Abstract
Tendon injuries are common and heal poorly, due in part to a lack of understanding of fundamental tendon cell biology. A major impediment to the study of tendon cells is the absence of robust, well-characterized in vitro models. Unlike other tissue systems, current tendon cell models do not account for how differences in isolation methodology may affect the activation state of tendon cells or the presence of various tendon cell sub-populations. The objective of this study was to characterize how common isolation methods affect the behavior, fate, and lineage composition of tendon cell cultures. Tendon cells isolated by explant exhibited reduced proliferative capacity, decreased expression of tendon marker genes, and increased expression of genes associated with fibroblast activation compared to digested cells. Consistently, explanted cells also displayed an increased propensity to differentiate to myofibroblasts compared to digested cells. Explanted cultures from multiple different tendons were substantially enriched for the presence of scleraxis-lineage (Scx-lin+) cells compared to digested cultures, while the overall percentage of S100a4-lineage (S100a4-lin+) cells was dependent on both isolation method and tendon of origin. Neither isolation method preserved the ratios of Scx-lin+ or S100a4-lin+ to non-lineage cells seen in tendons in vivo. Combined, these data indicate that further refinement of in vitro cultures models is required in order to more accurately understand the effects of various stimuli on tendon cell behavior.
Keywords: tendon, cell culture, fibroblast activation, lineage
INTRODUCTION
Following acute injury, tendons heal by the formation of a fibrotic scar. Though the scar restores tissue continuity, it can also severely limit functionality and can predispose the tendon to further injury and degeneration (1). Despite the frequency at which these injuries occur and the associated long-term complications, there are currently no treatments available that can effectively promote improved tendon healing due in part of a lack of understanding of the specific cellular mechanisms that mediate either regenerative tenogenic processes, or the pathological fibrotic response.
In an effort to improve tendon healing, many studies have employed in vitro studies to better understand tendon cell behavior and to identify potential targets for therapeutic intervention, an approach which has been successfully employed in other musculoskeletal tissues such as bone, cartilage, and muscle. In these tissues, well-characterized in vitro model systems have led to fundamental discoveries regarding the mechanisms regulating cell differentiation (2–5) and the important role of mechanical stimuli on tissue homeostasis (6–9). These in vitro findings can display a high degree of translatability and have informed in vivo studies that now underpin our basic understanding of these tissues. Despite the critical need for robust in vitro model systems, there is currently no standard for the study of tendon cells. Due to a lack of suitable tendon cell lines, the field relies on the isolation and culture of primary tendon cells; however there is still no standardized protocol for tendon cell isolation, with isolation methods (explant culture (10–12) or enzymatic digest (13, 14)) and cells derived from different tendons assumed to be equivalent and frequently used interchangeably.
It has long been understood that once isolated, tendon cells from many different species do not retain their in vivo characteristics, a phenomenon referred to as “phenotypic drift” (15). This process is typically described as a loss of tenogenic markers, including scleraxis (Scx), tenomodulin, Mohawk (Mkx), and collagen type 1 (Col1), increases in the expression of injury-associated collagens including collagen type III (Col3), alterations in cell morphology away from the typical spindle-shape, and changes in proliferative capacity over time in culture (15–19). This has traditionally been viewed as a negative outcome, and investigators have been advised to use isolated tendon cells at early passages in an attempt to preserve the tendon cell phenotype seen in vivo (17).
Though they are referred to by many different names, the majority of the cells that reside in tendons are fibroblasts. In general, when subjected to injury or stress, tissue-resident fibroblasts lose their quiescent, homeostatic phenotype and can activate to a proliferative, secretory phenotype in a well-defined process called fibroblast activation (20); however, the exact manner in which this occurs as well as the ultimate fate of activated resident fibroblasts is specific to a given tissue. In general, fibroblast activation is characterized by decreased expression of tissue-specific markers and increased expression of activation markers including periostin (Postn) (21), S100a4 (fibroblast-specific protein-1; Fsp-1) (22), CD248 (23), and CD106 (24). While there is evidence that tendon cells activate following acute injury in vivo (25–27), the degree to this process is conserved in tendon cells following isolation is unknown.
Fibroblast activation following injury can ultimately lead resident fibroblasts to differentiate to a myofibroblast phenotype (28). Myofibroblasts are contractile cells that are defined by a highly organized cytoskeleton that includes the presence of alpha smooth actin (α-SMA) containing stress fibers. While myofibroblasts are crucial for wound healing to occur, prolonged or aberrant myofibroblast activity is associated with fibrotic outcomes (29), therefore making the process of fibroblast activation to a myofibroblast phenotype an attractive target for modulating healing outcomes. Recent lineage-tracing studies have shown that some tendon cells can become α-SMA+ myofibroblasts following injury in vivo (25) and that the prolonged presence of myofibroblasts is associated with fibrotic tendon healing (26, 30, 31). However, the specific mechanisms governing tendon cell activation and differentiation to myofibroblasts are largely undetermined.
Though tendon cells have traditionally been seen as a homogenous population, recent studies have revealed that tendon cells exhibit remarkable heterogeneity, both within a given tendon (25, 32) and between different tendons based on their anatomical location (33). In addition to a lack of understanding of how various isolation methods affect the activation status of tendon cells, there has been no characterization of how in vivo heterogeneity is reflected in in vitro tendon cell cultures to date. Conflicting data on the tendon cell response to various stimuli may be partially explained by this lack of understanding of existing in vitro cultures systems. Moreover, as seen with other tissue-specific culture systems, a more thorough characterization of tendon cell behavior in vitro may lead to an improved understanding of tendon cell function in vivo. The purpose of this study was therefore twofold: 1) to investigate how different isolation methods affect tendon cell activation and fate in vitro and 2) to determine whether the isolation methodology affects the tendon cell populations present in in vitro cultures.
METHODS
Mice:
All animal studies were approved by the University of Rochester Committee for Animal Resources. C57BL6/J mice (000664), S100a4-Cre (012641), and ROSA-Ai9 F/F (007909) were purchased from The Jackson Laboratory (Bar Harbor, ME). ScxCreERT2 and Scx-GFP reporter mice were a gift from Dr. Ronen Schweitzer (Oregon Health and Science University, Portland, OR). ScxCreERT2 and S100a4-Cre mice were crossed to the ROSA-Ai9 F/F strain (ScxCreERT2;Ai9 F/-; referred to as Scx;Ai9, and S100a4Cre;Ai9 F/F; referred to as S100a4;Ai9 mice, respectively) as previously described (25, 31) to facilitate identification of the tendon cell lineages present following isolation. Cre+ Scx;Ai9 mice were treated with Tamoxifen (TMX; 100mg/kg; Sigma-Aldrich, St. Louis, MO) via intraperitoneal injection for three consecutive days, five days prior to isolation. Using this TMX regimen, only cells expressing Scx in intact tendon, at the time of TMX labeling, are labeled red (Scx-lineage [Scx-lin+]). In S100a4;Ai9 mice, all cells that express/have expressed S100a4 at any point are labeled red (S100a4-lin+). All isolations were performed from the tendons of 10–12 week old male and female mice. In order to quantify the efficiency of the Scx;Ai9 labeling, we examined contralateral hindpaws from ScxCreERT2; Ai9 F/-;Scx-GFP+ mice (referred to as Scx;Ai9;GFP mice) that were generated for an experiment unrelated to this study. Mice received TMX as described above, and hindpaws were harvested 17 days after the final injection. In these mice, Scx-lin cells are labeled red and cells that actively express Scx at the time of harvest fluoresce green.
Tendon cell isolation:
Following sacrifice, both flexor digitorum longus (FDL) tendons from three C57BL6/J mice (6 tendons total) were harvested. Tendons were carefully dissected from the surrounding tissue and the region from the heel to just proximal to the digit bifurcation was excised. For digested cultures, three tendons were placed into 1mL of complete Fibroblast Growth Medium-2 (FGM; #CC-3132, Lonza, Basel, Switzerland) containing 0.075% w/v collagenase type 2 (C6885, Sigma), minced, and incubated with spinning for 45 minutes at 37°C. The tissue isolate was then filtered through a 70 μm filter (#43-10070-50, Pluriselect-USA, El Cajon, CA) to remove any remaining debris, pelleted, resuspended in FGM, and plated into collagen-coated (50 μg/cm2 Type I Rat Tail Collagen, #354236, Corning, Bedford, MA) 35mm culture dishes. To generate explant cultures, three tendons were each cut into three pieces and placed on the bottom of collagen-coated 35mm culture dishes. Explants were allowed to adhere for 4–6 hours before being covered with FGM. Within two days, cells migrated from the explanted tendon tissue onto the bottom of the culture dish. The explant tissue was removed from the cultures at the first passaging, and monolayers derived from explanted cells were cultured as described below. Cell isolations were repeated from four different cohorts of mice for a biological n=4.
Tendon cell culture and serial passaging:
Cultures were maintained in FGM at 37 °C, 5% CO2, and 90% humidity with complete media exchanges every two days. At 70% confluence, cells were passaged (0.05% Trypsin EDTA, #25300–054, Gibco, Waltham, MA and 5mg/mL soybean Trypsin inhibitor, #9035-81-8, Gibco) and seeded at 4,000 cells/cm2 into 1× 60 mm and 1× 35 mm collagen-coated culture dishes, and 1x collagen-coated chamber slide (Lab-TekII Chamber Side 154526, Thermo Fisher Scientific, Waltham, MA). At each subsequent passage, the 60 mm plate was used both to quantify the population doubling time and to seed plates for the subsequent passage, 1× 35 mm plate was harvested for RNA isolation, and the chamber slide was fixed in 4% paraformaldehyde for 15 minutes to evaluate changes in cell morphology as well as immunohistochemical staining for Ki67 and α-SMA as described below.
Population doubling time:
At each passage, the total number of cells per 60 mm plate was quantified using a hemocytometer and used to calculate the doubling time according to the following equation:
where T = incubation time (days), Xb = the number of cells seeded, and Xe the number of cells at passaging.
RNA isolation and gene expression analysis:
Both intact FDL tendons from six, 10–12 week old male and female C57BL6/J (two mice pooled per sample, n=4 samples) were snap frozen in liquid nitrogen, placed into a guanidine isothiocyanate-phenol solution (TRIzol® Reagent, Invitrogen, Carlsbad, CA) and homogenized using 0.5 mm zirconium oxide beads (# ZROB05, Next Advance Inc., Troy, NY) and a Bullet Blender Gold Cell Disrupter (Next Advance). Cultured cell monolayers from each passage were collected directly into TRIzol®. Total RNA from all samples was isolated by column purification (Direct-zol RNA Microprep kit, #R2061, Zymo Research, Irvine, CA) and converted to cDNA (qScript; #84034, Quantabio, Beverly, MA). Primers were designed (Primer Express, Applied Biosystems, Foster City, CA) validated, and used for qPCR (PerfeCTa SYBR Green; #84069, Quantabio, CFX Connect Real-Time System; Bio-Rad). Primer sequences are provided in Table 1. Fold changes were calculated using the ΔΔCt method with β-actin (Actb) as an internal control (34). Data are shown normalized to the average gene expression levels in digested cells unless otherwise stated.
Table 1:
qPCR Primer Sequences
| Gene | Sequence (5’->3’) | Reference | |
|---|---|---|---|
| Actb | Fwd | AGATGTGCATCAGCAAGCAG | NM_007393.5 |
| Rev | GCGCAAGTTAGGTTTTGTCA | ||
| Scx | Fwd | TGGCCTCCAGCTACATTTCT | NM_198885.3 |
| Rev | TGTCACGGTCTTTGCTGAAC | ||
| Mkx | Fwd | CACCGTGACAACCCGTACC | NM_177595.4 |
| Rev | GCACTAGCGTCATCTGCGAG | ||
| Col1a1 | Fwd | GCTCCTCTTAGGGGCCACT | NM_007742.4 |
| Rev | CCACGTCTCACCATTGGGG | ||
| Col3a1 | Fwd | ACGTAGATGAATTGGGATGCAG | NM_009930.2 |
| Rev | GGGTTGGGGCAGTCTAGTG | ||
| Fsp1 | Fwd | AAGCTGAACAAGACAGAGCTCAAG | NM_011311.2 |
| Rev | GTCCTTTTCCCCAGGAAGCTA | ||
| Postn | Fwd | CAGGAGAGACCTTGCAGAAATTC | NM_001198765.1 |
| Rev | GAACGGCCTTCTCTTGATCGT | ||
| CD248 | Fwd | CACGGCCCATGGTGATCT | NM_054042.2 |
| Rev | TGTAGCTGAAATAACAGAGGTCTTGTG | ||
| CD106 | Fwd | ACAAGTCTACATCTCTCCCAGGAATAC | NM_011693.3 |
| Rev | CACAGCACCACCCTCTTGAA | ||
Lineage tracing:
Following sacrifice, explant and digested cultures were generated from FDL, flexor carpus ulnaris (FCU), and Achilles tendons of Scx;Ai9 and S100a4;Ai9 mice as described for the FDL tendon above. For tail tendons, bundles of fascicles were removed by pulling from the base of the tail tendon with sterile forceps. Explant and digested cultures were imaged every two days, and the number of lineage (red fluorescent) vs non-lineage (non-fluorescent) cells present was quantified by counting (ImageJ, National Institutes of Health, Bethesda, MD) for three random fields and averaged per culture. The lineage-tracing experiment was repeated three times (n=3).
Immunohistochemistry (IHC):
Serially passaged cell monolayers grown on chamber slides were fixed in 4% PFA, permeabilized with 0.1% TX100, and stained with antibodies to Ki67 (1:250, ab16667, Abcam, Cambridge, MA) to evaluate cell proliferation and FITC-α-SMA (1:200, F3777, Sigma) to determine myofibroblast content. Intact front paws, hindlimbs, and tails from Scx;Ai9 (n=4) and S100a4;Ai9 (n=4) mice, as well as intact hindpaws from Scx;Ai9;GFP (n=4) mice, were harvested, fixed in 10% neutral buffered formalin (72 hours), decalcified in Webb Jee EDTA (14 days), processed, and embedded in paraffin. Three-micron sagittal sections were cut and exposed to primary antibodies for red fluorescent protein (1:500, AB8181–200, SICGEN, Cantanhede, Portugal) to detect the Ai9 label. Nuclei were counterstained with DAPI. Slides were scanned with a VS120 Virtual Slide Microscope and OlyVIA software (Olympus Life Science, Shinjuku, Tokyo, Japan). The number of lineage (red and blue colocalization) vs. non-lineage (blue only) nuclei within the tendon tissue were quantified using Visiopharm (Visiopharm Corporation, Westminster, CO). To confirm the localization of lineage cells within the tendon tissue, following IHC for the Ai9 label, the coverslips were carefully removed from the slides and sections were with stained with Masson’s Trichrome. For in vitro lineage-tracing studies, the number of Ki67+ and α-SMA+ cells present in cell monolayers were quantified using a custom MATLAB script (code available upon request; MathWorks, Natick, MA).
Statistical analysis:
All statistical analyses were performed in Prism 8 (GraphPad Software, LLC., San Diego, CA). Changes in gene expression, α-SMA+ myofibroblasts, and number of Ki67+ cells between isolation methods were evaluated by unpaired t test at each timepoint. Gene expression changes between isolated cells and intact FDL tendon were analyzed by the Kruskal-Wallis and Dunn’s multiple comparisons tests. All data are shown as the mean ± standard deviation. Statistical significance was set at p ≤ 0.05.
RESULTS
Tendon cell morphology and proliferative capacity differ between isolation methods
Tendon cells isolated by digest exhibited a flat, spread morphology with a clearly defined nucleus (Figure 1A, top). In contrast, the majority of tendon cells that migrated out of the explanted tissue were long, thin, and more cylindrically shaped (Figure 1A, bottom). In addition to changes in cell morphology, there was a substantial decrease in the proliferative capacity of the explanted cells compared to the digested cells, indicated by increased doubling time at every passage (Figure 1B) as well as a significantly reduced percentage of Ki67+ cells in explanted versus digested cultures at each passage beyond passage 1, with the exception of passage 3 (Figure 1C; p1: p= 0.384, p2: p=<0.001, p3: p=0.867, p4: p=0.004, p5: p<0.001). Tendon cells isolated by explant stopped actively proliferating at passage 4, though cultures could continue to be replated and kept in culture through at least passage 6.
Figure 1: Isolation method affects tendon cell morphology and proliferation rates.
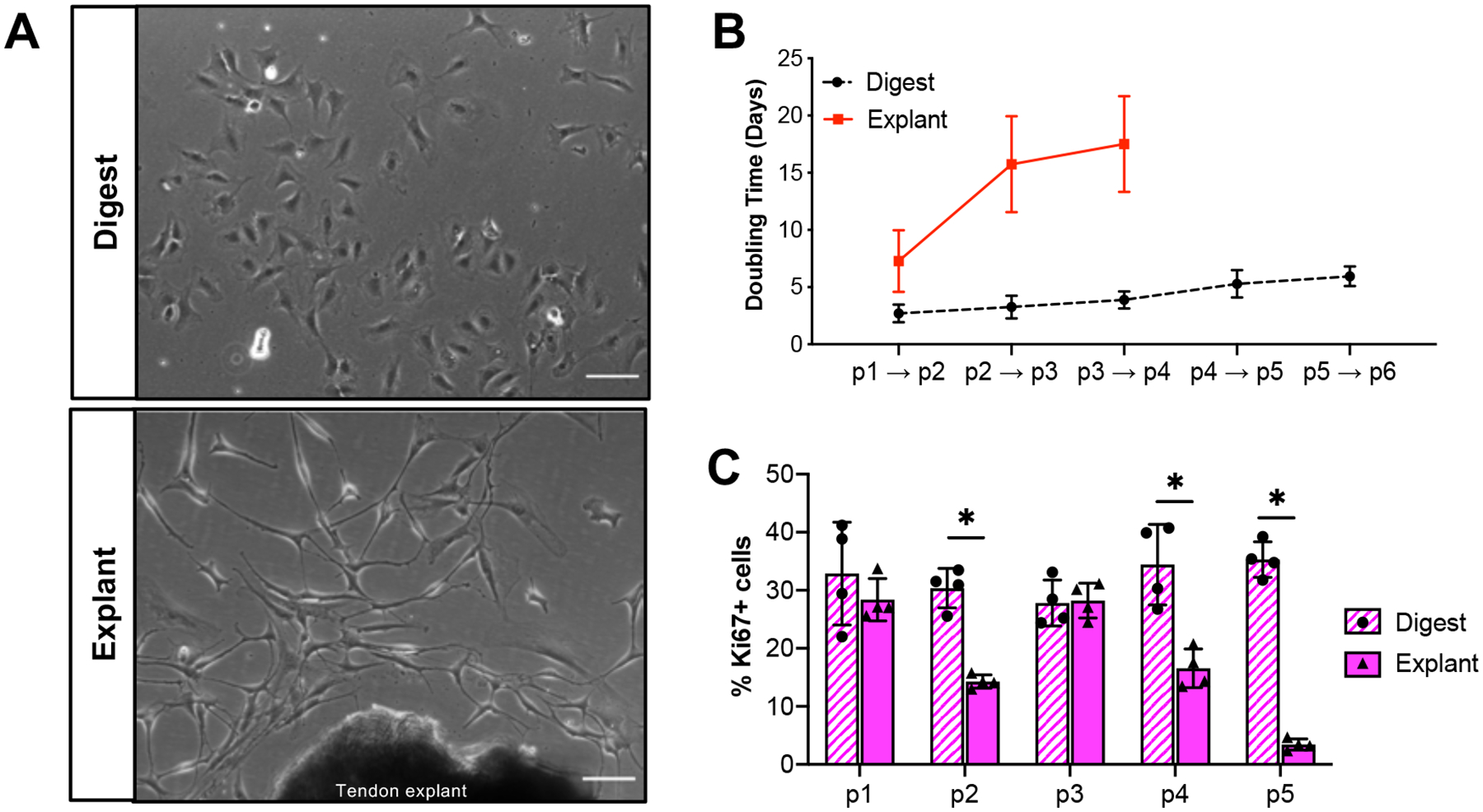
(A) Representative images of tendon cells isolated by collagenase digest (top) or explant culture (bottom) at day 4 post-isolation. Proliferation rates of digested and explanted cells as quantified by doubling time (B) or Ki67+ staining (C) over time in culture. Scale bars = 100 μm. *p<0.05. n=4.
Explanted cells exhibit a more activated gene expression profile compared to digested cells
To determine whether the changes in cell morphology and proliferative capabilities between isolation methods were reflective of changes in cell activation state, we examined the expression of tenogenic genes and genes associated with fibroblast activation at passage 1. Compared to cells isolated by digest, explanted cells exhibited significantly decreased expression of the tendon markers Scx (p=0.018), Mkx (p=0.030), and Col1a1 (p=0.031), along with significantly increased expression of the activation markers Col3a1 (p= 0.012), Postn (p=0.017) and CD248 (p=0.015). Expression of activation markers Fsp1 and CD106 were also increased in explanted cells compared to digested cells (~2-fold, p= 0.071 and ~4-fold, p=0.066, respectively), though these changes were not statistically significant. Digested cells also show alterations in the expression of both tenogenic markers and markers of fibroblast activation when compared to intact FDL tendon (Supplemental Figure S1) suggesting that isolation by any method results in cell activation, though removal of all matrix cues via digestion results in a different activation profile than forcing cells to migrate out of explanted tissue.
Cultures isolated by explant have a higher percentage of myofibroblasts than digested cultures
Given that one of the consequences of fibroblast activation is differentiation into a contractile myofibroblast phenotype, we next wanted to examine the effect of isolation method on myofibroblast content to determine whether the early increases in the expression of activation markers in explanted cultures relative to digested cultures would also result in increased myofibroblast differentiation. Cultures isolated by each method at each passage were stained with antibodies to the myofibroblast marker α-SMA (Figure 3A). Though the overall percentage of myofibroblasts remained low, explanted cultures contained a significantly higher percentage of α-SMA+ myofibroblasts than digested cultures at all passages examined (Figure 3B; p1: p= 0.003, p2: p=<0.001, p3: p=<0.001, p4: p<0.001, p5: p<0.001).
Figure 3: Explanted cultures contain more myofibroblasts compared to digested cultures.
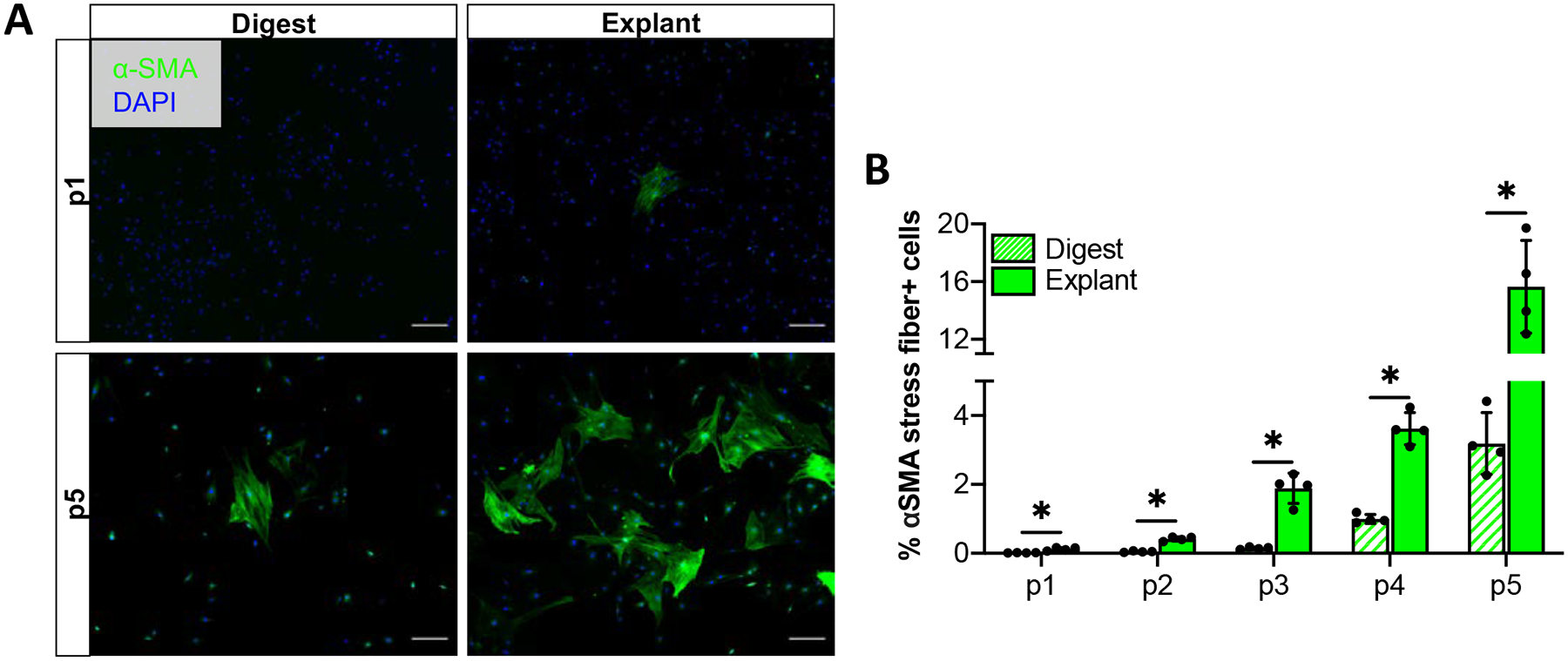
Representative images (A) and quantification (B) of α-SMA stress fiber (myofibroblast marker) staining over time in culture. Scale bars = 200 μm. *p<0.05, n=4.
The composition of tendon cell lineages in vitro is dependent on isolation method
Given the recent appreciation for the heterogeneity of tendon cells (25, 32, 35), we next sought to determine if the differences in cell morphology, proliferation, activation profile and myofibroblast content between isolation methods could be due to selective isolation of different tendon cell populations. As Scx-expressing cells have traditionally been seen as the predominant resident tendon cell population, we first examined whether Scx-lineage cells made up the majority of cells in tendon cell cultures. To facilitate labeling of Scx-lineage tendon cells, ScxCreERT2+ mice were crossed to the Rosa Ai9 reporter strain (Scx-lin+). Mice were given tamoxifen injections for three consecutive days, allowing a five day washout period prior to isolation (Figure 4A). This tamoxifen dosing regimen labeled 56.49 ± 5.14% of cells in the intact adult FDL tendon (Figure 4B). Following isolation by collagenase digest, Scx-lin+ cells comprised approximately 50% of the cells present through the 16 day culture period (Figure 4C). In contrast, Scx-lin+ cells made up 29.50 ± 15.49% of the cells in explanted cultures at day 2 but 93.37 ± 5.13% of cells present by day 8 post-isolation, remaining relatively unchanged from day 8 through day 16 (94.33 ± 1.55%) (Figure 4D). Given the fact that the labeling efficiency of inducible Cre drivers is rarely 100%, we wanted to determine whether the non-lineage cells present in culture could simply be due to inefficiency of the inducible ScxCre used to label Scx-lin cells in this study (i.e. the non-lineage cells are actually Scx-lin cells but were not labeled in vivo). To evaluate the efficiency of the Scx;Ai9 labeling, we examined intact FDL tendons from Scx;Ai9;GFP mice (Supplemental Figure S2). The majority of Scx-lin+ cells in Scx;Ai9;GFP mice are also Scx-GFP+ (60.77 ± 3.34%). However, 20.75 ± 5.28% of cells were only Scx-GFP+ suggesting that the ScxCreERT2 driver is approximately 75% efficient at labeling Scx-expressing cells in the adult mouse. Nonetheless, we observed that 15.36 ± 1.22% of tendon cells were neither Scx-lin+ nor Scx-GFP+ which supports the presence of true Scx-lin- cells in in vitro cultures.
Figure 4: Ratio of of Scx-lin+/Scx-lin- cells in culture depends on isolation method.
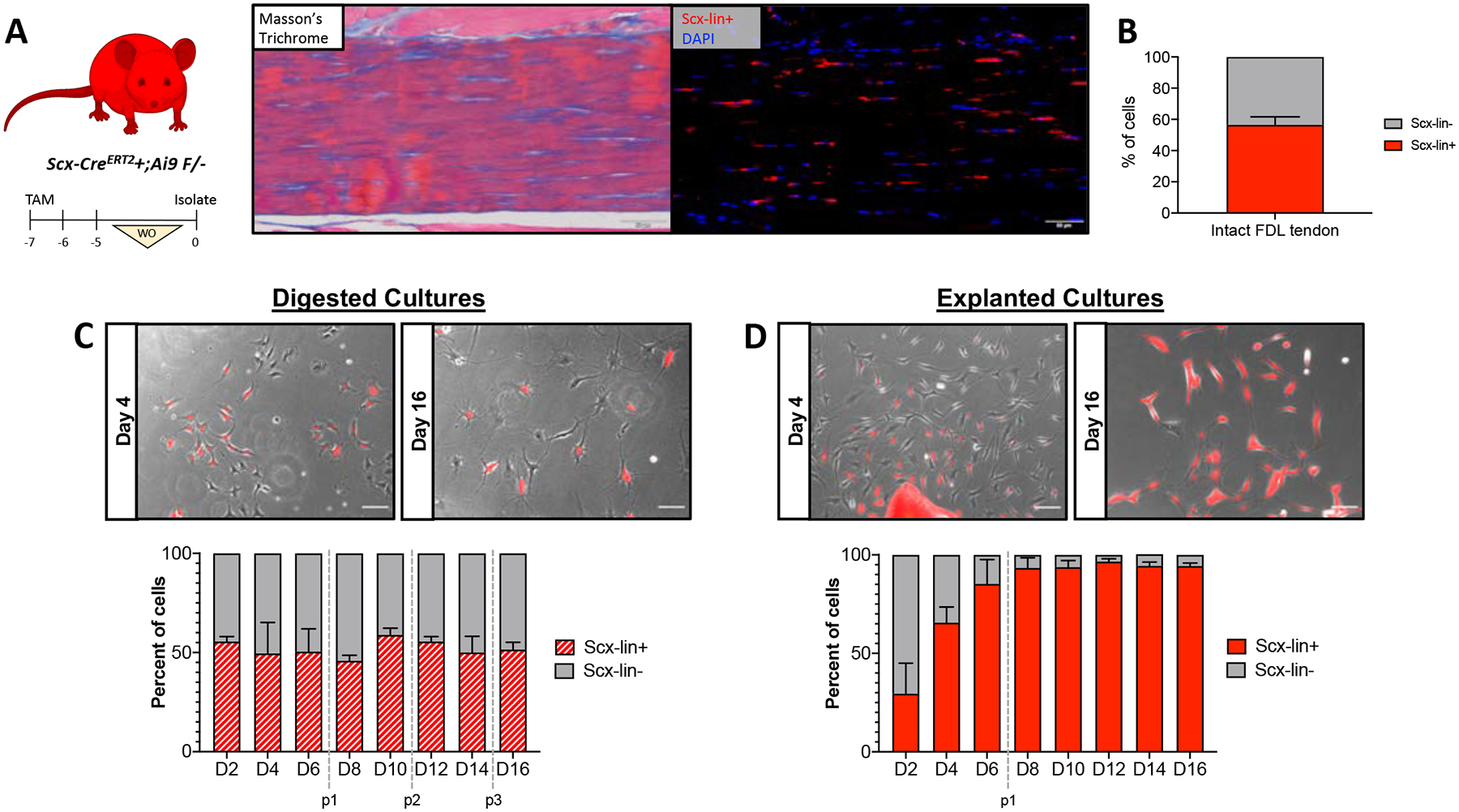
Scx;Ai9 mice were injected with TMX to label Scx-lin+ cells (A). Representative images showing intact FDL tendon (Masson’s Trichrome; left) and Ai9 labeling (immunofluorescence; right) of tendon cells in the same area using the described TMX regimen. Scale bars = 50 μm. Quantification (B) of Scx-lin+ and Scx-lin- tendon cells in intact FDL tendons. n=4. Representative images (C,D; top) and quantification (bottom) of Scx-lin+ (red) and Scx-lin- (gray) tendon cells isolated by digest (C) or explant culture (D). Passages are indicated by vertical gray dotted lines. Scale bars = 100 μm. n=3. Mouse model schematic was created using www.biorender.com.
Previous work has shown that S100a4 also marks a population of resident tendon cells (31), therefore we next wanted to determine the extent to which the isolation method affects the presence of S100a4-lineage cells. S100a4-Cre mice were crossed to the Rosa-Ai9 reporter strain (Figure 5A) which labels 70.56 ± 6.13% of cells (S100a4-lin+) in the intact FDL tendon (Figure 5B). Similar to the Scx-lin+ cells, the percentage of S100a4-lin+ (approximately 80–90%) cells did not change over time in digested cultures (Figure 5C). In contrast, the percentage of S100a4-lin+ cells increased over time from 43.93 ± 8.17% at day 2 to 80.62 ± 2.37 % by day 6, remaining above 80% through day 16 post-isolation in explanted cultures (Figure 5D). Interestingly, cultures derived from both reporter strains contain a population of non-lineage cells whose presence is both isolation method and time-dependent (Figures 4C–D, 5C–D).
Figure 5: Ratio of S100a4-lin+/S100a4-lin- cells in culture depends on isolation method.
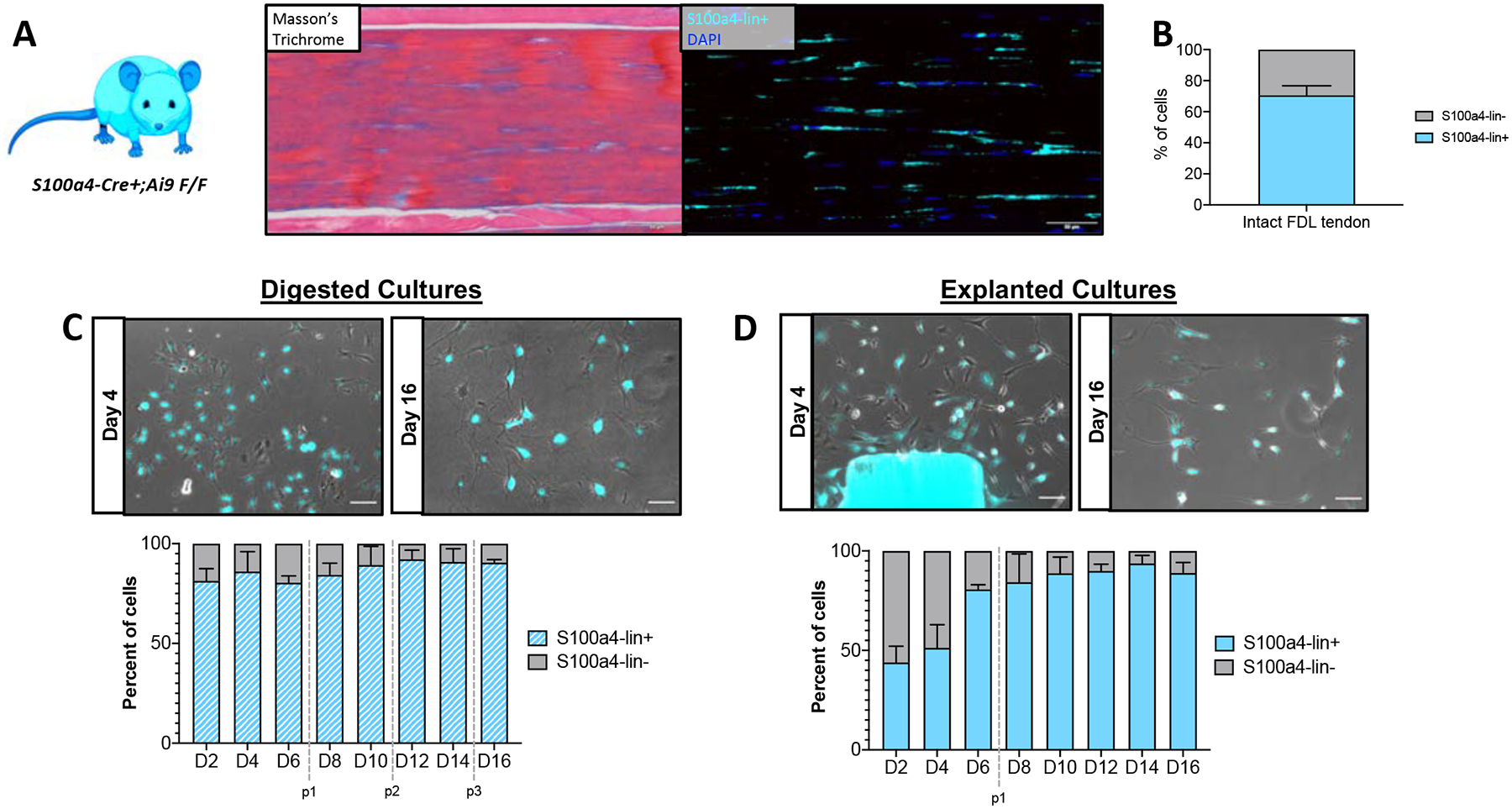
Representative images (A) showing intact FDL tendon from S100a4;Ai9 mice (Masson’s Trichrome; left) and Ai9 labeling (immunofluorescence, pseudo-colored cyan; right) of tendon cells in the same area. Scale bars = 50 μm. Quantification (B) of S100a4-lin+ and S100a4-lin- tendon cells in intact FDL tendons. n=4. Representative images (C,D; top) and quantification (bottom) of S100a4-lin+ (red) and S100a4-lin- (gray) tendon cells isolated by digest (C) or explant culture (D). Passages are indicated by vertical gray dotted lines. Scale bars = 100 μm. n=3. Mouse model schematic was created using www.biorender.com.
Scx-lin+ cell proliferation status is dependent on isolation method
Given the dramatic increase in the percentage of Scx-lin+ cells in explant cultures over time compared to the relatively steady Scx-lin+ percentage seen in digested cultures, we wanted to determine whether this difference was due to increased proliferation of Scx-lin+ cells, relative to non-lineage cells in explant cultures. Serially passaged explanted and digested cultures from Scx;Ai9 mice were stained for the proliferation marker Ki67 and the percentage of Ki67+ cells that were Scx-lin+ or Scx-lin- was quantified at each passage. Consistent with the steady proliferation seen in digested cultures over time independent of cell lineage (Figure 1C), the percentage of Ki67+ cells that were Scx-lin+ remained relatively constant, decreasing slightly over time in culture, from 86.99 ± 8.06% at passage 1 to 50.04 ± 7.84% at passage 5 (Figure 6A,B). In explanted cultures, Scx-lin+ cells made up 83.17 ± 8.40% of Ki67+ cells at passage 1, but this percentage decreased substantially over time to 19.03 ± 2.57% of cells at passage 5 (Figure 6C,D). Additionally, the percentage of Scx-lin+ Ki67+ cells was significantly lower in explanted cultures compared to digested cultures at each passage beyond passage 2 (Figure 6D, p1: p=0.535, p2: p=0.394, p3: p=0.006, p4: p=<0.001, p5: p=<0.001). Combined with the overall decrease in the proliferation of explanted cells, this similar decrease in the proliferation of explanted Scx-lin+ cells indicates that the increase in the number Scx-lin+ cells seen in explanted cultures over time is not due to on-going proliferation of Scx-lin+ cells relative to non-lineage cells.
Figure 6: Scx-lin+ cell proliferation status is dependent on isolation method.
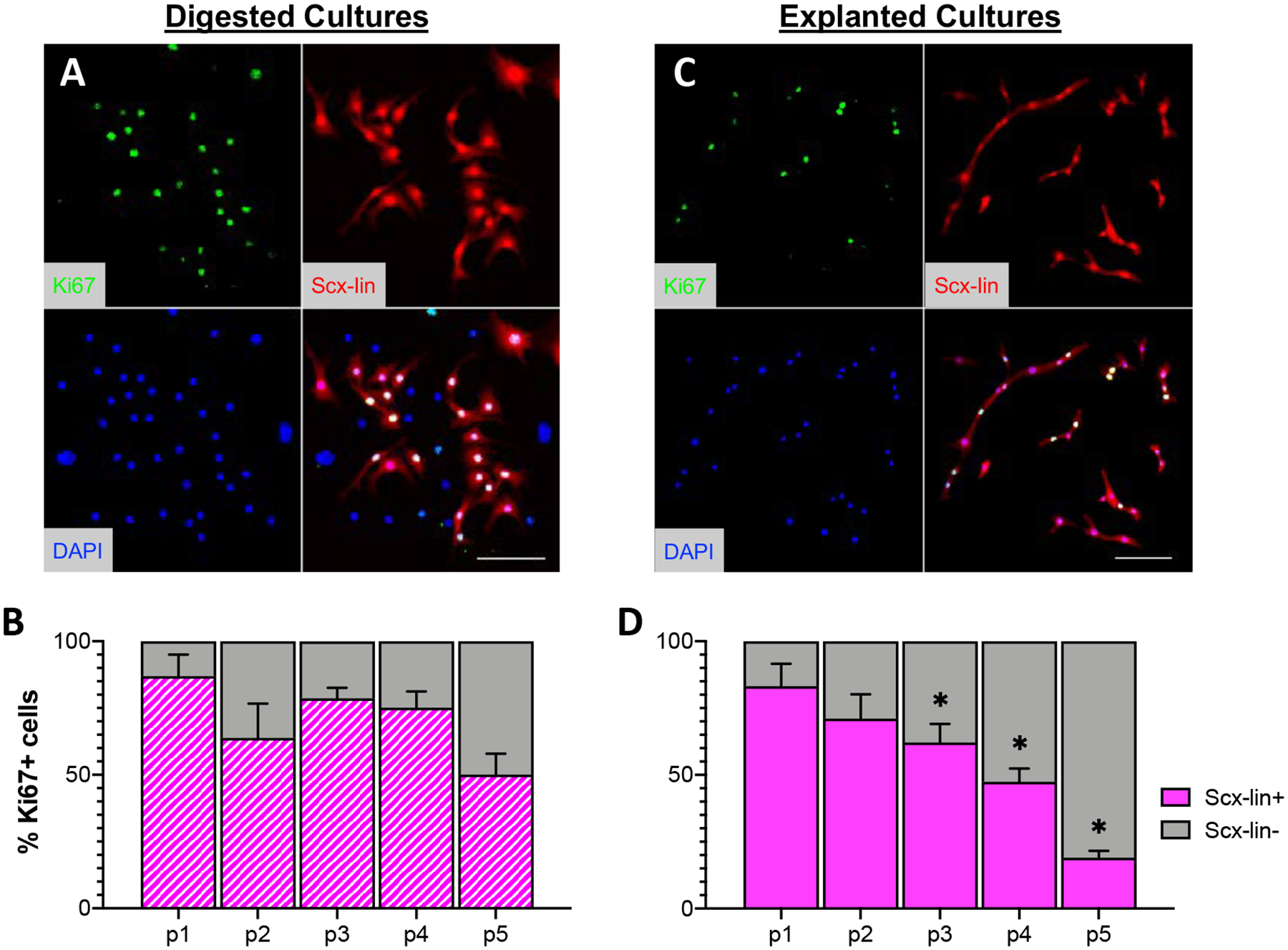
Representative images of Scx-lin+ Ki67+ cells from digested (A) or explanted (B) cultures at passage 1. Quantification of Scx-lin+ (magenta) or Scx-lin- (gray) present over time in cultures isolated by digest (B) or explant (D) culture. Scale bars = 100 μm. *p<0.05 compared to digested cultures at the same passage. n=4.
The majority of the myofibroblasts present in tendon cell cultures are not derived from in vivo Scx-lin+ cells
Using the same mouse model and labeling strategy employed in the current study, it has previously been reported that the majority of myofibroblasts present in the scar area of healing tendons in vivo are not derived from adult Scx-lin+ cells (25). To determine the Scx-lineage status of myofibroblasts present in in vitro cultures, explanted and digested cultures from Scx;Ai9 mice were stained for the myofibroblast marker α-SMA to quantify the percentage of α-SMA+ myofibroblasts that were derived from Scx-lin+ cells at each passage (Figure 7A,B; shown at passage 5). Similar to what is seen in vivo, the majority of α-SMA+ myofibroblasts in vitro are not derived from Scx-lin+ cells in either digested (Figure 7C) or explanted cultures at any passage (Figure 7D); however, the percentage of Scx-lin+ myofibroblasts present did differ significantly between isolation methods. At each passage, there were significantly fewer Scx-lin+ myofibroblasts in explanted cultures compared to digested cultures (Figure 7D, p1: p=0.005 p2: 0.001, p3:<0.001, p4: 0.017, p5: <0.001).
Figure 7: The majority of the myofibroblasts present in tendon cell cultures are not derived from in vivo Scx-lin+ cells.
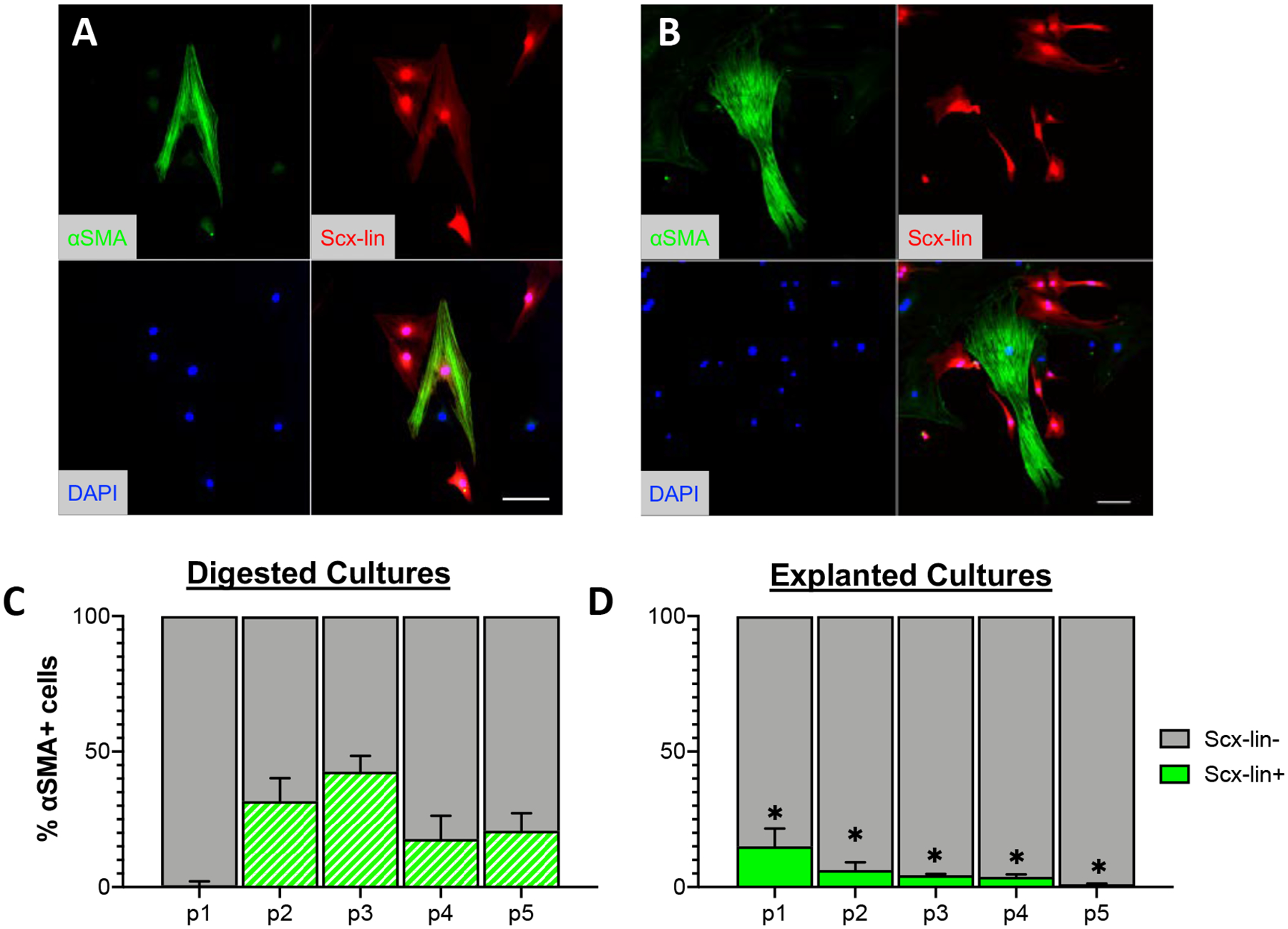
Representative images of Scx-lin+ SMA+ (A) and Scx-lin- SMA+ (B) cells from passage 5 cultures. Quantification of Scx-lin+ SMA+ (green) or Scx-lin- SMA+ (gray) present over time in cultures isolated by digest (C) or explant (D) culture. Scale bars = 100 μm. *p<0.05 compared to digested cultures at the same passage. n=4.
Tendon cell populations present in in vitro cultures are highly dependent on isolation method and the tendon of origin
Finally, we wanted to determine whether our findings regarding the effects of isolation method on tendon cell lineages present in in vitro cultures were specific to the FDL tendon or could also be observed across multiple tendons. We therefore performed the lineage tracing experiments with tendon cells isolated by digest or explant culture from the Achilles, flexor carpi ulnaris (FCU), and tail tendons of Scx;Ai9 and S100a4;Ai9 mice. To determine the in vivo labeling efficiency prior to isolation, sagittal sections were used to quantify the number of lin+ cells from each tendon (Figure 8). In the Achilles tendon, Scx-lin+ cells comprised 30.36 ± 5.83%, whereas S100a4-lin+ cells were 86.74 ± 1.56% of the total tendon cell population (Figure 8A–C). In the FCU, 41.55 ± 2.33% of cells were Scx-lin+ compared to 75.57 ± 4.13% that were S100a4-lin+ (Figure 8D–F). Finally, in the tail tendon, 69.40 ± 3.81% of tendon cells were Scx-lin+ with a similar 71.17 ± 2.80 cells being S100a4-lin+ (Figure 8G–I).
Figure 8: In vivo labeling efficiency of Scx-CreERT2+;Ai9 F/- and S100a4-Cre+;Ai9 F/F in multiple tendons.
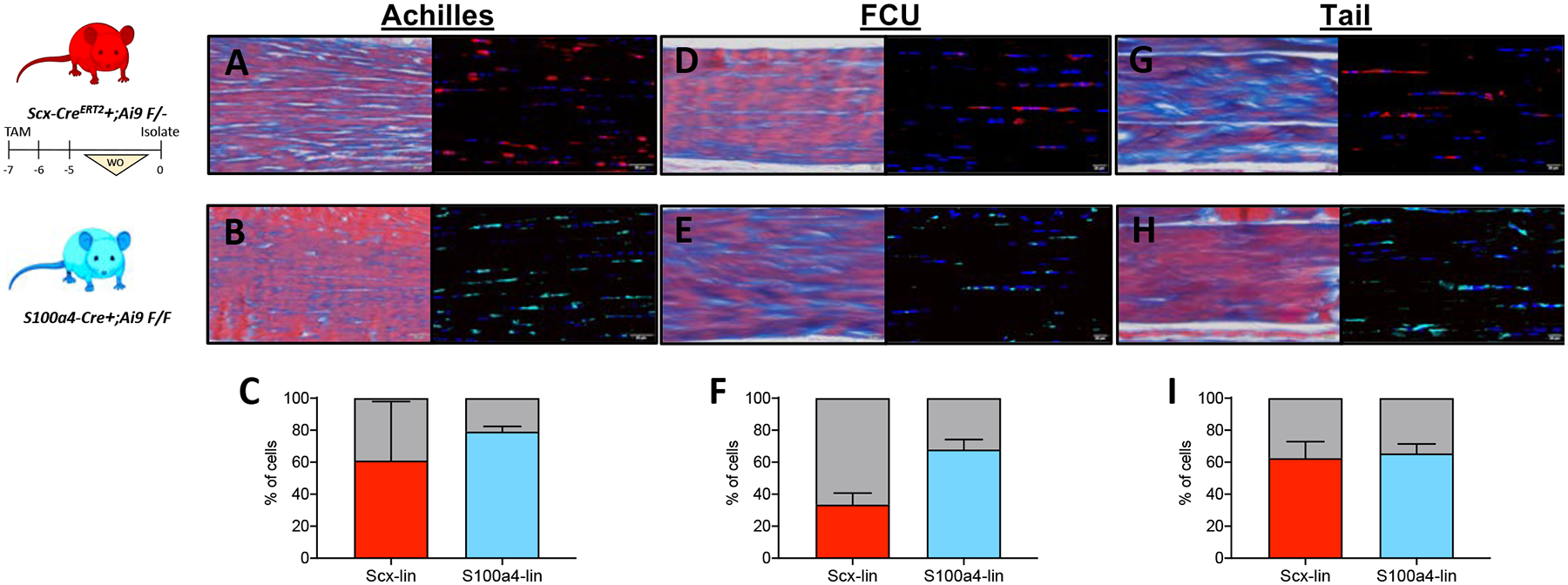
Representative images of in vivo labeling (left: Masson’s Trichrome, right: immunofluorescence) efficiency of Scx-CreERT2+;Ai9 F/- (A, D, G) and S100a4-Cre+;Ai9 F/F (B, E, H) in intact Achilles (A, B), flexor carpi ulnaris (FCU; D, E), and tail (G,H) tendons. Quantification of Scx-lin+ (red) and S100a4-lin+ (blue) in from Achilles (C), FCU (F), and tail tendons (I). Scale bars: A,B: 50 μm; D,E,G,H : 20 μm. n=4 mice for each tendon and lineage trace. Mouse model schematic was created using www.biorender.com.
Following isolation from the Achilles tendon (Figure 9A–C), a significantly higher (p<0.001) percentage of Scx-lin+ cells were present in digested (25.63 ± 2.66%) compared to explanted cultures (2.24 ± 2.67%) at day 4, but by day 16, there was a significantly higher percentage (p=0.004) of Scx-lin+ cells present in explanted cultures (58.17 ± 8.85%) compared to digested cultures (14.29 ± 9.33%). In contrast, at day 4 post-isolation from the Achilles tendon in both digested and explanted cultures, S100a4-lin+ cells comprised ~90% of the total cell population (D4 explant: 90.08 ± 1.87%, D4 digest: 92.50 ± 1.88%). By day 16 in culture, nearly all of the cells present in Achilles tendon cell cultures were S100a4-lin+ (D16 explant: 100 ± 0%, D16 digest: 96.77 ± 1.65).
Figure 9: Tendon cell populations present in in vitro cultures are highly dependent on isolation method and the tendon of origin.
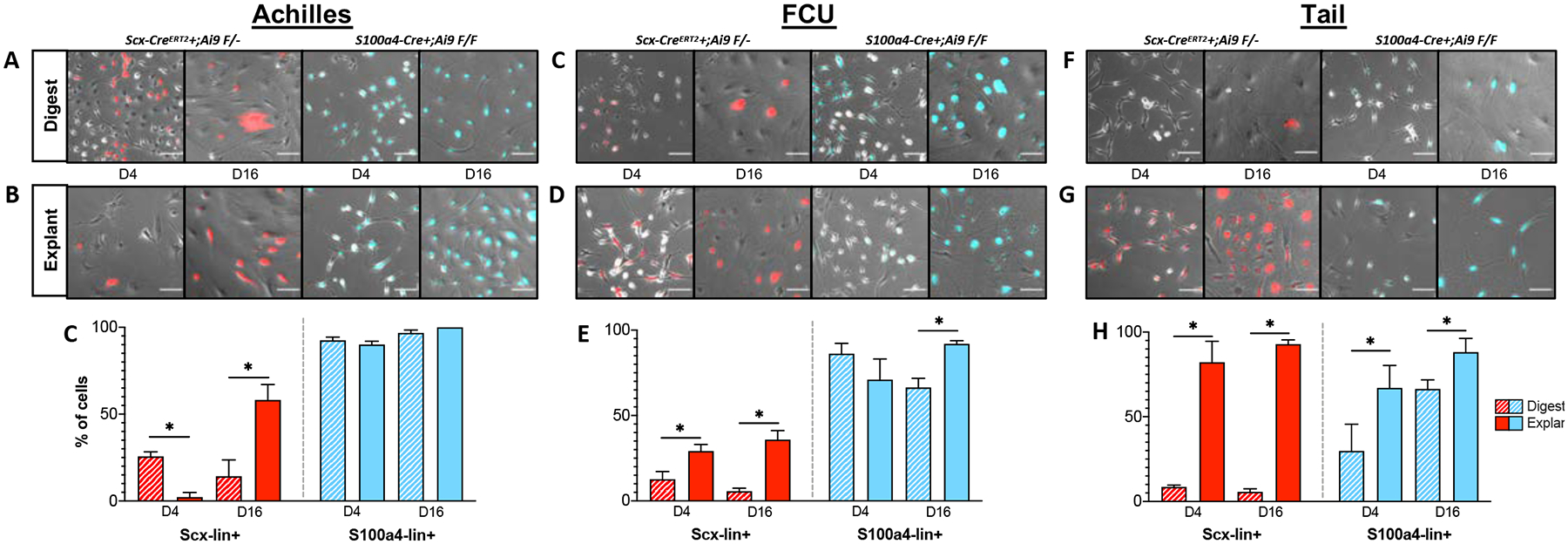
Representative images of Scx-lin+ (red) and S100a4-lin+ (blue) cells isolated from murine Achilles, flexor carpi ulnaris (FCU), and tail tendons by digest (A) or explant (B) culture. Quantification of Scx-lin+ (red) and S100a4-lin+ (blue) cells by digest (striped bars) or explant culture (solid bars) at days 4 and 16 post-isolation from Achilles (C), FCU (D), and tail (E) tendons. *p<0.05 between isolation methods at each timepoint. n=3 mice for each tendon and lineage trace. Scale bar = 100 μm.
In cultures derived from FCU tendons (Figure 9C–E), explanted cultures contained a significantly higher percentage of Scx-lin+ cells compared to digested at both day 4 (p=0.008; explant: 29.20 ± 3.84%, digest: 12.67 ± 4.50%) and day 16 (p=<0.001; explant: 35.90 ± 5.25%, digest: 5.67 ± 1.80%). The percentage of S100a4lin+ cells was not significantly different (p=0.123) between explanted (70.99 ± 12.07%) and digested (86.18 ± 6.08%) at day 4 post-isolation, however at day 16, there was a significantly higher (p=0.001) percentage of S100a4-lin+ cells in explanted (91.97 ± 1.80%) compared to digested (66.43 ± 5.35%) cultures.
Following isolation from tail tendons (Figure 9F–H), explanted cultures contained a significantly higher (p<0.001) percentage of Scx-lin+ cells (82.16 ± 12.35%) than digested cultures (8.56 ± 1.05%) at day 4 which persisted through day 16 (p<0.001; explant: 92.87 ± 2.54% vs digest: 5.67 ± 1.80%). Similarly, explanted cultures contained a significantly higher proportion of S100a4-lin+ cells than digested cultures at both day 4 (p=0.035; explant: 67.07 ± 13.25, digest: 29.82 ± 15.77%) and day 16 (p=0.018; explant: 88.17 ± 8.15, digest: 66.43 ± 5.25). Combined, these data indicate that both the isolation method and tendon of origin have an enormous effect on the tendon cell populations present in in vitro cultures.
DISCUSSION
To date, in vitro tendon cell culture systems have not taken into account how different isolations methods and source tendons may affect the ultimate phenotype and tendon cell lineage composition of cultured cells. In the present study, we examined how isolation by enzymatic digest or explant culture affect the proliferation, fate, and lineage of tendon cells isolated from the FDL tendon, demonstrating extensive differences in cellular behavior between isolation methods. Moreover, we establish that both isolation method and tendon of origin have a substantial effect on the presence of Scx-lin+ and S100a4-lin+ cells. Combined, these results suggest that further refinement of tendon cell isolation and culture protocols is required to ensure that in vitro studies more accurately reflect the desired tendon cell state and lineage composition.
Many previous studies have documented changes in the behavior of tendon cells following isolation, though these studies have typically been restricted to examination of tendon-specific genes in digested cell cultures. Yao et al. described that tendon cells isolated by collagenase digest from human Achilles tendons exhibit changes in morphology over time in culture, along with an increase in the collagen III to collagen I ratio and decreased expression of decorin (15). Cultured human tendon cells isolated by collagenase digest from the biceps long head tendon have also been shown to exhibit decreased expression of the tendon-related genes Scx, tenomodulin, thrombospondin-4, and Col3 along with increased expression of Col1 and tenascin-C compared to intact tendon (36). In contrast to the number of studies which examine changes in digested cell cultures, we are unaware of any study that specifically examine the fate of explanted cells over time in culture. In the present study, we took inspiration from other organ-specific fibroblast culture systems and looked specifically at changes in cell behavior associated with the process of fibroblast activation. Explanted cells in our study exhibited classic hallmarks of fibroblast activation, including reduced proliferation, increased expression of activation marker genes along with decreased expression of tissue-specific genes, and increased differentiation to α-SMA+ myofibroblasts. Moreover, explanted cultures were enriched for Scx-lin+ cells in all tendons examined, suggesting that adult Scx-lin+ cells are the predominant migratory cell population present in tendon. Similar to what was observed in explanted cultures in this study, Best et al. demonstrated that adult Scx-lin+ cells migrate from the tendon ends following acute injury of the FDL tendon to form a cellular bridge and that the majority of these Scx-lin+ in vivo do not differentiate into myofibroblasts (25). Despite the fact that more Scx-lin+ cells were present in explanted cultures compared to digested, explanted cultures had decreased expression of Scx, suggesting that, as occurs following injury in vivo (37), activation of tendon resident Scx-lin+ cells in vitro involves downregulation of Scx. Taken together, our data suggest that explanted cell behavior in vitro replicates at least some aspects of the behavior of cells that migrate out of the tendon into the scar tissue in vivo, indicating that in vivo study of explanted cells could provide a valuable tool to understand tendon cell behavior following acute injury.
Importantly, while explanted cells behaved similarly in vitro as is seen in vivo following acute injury, digested cells in our study also display an altered gene expression profile following isolation, though how this relates to any potential in vivo cell behavior is less clear. It may be that the behavior of digested cells in culture (i.e. proliferation, altered expression of activation markers relative to intact tendon) is reflective of tendon cell behavior seen in other tendinopathies in vivo (27), though further work is needed to confirm this. It has previously been suggested that isolation of tendon cells can result in their dedifferentiation to a more stem-like state (38), evidenced by decreased expression of tendon markers along with de novo expression of stem markers (39). In our study, we did not observe loss of Scx expression in digested cells compared to intact FDL tendon, and actually observed a slight (though non-significant) increase in the expression of Col1a1, which suggests that digested cells do not dedifferentiate upon isolation. It is possible that digested cells undergo some sort of differentiation event over time in culture- however, since we did not specifically examine whether isolated cells express stem cell markers or have increased clonogenic capacity following isolation in this system, we cannot say for certain. In any case, it is clear that cells isolated by either isolation method do not accurately represent the behavior of tendon cells during in vivo homeostasis.
Though previous studies have explored various isolation methods to selectively isolate different tendon cell populations based on their location within the tendon (40, 41) or their behavior in vitro (42–44) to our knowledge, this is the first study to utilize lineage tracing of known in vivo tendon cell populations to identify the tendon cell lineages present in in vitro cultures. Moreover, we directly compared the lineages present in cultures derived from multiple tendons isolated by both digest and explant culture. Strikingly, we observed that both isolation method and the tendon of origin have an enormous impact on the lineage of tendon cells present in in vitro cultures. In all tendons examined, explanted cultures were enriched for Scx-lin+ cells compared to digested cultures, with the effects of isolation methodology on the presence of S100a4-lin+ cells being dependent on the tendon of origin. Combined with the differential effects of isolation method on the activation status and fate of tendon cells in vitro, our lineage-tracing experiments suggest that cultures derived from different tendons by different isolation methodologies cannot, and should not, be directly compared.
Interestingly, we also identified a persistent non-lineage (lin-) cell population in cultures derived from both reporter strains whose percent of the total cell population varied depending isolation method and time post-isolation. This is particularly interesting given the differences in labeling between the two different Cre drivers: in cultures derived from the Scx;Ai9 mice, the presence of lin- cells is consistent with the fact that not all tendon cells are Scx-lin+ in vivo, however in cultures derived from S100a4;Ai9 mice, despite Fsp-1 (also known as S100a4) being a general marker of cell activation, the presence of lin- cells (i.e. cells that have never expressed Fsp-1 either in vivo or in vitro) suggests that Fsp-1 is not a marker of activation in this particular population. Future studies will clarify both the identity and role of this lin- population.
Though we have documented the effects of isolation method and tendon of origin on in vitro tendon cell cultures, this study is not without limitations. In all tendons examined, neither isolation method preserved the ratio of lineage to non-lineage cells seen in vivo, suggesting that the isolation and culture methods employed in this study select for various tendon cell populations. Furthermore, differences in the type of cell labeling between Cre drivers (inducible vs. non-inducible) makes determining the exact relationship between the Scx-lin and S100a4-lin populations identified in this study somewhat difficult, and there is most likely substantial overlap between the two populations as demonstrated previously using Scx;Ai9 mice crossed to an S100a4-GFP+ reporter strain (25). Future studies will clarify the extent to which this overlap persists during culture and will further define the relationship between the cell populations identified in this study. Though we only examined the consequence of culture in a particular culture media at a defined cellular density, changing either could provide an avenue to intentionally select for specific tendon cell lineages. We also chose to examine tendon cells isolated from adult mice (10–12 weeks old) in order to be able to compare our in vitro findings on cell fate with previous in vivo work using the same genetic models (25, 31). Isolating tendon cells by either method from significantly younger or older mice may result in the isolation of very different cell populations given that the number of cells that would be considered Scx-lin+ or S100a4-lin+ likely changes with age (45). Moreover given the indelible nature of the adult Scx-lin+ labeling regimen, we are unable to assess any natural fluctuations in Scx expression in order to further delineate subpopulations within the broader adult Scx-lin+ population, including potential Scx-expressing tendon stem/progenitor cell populations. In an attempt to simulate standard tissue culture practices, we relied on passaging cells from both explanted and digested cultures at 70% confluence. Due to the decreased proliferative capacity of explanted cells relative to digested cells, this resulted in a disparity between the total time in culture between isolation methods at all passages beyond passage 1, with explanted cells at each subsequent passage taking longer to reach 70% confluence than their digested counterparts. Nonetheless, we believe that this simply highlights another issue with the lack of standardization of tendon cell culture, as passage is the most common method of reporting the ‘age’ of cell cultures, regardless of isolation method. In this study, we found stark differences between the behavior, fate, and cell lineage proportions at each passage due to isolation method, suggesting that the use of passage to report the ‘age’ of primary tendon cells in culture requires further thought and refinement in order to be able to more accurately compare the results of different studies.
In summary, the data from this study demonstrate that the both the isolation method and tendon of origin have a substantial effect on the identify and behavior of tendon cells in culture. Especially in cultures derived from explanted cells, rather than considering the changes that occur in tendon cells post-isolation as a “phenotypic drift” towards an unknown or unnatural phenotype, we propose that explanted tendon cells are in fact mimicking the natural process that occurs following injury, resulting in activation and eventual progression to a myofibroblast fate depending on the lineage from which a cell derives. The differences in activation state and the subpopulations present between isolation methods identified here may help explain discrepancies in the existing literature and should be considered when designing future in vitro studies of tendon cell behavior.
Supplementary Material
Figure 2: Explanted tendon cells exhibit decreased expression of tendon markers, increased expression of activation markers.
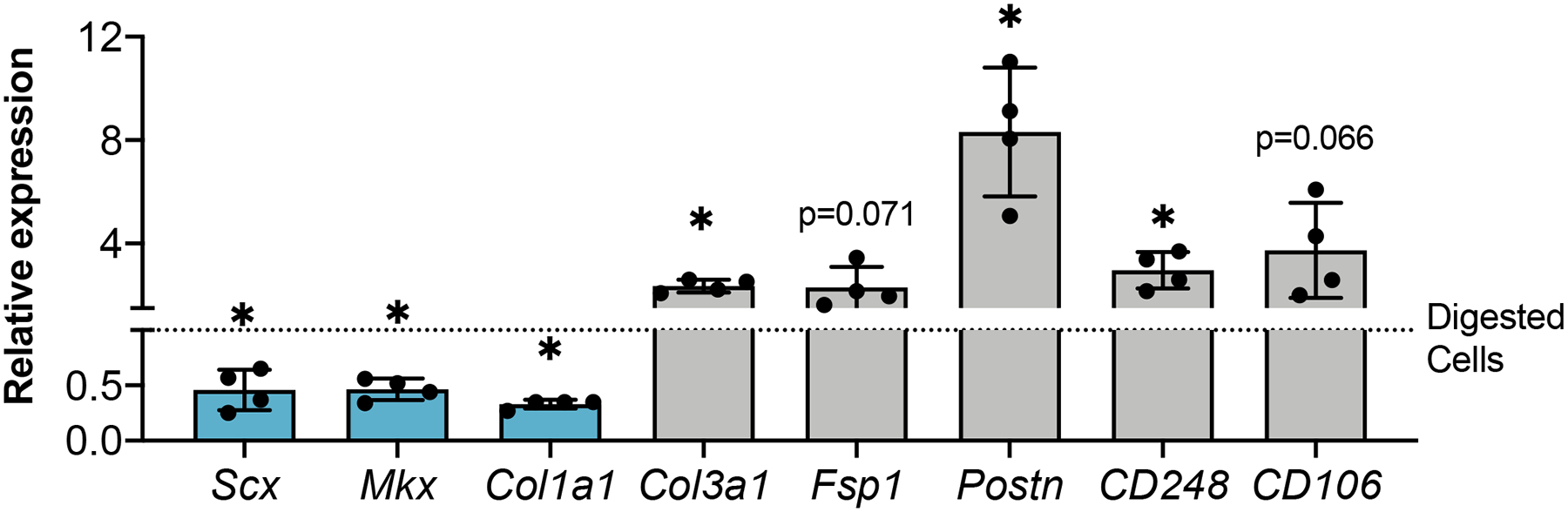
Expression of tendon-related (blue) and activation marker (gray) genes in explanted tendon cells shown relative to digested tendon cells at passage 1 (dotted line). *p<0.05 compared to digested TF. n=4.
Statement of clinical significance:
The development of informed in vitro tendon cell models will facilitate enhanced screening of potential therapeutic candidates to improve tendon healing.
ACKNOWLEDGEMENTS:
The authors thank the Histology, Biochemistry and Molecular Imaging (HBMI; University of Rochester, Rochester, NY, USA) Core for technical assistance. This work was supported in part by grants from the U.S. National Institutes of Health (NIH), National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) (AEL: K01AR068386 and R01AR073169, AECN: T32AR076950). The HBMI Core is supported by NIH/NIAMS Grant P30AR069655. The authors declare no competing interests.
Non-standard abbreviations
- Scx
Scleraxis
- Mkx
Mohawk
- Col1
Collagen type I
- Col3
Collagen type III
- Postn
Periostin
- Fsp-1
Fibroblast-specific protein 1, also known as S100a4
- S100a4
S100 calcium-binding protein A4
- αSMA
Alpha smooth muscle actin
- Scx;Ai9
ScxCreERT2;Ai9 F/- mice
- Scx;Ai9;Scx-GFP
ScxCreERT2;Ai9 F/-;Scx-GFP+ mice
- S100a4;Ai9
S100a4Cre;Ai9 F/F mice
- TMX
Tamoxifen
- Scx-lin+
Adult scleraxis lineage cells
- S100a4-lin+
S100a4 lineage cells
- FDL
Flexor Digitorum Longus tendon
- FGM
Fibroblast Growth Medium
- Ki67
Marker Of Proliferation Ki-67
- FCU
Flexor Carpus Ulnaris tendon
- PFA
Paraformaldehyde
- TX100
Triton X-100
- FITC
Fluorescein isothiocyanate
- NBF
Neutral buffered formalin
- EDTA
Ethylenediaminetetraacetic acid
- DAPI
4′,6-diamidino-2-phenylindole
REFERENCES
- 1.Nichols AEC, Best KT, and Loiselle AE (2019) The cellular basis of fibrotic tendon healing: challenges and opportunities. Translational research : the journal of laboratory and clinical medicine 209, 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, and Kambadur R (2002) Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem 277, 49831–49840 [DOI] [PubMed] [Google Scholar]
- 3.Sudo H, Kodama HA, Amagai Y, Yamamoto S, and Kasai S (1983) In vitro differentiation and calcification in a new clonal osteogenic cell line derived from newborn mouse calvaria. J Cell Biol 96, 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith CO, and Eliseev RA (2021) Energy Metabolism During Osteogenic Differentiation: The Role of Akt. Stem Cells Dev 30, 149–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buxton AN, Bahney CS, Yoo JU, and Johnstone B (2011) Temporal exposure to chondrogenic factors modulates human mesenchymal stem cell chondrogenesis in hydrogels. Tissue Eng Part A 17, 371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang C-M, Chien C-S, Yao C-C, Hsiao L-D, Huang Y-C, and Wu CB (2004) Mechanical strain induces collagenase-3 (MMP-13) expression in MC3T3-E1 osteoblastic cells. In J. Biol. Chem Vol. 279 pp. 22158–22165 [DOI] [PubMed] [Google Scholar]
- 7.Chang YJ, Chen YJ, Huang CW, Fan SC, Huang BM, Chang WT, Tsai YS, Su FC, and Wu CC (2016) Cyclic Stretch Facilitates Myogenesis in C2C12 Myoblasts and Rescues Thiazolidinedione-Inhibited Myotube Formation. Front Bioeng Biotechnol 4, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ponik SM, Triplett JW, and Pavalko FM (2007) Osteoblasts and osteocytes respond differently to oscillatory and unidirectional fluid flow profiles. J Cell Biochem 100, 794–807 [DOI] [PubMed] [Google Scholar]
- 9.Hecht N, Johnstone B, Angele P, Walker T, and Richter W (2019) Mechanosensitive MiRs regulated by anabolic and catabolic loading of human cartilage. Osteoarthritis Cartilage 27, 1208–1218 [DOI] [PubMed] [Google Scholar]
- 10.Schulze-Tanzil G, Mobasheri A, Clegg PD, Sendzik J, John T, and Shakibaei M (2004) Cultivation of human tenocytes in high-density culture. Histochemistry and Cell Biology 122, 219–228 [DOI] [PubMed] [Google Scholar]
- 11.Pietschmann MF, Wagenhäuser MU, Gülecyüz MF, Ficklscherer A, Jansson V, and Müller PE (2014) The long head of the biceps tendon is a suitable cell source for tendon tissue regeneration. Arch Med Sci 10, 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evrova O, Kellenberger D, Calcagni M, Vogel V, and Buschmann J (2020) Supporting Cell-Based Tendon Therapy: Effect of PDGF-BB and Ascorbic Acid on Rabbit Achilles Tenocytes in Vitro. Int J Mol Sci 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nichols AEC, Settlage RE, Werre SR, and Dahlgren LA (2018) Novel roles for scleraxis in regulating adult tenocyte function. BMC Cell Biol 19, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, and Wildemann B (2010) Characterization of tendon cell cultures of the human rotator cuff. Eur Cell Mater 20, 84–97 [DOI] [PubMed] [Google Scholar]
- 15.Yao L, Bestwick CS, Bestwick LA, Maffulli N, and Aspden RM (2006) Phenotypic drift in human tenocyte culture. Tissue Eng 12, 1843–1849 [DOI] [PubMed] [Google Scholar]
- 16.van Vijven M, Wunderli SL, Ito K, Snedeker JG, and Foolen J (2020) Serum deprivation limits loss and promotes recovery of tenogenic phenotype in tendon cell culture systems. J Orthop Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazzocca AD, Chowaniec D, McCarthy MB, Beitzel K, Cote MP, McKinnon W, and Arciero R (2012) In vitro changes in human tenocyte cultures obtained from proximal biceps tendon: multiple passages result in changes in routine cell markers. Knee Surg Sports Traumatol Arthrosc 20, 1666–1672 [DOI] [PubMed] [Google Scholar]
- 18.Bernard-Beaubois K, Hecquet C, Houcine O, Hayem G, and Adolphe M (1997) Culture and characterization of juvenile rabbit tenocytes. Cell Biol Toxicol 13, 103–113 [DOI] [PubMed] [Google Scholar]
- 19.Herrmann H, Dessau W, Fessler LI, and von der Mark K (1980) Synthesis of types I, III and AB2 collagen by chick tendon fibroblasts in vitro. Eur J Biochem 105, 63–74 [DOI] [PubMed] [Google Scholar]
- 20.Darby IA, Laverdet B, Bonté F, and Desmoulière A (2014) Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 7, 301–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott CG, Wang J, Guo X, Xu S. w., Eastwood M, Guan J, Leask A, Conway SJ, and Hamilton DW (2012) Periostin modulates myofibroblast differentiation during full-thickness cutaneous wound repair. Journal of Cell Science 125, 121–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Chen L, Xiao M, Wang C, and Qin Z (2011) FSP1+ fibroblasts promote skin carcinogenesis by maintaining MCP-1-mediated macrophage infiltration and chronic inflammation. Am J Pathol 178, 382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Teicher BA (2019) CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget 10, 993–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li P, Sanz I, O’Keefe RJ, and Schwarz EM (2000) NF-κB Regulates VCAM-1 Expression on Fibroblast-Like Synoviocytes. The Journal of Immunology 164, 5990–5997 [DOI] [PubMed] [Google Scholar]
- 25.Best KT, and Loiselle AE (2019) Scleraxis lineage cells contribute to organized bridging tissue during tendon healing and identify a subpopulation of resident tendon cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 33, 8578–8587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Best KT, Lee FK, Knapp E, Awad HA, and Loiselle AE (2019) Deletion of NFKB1 enhances canonical NF-κB signaling and increases macrophage and myofibroblast content during tendon healing. Scientific Reports 9, 10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dakin SG, Buckley CD, Al-Mossawi MH, Hedley R, Martinez FO, Wheway K, Watkins B, and Carr AJ (2017) Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Res Ther 19, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hinz B (2007) Formation and function of the myofibroblast during tissue repair. The Journal of investigative dermatology 127, 526–537 [DOI] [PubMed] [Google Scholar]
- 29.Darby IA, Zakuan N, Billet F, and Desmoulière A (2016) The myofibroblast, a key cell in normal and pathological tissue repair. Cell Mol Life Sci 73, 1145–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Best KT, Nichols AEC, Knapp E, Hammert WC, Ketonis C, Jonason JH, Awad HA, and Loiselle AE (2020) NF-κB activation persists into the remodeling phase of tendon healing and promotes myofibroblast survival. Sci Signal 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ackerman JE, Nichols AE, Studentsova V, Best KT, Knapp E, and Loiselle AE (2019) Cell non-autonomous functions of S100a4 drive fibrotic tendon healing. eLife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Micheli AJ, Swanson JB, Disser NP, Martinez LM, Walker NR, Oliver DJ, Cosgrove BD, and Mendias CL (2020) Single-cell transcriptomic analysis identifies extensive heterogeneity in the cellular composition of mouse Achilles tendons. Am J Physiol Cell Physiol 319, C885–c894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Disser NP, Ghahramani GC, Swanson JB, Wada S, Chao ML, Rodeo SA, Oliver DJ, and Mendias CL (2020) Widespread diversity in the transcriptomes of functionally divergent limb tendons. J Physiol 598, 1537–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, and Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods (San Diego, Calif.) 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 35.Kendal A, Layton T, Al-Mossawi H, Brown R, Loizou C, Rogers M, Sharp M, Dakin S, Appleton L, and Carr A (2019) Identification of human tendon cell populations in healthy and diseased tissue using combined single cell transcriptomics and proteomics. bioRxiv, 2019.2012.2009.869933 [Google Scholar]
- 36.Jo CH, Lim HJ, and Yoon KS (2019) Characterization of Tendon-Specific Markers in Various Human Tissues, Tenocytes and Mesenchymal Stem Cells. Tissue Eng Regen Med 16, 151–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dyment NA, Liu C-F, Kazemi N, Aschbacher-Smith LE, Kenter K, Breidenbach AP, Shearn JT, Wylie C, Rowe DW, and Butler DL (2013) The Paratenon Contributes to Scleraxis-Expressing Cells during Patellar Tendon Healing. In PLoS ONE Vol. 8 p. e59944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagenhäuser MU, Pietschmann MF, Sievers B, Docheva D, Schieker M, Jansson V, and Müller PE (2012) Collagen type I and decorin expression in tenocytes depend on the cell isolation method. BMC musculoskeletal disorders 13, 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tan GK, Pryce BA, Stabio A, Brigande JV, Wang C, Xia Z, Tufa SF, Keene DR, and Schweitzer R (2020) Tgfβ signaling is critical for maintenance of the tendon cell fate. eLife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Banes AJ, Donlon K, Link GW, Gillespie Y, Bevin AG, Peterson HD, Bynum D, Watts S, and Dahners L (1988) Cell populations of tendon: a simplified method for isolation of synovial cells and internal fibroblasts: confirmation of origin and biologic properties. J Orthop Res 6, 83–94 [DOI] [PubMed] [Google Scholar]
- 41.Taylor SH, Al-Youha S, Van Agtmael T, Lu Y, Wong J, McGrouther DA, and Kadler KE (2011) Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS One 6, e16337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang J, and Wang JH (2010) Characterization of differential properties of rabbit tendon stem cells and tenocytes. BMC musculoskeletal disorders 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang J, and Wang JH (2013) Human tendon stem cells better maintain their stemness in hypoxic culture conditions. PLoS One 8, e61424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu YF, Chen C, Tang JB, and Mao WF (2020) Growth and Stem Cell Characteristics of Tendon-Derived Cells with Different Initial Seeding Densities: An In Vitro Study in Mouse Flexor Tendon Cells. Stem Cells Dev 29, 1016–1025 [DOI] [PubMed] [Google Scholar]
- 45.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, and Huang AH (2017) Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Scientific Reports 7, 45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


