Abstract
More than 1 year has passed since social activities have been restricted due to the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). More recently, novel SARS-CoV-2 variants have been spreading around the world, and there is growing concern that they may have higher transmissibility and that the protective efficacy of vaccines may be weaker against them. Immediate measures are needed to reduce human exposure to the virus. In this study, the antiviral efficacy of deep-ultraviolet light-emitting diode (DUV-LED) irradiation (280 ± 5 nm, 3.75 mW/cm2) against three SARS-CoV-2 variants was evaluated. For the B.1.1.7, B.1.351, and P.1 variant strains, irradiation of the virus stocks for 1 s resulted in infectious titer reduction rates of 96.3%, 94.6%, and 91.9%, respectively, and with irradiation for 5 s, the rates increased to 99.9%, 99.9%, and 99.8%, respectively. We also tested the effect of pulsed DUV-LED irradiation (7.5 mW/cm2, duty rate: 50%, frequency: 1 kHz) under the same output conditions as for continuous irradiation and found that the antiviral efficacy of pulsed and continuous irradiation was the same. These findings suggest that by further developing and optimizing the DUV-LED device to increase its output, it may be possible to instantly inactivate SARS-CoV-2 with DUV-LED irradiation.
Keywords: SARS-CoV-2, variants, UV-LED, viral inactivation, COVID-19
1. Introduction
The global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has placed countries in a difficult and ever-evolving situation for over a year. More than 169 million cases of coronavirus disease (COVID-19) and 3.5 million deaths due to COVID-19 have been reported to the World Health Organization (WHO) as of 30 May 2021 [1]; these numbers represent increases of more than approximately 30- and 10-fold, respectively, when compared to the numbers last year. Although vaccinations have begun around the world, COVID-19 has not yet been completely suppressed. At the end of last year, three variants of SARS-CoV-2, that is, the United Kingdom (UK) strain (B.1.1.7) [2,3], South African strain (B.1.351) [4,5], and Brazilian strain (P.1) [6,7], were confirmed, and they have recently spread all over the world. These variants threaten society as a whole since they may have higher transmissibility [2,3,8,9,10,11,12], the protective efficacy of vaccines may be weaker against the variants [10,13,14,15,16,17], and patients infected with the variants may be more likely to develop severe medical conditions [18,19].
However, governments worldwide are attempting to balance economic activity and medical care as much as possible. Although the development of therapeutic agents and vaccines is an important strategy for bringing an end to the pandemic, it is also necessary to devise measures to reduce virus exposure to prevent the spread of infection due to droplets and droplet nuclei.
A deep-ultraviolet light-emitting diode (DUV-LED) instrument that generates around 250- to 300-nm wavelengths has been reported to effectively inactivate microorganisms, including SARS-CoV-2 [20,21,22,23,24,25]. Although the inactivating effect of DUV-LED irradiation is expected to be similar on wild-type SARS-CoV-2 and the new variants, the effect on the variants has not yet been confirmed, at least not with irradiation at a wavelength of 280 nm. UV-LED devices can emit pulsed irradiation as the radiation can be turned on and off at a high frequency. Recently, pulsed irradiation from UV-LED devices has been shown to be as effective as continuous irradiation for inactivating microorganisms [26]. As pulsed irradiation consumes less energy than continuous irradiation, it may be useful for the development of a more efficient microbe-inactivating device. In this study, we examined whether continuous and intermittent (pulsed) DUV-LED irradiation can inactivate three types of SARS-CoV-2 variants (B.1.1.7, B.1.351, and P.1).
2. Results
2.1. Inactivating Effects of Continuous Irradiation with a DUV-LED Device
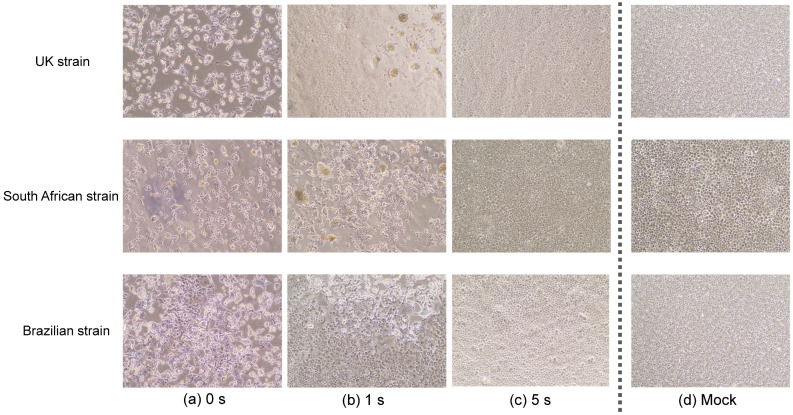
We observed a marked cytopathic effect in all cells that were infected with the UK, South African, or Brazilian strain and not irradiated with DUV-LED light (Figure 1a). The infected cells that were irradiated for 1 s showed an obvious reduction in the cytopathic effect (Figure 1b), and the morphology of the cells that were irradiated for 5 s was largely comparable to that of the mock cells (Figure 1c,d).
Figure 1.
Cytopathic changes in VeroE6/TMPRSS2 cells. Virus solutions of three types of SARS-CoV-2 variants (the UK, South African, and Brazilian strains) were treated with continuous DUV-LED irradiation for 0, 1, or 5 s, then diluted 10-fold and inoculated onto VeroE6/TMPRSS2 cells. Representative results are shown. Cytopathic changes in cells infected by virus without irradiation (a), or by virus with irradiation for 1 s (b) or 5 s (c), which corresponds to 3.75 or 18.75 mJ/cm2, respectively. (d) Mock cells.
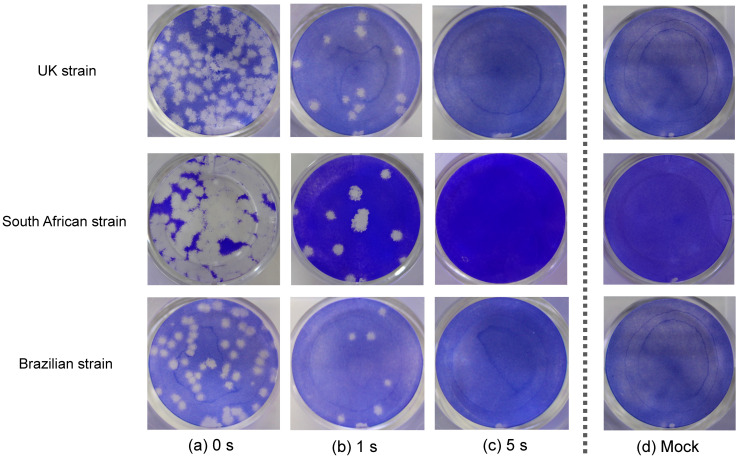
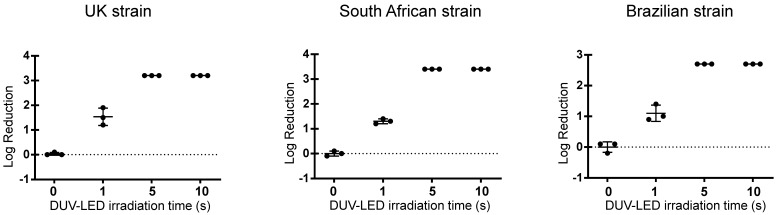
The plaque assay revealed that a short irradiation time inactivated SARS-CoV-2 variants rapidly (Figure 2). For the virus stocks of the UK, South African, and Brazilian strains, 1 s of continuous irradiation resulted in infectious titer reduction rates of 96.3%, 94.6%, and 91.9%, respectively, whereas 5 s of continuous irradiation resulted in infectious titer reduction rates of 99.9%, 99.9%, and 99.8%, respectively (Table 1 and Figure 3). These results suggested that continuous DUV-LED irradiation for a very short time can drastically inactivate SARS-CoV-2 variants.
Figure 2.
Plaque formation in VeroE6/TMPRSS2 cells. Virus solutions of three types of SARS-CoV-2 variants (the UK, South African, and Brazilian strains) were treated with continuous DUV-LED irradiation for 0, 1, or 5 s, then diluted 100-fold and inoculated onto VeroE6/TMPRSS2 cells. Representative results are shown. Plaque formation in cells infected by virus without irradiation (a), or by virus with irradiation for 1 s (b) or 5 s (c), which corresponds to 3.75 or 18.75 mJ/cm2, respectively. (d) Mock cells.
Table 1.
Differences in the infectious titer after continuous and pulsed DUV-LED irradiation for the UK, South African, and Brazilian strains irradiated with different patterns of DUV-LED light for 0, 1, 5, or 10 s.
| Control (No Irradiation) |
DUV-LED Irradiation Time (n = 3, Each) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 s | 5 s | 10 s | ||||||
| Continuous Irradiation |
Pulsed Irradiation |
Continuous Irradiation |
Pulsed Irradiation |
Continuous Irradiation |
Pulsed Irradiation |
|||
| UK strain | PFU (PFU/mL) | 3.5 × 104 | 1.3 × 103 | 1.9 × 103 | <20 | 4.0 × 101 | <20 | <20 |
| Log PFU ratio a | - | 1.5 ± 0.4 | 1.3 ± 0.1 | >3.2 | 3.0 ± 0.3 | >3.2 | >3.2 | |
| Infectious titer reduction rate b (%) | - | 96.3 | 94.4 | >99.9 | 99.9 | >99.9 | >99.9 | |
| South African strain | PFU (PFU/mL) | 5.3 × 104 | 2.9 × 103 | 3.5 × 103 | <20 | 5.3 × 101 | <20 | <20 |
| Log PFU ratio a | - | 1.3 ± 0.1 | 1.2 ± 0.1 | >3.4 | 3.1 ± 0.4 | >3.4 | >3.4 | |
| Infectious titer reduction rate b (%) | - | 94.6 | 93.4 | >99.9 | 99.9 | >99.9 | >99.9 | |
| Brazilian strain | PFU (PFU/mL) | 1.1 × 104 | 8.7 × 102 | 1.7 × 103 | <20 | <20 | <20 | <20 |
| Log PFU ratio a | - | 1.1 ± 0.3 | 0.8 ± 0.0 | >2.7 | >2.7 | >2.7 | >2.7 | |
| Infectious titer reduction rate b (%) | - | 91.9 | 84.4 | >99.8 | >99.8 | >99.8 | >99.8 | |
a log10 (Nt/N0) where Nt is the PFU count of the UV-irradiated sample and N0 is the PFU count of the sample without UV irradiation. Data are shown as mean ± SD. b (1 − 1/10log PFU ratio) × 100 (%).
Figure 3.
Log reduction in the infectious titer by continuous DUV-LED irradiation (current: 0.35 A) for the UK, South African, and Brazilian strains. Time-dependent inactivation of SARS-CoV-2 by irradiation. The results shown are the means and standard deviations of triplicate measurements.
2.2. Inactivating Effects of Pulsed Irradiation with a DUV-LED Device
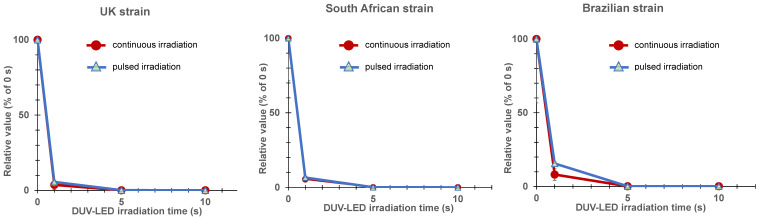
For the virus stocks of the UK, South African, and Brazilian strains, 1 s of pulsed irradiation resulted in infectious titer reduction rates of 94.4%, 93.4%, and 84.4%, respectively, whereas 5 s of pulsed irradiation resulted in infectious titer reduction rates of 99.9%, 99.9%, and 99.8%, respectively (Table 1 and Figure 4). These results were almost the same as those obtained with continuous irradiation.
Figure 4.
Relative value (percentage of the virus titer of the negative control sample irradiated for 0 s) of the infectious titer after continuous (current: 0.35 A) or pulsed (current: 0.7 A; duty ratio: 50%; frequency: 1 kHz) DUV-LED irradiation for the UK, South African, and Brazilian strains. The inactivating effects were almost the same between the continuous and pulsed irradiation.
3. Discussion
The present study demonstrated for the first time that DUV-LED irradiation can rapidly inactivate three types of SARS-CoV-2 variants; that is, the ones that were first described in the UK, South Africa, and Brazil [2,3,4,5,6,7,9,10,27]. Additionally, continuous and pulsed DUV-LED irradiation showed similar degrees of rapid virus inactivation.
UV-LED devices that can provide irradiation at various peak emission wavelengths, such as UV-A (320–400 nm), UV-B (280–320 nm), and UV-C (100–280 nm), have been adopted to inactivate various pathogenic species, including bacteria, viruses, and fungi. UV-C is considered to be the most effective germicidal region of the UV spectrum as it causes the formation of photoproducts in DNA and RNA [28]. These pyrimidine dimers interrupt the transcription, translation, and replication of DNA and RNA, and eventually lead to the death of the microorganism [29]. Last year, we reported for the first time that irradiation with DUV-LED at a wavelength of 280 ± 5 nm rapidly inactivated wild-type SARS-CoV-2 that was obtained from a COVID-19 patient [24]. The effect of DUV-LED irradiation on the wild-type SARS-CoV-2 (infectious titer reduction rates of 87.4% and 99.9% with 1 s and 10 s of irradiation, respectively) was similar to that on the SARS-CoV-2 variants in the present study. Since UV irradiation targets the genomic RNA of SARS-CoV-2 by inducing RNA degradation, the inactivating effects of DUV-LED irradiation on the UK, South African, and Brazilian variants were as expected, and similar effects are also expected for other variants that may emerge in the future. In addition, non-enveloped viruses that are highly resistant to disinfectants, including norovirus, are also expected to be susceptible to DUV-LED irradiation (unpublished observations).
In this study, we also tested the effect of pulsed irradiation with a DUV-LED device. As shown in Table 1 and Figure 4, the degree of virus inactivation by continuous and intermittent irradiation was comparable when the device outputs were the same (power X radiation time). This suggested that SARS-CoV-2 may be instantly inactivated by DUV-LED irradiation if the DUV-LED device is further developed and optimized to increase its output [26].
Despite the significant inactivating effects of DUV-LED reported here, this study has some limitations. First, these effects may be limited to the test conditions applied in this study, including the irradiation distance and output (working distance of 20 mm; irradiation at 3.75 mW/cm2 for continuous irradiation and 7.5 mW/cm2 for pulsed irradiation (duty rate: 50%; frequency: 1 kHz)). The irradiation distance was set to 20 mm, because it was assumed that in practical settings, the irradiation would be performed in a contained device to prevent human exposure. In addition, it is necessary to also evaluate multiple parameters, such as the frequency and duty ratio, to clarify the effectiveness of pulsed irradiation. Additionally, the influence of the material and the power consumption for the high amplitude were not evaluated. In the future, we will examine in more detail whether various conditions of DUV-LED irradiation may affect the degree of the inactivation of microorganisms.
In addition to community settings, healthcare settings are also vulnerable to the invasion and spread of SARS-CoV-2 and its variants. The stability of SARS-CoV-2 in aerosols and on surfaces [30] likely contributes to the transmission of the virus in medical environments. It is important to create an environment that minimizes virus exposure to suppress the spread of SARS-CoV-2 in a sustainable and efficient manner. It was confirmed in our study that SARS-CoV-2, including its variants, is highly susceptible to DUV-LED irradiation. By devising appropriate and optimized irradiation methods, it is conceivable that DUV-LED irradiation can be adapted and applied in various settings. This study provides useful baseline data for securing a safer community and medical environment. The development of devices equipped with DUV-LED is expected to prevent virus spread through the air and from contaminated surfaces.
4. Materials and Methods
4.1. Materials
1. Cells: VeroE6/TMPRSS2 cells were obtained from the Japanese Collection of Research Bioresources (JCRB) Cell Bank in Japan (https://cellbank.nibiohn.go.jp/english/; JCRB no. JCRB1819. Accessed on 31 May 2021). The cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 10% fetal bovine serum (FBS), penicillin/streptomycin, and 1 mg/mL G418 (Thermo Fisher Scientific, Tokyo, Japan).
2. Virus stocks: Three types of SARS-CoV-2 variants, that is, the variants that were first described in the UK (hCoV-19/Japan/QHN001/2020 (B.1.1.7)), South Africa (hCoV-19/Japan/TY8-612/2021 (B.1.351)), and Brazil (hCoV-19/Japan/TY7-501/2020 (P.1)), were obtained from the National Institute of Infectious Diseases of Japan. These viruses were propagated in VeroE6/TMPRSS2 cells cultured in DMEM containing 10% FBS and penicillin/streptomycin. At 48 h or 72 h after infection, virus stocks were collected by centrifuging the culture supernatants at 3000 rpm for 10 min. Clarified supernatants were kept at −80 °C until use.
3. DUV-LED: The DUV-LED apparatus, which generates a narrow-range wavelength (280 ± 5 nm), was obtained from Nikkiso Co. Ltd. (Tokyo, Japan). This wavelength was selected in consideration of its practicality due to the high output (radiation) power and increased durability of the LED device.
In addition to conventional continuous irradiation, this DUV-LED instrument enables pulsed irradiation as the radiation can be turned on and off at a high frequency. We used a signal generator (AIMEX Corporation, Tokyo, Japan) to irradiate DUV-LED light.
For the evaluation of DUV-LED inactivation of the target virus, aliquots of virus stock (150 μL) adjusted to 5.0 × 104 PFU/mL were placed in the center of a 60-mm Petri dish and irradiated with 3.75 mW/cm2 of continuous irradiation or with 7.5 mW/cm2 of pulsed irradiation (duty rate: 50%; frequency: 1 kHz) at a working distance of 20 mm for various times (1, 5, or 10 s; n = 3 each). We set the duty rate and frequency of the pulsed irradiation to match the output per time of the continuous irradiation (Supplementary Materials).
4.2. Methods
The antiviral efficacy of DUV-LED irradiation against the SARS-CoV-2 variants was evaluated. After DUV-LED irradiation, approximately 120 μL of each virus solution (adjusted to 5.0 × 104 PFU/mL) was collected with a 200-μL tip. Virus solutions were serially diluted in 10-fold steps in serum-free DMEM in a 1.5 mL tube, then inoculated onto VeroE6/TMPRSS2 monolayers in a 12-well plate. After the adsorption of virus for 2 h, cells were overlaid with MEM containing 1% carboxymethyl cellulose and 2% FBS (final concentration). The cells were incubated for 72 h in a CO2 incubator, then observed under a microscope for cytopathic effects. A non-irradiated virus suspension was used as a negative control. To calculate the PFU, cells were fixed with 10% formalin for 30 min, and stained with a 2% crystal violet solution.
The antiviral effects of DUV-LED irradiation were assessed using the logPFU ratio calculated as log10 (Nt/N0), where Nt is the PFU count of the UV-irradiated sample, and N0 is the PFU count of the sample without UV irradiation. In addition, the infectious titer reduction rate was calculated as (1 − 1/10 log PFU ratio) × 100 (%). All experiments were performed in a biosafety level 3 laboratory.
Acknowledgments
We wish to thank the National Institute of Infectious Diseases, Japan for providing the hCoV-19/Japan/TY7-501/2020, hCoV-19/Japan/TY8-612/2021, and hCoV-19/Japan/QHN001/2020 strains.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10060754/s1. Scheme S1: Output diagrams of two patterns of DUV-LED irradiation. (A) Continuous irradiation with a current of 0.35 A for 1, 5, or 10 s. (B) Intermittent (pulsed) irradiation with a current of 0.7 A for 1, 5, or 10 s (frequency: 1 kHz; duty rate: 50%). The output per time was set to be the same between continuous irradiation (A: 3.75 mW/cm2 × time) and intermittent irradiation (B: 7.5 mW/cm2 × time).
Author Contributions
H.I., H.S. and A.S. conceived the study and wrote the manuscript. A.S., C.K. and T.O. conducted the experiments dealing with the viruses. S.F. contributed to the study design, study supervision, and manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported in part by the Japan Agency for Medical Research and Development Research Program on Emerging and Re-emerging Infectious Diseases (20fk0108163 and 20fk0108518 to A.S.); Japan Agency for Medical Research and Development Japan Program for Infectious Diseases Research and Infrastructure (20wm0325009 and 21wm0325009 to A.S.); Japan Society for the Promotion of Science (JSPS) KAKENHI Grant-in-Aid for Scientific Research (B) (21H02361 to T.O. and A.S.); JSPS KAKENHI Grant-in-Aid for Scientific Research (C) (19K06382 to A.S.); JSPS KAKENHI Grant-in-Aid for Early-Career Scientists (19K15984 to C.K.); and a Grant for Joint Research Projects of the Research Institute for Microbial Diseases, Osaka University (to T.O. and A.S.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
H.S. receives part of his salary from Nikkiso Co., Ltd., Tokyo, Japan. Nikkiso Co., Ltd. supplied the deep-ultraviolet light-emitting diode instrument for evaluation. Nikkiso Co., Ltd. had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The other authors declare no conflicts of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. [(accessed on 31 May 2021)]; Available online: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20210601_weekly_epi_update_42.pdf?sfvrsn=5b0bbc7c_5.
- 2.Wise J. Covid-19: New coronavirus variant is identified in UK. BMJ. 2020;371:m4857. doi: 10.1136/bmj.m4857. [DOI] [PubMed] [Google Scholar]
- 3.Volz E., Mishra S., Chand M., Barrett J.C., Johnson R., Geidelberg L., Hinsley W.R., Laydon D.J., Dabrera G., O’Toole Á., et al. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021;593:266–269. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 4. [(accessed on 31 May 2021)]; Available online: https://sacoronavirus.co.za/2020/12/18/new-covid-19-variant-identified-in-sa/
- 5.Tegally H., Wilkinson E., Giovanetti M., Iranzadeh A., Fonseca V., Giandhari J., Doolabh D., Pillay S., San E.J., Msomi N., et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Africa. medRxiv. 2020 doi: 10.1101/2020.12.21.20248640. [DOI] [Google Scholar]
- 6.Resende P.C., Bezerra J.F., Vasconcelos R.H.T., Arantes I., Appolinario L., Mendonça A.C., Paixao A.C., Duarte A.C., Silva T., Rocha A.S., et al. Severe acute respiratory syndrome coronavirus 2 P.2 lineage associated with reinfection case, Brazil, June-October 2020. Emerg. Infect. Dis. 2021;27 doi: 10.3201/eid2707.210401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria N.R., Mellan T.A., Whittaker C., Claro I.M., Candido D.D.S., Mishra S., Crispim M.A.E., Sales F.C., Hawryluk I., McCrone J.T., et al. Genomics and epidemiology of a novel SARS-CoV-2 lineage in Manaus, Brazil. medRxiv. 2021 doi: 10.1101/2021.02.26.21252554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies N.G., Abbott S., Barnard R.C., Jarvis C.I., Kucharski A.J., Munday J.D., Pearson C.A.B., Russell T.W., Tully D.C., Washburne A.D., et al. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021;372:eabg3055. doi: 10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung K., Shum M.H.H., Leung G.M., Lam T.T.Y., Wu J.T. Early transmissibility assessment of the N501Y mutant strains of SARS-CoV-2 in the United Kingdom, October to November 2020. Eurosurveillance. 2021;26:2002106. doi: 10.2807/1560-7917.ES.2020.26.1.2002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plante J.A., Mitchell B.M., Plante K.S., Debbink K., Weaver S.C., Menachery V.D. The variant gambit: COVID-19’s next move. Cell Host Microbe. 2021;29:508–515. doi: 10.1016/j.chom.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kissler S.M., Fauver J.R., Mack C., Tail C.G., Breban M.I., Watkins A.E., Samant R.M., Anderson D.J., Ho D.D., Grubaugh N.D., et al. Densely sampled viral trajectories suggest longer duration of acute infection with B. 1.1. 7 variant relative to non-B. 1.1. 7 SARS-CoV-2. medRxiv. 2021 doi: 10.1101/2021.02.16.21251535. [DOI] [Google Scholar]
- 12.Korber B., Fischer W.M., Gnanakaran S., Yoon H., Theiler J., Abfalterer W., Hengartner N., Giorgi E.E., Bhattacharya T., Foley B., et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell. 2020;182:812–827. doi: 10.1016/j.cell.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madhi S.A., Baillie V., Cutland C.L., Voysey M., Koen A.L., Fairlie L., Padayachee S.D., Dheda K., Barnabas S.L., Bhorat Q.E., et al. Efficacy of the ChAdOx1 nCoV-19 Covid-19 Vaccine against the B.1.351 Variant. N. Engl. J. Med. 2021;384:1885–1898. doi: 10.1056/NEJMoa2102214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Z., VanBlargan L.A., Bloyet L.-M., Rothlauf P.W., Chen R.E., Stumpf S., Zhao H., Errico J.M., Theel E.S., Liebeskind M.J., et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29:477–488. doi: 10.1016/j.chom.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang P., Casner R.G., Nair M.S., Wang M., Yu J., Cerutti G., Liu L., Kwong P.D., Huang Y., Shapiro L., et al. Increased resistance of SARS-CoV-2 variant P.1 to antibody neutralization. Cell Host Microbe. 2021;29:747–751. doi: 10.1016/j.chom.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weisblum Y., Schmidt F., Zhang F., DaSilva J., Poston D., Lorenzi J.C.C., Muecksch F., Rutkowska M., Hoffmann H.-H., Michailidis E., et al. Escape from neutralizing antibodies by SARS-CoV-2 spike protein variants. eLife. 2020;9:e61312. doi: 10.7554/eLife.61312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacisuleyman E., Hale C., Saito Y., Blachere N.E., Bergh M., Conlon E.G., Schaefer-Babajew D.J., DaSilva J., Muecksch F., Gaebler C., et al. Vaccine Breakthrough Infections with SARS-CoV-2 Variants. N. Engl. J. Med. 2021;384:2212–2218. doi: 10.1056/NEJMoa2105000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davies N.G., Jarvis C.I., CMMID COVID-19 Working Group. Edmunds W.J., Jewell N.P., Diaz-Ordaz K., Keogh R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Challen R., Brooks-Pollock E., Read J.M., Dyson L., Tsaneva-Atanasova K., Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: Matched cohort study. BMJ. 2021;372:n579. doi: 10.1136/bmj.n579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rattanakul S., Oguma K. Inactivation kinetics and efficiencies of UV-LEDs against Pseudomonas aeruginosa, Legionella pneumophila, and surrogate microorganisms. Water Res. 2018;130:31–37. doi: 10.1016/j.watres.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 21.Nishisaka-Nonaka R., Mawatari K., Yamamoto T., Kojima M., Shimohata T., Uebanso T., Nakahashi M., Emoto T., Akutagawa M., Kinouchi Y., et al. Irradiation by ultraviolet light-emitting diodes inactivates influenza a viruses by inhibiting replication and transcription of viral RNA in host cells. J. Photochem. Photobiol. B. 2018;189:193–200. doi: 10.1016/j.jphotobiol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Kim D.-K., Kang D.-H. UVC LED irradiation effectively inactivates aerosolized viruses, bacteria, and fungi in a chamber-type air disinfection system. Appl. Environ. Microbiol. 2018;84:e00944-18. doi: 10.1128/AEM.00944-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng Y., Chen H., Basurto L.A.S., Protasenko V.V., Bharadwaj S., Islam M., Moraru C.I. Inactivation of Listeria and E. coli by Deep-UV LED: Effect of substrate conditions on inactivation kinetics. Sci Rep. 2020;10:3411. doi: 10.1038/s41598-020-60459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Inagaki H., Saito A., Sugiyama H., Okabayashi T., Fujimoto S. Rapid inactivation of SARS-CoV-2 with deep-UV LED irradiation. Emerg. Microbes Infect. 2020;9:1744–1747. doi: 10.1080/22221751.2020.1796529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heßling M., Hönes K., Vatter P., Lingenfelder C. Ultraviolet irradiation doses for coronavirus inactivation–review and analysis of coronavirus photoinactivation studies. GMS Hyg. Infect. Control. 2020;15:Doc08. doi: 10.3205/dgkh000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Song K., Taghipour F., Mohseni M. Microorganisms inactivation by continuous and pulsed irradiation of ultraviolet light-emitting diodes (UV-LEDs) Chem. Eng. J. 2018;343:362–370. doi: 10.1016/j.cej.2018.03.020. [DOI] [Google Scholar]
- 27.European Centre for Disease Prevention and Control Risk Assessment: Risk Related to Spread of New SARS- CoV-2 Variants of Concern in the EU/EEA. 29 December 2020. [(accessed on 31 May 2021)]; Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-risk-assessment-spread-new-sars-cov-2-variants-eueea.
- 28.Bintsis T., Litopoulou-Tzanetaki E., Robinson R.K. Existing and potential applications of ultraviolet light in the food industry —A critical review. J. Sci. Food Agric. 2000;80:637–645. doi: 10.1002/(SICI)1097-0010(20000501)80:6<637::AID-JSFA603>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 29.Kim S.-J., Kim D.-K., Kang D.-H. Using UVC light-emitting diodes at wavelengths of 266 to 279 nanometers to inactivate foodborne pathogens and pasteurize sliced cheese. Appl. Environ. Microbiol. 2016;82:11–17. doi: 10.1128/AEM.02092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.