PURPOSE
ATM, a gene that controls repair of DNA double-strand breaks, confers an excess lifetime risk of breast cancer among carriers of germline pathogenic variants (PV). ATM PV homozygotes are particularly sensitive to DNA damage caused by ionizing radiation. Consequently, there is concern that adjuvant radiotherapy (RT) may cause excess morbidity among heterozygous carriers of ATM PV. We evaluated the tolerability of breast RT among carriers of ATM germline variants.
METHODS
Of 167 patients with ATM germline variants presenting to our institution with breast cancer, 91 received RT. Treatment-related toxicity was ascertained from medical records and graded across organ systems. Toxicities grade > 2 were recorded from the end of treatment to last evaluable follow-up and were analyzed according to ATM variant pathogenicity.
RESULTS
Of 91 evaluable carriers of ATM variants, with a median follow-up of 32 months following RT, 25% (n = 23) harbored a PV, whereas 75% (n = 68) harbored a variant of uncertain significance (VUS). Prevalence of grade ≥ 2 toxicity unrelated to post-mastectomy reconstruction among patients with ATM PV was: 32% at the end of treatment (v 34% for VUS carriers), 11% at 1 year of follow-up (v 4% for VUS carriers), and 8% at the last follow-up (v 13% for VUS carriers), consistent with previous studies of RT among unselected populations. No grade 4 or 5 toxicities were observed. ATM variant pathogenicity was not associated with local toxicity, contralateral breast cancer, or secondary malignancy in this limited cohort of patients who received breast RT.
CONCLUSION
We found no evidence of excess RT-associated toxicity among carriers of pathogenic ATM germline variants. Breast-conserving therapy and adjuvant RT may be safely considered among appropriately selected carriers of ATM germline variants.
INTRODUCTION
Syndromes of DNA repair deficiency may confer both cancer predisposition1 and increased sensitivity to DNA-damaging agents, such as ionizing radiation.2 A master regulator of DNA double-strand break repair, the ataxia telangiectasia mutated (ATM) gene, encodes a kinase with downstream targets, including BRCA1, BRCA2, CHEK2, and p53.3 Homozygous deficiency of ATM causes the rare, autosomal-recessive ataxia-telangiectasia syndrome (A-T), characterized by cerebellar ataxia, oculocutaneous telangiectasias, reduced life expectancy, and an increased predisposition to cancers of the breast4 and lymphoid system.5 Patients with A-T also exhibit profound sensitivity to ionizing radiation, with reports of patients with pediatric A-T lymphoma sustaining severe radiation-associated pneumonitis and ulcerative dermatitis following radiotherapy (RT).6,7 Heterozygous carriers of ATM germline variants comprise 1%-2% of the US population8 and exhibit increased rates of breast, pancreas, and prostate cancers,9 all of which may typically be treated with RT.
CONTEXT
Key Objective
Given the role of ATM in DNA damage repair, do patients with breast cancer who carry ATM germline pathogenic variants exhibit excess toxicity following adjuvant radiotherapy?
Knowledge Generated
Among 91 carriers of germline ATM variants with breast cancer (23 pathogenic and 68 variants of uncertain significance), and at a median follow-up of nearly 3 years, we found no increased toxicity between the groups, nor in either group when compared with historical nongenetically selected controls. Rates of contralateral cancers and secondary malignancies were similarly rare and did not differ from those seen in unselected populations.
Relevance
Appropriately selected patients with breast cancer who carry germline pathogenic variants of ATM can be safely considered for breast-conserving therapy, inclusive of adjuvant radiotherapy.
ATM functions in repairing the main therapeutic lesion of RT: the DNA double-strand break. Consequently, there is concern that heterozygous carriers of ATM variants may be susceptible to excess RT-associated toxicity, not only manifesting as local tissue injury but also leading to an elevated risk of RT-induced secondary malignancies. The WECARE study was among the largest analyses to date positing that certain ATM alleles, including those yet to be characterized as pathogenic, may confer an elevated risk of contralateral breast cancer (CBC) following breast RT.1,10 Moreover, a haploinsufficient radiosensitive phenotype has been suggested by in vitro assays in the setting of ATM heterozygosity,11 but the degree to which these preclinical findings apply to clinical practice remains unclear. Therefore, we evaluated the safety and tolerability of adjuvant breast RT among carriers of germline ATM variants following breast RT by analyzing acute and late RT-associated toxicity.
METHODS
We identified patients who presented to our center with a diagnosis of breast cancer, 167 of whom were found to carry ATM germline variants by standard-of-care panel-based genetic testing. Of these patients, 91 received RT between 1992 and 2018 and had evaluable records. ATM variants were classified as pathogenic for this study per the interpretation of the diagnostic laboratory and reviewed in ClinVar to be listed as pathogenic or likely pathogenic.12 Those with benign or likely benign variants were excluded. The remaining ATM variants (uncertain significance or conflicting interpretations of pathogenicity) were classified in this analysis as variants of uncertain significance (VUS).
Toxicities were ascertained from medical records and graded per Common Terminology Criteria for Adverse Events (CTCAE) v5,13 Baker capsular contracture scale,14 and International Society for Lymphology lymphedema scale.15 Toxicities of grade 2 or above were recorded at three time points: end of treatment visit (acute), 1 year (chronic), and at the last follow-up (only ascertained if at least 2 years after completing RT). Additional relevant clinicopathologic parameters were collected, including age, body mass index (BMI), comorbidities, and smoking history, as were treatment characteristics such as RT dose, fractionation regimen, boost RT, use of bolus, and RT treatment modality. Associations between clinicopathologic features, treatment characteristics, and toxicity were evaluated by univariable and multivariable logistic regression models. Overall survival and recurrence events were estimated using the Kaplan-Meier method. Secondary malignancy and CBC were evaluated using Fine-Gray competing risks methodology, with death as a competing event.
RESULTS
Of 91 ATM germline variant carriers who received breast RT, median age was 48 years (28-73 years), with 25% (n = 23) harboring a pathogenic variant (PV) and 75% (n = 68) harboring a VUS (Table 1). Median follow-up was 32 months following RT (range, 7-303); 73% (n = 72) underwent lumpectomy and adjuvant whole-breast RT, and 27% (n = 26) underwent mastectomy and post-mastectomy RT. Twenty-one (81%) of the mastectomy patients underwent reconstruction before RT, primarily immediate-delayed implant-based reconstructions in which the tissue expander was irradiated (n = 14). Seven patients received bilateral RT and two received re-irradiation to the same breast for a total of 100 RT courses; the re-irradiation course toxicities were excluded from toxicity analysis for a total of 98 primary courses. Thirty-two percent of patients (n = 31) received hypofractionated RT; none of the patients (n = 43) who received regional nodal irradiation (RNI) received hypofractionated RT.
TABLE 1.
Patient and Treatment Characteristics
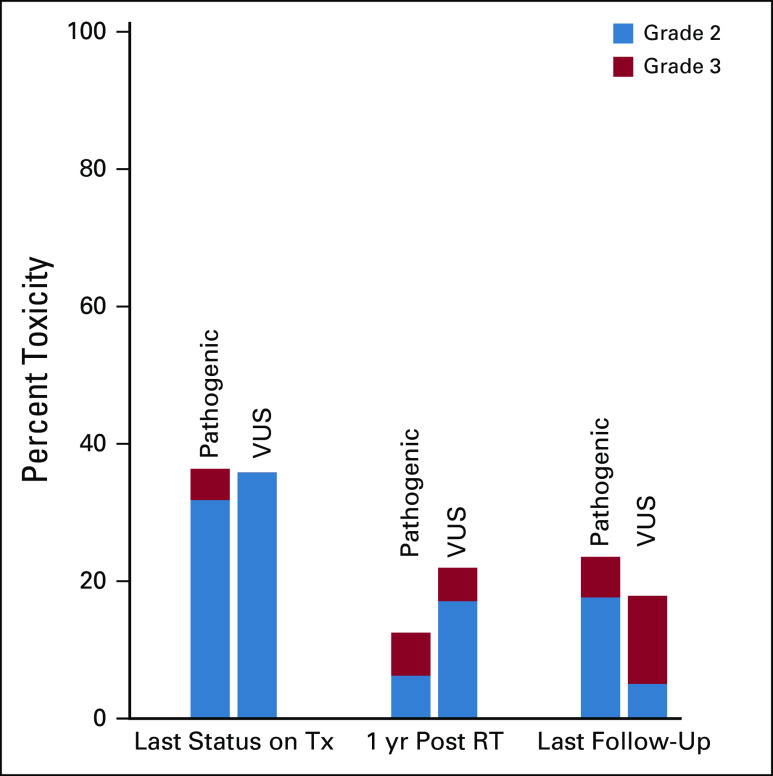
At the final on-treatment visit, 37% (n = 33/89) exhibited grade ≥ 2 toxicity: 30% (n = 27) with grade 2 and 1% (n = 1) with grade 3 dermatitis, and 6% with other grade 2 events (fatigue, seroma, decreased range of motion, or pain) (Fig 1). No difference in toxicity was observed between those harboring pathogenic or VUS ATM variants on univariate and multivariable analyses (Table 2), and toxicity rates for both groups were consistent with those of adjuvant breast RT among genotypically unselected cohorts (Table 3). On multivariable analysis, breast-only versus comprehensive regional nodal radiation was the only significant predictor of toxicity (odds ratio [OR], 6.29; P < .001).
FIG 1.
Proportion of grade 2 and 3 toxicity among carriers of ATM germline variants as stratified by variant pathogenicity. RT, radiotherapy; tx, treatment; VUS, variant of uncertain significance.
TABLE 2.
Univariate and Multivariable Regression Models of Clinicopathologic or Treatment Characteristics and Their Association With Toxicity Grade ≥ 2
TABLE 3.
Acute and Late Toxicities Compared With Historical Controls From Breast RT Studies Among Genetically Unselected Patients
At 1 year following RT, 18% (n = 10/57) had grade ≥ 2 toxicity: grade 2a lymphedema of the upper extremity (n = 3), grade 2b lymphedema of the breast (n = 1), grade 3 skin necrosis (n = 1), and capsular contracture of grade 2 (n = 3) or grade 3 (n = 2). Use of bolus was associated with any grade 2-3 toxicity at 1 year (OR, 12.1; P = .004), but ATM variant status was not (Fig 1; Table 2).
The last follow-up visit, analyzed only if occurring at least 2 years following RT, was a median of 60 months following RT (range 27-334) and demonstrated 20% (n = 11/56) grade > 2 toxicity, entirely comprising lymphedema or capsular contracture: grade 2a lymphedema of the upper extremity (n = 4), grade 2b lymphedema of the breast (n = 1), and grade 2 (n = 1) or grade 3 (n = 5) capsular contracture. Incidentally, all five patients with grade 3 capsular contracture had a contralateral prophylactic mastectomy with reconstruction that also exhibited grade 2-3 contracture of the nonirradiated breast. Use of bolus was associated with toxicity at this timepoint (OR, 3.87; P = .049), but ATM variant status was not (Fig 1; Table 2). Of the 35 patients who were excluded from last follow-up analysis, only one patient was lost to follow-up approximately 3 years post-RT; the remaining 34 had not reached 2 years post-RT.
Among carriers of pathogenic ATM germline variants, the proportion of grade ≥ 2 toxicity unrelated to post-mastectomy reconstruction was 32% at the end of treatment, 11% at 1 year of follow-up, and 8% at the last follow-up. Among VUS carriers, the proportion of grade ≥ 2 toxicity unrelated to post-mastectomy reconstruction was: 34% at the end of treatment, 4% at 1 year of follow-up, and 13% at the last follow-up.
Overall, no patients exhibited significant (grade ≥ 2) telangiectasias, fibrosis, or fat necrosis at any time point, and no grade 4 or 5 toxicities were observed. There were no discernible differences in local toxicity or RT tolerability when comparing pathogenic ATM variants versus VUS.
CBCs were observed in seven patients (PV: n = 1; VUS: n = 6) at a median of 8 years (range: 1-16) after breast RT, yielding a 10-year cumulative incidence of CBC of 4.5% (Table 4). Detailed radiation records of the initial treatment were unavailable to retrospectively calculate dose to the contralateral breast. Metachronous nonbreast malignancies were observed in 12 patients at a median of 8 years (range, 0.1-16.8) following RT. Four of these arose in carriers of pathogenic ATM germline variants, and none were in the RT treatment field. There was no significant difference in the rate of secondary malignancies in pathogenic versus VUS carriers (n = 4 pathogenic, n = 8 VUS; P = .74).
TABLE 4.
Contralateral Breast Cancer Cases
Overall, seven local recurrence events were observed, and two patients were treated with repeat RT (pathogenic n = 1, VUS n = 1), neither of whom has exhibited grade ≥ 2 toxicity to date. For the overall cohort, 5-year recurrence-free survival was 89% (95% CI, 81 to 98) and 5-year overall survival was 91% (95% CI, 83 to 99).
DISCUSSION
Among this cohort of heterozygous carriers of ATM germline variants, all of whom were treated with adjuvant RT for breast cancer, we found no association between pathogenicity of the germline variants and excess local toxicities. Although seven patients developed CBCs at a median of 8 years following RT, only one of these patients carried a pathogenic ATM variant and the overall rate of CBC was comparable to that seen in unselected populations of patients with breast cancer.
As expected, bolus was significantly correlated with toxicity at both 1-year follow-up and last follow-up in our cohort. Unlike other studies of RT among unselected patients, BMI,23 age,24 and smoking history25 were not significant predictors for RT-associated local toxicity. The single patient in our cohort who developed grade 3 skin necrosis at 1 year also had grade 3 dermatitis at last on-treatment visit. This was an unusual case in which capecitabine was administered concurrently with RT. No other patients in this cohort received chemotherapy concurrent with RT.
The average age of breast cancer onset in our cohort was 48, younger than the average breast cancer diagnosis age and similar to those with BRCA1/2 variants.26
Although the current study does not show a direct association between pathogenicity and RT toxicity among carriers of ATM variants, others have found an increased risk of grades 3-4 subcutaneous late toxicities after breast RT in women harboring two ATM variants (n = 3) but not one ATM variant (n = 3) (whether those with two variants harbored them on the same allele or in a homozygous/biallelic fashion was not reported).27
Consistent with our findings, another reported cohort of 14 patients with breast cancer carrying pathogenic germline ATM variants showed no excessive toxicity and demonstrated superior local control and survival with RT compared with nonirradiated patients.28 Indeed, drawing from this and other studies, the American Society for Radiation Oncology (ASTRO) recently developed guidelines suggesting that carriers of pathogenic ATM variants do not face inordinate risk of RT-associated toxicity and can feasibly receive adjuvant radiotherapy.29
The likelihood of acute radiation dermatitis in this series is similar to that seen on either arm of MA.20, a randomized controlled trial that evaluated the addition of RNI to whole breast radiation among genetically unselected patients who underwent lumpectomy,16 in addition to several other landmark breast RT studies (Table 3). Similarly, the rates of lymphedema in the current series are similar to those from the RNI arm of MA.20 and the nodal RT (without axillary dissection) arm of AMAROS.21 The rates of capsular contracture in our series are also consistent with previous literature among genetically unselected cohorts (Benediktsson et al, 2006), and all patients in our series with grade 3 capsular contracture also had grade 2-3 capsular contracture of the nonirradiated reconstructed contralateral breast. For comparison, a prospective trial monitoring capsular contracture following implant reconstruction showed grades 3 and 4 capsular contracture in as many as 42% of patients following RT and 15% without RT (Benediktsson et al., 2006).
Concern has been raised over increased CBC risk among heterozygous carriers of ATM variants treated with RT. The WECARE study ascertained 708 patients with CBC who were matched to 1,399 controls with unilateral cancer diagnosed in 1985-2000. In a 2010 analysis among those carrying putatively deleterious ATM missense variants, mean contralateral breast dose of ≥ 1.0Gy was associated with an increased risk of CBC both compared to those with wild-type ATM (rate ratio [RR], 3.3) and compared with ATM variant carriers who did not receive RT (RR, 5.8).1 More recently, the WECARE investigators further demonstrated that RT significantly modified CBC risk among carriers of ATM rare missense VUSs (risk of CBC without RT, RR = 0.38; 95% CI, 0.09 to 1.15; v with RT, RR = 2.98; 95% CI, 1.31 to 6.80).10 Contemporary RT simulation and treatment planning approaches may mitigate the risk of this otherwise rare event by limiting contralateral breast radiation exposure to far less than historical levels. Notably, whereas seven women in our series were diagnosed with CBC at a median 8 years following RT (10-year CBC risk = 4.5%), VUS carriers accounted for 6 of these 7 patients.
Of additional interest, we were unable to elaborate on differences in acute or chronic cardiovascular toxicity, although a meta-analysis of ATM carriers found an increased risk of mortality from ischemic heart diseases (RR, 1.7) relative to non-ATM carriers.9 More detailed prospective monitoring of ATM variant carriers will be needed to further elucidate the potential for RT to influence cardiovascular outcomes.
These findings must be interpreted in the context of the study design. Although our analyses benefit from a large, uniformly treated cohort of patients with breast cancer at a single institution over several years of follow-up, these retrospective data are necessarily limited by the potential for bias and confounding in our report. Efforts were made to mitigate these concerns by comprehensively including all carriers of ATM germline variants presenting to our center who received RT, and by performing multivariable analyses that incorporate clinicopathologic and treatment parameters alongside ATM pathogenicity and VUS status. It also remains possible that rapidly transient toxicities that arose and resolved between our analytical time points were not captured (i.e. between last on-treatment visit and 1-year, or between 1-year follow-up and last follow-up), although the clinical relevance of these short-lived findings may be of limited interest.
Thus, in this cohort of patients with breast cancer harboring ATM germline variants classified as pathogenic or VUS, we found no evidence of excess radiation-associated toxicity or an elevated risk of radiation-associated secondary malignancies. Although larger studies with longer follow-up are needed to fully characterize the effects of RT in this population, our findings suggest that breast-conserving therapy and adjuvant RT may be safely considered among carriers of ATM germline variants.
PRIOR PRESENTATION
Presented as an Oral Abstract at 2019 ASCO Annual Meeting, Chicago, IL, May 31-June 4, 2019.
SUPPORT
Partially supported by the NIH/NCATS Grant # TL1-TR-002386 (L.A.M.); ASCO Conquer Cancer Merit Award 2019 (L.A.M.); NIH/NCI Cancer Center Support Grant (P30 CA008748).
AUTHOR CONTRIBUTIONS
Conception and design: Leslie A. Modlin, Atif J. Khan, Beryl McCormick, Simon N. Powell, Lior Z. Braunstein
Financial support: Leslie A. Modlin, Lior Z. Braunstein
Administrative support: Leslie A. Modlin, Lior Z. Braunstein
Provision of study materials or patients: Leslie A. Modlin, Lior Z. Braunstein
Collection and assembly of data: Leslie A. Modlin, Boris Mueller, Erin F. Gillespie, Lior Z. Braunstein
Data analysis and interpretation: Leslie A. Modlin, Jessica Flynn, Zhigang Zhang, Oren Cahlon, Atif J. Khan, Zsofia K. Stadler, Mark E. Robson, Lior Z. Braunstein
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Atif J. Khan
Employment: Memorial Sloan-Kettering Cancer Center
Research Funding: Clovis Oncology
Erin F. Gillespie
Other Relationship: eContour
Beryl McCormick
Stock and Other Ownership Interests: Varian Medical Systems
Zsofia K. Stadler
Consulting or Advisory Role: Allergan, Genentech/Roche, Regeneron, Optos, Adverum, Biomarin, Alimera Sciences, Novartis, Spark Therapeutics, Fortress Biotech, Regenxbio
Mark E. Robson
Consulting or Advisory Role: Change HealthCare
Research Funding: AstraZeneca, Abbvie, Pfizer, Merck
Travel, Accommodations, Expenses: AstraZeneca, Merck
Other Relationship: Research to Practice, Clinical Care Options, Physicans' Education Resource, Invitae, Pfizer
(OPTIONAL) Uncompensated Relationships: Merck, Pfizer, Daiichi Sankyo, Epic Sciences
(OPTIONAL) Open Payments Link: https://openpaymentsdata.cms.gov/physician/612669/summary
Simon N. Powell
Consulting or Advisory Role: Varian Medical Systems, Philips Healthcare
Research Funding: Philips Healthcare
No other potential conflicts of interest were reported.
REFERENCES
- 1.Bernstein JL, Haile RW, Stovall M, et al. : Radiation exposure, the ATM gene, and contralateral breast cancer in the women's environmental cancer and radiation epidemiology study. J Natl Cancer Inst 102:475-483, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma J, Setton J, Morris L, et al. : Genomic analysis of exceptional responders to radiotherapy reveals somatic mutations in ATM. Oncotarget 8:10312-10323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Awasthi P, Foiani M, Kumar A: ATM and ATR signaling at a glance. J Cell Sci 129:1285, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Swift M, Reitnauer PJ, Morrell D, et al. : Breast and other cancers in families with ataxia-telangiectasia. N Engl J Med 316:1289-1294, 1987 [DOI] [PubMed] [Google Scholar]
- 5.Stankovic T, Kidd AM, Sutcliffe A, et al. : ATM mutations and phenotypes in ataxia-telangiectasia families in the British Isles: Expression of mutant ATM and the risk of leukemia, lymphoma, and breast cancer. Am J Hum Genet 62:334-345, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gotoff SP, Amirmokri E, Liebner EJ: Ataxia telangiectasia. Neoplasia, untoward response to x-irradiation, and tuberous sclerosis. Am J Dis Child 114:617-625, 1967 [DOI] [PubMed] [Google Scholar]
- 7.Morgan JL, Holcomb TM, Morrissey RW: Radiation reaction in ataxia telangiectasia. Am J Dis Child 116:2, 1968 [DOI] [PubMed] [Google Scholar]
- 8.Swift M, Morrell D, Cromartie E, et al. : The incidence and gene frequency of ataxia-telangiectasia in the United States. Am J Hum Genet 39:573-583, 1986 [PMC free article] [PubMed] [Google Scholar]
- 9.van Os NJ, Roeleveld N, Weemaes CM, et al. : Health risks for ataxia-telangiectasia mutated heterozygotes: A systematic review, meta-analysis and evidence-based guideline. Clin Genet 90:105-117, 2016 [DOI] [PubMed] [Google Scholar]
- 10.Reiner AS, Robson ME, Mellemkjaer L, et al. : Radiation treatment, ATM, BRCA1/2, and CHEK2*1100delC pathogenic variants, and risk of contralateral breast cancer. J Natl Cancer Inst 112:1275-1279, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paterson MC, Anderson AK, Smith BP, et al. : Enhanced radiosensitivity of cultured fibroblasts from ataxia telangiectasia heterozygotes manifested by defective colony-forming ability and reduced DNA repair replication after hypoxic gamma irradiation. Cancer Res 29:10, 1979 [PubMed] [Google Scholar]
- 12.Landrum MJ, Lee JM, Benson M, et al. : ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res 46:D1062-D1067, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.United States Department of Health and Human Services : Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017 [Google Scholar]
- 14.Spear SL, Baker JL Jr: Classification of capsular contracture after prosthetic breast reconstruction. Plast Reconstr Surg 96:1119-1123, 1995; discussion 1124 [PubMed] [Google Scholar]
- 15.International Society of Lymphology : The diagnosis and treatment of peripheral lymphedema: 2016 Consensus Document of the International Society of Lymphology. Lymphology 49:170-184, 2016 [PubMed] [Google Scholar]
- 16.Whelan TJ, Olivotto IA, Parulekar WR, et al. : Regional nodal irradiation in early-stage breast cancer. N Engl J Med 373:307-316, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patel AK, Ling DC, Richman AH, et al. : Hypofractionated whole-breast irradiation in large-breasted women-is there a dosimetric predictor for acute skin toxicities? Int J Radiat Oncol Biol Phys 103:71-77, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Pignol JP, Olivotto I, Rakovitch E, et al. : A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 26:2085-2092, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Whelan TJ, Pignol JP, Levine MN, et al. : Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 362:513-520, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Matzinger O, Heimsoth I, Poortmans P, et al. : Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol 49:24-34, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Donker M, van Tienhoven G, Straver ME, et al. : Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): A randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol 15:1303-1310, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benediktsson K, Perbeck L: Capsular contracture around saline-filled and textured subcutaneously-placed implants in irradiated and non-irradiated breast cancer patients: Five years of monitoring of a prospective trial. J Plast Reconstr Aesthet Surg 59:27-34, 2006 [DOI] [PubMed] [Google Scholar]
- 23.Parekh A, Dholakia AD, Zabranksy DJ, et al. : Predictors of radiation-induced acute skin toxicity in breast cancer at a single institution: Role of fractionation and treatment volume. Adv Radiat Oncol 3:8-15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright JL, Takita C, Reis IM, et al. : Racial variations in radiation-induced skin toxicity severity: Data from a prospective cohort receiving postmastectomy radiation. Int J Radiat Oncol Biol Phys 90:335-343, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Twardella D, Popanda O, Helmbold I, et al. : Personal characteristics, therapy modalities and individual DNA repair capacity as predictive factors of acute skin toxicity in an unselected cohort of breast cancer patients receiving radiotherapy. Radiother Oncol 69:145-153, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Kuchenbaecker KB, Hopper JL, Barnes DR, et al. : Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 317:2402-2416, 2017 [DOI] [PubMed] [Google Scholar]
- 27.Iannuzzi CM, Atencio DP, Green S, et al. : ATM mutations in female breast cancer patients predict for an increase in radiation-induced late effects. Int J Radiat Oncol Biol Phys 52:8, 2002 [DOI] [PubMed] [Google Scholar]
- 28.Su Y, Swift M: Outcomes of adjuvant radiation therapy for breast cancer in women with Ataxia-Telangiectasia mutations. JAMA 286:2, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Bergom C, West CM, Higginson DS, et al. : The implications of genetic testing on radiotherapy decisions: A guide for radiation oncologists. Int J Radiat Oncol Biol Phys 317:105-712, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]