J., Zhao L., Li Y., He Q., Huang H. Exosome secreted from adipose-derived stem cells attenuates diabetic nephropathy by promoting autophagy flux and inhibiting apoptosis in podocyte. Stem Cell Res. Ther. 2019;10:95. doi: 10.1186/s13287-019-1177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Duan Y., Luo Q., Wang Y., Ma Y., Chen F., Zhu X., Shi J. Adipose mesenchymal stem cell-derived extracellular vesicles containing microRNA-26a-5p target TLR4 and protect against diabetic nephropathy. J. Biol. Chem. 2020;295:12868–12884. doi: 10.1074/jbc.RA120.012522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gao F., Zuo B., Wang Y., Li S., Yang J., Sun D. Protective function of exosomes from adipose tissue-derived mesenchymal stem cells in acute kidney injury through SIRT1 pathway. Life Sci. 2020;255:117719. doi: 10.1016/j.lfs.2020.117719. [DOI] [PubMed] [Google Scholar]
- 159.Matthay M.A., Zemans R.L., Zimmerman G.A., Arabi Y., Beitler J.R., Mercat A., Herridge M., Randolph A.G., Calfee C.S. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Brave H., MacLoughlin R. State of the Art Review of Cell Therapy in the Treatment of Lung Disease, and the Potential for Aerosol Delivery. Int. J. Mol. Sci. 2020;21:6435. doi: 10.3390/ijms21176435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shigemura N., Okumura M., Mizuno S., Imanishi Y., Nakamura T., Sawa Y. Autologous transplantation of adipose tissue-derived stromal cells ameliorates pulmonary emphysema. Am. J. Transplant. 2006;6:2592–2600. doi: 10.1111/j.1600-6143.2006.01522.x. [DOI] [PubMed] [Google Scholar]
- 162.Kim Y.-S., Kim J.-Y., Cho R., Shin D.-M., Lee S.W., Oh Y.-M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017;49:e284. doi: 10.1038/emm.2016.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang S., Danchuk S.D., Bonvillain R.W., Xu B., Scruggs B.A., Strong A.L., Semon J.A., Gimble J.M., Betancourt A.M., Sullivan D.E., et al. Interleukin 6 Mediates the Therapeutic Effects of Adipose-Derived Stromal/Stem Cells in Lipopolysaccharide-Induced Acute Lung Injury. Stem Cells. 2014;32:1616–1628. doi: 10.1002/stem.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sánchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J.-M., Guerrero J.E., Pérez-Calvo C., et al. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020;25:100454. doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Mitchell R., Mellows B., Sheard J., Antonioli M., Kretz O., Chambers D., Zeuner M.-T., Tomkins J.E., Denecke B., Musante L., et al. Secretome of adipose-derived mesenchymal stem cells promotes skeletal muscle regeneration through synergistic action of extracellular vesicle cargo and soluble proteins. Stem Cell Res. Ther. 2019;10:116. doi: 10.1186/s13287-019-1213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhu M., Liu Y., Qin H., Tong S., Sun Q., Wang T., Zhang H., Cui M., Guo S. Osteogenically-induced exosomes stimulate osteogenesis of human adipose-derived stem cells. Cell Tissue Bank. 2021;22:77–91. doi: 10.1007/s10561-020-09867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhao C., Chen J., Peng W., Yuan B., Bi Q., Xu Y. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020;21:1881–1889. doi: 10.3892/mmr.2020.10982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Tofiño-Vian M., Guillén M.I., Del Caz M.D.P., Silvestre A., Alcaraz M.J. Microvesicles from Human Adipose Tissue-Derived Mesenchymal Stem Cells as a New Protective Strategy in Osteoarthritic Chondrocytes. Cell. Physiol. Biochem. 2018;47:11–25. doi: 10.1159/000489739. [DOI] [PubMed] [Google Scholar]
- 169.Woo C.H., Kim H.K., Jung G.Y., Jung Y.J., Lee K.S., Yun Y.E., Han J., Lee J., Kim W.S., Choi J.S., et al. Small extracellular vesicles from human adipose-derived stem cells attenuate cartilage degeneration. J. Extracell. Vesicles. 2020;9:1735249. doi: 10.1080/20013078.2020.1735249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang C., Song W., Chen B., Liu X., He Y. Exosomes Isolated from Adipose-Derived Stem Cells: A New Cell-Free Approach to Prevent the Muscle Degeneration Associated with Torn Rotator Cuffs. Am. J. Sports Med. 2019;47:3247–3255. doi: 10.1177/0363546519876323. [DOI] [PubMed] [Google Scholar]
- 171.Van Dongen J.A., Harmsen M.C., Van Der Lei B., Stevens H.P. Augmentation of Dermal Wound Healing by Adipose Tissue-Derived Stromal Cells (ASC) Bioengineering. 2018;5:91. doi: 10.3390/bioengineering5040091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Stessuk T., Puzzi M.B., Chaim E.A., Alves P.C.M., De Paula E.V., Forte A., Izumizawa J.M., Oliveira C.C., Frei F., Ribeiro-Paes J.T. Platelet-rich plasma (PRP) and adipose-derived mesenchymal stem cells: Stimulatory effects on proliferation and migration of fibroblasts and keratinocytes in vitro. Arch. Dermatol. Res. 2016;308:511–520. doi: 10.1007/s00403-016-1676-1. [DOI] [PubMed] [Google Scholar]
- 173.Seo G., Oh E., Yun M., Lee J.-Y., Bae J.S., Joo K., Chae G.T., Lee S.-B. Adipose-derived stem cell conditioned medium accelerates keratinocyte differentiation via the upregulation of miR-24. Exp. Dermatol. 2015;24:792–793. doi: 10.1111/exd.12754. [DOI] [PubMed] [Google Scholar]
- 174.Pu C.-M., Chen Y.-C., Chen Y.-C., Lee T.-L., Peng Y.-S., Chen S.-H., Yen Y.-H., Chien C.-L., Hsieh J.-H., Chen Y.-L. Interleukin-6 from Adipose-Derived Stem Cells Promotes Tissue Repair by the Increase of Cell Proliferation and Hair Follicles in Ischemia/Reperfusion-Treated Skin Flaps. Mediat. Inflamm. 2019;2019:2343867. doi: 10.1155/2019/2343867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bai Y., Han Y.-D., Yan X.-L., Ren J., Zeng Q., Li X.-D., Pei X.-T., Han Y. Adipose mesenchymal stem cell-derived exosomes stimulated by hydrogen peroxide enhanced skin flap recovery in ischemia-reperfusion injury. Biochem. Biophys. Res. Commun. 2018;500:310–317. doi: 10.1016/j.bbrc.2018.04.065. [DOI] [PubMed] [Google Scholar]
- 176.Yang C., Luo L., Bai X., Shen K., Liu K., Wang J., Hu D. Highly-expressed micoRNA-21 in adipose derived stem cell exosomes can enhance the migration and proliferation of the HaCaT cells by increasing the MMP-9 expression through the PI3K/AKT pathway. Arch. Biochem. Biophys. 2020;681:108259. doi: 10.1016/j.abb.2020.108259. [DOI] [PubMed] [Google Scholar]
- 177.Mazini L., Rochette L., Malka G. Adipose-Derived Stem Cells (ADSCs) and Growth Differentiation Factor 11 (GDF11): Regenerative and Antiaging Capacity for the Skin. Regen. Med. 2020 doi: 10.5772/intechopen.91233. [DOI] [Google Scholar]
- 178.Lv Q., Deng J., Chen Y., Wang Y., Liu B., Liu J. Engineered Human Adipose Stem-Cell-Derived Exosomes Loaded with miR-21-5p to Promote Diabetic Cutaneous Wound Healing. Mol. Pharm. 2020;17:1723–1733. doi: 10.1021/acs.molpharmaceut.0c00177. [DOI] [PubMed] [Google Scholar]
- 179.Cho B.S., Kim J.O., Ha D.H., Yi Y.W. Exosomes derived from human adipose tissue-derived mesenchymal stem cells alleviate atopic dermatitis. Stem Cell Res. Ther. 2018;9:187. doi: 10.1186/s13287-018-0939-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shin K.-O., Ha D.H., Kim J.O., Crumrine D.A., Meyer J.M., Wakefield J.S., Lee Y., Kim B., Kim S., Kim H.-K., et al. Exosomes from Human Adipose Tissue-Derived Mesenchymal Stem Cells Promote Epidermal Barrier Repair by Inducing De Novo Synthesis of Ceramides in Atopic Dermatitis. Cells. 2020;9:680. doi: 10.3390/cells9030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kim K., Fan Y., Lin G., Park Y.K., Pak C.S., Jeong J.H., Kim S. Synergistic Effect of Adipose-Derived Stem Cells and Fat Graft on Wrinkles in Aged Mice. Plast. Reconstr. Surg. 2019;143:1637–1646. doi: 10.1097/PRS.0000000000005625. [DOI] [PubMed] [Google Scholar]
- 182.Guo S., Wang T., Zhang S., Chen P., Cao Z., Lian W., Guo J., Kang Y. Adipose-derived stem cell-conditioned medium protects fibroblasts at different senescent degrees from UVB irradiation damages. Mol. Cell. Biochem. 2020;463:67–78. doi: 10.1007/s11010-019-03630-8. [DOI] [PubMed] [Google Scholar]
- 183.Li L., Ngo H.T., Hwang E., Wei X., Liu Y., Liu J., Yi T.-H. Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cell Culture Prevents UVB-Induced Skin Aging in Human Keratinocytes and Dermal Fibroblasts. Int. J. Mol. Sci. 2019;21:49. doi: 10.3390/ijms21010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.