Abstract
Simple Summary
An increasing number of cancer cases has been reported throughout the years. Most cancers are linked to unhealthy lifestyles and genetic inheritance. Nevertheless, unknown to many, infection from microorganisms (bacteria, viruses, fungi) and sometimes, parasites, can also lead to cancer development. For these cancers, the infection may inflict mechanical injury on host cells, whilst gene products or protein secretion from the microorganism further alters host cell activity, leading to abnormal cell development and growth. Due to the cancer-causing characteristic of these microorganisms, they have been classified as definite biological agents that cause cancer. This review describes the cancer development process caused by some of these microorganisms and highlights strategies to prevent or treat the associated cancers.
Abstract
Cancer is a global health problem associated with genetics and unhealthy lifestyles. Increasingly, pathogenic infections have also been identified as contributors to human cancer initiation and progression. Most pathogens (bacteria, viruses, fungi, and parasites) associated with human cancers are categorized as Group I human carcinogens by the International Agency for Research on Cancer, IARC. These pathogens cause carcinogenesis via three known mechanisms: persistent infection that cause inflammation and DNA damage, initiation of oncogene expression, and immunosuppression activity of the host. In this review, we discuss the carcinogenesis mechanism of ten pathogens, their implications, and some future considerations for better management of the disease. The pathogens and cancers described are Helicobacter pylori (gastric cancer), Epstein-Barr virus (gastric cancer and lymphoma), Hepatitis B and C viruses (liver cancer), Aspergillus spp. (liver cancer), Opisthorchis viverrine (bile duct cancer), Clonorchis sinensis (bile duct cancer), Fusobacterium nucleatum (colorectal cancer), Schistosoma haematobium (bladder cancer); Human Papillomavirus (cervical cancer), and Kaposi’s Sarcoma Herpes Virus (Kaposi’s sarcoma).
Keywords: infections, pathogens, carcinogenesis
1. Introduction
Cancer is a disease in which cells divide in an uncontrolled manner and have the ability to invade nearby tissues. The disease is a significant cause of morbidity and mortality. From the latest annual cancer case report of 2020, the World Health Organization (WHO) estimated more than 19.2 million new cases being diagnosed and 9.9 million mortalities from cancer [1]. Carcinogenesis and the progression of cancer are usually undetectable externally; many cancers, such as pancreatic and colorectal cancers (CRC) are undiagnosed until they reach a later stage [2,3]. Risk factors associated with lifestyle, diet, and genetic predisposition have been identified to contribute to carcinogenesis [4]. Increasingly, infections caused by pathogenic microorganisms and parasites have also been linked to cancer [5,6,7,8,9].
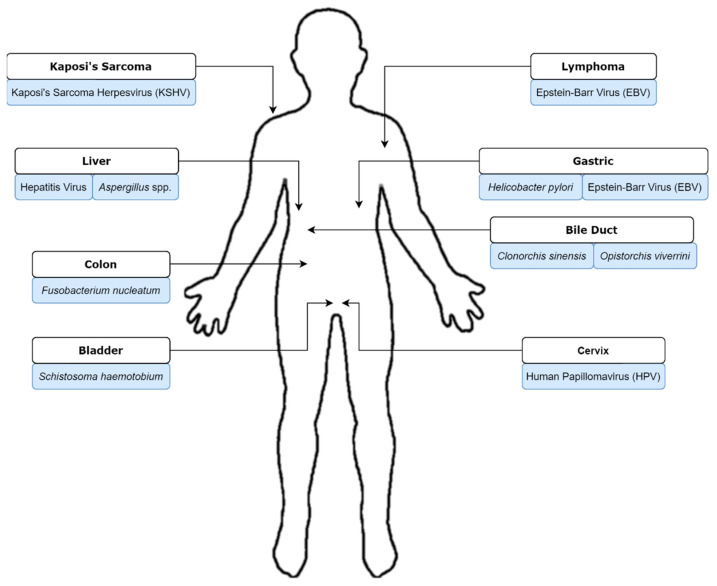
Microorganisms have been primarily studied for their roles in causing infection. However, since the early 20th century, the association of some infectious agents with cancer has been reported [10]. Beginning in the 1900s with a report by Askanazy, the link between Opisthorchis felineus infection and liver cancer, in addition to the discovery of bladder cancer-causing Bilharzia infections (schistosomiasis), have been highlighted [11]. Subsequently, the remarkable finding of oncolytic viruses was reported by Peyton Rous in 1911 in an avian model [12]. Fifty years later, Anthony Epstein proposed and proved the association of the Epstein-Barr virus (EBV with Burkitt lymphoma [13,14]). Infectious agents cause carcinogenesis largely via three mechanisms [15]. The first mechanism is via persistent infection which gives rise to inflammation and cell damage, where accumulative cell damage leads to mutations and carcinogenesis. Alternatively, infectious agents might cause the expression of oncogenic genes of their host and lead to cancer. In the third scenario, immunosuppression caused by infectious agents leads to carcinogenesis in the host via immunologic recognition disruption [15]. Carcinogenesis tropism is observed in cancers caused by infectious agents, whereby different agents are associated with different types of cancer (Figure 1). This review will describe ten infectious agents whose roles have been confirmed in carcinogenesis.
Figure 1.
Tropism of pathogens associated with human cancer development described in this review.
2. Gastric Cancer
Gastric cancer (GC) ranked fifth in worldwide cancer incidence (1,089,103 new cases) and third in cancer-related mortality (768,793 mortalities) in the year 2020 [1]. Risk factors for GC include lifestyle modifiers such as dietary habits and smoking, family history, and socioeconomic status [16,17,18]. In addition, infection with Helicobacter pylori and EBV have also been reported to be risk factors for the occurrence of GC [19]. H. pylori infection is attributed to almost all GC cases; EBV contributes to less than 10% of incidences [20,21].
2.1. Helicobacter pylori
Helicobacter pylori is a Gram-negative microaerophilic bacterium that could be isolated from the upper gastrointestinal tract of more than 50% of the population [22]. It can be transmitted through saliva, vomit material, and feces [23]. The bacteria’s chemotaxis properties allow it to detect pH changes in the stomach and subsequently burrow into the epithelial layer to escape the acidic mucosal lining [24]. In addition, H. pylori secrete urease which breaks down gastric urea to produce carbon dioxide and ammonia, neutralizing the acidic pH of the environment [25]. While these properties allow the bacteria to survive in the stomach and duodenum, the production of ammonia is toxic to host epithelial cells, whereby long-term colonization of H. pylori causes inflammation and results in chronic gastritis [26,27].
For hosts who carry the cag pathogenicity island (PAI)-positive H. pylori, secreted CagA disrupts cellular processes and host cell gene transcription via tyrosine phosphorylation of SHP-2 and kinases, causing neoplastic morphological changes and cell proliferation [28,29,30]. At the same time, the type IV secretion system expressed by cag PAI injects bacterial peptidoglycan into gastric epithelial cells, stimulating cytokine expression and further inflammation [31]. In addition, 50% of all H. pylori strains secrete VacA, a virulence factor that induces epithelial cell vacuolization and inhibits T-cell activation and proliferation, aiding the bacteria’s gastric colonization and leading to peptic ulceration [29,32]. Subsequently, if lifestyle or genetic risk factors are present, for hosts who are low acid producers, gastric cancer might occur, while high acid producers might develop duodenal cancer [33]. Association of H. pylori and gastric inflammation has been reported since 1982, and the bacteria was later categorized as a group I (definite) carcinogen by the IARC in 1994 [34,35].
2.2. Epstein-Barr Virus (EBV)
The Epstein-Barr virus (Human herpesvirus 4) is usually associated with Burkitt’s lymphoma [14] and is the etiological agent of infectious mononucleosis. It is a capsulated DNA virus and spreads via body fluids such as saliva and genital secretions. It has been reported that most people will be infected with the virus at some point in their lives. After infection, the virus may remain dormant in the B cells of its immunocompetent host, until it is reactivated [36]. Besides lymphoma, EBV infection has also been associated with the occurrence of GC [37]. About 9% of GCs reported an EBV etiology, where meta-analyses on EBV-associated gastric cancers (EBVaGC) showed, intriguingly, slightly higher prevalence in young males of American and European populations [21,38] and a lower incidence in China [39].
While H. pylori infection is mostly associated with tumors in the gastric antrum, EBVaGCs are usually located at non-antrum sites of the stomach [40]. Compared to H. pylori-associated gastric cancer in which carcinogenesis is caused by the bacteria’s toxin secretion and ammonia production, EBVaGCs are associated with host genome methylation via EBV modulation [41,42,43]. In EBVaGC, the virus is orally ingested and reaches gastric epithelial cells via saliva carriage. The virus enters epithelial cells via the host cell receptors with B cell mediation, and is subsequently assembled into circular mini chromosomes (“episomes”) [44,45].
Once latent infection is established, viral transcripts and proteins contribute towards carcinogenesis in the stomach [45]. Viral non-coding RNAs, EBER-1 and -2, promote tumor cell proliferation and migration, while the protein EBV-determined nuclear antigen-1 (EBNA1) induces reactive oxygen species (ROS) accumulation and at the same time impairs the host response towards DNA damage [46,47,48,49]. Importantly, EBV latent membrane protein 2A (LMP2A) induces epigenetic changes to the host genome via methylation of CpG islands, inactivating tumor suppressor genes such as PTEN and tumor-associated antigens [41,50]. In fact, host genome methylation is a common characteristic of EBVaGC, in which besides PTEN, promoter hypermethylation of CDH1, p14ARF, p15, p16INK4a, and p73 tumor suppressor genes has also been associated with EBV infection [43]. In addition to host genome methylation, viral genome methylation further allows the pathogen to escape host immune detection. Recent research shows the involvement of EBV miRNAs, where the non-coding RNAs have been reported to upregulate cancer cell proliferation, inhibit apoptosis, and suppress interferons (IFN) signaling [51,52,53].
3. Liver Cancer
Hepatocellular carcinoma (HCC), commonly known as primary liver cancer has a survival rate of 6 to 20 months, and is one of the cancers with high mortality rates [54,55] with 830,180 recorded mortalities and 905,677 incidents in 2020 [1]. The disease occurs most often in individuals who have a history of chronic liver diseases such as cirrhosis [56,57]. Alcohol consumption, smoking, and metabolic conditions such as obesity and type 2 diabetes are HCC co-clinical factors. In addition, chronic infection by the hepatitis virus and consumption of food contaminated with aflatoxin from the Aspergillus fungi confers a high risk towards the development of HCC [58]. All the above risk factors subject the liver to a state of chronic inflammation, with ongoing cycles of oxidative stress, DNA damage, hepatocyte turnover, and fibrosis [59].
3.1. Hepatitis Virus
There are five hepatitis viruses (A–E); two of them, hepatitis B (HBV) and C (HCV), are associated with HCC [56]. Despite their tropism for the liver, HBV and HCV are from different families, namely, Hepadnaviridae for HBV and Flaviviridae for HCV. The HBV genome consists of double-stranded DNA with reverse transcriptase, while HCV is a single-strand RNA virus. These two viruses are transmitted through blood. Nevertheless, HBV can also spread via body fluids during sexual intercourse or vertical mother-to-infant transmission [60].
Epidemiological studies suggest chronic HBV infection as the main risk factor in HCC development [61,62,63], where low copy numbers of the virions are sufficient to initiate infection [64]. Once it enters the host, the virus makes its way to the liver, binds to hepatocytes via the NTCP (sodium taurocholate co-transporting polypeptide) receptor, enters the cell via endocytosis, and proceeds to the nucleus [65]. Viral DNA transcription and protein translation are then initiated [63]. During active infection, the newly generated viral DNA will be integrated into the host genome, leading to chromosomal instability (CIN), insertional mutagenesis, and cis-activation of tumor-associated genes. Interestingly, no consistent singular target gene for HBV DNA integration has been identified; though pathways associated with AKT activation, mitotic cell cycle, AXIN1, and DNA imprinting have been reported to be dysregulated by the infection [59]. In addition, binding of the Hepatitis B X protein (HBx), to the host genome changes the expression of miRNAs and further disrupts histone methyltransferases activity, leading to cell expression pattern changes in HCC pathophysiology [66,67]. In addition, although the full extent of Hepatitis D virus (HDV)-associated HCC pathogenesis remains to be investigated, studies so far have found that co-infection of the virus with HBV will increase hepatocyte necro-inflammation, leading to cirrhosis and HCC [68,69].
In chronic HBV infection, the host experiences phases of “immune tolerance,” “immune reactive,” “inactive carrier,” “chronic hepatitis,” and “HBV surface antigen-negative” [70]. The risk for HCC is higher at both “immune reactive” and “chronic hepatitis” phases. During the “immune reactive” phase, the virus infects hepatocytes and integrates into host DNA. Subsequently, during “chronic hepatitis,” HBV replication is lowered, allowing viral mutants to escape host immune response. Nevertheless, this will still drive inflammation and hepatitis progression in the host via continuous activation of impaired anti-viral immune response, which in turn exacerbates inflammation and hepatocyte turnover, causing clonal expansion of premalignant cells containing HBV-integrated host DNA and HCC [71].
Compared to HBV infection, HCV does not integrate into the host genome. There are two phases of HCV infection: acute and chronic [59]. The risk for HCC increases during the chronic phase, and may increase as much as 17-fold [72]. The virus is transcribed once it reaches and enters the host’s hepatocytes [73,74]. HCV viral factors are then implicated in the interference of a variety of molecular pathways, including cell metabolism, genetic repair, apoptosis, and induction of ROS activity [75].
Progression of HCV infection induces metabolic reprogramming of hepatocytes, causing hepatosteatosis where there is a deposition of excessive triglycerides in cells [76]. Viral phosphoprotein NS5A further activates the PI3K/AKT signaling pathway, which is integral to HCC development. Viral proteins and their genome block tumor suppressors such as p53 and the epidermal growth factor receptor (EGFR), and induces ROS activity via the mediation of NADPH oxidase-1 and -4 (NOX) [77]. Chronic HCV infection also triggers both innate and adaptive immunity of the host, where inflammatory cytokines including tumor necrosis factors (TNF), interleukin (IL), and lymphotoxins (LT) are increased due to activation of inflammatory pathways such as NF-κB [78]. All the above produces a carcinogenic microenvironment promoting genetic instability and the development of hepatic stellate cells (HSCs) [79,80]. Epithelial to mesenchymal trans-differentiation (EMT) changes in HSCs are regulated by TGF-β growth factor then further contributes to carcinogenesis [81].
3.2. Aspergillus spp.
Two species from the Aspergillus fungus, namely Aspergillus flavus and Aspergillus parasiticus produce the genotoxic compound aflatoxin, which can be found in improperly stored food crops, such as rice, wheat, millet, corn, and peanuts [82,83,84]. The toxin, when ingested via food supplies, may cause acute aflatoxin poisoning and lead to abdominal pain and vomiting. Serious cases of acute exposure have been reported to cause pulmonary edema, fatty liver, liver necrosis, and even death [85]. Intriguingly, the toxin has a tropism for the liver, where chronic exposure to the toxin has been proven to increase HCC risk in humans and many species of animals. Epidemiologically, aflatoxin exposure is linked to HCC in many West African countries due to inappropriate post-harvest processing; in addition, developing countries have a higher incidence rate, where low-income populations sometimes resort to long-term consumption of moldy food produce to avoid starvation [86,87,88].
Although there are four aflatoxins (AFB1, AFB2, AFG1, and AFG2), AFB1 is the most common and has been strongly associated with HCC [85]. Once ingested, AFB1 will find its way to the liver and is subsequently activated by microsomal enzymes, forming DNA adducts of trans-8, 9-dihydro-8- (N7-guanyl)-9-hydroxyaflatoxin B1 (AFB1-N7-dG), and trans-8, 9-dihydro-8- (2, 6-diamino-4-oxo-3, 4-dihydropyrimid-5-yl-formamido) -9-hydroxy aflatoxin B1 (AFB1-Fapy-dG) [89]. AFB1-N7-dG has been reported to cause G > T mutagenesis [90], while AFB1-Fapy-dG may cause all G > T, G > A, G > C and single nucleotide deletions in p53 [91,92]. In addition to the formation of DNA adducts, AFB1 has also been found to cause mutations (AGG > AGT) at codon 249 of p53, leading to arginine substitution with serine (R249S) [92,93]. Hosts without proficient nucleotide or base excision repair mechanisms, together with by-pass by the error-prone DNA polymerase ζ during DNA replication will lead to clonal expansion of hepatocytes with p53 allelic deletions. Coupled with chronic inflammation due to HBV or HCV infections, chronic hepatitis and/or liver cirrhosis will occur, with HCC as a sequela.
4. Bile Duct Cancer
Risk factors for bile duct cancer or also known as cholangiocarcinoma (CCA) include older age, smoking, chronic liver disease, and primary sclerosing cholangitis (PSC) [94]. The cancer remains rare in the western hemisphere. Nevertheless, in southeast Asia, the prevalence is higher, where the disease is usually caused by helminth (parasite) infection. Opisthorchis viverrini and Clonorchis sinensis, two species of liver flukes, are the causative pathogens via food contamination [95]. Duration and intensity of the infection, host and liver parasite genetics, diet, and environmental exposure determine if the infection will lead to CCA [96]. Even though the etiology of liver fluke-associated CCA is known, many patients present at the later stages of III and IV with unresectable tumors, rendering the disease with poor clinical outcomes [95]. Both parasites are classified as group I human carcinogens by the IARC in 2012.
4.1. Opisthorchis viverrini
Opisthorchisviverrini was first discovered in Southeast Asia in 1886 in a fish by the parasitologist Jules Poirier [97]; it is prevalent in Thailand, Laos, Vietnam, and Cambodia. It is a monoecious hermaphrodite [98] and requires three different hosts (two intermediate and one definitive) to complete its life cycle. O. viverrini miracidia larvae infect freshwater snails (Bithynia spp.) and grow into sporocysts in snail tissues. These sporocysts become cercaria larvae, escape from snail tissues, and migrate towards fish, their second intermediate host. The flukes will then develop into metacercaria in the flesh of the fish. Ingestion of raw, contaminated fish frees O. viverrini into their final host, where they migrate towards the biliary tree and dominate the bile duct [99,100]. The flukes then cause CCA via three mechanisms: mechanical and chronic injury to biliary epithelial cells, immunologic inflammation via release of reactive oxygen intermediates and nitric oxide, and host cell proliferation via parasite secretion products. In chronic infection, these will cause DNA damage to the host cells and lead to tumorigenesis [101].
The parasites establish localization in biliary cells via securing their oral and ventral suckers into the cell epithelia. This damages the host bile ducts, with the development of ulcers as the infection progresses. The presence of the parasite and its secretion products induce an immune reaction from its host, prompting the release of pro-inflammatory cytokines mediated by toll-like receptor (TLR) signaling. Secretion products from the fluke are usually proteins for nutrient digestion and host tissue invasion. Proteomic investigations identified one of these proteins as Ov-GRN-1, a granulin-like parasite growth factor that has been shown to cause aberrant growth of the biliary cells [102]. In addition, thioredoxin and thioredoxin peroxidase are produced by this parasite to induce an anti-apoptotic mechanism [103]. Intriguingly, secretion products from O. viverrini contribute towards wound healing processes in the host to counter mechanical injuries caused by its suckers on the cells. However, as the parasites feed continuously, complete recovery of the biliary epithelial is not achieved.
Repeated cycles of cell division during incomplete wound healing subsequently leads to DNA damage and genomic instability of the host. Whole exome sequencing studies in O. viverrini-associated CCA patients revealed mutations in genes of canonical carcinogenesis pathways such as TP53, KRAS, and SMAD4 [104]. Mutations in genes associated specifically with CCA were also identified, these include RNF43, PEG3, BAP1, ARID1A, MLL3, IDH1/2, GNAS, and ROBO2 [105]. Host genomic instability, together with the presence of other carcinogenetic factors such as dietary nitrosamines (found in salted or fermented fish, a common dish in southeast Asia), conduce a microenvironment that is favorable for malignancies [101,105,106]. Of note, besides dietary nitrosamine, O. viverrini-associated CCA cases carrying active H. pylori infections have been observed, and hamster infection models showed an obligatory mutual relationship of the fluke with the carcinogenic bacteria [107].
4.2. Clonorchis sinensis
Clonorchis sinensis, the Chinese liver fluke, is mainly found in East Asian countries such as China, Taiwan, Korea, and Northern Vietnam [108]. Like O. viverrini, the parasite is digenetic, with snails and cyprinid fish as intermediate hosts. It shares a similar mechanism of infection and carcinogenesis as O. viverrini—by causing mechanical damage and chemical irritation to its host.
In addition to mechanical damage, feeding of the frequently propagating parasites at the bile ducts serves as mechanical obstruction, leading to metaplasia of the biliary epithelial cells. These cells will transform into mucin-producing cells that produce excretory-secretory products (ESPs) and mucus in the bile [109]. At the same time, the presence of the parasite at the biliary tree is recognized by host TLR-2 and -4, resulting in the production of inflammatory cytokines and chemokines. These peptides were originally intended for fluke elimination, however, they now contribute towards disease progression, causing toxicity towards the host, and cholangiocyte damage [110].
Production of ESPs from the parasite was found to induce metabolic oxidative stress [103,110], activating inflammatory mediators such as NADPH oxidase, nitric oxide synthase, lipoxygenase, cyclooxygenase, along with xanthine oxidase to generate free radicals, exacerbating the inflammatory response mediated by NF-κB [111,112]. In particular, the production of nitric oxide leads to host DNA damage by DNA repair inhibition and cyclooxygenase stimulation [113,114]. Generated free radicals will subsequently cause lipid peroxidation (LPO), a process that increases cell proliferation and deactivates cell apoptosis. In addition, ESPs might trigger host transcriptome, proteome, and miRNA expression changes via processes such as histone modification and mini-chromosome maintenance (MCM) regulation [115]. In chronic infection, all the above factors contribute to host genome instability and increased fragility to carcinogens, which, coupled with environmental risk factors such as the consumption of dietary nitrosamines, will lead to CCA.
5. Colorectal Cancer
Colorectal cancer (CRC) has affected more than 1.9 million people worldwide in the year 2020 with 935,173 mortalities [1]. While diet such as frequent red meat consumption and family history were reported to be associated with CRC, gut microbiome dysbiosis was recently identified as a risk factor for CRC [116]. Several bacteria, such as Bacteroides fragilis, Streptococcus gallolyticus, Streptococcus bovis, and Fusobacterium nucleatum have been reported to have a higher abundance in CRC patients. Among them, F. nucleatum has been suggested as a potential microbial carcinogen that initiates the development of CRC [117,118].
Fusobacterium nucleatum
Fusobacterium nucleatum are anaerobic Gram-negative bacteria that were first isolated from the oral cavity. With the advent of gut microbiome profiling, it was discovered that the bacteria can also colonize human intestines [119]; nevertheless, the movement of F. nucleatum from the mouth to colon remains unclear. In 2013, Kostic et al. proved that infection by F. nucleatum increases tumor cell multiplicity and recruits tumor-infiltrating myeloid cells in an in vivo model. Accordingly, carcinogenic properties of the bacteria were then reported, where the bacteria were found to enhance the proliferation of normal human colon cells, subsequently triggering the epithelial-mesenchymal transition pathway [120].
Fusobacterium nucleatum contributes toward the development of CRC via a few pathways. The bacteria attach and invade colon endothelial cells via the FadA adhesion protein. This causes the secretion of cytokines (IL-6, 8, 10, 18; TNF-α) and the expression of NF-κB, creating a pro-inflammatory environment in the colon [121]. At the same time, macrophage infiltration and methylation of the cyclin-dependent kinase inhibitor 2A, CDKN2A [122] occurs in the tumor microenvironment. In addition, FadA binding of the host cell E-cadherin receptor activates β-catenin signaling. This promotes tumor cell proliferation via increased expression of oncogenes of the Wnt pathway and their transcription factors [118]. Chronic infection activates the p38 gene which is crucial in the production of matrix metalloproteinase (MMP)-1, -9, and -13 for invasion as well as metastasis properties [123]. Besides FadA, F. nucleatum harbors another virulence factor, Fap2, which binds to TIGIT, an inhibitory receptor on T cells and natural killer cells, protecting tumor cells from the host immune system. Indeed, the bacteria were found to inhibit human T-cell responses toward mitogens and antigens (immunosuppressive activities) [124], most probably via blockage of the cell cycle mid-G1 phase [125].
Of note, in the case of CRC, cross-talk between microbial species might be important in causing cancer. Other than F. nucleatum, bacteria such as Peptostreptococcus stomatis, Parvimonas micra, and Akkermansia muciniphila have been found to be over-represented in the gut mucosa of CRC patients [126,127,128,129]. The exact role of these bacteria in CRC pathogenesis, however, remains to be investigated.
6. Bladder Cancer
In 2020, more than 573,000 cases of urinary bladder carcinoma were newly reported worldwide, followed by 212,536 mortalities [1]. The cancer includes urothelial carcinoma, squamous cell carcinoma, and adenocarcinoma; some can involve more than one cell type. In many parts of the world, squamous cell carcinoma can be caused by chronic irritation to the bladder as a result of prolonged urinary catheter usage. Nevertheless, in the Middle East and Africa regions, the cancer is associated with urogenital schistosomiasis caused by Schistosoma haematobium parasites [130].
Schistosoma haematobium
Urogenital schistosomiasis is a medical condition caused by S. haematobium infections. This infection leads to chronic inflammation and the presence of blood in the urine—hematuria [131], a condition associated with the development of bladder cancer. S. haematobium was first discovered in the 1850s in Cairo, Egypt, and carcinogenic properties of the parasite were later reported in the late 1880s [132,133]. Compared to other parasites, this trematode lives in pairs (male and female) and undergoes sexual reproduction during their life cycle. The free-swimming parasite could be acquired from freshwater environments, where it enters human hosts via skin penetration. This process is mediated by the secretion of proteolytic enzymes [134]. Following this, the cercaria larvae will migrate to its favorable site of infection (uterus, bladder, and prostate) for reproduction. Eggs of S. haematobium can be traced from urine samples of infected patients; eggs that are deposited in the bladder wall will cause damage and inflammation to the bladder lumen [135], increasing the risk of bladder cancer.
In chronic infection, the stuck eggs induce a granulomatous host T helper 2 (TH2) immune response due to prolonged inflammation, and urinary bladder irritation [136,137]. H03-H-IPSE, a major ortholog of the interleukin-4-inducing principle (IPSE) protein secreted by S. haematobium eggs, was found to induce urothelial cell proliferation in mouse models with nuclear localization, driving the cells towards the S-phase of the cell cycle. The protein also induces bladder angiogenesis [135] and allows the eggs to escape the host immune system. All the above leads to urothelial hyperplasia, a pre-cancerous lesion. In addition to its eggs, adult parasites have also been shown in xenogeneic animal models to increase cell proliferation and migration, as well as to decrease apoptosis [135]. Nevertheless, a single exposure to a parasite antigen will not contribute to tumorigenesis, suggesting the need for chronic infection to cause cancer in hosts. Interestingly, bacterial and parasite co-infection in females was reported to increase bladder cancer risk, though the mechanism of carcinogenesis is still unclear [130]. Indeed, urine microbiome dysbiosis in urogenital schistosomiasis has also been reported in bladder cancer and other pathologies of the organ [138].
In hosts, metabolism of parasite molecules, such as catechol estrogens and guanine-derived oxidation products will lead to genotoxic effects such as mutation, DNA strand breakage, and sister chromatid exchanges induced by the hydroxyl radical from inflammatory cells [130,139,140]. Chromosomes 1, 3, 5, 6, 7, 8, 9, 11, 14, 15, 17, 18, and Y are the most frequent site with abnormalities observed in bladder cancer development [141,142], where deletion in chromosome 9 has been associated with S. haematobium infections, leading to the loss of important protein function crucial in activating p53 and retinoblastoma (Rb) pathways and anti-apoptotic programs [143,144]. Urogenital schistosomiasis will also cause molecular perturbation via overexpression of the fibroblast growth factor receptor protein 3, causing the aggressive proliferation of cells [143]. In addition, mutations in KRAS have also been observed [145].
Due to its propensity to cause genetic and epigenetic changes that lead to cell hyperplasia and cancer, S. haematobium has been classified as a group I definitive biological carcinogenic agent by the IARC in 2012 [146]. Moreover, exposure to pro-carcinogenic factors such as smoking and N-nitroso compounds (either from diet, or from dysbiosis of urine microbiome in schistosomiasis) will increase bladder cancer risk in urogenital schistosomiasis [130,147,148].
7. Cervical Cancer
Cervical cancer is mostly diagnosed among women at the age of 35 to 44 years old. It is the fourth most frequent cancer reported globally with 604,127 cases in 2020 [1]. The burden of cervical cancer faced by low- and middle-income countries is significantly greater than in high-income countries, and contributed to 341,831 deaths in 2020 [1,149]. Interestingly, cervical cancer is usually caused by infectious agents, namely the human papillomavirus (HPV). Co-infections by Chlamydia trachomatis can initiate chronic infection on the endocervical cells at the transformation zone, which exposes the cells to oncogenic HPV infections [150].
Human Papillomavirus (HPV)
Human Papillomavirus is considered the principal etiological agent [151] and classified as a carcinogen for cervical cancer. There are currently more than 200 HPV subtypes, where they are classified as group I, IIA, IIB, and III carcinogens by the IARC. Amongst the HPV subtypes, HPV 16 and HPV 18 have been described as potential human carcinogens since 1983 [152]. Risk factors for HPV-associated cervical cancer include smoking and sexual exposure with multiple sexual partners, where these partners in turn, also have multiple partners [153].
All HPV subtypes are naked (non-enveloped) viruses with a small diameter (~55 nm) and a circular double-stranded DNA genome [154]. The virus is epitheliotrophic, where, after sexual contact (vaginal, anal, or oral) with an infected person, it will bind to heparin sulfate proteoglycans on the cervical basal basement membrane through breaks in the epithelium [155,156]. The virus will then be uncoated and directly transported to the host nucleus for replication, where early proteins (E1–E7) and late capsid proteins (L1 and L2) will be synthesized [157]. Episomal copies of the HPV genome will remain inside the infected cells [158,159]. E1 and E2 will be expressed for the production of virions to invade other neighboring cells while viral capsids aid viral movement inside the host cell during infection [160]. The oncogenic properties of HPV are mostly conferred via E6 and E7.
During chronic HPV infection, integration of the virus genome inside the host cell might occur, where cells will transition into cervical intraepithelial neoplasia grade I (CIN-1). E6 and E7 proteins interfere with cell proliferation activity controlled by p53 and pRb, [161] and also inhibit cell cycle checkpoint control via cyclin-dependent kinase (CDK) inhibitors (p21, p27, p16) [162]. These molecular events facilitate anti-apoptosis activity, disrupt the DNA repair mechanism, and deregulate cell cycle control, driving differentiating cells into the S-phase and rapid proliferation.
Lesions from CIN-1 may develop into CIN-2 or -3 in the space of 2 to 3 years. During this development, abnormal proliferation of cervical cells will occur, driven by the upregulation of genomics signatures such as those from the Kinesin family member (KIF23), Integrin subunit alpha V (ITGAV), CDKN2A, and Centromere Protein E (CENPE). Particularly, during the later CIN stages, proteins such as BUB1 mitotic checkpoint serine/threonine kinase B (BUB1B), mitotic arrest-deficient 2 (MAD2L1), checkpoint kinase 1 (CHEK1), cyclins, and proteins involved in the cell division cycle are found to be highly regulated [163]. From CIN-3, further failure to prohibit cell proliferation will lead to invasive carcinoma.
8. Kaposi’s Sarcoma
Kaposi’s sarcoma (KS) is a rare cancer lesion that appears on the skin, mouth, and nose lining, lymph nodes, or other vital organs. Globally, KS contributed 34,370 new cases with 15,084 mortalities in 2020 [1]. The lesions are usually purple in color, and consist of lymphatic endothelial cells or their precursors, which will develop into clonal metastases of spindle cells in the advanced stage [164]. KS cases can be divided into four subtypes: (1) epidemic, (2) classic, (3) endemic, and (4) iatrogenic [165]. Epidemic KS is associated with the HIV/AIDS epidemic [166]. Classic and endemic KS refer respectively to cases that occurred in elderly southern European and Middle Eastern males, and children in equatorial, eastern, and southern African countries. Iatrogenic KS mostly occurs in organ transplant patients receiving immunosuppressive therapy. The causative pathogen in KS has been identified as Kaposi’s sarcoma herpesvirus (KSHV), also referred to as human herpesvirus 8 (HHV8) [167]. The virus was originally thought to co-evolve with the human population; nevertheless, studies identified higher seroprevalence amongst sub-Saharan Africans and Mediterranean populations compared to northern Europeans, northern Americans, and Asians [168]. KSHV mother-to-child transmission or transmission between siblings and playmates is common in endemic countries. On the other hand, viral transmission has been reported to be more common in homosexual men with multiple partners in non-endemic countries [169].
Kaposi’s Sarcoma Herpesvirus (KSHV)/Human Herpesvirus 8 (HHV8)
Following the discovery of its genome sequences in biopsies, KSHV was identified as the causative agent for KS (IARC Class I carcinogen) [167]. The virus is a gammaherpesvirus with five major subtypes (A, A5, B, C, and D). Interestingly, the virus encodes proteins homologous to its human host, such as cyclin, viral FLICE inhibitory protein (vFLIP), B cell lymphoma 2 (BCL-2), IL-6, interferon regulatory factors, and chemokines, where viral cyclin and vFLIP promote the proliferation of infected tumor cells during latent infection [164]. Besides KS, KSHV is also associated with Castleman’s disease and primary effusion lymphoma, both neoplasms of the lymphatic system.
Routes of KSHV transmission are still not elucidated, though it has been postulated that the virus is transmitted via saliva, and upon host entry, infects a variety of cells, such as endothelial cells, epithelial cells, and fibroblasts [170]. The virus can also infect cells of the immune system including monocytes, B cells, and dendritic cells [171]. Once it enters the target cell, KSHV will be uncoated, with its genome circularizing to become an episome in the nucleus. It will then either become latent (causing lifelong infection) or undergo cycles of lytic reactivation. Proteins from both stages can contribute to tumorigenesis. vFLIP and viral miRNAs from the latent stage stimulate pathways such as NF-κB to increase host cell survival and prevent apoptosis, while promoting vascular proliferation and leading to inflammation [172,173]. These changes can also be caused by lytic proteins such as KSHV vIL-6 via the expression of vascular endothelial growth factor (VEGF) and platelet-derived growth factor (PDGF) [174,175,176].
Of note, although KSHV oncogenic proteins have been shown to prevent apoptosis in experimental models, complete tumorigenesis events require the existence of co-factors, such as intake of immunosuppression drugs by the host, or the presence of HIV virus co-infection. HIV-1 encodes a transcriptional trans-activator, Tat, which increases KSHV infectivity [177] and induces apoptosis of CD4+ T cells [178]. Another HIV protein, Nef, regulates the AKT signaling pathway, facilitating angiogenesis and KSHV oncogenesis by boosting levels of vIL-6 [179,180] and other cytokines (IFN-γ, TNF-α, IL-1), causing reactivation of the KSHV life cycle via the JAK/STAT pathway [180,181].
9. Lymphoma
Lymphoma refers to blood malignancies that arise from lymphocytes (B cells, T cells, and natural killer cells), with most lymphomas from B cell origin (90%) [182]. The cancer is broadly divided into Hodgkin (10%) and non-Hodgkin (90%) lymphoma. The total cases reported for lymphoma in 2020 is worrying, with 627,439 newly diagnosed cases and 283,169 fatalities [1]. Patients usually experience enlarged lymph nodes, night sweats, weight loss, and tiredness. Oncogenic mutation(s) in lymphocytes followed by clonal propagation has been reported in lymphomas [183], where age, male gender, and an impaired immune system have been identified as risk factors [184,185]. In addition, infection by pathogens such as human T-cell leukemia virus, HHV8, H. pylori, HCV, and EBV has been reported to be associated with lymphoma. Among these, EBV has been shown to cause a variety of lymphomas, including Burkitt’s lymphoma (BL) and Hodgkin lymphoma (HL) [186].
Epstein-Barr Virus (EBV)
Epstein-Barr virus-associated lymphomas usually occur during childhood (asymptomatic) with a progression towards infectious mononucleosis during adolescence. Primary infection occurs via the oral route, leading to viral entry and replication in the oral mucosal epithelium and B cells, where it enters the lytic phase. EBV may also enter pharyngeal lymphoid tissues, where the virus will switch to its latent phase [187] as episomes, and lifelong infections occur in the presence of latent membrane proteins (LMP) expression [11,15]. Infected cells carry EBNA, LMP, EBV-encoded RNA (EBER), and EBV miRNAs [188,189].
Cases of EBV-associated BL are mostly reported in regions where malaria is hyperendemic [190]. Malaria infection activates the host immune response to produce B cells and translocation of c-MYC, leading to increased B cell proliferation [191]. Together with mutations in ARF-MDM2-p53 pathways, p53-dependent apoptosis events are prevented [188]. EBNA-1 possesses oncogenic properties which cause high levels of ROS production and increased NOX2 catalytic subunits of the NADPH oxidase, leading to genetic instability [192]. Lymphocyte immortalization is further controlled by EBNA-2 nuclear protein via the expression of cellular proteins such as EBV receptor/CR2 (CD21) [193]. During latent infection, EBV has been reported to convert human B cells to become lymphoblastic cell lines (latency III infection) via aggressive lymphoblast proliferation [194,195].
In contrast, EBV-associated HL is characterized by the formation of multinucleated Reed-Sternberg (HRS) cells or mononucleated Hodgkin cells derived from B lymphocytes [188]. Most HRS cells possess a defective rearrangement of their B cell surface, disrupting normal cell signaling functions [196]. LMP-1 and LMP-2 are highly expressed in HRS, where these proteins complement cell surface defects and play a vital role in their survival [197,198]. In addition, genetic mutations such as translocation of CIITA (MHC2TA) have been observed in 15% of HL cases [199]. These mutations cause further disruptions in lymph node structure and cause the infiltration of non-neoplastic inflammatory-cells, increasing the release of various inflammatory cytokines (IL-1, IL-6, TGF-β, and TNFα) [188].
10. Implication of Infectious Agents in Carcinogenesis and Future Considerations
It is now evident that some cancers, even though not categorized as infectious diseases, can be caused or further exacerbated by infectious agents via various carcinogenesis pathways (Table 1). This discovery has a few implications. Firstly, some of these cancers may be prevented with vaccinations or other public health measures that prevent the human host from coming into contact with cancer-causing pathogens. In addition, periodical screening or infection surveillance in patients and intake of drugs, such as antimicrobials may help to prevent pre-cancerous lesions from worsening.
Table 1.
Carcinogenesis mechanism and prevention/treatment strategies for infection-associated cancers.
| Pathogen | Type of Cancer | Carcinogenesis Mechanism | Prevention/Treatment |
|---|---|---|---|
| Helicobacter pylori | Gastric |
|
|
| Epstein-Barr Virus (EBV) | Gastric |
|
|
| Hepatitis B Virus (HBV) | Liver |
|
|
| Hepatitis C Virus (HCV) | Liver |
|
|
| Aspergillus spp. | Liver |
|
|
| Opisthorchis viverrini | Bile Duct |
|
|
| Clonorchis sinensis | Bile Duct |
|
|
| Fusobacterium nucleatum | Colorectal |
|
|
| Schitosoma haematobium | Bladder |
|
|
| Human Papillomavirus (HPV) | Cervical |
|
|
| Kaposi’s Sarcoma Herpesvirus (KSHV) | Kaposi’s Sarcoma |
|
|
| Epstein-Barr Virus (EBV) | Lymphoma |
Burkitt’s Lymphoma
|
|
Hodgkin’s Lymphoma
|
Vaccinations against HBV and HPV infections have contributed to the lowering of hepatitis (and subsequently, HCC) and cervical cancer incidences. The implementation of infant HBV vaccination programs in many countries since 1995 has significantly reduced the incidence of pediatric hepatitis and primary liver cancer [200]. On the other hand, as cervical cancer is mostly a sequela of HPV infection, it is projected that high vaccine coverage will enable the global elimination of this cancer [201]. Other public health measures have also proved important in controlling infections by cancer-associated pathogens and thereby lowering cancer incidence. Establishing good regulating systems of staple dietary foods and improving storage conditions of crops will reduce Aspergillus transmission and GC [84,85,86]. Snail control to prevent schistosomiasis infections has been shown to be effective in reducing parasite infection [202,203]. In Thailand, behavioral-psycho-social interventions are being carried out to increase public awareness of the risks of raw fish consumption to reduce fluke infections and CCA [204].
Pathogen biomarker-based screening for patients has been integral in the detection and monitoring of infected patients, paving the way for early intervention to prevent tumorigenesis. Prior to HPV vaccination, Papanicolaou (Pap) smears with HPV tests allow for the detection of precancerous lesions and contribute to the lessening of cervical cancer burden. Indeed, Pap smear screening is still important even after HPV vaccination and is recommended to be carried out every 3 or 5 years, according to risk factors of the population [205]. Endoscopic surveillance involves the monitoring of the gastric environment to detect cellular changes, due to H. pylori and EBV infections [20,21], where the treatment of patients using antibiotics (H. pylori) to lower GC risk [206], or immunotherapy using checkpoint inhibitors (EBV) for treatment of GC [45] (which is currently being tested in clinical trials), can ensue for positive cases. Besides antibiotics, antimicrobials such as antivirals have been found to prevent pre-malignant stages of virus-associated cancers, such as IFN-free anti-viral therapies for HCV [207], zidovudine and valganciclovir for KSHV [208], and anti-worm medication such as praziquantel or albendazole to eliminate flukes and schistosomiasis [209].
Moving forward, multiomics studies into pathogens coupled with molecular editing will further contribute towards the discovery of better vaccination and treatment targets, along with improved strategies for pathogen detection, control, and elimination. This will enhance the prevention, early detection, and treatment of associated cancers. In addition, treatment using oncolytic viruses that infect and kill targeted cancer cells will also be a new paradigm in cancer therapy.
11. Conclusions
Infection by some pathogens may trigger carcinogenesis pathways that lead to cancer in susceptible individuals. Identification of pathogens that can function as human carcinogens, understanding how exposure to these pathogens occurs, and the subsequent carcinogenic mechanisms they trigger will be important. Knowledge in these areas will provide useful clues for successful pathogen-associated cancer management, control, and ultimately, prevention.
Author Contributions
H.-m.N. conceived of the idea for the article. M.N.A.H. and H.-m.N. performed the literature search, drafted the manuscript, and prepared the figures. M.N.A.H., E.A.M.H., S.-F.C., and H.-m.N. critically revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Fundamental Research Grant Scheme, (FRGS/1/2018/SKK11/UKM/02/2) awarded by the Ministry of Higher Education, Malaysia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty S., Rahman T. The Difficulties in Cancer Treatment. Ecancermedicalscience. 2012;6:ed16. doi: 10.3332/ecancer.2012.ed16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeller G., Tap J., Voigt A.Y., Sunagawa S., Kultima J.R., Costea P.I., Amiot A., Böhm J., Brunetti F., Habermann N., et al. Potential of fecal microbiota for early-stage detection of colorectal cancer. Mol. Syst. Biol. 2014;10:766. doi: 10.15252/msb.20145645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan N., Afaq F., Mukhtar H. Lifestyle as risk factor for cancer: Evidence from human studies. Cancer Lett. 2010;293:133–143. doi: 10.1016/j.canlet.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldszmid R.S., Dzutsev A., Trinchieri G. Host Immune Response to Infection and Cancer: Unexpected Commonalities. Cell Host Microbe. 2014;15:295–305. doi: 10.1016/j.chom.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser M.J. Understanding Microbe-Induced Cancers. Cancer Prev. Res. 2008;1:15–20. doi: 10.1158/1940-6207.CAPR-08-0024. [DOI] [PubMed] [Google Scholar]
- 7.Tjalsma H., Boleij A., Marchesi J., Dutilh B.E. A bacterial driver–passenger model for colorectal cancer: Beyond the usual suspects. Nat. Rev. Genet. 2012;10:575–582. doi: 10.1038/nrmicro2819. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z., Chen J., Yao H., Hu H. Fusobacterium and Colorectal Cancer. Front. Oncol. 2018;8:371. doi: 10.3389/fonc.2018.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang A.H., Parsonnet J. Role of Bacteria in Oncogenesis. Clin. Microbiol. Rev. 2010;23:837–857. doi: 10.1128/CMR.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howley P.M. Gordon Wilson Lecture: Infectious Disease Causes of Cancer: Opportunities for Prevention and Treatment. Trans. Am. Clin. Clim. Assoc. 2015;126:117–132. [PMC free article] [PubMed] [Google Scholar]
- 11.Hausen H.Z., Fox J.G., Wang T.C., Parsonnet J. Infections Causing Human Cancer. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2006. pp. 468–484. [DOI] [Google Scholar]
- 12.Kumar P., Murphy F.A. Francis Peyton Rous. Emerg. Infect. Dis. 2013;19:660–663. doi: 10.3201/eid1904.130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pannone G., Zamparese R., Pace M., Pedicillo M.C., Cagiano S., Somma P., Errico M.E., Donofrio V., Franco R., De Chiara A., et al. The role of EBV in the pathogenesis of Burkitt’s Lymphoma: An Italian hospital based survey. Infect. Agents Cancer. 2014;9:1–11. doi: 10.1186/1750-9378-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epstein M., Achong B., Barr Y. Virus Particles in Cultured Lymphoblasts from Burkitt’s Lymphoma. Lancet. 1964;283:702–703. doi: 10.1016/S0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 15.Dalton-Griffin L., Kellam P. Infectious causes of cancer and their detection. J. Biol. 2009;8:67. doi: 10.1186/jbiol168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckland G., Travier N., Huerta J., Bueno-De-Mesquita H., Siersema P., Skeie G., Weiderpass E., Engeset D., Ericson U., Ohlsson B., et al. Healthy lifestyle index and risk of gastric adenocarcinoma in the EPIC cohort study. Int. J. Cancer. 2014;137:598–606. doi: 10.1002/ijc.29411. [DOI] [PubMed] [Google Scholar]
- 17.Ladeiras-Lopes R., Pereira A.K., Nogueira A., Pinheiro-Torres T., Pinto I., Santos-Pereira R., Lunet N. Smoking and gastric cancer: Systematic review and meta-analysis of cohort studies. Cancer Causes Control. 2008;19:689–701. doi: 10.1007/s10552-008-9132-y. [DOI] [PubMed] [Google Scholar]
- 18.Nagel G., Linseisen J., Boshuizen H.C., Pera G., Giudice G.D., Westert G.P., Bueno-De-Mesquita H.B., Allen N.E., Key T.J., Numans M.E., et al. Socioeconomic position and the risk of gastric and oesophageal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC-EURGAST) Int. J. Epidemiol. 2007;36:66–76. doi: 10.1093/ije/dyl275. [DOI] [PubMed] [Google Scholar]
- 19.Sitarz R., Skierucha M., Mielko J., Offerhaus J., Maciejewski R., Polkowski W. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag. Res. 2018;10:239–248. doi: 10.2147/CMAR.S149619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stewart O.A., Wu F., Chen Y. The role of gastric microbiota in gastric cancer. Gut Microbes. 2020;11:1220–1230. doi: 10.1080/19490976.2020.1762520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy G., Pfeiffer R., Camargo M.C., Rabkin C.S. Meta-analysis Shows That Prevalence of Epstein–Barr Virus-Positive Gastric Cancer Differs Based on Sex and Anatomic Location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amieva M., Peek R.M. Pathobiology of Helicobacter pylori–Induced Gastric Cancer. Gastroenterology. 2016;150:64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kusters J.G., van Vliet A.H.M., Kuipers E.J. Pathogenesis of Helicobacter pylori Infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amieva M.R., El–Omar E.M. Host-Bacterial Interactions in Helicobacter pylori Infection. Gastroenterology. 2008;134:306–323. doi: 10.1053/j.gastro.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 25.Graham D.Y., Miftahussurur M. Helicobacter pylori urease for diagnosis of Helicobacter pylori infection: A mini review. J. Adv. Res. 2018;13:51–57. doi: 10.1016/j.jare.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dumrese C., Slomianka L., Ziegler U., Choi S.S., Kalia A., Fulurija A., Lu W., Berg D.E., Benghezal M., Marshall B., et al. The secreted Helicobacter cysteine-rich protein A causes adherence of human monocytes and differentiation into a macrophage-like phenotype. FEBS Lett. 2009;583:1637–1643. doi: 10.1016/j.febslet.2009.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiotani A., Graham D.Y. Pathogenesis and therapy of gastric and duodenal ulcer disease. Med. Clin. N. Am. 2002;86:1447–1466. doi: 10.1016/S0025-7125(02)00083-4. [DOI] [PubMed] [Google Scholar]
- 28.Backert S., Selbach M. Role of type IV secretion in Helicobacter pyloripathogenesis. Cell. Microbiol. 2008;10:1573–1581. doi: 10.1111/j.1462-5822.2008.01156.x. [DOI] [PubMed] [Google Scholar]
- 29.Kao C.-Y., Sheu B.-S., Wu J.-J. Helicobacter pylori infection: An overview of bacterial virulence factors and pathogenesis. Biomed. J. 2016;39:14–23. doi: 10.1016/j.bj.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Backert S., Neddermann M., Maubach G., Naumann M. Pathogenesis of Helicobacter pylori infection. Helicobacter. 2016;21:19–25. doi: 10.1111/hel.12335. [DOI] [PubMed] [Google Scholar]
- 31.Viala J., Chaput C., Boneca I.G., Cardona A., Girardin S.E., Moran A.P., Athman R., Mémet S., Huerre M.R., Coyle A.J., et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat. Immunol. 2004;5:1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 32.Cover T.L., Blanke S.R. Helicobacter pylori VacA, a paradigm for toxin multifunctionality. Nat. Rev. Genet. 2005;3:320–332. doi: 10.1038/nrmicro1095. [DOI] [PubMed] [Google Scholar]
- 33.Zali H., Rezaei-Tavirani M., Azodi M.Z. Gastric cancer: Prevention, risk factors and treatment. Gastroenterol. Hepatol. Bed Bench. 2011;4:175–185. [PMC free article] [PubMed] [Google Scholar]
- 34.Marshall B.J., Warren J.R. Unidentified Curved Bacilli in the Stomach Of Patients with Gastritis and Peptic Ulceration. Lancet. 1984;323:1311–1315. doi: 10.1016/S0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 35.Ahn H.J., Lee D.S. Helicobacter pylori in gastric carcinogenesis. World J. Gastrointest. Oncol. 2015;7:455–465. doi: 10.4251/wjgo.v7.i12.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trivedi P., Slack F.J., Anastasiadou E. Epstein-Barr virus: From kisses to cancer, an ingenious immune evader. Oncotarget. 2018;9:36411–36412. doi: 10.18632/oncotarget.26381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burke A.P., Yen T.S., Shekitka K.M., Sobin L.H. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod. Pathol. 1990;3:377–380. [PubMed] [Google Scholar]
- 38.Camargo M.C., Murphy G., Koriyama C., Pfeiffer R.M., Kim W.H., Herrera-Goepfert R., Corvalan A.H., Carrascal E., Abdirad A., Anwar M., et al. Determinants of Epstein-Barr virus-positive gastric cancer: An international pooled analysis. Br. J. Cancer. 2011;105:38–43. doi: 10.1038/bjc.2011.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iizasa H., Nanbo A., Nishikawa J., Jinushi M., Yoshiyama H. Epstein-Barr Virus (EBV)-associated Gastric Carcinoma. Viruses. 2012;4:3420–3439. doi: 10.3390/v4123420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akiba S., Koriyama C., Herrera-Goepfert R., Eizuru Y. Epstein-Barr virus associated gastric carcinoma: Epidemiological and clinicopathological features. Cancer Sci. 2008;99:195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shinozaki-Ushiku A., Kunita A., Aya S.-U. Update on Epstein-Barr virus and gastric cancer (Review) Int. J. Oncol. 2015;46:1421–1434. doi: 10.3892/ijo.2015.2856. [DOI] [PubMed] [Google Scholar]
- 42.Fukayama M., Abe H., Kunita A., Shinozaki-Ushiku A., Matsusaka K., Ushiku T., Kaneda A. Thirty years of Epstein-Barr virus-associated gastric carcinoma. Virchows Archiv. 2020;476:353–365. doi: 10.1007/s00428-019-02724-4. [DOI] [PubMed] [Google Scholar]
- 43.Qu Y., Dang S., Hou P. Gene methylation in gastric cancer. Clin. Chim. Acta. 2013;424:53–65. doi: 10.1016/j.cca.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Imai S., Nishikawa J., Takada K. Cell-to-Cell Contact as an Efficient Mode of Epstein-Barr Virus Infection of Diverse Human Epithelial Cells. J. Virol. 1998;72:4371–4378. doi: 10.1128/JVI.72.5.4371-4378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Y., Zhang J., Cheng A.S.L., Yu J., To K.F., Kang W. Gastric cancer: Genome damaged by bugs. Oncogene. 2020;39:3427–3442. doi: 10.1038/s41388-020-1241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Iwakiri D., Eizuru Y., Tokunaga M., Takada K. Autocrine growth of Epstein-Barr virus-positive gastric carcinoma cells mediated by an Epstein-Barr virus-encoded small RNA. Cancer Res. 2003;63:7062–7067. [PubMed] [Google Scholar]
- 47.Banerjee A.S., Pal A.D., Banerjee S. Epstein–Barr virus-encoded small non-coding RNAs induce cancer cell chemoresistance and migration. Virology. 2013;443:294–305. doi: 10.1016/j.virol.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Sivachandran N., Dawson C.W., Young L.S., Liu F.-F., Middeldorp J., Frappier L. Contributions of the Epstein-Barr Virus EBNA1 Protein to Gastric Carcinoma. J. Virol. 2011;86:60–68. doi: 10.1128/JVI.05623-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim S.-M., Hur D.Y., Hong S.-W., Kim J.H. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem. Biophys. Res. Commun. 2017;494:550–555. doi: 10.1016/j.bbrc.2017.10.095. [DOI] [PubMed] [Google Scholar]
- 50.Hino R., Uozaki H., Murakami N., Ushiku T., Shinozaki A., Ishikawa S., Morikawa T., Nakaya T., Sakatani T., Takada K., et al. Activation of DNA Methyltransferase 1 by EBV Latent Membrane Protein 2A Leads to Promoter Hypermethylation of PTEN Gene in Gastric Carcinoma. Cancer Res. 2009;69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 51.Tsai C.-Y., Liu Y.Y., Liu K.-H., Hsu J.-T., Cheng-Tang C., Chiu C.-T., Yeh T.-S. Comprehensive profiling of virus microRNAs of Epstein-Barr virus-associated gastric carcinoma: Highlighting the interactions of ebv-Bart9 and host tumor cells. J. Gastroenterol. Hepatol. 2017;32:82–91. doi: 10.1111/jgh.13432. [DOI] [PubMed] [Google Scholar]
- 52.Zheng X., Wang J., Wei L., Peng Q., Gao Y., Fu Y., Lu Y., Qin Z., Zhang X., Lu J., et al. Epstein-Barr Virus MicroRNA miR-BART5-3p Inhibits p53 Expression. J. Virol. 2018;92:92. doi: 10.1128/JVI.01022-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hooykaas M.J.G., van Gent M., Soppe J.A., Kruse E., Boer I.G.J., Van Leenen D., Koerkamp M.J.A.G., Holstege F.C.P., Ressing M.E., Wiertz E.J.H.J., et al. EBV MicroRNA BART16 Suppresses Type I IFN Signaling. J. Immunol. 2017;198:4062–4073. doi: 10.4049/jimmunol.1501605. [DOI] [PubMed] [Google Scholar]
- 54.Byam J., Renz J., Millis J.M. Liver transplantation for hepatocellular carcinoma. HepatoBiliary Surg. Nutr. 2013;2:22–30. doi: 10.3978/j.issn.2304-3881.2012.11.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 56.Chuang S.-C., Vecchia C.L., Boffetta P. Liver cancer: Descriptive epidemiology and risk factors other than HBV and HCV infection. Cancer Lett. 2009;286:9–14. doi: 10.1016/j.canlet.2008.10.040. [DOI] [PubMed] [Google Scholar]
- 57.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fattovich G., Stroffolini T., Zagni I., Donato F. Hepatocellular Carcinoma in Albania: Incidence and Risk Factors. Int. J. Sci. Res. 2016;5:1178–1183. doi: 10.21275/v5i1.nov152992. [DOI] [PubMed] [Google Scholar]
- 59.Tu T., Bühler S., Bartenschlager R. Chronic viral hepatitis and its association with liver cancer. Biol. Chem. 2017;398:817–837. doi: 10.1515/hsz-2017-0118. [DOI] [PubMed] [Google Scholar]
- 60.Handsfield H.H. Hepatitis A and B immunization in persons being evaluated for sexually transmitted diseases. Am. J. Med. 2005;118:69–74. doi: 10.1016/j.amjmed.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 61.El-Serag H.B. Epidemiology of Viral Hepatitis and Hepatocellular Carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.El–Serag H.B., Rudolph K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 63.Lamontagne R.J., Bagga S., Bouchard M.J. Hepatitis B virus molecular biology and pathogenesis. Hepatoma Res. 2016;2:163–186. doi: 10.20517/2394-5079.2016.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ringelhan M., McKeating J.A., Protzer U. Correction to ‘Viral hepatitis and liver cancer’. Philos. Trans. R. Soc. B Biol. Sci. 2018;373:20170339. doi: 10.1098/rstb.2017.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Watashi K., Urban S., Li W., Wakita T. NTCP and Beyond: Opening the Door to Unveil Hepatitis B Virus Entry. Int. J. Mol. Sci. 2014;15:2892–2905. doi: 10.3390/ijms15022892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kgatle M.M., Spearman C.W., Kalla A.A., Hairwadzi H.N. DNA Oncogenic Virus-Induced Oxidative Stress, Genomic Damage, and Aberrant Epigenetic Alterations. Oxidative Med. Cell. Longev. 2017;2017:1–16. doi: 10.1155/2017/3179421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rivière L., Gerossier L., Ducroux A., Dion S., Deng Q., Michel M.-L., Buendia M.-A., Hantz O., Neuveut C. HBx relieves chromatin-mediated transcriptional repression of hepatitis B viral cccDNA involving SETDB1 histone methyltransferase. J. Hepatol. 2015;63:1093–1102. doi: 10.1016/j.jhep.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Fattovich G., Bortolotti F., Donato F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 69.Verme G., Amoroso P., Lettieri G., Pierri P., David E., Sessa F., Rizzi R., Bonino F., Recchia S., Rizzetto M. A histological study of hepatitis delta virus liver disease. Hepatology. 1986;6:1303–1307. doi: 10.1002/hep.1840060613. [DOI] [PubMed] [Google Scholar]
- 70.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of chronic hepatitis B virus infection. J. Hepatol. 2012;57:167–185. doi: 10.1016/j.jhep.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 71.Mason W.S., Gill U.S., Litwin S., Zhou Y., Peri S., Pop O., Hong M., Naik S., Quaglia A., Bertoletti A., et al. HBV DNA Integration and Clonal Hepatocyte Expansion in Chronic Hepatitis B Patients Considered Immune Tolerant. Gastroenterology. 2016;151:986–998.e4. doi: 10.1053/j.gastro.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lin M.V., King L.Y., Chung R.T. Hepatitis C Virus–Associated Cancer. Annu. Rev. Pathol. Mech. Dis. 2015;10:345–370. doi: 10.1146/annurev-pathol-012414-040323. [DOI] [PubMed] [Google Scholar]
- 73.Shi G., Suzuki T. Molecular Basis of Encapsidation of Hepatitis C Virus Genome. Front. Microbiol. 2018;9:396. doi: 10.3389/fmicb.2018.00396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ashfaq U.A., Javed T., Rehman S., Nawaz Z., Riazuddin S. An overview of HCV molecular biology, replication and immune responses. Virol. J. 2011;8:161. doi: 10.1186/1743-422X-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartosch B., Thimme R., Blum H.E., Zoulim F. Hepatitis C virus-induced hepatocarcinogenesis. J. Hepatol. 2009;51:810–820. doi: 10.1016/j.jhep.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Pekow J.R., Bhan A.K., Zheng H., Chung R.T. Hepatic steatosis is associated with increased frequency of hepatocellular carcinoma in patients with hepatitis C-related cirrhosis. Cancer. 2007;109:2490–2496. doi: 10.1002/cncr.22701. [DOI] [PubMed] [Google Scholar]
- 77.De Mochel N.S.R., Seronello S., Wang S.H., Ito C., Zheng J.X., Liang T.J., Lambeth J.D., Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Irshad M., Gupta P., Irshad K. Molecular basis of hepatocellular carcinoma induced by hepatitis C virus infection. World J. Hepatol. 2017;9:1305–1314. doi: 10.4254/wjh.v9.i36.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGivern D.R., Lemon S.M. Virus-specific mechanisms of carcinogenesis in hepatitis C virus associated liver cancer. Oncogene. 2011;30:1969–1983. doi: 10.1038/onc.2010.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Okuda M., Li K., Beard M.R., Showalter L.A., Scholle F., Lemon S.M., Weinman S.A. Mitochondrial injury, oxidative stress, and antioxidant gene expression are induced by hepatitis C virus core protein. Gastroenterology. 2002;122:366–375. doi: 10.1053/gast.2002.30983. [DOI] [PubMed] [Google Scholar]
- 81.Hoshida Y., Fuchs B.C., Bardeesy N., Baumert T.F., Chung R.T. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J. Hepatol. 2014;61:S79–S90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kew M.C. Aflatoxins as a cause of hepatocellular carcinoma. J. Gastrointest. Liver Dis. 2013;22:305–310. [PubMed] [Google Scholar]
- 83.Jalili M. A Review on Aflatoxins Reduction in Food. Iran. J. Health Saf. Environ. 2016;3:445–459. [Google Scholar]
- 84.Waliyar F., Osiru M., Ntare B., Kumar K.V.K., Sudini H., Traore A., Diarra B. Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin J. 2015;8:245–252. doi: 10.3920/WMJ2014.1766. [DOI] [Google Scholar]
- 85.Kensler T.W., Roebuck B.D., Wogan G.N., Groopman J.D. Aflatoxin: A 50-Year Odyssey of Mechanistic and Translational Toxicology. Toxicol. Sci. 2010;120:S28–S48. doi: 10.1093/toxsci/kfq283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Strosnider H., Azziz-Baumgartner E., Banziger M., Bhat R.V., Breiman R., Brune M.-N., Decock K., Dilley A., Groopman J., Hell K., et al. Workgroup Report: Public Health Strategies for Reducing Aflatoxin Exposure in Developing Countries. Environ. Health Perspect. 2006;114:1898–1903. doi: 10.1289/ehp.9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Phillips T.D., Afriyie-Gyawu E., Williams J., Huebner H., Ankrah N.-A., Ofori-Adjei D., Jolly P., Johnson N., Taylor J., Marroquin-Cardona A., et al. Reducing human exposure to aflatoxin through the use of clay: A review. Food Addit. Contam. Part. A. 2008;25:134–145. doi: 10.1080/02652030701567467. [DOI] [PubMed] [Google Scholar]
- 88.Yang J.D., Hainaut P., Gores G.J., Amadou A., Plymoth A., Roberts L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019;16:589–604. doi: 10.1038/s41575-019-0186-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McCullough A.K., Lloyd R.S. Mechanisms underlying aflatoxin-associated mutagenesis—Implications in carcinogenesis. DNA Repair. 2019;77:76–86. doi: 10.1016/j.dnarep.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin Y.-C., Li L., Makarova A.V., Burgers P.M., Stone M., Lloyd R.S. Error-prone Replication Bypass of the Primary Aflatoxin B1 DNA Adduct, AFB1-N7-Gua. J. Biol. Chem. 2014;289:18497–18506. doi: 10.1074/jbc.M114.561563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin Y.-C., Li L., Makarova A.V., Burgers P.M., Stone M., Lloyd R.S. Molecular basis of aflatoxin-induced mutagenesis-role of the aflatoxin B1-formamidopyrimidine adduct. Carcinogenesis. 2014;35:1461–1468. doi: 10.1093/carcin/bgu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamid A.S., Tesfamariam I.G., Zhang Y., Zhang Z.G. Aflatoxin B1-induced hepatocellular carcinoma in developing countries: Geographical distribution, mechanism of action and prevention. Oncol. Lett. 2013;5:1087–1092. doi: 10.3892/ol.2013.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huang X.-H., Sun L.-H., Lu D.-D., Sun Y., Ma L.-J., Zhang X.-R., Huang J., Yu L. Codon 249 mutation in exon 7 ofp53gene in plasma DNA: Maybe a new early diagnostic marker of hepatocellular carcinoma in Qidong risk area, China. World J. Gastroenterol. 2003;9:692–695. doi: 10.3748/wjg.v9.i4.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Khan S.A., Tavolari S., Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019;39:19–31. doi: 10.1111/liv.14095. [DOI] [PubMed] [Google Scholar]
- 95.Prueksapanich P., Piyachaturawat P., Aumpansub P., Ridtitid W., Chaiteerakij R., Rerknimitr R. Liver Fluke-Associated Biliary Tract Cancer. Gut Liver. 2018;12:236–245. doi: 10.5009/gnl17102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Suk W.A., Bhudhisawasdi V., Ruchirawat M. The Curious Case of Cholangiocarcinoma: Opportunities for Environmental Health Scientists to Learn about a Complex Disease. J. Environ. Public Health. 2018;2018:1–7. doi: 10.1155/2018/2606973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Saijuntha W., Sithithaworn P., Kaitsopit N., Andrews R.H., Petney T.N. Advances in Experimental Medicine and Biology. Volume 766. Springer Science and Business Media LLC; New York, NY, USA: 2014. Liver Flukes: Clonorchis and Opisthorchis; pp. 153–199. [DOI] [PubMed] [Google Scholar]
- 98.Jex A.R., Young N., Sripa J., Hall R.S., Scheerlinck J.-P., Laha T., Sripa B., Gasser R.B. Molecular Changes in Opisthorchis viverrini (Southeast Asian Liver Fluke) during the Transition from the Juvenile to the Adult Stage. PLoS Negl. Trop. Dis. 2012;6:e1916. doi: 10.1371/journal.pntd.0001916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaewpitoon N. Opisthorchis viverrini: The carcinogenic human liver fluke. World J. Gastroenterol. 2008;14:666–674. doi: 10.3748/wjg.14.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young N.D., Campbell B.E., Hall R.S., Jex A.R., Cantacessi C., Laha T., Sohn W.-M., Sripa B., Loukas A., Brindley P.J., et al. Unlocking the Transcriptomes of Two Carcinogenic Parasites, Clonorchis sinensis and Opisthorchis viverrini. PLoS Negl. Trop. Dis. 2010;4:e719. doi: 10.1371/journal.pntd.0000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sripa B., Brindley P.J., Mulvenna J., Laha T., Smout M., Mairiang E., Bethony J.M., Loukas A. The tumorigenic liver fluke Opisthorchis viverrini—Multiple pathways to cancer. Trends Parasitol. 2012;28:395–407. doi: 10.1016/j.pt.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Smout M., Laha T., Mulvenna J., Sripa B., Suttiprapa S., Jones A., Brindley P.J., Loukas A. A Granulin-Like Growth Factor Secreted by the Carcinogenic Liver Fluke, Opisthorchis viverrini, Promotes Proliferation of Host Cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.van Tong H., Brindley P.J., Meyer C.G., Velavan T.P. Parasite Infection, Carcinogenesis and Human Malignancy. EBioMedicine. 2017;15:12–23. doi: 10.1016/j.ebiom.2016.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chan-On W., Nairismägi M.-L., Ong C.K., Lim W.K., Dima S., Pairojkul C., Lim K.H., McPherson J.R., Cutcutache I., Heng H.L., et al. Exome sequencing identifies distinct mutational patterns in liver fluke–related and non-infection-related bile duct cancers. Nat. Genet. 2013;45:1474–1478. doi: 10.1038/ng.2806. [DOI] [PubMed] [Google Scholar]
- 105.Jusakul A., Kongpetch S., Teh B.T. Genetics of Opisthorchis viverrini-related cholangiocarcinoma. Curr. Opin. Gastroenterol. 2015;31:258–263. doi: 10.1097/MOG.0000000000000162. [DOI] [PubMed] [Google Scholar]
- 106.Flavell D.J., Lucas S.B. Potentiation by the human liver fluke, Opisthorchis viverrini, of the carcinogenic action of N-nitrosodimethylamine upon the biliary epithelium of the hamster. Br. J. Cancer. 1982;46:985–989. doi: 10.1038/bjc.1982.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Deenonpoe R., Chomvarin C., Pairojkul C., Chamgramol Y., Loukas A., Brindley P.J., Sripa B. The Carcinogenic Liver Fluke Opisthorchis viverrini is a Reservoir for Species of Helicobacter. Asian Pac. J. Cancer Prev. 2015;16:1751–1758. doi: 10.7314/APJCP.2015.16.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chai J.-Y., Murrell K.D., Lymbery A.J. Fish-borne parasitic zoonoses: Status and issues. Int. J. Parasitol. 2005;35:1233–1254. doi: 10.1016/j.ijpara.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 109.Kim T.-S., Pak J.H., Kim J.-B., Bahk Y.Y. Clonorchis sinensis, an oriental liver fluke, as a human biological agent of cholangiocarcinoma: A brief review. BMB Rep. 2016;49:590–597. doi: 10.5483/BMBRep.2016.49.11.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoo K.-S., Lim W.T., Choi H.S. Biology of Cholangiocytes: From Bench to Bedside. Gut Liver. 2016;10:687–698. doi: 10.5009/gnl16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nam J.-H., Moon J.H., Kim I.K., Lee M.-R., Hong S.-J., Ahn J.H., Chung J.W., Pak J.H. Free radicals enzymatically triggered by Clonorchis sinensis excretory–secretory products cause NF-κB-mediated inflammation in human cholangiocarcinoma cells. Int. J. Parasitol. 2012;42:103–113. doi: 10.1016/j.ijpara.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 112.Won J., Ju J.-W., Kim S.M., Shin Y., Chung S., Pak J.H. Clonorchis sinensis Infestation Promotes Three-Dimensional Aggregation and Invasion of Cholangiocarcinoma Cells. PLoS ONE. 2014;9:e110705. doi: 10.1371/journal.pone.0110705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Han C., Leng J., Demetris A.J., Wu T. Cyclooxygenase-2 Promotes Human Cholangiocarcinoma Growth. Cancer Res. 2004;64:1369–1376. doi: 10.1158/0008-5472.CAN-03-1086. [DOI] [PubMed] [Google Scholar]
- 114.Jaiswal M., LaRusso N.F., Shapiro R.A., Billiar T.R., Gores G.J. Nitric oxide–mediated inhibition of DNA repair potentiates oxidative DNA damage in cholangiocytes. Gastroenterology. 2001;120:190–199. doi: 10.1053/gast.2001.20875. [DOI] [PubMed] [Google Scholar]
- 115.Kim N.-W., Kim J.-Y., Moon J.H., Kim K.-B., Kim T.-S., Hong S.-J., Cheon Y.P., Pak J.H., Seo S.-B. Transcriptional induction of minichromosome maintenance protein 7 (Mcm7) in human cholangiocarcinoma cells treated with Clonorchis sinensis excretory–secretory products. Mol. Biochem. Parasitol. 2010;173:10–16. doi: 10.1016/j.molbiopara.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 116.Shang F.-M., Liu H.-L. Fusobacterium nucleatum and colorectal cancer: A review. World J. Gastrointest. Oncol. 2018;10:71–81. doi: 10.4251/wjgo.v10.i3.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kostic A., Chun E., Robertson L., Glickman J.N., Gallini C.A., Michaud M., Clancy T.E., Chung D.C., Lochhead P., Hold G., et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rubinstein M.R., Wang X., Liu W., Hao Y., Cai G., Han Y.W. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/β-Catenin Signaling via its FadA Adhesin. Cell Host Microbe. 2013;14:195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Castellarin M., Warren R., Freeman J.D., Dreolini L., Krzywinski M., Strauss J., Barnes R., Watson P., Allen-Vercoe E., Moore R.A., et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2011;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ma C., Luo H., Gao F., Tang Q., Chen W. Fusobacterium nucleatum promotes the progression of colorectal cancer by interacting with E-cadherin. Oncol. Lett. 2018;16:2606–2612. doi: 10.3892/ol.2018.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dharmani P., Strauss J., Ambrose C., Allen-Vercoe E., Chadee K. Fusobacterium nucleatum Infection of Colonic Cells Stimulates MUC2 Mucin and Tumor Necrosis Factor Alpha. Infect. Immun. 2011;79:2597–2607. doi: 10.1128/IAI.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Park H.E., Kim J.H., Cho N.-Y., Lee H.S., Kang G.H. Intratumoral Fusobacterium nucleatum abundance correlates with macrophage infiltration and CDKN2A methylation in microsatellite-unstable colorectal carcinoma. Virchows Archiv. 2017;471:329–336. doi: 10.1007/s00428-017-2171-6. [DOI] [PubMed] [Google Scholar]
- 123.Koul H.K., Pal M., Koul S. Role of p38 MAP Kinase Signal Transduction in Solid Tumors. Genes Cancer. 2013;4:342–359. doi: 10.1177/1947601913507951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huynh T., Kapur R.V., Kaplan C.W., Cacalano N., Haake S.K., Shi W., Sieling P., Jewett A. The Role of Aggregation in Fusobacterium nucleatum–Induced Immune Cell Death. J. Endod. 2011;37:1531–1535. doi: 10.1016/j.joen.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 125.Shenker B.J., Datar S. Fusobacterium nucleatum inhibits human T-cell activation by arresting cells in the mid-G1 phase of the cell cycle. Infect. Immun. 1995;63:4830–4836. doi: 10.1128/iai.63.12.4830-4836.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nistal E., Fernández-Fernández N., Vivas S., Olcoz J.L. Factors Determining Colorectal Cancer: The Role of the Intestinal Microbiota. Front. Oncol. 2015;5:220. doi: 10.3389/fonc.2015.00220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Osman M.A., Neoh H.-M., Mutalib N.-S.A., Chin S.-F., Mazlan L., Ali R.A.R., Zakaria A.D., Ngiu C.S., Ang M.Y., Jamal R. Parvimonas micra, Peptostreptococcus stomatis, Fusobacterium nucleatum and Akkermansia muciniphila as a four-bacteria biomarker panel of colorectal cancer. Sci. Rep. 2021;11:1–12. doi: 10.1038/s41598-021-82465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Drewes J.L., White J.R., Dejea C.M., Fathi P., Iyadorai T., Vadivelu J., Roslani A.C., Wick E.C., Mongodin E.F., Loke M.F., et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes. 2017;3:1–12. doi: 10.1038/s41522-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Baxter N.T., Iv M.T.R., Rogers M.A.M., Schloss P.D. Microbiota-based model improves the sensitivity of fecal immunochemical test for detecting colonic lesions. Genome Med. 2016;8:1–10. doi: 10.1186/s13073-016-0290-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mostafa M.H., Sheweita S., O’Connor P.J. Relationship between Schistosomiasis and Bladder Cancer. Clin. Microbiol. Rev. 1999;12:97–111. doi: 10.1128/CMR.12.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gray D., Ross A.G., Li Y.-S., McManus D.P. Diagnosis and management of schistosomiasis. BMJ. 2011;342:d2651. doi: 10.1136/bmj.d2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bella S.D., Riccardi N., Giacobbe D.R., Luzzati R. History of schistosomiasis (bilharziasis) in humans: From Egyptian medical papyri to molecular biology on mummies. Pathog. Glob. Health. 2018;112:268–273. doi: 10.1080/20477724.2018.1495357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barakat R.M. Epidemiology of Schistosomiasis in Egypt: Travel through Time: Review. J. Adv. Res. 2013;4:425–432. doi: 10.1016/j.jare.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ross A.G., Inobaya M.T., Olveda R.M., Chau T.N., Olveda D.U. Prevention and control of schistosomiasis: A current perspective. Res. Rep. Trop. Med. 2014;5:65–75. doi: 10.2147/RRTM.S44274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ishida K., Hsieh M.H. Understanding Urogenital Schistosomiasis-Related Bladder Cancer: An Update. Front. Med. 2018;5:223. doi: 10.3389/fmed.2018.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosin M.P., Zaki S.S.E.D., Ward A.J., Anwar W.A. Involvement of inflammatory reactions and elevated cell proliferation in the development of bladder cancer in schistosomiasis patients. Mutat. Res. Mol. Mech. Mutagen. 1994;305:283–292. doi: 10.1016/0027-5107(94)90248-8. [DOI] [PubMed] [Google Scholar]
- 137.Pearce E.J., Macdonald A.S. The immunobiology of schistosomiasis. Nat. Rev. Immunol. 2002;2:499–511. doi: 10.1038/nri843. [DOI] [PubMed] [Google Scholar]
- 138.Adebayo A.S., Survayanshi M., Bhute S., Agunloye A.M., Isokpehi R.D., Anumudu C.I., Shouche Y.S. The microbiome in urogenital schistosomiasis and induced bladder pathologies. PLoS Neglected Trop. Dis. 2017;11:e0005826. doi: 10.1371/journal.pntd.0005826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Botelho M.C., Alves H., Richter J. Halting Schistosoma haematobium—Associated Bladder Cancer. Int. J. Cancer Manag. 2017;10:9430. doi: 10.5812/ijcm.9430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gouveia M.J., Santos J., Brindley P.J., Rinaldi G., Lopes C., Santos L.L., Costa J.M.C.D., Vale N. Estrogen-like metabolites and DNA-adducts in urogenital schistosomiasis-associated bladder cancer. Cancer Lett. 2015;359:226–232. doi: 10.1016/j.canlet.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 141.Panani A.D., Ferti A.D., Raptis S.A., Roussos C. Novel recurrent structural chromosomal aberrations in primary bladder cancer. Anticancer. Res. 2004;24:2967–2974. [PubMed] [Google Scholar]
- 142.Fadl-Elmula I. Chromosomal changes in uroepithelial carcinomas. Cell Chromosom. 2005;4:1. doi: 10.1186/1475-9268-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aly M.S., Khaled H.M., Emara M., Hussein T.D. Cytogenetic profile of locally advanced and metastatic schistosoma-related bladder cancer and response to chemotherapy. Cancer Genet. 2012;205:156–162. doi: 10.1016/j.cancergen.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 144.McConkey D.J., Lee S., Choi W., Tran M., Majewski T., Lee S., Siefker-Radtke A., Dinney C., Czerniak B. Molecular genetics of bladder cancer: Emerging mechanisms of tumor initiation and progression. Urol. Oncol. Semin. Orig. Investig. 2010;28:429–440. doi: 10.1016/j.urolonc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Botelho M.C., Veiga I., Oliveira P.A., Lopes C., Teixeira M., Costa J.M.C.D., Machado J.C. Carcinogenic ability of Schistosoma haematobium possibly through oncogenic mutation of KRAS gene. Adv. Cancer Res. Treat. 2013;2013:876585. [PMC free article] [PubMed] [Google Scholar]
- 146.Zaghloul M.S. Bladder cancer and schistosomiasis. J. Egypt. Natl. Cancer Inst. 2012;24:151–159. doi: 10.1016/j.jnci.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 147.Chala B., Choi M.-H., Moon K.C., Kim H.S., Kwak C., Hong S.-T. Development of Urinary Bladder Pre-Neoplasia by Schistosoma haematobium Eggs and Chemical Carcinogen in Mice. Korean J. Parasitol. 2017;55:21–29. doi: 10.3347/kjp.2017.55.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Honeycutt J., Hammam O., Fu C.-L., Hsieh M.H. Controversies and challenges in research on urogenital schistosomiasis-associated bladder cancer. Trends Parasitol. 2014;30:324–332. doi: 10.1016/j.pt.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Vu M., Yu J., Awolude O.A., Chuang L. Cervical cancer worldwide. Curr. Probl. Cancer. 2018;42:457–465. doi: 10.1016/j.currproblcancer.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 150.Madeleine M.M., Anttila T., Schwartz S.M., Saikku P., Leinonen M., Carter J.J., Wurscher M., Johnson L.G., Galloway D.A., Daling J.R. Risk of cervical cancer associated withChlamydia trachomatis antibodies by histology, HPV type and HPV cofactors. Int. J. Cancer. 2007;120:650–655. doi: 10.1002/ijc.22325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schiffman M., Wentzensen N., Wacholder S., Kinney W., Gage J.C., Castle P.E. Human Papillomavirus Testing in the Prevention of Cervical Cancer. J. Natl. Cancer Inst. 2011;103:368–383. doi: 10.1093/jnci/djq562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.zur Hausen H., Francoise B.-S. The Discoveries of Human Papilloma Viruses That Cause Cervical Cancer and of Human Immunodeficiency Virus. [(accessed on 24 January 2021)];Nobel Prize. 2008 8:1–26. Available online: https://www.nobelprize.org/uploads/2018/06/advanced-medicineprize2008.pdf. [Google Scholar]
- 153.Gravitt P.E. The known unknowns of HPV natural history. J. Clin. Investig. 2011;121:4593–4599. doi: 10.1172/JCI57149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Okunade K.S. Human papillomavirus and cervical cancer. J. Obstet. Gynaecol. 2020;40:602–608. doi: 10.1080/01443615.2019.1634030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Schiller J.T., Day P.M., Kines R.C. Current understanding of the mechanism of HPV infection. Gynecol. Oncol. 2010;118:S12–S17. doi: 10.1016/j.ygyno.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kines R.C., Cerio R.J., Roberts J.N., Thompson C.D., Pinos E.D.L., Lowy D.R., Schiller J.T. Human papillomavirus capsids preferentially bind and infect tumor cells. Int. J. Cancer. 2016;138:901–911. doi: 10.1002/ijc.29823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Doorbar J., Egawa N., Griffin H., Kranjec C., Murakami I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015;25:2–23. doi: 10.1002/rmv.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McMurray H.R., Nguyen D., Westbrook T.F., Mcance D.J. Biology of human papillomaviruses. Int. J. Exp. Pathol. 2001;82:15–33. doi: 10.1046/j.1365-2613.2001.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Doorbar J., Quint W., Banks L., Bravo I.G., Stoler M., Broker T.R., Stanley M.A. The Biology and Life-Cycle of Human Papillomaviruses. Vaccine. 2012;30:F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- 160.Horvath C.A.J., Boulet G.A.V., Renoux V.M., Delvenne P.O., Bogers J.-P.J. Mechanisms of cell entry by human papillomaviruses: An overview. Virol. J. 2010;7:11. doi: 10.1186/1743-422X-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yeo-Teh N.S.L., Ito Y., Jha S. High-Risk Human Papillomaviral Oncogenes E6 and E7 Target Key Cellular Pathways to Achieve Oncogenesis. Int. J. Mol. Sci. 2018;19:1706. doi: 10.3390/ijms19061706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Longworth M.S., Laimins L.A. Pathogenesis of Human Papillomaviruses in Differentiating Epithelia. Microbiol. Mol. Biol. Rev. 2004;68:362–372. doi: 10.1128/MMBR.68.2.362-372.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Balasubramaniam S.D., Balakrishnan V., Oon C.E., Kaur G. Key Molecular Events in Cervical Cancer Development. Medicine. 2019;55:384. doi: 10.3390/medicina55070384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Boshoff C. Kaposi’s Sarcoma Biology. IUBMB Life. 2002;53:259–261. doi: 10.1080/15216540212645. [DOI] [PubMed] [Google Scholar]
- 165.Torrence G.M., Wrobel J.S. A case of mistaken identity: Classic Kaposi sarcoma misdiagnosed as a diabetic foot ulcer in an atypical patient. Clin. Diabetes Endocrinol. 2019;5:1–6. doi: 10.1186/s40842-019-0083-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cesarman E., Damania B., Krown S.E., Martin J., Bower M., Whitby D. Kaposi sarcoma. Nat. Rev. Dis. Prim. 2019;5:1–21. doi: 10.1038/s41572-019-0060-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chang Y., Cesarman E., Pessin M., Lee F., Culpepper J., Knowles D., Moore P. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi’s sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 168.Schulz T.F. The pleiotropic effects of Kaposi’s sarcoma herpesvirus. J. Pathol. 2005;208:187–198. doi: 10.1002/path.1904. [DOI] [PubMed] [Google Scholar]
- 169.Henke-Gendo C., Schulz T. Transmission and disease association of Kaposi’s sarcoma-associated herpesvirus: Recent developments. Curr. Opin. Infect. Dis. 2004;17:53–57. doi: 10.1097/00001432-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 170.You H., Asiimwe S., Byakwaga H., Glidden D., Shiboski C., Dollard S., Martin J. Low Prevalence of KSHV DNA in Female Genital Fluid: Findings from Zimbabwean Women and Systematic Review of the Literature of KSHV Shedding in Body Fluids and Mucous Membranse Surfaces; Proceedings of the 16th International Conference on Malignancies in HIV/AIDS; Bethesda, Maryland. 23–24 October 2017; p. 115. [Google Scholar]
- 171.Bechtel J.T., Liang Y., Hvidding J., Ganem D. Host Range of Kaposi’s Sarcoma-Associated Herpesvirus in Cultured Cells. J. Virol. 2003;77:6474–6481. doi: 10.1128/JVI.77.11.6474-6481.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Dittmer D.P., Damania B. Kaposi sarcoma–associated herpesvirus: Immunobiology, oncogenesis, and therapy. J. Clin. Investig. 2016;126:3165–3175. doi: 10.1172/JCI84418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ballon G., Akar G., Cesarman E. Systemic Expression of Kaposi Sarcoma Herpesvirus (KSHV) Vflip in Endothelial Cells Leads to a Profound Proinflammatory Phenotype and Myeloid Lineage Remodeling In Vivo. PLoS Pathog. 2015;11:e1004581. doi: 10.1371/journal.ppat.1004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aoki Y., Yarchoan R., Wyvill K., Okamoto S.-I., Little R.F., Tosato G. Detection of viral interleukin-6 in Kaposi sarcoma–associated herpesvirus–linked disorders. Blood. 2001;97:2173–2176. doi: 10.1182/blood.V97.7.2173. [DOI] [PubMed] [Google Scholar]
- 175.Arvanitakis L., Geras-Raaka E., Varma A., Gershengorn M.C., Cesarman E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nat. Cell Biol. 1997;385:347–350. doi: 10.1038/385347a0. [DOI] [PubMed] [Google Scholar]
- 176.Cavallin L.E., Ma Q., Naipauer J., Gupta S., Kurian M., Locatelli P., Romanelli P., Nadji M., Goldschmidt-Clermont P.J., Mesri E.A. KSHV-induced ligand mediated activation of PDGF receptor-alpha drives Kaposi’s sarcomagenesis. PLoS Pathog. 2018;14:e1007175. doi: 10.1371/journal.ppat.1007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thakker S., Verma S.C. Co-infections and Pathogenesis of KSHV-Associated Malignancies. Front. Microbiol. 2016;7:151. doi: 10.3389/fmicb.2016.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dabrowska A., Kim N., Aldovini A. Tat-Induced FOXO3a Is a Key Mediator of Apoptosis in HIV-1-Infected Human CD4+T Lymphocytes. J. Immunol. 2008;181:8460–8477. doi: 10.4049/jimmunol.181.12.8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhu X., Guo Y., Yao S., Yan Q., Xue M., Hao T., Zhou F., Zhu J., Qin D., Lu C. Synergy between Kaposi’s sarcoma-associated herpesvirus (KSHV) vIL-6 and HIV-1 Nef protein in promotion of angiogenesis and oncogenesis: Role of the AKT signaling pathway. Oncogene. 2013;33:1986–1996. doi: 10.1038/onc.2013.136. [DOI] [PubMed] [Google Scholar]
- 180.Qin J., Lu C. Infection of KSHV and Interaction with HIV: The Bad Romance. In: Cai Q., Yuan Z., Lan K., editors. Infectious Agents Associated Cancers: Epidemi-ology and Molecular Biology. Springer Nature Singapore Pte. Ltd.; Singapore: 2017. p. 237. [Google Scholar]
- 181.Zeng Y., Zhang X., Huang Z., Cheng L., Yao S., Qin D., Chen X., Tang Q., Lv Z., Zhang L., et al. Intracellular Tat of Human Immunodeficiency Virus Type 1 Activates Lytic Cycle Replication of Kaposi’s Sarcoma-Associated Herpesvirus: Role of JAK/STAT Signaling. J. Virol. 2006;81:2401–2417. doi: 10.1128/JVI.02024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Shannon-Lowe C., Rickinson A.B., Bell A.I. Epstein–Barr virus-associated lymphomas. Philos. Trans. R. Soc. B Biol. Sci. 2017;372:20160271. doi: 10.1098/rstb.2016.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Malcolm T.I.M., Hodson D.J., Macintyre E.A., Turner S.D. Challenging perspectives on the cellular origins of lymphoma. Open Biol. 2016;6:160232. doi: 10.1098/rsob.160232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chihara D., Nastoupil L.J., Williams J.N., Lee P., Koff J.L., Flowers C.R. New insights into the epidemiology of non-Hodgkin lymphoma and implications for therapy. Expert Rev. Anticancer. Ther. 2015;15:531–544. doi: 10.1586/14737140.2015.1023712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zhang Y., Dai Y., Zheng T., Ma S. Risk factors of non-Hodgkin’s lymphoma. Expert Opin. Med. Diagn. 2011;5:539–550. doi: 10.1517/17530059.2011.618185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Farrell P.J. Epstein–Barr Virus and Cancer. Annu. Rev. Pathol. Mech. Dis. 2019;14:29–53. doi: 10.1146/annurev-pathmechdis-012418-013023. [DOI] [PubMed] [Google Scholar]
- 187.Thorley-Lawson D.A., Hawkins J.B., Tracy S.I., Shapiro M. The pathogenesis of Epstein–Barr virus persistent infection. Curr. Opin. Virol. 2013;3:227–232. doi: 10.1016/j.coviro.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Pattle S.B., Farrell P. The role of Epstein–Barr virus in cancer. Expert Opin. Biol. Ther. 2006;6:1193–1205. doi: 10.1517/14712598.6.11.1193. [DOI] [PubMed] [Google Scholar]
- 189.Klein G., Klein E., Kashuba E. Interaction of Epstein-Barr virus (EBV) with human B-lymphocytes. Biochem. Biophys. Res. Commun. 2010;396:67–73. doi: 10.1016/j.bbrc.2010.02.146. [DOI] [PubMed] [Google Scholar]
- 190.Thorley-Lawson D.A., Allday M.J. The curious case of the tumour virus: 50 years of Burkitt’s lymphoma. Nat. Rev. Genet. 2008;6:913–924. doi: 10.1038/nrmicro2015. [DOI] [PubMed] [Google Scholar]
- 191.McMahon S.B. MYC and the Control of Apoptosis. Cold Spring Harb. Perspect. Med. 2014;4:a014407. doi: 10.1101/cshperspect.a014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gruhne B., Sompallae R., Marescotti D., Kamranvar S.A., Gastaldello S., Masucci M.G. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proc. Natl. Acad. Sci. USA. 2009;106:2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Larcher C., Kempkes B., Kremmer E., Prodinger W.M., Pawlita M., Bornkamm G.W., Dierich M.P. Expression of Epstein-Barr virus nuclear antigen-2 (EBNA2) induces CD21/CR2 on B and T cell lines and shedding of soluble CD21. Eur. J. Immunol. 1995;25:1713–1719. doi: 10.1002/eji.1830250634. [DOI] [PubMed] [Google Scholar]
- 194.Hatton O.L., Harris-Arnold A., Schaffert S., Krams S.M., Martinez O.M. The interplay between Epstein–Barr virus and B lymphocytes: Implications for infection, immunity, and disease. Immunol. Res. 2014;58:268–276. doi: 10.1007/s12026-014-8496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lamb R., Parks G. Paramyxoviridae: The viruses and their replication. In: Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. Fields Virology. 5th ed. Volume 1. Lippincott Williams & Wilkins; Philadelphia, PA, USA: 2007. pp. 1449–1496. [Google Scholar]
- 196.Küppers R. Molecular biology of Hodgkin lymphoma. Hematol. Am. Soc. Hematol. Educ. Progr. 2009;2009:491–496. doi: 10.1182/asheducation-2009.1.491. [DOI] [PubMed] [Google Scholar]
- 197.de la Cruz-Merino L., Lejeune M., Fernández E.N., Carrasco F.H., López A.G., Vacas A.I., Pulla M.P., Callau C., Álvaro T. Role of Immune Escape Mechanisms in Hodgkin’s Lymphoma Development and Progression: A Whole New World with Therapeutic Implications. Clin. Dev. Immunol. 2012;2012:1–24. doi: 10.1155/2012/756353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.White M.K., Pagano J.S., Khalili K. Viruses and Human Cancers: A Long Road of Discovery of Molecular Paradigms. Clin. Microbiol. Rev. 2014;27:463–481. doi: 10.1128/CMR.00124-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Steidl C., Shah S.P., Woolcock B.W., Rui L., Kawahara M., Farinha P., Johnson N.A., Zhao Y., Telenius A., Neriah S.B., et al. MHC class II transactivator CIITA is a recurrent gene fusion partner in lymphoid cancers. Nat. Cell Biol. 2011;471:377–381. doi: 10.1038/nature09754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lin C.-L., Kao J.-H. Hepatitis B: Immunization and Impact on Natural History and Cancer Incidence. Gastroenterol. Clin. N. Am. 2020;49:201–214. doi: 10.1016/j.gtc.2020.01.010. [DOI] [PubMed] [Google Scholar]
- 201.Bigaard J., Franceschi S. Vaccination against HPV: Boosting coverage and tackling misinformation. Mol. Oncol. 2020;15:770–778. doi: 10.1002/1878-0261.12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhou Y.-B., Chen Y., Liang S., Song X.-X., Chen G.-X., He Z., Cai B., Yihuo W.-L., He Z.-G., Jiang Q.-W. Multi-host model and threshold of intermediate host Oncomelania snail density for eliminating schistosomiasis transmission in China. Sci. Rep. 2016;6:31089. doi: 10.1038/srep31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Lo N.C., Gurarie D., Yoon N., Coulibaly J.T., Bendavid E., Andrews J.R., King C. Impact and cost-effectiveness of snail control to achieve disease control targets for schistosomiasis. Proc. Natl. Acad. Sci. USA. 2018;115:E584–E591. doi: 10.1073/pnas.1708729114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Suwannahitatorn P., Webster J., Riley S., Mungthin M., Donnelly C.A. Uncooked fish consumption among those at risk of Opisthorchis viverrini infection in central Thailand. PLoS ONE. 2019;14:e0211540. doi: 10.1371/journal.pone.0211540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.MacLaughlin K.L., Jacobson R.M., Breitkopf C.R., Wilson P.M., Jacobson D.J., Fan C., Sauver J.L.S., Rutten L.J.F. Trends Over Time in Pap and Pap-HPV Cotesting for Cervical Cancer Screening. J. Women’s Health. 2019;28:244–249. doi: 10.1089/jwh.2018.7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wroblewski L.E., Peek R.M., Wilson K.T. Helicobacter pylori and Gastric Cancer: Factors That Modulate Disease Risk. Clin. Microbiol. Rev. 2010;23:713–739. doi: 10.1128/CMR.00011-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pawlotsky J.-M., Feld J.J., Zeuzem S., Hoofnagle J.H. From non-A, non-B hepatitis to hepatitis C virus cure. J. Hepatol. 2015;62:S87–S99. doi: 10.1016/j.jhep.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 208.Krown S.E., Dittmer D.P., Cesarman E. Pilot Study of Oral Valganciclovir Therapy in Patients with Classic Kaposi Sarcoma. J. Infect. Dis. 2011;203:1082–1086. doi: 10.1093/infdis/jiq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hong S.-T. Albendazole and Praziquantel: Review and Safety Monitoring in Korea. Infect. Chemother. 2018;50:1–10. doi: 10.3947/ic.2018.50.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.