Abstract
Problem:
A successful outcome to pregnancy is critically dependent on the initiation of maternal immune tolerance before embryo implantation. Cells of embryonic origin that come in contact with the uterine microenvironment can exert influence over the phenotype and function of immune cells to facilitate robust implantation; however, what influence they may have on B cells remains unknown. In this study, we investigate the effect of human trophoblast cells on B-cell phenotype and the subsequent effect on peri-implantation events.
Method of study:
We cultured purified human B cells with the first-trimester human trophoblast cell line Swan 71 to investigate trophoblast-B-cell interactions and utilized trophoblast spheroids in an in vitro implantation model of migration and invasion.
Results:
Trophoblast-educated B cells or TE-B cells were found to consist of B cells in committed lineages such as plasmablasts and memory B cells, as well as increased proportions in subsets of CD24hiCD27+ regulatory B cells and CD19+IL-10+ B cells. Conditioned media from the TE-B cells showed reduced production of pro-inflammatory cytokines that influenced the T-cell proliferation and cytokine production. Using trophoblast spheroids, we assessed the role of TE-B cells in trophoblast invasion and migration. Our results demonstrate a protective effect of TE-B-conditioned media against deleterious inflammation as evidenced by survival of the trophoblast spheroid in the presence of an immune assault and promotion of a migratory phenotype.
Conclusion:
We posit that trophoblast-mediated education of B cells leads to their acquisition of properties capable of modulating inflammation in the uterine environment during the peri-implantation period.
Keywords: B cells, inflammation, regulatory phenotype, spheroid, trophoblast
1 |. INTRODUCTION
In pregnancy, maternal immune cells in the local uterine microenvironment are critical players in the physiological processes that lead to successful conception, implantation, gestation, and ultimately, parturition.1 At the outset of pregnancy, the foremost objective is to build and foster a coordinated interplay of immune cells, hormones, and cytokines that is oriented toward securing blastocyst attachment and implantation into the uterine decidua. At periimplantation, innate and adaptive immune cells including macrophages, natural killer cells, dendritic cells, and T cells accumulate in the endometrium. Interestingly, the phenotypes of endometrial and decidual immune cells in early pregnancy are altered compared with the non-pregnant state; that is, uterine dendritic cells (uDC) retain an immature tolerogenic state,2 uterine natural killer cells (uNK) exhibit reduced cytotoxic capability,3 and CD8+ T cells in the decidua have reduced expression of perforin and granzyme B.4 These modified functional roles are recognized as beneficial and contributory to a receptive uterus and vital for the development of the placenta.5
The first point of contact between embryonic cells and the maternal uterine microenvironment is via the invading trophoblast cells of the blastocyst. Trophoblast cells present an interesting immunologic occurrence in that they are semi-allogenic and should, in principle, trigger an adverse inflammatory response. Instead, the trophoblast thrives in the tolerant immunological milieu at the implantation site. Previous studies have shown that trophoblast cells can regulate immune cell function through various pathways. First-trimester trophoblast cells secrete high levels of TGF-β and thymic stromal lymphopoietin (TSLP), and have been observed to actively recruit and differentiate regulatory T cells (Tregs).2,6 Exosomes produced by trophoblast cells can act as recruiting agents for monocytes and downregulate NKG2D receptors on NK, CD8+, and γδ T cells to reduce their cytotoxic capacity.7,8 Trophoblast cells have also been shown to induce a predominantly tolerogenic profile in dendritic cells in vitro.9 Trophoblast cells, therefore, are suggested to be paramount in shaping the manner in which maternal uterine immune cells respond to invasion of the blastocyst.
So far, studies on lymphocytes within the uterine microenvironment have been largely limited to T cells, which contribute immensely to the controlled inflammatory environment requisite for uterine receptivity leading to implantation and placentation.10 However, there are now a number of studies outlining an emerging role for B cells as being active participants in pregnancy. Recent studies have shown a protective effect of IL-10-producing B cells (B10) in restoring tolerance and rescuing pregnancy. Mouse splenic B10 cells are expanded during normal pregnancy, and their adoptive transfer to abortion-prone mice rescued pregnancies.11 Similarly in humans, IL-10-producing CD19+CD24hiCD27+ regulatory B cells (Bregs) are significantly higher in women undergoing normal pregnancies in their first trimester as opposed to non-pregnant women, or women who suffered spontaneous abortions.12 These studies suggest that B cells play a role in early pregnancy; thus, there is an impetus to investigate events critical at this stage.
In this study, we aimed to investigate the effect of first-trimester trophoblast cells on the human B-cell phenotype and function. Here, we provide in vitro evidence that trophoblast cells can promote the differentiation of regulatory B cells, transitional B cells, IgM-memory B cells, and plasmablasts, which subsequently produced a dampened inflammatory cytokine profile. We also show that these trophoblast-educated B (TE-B) cells can regulate T-cell proliferation and cytokine production. Lastly, using trophoblast spheroids as an in vitro implantation model, we demonstrate that the addition of conditioned media from TE-B cells protect the spheroid from damage and aid trophoblast migration, despite the presence of deleterious cytokines from highly activated T cells. Thus, we propose that first-trimester trophoblast cells can educate B cells to acquire phenotypes and functional properties that contribute in calibrating inflammation that otherwise could be detrimental to a successful implantation event.
2 |. METHODS
2.1 |. Cell lines and culture conditions
The human first-trimester trophoblast cell line, SWAN 71 (Sw.71), was derived from a 7-week placenta and immortalized by ectopic expression of the catalytic subunit of the human telomerase (hTERT)13 and used for all experiments performed. Cells were maintained in DMEM/F12 (Gibco Invitrogen) and passaged when approximately 85% confluent. For some experiments, hTERT-immortalized human endometrial stromal cells (HESC)14 were used and maintained in DMEM (Gibco Invitrogen) and passaged every 3 days. All culture media were supplemented with 2 mmol/L L-glutamine, 10% fetal bovine serum (FBS), 1 mmol/L sodium pyruvate, 0.1 mmol/L non-essential amino acids, 1 mmol/L sodium pyruvate, 100 units/mL penicillin-streptomycin, and kept at 37°C and 5% CO2.
2.2 |. Peripheral blood mononuclear cells (PBMC) and B-cell isolation
Human peripheral blood mononuclear cells (PBMCs) were isolated from whole blood obtained from consenting healthy non-pregnant female donors as approved by the Human Investigation Committee of the Yale University Institutional Review Board (IRB). Briefly, whole blood collected in EDTA-coated vacutainers was diluted 1:2 with PBS and carefully overlaid into a tube containing equal volume of Lymphoprep™ (StemCell Tech). Cells were spun at 750 g without brake and acceleration for 25 minutes at room temperature with resultant mononuclear cells harvested from the interface of plasma and Lymphoprep™ and washed twice with large volumes of PBS. B cells were then purified by negative selection from the PBMCs using the MojoSort™ Human Pan B Cell Isolation Kit (BioLegend) following the manufacturer’s instructions, resulting in highly purified population of CD19+ Cells (>90% purity).
2.3 |. Collection of conditioned media
To obtain conditioned media from trophoblast cells, Sw.71 cells were plated at 25% confluency into T-175 flasks. Cells were incubated for 48 hours after which the cell supernatant was collected and centrifuged to remove all cellular debris. Cell-free supernatant was then aliquoted and stored at −80°C until further use.
Highly purified CD19+ B cells were resuspended in RPMI-1640 media (Gibco Invitrogen) and seeded at 1 × 106 cells/well in a 24-well flat-bottom plate (Sigma-Aldrich). Cells were incubated either unstimulated for the non-treated control or stimulated with Sw.71 conditioned media diluted 50% in RPMI medium for 48 hours at 37°C and 5% CO2. Cell supernatant was collected and spun down to exclude cellular debris in the collection of cell-free supernatant. Aliquots were prepared and stored at −80°C until further use.
2.4 |. Preparation of differentiated and activated T cells
T cells were purified from isolated PBMCs by negative selection using the EasySep™ Human T cell Isolation Kit (StemCell Tech) following the manufacturer’s instructions resulting in purity of >95% CD3+ cells. For non-specific T-cell receptor (TCR) stimulation, 1 × 105 cells T cells were seeded in 96-well flat-bottom plate pre-coated with anti-CD3 (10 ug/mL, BD Biosciences) and anti-CD28 (1 ug/mL, BD Biosciences) antibodies in RPMI medium supplemented with 10% FBS and IL-2 (2 ng/mL, PeproTech). The cells were left to incubate at 37°C and 5% CO2 for 3 days after which the stimulated cells were transferred to a new plate in normal 10% RPMI medium and rested for 2 more days. In some experiments, these pre-primed T cells were activated with Cell Stimulation Cocktail (eBioscience) containing phorbol 12-myristate 13-acetate (PMA) and ionomycin for 5 hours, to induce differentiation into activated T cells.
2.5 |. Flow cytometry
Multi-parameter flow cytometry was used to assess B cell phenotypes using a panel of conjugated mouse antihuman monoclonal antibodies (mAb) specific for the following markers: CD19-FITC Clone HIB19 (BD Biosciences), CD24-PerCP Clone ML5 (BD Biosciences), CD27-APC Clone O323 (BioLegend), CD38-PE Clone HIT2 (BD Biosciences), IgM-PECy7 Clone G20–127 (BD Biosciences), and IL-10-FITC Clone JES3–9D7 (BioLegend). B cells were collected, washed twice, and then incubated with heat-inactivated human serum for 20 minutes on ice. Cells were washed and stained with the cocktail of mAb in FACS buffer for 30 minutes in the dark on ice. For IL-10 intracellular staining, cells were stimulated using a Cell Stimulation Cocktail (plus protein transport inhibitors; eBioscience) for 5 hours prior to fixing and permeabilizing using the BD Cytofix/Cytoperm Kit (BD Biosciences). The cells were then stained with IL-10-FITC for 30 minutes at room temperature. Cells were washed and transferred to FACS tubes for flow cytometry analysis. For T cells, the following markers were used: CD4-APC Clone OKT4 (SunGene Biotech), CD25-PE Clone HI25A (SunGene Biotech), and Foxp3-FITC Clone PCH101 (eBioscience).
Cell phenotype was determined by four-color flow cytometry analysis (FACSCalibur; BD Biosciences), and the data were analyzed using FlowJo software (Treestar). Unstained, single color, FMO, and isotype controls were used to set gates and identify the positive populations.
2.6 |. Classification of B cell subsets
The following B-cell subsets were identified by previous studies as regulatory in function via their production of IL-10 and thus were used to classify the B cells acquired: regulatory B cells (CD19+CD24+CD27+), transitional B cells (CD19+CD24hiCD38hi), IgM-memory B cells (CD19+IgM+CD27+), and plasmablasts (CD19+CD27hiCD38hi).
2.7 |. Cytokine/chemokine analysis
The effect of trophoblast supernatant on immune cell chemokine and cytokine production was determined using Bio-Plex Pro™ Human Cytokine 17-plex Assay (Bio-Rad) following the manufacturer’s instructions. Briefly, wells of a 96-well filter plate were loaded with the prepared standard solution or cell-free supernatant and incubated with the 17-plex ProBead Mix at ±800 rpm for 30 minutes in the dark at room temperature. Wells were then vacuum-washed three times then incubated with 25 μl of biotinylated detection antibody at ±800 rpm for 30 minutes at room temperature in the dark. After three washes, streptavidin-phycoerythrin was added to each well and incubated for 10 minutes at ±800 rpm in the dark. After a final wash, the beads were resuspended in sheath buffer for measurement with the LUMINEX 200 (LUMINEX).
2.8 |. RNA isolation, RT-PCR, and quantitative PCR analysis
RNA was extracted from purified B cells cultured in TB-CM, or normal medium as controls, using the RNeasy kit (Qiagen). RNA concentration and purity were quantified via spectrophotometric analyses of 260/280 ratios with a cut-off ratio of 1.8 or above for samples to be used for analysis. Pellets were resuspended in 20 μL of DNAse/RNAse-free water and quantitation using NanoDrop 2000 (Thermo Fisher). For quantitative analysis of messenger RNA (mRNA), 1 μg of RNA from each sample was reverse-transcribed using iSCript reverse transcription Supermix (Bio-Rad). The complementary DNA (cDNA) was diluted to 1:5 in nuclease-free water, and 5 μL was used in the polymerase chain reaction (PCR). iTaq Universal SYBR green Supermix (Bio-Rad) and gene-specific primers were added to the reverse transcriptase reactions and run on the CFX96, C1000 system quantitative (qPCR) machine (Bio-Rad). The primer pair sequences are as follows: IL-10 forward 5′-GTGATGCCCCAAGCTGAGA-3′ and reverse 5′-CACGGCTTGCTCTTGTTTT-3′; internal normalizer gene GADPH forward 5′-TTAAAAGCAGCCCTGGTGAC-3′ and reverse 5′-CTCTGCTCCTCCTGTTCGAC-3′. Data were collected in the SYBR channel, and the final cycle was followed by a thermal melt curve. The relative expression to reference GAPDH levels were calculated using the ΔΔCt method (ΔCt = ΔCt treated−ΔCt control), and the results expressed as the fold change over control (Fold change = 2−ΔΔCt).
2.9 |. Formation of trophoblast spheroids
For formation of trophoblast spheroids, 4 × 105 Sw.71-GFP cells were seeded into each well of a 96-well ultra-low attachment plate (Sigma-Aldrich) and centrifuged at 400 g for 5 minutes to cluster the cells together. The plate was incubated for 48 hours to allow the clumped trophoblast to form into a spheroid. The spheroids were individually collected and transferred onto other plates for migration or invasion assays. This in vitro model of trophoblast implantation is described in full by You et al.15
2.10 |. Trophoblast spheroid invasion assay
The matrigel invasion assay directly measures the invasive capability of the trophoblasts as conditioned by the environment. Briefly, a trophoblast spheroid was carefully plated into the center of a well layered with a matrigel (Corning Inc) solution comprised of matrigel and the treatment medium. Serum-free media supplemented with treatment medium was used to drive trophoblast invasion as measured by the presence of cellular protrusions within the matrigel layer. Decidualized stromal cell-conditioned medium and serum-free culture medium were used as positive and negative controls for invasion, respectively. The invasion of trophoblast cells into the matrigel matrix was monitored from 2 to 4 days and visualized under the Revolve Pro microscope (Echo Laboratories). To indicate the level of activity of individual spheroids across biologically independent experiments, a scoring system was devised based on calculated area and visual characteristics of 2D images. Differences in spheroid activity assessed at 48 and 96 hours of culture were scored as follows: 0 = cell death; 1 = viable spheroid without change in area; 2 = >10% increase in area; 3 = >10% increase in area with peripheral protrusions; 4 = >10% increase in area and prominent peripheral protrusions. Area (A = πr2) was calculated from 2D images where radius (r) was set as half the average of two perpendicular diameter lengths of the horizontal and vertical axes.
2.11 |. Trophoblast spheroid migration assay
For the migration assay, trophoblast spheroids were individually seeded in a 96-well plate and grown in serum-free culture media supplemented with experimental treatment medium. The spheroids were monitored for 48 hours with time-lapse documentation at 2-hour intervals using the IncuCyte ZOOM System (Essen BioScience). Conditioned medium from decidualized stromal cells and normal culture medium were used as positive and negative controls, respectively. To indicate the level of activity of individual spheroids across biologically independent experiments, a scoring system was devised based on visual characteristics of 2D images. Differences in spheroid activity assessed at 0 and 48 hours of culture were scored as follows: 0 = cell death; 1 = viable spheroid; 2 = viable spheroid with migration of trophoblast cells; 3 = viable spheroid with extensive migration of trophoblast cells.
2.12 |. Statistical analysis
All experiments were performed in triplicate. The results were analyzed for significant differences using the Student’s t test or the Mann-Whitney test for parametric and nonparametric samples, respectively. For multiple comparisons, one-way ANOVA with Dunnett’s post hoc test was used. Data are presented as the mean ± standard error of the mean. Differences between groups were considered statistically significant at P-value <.05 as analyzed using GraphPad Prism 7.03 software (GraphPad).
3 |. RESULTS
3.1 |. Trophoblast cells modify the phenotype of B cells and reduce pro-inflammatory cytokine production
Our first objective was to determine whether trophoblast cells could influence B-cell differentiation. To test this, we cultured purified peripheral blood B cells with either immortalized first-trimester trophoblast cells, trophoblast-conditioned medium, or normal medium as controls for 48 hours. Cells were subsequently collected, and the B cell phenotype determined by flow cytometry. Figure 1A shows representative flow cytometry panels for the evaluated CD19+ B cells subsets: CD24hiCD27+ regulatory B cells, CD24hiCD38hi transitional B cells, CD27+IgM+ memory B cells, and CD38hiCD27hi plasmablasts. Our results indicate that trophoblast cells can directly, or indirectly by secreted factors, induce B cells to undergo differentiation characterized by a significant increase in the proportion of B regulatory, transitional, IgM-memory, and plasmablast subpopulations (Figure 1B). The fact that culture with trophoblast-conditioned medium or trophoblasts directly yielded similar alterations in phenotypes suggested that the greater part of the observed phenotypic changes can be incurred through soluble factors present in the conditioned medium rather than cognate interactions with the trophoblast cells. For this reason, in all following experiments we focused on using B cells cultured with trophoblast-conditioned medium and refer to them henceforth as trophoblast-educated B cells or TE-B cells.
FIGURE 1.
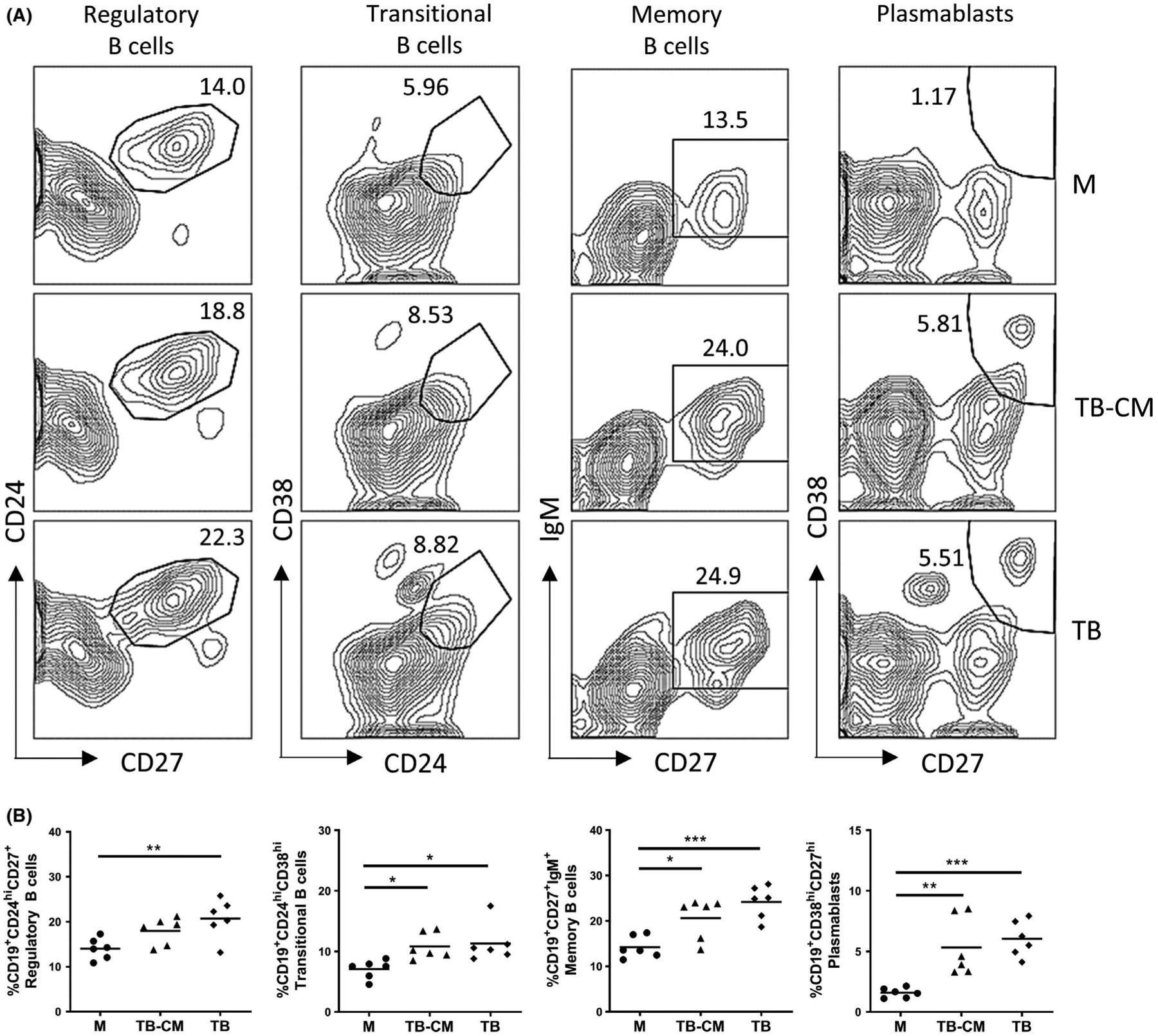
Trophoblast cells modify B-cell phenotype. Purified B cells (105 cells/100 μL medium) were cultured in normal medium (M), trophoblast-conditioned medium (TB-CM), or directly co-cultured with trophoblast (TB) cells. After 48 h, recovered B cells were stained with B cell lineage surface markers CD19, CD24, CD27, CD38, and IgM to discriminate between the Breg, transitional, IgM-memory, and plasmablast subpopulations. Representative flow cytometry panels indicating gating strategy shown in (A), with combined results from three independent experiments performed in duplicate shown in (B). *P < .05, **P < .01, ***P < .001
Next, we characterized the cytokine/chemokine profile of TE-B cells by multiplex assay analysis (Figure 2). Interestingly, B cells cultured in trophoblast-conditioned medium showed major differences in their secreted cytokine profile, with concentrations of the pro-inflammatory cytokines IL-17, IL-1β, IL-6, TNFα, and the chemokine RANTES, all significantly reduced compared to that of control medium. All other analytes were not significantly different between cultures. This indicated that secreted factors found in the conditioned medium from trophoblast cell cultures dampened the basal inflammatory cytokine profile in B cells.
FIGURE 2.
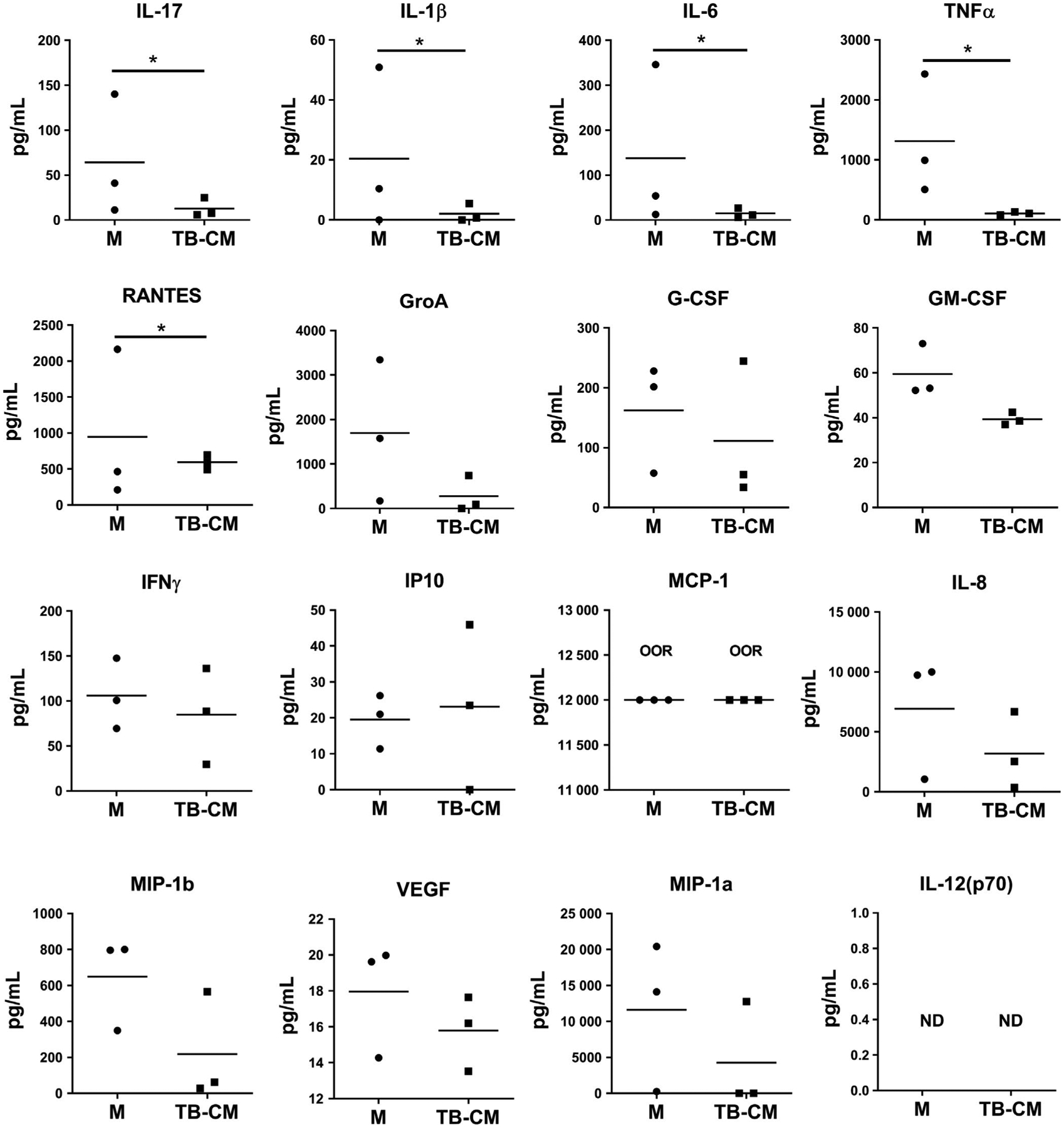
Trophoblast-educated B cells have diminished pro-inflammatory cytokine production. Cell-free supernatant from cultures of purified B cells incubated in trophoblast-conditioned medium (TB-CM) or control medium (M) for 48 h were quantified against TB-CM alone using Bio-Plex® multiplex assay. Results were expressed in pg/mL ± SEM with data presented as concentration minus concentrations found in TB-CM alone. IL-12 was not detected (ND) and MCP-1 readouts were out of range (OOR) and could not be extrapolated in the assay. Data represent pooled supernatants of duplicate wells from three independent experiments. *P < .05
The modification in phenotype and the general reduction in pro-inflammatory cytokines alluded to the induction of regulatory properties in these TE-B cells. To verify, we looked specifically at the capacity of these cells to generate the regulatory cytokine IL-10. Expression of IL-10 mRNA was significantly increased in B cells cultured in trophoblast-conditioned medium compared to control medium (Figure 3A). Similarly, assessment of intracellular IL-10 indicated that the percentage of IL-10-producing B cells increased with culture in trophoblast-conditioned medium. A further increase was shown in B cells cultured with trophoblast cells directly, which yielded similar percentages of IL-10+ cells to that observed with CpG (TLR9 agonist), a known factor responsible for the expansion of IL-10+ B cell frequencies in humans16 (Figure 3B). Lastly, analysis of surface markers showed that IL-10+ B cells have increased expression of CD24, CD38, and IgM, and intermediate expression of CD27, similar to that of CpG-generated IL-10+ B cells. The surface marker expression profile indicates that IL-10+ B cells are found in the suppressive B-cell subsets, namely within the groups of regulatory B cells (CD19+CD24+CD27+), transitional B cells (CD19+CD24hiCD38hi), and IgM-memory B cells (CD19+IgM+CD27+; Figure 3C).
FIGURE 3.
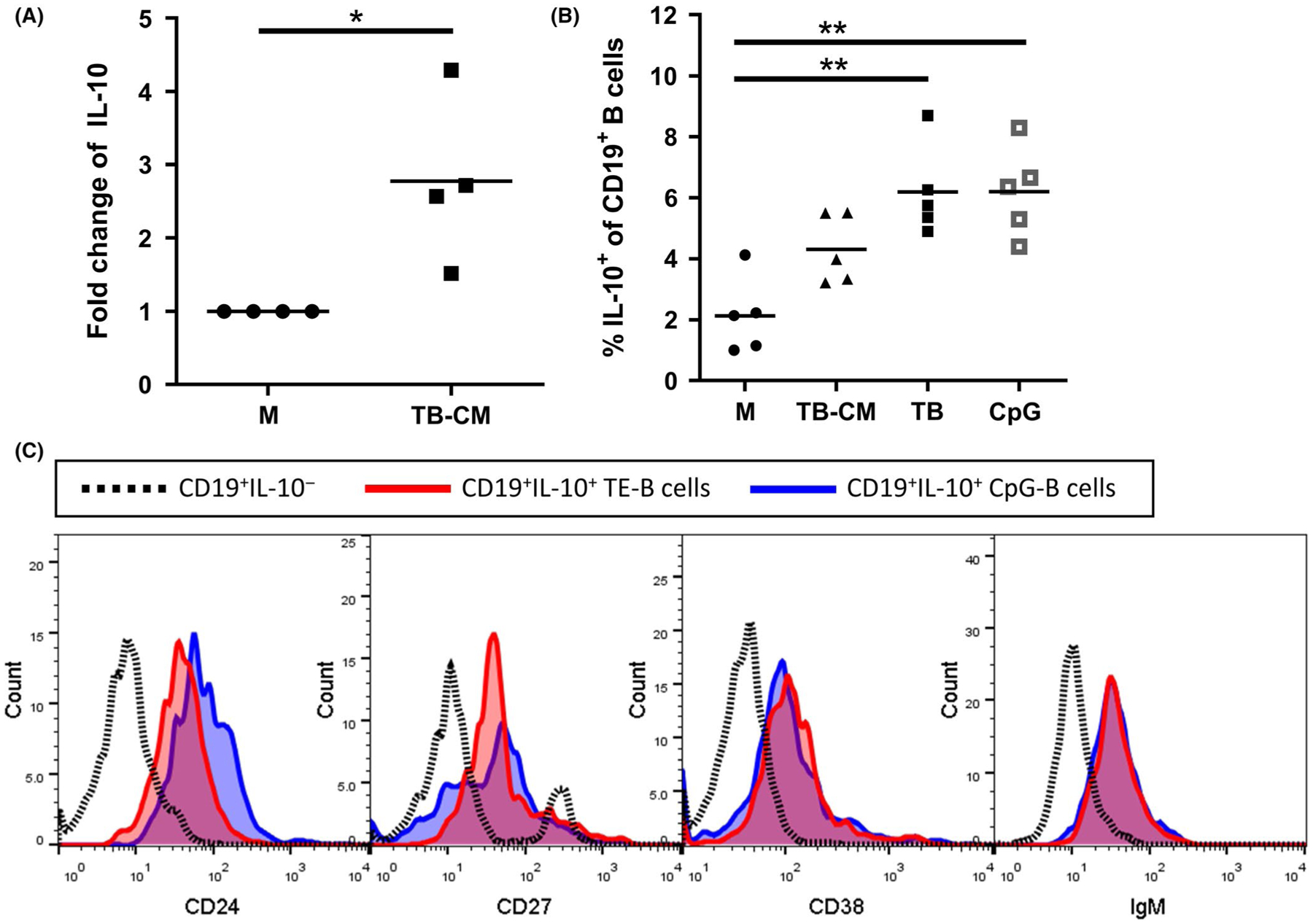
Trophoblast cells secrete factors that upregulate IL-10 expression in B cells. A, Purified B cells were cultured with trophoblast-conditioned medium (TB-CM) or normal medium (M) for 48 h after which B cells were processed for IL-10 mRNA expression by RT-qPCR analysis. Results are shown as fold increase over controls. B, Production of IL-10 by B cells cultured with M, TB-CM, or trophoblast cell (TB) cells directly was assessed by intracellular flow cytometric methods against positive control cultures stimulated with CpG. Results shown represent data collected from 4–5 independent experiments. C, Expression of surface markers CD38, CD24, CD27, and IgM on CD19+IL-10+ B cells after trophoblast education (red) and CpG treatment (blue) was compared to non-IL-10 producing B cells (dotted line). *P < .05, **P < .01
3.2 |. TE-B cells regulate T-cell proliferation and cytokine production
B cells perform a range of immunological functions, some of which require close interactions with T cells. At the implantation stage, various T-cell subsets participate in the development of a receptive endometrium. Critical events such as generation of Th1/Th2/Th17 cells, development of memory T cells, and induction of Treg cells require an expanded CD4+ T-cell subset to generate these lineages.17–19 Post-implantation, decidual CD4+ T cells are in much lower proportions due to the general predominance of CD8+ T cells.4 Here, we explored whether B cells differentiated under the influence of trophoblast secreted factors are functional and able to influence T-cell subset composition and T-cell cytokine production.
To test this hypothesis, we non-specifically stimulated purified T cells into proliferation with anti-CD3 and anti-CD28 antibodies alone, or in the presence of conditioned medium from cultures of normal B cells, trophoblast cells, and TE-B cells. Cells were then left to rest after which CD4 and CD8 T-cell subset composition and cytokine expression was analyzed (Figure 4A). Representative flow cytometric panels and gating strategy for the CD4+ and CD8+ T-cell subsets are shown in Figure 4B, as well as the tabulated CD4+/CD8+ ratios following differentiation. Our data indicated that exposure of proliferating T cells to trophoblast or TE-B-cell-conditioned media increased the proportion of CD4+ T cells compared to proliferating T cells cultured in medium from B cells alone (Figure 4C). We next examined whether the expansion in CD4+ T cells was a result of an increase in the Treg population. Whilst TGFβ, the positive control for CD4+Foxp3+ Treg expansion, generated the highest percentage of CD4+Foxp3+ Tregs, no such response was observed in our expanded populations suggesting that conditioned medium from TE-B cells are not responsible for Treg differentiation (Figure 4C).
FIGURE 4.
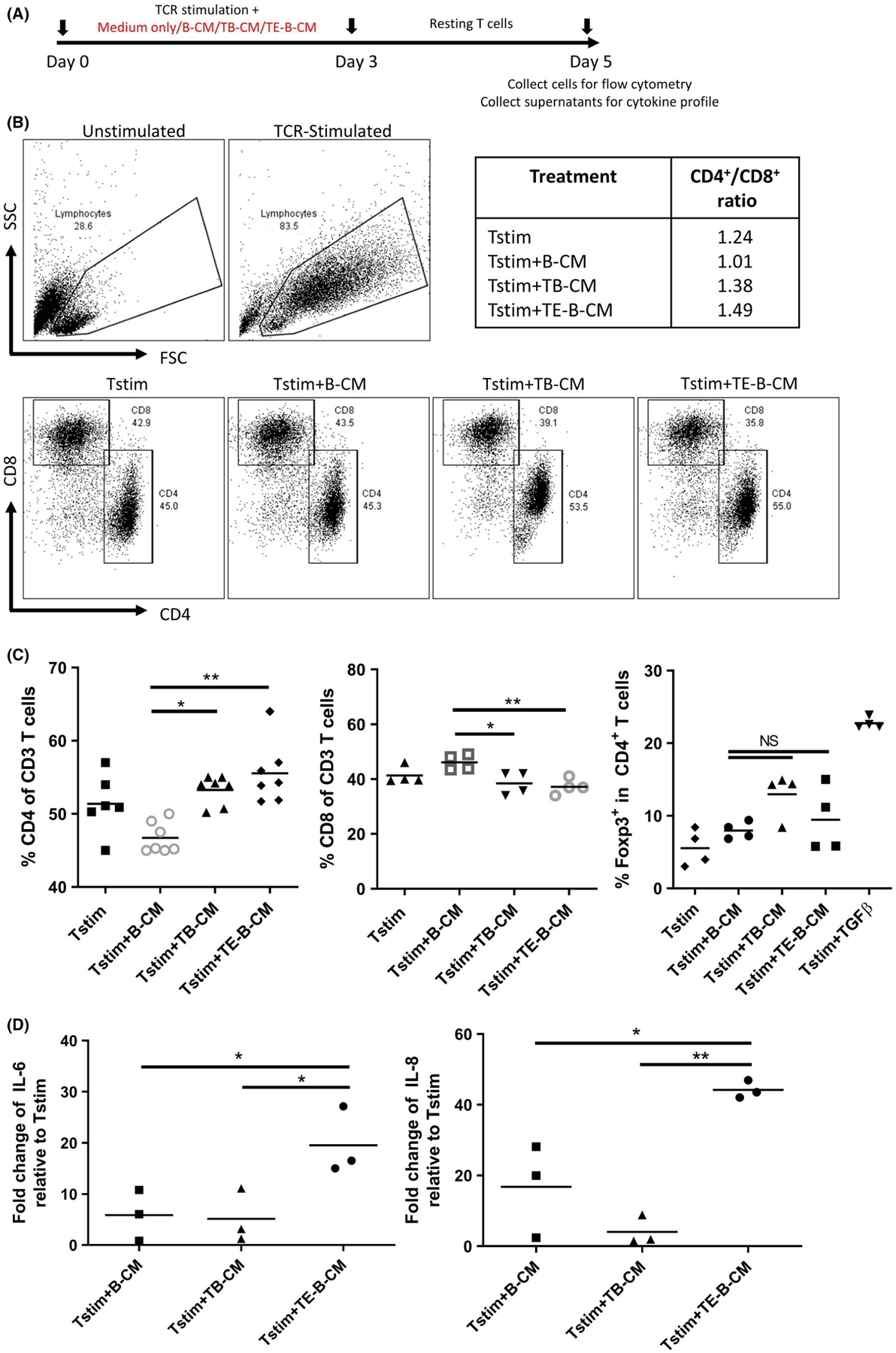
TE-B cells impact on T-cell proliferation and cytokine production. Purified T cells were (A) TCR-stimulated with anti-CD3 and anti-CD28 antibodies in normal medium alone (Tstim), or with addition of conditioned medium obtained from cultures of normal B cells (B-CM), trophoblast cells (TB-CM), or TE-B cells (TE-B-CM). B, Representative flow cytometry plots comparing side and forward scatter of unstimulated and TCR-stimulated T cells (upper panels), the gating strategy for CD4+ and CD8+ subsets (lower panels), and the summarized CD4+/CD8+ ratios. C, Graphical presentation of data combined from a minimum of four independent experiments showing CD4+ and CD8+ T cells as a percent of all CD3+ T cells and expression of transcription factor Foxp3 in CD4+ T cells. D, Fold change increase of IL-6 and IL-8 cytokine production by proliferating T cells cultured in indicated conditioned medium over normal medium controls. *P < .05, **P < .01
However, the presence of TE-B-cell-conditioned medium during TCR stimulation had an independent effect on the cytokine profile of proliferating T cells. We observed a significant increase in the expression of the pro-inflammatory cytokines IL-6 and IL-8 by T cells exposed to secreted factors from TE-B cell cultures compared to conditioned medium from cultures of trophoblasts and normal B cells (Figure 4D). Both IL-6 and IL-8 are cytokines that have been repeatedly shown as important factors in implantation as reduced levels have been correlated with a reduction in trophoblast invasion resulting in early miscarriages.20,21 These findings demonstrate a network of cell communication where trophoblast cells promote B-cell differentiation into unique B-cell subtypes with the capacity to regulate T-cell proliferation and cytokine production.
3.3 |. TE-B cells contribute to the control of inflammation at implantation
To investigate the potential functional role of TE-B cells at implantation, we utilized a novel 3D in vitro model to evaluate trophoblast migration and invasion.15 Trophoblast cells were grown as blastocyst-like spheroids and transferred onto matrigel-coated wells and assessed for evidence of cellular protrusions indicative of invasion into the matrigel matrix. Whilst stromal cell-conditioned medium clearly induced prominent protrusions as expected, conditioned medium from TE-B cells or control B cells elicited effects similar to that of media controls (Figure 5A,B, left panel). Similarly, stromal cell-conditioned medium induced rapid attachment and migration of trophoblast cells (from 12 hours), an effect not replicated with conditioned medium from TE-B-cell cultures. Furthermore, TE-B cells did not impact on trophoblast attachment and migration induced by stromal cell-conditioned medium (Figure 5B,C, right panel) indicating that TE-B cells did not secrete factors that directly or indirectly affected trophoblast attachment, migration, or invasion.
FIGURE 5.

TE-B cells do not express soluble factors that affect the invasion and migration activity of trophoblast spheroids. A, For invasion assays, trophoblast spheroids were cultured on matrigel solution combined with normal medium (negative control) or conditioned medium from decidualized stromal cell cultures (stromal CM) as a positive control, or cultures of TE-B cells (TE-B-CM) or normal B cells (B-CM). The formation of trophoblast cell protrusions indicative of invasion into the matrigel matrix was monitored for up to 96 h, with protrusions clearly generated by stromal CM as expected, but not from any other conditioned or control medium. Representative photographs at 48- and 96-h timepoints of the trophoblast spheroids in the invasion assay are shown. B, Independent invasion (left panel) and migration (right panel) experiments were assessed and given an activity score. Combined data from at least three biologically independent experiments are shown. C, For migration assays, trophoblast spheroids were individually seeded into 96-well plate with attachment and migration of trophoblast cells monitored for 48 h. Stromal CM induced cell attachment and migration; however, no such effect was seen with control or TE-B-cell-conditioned media. TE-B-CM was also not capable of inhibiting migration induced by stromal CM. Representative photographs of trophoblast spheroids at 0-, 12-, 24-, 36-, and 48-h timepoints in the migration assay are shown. All photographs were taken using the Revolve Pro microscope in (A), or the IncuCyte ZOOM System in (B) at 200× magnification
We then examined if TE-B cells might have an indirect effect on trophoblast invasion and migration via modification of T-cell activity. We collected the cell-free supernatant from rested T cells non-specifically stimulated in the presence of normal medium or conditioned medium from TE-B or normal B-cell cultures (Figure 6A). These supernatants were combined with matrigel and plated into individual wells for trophoblast invasion assays. Conditioned media from stimulated T cells alone or in combination with TE-B cell or B cell–conditioned mediums were able to induce protrusions from trophoblast cells with varying consistency (Figure 6B and 6C, left panel). However, no conditioned media could induce migration of trophoblast cells (Figure 6D and 6C, right panel). Taken together, these results suggest that soluble factors produced by T cells in a non-inflammatory state have a limited effect on the functional activity of trophoblast cells.
FIGURE 6.
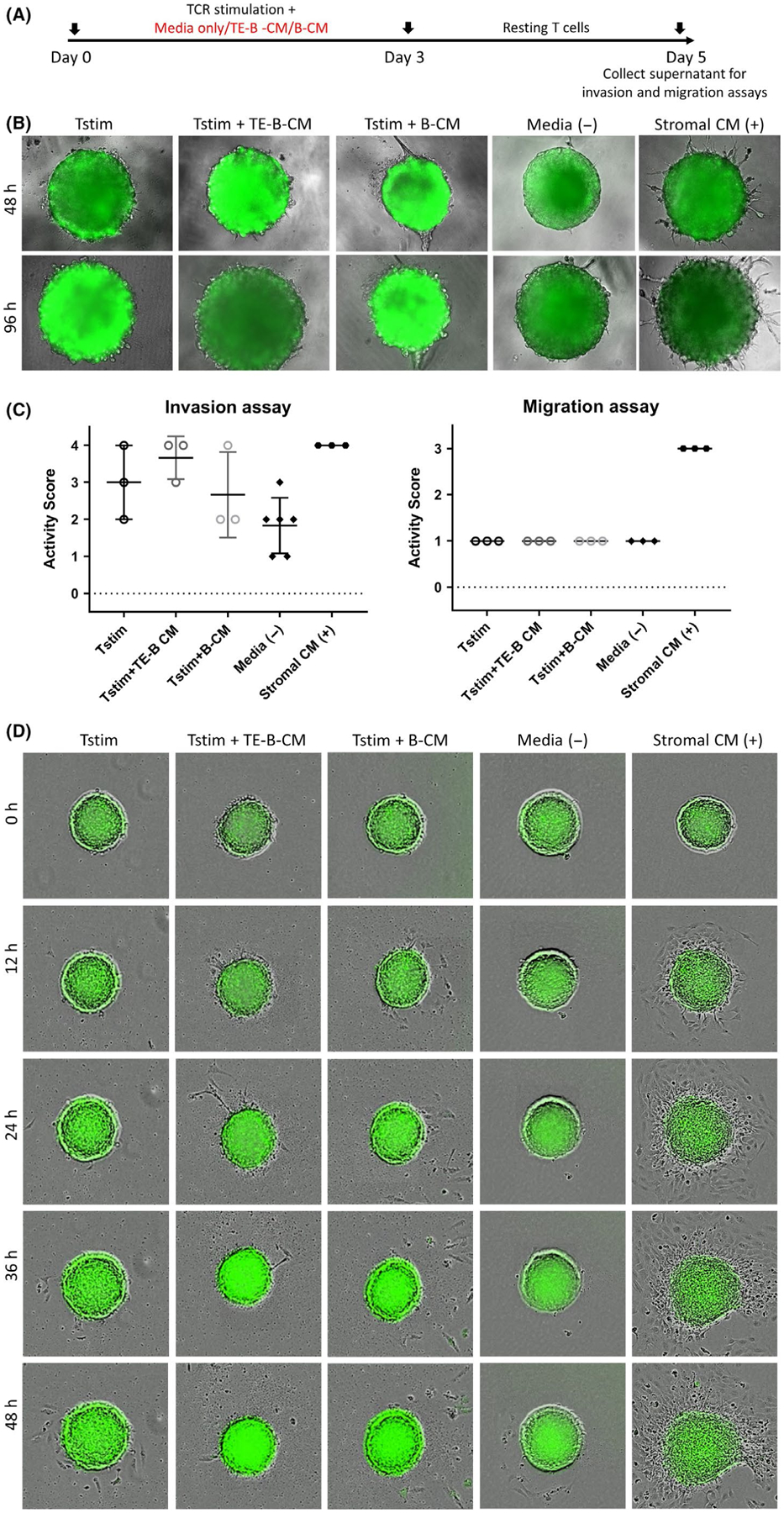
TE-B cells do not promote trophoblast invasion or migration by indirect effect on T cells. A, Timeline of T-cell stimulation, resting period, and collection of media is shown. B, For invasion assays, trophoblast spheroids were incubated on matrigel combined with medium from TCR-stimulated T cells alone (Tstim) or in the presence of conditioned medium from TE-B cells (TE-B_CM) or normal B cells (B-CM). Medium alone or stromal cell medium was used as negative and positive controls, respectively. No effect on invasion by any of the conditioned media was observed. Representative photographs at 48- and 96-h timepoints of the trophoblast spheroids in the matrigel invasion assay are shown. C, Independent invasion (left panel) and migration (right panel) experiments were assessed and given an activity score. Combined data from at least three biologically independent experiments are shown. D, For migration assays, trophoblast spheroids were individually monitored for attachment and migration of trophoblast cells for 48 h. No migration was induced by stimulated T-cell culture media with or without conditioned media from TE-B or normal B cells. Representative photographs of trophoblast spheroids at 0-, 12-, 24-, 36-, and 48-h timepoints in the migration assay are shown. All photographs were taken using the Revolve Pro microscope or the IncuCyte ZOOM System at 200× magnification and are representative of three other independent experiments performed in duplicate
Th1 inflammation is essential for implantation; however, the level of inflammation should fall within a range that is not detrimental to the embryo. In these experiments, we investigated whether TE-B cells could modulate the level of inflammation in a novel trophoblast model of deleterious inflammation where conditioned medium from cultures of PMA/ionomycin activated pre-primed T cells are added to trophoblast spheroids. By combining TCR stimulation by anti-CD3/CD28 antibodies with PMA/ionomycin stimulation which activates the cells through the Ca2+/calmodulin-dependent and PKC signaling pathways, we were able to induce heightened activation in T cells. The resultant conditioned medium derived from these supra-activated cells is predominantly comprised of pro-inflammatory cytokines. Incubation with the activated T cell–conditioned medium resulted in restricted spheroid growth and active disintegration of peripheral cells as visualized by a fuzzy outermost layer of dead trophoblast cells clearly identifiable by 96 hours in invasion assays and 48 hours in migration assays (Figure 7). This cell death was confirmed by CellTox green dye binding (Dimova T., Yuan Y., Aldo P., and Mor G., manuscript in preparation). To determine whether TE-B cells could dampen the deleterious inflammatory response, conditioned medium from TE-B cell or normal B cell cultures was added to rested TCR-stimulated T cells prior to PMA/ionomycin activation, with resultant supernatants added to wells containing trophoblast spheroids and observed for invasion and migration capacity (Figure 7A). Soluble factors from these activated T cells clearly inhibited generation of cellular protrusions; rather, death of the outer spheroid cells is evident (Figure 7B,C, left panel). However, activation of T cells in the presence of conditioned medium from TE-B cells could negate this effect, as indicated by increased survival of these trophoblast cells. This effect was also seen when conditioned medium from normal B cells was used. In a similar manner, medium from activated T cells inhibited spheroid growth, attachment, and migration; rather, death of peripheral trophoblast cells was observed (Figure 7C,D, right panel). This outcome could be inhibited by prior addition of conditioned medium from TE-B cells or normal B cell cultures, although conditioned medium from TE-B cells was also able to induce partial trophoblast migration. Together these results suggest that soluble factors produced by TE-B cells may play a role in the regulation of inflammation such that it can protect against embryo cell death and contribute to maintaining the cytokine environment necessary for progression of implantation.
FIGURE 7.
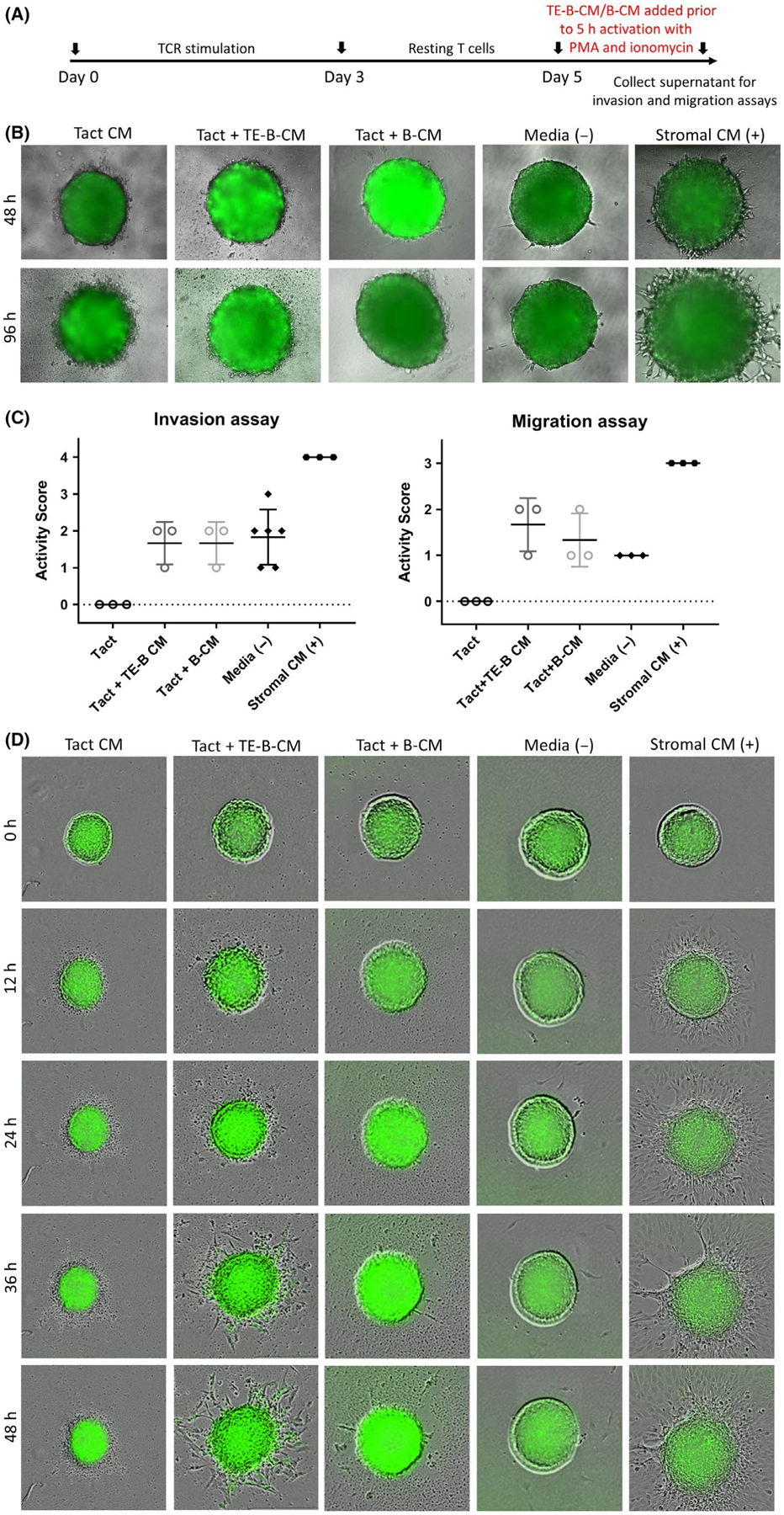
Soluble factors from TE-B cells can protect the trophoblast spheroid from cell damage and aid migration in conditions of deleterious inflammation. A, Timeline of non-specific T-cell stimulation, resting period, and T-cell activation by PMA/ionomycin in normal or conditioned media from cultures of TE-B cells (TE-B-CM) or normal B cells (B-CM). B, Trophoblast spheroid invasion assays. Trophoblast spheroids were plated onto matrigel matrix solutions containing conditioned medium from activated T cells (Tact CM), T cells activated in the presence of conditioned medium from TE-B cells (Tact + TE-B-CM) or normal B cells (Tact + B-CM) and assessed at 48 and 96 h. Normal medium and stromal cell medium acted as negative and positive controls, respectively. C, Independent invasion (left panel) and migration (right panel) experiments were assessed and given an activity score. Combined data from at least three biologically independent experiments are shown. D, Trophoblast migration assays. Individual trophoblasts were plated in the presence of conditioned medium from activated T cells as above and assessed every 12 h for 2 d. Photographs are representative of three independent experiments. All photographs were taken using the Revolve Pro microscope or the IncuCyte ZOOM System at 200× magnification
4 |. DISCUSSION
Pregnancy is a delicate condition requiring a complex and coordinated interplay of immune cells, endometrial cells, and endocrine factors. From the outset, the maternal immune system is tasked with protecting the mother against foreign assault as well as protecting the conceptus from damage. The conceptus on the other hand imposes an influence on the maternal immune system such that it is accepted into the uterine microenvironment despite its semi-allogeneic nature. At implantation, feto-maternal immune cross-talk is initiated by the trophoblast cells. Trophoblast-immune interactions are categorized into three stages: (a) recruitment—where the trophoblast transmits signals to encourage influx of immune cells in the implantation site, (b) education—where the trophoblast influences the phenotype and function of immune cells, and (c) response—where the immune cells educated by the trophoblast cells carry out functions that support implantation and placental formation.22 The trophoblast’s role in the recruitment and phenotype modification of some immune cells has been studied over the years, including the trophoblasts impact on the form and functions of monocytes, NK cells, regulatory T cells, macrophages, and dendritic cells.2,6,7,23,24 Interestingly, the B cells’ role in implantation has not been extensively explored. In this study, we focused on the effects of trophoblast cells on B-cell phenotype and function, and the subsequent role of these trophoblast-educated B cells (TE-B cells) in the context of implantation.
B-cell lymphopoiesis generates a diverse repertoire of peripheral B cells, starting from B cells exiting the bone marrow and entering peripheral circulation as immature transitional B cells and naïve B cells. Upon acquiring cognate antigens in secondary lymphoid locations, B cells become activated and differentiate into memory B cells, plasmablasts, or fully differentiated plasma cells bearing combinations of surface markers including CD21, CD24, CD27, CD38, and IgM.25 In recent years, B cells have been attributed a role in pregnancy due to their production of protective antibodies against paternal antigens as well as the production of damaging auto-antibodies that have been implicated in some pregnancy pathologies.26 IL-10-producing regulatory B cells have also been identified as important cells in pregnancy well-being.27 In our study, we show that trophoblast cells have a profound effect on the phenotype and cytokine production of B cells, mainly by inducing the differentiation of B cells into transitional B cells and committed B-cell lineage subsets such as regulatory B cells, IgM-only memory B cells, and plasmablasts. We hypothesize here that the Sw.71 trophoblast cells preferentially differentiate and expand B cells that can potentially function for immune regulation (regulatory B cells and IL-10+ transitional B cells) as well as protection against foreign antigen assaults (memory B cells and plasmablasts).
It is tempting to speculate that the observed effect on B cell phenotype and cytokine profiles may stem from the polarizing effect of Sw.71 trophoblast-derived exosomes, a phenomenon that has been previously reported in monocytes wherein their recruitment and differentiation were modified to support trophoblast function.7 In the same study, trophoblast-derived exosomes also decreased the production of pro-inflammatory cytokine IL-17 and chemokines CCL5 and macrophage migration inhibitory factor (MIF). Our results emulate their findings of cell-contact-independent modification of immune cells by Sw.71 cells, with B cells undertaking differentiation into specific B cell lineages and production of reduced levels of pro-inflammatory cytokines IL-17, IL-1β, IL-6, and TNF-α. Furthermore, Sw.71 cells are characterized as representing extravillous cytotrophoblasts that express fetal fibronectin secretin, cytokeratin 7, vimentin, and HLA-G, as well as low levels of human chorionic gonadotrophin (hCG).13 It is possible that hCG from the Sw.71 trophoblast cells may have also influenced the observed phenotypic changes in B cells. There is growing evidence that hCG can mediate immune-modulatory effects that promote tolerance at the maternal-fetal interface specifically in early pregnancy, and recent studies have demonstrated the capacity of hCG to expand the population of CD24hiCD27+ regulatory B cells as well as boost IL-10 production in CD19+ B cells.12,28–30 Additionally, hCG has been shown to alter the secretory profile of dendritic cells and human endometrial epithelial cells to support endometrial receptivity and blastocyst implantation.31,32
Feto-maternal cross-talk in early pregnancy is essential for the establishment of maternal immune tolerance, regulation of trophoblast activity, and remodeling of the uterine spiral arteries.33 Local immune cells in the endometrium are hypothesized to possess embryotrophic properties by providing a cytokine network that controls the immune and endocrine systems, as well as trophoblast function at implantation. The expression of cytokines mediates the embryo-maternal paracrine dialog necessary for the early stages of implantation and trophoblast invasion.34,35 Our findings demonstrate a modified cytokine profile of TE-B cells, suggesting their involvement in the balance of cytokines in the maternal-fetal interface which may contribute to implantation success. The decreased production of pro-inflammatory cytokines IL-17, IL-1β, IL-6, and TNF-α of TE-B cells can potentially downregulate inflammatory reactions and provide cytokine regulation in a predominantly pro-inflammatory environment required during implantation. Moreover, the increased mRNA expression of regulatory cytokine IL-10 indicates that the TE-B cells may also have the capacity to counteract inflammation in the microenvironment when needed. Recently, IL-10 produced by B cells has been demonstrated to provide protection against fetal death in murine pregnancies jeopardized by the presence of lipo-polysaccharide (LPS),36 further highlighting the influence of B cells in the immune balance at the maternal-fetal interface. The findings on B cell phenotype demonstrate that trophoblast education of B cells helps set the stage for a balanced immune condition that is conducive for implantation.
Although considered as scarce populations in the uterine microenvironment, B cells are present and active in the endometrium and decidua.17,37,38 In this study, we focused on the interaction of B cells and T cells, specifically the effect of TE-B cells on T-cell subset distribution and cytokine production. In most studies of pregnancy, the CD4+/CD8+ T-cell ratio has been reported to be approximately 1 or slightly decreased as the number of CD8+ T cells has been found to be higher than CD4+ T cells.39 However, in pre-pregnancy endometrium, which is arguably the best representation of the endometrium at the onset of pregnancy, there is a predominance of CD4+ T cells and an increased CD4+/CD8+ ratio compared to term decidua and peripheral blood17,40 presumably in preparation for differentiation into effector T cells necessary for inflammation. Treatment with TE-B-conditioned media alongside TCR stimulation maintained the high CD4+/CD8+ T-cell ratio. However, comparison with other controls such as trophoblast-conditioned medium treatment showed that this is likely a consequence of trophoblast factors and TE-B-conditioned media only enhances that effect. The increase in IL-6 and IL-8 production by TE-B treated T cells is interesting as these cytokines play critical roles in implantation events, particularly in spiral artery remodeling. Inadequate levels of IL-6 and IL-8 have been associated with reduced trophoblast invasion translating to spontaneous abortions.20,41 IL-8 in particular has been shown to stimulate extravillous trophoblast invasion and is involved in uterine natural killer cell-mediated stimulation of trophoblast invasion.42,43 The amplified production as per TE-B treatment is indicative of cytokine production being orchestrated to accommodate the invading trophoblasts. Lastly, we determined if TE-B cells can contribute toward expansion of the CD4+Foxp3+ regulatory T cells via induction of Foxp3 expression in CD4+ T cells. The results showed levels of Foxp3 expression similar to the treatment with just the trophoblast-conditioned medium, supporting the idea that Treg recruitment at implantation is achieved by fetal antigens,6 and so at this point there is no indication that soluble factors produced by TE-B cells are involved in Treg induction.
Uterine receptivity pertinent for embryo implantation is brought about by a Th1 inflammation in the endometrium, with the process of trophoblast migration and invasion dependent on the chemotactic gradients and cytokines conceived in the implantation milieu by resident immune cells. This inflammatory activity conditions the uterine microenvironment to receive the invading trophoblast and permit cells of embryonic origin to migrate into maternal tissue.44 The various regulatory mechanisms by both immune and decidualized endometrial cells to keep the pro-inflammatory cytokines and chemokines in check indicate that this inflammation is a tightly controlled process. Previous studies as well as the results thus far in this study provide evidence for a significant role of trophoblast cells in orchestrating control of inflammation. In this study, TE-B cells in a non-inflammatory, non-dangerous state by themselves do not have any significant effect on the activity of trophoblast spheroid cells (Figure 5), although combined with conditioned medium from TCR-stimulated T cells, TE-B cells may promote invasion of trophoblast cells (Figure 6). However, they do demonstrate functional activity within a system with prevailing inflammatory conditions (Figure 7). In our activated T cell–mediated inflammation model, we demonstrated a deleterious condition where the inflammation is elevated to toxic levels for the spheroid. This detrimental effect was negated upon the addition of TE-B-conditioned medium prior to PMA/ionomycin activation of the stimulated T cells. Furthermore, TE-B-conditioned medium treatment in the migration assay resulted in trophoblast cells migrating out of the spheroid in 2 out of 3 independent experiments, as opposed to the activated T-cell treatment alone or that with B cell–conditioned medium, suggesting that soluble factors from TE-B cells may have prevented and interfered with the normal expression and function of the activated T-cell cytokine profiles to a more tolerable level such that spheroid survival and subsequent activity was permitted. This may be due directly to increased levels of IL-6 and IL-8 cytokines found within the TE-B-conditioned medium, or indirectly by changes in the proportions of cytokines produced by activating T cells in the presence of TE-B-conditioned medium, thus creating a more favorable chemotactic gradient and prompting migration to occur. Whilst these effects demand further investigation in primary trophoblast cell lines and more physiological settings such as in vivo mouse models, the in vitro models used here imply that B cells may play a specific regulatory role in maintaining an environment conducive for trophoblast invasion and migration, a hallmark event in implantation.
The confirmation that TE-B cells can only partially restore normal migration activity is a testament that the process of implantation is regulated by multiple cellular players working together. The results gathered from the invasion and migration assays emphasize two things: (a) that controlled inflammation is necessary for implantation events to proceed and that (b) trophoblast-educated B cells contribute in calibrating the inflammation to a tolerable level.
5 |. CONCLUSION
In toto, we demonstrate in this study that the interactions and interplay of Sw.71 trophoblast cells and B cells influence the generation of a tolerable inflammatory environment that is favorable for trophoblast health and activity. Successful implantation is dependent on a network of immune cells and endometrial cells working together for a balanced inflammatory response necessary in achieving a receptive uterine microenvironment. Cells of embryonic origin, primarily trophoblasts, have been previously reported as significant modifiers of immune function in order to facilitate acceptance of the invading embryonic cells as well as prime cells to ensue immune reactions against foreign antigens should the need arise. In this study, we describe the effect of first-trimester trophoblast cells on B cells in vitro by showing the phenotypic and cytokine profile changes. TE-B cells are comprised of higher percentages of regulatory cells, IgM-memory cells, plasmablasts, and IL-10-producing immature/transitional cells that exhibit reduced cytokine/chemokine production. These TE-B cells were shown to help maintain an expanded CD4+ T-cell subset and stimulate the production of vital cytokines IL-6 and IL-8. Lastly, we demonstrate the role of B cells in counterbalancing inflammation due to activated T cells, enabling our in vitro implantation model of trophoblast spheroids to survive and undertake implantation hallmarks. Collectively, our results imply that trophoblast education of B cells may be one of the regulatory mechanisms in place ensuring the success of implantation in pregnancy.
ACKNOWLEDGMENTS
This work was supported by NHMRC Project (APP1020984) and Fellowship (APP1012386) grants to KD; and Research Training Program Domestic (RTPd) Scholarship and UniSA Research Degree Students International Travel Grant to RG-G. Additional support was provided by the Department of Obstetrics and Gynecology, Yale School of Medicine.
Footnotes
CONFLIC T OF INTEREST
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Robertson SA. Immune regulation of conception and embryo implantation-all about quality control? J Reprod Immunol. 2010;85(1):51–57. [DOI] [PubMed] [Google Scholar]
- 2.Du MR, Guo PF, Piao HL, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal-fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol. 2014;192(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Acar N, Ustunel I, Demir R. Uterine natural killer (uNK) cells and their missions during pregnancy: a review. Acta Histochem. 2011;113(2):82–91. [DOI] [PubMed] [Google Scholar]
- 4.Tilburgs T, Schonkeren D, Eikmans M, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. 2010;185(7):4470–4477. [DOI] [PubMed] [Google Scholar]
- 5.Zenclussen AC, Hammerling GJ. Cellular regulation of the uterine microenvironment that enables embryo implantation. Front Immunol. 2015;6:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramhorst R, Fraccaroli L, Aldo P, et al. Modulation and recruitment of inducible regulatory T cells by first trimester trophoblast cells. Am J Reprod Immunol. 2012;67(1):17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am J Reprod Immunol. 2011;65(1):65–77. [DOI] [PubMed] [Google Scholar]
- 8.Hedlund M, Stenqvist AC, Nagaeva O, et al. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J Immunol. 2009;183(1):340–351. [DOI] [PubMed] [Google Scholar]
- 9.Salamone G, Fraccaroli L, Gori S, et al. Trophoblast cells induce a tolerogenic profile in dendritic cells. Hum Reprod. 2012;27(9):2598–2606. [DOI] [PubMed] [Google Scholar]
- 10.Lee JY, Lee M, Lee SK. Role of endometrial immune cells in implantation. Clin Exp Reprod Med. 2011;38(3):119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jensen F, Muzzio D, Soldati R, Fest S, Zenclussen AC. Regulatory B10 cells restore pregnancy tolerance in a mouse model. Biol Reprod. 2013;89(4):90. [DOI] [PubMed] [Google Scholar]
- 12.Rolle L, Memarzadeh Tehran M, Morell-Garcia A, et al. Cutting edge: IL-10-producing regulatory B cells in early human pregnancy. Am J Reprod Immunol. 2013;70(6):448–453. [DOI] [PubMed] [Google Scholar]
- 13.Straszewski-Chavez SL, Abrahams VM, Alvero AB, et al. The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta. 2009;30(11):939–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krikun G, Mor G, Lockwood C. The immortalization of human endometrial cells. Methods Mol Med. 2006;121:79–83. [DOI] [PubMed] [Google Scholar]
- 15.You Y, Stelzl P, Zhang Y, et al. Novel 3D in vitro models to evaluate trophoblast migration and invasion. Am J Reprod Immunol. 2019;81(3):e13076. [DOI] [PubMed] [Google Scholar]
- 16.Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells. Blood. 2011;117(2):530–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feyaerts D, Benner M, van Cranenbroek B, van der Heijden O, Joosten I, van der Molen RG. Human uterine lymphocytes acquire a more experienced and tolerogenic phenotype during pregnancy. Sci Rep. 2017;7(1):2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kieffer TE, Faas MM, Scherjon SA, Prins JR. Pregnancy persistently affects memory T cell populations. J Reprod Immunol. 2017;119:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Polese B, Gridelet V, Araklioti E, Martens H, Perrier d’Hauterive S, Geenen V. The endocrine milieu and CD4 T-lymphocyte polarization during pregnancy. Front Endocrinol. 2014;5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pitman H, Innes BA, Robson SC, Bulmer JN, Lash GE. Altered expression of interleukin-6, interleukin-8 and their receptors in decidua of women with sporadic miscarriage. Hum Reprod. 2013;28(8):2075–2086. [DOI] [PubMed] [Google Scholar]
- 21.von Wolff M, Thaler CJ, Strowitzki T, Broome J, Stolz W, Tabibzadeh S. Regulated expression of cytokines in human endometrium throughout the menstrual cycle: dysregulation in habitual abortion. Mol Hum Reprod. 2000;6(7):627–634. [DOI] [PubMed] [Google Scholar]
- 22.Dekel N, Gnainsky Y, Granot I, Racicot K, Mor G. The role of inflammation for a successful implantation. Am J Reprod Immunol. 2014;72(2):141–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abumaree MH, Chamley LW, Badri M, El-Muzaini MF. Trophoblast debris modulates the expression of immune proteins in macrophages: a key to maternal tolerance of the fetal allograft? J Reprod Immunol. 2012;94(2):131–141. [DOI] [PubMed] [Google Scholar]
- 24.Sotnikova N, Voronin D, Antsiferova Y, Bukina E. Interaction of decidual CD56+ NK with trophoblast cells during normal pregnancy and recurrent spontaneous abortion at early term of gestation. Scand J Immunol. 2014;80(3):198–208. [DOI] [PubMed] [Google Scholar]
- 25.Kaminski DA, Wei C, Qian Y, Rosenberg AF, Sanz I. Advances in human B cell phenotypic profiling. Front Immunol. 2012;3:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marie-Cardine A, Divay F, Dutot I, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation. Clin Immunol. 2008;127(1):14–25. [DOI] [PubMed] [Google Scholar]
- 27.Guzman-Genuino RM, Diener KR. Regulatory B cells in pregnancy: lessons from autoimmunity, graft tolerance, and cancer. Front Immunol. 2017;8:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dauven D, Ehrentraut S, Langwisch S, Zenclussen AC, Schumacher A. Immune modulatory effects of human chorionic gonadotropin on dendritic cells supporting fetal survival in murine pregnancy. Front Endocrinol. 2016;7:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fettke F, Schumacher A, Canellada A, et al. Maternal and fetal mechanisms of B cell regulation during pregnancy: human chorionic gonadotropin stimulates B cells to produce IL-10 while alpha-fetoprotein drives them into apoptosis. Front Immunol. 2016;7:495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumacher A Human chorionic gonadotropin as a pivotal endocrine immune regulator initiating and preserving fetal tolerance. Int J Mol Sci. 2017;18(10):2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paiva P, Hannan NJ, Hincks C, et al. Human chorionic gonadotrophin regulates FGF2 and other cytokines produced by human endometrial epithelial cells, providing a mechanism for enhancing endometrial receptivity. Hum Reprod. 2011;26(5):1153–1162. [DOI] [PubMed] [Google Scholar]
- 32.Wan H, Versnel MA, Leijten LM, et al. Chorionic gonadotropin induces dendritic cells to express a tolerogenic phenotype. J Leukoc Biol. 2008;83(4):894–901. [DOI] [PubMed] [Google Scholar]
- 33.Lash GE. Cross-talk at the feto-maternal interface. Cold Spring Harb Perspect Med. 2015;5(12):a023010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol. 2008;20(4):462–469. [DOI] [PubMed] [Google Scholar]
- 35.Saito S Cytokine network at the feto-maternal interface. J Reprod Immunol. 2000;47(2):87–103. [DOI] [PubMed] [Google Scholar]
- 36.Busse M, Campe KJ, Nowak D, et al. IL-10 producing B cells rescue mouse fetuses from inflammation-driven fetal death and are able to modulate T cell immune responses. Sci Rep. 2019;9(1):9335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blois SM, Alba Soto CD, Tometten M, Klapp BF, Margni RA, Arck PC. Lineage, maturity, and phenotype of uterine murine dendritic cells throughout gestation indicate a protective role in maintaining pregnancy. Biol Reprod. 2004;70(4):1018–1023. [DOI] [PubMed] [Google Scholar]
- 38.Rinaldi SF, Makieva S, Saunders PT, Rossi AG, Norman JE. Immune cell and transcriptomic analysis of the human decidua in term and preterm parturition. Mol Hum Reprod. 2017;23(10):708–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Watanabe M, Iwatani Y, Hidaka Y, Mitsuda N, Amino N. Changes in soluble CD4 and CD8 proteins in healthy pregnant and postpartum women. Am J Reprod Immunol. 1996;36(4):220–227. [DOI] [PubMed] [Google Scholar]
- 40.Sabbaj S, Hel Z, Richter HE, Mestecky J, Goepfert PA. Menstrual blood as a potential source of endometrial derived CD3+ T cells. PLoS ONE. 2011;6(12):e28894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Champion H, Innes BA, Robson SC, Lash GE, Bulmer JN. Effects of interleukin-6 on extravillous trophoblast invasion in early human pregnancy. Mol Hum Reprod. 2012;18(8):391–400. [DOI] [PubMed] [Google Scholar]
- 42.De Oliveira LG, Lash GE, Murray-Dunning C, et al. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta. 2010;31(7):595–601. [DOI] [PubMed] [Google Scholar]
- 43.Jovanovic M, Stefanoska I, Radojcic L, Vicovac L. Interleukin-8 (CXCL8) stimulates trophoblast cell migration and invasion by increasing levels of matrix metalloproteinase (MMP)2 and MMP9 and integrins alpha5 and beta1. Reproduction. 2010;139(4):789–798. [DOI] [PubMed] [Google Scholar]
- 44.Mor G, Cardenas I, Abrahams V, Guller S. Inflammation and pregnancy: the role of the immune system at the implantation site. Ann NY Acad Sci. 2011;1221:80–87. [DOI] [PMC free article] [PubMed] [Google Scholar]


