Abstract
Active antibody-mediated rejection (AMR) is a potentially devastating complication and consistently effective treatment remains elusive. We hypothesized that the reversal of acute AMR requires rapid elimination of antibody-secreting plasma cells (PC) with a proteasome inhibitor, bortezomib, followed by the sustained inhibition of PC generation with CTLA4-Ig or belatacept (B/B). We show in mice that B/B therapy selectively depleted mature PC producing donor-specific antibodies (DSA) and reduced DSA, when administered after primary and secondary DSA responses had been established. A pilot investigation was initiated to treat 6 consecutive patients with active AMR with B/B. Compassionate use for the first patient after his 3rd kidney transplant, who developed early, severe acute AMR that did not respond to steroids, plasmapheresis and intravenous immunoglobulin. B/B treatment resulted in a rapid reversal of AMR, leading us to treat five additional patients who also resolved their acute AMR episode and had sustained disappearance of circulating DSA for ≤ 30 months. This study provides a proof-of-principle demonstration that mouse models can identify mechanistically rational therapies for the clinic. Follow-up investigations with a more stringent clinical design are warranted to test whether B/B improves on the standard of care for the treatment of acute AMR.
1. Introduction
Active acute antibody-mediated rejection is a potentially devastating complication that occurs in about 5–7% of all kidney transplant recipients, and is responsible for 20–48% of rejection episodes among pre-sensitized positive crossmatch patients(1). The standard of care for acute AMR is plasmapheresis and intravenous immunoglobulin, however, clinical evidence for the effectiveness of this treatment has been based on very limited numbers of controlled trials(2). Furthermore, the long-term efficacy of new agents that deplete B cells (rituximab), deplete PCs (bortezomib), and complement inhibitors (eculizumab) remains unclear, suggesting an opportunity for identifying improved therapies for acute AMR. Costimulation blockade has been shown to inhibit the generation of T cells that are necessary for productive DSA production(3, 4). We and others have shown in experimental rodent models that co-stimulation is necessary for sustaining germinal center (GC) B cell responses, and that late intervention with anti-CD154 or CTLA4-Ig rapidly collapses established GC responses, thereby preventing the further generation of PC and memory B cells(5–10). We also reported that treatment with CTLA4-Ig, starting at the time of transplantation in sensitized recipients prevented recall humoral responses(5, 7). Belatacept, a high-affinity mutated CTLA4-Ig is an FDA approved medication for the prevention of acute rejection (AR) in kidney transplant recipients(11). Consistent with experimental findings, patients on belatacept- compared to cyclosporine-based immunosuppression exhibited reduced rates of DSA development despite higher rejection rates, de novo conversion of donor-specific IgM to IgG, and pre-existing DSA levels(12–14).
Despite promising observations of its efficacy, CTLA4-Ig usually fails to halt DSA responses when administered after PCs have been generated(8). These observations led us to hypothesize that the elimination of PCs is necessary to effect a rapid reversal of established DSA responses. Bortezomib is a first generation proteasome inhibitor that induces PCs death by inducing endoplasmic reticulum stress, however, its repeated use is limited by gastrointestinal, neurological and hematological side effects(15). In a recent multicenter study of 33 pediatric kidney transplant recipients with biopsy-proven AMR, bortezomib (4–8 doses) in conjunction with intravenous immunoglobulin (IVIG) (90%), PP (78%) and rituximab (78%) resulted in a transient improvement or stabilization of estimated glomerular filtration rates, but 25% of patients developed DSA by 1 month post-bortezomib treatment and kidney function declined at 12 months post-bortezomib(16). In a non-human primates, Kwun et al.(17) reported that bortezomib monotherapy in a desensitization protocol reduced the numbers of antibody-producing cells in the bone marrow (BM), but did not significantly reduce DSA levels, but instead, transiently induced GC B cell and T follicular helper cell expansion. More recently, Tremblay et al.(18) reported that a second generation proteasome inhibitor, carfilzomib, alone or in combination with plasmapheresis, resulted in substantially reduced HLA antibodies but a regound and return to baseline between day 81–141 post-treatment. Furthermore, Woodle et al.(19) reported that the proteasome inhibitor carfilzomib induced proteasomal structural adaptations that contributed to drug resistance, thus limiting the efficacy of repeated treatments. Collectively, these observations underscore the modest and transient efficacy of monotherapy with proteasome inhibitors, and the need for improved strategies for preventing DSA responses long-term.
Here, we present studies from mice and transplant patients demonstrating the ability of B/B to reverse established DSA responses in mice, and preliminary observations that B/B may be able to rescue kidney allografts from active AMR or mixed rejection.
2. Materials and Methods
A description of materials, methods and statistical analysis is provided in the Supplemental Matierials.
Study approval.
All of the experiments were performed under protocols approved by the Institutional Animal Care and Use Committee at the University of Chicago, and the institutional review board of the Ohio State University (IRB study number: 2018H0432) with written informed consent obtained from all study patients, and in accordance with the principles in the Declaration of Helsinki.
3. Results
3.1. CTLA4-Ig plus Bortezomib is more effective than individual therapy at the inhibiting established primary DSA responses
Antibody-secreting cells in the human BM exists as three distinct subsets with different longevity (20) (21, 22) raising the possibility that partial effects of bortezomib could be explained by their selective impact on PCs subsets. Based on their expression of Transmembrane activator and CAML interactor (TACI) and CD138 and the sequential loss of B cell markers, B220 and CD19(20) (Fig 1A&B), antibody secreting cells can be divided into 3 main subsets: newly generated plasmablasts (PB) (TACI+CD138+CD19+B220+), early PCs (TACI+CD138+CD19+B220−) and mature PCs (TACI+CD138+CD19−B220−). Both the BM and spleen had more early and mature PCs compared to PBs. To define the immediate effects of bortezomib, we examined mice 2 days after a single treatment with bortezomib. A significant reduction of PBs and PCs in the BM and of PCs in the spleen after bortezomib (Fig1C&D), CTLA4-Ig did not reduce PB or PCs numbers in the BM or spleen. Long-lived plasma cells in human bone marrow have been characterized by the CD20−CD19−CD27−CD38+CD138+ phenotype(23). We examined the effect of bortezomib on LLPCs and observed that it has a comparable succeptibility to bortezomib as mature PC (Fig S1A)
Figure-1. Bortezomib preferentially depletes mature plasma cells (PC) from the bone marrow and spleen of naïve mice.
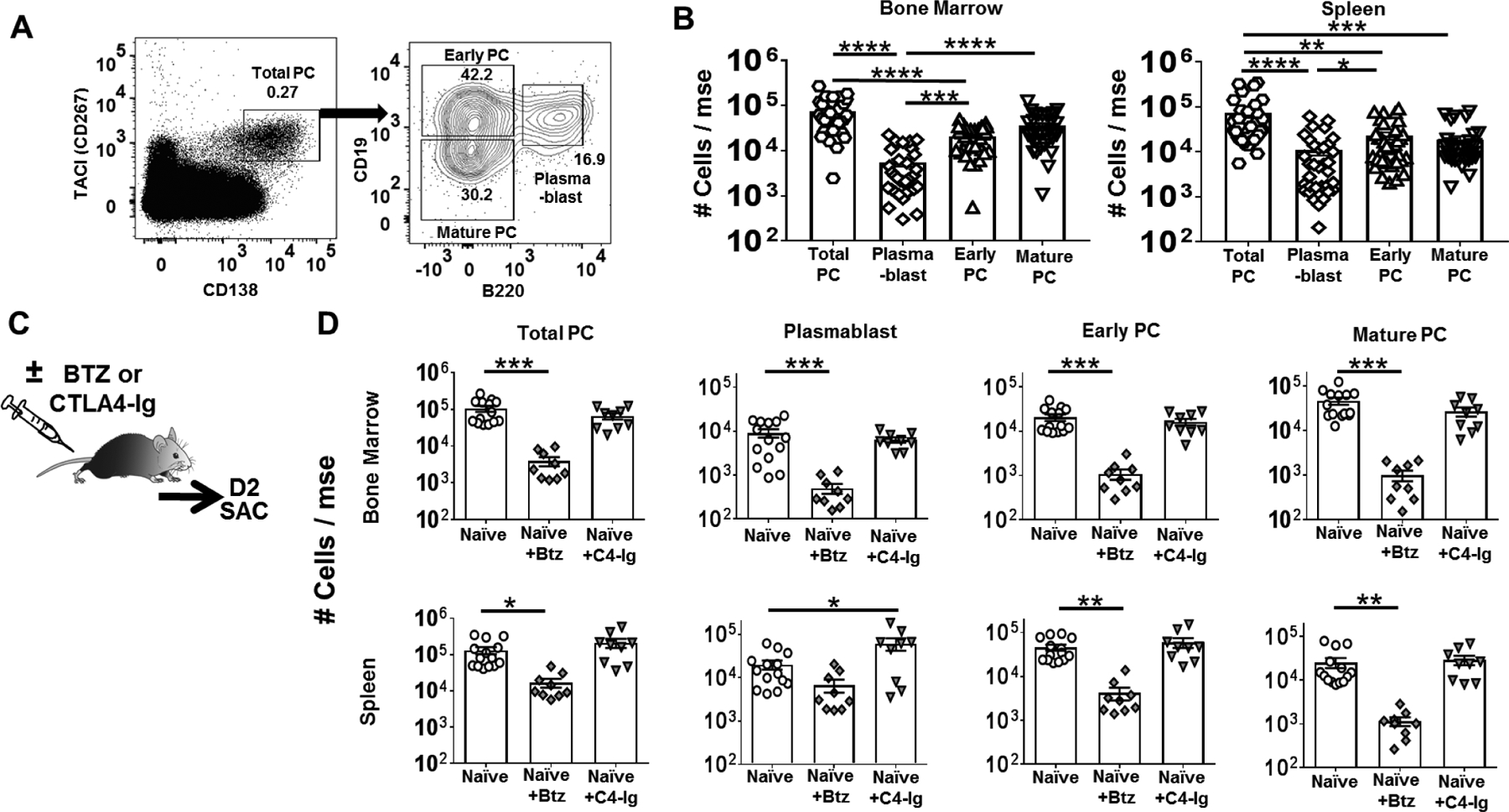
(A) Gating strategy used to identify total and subsets of plasma cells, namely, plasmablast, early PC and mature PC. (B) Quantification of total number of plasma cell subsets retrieved from the bone marrow (Left panel on B) and spleen (Right panel on B) of a naïve C57BL/6 WT mouse (N=28/group). (C) Experimental design. Two days post-treatment with Bortezomib or CTLA4-Ig, naïve C57BL/6 WT mouse were sacrificed. (D) Quantification of PC subsets in bone marrow (Top) and spleen (Bottom) harvested from C57BL/6 mice after Bortezomib (Btz) or CTLA-Ig (C4-Ig) treatment (N=9–16/group). Y-axis represents total cells retrieved per mouse from naïve or mice receiving Bortezomib or CTLA4-Ig. Data are pooled from ≥ two independent experiments and presented as Mean ± SEM. Statistically significant differences were assessed by one way ANOVA. (**P <0.01) (***P <0.001) (****P <0.0001).
We next exmined the effect of bortezomib, alone or in combination with CTLA-4Ig, could reverse an early ongoing primary immune response, by initiating bortezomib, alone or in combination with 2x/week CTLA4-Ig, on day 5 post-immunization with B/c spleen cells (DST; Fig 2A). When analyzed on day 10 post-DST, bortezomib monotherapy and B/B therapy significantly reduced total PCs as well as the early and mature PC subsets in the bone marrow (Fig 2B), while only the mature PC subset was depleted in the spleen (Fig 2C; Fig S1B). Similar trends were observed when the number of IgG-secreting cells in the BM and spleen were enumerated by IgG ELISPOT (Fig 2D). Finally, we tested whether B/B remained effective when treatment was initiated on day 14 post-DST, at the peak of DSA response (Fig S2A). Here we observed combination therapy was more effective than bortezomib or CTLA4-Ig monotherapy at reducing DSA responses measured by day 28 post-DST (Fig 2E).
Figure-2: Delayed treatment with CTLA4-Ig plus Bortezomib inhibits established primary humoral responses in mice immunized with donor spleen cells.
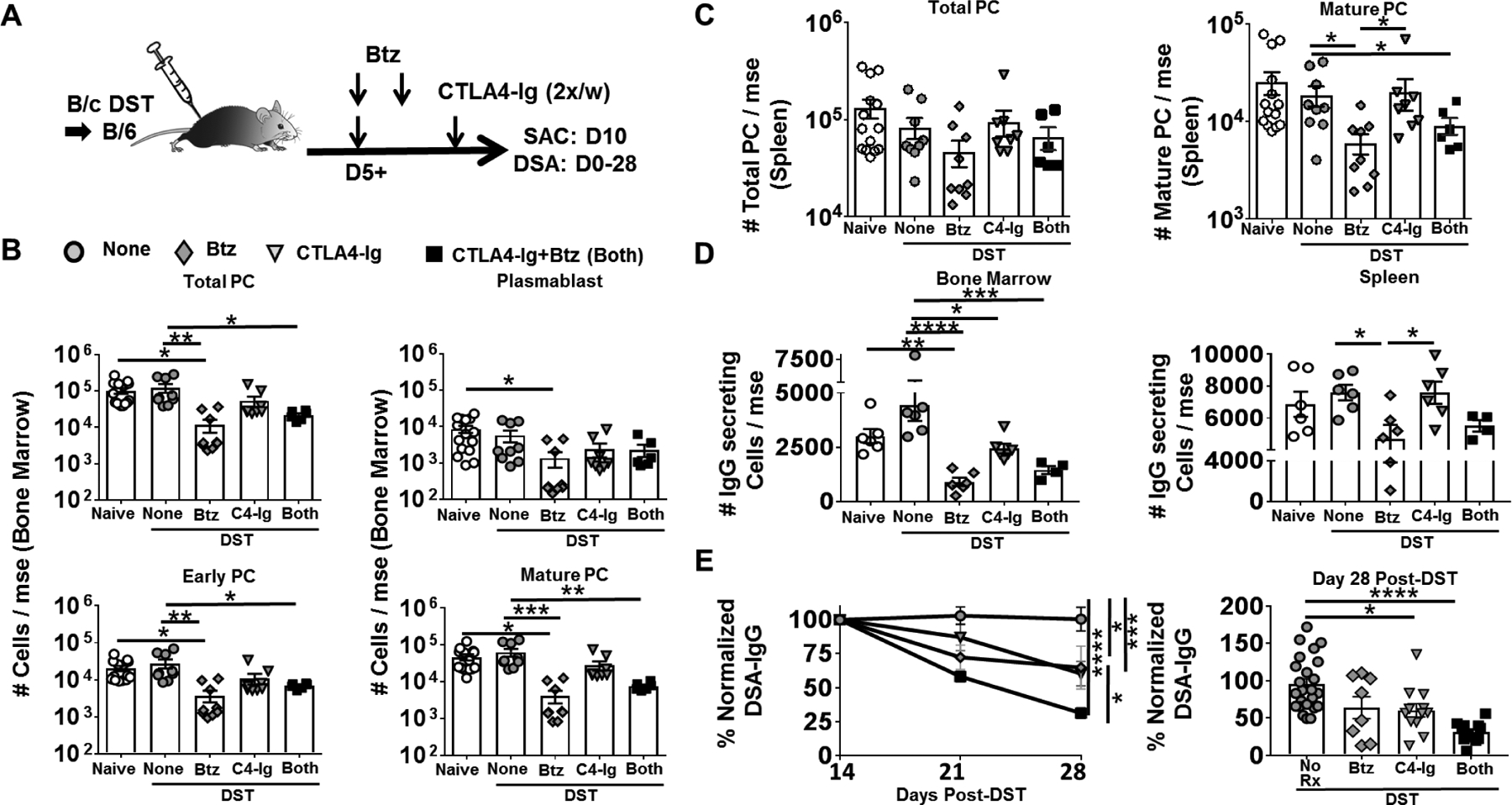
(A) Experimental design. C57BL/6 mice were immunized with BALB/c donor splenocytes (DST) and received no treatment (None), CTLA4-Ig (C4-Ig; from D5), Bortezomib (Btz; D5, D6) or CTLA4-Ig and Bortezomib (Both; from D5). (B) Quantification of plasma cell (PC) subsets in bone marrow; (C) and spleen harvested from naïve or D10 post-DST. (D) Total IgG secreting cells in the bone marrow (Left) and spleen (Right), harvested from naïve or D10 post-DST. Y-axis represents total cells retrieved per mouse, not receiving (None) or receiving CTLA4-Ig (POD 5+) or Bortezomib (D5, 6) or Both (N=5–8/group). (E) Anti-BALB/c IgG were assessed from D14–28 after DST immunization (Left panel) (N=6–25/group), and effectiveness of mono or combination therapy were compared on D28 post-DST (Right panel) (N=8–25/group). Each dot represents an individual mouse, pooled from >2 independent experiments. Data are presented as Mean ± SEM and statistically significant differences assessed by one way ANOVA. (*P <0.05) (**P <0.01) (***P <0.001) (****P <0.0001).
We also tested the efficacy of B/B treatment following B/c skin transplantation. We delayed start of treatment to day 14 post-skin transplant (Fig 3A) when the DSA response was detectable but still increasing, and focussed on the long-term effect of therapy by analyzing on day 28 post-skin transplant (Fig S2B). Monotherapy with bortezomib or CTLA4-Ig, as well as B/B treatment induced significant reduction in the total and the more mature subsets of PCs in the BM (Fig 3B). Similar but more modest trends were observed in the spleen (Fig S3). Notably, 2-weeks treatment with CTLA4-Ig resulted in a significant reduction in total PCs in the BM, in contrast to the 5-day CTLA4-Ig monotherapy which had no significant effect (Fig 2B). B/B tended to be more effective than monotherapy when the number of IgG-secreting cells in the BM and spleen was examined (Fig 3C), and in inhibiting DSA (Fig 3D). Thus, the complementary effects of bortezomib in depleting the more mature PC subsets, and CTLA4-Ig gradually depleting PCs in the spleen and BM and/or preventing the generation of new PB and PC(5–7, 21, 22) resulted in depletion of IgG-secreting cells in the BM and spleen as well as the rapid and long-term control of DSA responses. Finally, when the start of B/B treatment was delayed until the DSA response was at its peak, on day 35 post-skin transplantation, a partial reducting of DSA was observed but additional treatments with bortezomib on day 63 and 77 further reduced DSA (Fig 3E). Collectively, these data confirm the efficacy of delayed and repeated bortezomib treatment in combination with maintenance CTLA4-Ig therapy in reversing established DSA responses.
Figure-3: Delayed treatment with CTLA4-Ig plus Bortezomib inhibits established primary humoral responses in mice immunized with skin transplant.
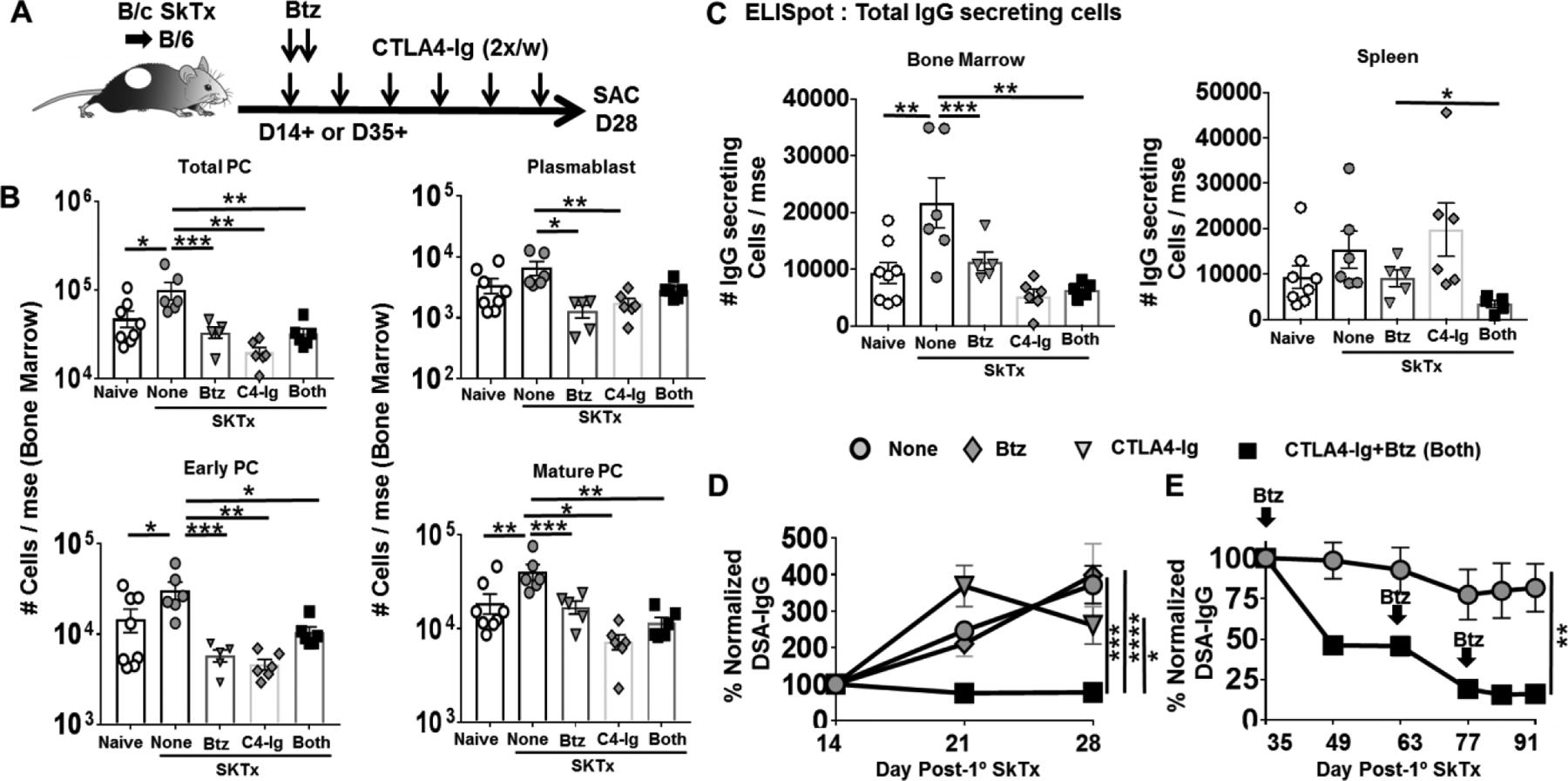
(A) Experimental design. C57BL/6 mice were immunized with BALB/c skin allografts and received no treatment (None), CTLA4-Ig (C4-Ig; from D14), Bortezomib (Btz; D14, D15) or CTLA4-Ig and Bortezomib (Both; from D14 or D35). (B) Quantification of plasma cell (PC) subsets in bone marrow; (C) and total IgG secreting cells in the bone marrow (Left) and spleen (Right) harvested from naïve or D28 post-skin transplant. Y-axis represents total cells retrieved per mouse from naïve, skin transplanted mice not receiving (None) or receiving CTLA4-Ig (POD 14+) or Bortezomib (D14, 15) or Both (from POD14) (N=5–8/group). (D) Anti-BALB/c IgG were assessed from D14–28 post-skin transplant (N=4–6/group). (E) Anti- BALB/c IgG from C57BL/6 WT mice post-skin transplant and received no treatment (None) or CTLA4-Ig and Bortezomib (Both) from POD35+. Repeated Bortezomib treatment on D63 and D77. (N=4–6/group). Each dot represents an individual mouse, pooled from >2 independent experiments. Data are presented as Mean ± SEM and statistically significant differences assessed by one way ANOVA. (*P <0.05) (**P <0.01) (***P <0.001) (****P <0.0001).
3.2. CTLA4-Ig plus Bortezomib reduce established DSA responses in sensitized recipients
The frequently observed late rebound of DSA observed in clinical studies with bortezomib monotherapy(16, 24, 25) can be explained by transient dosing. Indeed, sensitized mice treated with bortezomib and then rechallenged 2 weeks later with BALB/c spleen cells responded with a DSA response that was similar to untreated recipients, whereas mice treated with CTLA4-Ig alone or B/B, maintained low DSA responses (Fig 4A–B). These observations confirm the ability of CTLA4-Ig to inhibit DSA responses upon re-challenge, and underscore the necessity of sustained treatment with CTLA4-Ig to prevent rebound DSA responses(16).
Figure-4: Delayed treatment with CTLA4-Ig plus Bortezomib controls established humoral responses in sensitized mice.
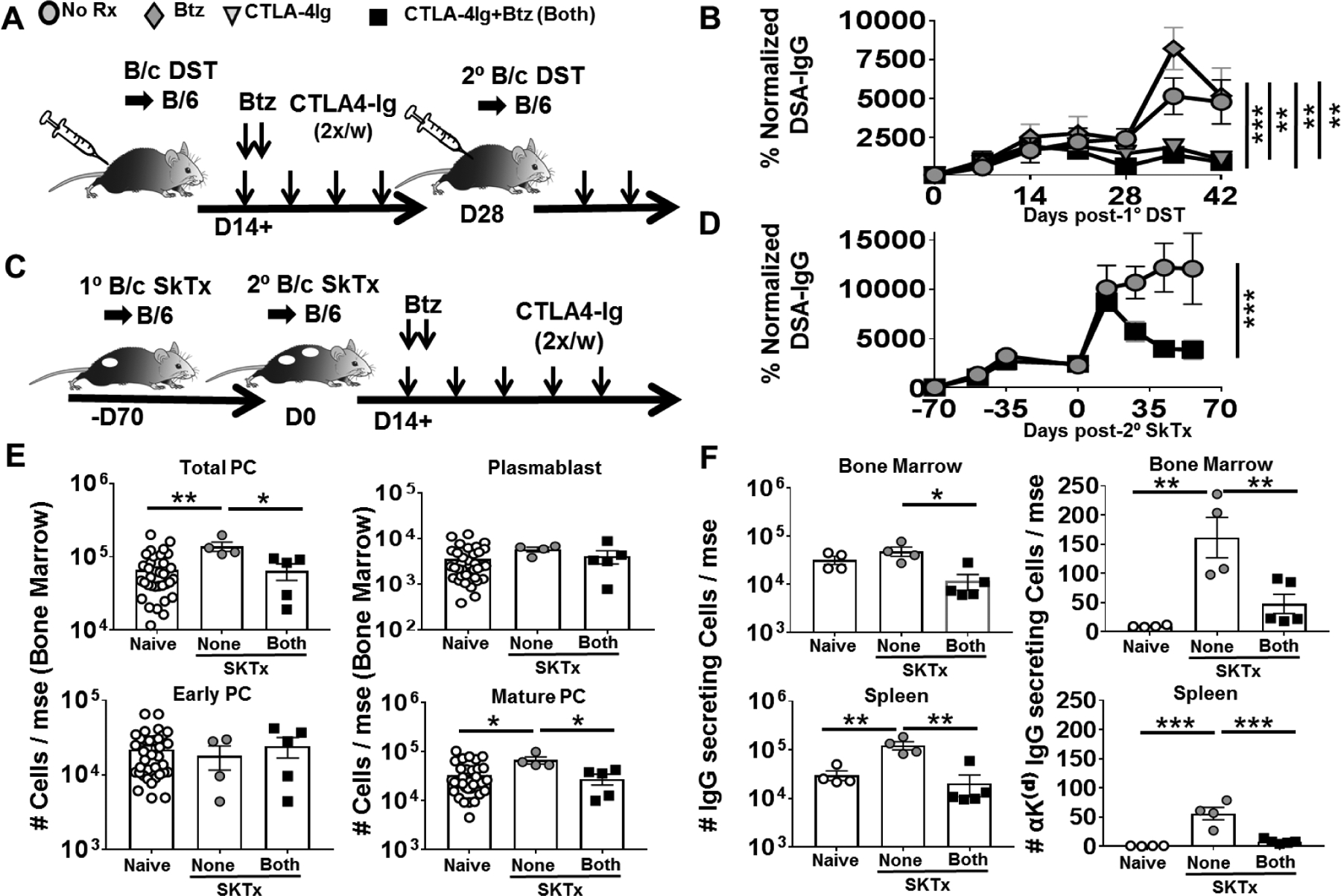
(A) Experimental design. C57BL/6 WT recipients were immunized with BALB/c donor splenocytes (DST) on D0 and D28. Mice received no treatment (None), CTLA4-Ig (C4-Ig; POD14+), Bortezomib (Btz; D14, D15) or CTLA4-Ig and Bortezomib (Both). (B) Anti-BALB/c IgG were assessed from D0–42 post-DST (N=4/group). (C) Experimental design. Mice received BALB/c skin transplants on POD-70 and POD0, and treated from POD14, as indicated. (D) Anti-BALB/c IgG from –D70 to D70 (N=4/group). (E) Quantification of PC subsets in bone marrow (N=4–36) and (F) total IgG secreting cells (Left) and anti Kd-IgG secreting cells (Right) (N=4–5) in the bone marrow of naïve or post-2° skin transplantation, on POD28. Experimental groups are: not receiving (None) or receiving CTLA4-Ig and Bortezomib both (Both; from POD14). Y axis represents total cells retrieved per mouse from at least two independent experiments. Data are presented as Mean ± SEM and statistically significant differences assessed by one way ANOVA. (*P <0.05) (**P <0.01) (***P<0.001).
De novo DSA responses and acute AMR are more likely to occur in sensitized recipients; we previously reported that CTLA4-Ig administered at the time of alloantigen challenge of sensitized recipients effectively prevents the recall antibody response(5, 7). Here we demonstrate that B/B successfully reversed established recall DSA responses, when administered from D14 post-secondary BALB/c skin transplantation in recipients sensitized with BALB/c skin allografts ~70 days previously (Fig 4C–D). Upon skin transplant rechallenge, a significant increase in only BM P3 subset was observed; B/B successfully depleted this P3 subset in the BM (Fig 4E) but not in the spleen (Fig S3). Nevertheless, total IgG-secreting cells as well as donor-specific (anti-Kd)-IgG secreting cells were significantly reduced in both the BM and spleen (Fig 4F). These observations underscore the superiority of quantifying donor-specific PC frequencies and DSA over total PC subsets to demonstrate the efficacy of B/B in reversing and inhibiting DSA responses long-term in non-sensitized and sensitized mice. These observations also prompted us to test whether bortezomib and CTLA4-Ig might be effective at reversing acute AMR in a series of six renal transplant patients undergoing acute ABMR or mixed rejection.
3.3. Clinical experience; (Table 1–3, Table S1–3)
Table 1.
Patient demographics of AMR patients treated with bortezomib and belatacept
| Patient | Age (yrs) | Gender | Donor Type | No. Previous KTx | Cause of ESRD | Induction | Immunosuppression before B/B |
|---|---|---|---|---|---|---|---|
| 1 | 39 | Male | Deceased | 2 | IgA Nephropathy | Basiliximab | Everolimus, Prednisolone, Mycophenolic acid |
| 2 | 55 | Female | Living | 2 | Reflux Nephropathy | ATG | Everolimus, CSA, Mycophenolic acid |
| 3 | 64 | Female | Deceased | 0 | Membranous Nephropathy | ATG | Everolimus, Mycophenolic acid |
| 4 | 26 | Male | Living | 0 | FSGS | ATG | Everolimus, CSA Mycophenolic acid |
| 5 | 60 | Female | Living | 0 | Contrast Induced Nephropathy | ATG | Everolimus, Mycophenolic acid |
| 6 | 35 | Male | Living | 0 | IgA Nephropathy | ATG | Everolimus, Mycophenolic acid |
Table 3.
Clinical outcomes of AMR patients treated with belatacept plus bortezomib
| Renal Function (sCr, eGFR) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | B/B start | Post-B/B nadir | Most recent (months post-Tx) | Biopsy Day post-Tx | Total IVIG doses | Total PP doses | Total Btz doses (start day) | Bela start Day post-Tx | DSA (Adays to loss) | Days to GL | Urine P/C Ratio (most recent) |
| 1 | 6.1, 10 | 1.2, 101 | 1.2, 101 (30 m) | 20, C4d+ | 7 | 12 | 3 (24) | 21 | A68 (21) B37 (23) DP3 (285) DQ5 (10) |
NA | 0.10 |
| 2 | 3.14, 15 | 0.79, 62 | 1.3, 43 (29 m) | 11, C4d+ | 2 | 4 | 2 (14) | 19 | A3 (6) DP1(22) DP3 (273) |
NA | 0.35 |
| 3 | 2.5, 24 | 0.8, 64 | 1.2, 56 (22 m) | None | 4 | 6 | 4 (32) | 32 | B8 (9) B51 (339) C1(622) DR53(63) |
NA | 0.49 |
| 4 1st episode |
2.1, 45 | 1.31, 98 | NA | 11, C4d+ | 1 | 2 | 4 (18) | 17 | DR7 (5) DR53 (110) DQ2 (320) DQ6 (15) |
NA | NA |
| 4 2nd episode |
1.3, 98 | 1.2, 118 | 1.3, 98 (18 m) | 82, C4d+ | 2 | 2 | 4 (115) | ongoing | DR7 (119) | NA | 0.07 |
| 5 | 8.7, 5 | 3.44, 14 | BHDD (10 m) | 6, C4d+ | 3 | 6 | 2 (9) | 14 | DQ7 (7) DRB1*04 (29) DRB1*14 (3) DR52 (3) |
96 | 4.92 |
| 6 | 2.3, 33 | 1.8, 65 | 1.8, 44 (26 m) | 291, C4d+ | 3 | 4 | 4 (292) | 292 | A1 (9) B8 (9) B44 (9) DQ2 (491) |
NA | 0.16 |
days to loss: calculated from day of detection to day of loss;
HHD: Hemodialysis
Case 1 (Fig 5) 39 year old highly sensitized Caucasian male (class I and class II PRA 79% and 37% respectively) with ESRD secondary to IgA nephropathy status post 2 previous renal transplants. The first transplant was performed in 2004, and he experienced delayed graft function and the graft was lost after a few months. The kidney was removed in 2008 due to AR of the non-functioning kidney. He received a second transplant in 2012, developed AMR that was unsuccessfully treated and was lost within 2 months. This kidney was removed in 2013 for AR of the non-functioning allograft. He received his third kidney transplant from a deceased donor in 2015. The patient has an unknown pre-transplant blood transfusion history but was sensitized during his previous transplant episodes, with antibodies to DP3 detected in 2009 and 2015 (5 months pre-transplant), and anti-DQ5 detected in 2009. Immediately pre-transplant his flow XM was negative and he had undetectable levels of pre-formed DSAs to DP3 and DQ5 by SAB. His initial urine output was good but declined to anuria within 12 hours. He received basiliximab induction, everolimus and steroid maintenance immunosuppression, while calcineurin inhibitor was withheld due to severe renal dysfunction. Dialysis was started on post-operative day (POD)1. An open kidney biopsy on POD2 showed ATN with thrombotic microangiopathy (TMA) and was C4d negative. No DSAs were detected on POD2 but PP, IVIG, and steroids were begun due to a high suspicion of acute AMR with associated TMA presumably resulting from preformed alloantibodies. Repeat biopsy on POD12 was consistent with C4d negative acute AMR and DSAs specific for A68, B37, DQ5 and DP3 were detected by POD17. A biopsy on POD20 showed AMR with TMA and diffuse C4d (Banff scoring in Table 2), and a follow-up POD35 biopsy on showed slightly less prominent histologic changes (Table S1). Bortezomib (POD24) and belatacept (POD20)(B/B) were initiated with resolution of anuria over the next week. His last dialysis and PP was POD35. DSA specific for A68, B37 and DQ5 was reduced to undetectable by POD35, whereas DP3-specific antibodies took 182 days to reach undetectable levels. At the 30 month follow-up, DSA remained undetectable, serum creatine and eGFR were 1.2 and 101, respectively, compared to 6.16 and 10 at B/B initiation. (Table 3)
Figure-5.
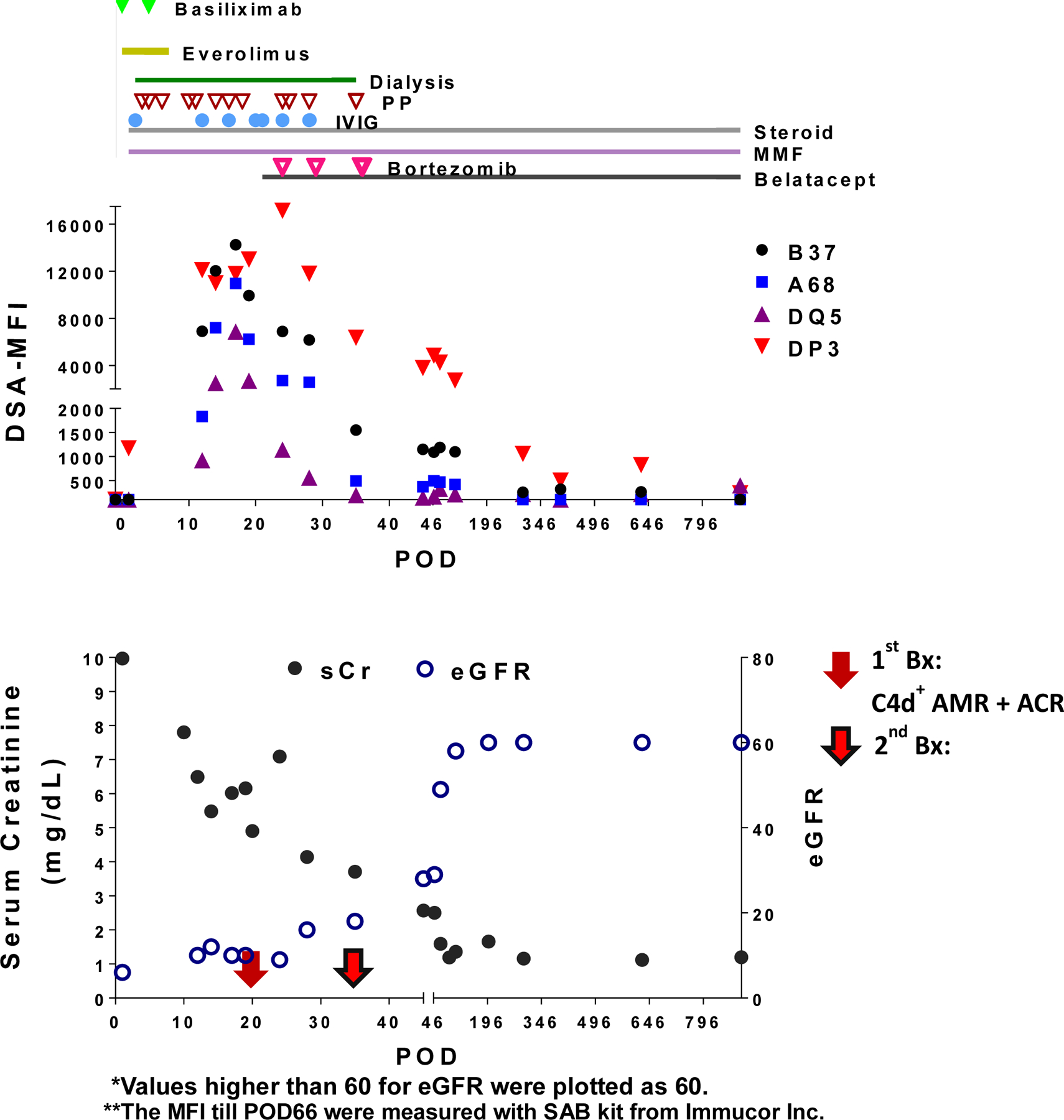
Clinical sequelae for Case 1
Table 2.
AMR characteristics of patients treated with belatacept plus bortezomib (B/B)
| Indication Biopsy Findings | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Days to dnDSA | Days to C4d+ Biopsy | Days to Bela | Days to Bort | Months Post-Tx Follow-up | DSAs (Peak MFI) | Prior AMR Treatment | t | v | i | g | ci | ct | cg | mm | cv | ah | ptc | C4d | other |
| 1 | 12 | 20 | 21 | 24 | 30 | A68 (10,959) B37 (14,251) DP3 (17,144) DQ5 (6,870) |
PP, IVIG, steroids | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 3 | ATN, TMA |
| 2 | 11 | 11 | 19 | 14 | 29 | A3 (2086) DP1(18452) DP3 (3,153) |
PP, ATG, IVIG, steroids | 3 | - | 2 | 2 | 0 | 0 | 0 | 0 | - | 0 | 3 | 3 | ATN, IH |
| 3 | 25 | ND | 32 | 32 | 22 | B8 (3994) B51 (8583) C1 (5015) DR53 (2564) |
PP, IVIG, steroids | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 4 1st episode |
0 | 11 | 17 | 21 | - | DR7 (6,803) DR53 (6568) DQ2 (12756) DQ6 (2949) |
PP, IVIG, steroids | 3 | - | 2 | 0 | 0 | 0 | 0 | 0 | - | 0 | 2 | 3 | ATN |
| 4 2nd episode |
31 | 82 | ongoing | 115 | 18 | DR7 (3,744) | PP, IVIG, steroids, B/B | 1 | - | 2 | 0 | 1 | 1 | 0 | 0 | - | 0 | 0 | 1 | - |
| 5 | 6 | 6 | 14 | 9 | 10 | DQ7 (6373) DRB1*04 (3751) DRB1*14 (2314) DR52 (2847) |
PP, IVIG, steroids | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ATN, IH, Necrosis |
| 6 | 290 | 291 | 292 | 292 | 26 | A1 (6861) B8 (4505) B44 (2,519) DQ2 (14178) |
PP, IVIG, steroids | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 2 | 0 | 2 | 3 | ATN, TMA |
Case 2 (Fig S5-A) 55 year old highly sensitized Caucasian female (cPRA=98) with ESRD due to vesicoureteral reflux post 2 previous renal transplants. The first transplant in 1983 lasted less than 1 year and was lost due to unknown reasons. The second kidney lasted 27 years, was never biopsied and possibly lost due to complications following ureteral strictures. She had 2 previous pregnancies and a 15 unit blood transfusion history. She received her third, living donor kidney transplant, which initially functioned well. Her flow XM was negative and she had no DSAs by SAB on day of transplant or through five years of pre-tranplant testing. She received ATG induction and everolimus plus cyclosporine for maintenance immunosuppression. On POD11, she returned to the hospital with elevated serum creatinine (sCr) and oliguria, and a biopsy demonstrated a mixed acute cellular and antibody mediated rejection (Banff scoring in Tables 2 & S1). Although her pre-transplant flow XM was negative and she had no DSAs by SAB, she was positive at the time of biopsy for anti-A3, DP1, and DP3 DSAs, and was presumed to have developed an anamnestic alloimmune response. She was treated with a course of ATG, PP, and IVIG followed by bortezomib (POD14, 17) and belatacept (POD19). She did not require dialysis due to the prompt return of renal function. DSA specific for A3 and DP1 rapidly reduced to MFI <2000 in 6–22 days following initial detection, while DSA specific for DP3 took 273 days. All specificities remained undetectable when last seen at 29 months post-transplantation. At 29 months her serum creatinine and eGFR were 1.3 and 43, respectively, compared to 3.14 and 15 at B/B initiation. (Table 3) Indication biopsies at 22 and 29 month post-transplant, showed mild transplant glomerulopathy, but no ACR or AMR, no acute inflammatory infiltrates, no interstitial fibrosis or tubular atrophy, and were C4d negative.
Case 3 (Fig S5-B) 64 year old highly sensitized African-American female (class I PRA=35%) with ESRD due to membranous glomerulonephritis. She had 1 previous pregnancy and a 4 unit blood transfusion history. She had no identified DSA by SAB on day of transplant or through two years of pre-tranplant testing. She received a deceased, brain dead donor renal transplant, experienced a slow return of renal function and was discharged from the hospital on POD8. She received ATG induction with everolimus plus mycophenolic acid (MMF) for maintenance immunosuppression, and returned 3 days later for a 4 day admission due to anemia and gross hematuria, with the hematuria resolving without intervention. She then returned on POD #21 with worsening renal function and was DSA positive on POD25 (anti-B8 and B51, Table S2), however a biopsy was not obtained due to ongoing oral antiplatelet therapy for the presence of cardiac stents. Treatment for AMR, presumed to be either a recall or de novo humoral or mixed alloimmune response, was begun on POD30 with apheresis and IVIG plus steroids, and B/B was initiated on POD32 when sCr was 2.49 mg/dL. Her renal function rapidly resolved to a new baseline sCr of 1.45 mg/dl on POD42, and was at 1.17 mg/dL at 22 months post-transplant. DSA specific for B8 rapidly declined, and B51 DSA dropped below our MFI 2000 positive cutoff on POD 364. There was a transient increase in anti-DR53 and anti-C1 DSA from POD58 that dropped below our MFI 2000 positive cutoff by POD121 and POD680 respectively. (Table S2).
Case 4 (Fig S5-C) 26 year old non-sensitized African-American male (cPRA=0) with ESRD due to focal segmental glomerular sclerosis and no blood transfusion history who underwent an uneventful living unrelated renal transplant. He had no detectable PRA on day of transplant or through two years of pre-tranplant testing. He received ATG induction and everolimus plus cyclosporine for maintenance immunosuppression. He experienced prompt return of renal function and was discharged from the hospital on POD5, but was readmitted on POD11 for fevers, with elevated sCr and fluid overload. Renal biopsy surprisingly revealed mixed acute cellular and antibody-mediated rejection and was diffusely C4d positive (Banff scoring Table 2 & S1, 1st episode). He was also DSA positive (DR7; MFI 6803) with a sCr of 5.6mg/dl. His presentation was most consistent with an anamnestic anti-donor immune response although he had no pre-transplant sensitizing events. He was immediately treated with apheresis, IVIG, bortezomib and steroids, while everolimus plus cyclosporine were switched to MMF and belatacept. His renal function quickly improved with his DSA becoming undetectable on POD17.
A return of DSA was observed by POD31, with new specificities (DR53, DQ2 and DQ6) and reappearance of DR7, however he maintained stable renal function of 1.52 mg/dl sCr. A follow-up biopsy was obtained on POD82 and showed patchy inflammation and mild, focal C4d staining (Table 2, 2nd episode). A second round of bortezomib was initiated on POD115, resulting in the loss of all DSAs by POD341. At last follow-up of 18 months post-transplantation, DSA remained undetectable and sCr at 1.57 mg/dl. (Table 3)
Case 5 (Fig S5-D) 60 year old non-sensitized (cPRA=0), non-transfused, nulliparous Caucasian female with Gitelman’s syndrome and ESRD due to progression of contrast- induced nephropathy, transplanted with a living unrelated renal transplant. She had no detectable HLA antibodies on day of transplant or through one year of pre-tranplant testing. She received ATG induction and everolimus plus MMF for maintenance immunosuppression. After 24 hours, renal function deteriorated culminating in a renal biopsy on POD6 revealing diffuse interstitial hemorrhage and focal necrosis with focal C4d staining (Banff scoring Table 2 &S1), and she was DSA positive (DQ7). Four days later she became positive for DR4, 14 and 52 specificities. This appeared to be due to a rapid recall response although by history she had no sensitizing events. Apheresis, IVIG, and steroids were initiated without improvement. Belatacept and bortezomib were started 3 days later. Her course was complicated by a wound hematoma and wound dehiscence due to infection. She remained hemodialysis dependent with an improving urine output for the next 10 days. The urine output again declined and a repeat biopsy on POD28 showed Banff grade III acute cellular rejection without AMR and was C4d negative. This rejection was treated with ATG and additional steroids, in addition to ongoing belatacept and MMF. There was no further detection of DSA after POD39 unitl POD300 (204 days after treatment was discontinued), however, the kidney function never returned as it progressed to infarction and was removed on POD221 (no C4d staining was performed due to complete parenchymal necrosis) (Table 3). These observations are consistent with the ability of belatacept-based therapy to prevent DSA development, but also its limitation in face of aggressive acute cellular rejection.
Case 6 (Fig S5-E) 35 year old non-sensitized Caucasian male (cPRA=0) with ESRD due to IgA nephropathy who underwent an uneventful living unrelated renal transplant. He had no detectable HLA antibodies on day of transplant or through 11 months of pre-tranplant testing. He received ATG induction and everolimus plus MMF for maintenance immunosuppression. On a routine outpatient visit on POD290 he had a slightly elevated sCr of 2.3, and was DSA positive (A1, B8, B44 and DQ2). An inpatient follow-up biopsy showed AMR with mild TMA and diffuse C4d positivity (Banff scoring Table 2 & S1). The histology favored acute AMR over chronic active AMR due to the absence of transplant glomerulopathy due to de novo DSA. Apheresis and steroids were initiated with B/B starting 2 days later and sCr improved to a new baseline of 1.99 mg/dl (from 2.3) 12 days later. Class I DSA rapidly declined to undetectable within 7 days of B/B treatment, while the DQ2 DSA became undetectable after 491 days. At 26 month post-transplantation follow-up, sCr was 1.78 mg/dl with an eGFR of 44. (Table 3)
Discussion
This is a mouse-to-human proof-of-principle study that collectively underscores the efficacy of B/B in reversing established DSA responses and preventing the return of DSA. In mouse models, we build on our previous observations that belatacept can reverse T cell- and GC-dependent B cell responses as well memory B cell recall DSA responses, but it does not readily reverse DSA responses once antibody-secreting cells have been generated(5–8). We now show that short-term treatment with bortezomib results in a significant reduction of early and mature PCs in the BM, with a similar trend with mature PCs in the spleen. These observations are consistent with mature PC producing larger amounts of antibodies and therefore being more susceptible to the inhibition of the ubiquitin proteasomal degradation pathway(15). We conclude that, as a consequence of the complementary mechanisms of action, B/B therapy was able to rapidly reverse DSA responses and prevent rebound, even upon re-challenge with donor-specific splenocytes or secondary allogeneic skin transplants. We also showed that when DSA responses induced with skin allografts had been established for 35 days, single treatment of bortezomib was not sufficiently effective, but that repeated dosing with bortezomib together with continued treatment with CTLA4-Ig resulted in a gradual decline in circulating DSA. These observatations suggest that B/B therapy may be successful not only at reversing recently established DSA responses, also in established high-titer DSA recipients and in desensitization, consistent with recent observations in non-human primates (NHP)(17, 26). Finally, we noted that enumeration of IgG by ELISPOT, and of DSA-IgG secreting cells in particular, more accurately predicted the impact of treatment on circulating DSA levels compared to the assessment of total PC numbers by flow cytometry. These observations underscore the superiority of quantifying donor-specific cells compared to total numbers of plasma cell or their subsets.
In six human kidney transplant recipeints, B/B therapy was associated with the rescue from early acute AMR or mixed rejection, although it is possible that co-administered IVIG and PP also contributed to the observed efficacy. The ability to maintain low-to-undetectable DSA levels with a follow up of 10–30 months was an equally important observation. While the majority of successful treatment with B/B were for early active AMR, treatment of case 6 was initiated on POD 292 upon routine followup was associated with a loss of DSA and a significant and sustained improvement of sCr and eGFR for ~16 months (Table S3). Additionally, for patient 4, initial successful B/B treatment starting on POD11 was associated with the return of DR7 on POD31 and reappearance of new specificities (DR53 DQ2, and DQ6). The observation that a second round of bortezomib initiated on POD115 was associated with the loss of all DSAs within 131 days suggests that B/B may successfully reverse more established DSA responses and preventing their rebound. These observations are congruent with the data from the mouse models, that CTLA4-Ig/belatacept is necessary for the long-term control of DSA and that repeated bortezomib treatment can progressively reduce circulating DSA. Finally,five DSA (B51, C1, DR53, DP3, and DQ2) became undetectable after 100–622 days on belatacept-based immunosuppression, without additional bortezomib treatment. These observations are also consistent with the reports by Bray et al.(12, 13) that the levels of pre-transplant and de novo DSA are reduced by belatacept-based immunosuppression, and by the observations by Rozanski et al. (21, 23) that mature PCs in the BM express CD28 and receive survival signals from CD28:B7 interactions.
Our findings are also complementary to recent reports in non-human primates(17, 26, 27), where the combined treatment of costimulation blockade and proteasome inhibitor reduced DSA (but not anti-CMV and anti-tetanus IgG) in a skin-sensitized non-human primates, and also reduced BM PCs, lymph node follicular helper T cells and memory B cell proliferation. Furthermore, desensitization followed by allogeneic kidney transplantation resulted in the avoidance of AMR and prolonged graft survival under conventional maintenance immunosuppression with tacrolimus, MMF and steroids. Notably, T cell infiltration into the allografts, as well as GC and DSA responses were significantly reduced post-transplantation in the recipents underscoring the efficacy of the desensitization protocol.
There are critical limitations to our study. First, the observations involve a very small cohort of a non-randomized patients diagnosed with a spectrum of AMR or mixed rejection. There was no control group that received standard of care without B/B, so we cannot conclude definitively that the salutary effects observed were due to B/B therapy. The absence of follow-up protocol biopsies in the majority of cases is another serious limitation. Finally, there are no mechanistic studies to explain why this therapy was successful in the treated patients or why some DSA specificities took a much longer time to clear. Overall, Class I tended to be more rapidly reduced, within 25 days of treatment compared to Class II (7 of 9 (78%) vs. 7 of 14 (46%) DSA, while MFI of the DSA was not predictive of kinetics of disappearance (Fig S5). These limitations point to the need for a randomized control study with larger numbers of patients with active AMR to rigorously define the efficacy of B/B in patients where standard of care regimens have failed. Despite this need, in reality the limited numbers of patients succumbing to early active AMR in the current clinical climate makes such trials extremely challenging to execute successfully. Indeed, Montgomery et al.(28) discussed that “treatment for AMR is not standardized, and there is little in the way of evidence‐based treatment guidelines” underscore the challenges facing such studies.
Basic immunologists have long argued for the utility of murine models, and the ability to leverage mechanistic findings made in murine models into a rational basis for a clinical studies that demonstrate safety and efficacy. Despite the caveats of our clinical observations, this study supports the notion that experimentation in mice may be useful for identifying therapies for clinical events that are infrequent and often unanticipated. Taken together, these preliminary clinical data support the need for future prospective randomized clinical trials using B/B for treating active AMR or mixed rejection, and potentially, for reversing established DSA responses in sensitized patients on the transplant waitlist.
Supplementary Material
Acknowledgments
The experimental studies were supported in part by grants (1R01AI110513, P01AI-97113) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. JSY was supported in part by a grant from the American Heart Association and Enduring Hearts (15POST25700452) and NIH Respiratory Biology Training Grant T32 HL07605. MHC monomers were provided by the NIH Tetramer Core Facility (contract HHSN272201300006C).
Abbreviations:
- AMR
antibody mediated rejection
- AR
acute rejection
- B/B
bortezomib plus belatacept treatment
- BM
bone marrow
- CoB
costimulation blockade
- DSA
donor specific antibodies
- eGFR
estimated glomerular filtration rate
- GC
germinal center
- HLA
human leukocyte antigen
- IVIG
intravenous immunoglobulin
- meCSA
microemulsion Cyclosporin A
- MFI
mean fluorescence intensity
- PC
plasma cells
- PB
plasmablast
- POD
post-operative day
- TMA
thrombotic microangiopathy
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant 2012;2012:193724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan SS, Ying TD, Wyburn K, Roberts DM, Wyld M, Chadban SJ. The Treatment of Antibody-Mediated Rejection in Kidney Transplantation: An Updated Systematic Review and Meta-Analysis. Transplantation 2018;102(4):557–568. [DOI] [PubMed] [Google Scholar]
- 3.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol 1993;11:191–212. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz RH. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992;71(7):1065–1068. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Wang Q, Yin D, Vu V, Sciammas R, Chong AS. Cutting Edge: CTLA-4Ig Inhibits Memory B Cell Responses and Promotes Allograft Survival in Sensitized Recipients. J Immunol 2015;195(9):4069–4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. Am J Transplant 2013;13(9):2280–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J, Chen J, Young JS, Wang Q, Yin D, Sciammas R et al. Tracing Donor-MHC Class II Reactive B cells in Mouse Cardiac Transplantation: Delayed CTLA4-Ig Treatment Prevents Memory Alloreactive B-Cell Generation. Transplantation 2016;100(8):1683–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young JS, Chen J, Miller ML, Vu V, Tian C, Moon JJ et al. Delayed Cytotoxic T Lymphocyte-Associated Protein 4-Immunoglobulin Treatment Reverses Ongoing Alloantibody Responses and Rescues Allografts From Acute Rejection. Am J Transplant 2016;16(8):2312–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. J Exp Med 2012;209(3):597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. J Exp Med 2012;209(11):2079–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vincenti F. Belatacept and Long-Term Outcomes in Kidney Transplantation. N Engl J Med 2016;374(26):2600–2601. [DOI] [PubMed] [Google Scholar]
- 12.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L et al. Posttransplant reduction in preexisting donor-specific antibody levels after belatacept- versus cyclosporine-based immunosuppression: Post hoc analyses of BENEFIT and BENEFIT-EXT. Am J Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bray RA, Gebel HM, Townsend R, Roberts ME, Polinsky M, Yang L et al. De novo donor-specific antibodies in belatacept-treated vs cyclosporine-treated kidney-transplant recipients: Post hoc analyses of the randomized phase III BENEFIT and BENEFIT-EXT studies. Am J Transplant 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everly MJ, Roberts M, Townsend R, Bray RA, Gebel HM. Comparison of de novo IgM and IgG anti-HLA DSAs between belatacept- and calcineurin-treated patients: An analysis of the BENEFIT and BENEFIT-EXT trial cohorts. Am J Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 15.Woodle ES, Alloway RR, Girnita A. Proteasome inhibitor treatment of antibody-mediated allograft rejection. Curr Opin Organ Transplant 2011;16(4):434–438. [DOI] [PubMed] [Google Scholar]
- 16.Kizilbash S, Claes D, Ashoor I, Chen A, Jandeska S, Matar RB et al. Bortezomib in the treatment of antibody-mediated rejection in pediatric kidney transplant recipients: A multicenter Midwest Pediatric Nephrology Consortium study. Pediatr Transplant 2017;21(3). [DOI] [PubMed] [Google Scholar]
- 17.Kwun J, Burghuber C, Manook M, Ezekian B, Park J, Yoon J et al. Successful desensitization with proteasome inhibition and costimulation blockade in sensitized nonhuman primates. Blood Adv 2017;1(24):2115–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tremblay S, Driscoll JJ, Rike-Shields A, Hildeman DA, Alloway RR, Girnita AL et al. A prospective, iterative, adaptive trial of carfilzomib-based desensitization. Am J Transplant 2020;20(2):411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodle ES, Tremblay S, Brailey P, Girnita A, Alloway RR, Aronow B et al. Proteasomal adaptations underlying carfilzomib-resistance in human bone marrow plasma cells. Am J Transplant 2020;20(2):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pracht K, Meinzinger J, Daum P, Schulz SR, Reimer D, Hauke M et al. A new staining protocol for detection of murine antibody-secreting plasma cell subsets by flow cytometry. Eur J Immunol 2017;47(8):1389–1392. [DOI] [PubMed] [Google Scholar]
- 21.Rozanski CH, Arens R, Carlson LM, Nair J, Boise LH, Chanan-Khan AA et al. Sustained antibody responses depend on CD28 function in bone marrow-resident plasma cells. J Exp Med 2011;208(7):1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rozanski CH, Utley A, Carlson LM, Farren MR, Murray M, Russell LM et al. CD28 Promotes Plasma Cell Survival, Sustained Antibody Responses, and BLIMP-1 Upregulation through Its Distal PYAP Proline Motif. J Immunol 2015;194(10):4717–4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halliley JL, Tipton CM, Liesveld J, Rosenberg AF, Darce J, Gregoretti IV et al. Long-Lived Plasma Cells Are Contained within the CD19(−)CD38(hi)CD138(+) Subset in Human Bone Marrow. Immunity 2015;43(1):132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodle ES, Shields AR, Ejaz NS, Sadaka B, Girnita A, Walsh RC et al. Prospective iterative trial of proteasome inhibitor-based desensitization. Am J Transplant 2015;15(1):101–118. [DOI] [PubMed] [Google Scholar]
- 25.Eskandary F, Regele H, Baumann L, Bond G, Kozakowski N, Wahrmann M et al. A Randomized Trial of Bortezomib in Late Antibody-Mediated Kidney Transplant Rejection. J Am Soc Nephrol 2018;29(2):591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burghuber CK, Manook M, Ezekian B, Gibby AC, Leopardi FV, Song M et al. Dual targeting: Combining costimulation blockade and bortezomib to permit kidney transplantation in sensitized recipients. Am J Transplant 2019;19(3):724–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ezekian B, Schroder PM, Mulvihill MS, Barbas A, Collins B, Freischlag K et al. Pretransplant Desensitization with Costimulation Blockade and Proteasome Inhibitor Reduces DSA and Delays Antibody-Mediated Rejection in Highly Sensitized Nonhuman Primate Kidney Transplant Recipients. J Am Soc Nephrol 2019;30(12):2399–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery RA, Loupy A, Segev DL. Antibody-mediated rejection: New approaches in prevention and management. Am J Transplant 2018;18 Suppl 3:3–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


