Abstract
Recently in Korea, where triple therapy is accepted as the first-line Helicobacter pylori (H. pylori) eradication treatment, antibiotic resistance to clarithromycin has increased considerably, resulting in eradication rates of less than 80%. We investigated the efficacy of tailored therapy after a clarithromycin resistance test compared with empirical therapy for H. pylori eradication. The cost-effectiveness of H. pylori eradication success was evaluated according to the average medical cost per patient. A total of 364 patients were enrolled in the study. The first-line H. pylori eradication rate was significantly higher in patients who received tailored therapy than in those who received empirical therapy. The total medical costs for the tailored and empirical groups were 46,374 Won and 53,528 Won. The total treatment period for each ultimately successful eradication in the tailored group was 79.8 ± 2.8 days, which is shorter than that of the empirical group (99.2 ± 7.4 days). The rate of eradication-related adverse events for the tailored group and empirical group was 12.9% and 14.8%, respectively. Tailored therapy could be a useful option to achieve a higher successful eradication rate, shorter treatment periods, and lower medical costs than empirical therapy in the era of increasing antibiotic resistance.
Keywords: Helicobacter pylori, eradication, antibiotic resistance, tailored, empirical
1. Introduction
Helicobacter pylori (H. pylori) is associated with peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric cancer [1,2,3], and Korean guidelines recommend triple therapy as the first-line eradication treatment for H. pylori infection [4]. However, due to the increase of antibiotic-resistant strains, the eradication rate has gradually decreased [5,6], and several clinical studies have reported that the eradication rate of triple therapy is less than 80% [7,8,9]. The causes of this overall decrease in eradication rate include antibiotic resistance, age, smoking status, the difference in host immunity, underlying disease, and poor drug compliance [10,11], but antibiotic resistance is known as the most important factor among them [12].
Kuo et al. recently reported on patients with refractory H. pylori infection in Taiwan. Dual resistance to both clarithromycin and levofloxacin was found in 73.2%. This study highlighted ways to decide the optimum H. pylori eradication strategy according to the results of antibacterial susceptibility analysis [13]. In Italy, current guidelines recommend 10-day bismuth-based or sequential and concomitant regimens for first-line H. pylori eradication. Bismuth-based and bismuth-free therapies are equally effective for first-line H. pylori eradication [14]. Therefore, in areas with high clarithromycin resistance, opinions have arisen that the eradication treatment should be improved by adding more antibiotics instead of the triple therapy as the primary treatment, or changing to a new antibiotic of another class [15].
An understanding of the mechanism of H. pylori resistance is extremely complex. The H. pylori virulence factors are involved in the induction of inflammatory responses, and control and regulate those responses, maintaining chronic inflammation [16]. The H. pylori exhibit an expanded complex of mechanisms that alter host cellular responses and signaling pathways. H. pylori elicit numerous adaptive mechanisms that enable effective bacterial adherence, colonization, and cellular alterations that provide the induction of further premalignant changes in the gastric microenvironment [17].
In the Maastricht V/Florence guidelines, bismuth-containing quadruple therapy or concomitant therapy is recommended in areas where the clarithromycin resistance rate is higher than 15% [18]. Recently, the clarithromycin resistance rate in Korea has been about 30% [19]. Based on the aforementioned guidelines, it is necessary to establish a treatment plan based on the results of antibiotic susceptibility tests rather than maintaining the existing standard triple therapy that is based on clarithromycin as the primary treatment. Resistance to clarithromycin is mostly caused by a point mutation at position 2142 or 2143 of the 23S ribosomal RNA gene [20], and antibiotic resistance can also be predicted by using a dual-priming oligonucleotide (DPO)-based multiplex polymerase chain reaction (PCR) test [21]. This method is an excellent test that shows a relatively high sensitivity of 82% to 90% and a specificity of 95% or more. Moreover, it has the advantage of being able to confirm the resistance to clarithromycin in addition to other antibiotics [22]. Therefore, it is possible to check the individual’s resistance to antibiotics and then perform tailored therapy for H. pylori.
Tailored therapy based on the DPO-PCR test could be a useful regimen to increase the eradication rate of H. pylori infection. However, the cost-effectiveness of this test has not yet been definitively identified. The DPO-based multiplex PCR is more expensive than the Giemsa stain and rapid urease tests. It is difficult to perform this test routinely in clinical practice before the eradication of H. pylori. Therefore, we aimed to evaluate the eradication rate, adverse drug events, and cost-effectiveness of empirical and tailored therapies for the treatment of H. pylori infection.
2. Materials and Methods
2.1. Study Design and Subjects
This retrospective study was performed at two university hospitals in South Korea (Ewha Womans University Mokdong Hospital and Ewha Womans University Seoul Hospital) from January 2017 to December 2019. We included 435 subjects who met the following criteria: (1) the presence of H. pylori was confirmed by rapid urease test, histology such as Giemsa stain, urea breath test (UBT), or DPO-PCR test; (2) patients receiving empirical therapy or tailored therapy based on the DPO-PCR results. Subjects (n = 71) were excluded in the following conditions: (1) patients aged >80 years (n = 13); (2) history of gastrectomy (n = 18); (3) severe systemic illness, such as severe cardiopulmonary dysfunction, liver cirrhosis, or renal failure (n = 17); (4) history of any allergic reaction to antibiotics (n = 8); (5) loss to follow-up (n = 15).
A total of 364 patients who received H. pylori eradication treatment (n = 121 in Ewha Womans University Mokdong Hospital and n = 243 in Ewha Womans University Seoul Hospital) were enrolled in this study. Data for January 2017 and January 2018 were collected through chart review in Ewha Womans University Mokdong Hospital, and data for February 2018 and December 2019 were collected using data extracted from the Clinical Data Warehouse of the Ewha Womans University Seoul Hospital. This study was approved by the Institutional Review Board (IRB approval number: 2020-09-013).
2.2. H. Pylori Diagnosis and DPO-Based Multiplex PCR
Infection with H. pylori was regarded as positive when at least one positive result was obtained in UBT (Otsuka®, Tokyo, Japan), rapid urease test (CLOtest®; Delta West, Bentley, Australia), or histologic assessment (Giemsa staining) conducted using gastric biopsy specimens from the antrum and greater curvature of the body. In the tailored therapy group, DNA was extracted from frozen gastric biopsy specimens to detect clarithromycin-resistant H. pylori mutants. DPO-based multiplex PCR (Seeplex® H. pylori-ClaR ACE Detection; Seegene, Inc., Seoul, Korea) was performed. Point mutations were identified by PCR amplification of a portion of the 23S ribosomal RNA gene. The amplified DNA products were visualized on a UV transilluminator after electrophoresis on a 2% agarose gel. The amplified DNA products were determined to have point mutations.
2.3. H. Pylori Eradication Therapy Regimen
There were a total of three regimens for empirical therapy, which depended on the doctor’s preference: (1) triple therapy (proton-pump inhibitor bid, amoxicillin 1 g bid, clarithromycin 500 mg bid) for 14 days; (2) sequential therapy (proton-pump inhibitor bid and amoxicillin 1 g for 5 days followed by proton-pump inhibitor bid, clarithromycin 500 mg, and metronidazole 500 mg for 5 days) for 10 days; (3) bismuth-containing quadruple therapy (proton-pump inhibitor bid, bismuth subcitrate 300 mg qid, metronidazole 500 mg tid, tetracycline 500 mg qid) for 14 days. In the tailored therapy group, patients received the eradication regimen based on the results of the DPO-PCR test. Six weeks after the end of the treatment, compliance with therapy and side effects were assessed through personal interviews. An adverse event was defined as an unscheduled early visit to the outpatient clinic with symptoms during eradication or when the H. pylori eradication treatment was stopped due to adverse drug effects. UBT, rapid urease test, or Giemsa staining of gastric biopsy specimens were performed to confirm successful H. pylori eradication.
2.4. Medical Cost
The total medical cost per patient was assessed as the sum of the diagnostic and regimen costs. The cost of the DPO-PCR test was 38,350 Won, while those of the Giemsa stain and rapid urease test were 11,427 Won and 10,504 Won, respectively. The cost of 14-day triple therapy was calculated to be 34,300 Won. The cost of 10 day sequential therapy was 41,800 Won and that of the 14-day quadruple therapy was 25,746 Won.
2.5. Statistical Analysis
Continuous variables were presented as mean ± standard deviation, and categorical variables were presented as the number of subjects and percentage. Group comparisons were performed using independent samples t-tests or Mann–Whitney U tests for continuous variables and Pearson’s chi-squared tests or Fisher’s exact tests for categorical variables. Categorical variables are presented as numbers and proportions. All statistical analyses were 2-sided, and results were considered statistically significant at p < 0.05. The Statistical Package for the Social Science (SPSS) software (version 21.0; SPSS Inc., Chicago, IL, U.S.) was used for statistical analysis.
3. Results
3.1. Baseline Characteristics of Study Subjects
During the study period, 435 patients received H. pylori eradication treatment. After excluding 71 patients, 364 patients were finally enrolled in the study. Empirical therapy was given to 155 patients, and 209 patients received tailored therapy. There were older and more current smokers in the tailored therapy group. The most common cause of need for H. pylori eradication was H. pylori associated gastritis in both groups (Table 1).
Table 1.
Baseline characteristics of empirical and tailored therapy groups.
| Total (n = 364) |
Empirical Therapy (n = 155) |
Tailored Therapy (n = 209) |
p Value | |
|---|---|---|---|---|
| Age (years), mean ± SD | 56.0 ± 12.5 | 54.8 ± 11.7 | 56.9 ± 13.0 | 0.117 |
| Sex, n (%) | ||||
| Male | 197 (54.1) | 79 (51.0) | 118 (56.5) | 0.298 |
| Female | 167 (45.9) | 76 (49.0) | 91 (43.5) | |
| Smoking, n (%) | 108 (29.7) | 37 (23.9) | 71 (34.0) | 0.037 |
| Alcohol drinking, n (%) | 110 (30.2) | 56 (36.1) | 54 (25.8) | 0.034 |
| Disease for H. pylori eradication, n (%) | <0.001 | |||
| Peptic ulcer | 109 (29.9) | 39 (25.2) | 70 (33.5) | |
| Post-ESD for EGC | 24 (6.6) | 5 (3.2) | 19 (9.1) | |
| Post-ER for adenoma | 35 (9.6) | 11 (7.1) | 24 (11.5) | |
| MALT Lymphoma | 4 (1.1) | 2 (1.3) | 2 (1.0) | |
| H. pylori gastritis | 175 (48.1) | 93 (60.0) | 82 (39.2) | |
| Lymphoid follicular gastritis | 13 (3.6) | 4 (2.6) | 9 (4.3) | |
| Family history of gastric cancer | 4 (1.1) | 1 (0.6) | 3 (1.5) |
Abbreviations: SD, standard deviation; ESD, endoscopic submucosal dissection; EGC, early gastric cancer; ER, endoscopic resection; MALT lymphoma, mucosa-associated lymphoid tissue lymphoma.
3.2. H. pylori Eradication Rate of Study Subjects
Of 155 patients in the empirical therapy group, 111 (71.6%) received sequential therapy as the first-line H. pylori eradication regimen. Of 209 patients in the tailored therapy group, 133 (66.5%) received 7-day triple therapy as the first-line H. pylori eradication regimen. As for the eradication rate according to regimen, the 14-day triple therapy was 81.5%, bismuth-containing quadruple therapy was 88.2%, and sequential therapy was 82.0% in the empirical therapy group. In the tailored therapy group, the eradication rate for 7-day triple therapy was 89.2%, bismuth-containing quadruple therapy was 91.7%, and sequential therapy was 80.0%. The eradication rate was significantly higher in those who received tailored therapy than in those who received empirical therapy (82.6% vs. 91.2%; p = 0.023) (Table 2).
Table 2.
H. pylori eradication rate of study subjects.
| Empirical Therapy (n = 155) |
Tailored Therapy (n = 209) |
p Value | ||
|---|---|---|---|---|
| 1st line eradication regimen, n (%) | Triple | 27 (17.4) | 139 (66.5) | - |
| Quadruple | 17 (11.0) | 60 (28.7) | - | |
| Sequential | 111 (71.6) | 10 (4.8) | - | |
| 1st line eradication rate according to regimen, n (%) | Triple | 22/27 (81.5) | 124/139 (89.2) | 0.328 |
| Quadruple | 15/17 (88.2) | 55/60 (91.7) | 0.646 | |
| Sequential | 91/111 (82.0) | 8/10 (80.0) | 1.000 | |
| Outcome of H. pylori 1st line eradication, % (n/N) | ||||
| Eradication rate in analysis | 82.6 (128/155) | 91.7 (187/204) | 0.023 | |
3.3. Medical Cost of Study Subjects
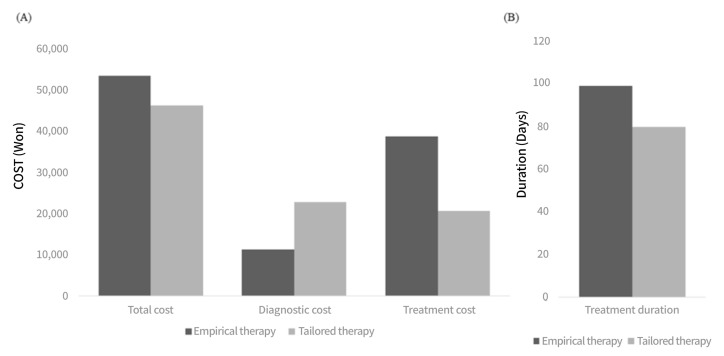
The total medical costs per tailored group were 46,374 Won and those for the empirical group were 53,528 Won (p < 0.001). The cost of the diagnostic method was higher in the tailored therapy group (22,914 Won vs. 11,282 Won; p < 0.001). The cost of the eradication regimen was higher in the empirical group (38,746 Won vs. 20,704 Won; p < 0.001). The total treatment duration for each ultimately successful eradication in the tailored therapy group was 79.8 ± 2.8 days, which is significantly shorter than that of the empirical group’s 99.2 ± 7.4 days (p = 0.013). (Figure 1)
Figure 1.
The total medical cost and treatment duration in patients on tailored therapy vs. empirical therapy (A) The total medical cost consisting of diagnostic and treatment cost in patients treated with tailored and empirical therapy. (B) The duration of treatment for each ultimately successful eradication in patients treated with tailored and empirical therapy.
3.4. Adverse Effects Seen in Study Subjects
Adverse drug events were more common in the empirical therapy group than in the tailored therapy group (14.8% vs. 12.9%, p = 0.028). The most common adverse event was nausea or vomiting in both groups (26.1% vs. 29.7%) (Table 3). Five patients in the empirical and three patients in the tailored therapy group discontinued H. pylori eradication treatment due to side effects. After H. pylori eradication failure, there were 6 patients (3.9%) in the empirical therapy group who did not want the next eradication treatment, but none of the patients in the tailored therapy group denied further treatment.
Table 3.
H. pylori eradication related adverse effects of study subjects.
| Empirical Therapy | Tailored Therapy | p Value | |
|---|---|---|---|
| Adverse event for eradication treatment, n (%) | 23 (14.8) | 27 (12.9) | 0.028 |
| Abdominal pain | 5 (21.8) | 2 (7.4) | |
| Nausea/Vomiting | 6 (26.1) | 8 (29.7) | |
| Headache | 4 (17.4) | 2 (7.4) | |
| Diarrhea | 5 (21.7) | 4 (14.8) | |
| Dyspepsia | 0 (0.0) | 7 (25.9) | |
| Metallic taste | 3 (13.0) | 4 (14.8) | |
| No further treatment after eradication fail, n (%) | 6 (3.9) | 0 (0.0) | NA |
4. Discussion
In this study, the eradication rate of H. pylori was significantly higher in those who received tailored therapy as their first-line treatment in comparison to those who received empirical therapy. The H. pylori eradication regimen is more cost-effective in tailored therapy than empirical therapy. The total treatment periods for ultimately successful eradication were shorter in the tailored therapy group, and fewer eradication-related adverse drug events were observed compared to the empirical therapy group.
The first-line H. pylori eradication rate was significantly higher with tailored therapy than with empirical therapy. Recently, as the clarithromycin resistance rate of H. pylori has increased, the eradication rate of the existing triple therapy for seven days has decreased. To overcome this situation, prolonged treatment periods, various regimens such as quadruple therapy with bismuth, sequential and concurrent therapy, or tailored therapy may be used [8,23,24,25]. It is important to succeed as a first-line eradication treatment because the H. pylori eradication regimen requires a large amount of medicine, including two antibiotics to be taken. In particular, if you are already taking other drugs due to a comorbid disease, the H. pylori eradication treatment might a great burden. Moreover, antibiotic resistance is the leading cause of failure of the H. pylori eradication treatment, and we should try to reduce antibiotic resistance. Empirical quadruple therapy with bismuth as the first-line therapy raises public health concerns regarding increasing resistance to the constituent antibiotics. Finally, in tailored therapy, the risk of misuse of the antibiotics is lower due to reduced H. pylori eradication retreatment.
The H. pylori eradication regimen was more cost-effective in the tailored therapy than in the empirical therapy in this study. Different studies that have evaluated H. pylori tailored therapy have achieved contradictory results. Liou et al. in Taiwan found that 6920 USD would be required to additionally cure one patient using the genotype resistance guide therapy, compared to empirical therapy, which is clearly not a cost-effective option [26]. Chang et al. in South Korea evaluated the cost-effectiveness of tailored therapy, and compared the results of standard triple therapy with those of empirical bismuth quadruple therapy. Total per capita medical costs were 503.50 USD in the tailored group and 406.50 USD in the empirical group [27]. However, Cosme et al. reported that in Spain, the culture-based approach was more cost-effective than standard first-line therapy given empirically [28]. Gweon et al. showed that the cost for a successful eradication using DPO-based PCR would be similar or superior to the expected cost of a successful eradication with a 14-day empirical treatment when the first-line eradication rate is ≤80% [29]. Since H. pylori antibiotic resistance varies among different geographic areas, the cost-effectiveness may vary according to the cost of care in a given country, and therefore the same conclusion may not be applicable to other healthcare systems [30].
A novel view of H. pylori infections is emerging in microbiological point. The changes of gut microbiota are greatly implicated in the pathogenicity of H. pylori Infections. The antimicrobial peptides (AMPs) have great importance in the innate immune reactions to H. pylori and participate in conservative co-evolution with an intricate microbiome. During H. pylori infections, AMP expression is able to eradicate the bacteria, thereby preventing H. pylori infections in the gastrointestinal tract [31]. The β-Defensins which belong to the AMP group expression, are enhanced during H. pylori infection [32].
In chronic inflammation induced by H. pylori infection, COX-2 is modulated by DNA methylation. The DNA methylation changes at the COX-2 promoter are associated with transcriptional activation and precede histone modifications in gastric cells exposed to H. pylori [33]. Woo et al. reported that the genome-wide methylation profiles associated with H. pylori infection. The gastric cancer is regulated by methylation mechanism rather than genetic linkage, and H. pylori leads DNA methylation [34]. Therefore, methylation-based biomarkers could be used for monitoring the prognosis of treatment, drug response, and recurrence in gastric cancer.
The prevalence of 23S rRNA point mutations was 28.7% in the study population. A total of 139 patients (66.5%) who received 7-day triple therapy were included in the tailored therapy group. This regimen might decrease drug-related adverse events and result in shorter treatment duration and minimal antibiotic overuse. Better compliance and fewer adverse effects were observed in patients in the tailored therapy group, similar to those reported in a previous study [35,36]. Choi et al. showed that the rate of eradication–related side effects for tailored regimens was 12.0%, which differed significantly from that of empirical bismuth quadruple therapy for first-line H. pylori treatment [36]. Therefore, tailored therapy with DPO-based multiplex PCR for H. pylori eradication may be superior in quality, with fewer adverse events, compared to empirical therapy in Korea, where clarithromycin resistance is high.
There are several limitations in this study. First, patients with a negative H. pylori infection were not enrolled in this study, and the total medical cost would be underestimated. Second, as this study was conducted retrospectively, there were limitations in obtaining detailed medical information that could have an influence on eradication failure or diagnosis of infection. Third, this study enrolled patients from only two medical centers, and a majority of them resided in Seoul. This study may have been subject to a selection bias. Fourth, DPO-based multiplex PCR could determine only the presence of clarithromycin resistance, and we did not check for resistance to other antibiotics such as A2115G, G2141A, A2142T, and T2182C.
5. Conclusions
In conclusion, this study showed that tailored therapy could be a useful option to achieve a higher successful eradication rate, shorter treatment periods, and lower medical costs than empirical therapy in the era of increasing antibiotic resistance.
Author Contributions
Conceptualization, A.R.C., K.-N.S., and C.H.T.; data curation, A.R.C., Y.P., and E.-M.S.; formal analysis, A.R.C., and K.-N.S.; investigation, K.-N.S., Y.P., E.-M.S., and S.-A.J.; methodology, C.H.T. and S.-A.J.; project administration, K.-N.S.; software, Y.P., E.-M.S., C.H.T., and S.-A.J.; supervision, K.-N.S.; visualization, S.-A.J.; writing—original draft, A.R.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Ewha Womans University hospital (SEUMC 2020-09-013).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to our IRB policy but are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Farinha P., Gascoyne R.D.J.G. Helicobacter pylori and MALT lymphoma. Gastroenterology. 2005;128:1579–1605. doi: 10.1053/j.gastro.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 2.Huang J.-Q., Sridhar S., Hunt R.H.J.T.L. Role of Helicobacter pylori infection and non-steroidal anti-inflammatory drugs in peptic-ulcer disease: A meta-analysis. Lancet. 2002;359:14–22. doi: 10.1016/S0140-6736(02)07273-2. [DOI] [PubMed] [Google Scholar]
- 3.Wroblewski L.E., Peek R.M., Wilson K.T. Helicobacter pylori and gastric cancer: Factors that modulate disease risk. Clin. Microbiol. Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung H.K., Kang S.J., Lee Y.C., Yang H.-J., Park S.-Y., Shin C.M., Kim S.E., Lim H.C., Kim J.-H., Nam S.Y., et al. Evidence-based Guidelines for the Treatment of Helicobacter pylori Infection in Korea: 2020 Revised Edition. Korean J. Helicobacter Up. Gastrointest. Res. 2020;20:261–287. doi: 10.7704/kjhugr.2020.0045. [DOI] [Google Scholar]
- 5.Kim J.M., Kim J.S., Jung H.C., Kim N., Kim Y.-J., Song I.S. Distribution of antibiotic MICs for Helicobacter pylori strains over a 16-year period in patients from Seoul, South Korea. Antimicrob. Agents Chemother. 2004;48:4843–4847. doi: 10.1128/AAC.48.12.4843-4847.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang T.J., Kim N., Kim H.B., Lee B.H., Nam R.H., Park J.H., Lee M.K., Park Y.S., Lee D.H., Jung H.C., et al. Change in antibiotic resistance of Helicobacter pylori strains and the effect of A2143G point mutation of 23S rRNA on the eradication of H. pylori in a single center of Korea. J. Clin. Gastroenterol. 2010;44:536–543. doi: 10.1097/MCG.0b013e3181d04592. [DOI] [PubMed] [Google Scholar]
- 7.De Francesco V., Giorgio F., Hassan C., Manes G., Vannella L., Panella C., Ierardi E., Zullo A. Worldwide H. pylori antibiotic resistance: A systematic review. J. Gastrointest. Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- 8.Kim B.J., Lee H., Lee Y.C., Jeon S.W., Kim G.H., Kim H.-S., Sung J.K., Lee D.H., Kim H.U., Park M.I., et al. Ten-Day Concomitant, 10-Day Sequential, and 7-Day Triple Therapy as First-Line Treatment for Helicobacter pylori Infection: A Nationwide Randomized Trial in Korea. Gut Liver. 2019;13:531–540. doi: 10.5009/gnl19136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.S., Kim B.W., Hong S.J., Kim J.I., Shim K.-N., Kim J.-H., Baik G.H., Kim S.W., Song H.J., Kim J.H. Sequential Therapy versus Triple Therapy for the First Line Treatment of Helicobacter pylori in Korea: A Nationwide Randomized Trial. Gut Liver. 2016;10:556–561. doi: 10.5009/gnl15470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yan T.L., Gao J.G., Wang J.H., Chen D., Lu C., Xu C.-F. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J. Gastroenterol. 2020;26:4846–4856. doi: 10.3748/wjg.v26.i32.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Y., Tang G., Pan L., Zhu H., Zhou S., Wei Z. Clinical factors associated with initial Helicobacter pylori eradication therapy: A retrospective study in China. Sci. Rep. 2020;10:1–5. doi: 10.1038/s41598-020-72400-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liou J.M., Chen P.Y., Kuo Y.T., Wu M.-S. The Taiwan Gastrointestinal Disease and Helicobacter Consortium. Toward population specific and personalized treatment of Helicobacter pylori infection. J. Biomed. Sci. 2018;25:70. doi: 10.1186/s12929-018-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuo C.J., Lee C.H., Chang M.L., Lin C.-Y., Lin W.-R., Su M.-Y., Chiu C.-H., Tseng C.-N., Wu Y.-S., Chiu C.-T., et al. Multidrug resistance: The clinical dilemma of refractory Helicobacter pylori infection. J. Microbiol. Immunol. Infect. 2021 doi: 10.1016/j.jmii.2021.03.006. [DOI] [PubMed] [Google Scholar]
- 14.De Francesco V., Pontone S., Bellesia A., Serviddio G., Panetta C., Palma R., Zullo A. Quadruple, sequential, and concomitant first-line therapies for H. pylori eradication: A prospective, randomized study. Dig. Liver Dis. 2018;50:139–141. doi: 10.1016/j.dld.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Chey W.D., Leontiadis G.I., Howden C.W., Moss S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017;112:212–239. doi: 10.1038/ajg.2016.563. [DOI] [PubMed] [Google Scholar]
- 16.Baj J., Forma A., Sitarz M., Portincasa P., Garruti G., Krasowska D., Maciejewski R. Helicobacter pylori Virulence Factors-Mechanisms of Bacterial Pathogenicity in the Gastric Microenvironment. Cells. 2020;10:27. doi: 10.3390/cells10010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imkamp F., Lauener F.N., Pohl D., Lehours P., Vale F.F., Jehanne Q., Zbinden R., Keller P.M., Wagner K. Rapid Characterization of Virulence Determinants in Helicobacter pylori Isolated from Non-Atrophic Gastritis Patients by Next-Generation Sequencing. J. Clin. Med. 2019;8:1030. doi: 10.3390/jcm8071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 19.Lee J.Y., Kim N., Nam R.H., Choi S.I., Lee J.W., Lee D.H. Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 2019;24:e12660. doi: 10.1111/hel.12660. [DOI] [PubMed] [Google Scholar]
- 20.Seo S.I., Do B.J., Kang J.G., Kim H.S., Jang M.K., Kim H.Y., Shin W.G. Helicobacter pylori eradication according to sequencing-based 23S ribosomal RNA point mutation associated with clarithromycin resistance. J. Clin. Med. 2020;9:54. doi: 10.3390/jcm9010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim J.L., Cho S.-J., Chung S.J., Lee A., Choi J., Chung H., Kim S.G. Empiric Versus Clarithromycin Resistance-Guided Therapy for Helicobacter pylori Based on Polymerase Chain Reaction Results in Patients with Gastric Neoplasms or Gastric Mucosa-Associated Lymphoid Tissue Lymphoma: A Randomized Controlled Trial. Clin. Transl. Gastroenterol. 2020;11:e00194. doi: 10.14309/ctg.0000000000000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skrebinska S., Mégraud F., Bessède E.J.H. Diagnosis of Helicobacter pylori infection. Helicobacter. 2018;23:e12515. doi: 10.1111/hel.12515. [DOI] [PubMed] [Google Scholar]
- 23.Jung Y.S., Park C.H., Park J.H., Nam E., Lee H.L. Efficacy of Helicobacter pylori eradication therapies in Korea: A systematic review and network meta-analysis. Helicobacter. 2017;22 doi: 10.1111/hel.12389. [DOI] [PubMed] [Google Scholar]
- 24.Liu W.Z., Xie Y., Lu H., Cheng H., Zeng Z.R., Zhou L.Y., Chen Y., Wang J.B., Du Y.Q., Lu N.H., et al. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. doi: 10.1111/hel.12475. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y.K., Wu M.C., Wang S.S., Kuo C.-H., Lee Y.-C., Chang L.-L., Wang T.-H., Chen Y.-H., Wang W.-M., Wu D.-C., et al. Lansoprazole-based sequential and concomitant therapy for the first-line Helicobacter pylori eradication. J. Dig. Dis. 2012;13:232–238. doi: 10.1111/j.1751-2980.2012.00575.x. [DOI] [PubMed] [Google Scholar]
- 26.Liou J.M., Chen P.Y., Luo J.C., Lee J.-Y., Chen C.-C., Fang Y.-J., Yang T.-H., Chang C.-Y., Bair M.-J., Chen M.-J., et al. Efficacies of Genotypic Resistance-Guided vs Empirical Therapy for Refractory Helicobacter pylori Infection. Gastroenterology. 2018;155:1109–1119. doi: 10.1053/j.gastro.2018.06.047. [DOI] [PubMed] [Google Scholar]
- 27.Chang Y.W., Shin G.Y., Kim J.W., Moon J.-C., Chang E.J., Oh C.H., Jang J.-Y. Cost-Effectiveness of Empirical Bismuth-Based Quadruple Therapy and Tailored Therapy After Clarithromycin Resistance Tests for Helicobacter pylori Eradication. Dig. Dis. Sci. 2021 doi: 10.1007/s10620-021-06938-y. [DOI] [PubMed] [Google Scholar]
- 28.Cosme A., Montes M., Martos M., Gil I., Mendarte U., Salicio Y., Piñeiro L., Recasens M.T., Ibarra B., Sarasqueta C., et al. Usefulness of antimicrobial susceptibility in the eradication of Helicobacter pylori. Clin. Microbiol. Infect. 2013;19:379–383. doi: 10.1111/j.1469-0691.2012.03844.x. [DOI] [PubMed] [Google Scholar]
- 29.Gweon T.G., Kim J.S., Kim B.W. An Economic Modeling Study of Helicobacter pylori Eradication: Comparison of Dual Priming Oligonucleotide-Based Multiplex Polymerase Chain Reaction and Empirical Treatment. Gut Liver. 2018;12:648–654. doi: 10.5009/gnl18079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gisbert J.P. Empirical or susceptibility-guided treatment for Helicobacter pylori infection? A comprehensive review. Ther. Adv. Gastroenterol. 2020;13:1756284820968736. doi: 10.1177/1756284820968736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pero R., Brancaccio M., Laneri S., De Biasi M.G., Lombardo B., Scudiero O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules. 2019;9:237. doi: 10.3390/biom9060237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pero R., Coretti L., Nigro E., Lembo F., Laneri S., Lombardo B., Daniele A., Scudiero O. β-Defensins in the Fight against Helicobacter pylori. Molecules. 2017;22:424. doi: 10.3390/molecules22030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angrisano T., Pero R., Brancaccio M., Coretti L., Florio E., Pezone A., Calabrò V., Falco G., Keller S., Lembo F., et al. Cyclical DNA Methylation and Histone Changes Are Induced by LPS to Activate COX-2 in Human Intestinal Epithelial Cells. PLoS ONE. 2016;11:e0156671. doi: 10.1371/journal.pone.0156671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woo H.D., Fernandez-Jimenez N., Ghantous A., Degli Esposti D., Cuenin C., Cahais V., Choi I.J., Kim Y.-I., Kim J., Herceg Z. Genome-wide profiling of normal gastric mucosa identifies Helicobacter pylori- and cancer-associated DNA methylome changes. Int. J. Cancer. 2018;143:597–609. doi: 10.1002/ijc.31381. [DOI] [PubMed] [Google Scholar]
- 35.Zhou L., Zhang J., Song Z., He L., Li Y., Qian J., Bai P., Xue Y., Wang Y., Lin S. Tailored versus Triple plus Bismuth or Concomitant Therapy as Initial Helicobacter pylori Treatment: A Randomized Trial. Helicobacter. 2016;21:91–99. doi: 10.1111/hel.12242. [DOI] [PubMed] [Google Scholar]
- 36.Choi Y.I., Chung J.W., Park D.K., Kim K.O., Kwon K.A., Kim Y.J., Seo J.Y. Tailored eradication vs empirical bismuth-containing quadruple therapy for first-line Helicobacter pylori eradication: A comparative, open trial. World J. Gastroenterol. 2019;25:6743–6751. doi: 10.3748/wjg.v25.i46.6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to our IRB policy but are available from the corresponding author upon reasonable request.