Abstract
PURPOSE
Many novel therapies for relapsed and refractory neuroblastoma require tumor tissue for genomic sequencing. We analyze our experience with image-guided biopsy in these patients, focusing on safety, yield, adequacy for next-generation sequencing (NGS), and correlation of tumor cell percent (TC%) with quantitative uptake on 123I-meta-iodobenzylguanidine (MIBG) single-photon emission computed tomography with computed tomography (SPECT/CT).
MATERIALS AND METHODS
An 11-year retrospective review of image-guided biopsy on 66 patients (30 female), with a median age of 8.7 years (range, 0.9-49 years), who underwent 95 biopsies (55 bone and 40 soft tissue) of relapsed or refractory neuroblastoma lesions was performed.
RESULTS
There were seven minor complications (7%) and one major complication (1%). Neuroblastoma was detected in 88% of MIBG- or fluorodeoxyglucose-avid foci. The overall NGS adequacy was 69% (64% in bone and 74% in soft tissue, P = .37). NGS adequacy within neuroblastoma-positive biopsies was 88% (82% bone and 96% soft tissue, P = .11). NGS-adequate biopsies had a greater mean TC% than inadequates (51% v 18%, P = .03). NGS-adequate biopsies had a higher mean number of needle passes (7.5 v 3.4, P = .0002). The mean tissue volume from NGS-adequate soft-tissue lesions was 0.16 cm3 ± 0.12. Lesion:liver and lesion:psoas MIBG uptake ratios correlated with TC% (r = 0.74, r = 0.72, and n = 14). Mean TC% in NGS-adequate samples was 51%, corresponding to a lesion:liver ratio of 2.9 and a lesion:psoas ratio of 9.0. Thirty percent of biopsies showed an actionable ALK mutation or other therapeutically relevant variant.
CONCLUSION
Image-guided biopsy for relapsed or refractory neuroblastoma was safe and likely to provide NGS data to guide therapy decisions. A lesion:liver MIBG uptake ratio of ≥ 3 or a lesion:psoas ratio of > 9 was associated with a TC% sufficient to deliver NGS results.
INTRODUCTION
Neuroblastoma is a pediatric cancer arising from the peripheral sympathetic nervous system comprising 12% of childhood cancer deaths.1,2 Survival rates vary with patient and tumor characteristics described in the International Neuroblastoma Risk Group classification system,3-5 which assigns groups based on age, International Neuroblastoma Risk Group tumor stage, histopathology, MYCN oncogene copy number, chromosome 11q copy number, and tumor ploidy. Neuroblastoma risk groups are deemed very low-, low-, intermediate-, and high-risk, corresponding to 5-year survival rates of > 85%, 75%-85%, 50%-75%, and < 50%, respectively. High-risk patients have a 50%-60% incidence of disease progression during or after initial therapy.3
CONTEXT
Key Objective
Ongoing investigation and implementation of genetically targeted therapies for relapsed and refractory neuroblastoma requires procurement of adequate tumor tissue for next-generation sequencing (NGS). This study investigated the use of image-guided core needle biopsy for this purpose, with a focus on 123I-meta-iodobenzylguanidine (MIBG) scintigraphy variables that were associated with sample adequacy for NGS.
Knowledge Generated
Image-guided core needle biopsy for relapsed and refractory neuroblastoma is a safe and effective means of procuring NGS-adequate tissue from bone and soft-tissue masses. Adequate tissue samples contained an estimated tumor cell volume of 0.10cm3, with an average sample composition of 50% tumor cells, which in this study corresponded to a lesion:liver 123I-MIBG avidity ratio of approximately 3.0.
Relevance
Needle biopsy represents a less invasive alternative to open or laparoscopic biopsy of relapsed and refractory neuroblastoma. Quantification of 123I-MIBG avidity can aid in the selection of biopsy sites in the setting of multiple metastases.
Relapsed neuroblastoma remains a largely incurable disease. This intractability is thought to be due to significant clonal evolution over the course of genotoxic therapy, with relapsed tumors often exhibiting a set of mutations highly divergent from those present at primary diagnosis.6-10 Given this, clinical trials have sought to target new pharmacotherapies against specific genetic aberrations in patients with relapsed neuroblastoma, such as mutations in the ALK gene (ClinicalTrials.gov identifier: NCT02780128).11 Institution of these therapies is dependent on successful genetic profiling of the disease at study entry.
Successful next-generation sequencing (NGS) of primary refractory (disease does not regress or progresses during frontline chemotherapy) or relapsed neuroblastomas requires biopsy providing adequate lesional tissue for DNA extraction. Adequacy might be influenced by numerous factors including sample tumor cell percent (TC%), sample volume, and origin of the sample (bone v soft tissue). Here, we summarize our experience with biopsy of relapsed and refractory high-risk neuroblastoma, focusing on safety, yield, sample adequacy for NGS and assessing for possible factors that may predict adequacy, including quantitative 123I-meta-iodobenzylguanidine (MIBG) uptake within the target lesion. MIBG single-photon emission computed tomography with computed tomography (SPECT/CT) has been regarded as a nonquantitative imaging modality because of a lack of correction for photon absorption and photon scatter. A recent demonstration of quantitative MIBG SPECT/CT with low-intrapatient variability and high accuracy in patients12 with neuroblastoma has now provided the opportunity to use this tool for biopsy site selection.
MATERIALS AND METHODS
Subject Enrollment
Imaging, pathology, and clinical data were obtained by review of the electronic medical record (Epic Systems, Verona, WI) and radiology imaging archive (iSite PACS; Philips Healthcare, Eindhoven, Netherlands). All patients who underwent image-guided biopsy for relapsed or primary refractory neuroblastoma over an 11-year period (January 1, 2009, to January 1, 2020) were included. The Children's Hospital Institutional Review Board reviewed and approved this research project.
Prebiopsy Imaging and Biopsy Planning
Biopsy site selection was directed by MIBG imaging in all but nine patients who did not have known MIBG-avid disease; of these nine, five were guided by 18F-fluorodeoxyglucose (FDG) positron emission tomography, whereas four had no prebiopsy nuclear imaging available. Nuclear imaging was used in conjunction with CT and/or magnetic resonance imaging (MRI); 56 biopsies were preceded by MRI. Planar MIBG scans were used in 37 of 95 biopsy procedures, whereas SPECT/CT was used for 54 of 95 biopsies. MIBG imaging was performed 18-24 hours following intravenous administration of 0.14 mCi/kg 123I-MIBG. Images were acquired on a Siemens Symbia Intevo (Munich, Germany) dual head SPECT/CT and processed using a Siemens Syngovia quantitative image analysis package using an ordered subset expectation maximization reconstruction algorithm with attenuation correction resolution recovery and scatter compensation.12 Regions evaluated for MIBG standard uptake value (SUV) included right lobe of the liver, right psoas muscle, and site(s) chosen for biopsy. Regions of interest were drawn by the attending radiologist or a research assistant and confirmed by the attending radiologist (Fig 1). Lesion MIBG uptake was quantified as a ratio of lesion maximum SUV to right lobe of liver (or right psoas) mean SUV. Lesion:liver (and lesion:psoas) MIBG SUV ratios were quantified in 14 cases.
FIG 1.
Eleven-year-old boy underwent 123I meta-iodobenzylguanidine (MIBG) imaging with single-photon emission computed tomography with computed tomography (SPECT/CT) of the chest, abdomen, and pelvis. (A) Planar anterior and posterior images show focal uptake in the left supraclavicular region, thoracic spine, right pelvis, multiple retroperitoneal paraspinal lesions, and bilateral proximal femurs. (B) Axial fused 123I MIBG SPECT/CT shows a left anterior paraspinal lesion with increased activity. (C-E) Axial reprocessed images for standard uptake value analysis show regions of interest placed on the lesion in (C), right lobe of liver in (D), and right psoas in (E).
Biopsy
Image guidance for biopsy varied with anatomic location of the target (Figs 2A-2F). Ultrasound guidance was preferred for soft-tissue masses. Fluoroscopic guidance (Siemens Artis Zeego; Siemens, Munich, Germany) was preferred for large, radiographically visible lesions in the appendicular skeleton or ribs, in which respiratory motion was a concern. Conventional CT (Siemens SOMATOM Definition Flash) and later C-arm CT with navigational guidance software (Siemens Artis Zeego with Syngo iGuide and Syngo DynaCT) were used for radiographically visible small bone lesions of the axial skeleton. For radiographically occult but MRI-visible bone lesions, magnetic resonance fusion software was used when possible. MRI overlaid on fluoroscopy (Siemens Syngo InSpace 3-D/3-D fusion software with MRI preprocessing on Syngo XWP workstation) was preferred for large lesions in the extremities, and MRI fused to C-arm CT was used for small lesions or those in the axial skeleton13,14 (Figs 2A-2F). Bone biopsies were performed with a manual 15-gauge (G) Bonopty coaxial biopsy system (Bonopty; AprioMed AB, Uppsala, Sweden) or a drill-based 13-G biopsy system (OnControl; Teleflex, Wayne, PA). Soft-tissue lesions were obtained using 16-G and 18-G full-core biopsy instruments (BioPince; Argon Medical, Frisco, TX) or a half-core 18-G system (Bard Mission needle; Bard Biopsy, Tempe, AZ). Parallel standard iliac crest marrow aspirates were performed by an oncologist during bone biopsies in 30 of 55 cases. Biopsy locations are described in Appendix Figure A1. Total volume of biopsy tissue was derived from instrument gauge and trough length (using the cylinder formula Vp = πr2l, where Vp is the pass volume, r is the instrument radius, and l is the trough length) multiplied by the number of passes made. This formula was used for BioPince needles, whereas for half-core Bard Mission needles, Vp was halved. This analysis was performed on soft-tissue masses only, as radiographic visibility of bone lesions was variable, preventing consistent measurement of trough length passing through tumor. Procedural complications were classified according to the guidelines defined in the Society of Interventional Radiology (SIR) Clinical Practice Guidelines.15 Technical success was defined as successful procurement of tissue suitable for pathologic analysis.
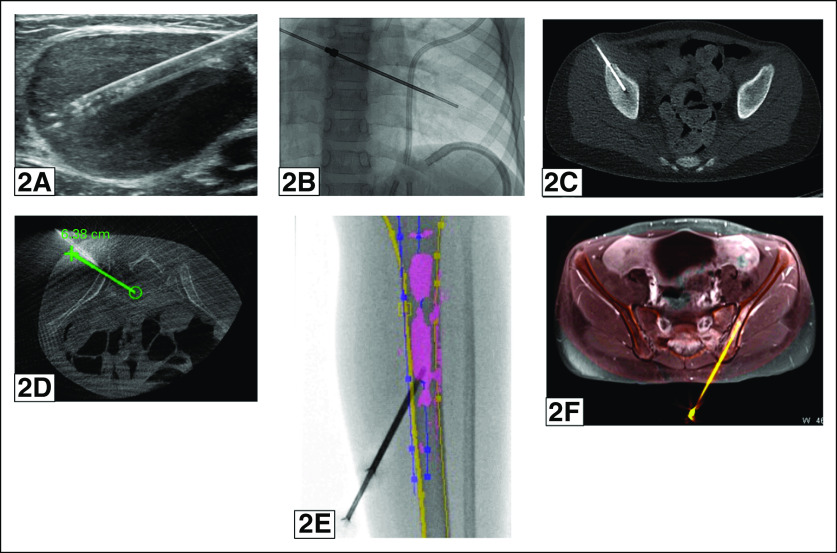
FIG 2.
(A) A 3-year-old patient underwent ultrasound-guided biopsy of a left inguinal lymph node using a 16G Biopince biopsy instrument, yielding an next-generation sequencing (NGS)–adequate neuroblastoma sample. (B) A 6-year-old patient underwent fluoroscopically guided biopsy of a radiologically evident right 8th rib lesion using a 15G Bonopty biopsy instrument, yielding an NGS-adequate neuroblastoma sample. (C) A 9-year-old patient underwent conventional computed tomography (CT)–guided biopsy of a radiologically evident right iliac lesion using a 15G Bonopty instrument, yielding an NGS-adequate neuroblastoma sample. (D) An 8-year-old patient underwent biopsy of a radiologically evident left sacral lesion with guidance from C-arm CT with iGuide software using a 13G OnControl biopsy instrument, yielding an NGS-adequate neuroblastoma sample. (E) An 8-year-old patient underwent biopsy of a radiologically occult left tibial lesion with guidance from fluoroscopy with 2D magnetic resonance (MR) overlay using a 13G OnControl biopsy instrument, yielding an NGS-adequate neuroblastoma sample (lesion outlined in pink). (F) A 13-year-old patient underwent biopsy of a radiologically occult left iliac lesion with guidance from C-arm CT with 3D MR overlay using a 15G Bonopty biopsy instrument, yielding a sample that was neuroblastoma-positive but inadequate for NGS.
Pathology and Genetic Analysis
Tissue was formalin-fixed, decalcified as needed using EDTA, and paraffin-embedded following standard laboratory procedures. Information on TC% estimates is provided in the Appendix. A sample positive for neuroblastoma from immature to mature was counted as tumor-positive. Thus, MIBG- or FDG-avid tissue may, on pathologist review, represent active neuroblastoma (warranting NGS) or post-treatment, mature ganglioneuromatous tissue (in which case, the tissue was not sent for NGS, but was also not counted as a specimen failure). Marrow aspirates were not sent for NGS, except in one instance where marrow was tumor-positive, but the bone cores were negative.
NGS was performed at either Foundation Medicine16 or the Children's Hospital of Philadelphia Division of Genomic Diagnostics,17 depending on era of biopsy and/or clinical trial requirements.
The treating oncologist, in consultation with the pathologist, made the final decision on sending a biopsy specimen for sequencing (Fig 3). Although both NGS platforms request a minimum of 20% TC, in cases where rebiopsy was not feasible, NGS was attempted at TC% as low as 5% with success. Thus, a TC% cutoff of < 5% was used to define an NGS-inadequate sample that never reached the genomics lab. NGS-inadequate samples were thus defined as any in-lab sample failures, biopsies negative for neuroblastic tissue, and biopsies yielding < 5% TC. NGS-adequate samples were defined as returning valid sequencing results from the genomic laboratory. Further discussion of analytic subgroups is included in the Appendix.
FIG 3.
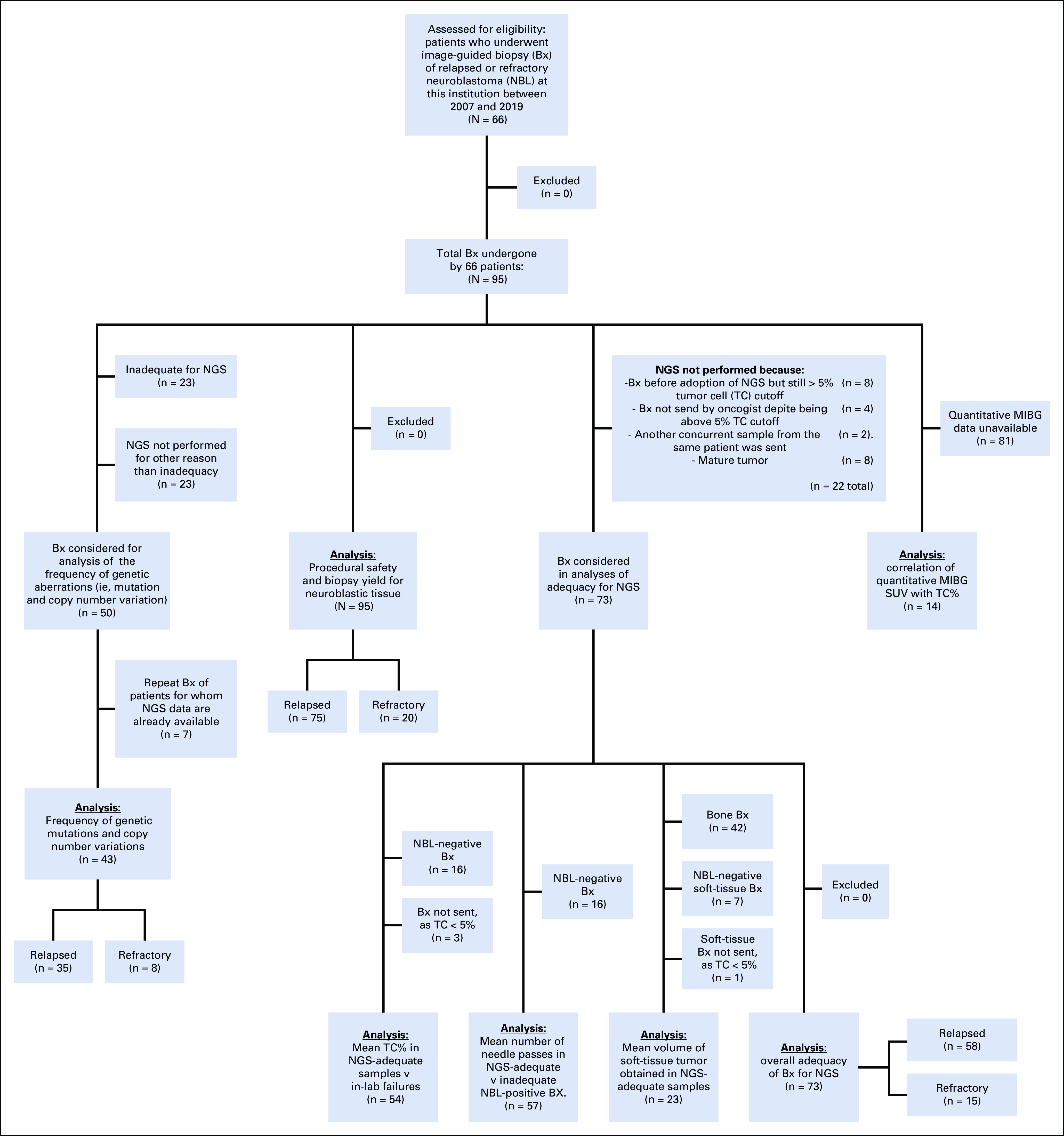
CONSORT diagram describing the subgroups involved in the various analyses within the study, including the analysis of NGS findings and their frequency; analysis of procedural safety and biopsy yield; analyses of NGS adequacy and mean TC%, needle passes, and procured tissue volume; overall NGS adequacy; and analysis of MIBG uptake versus TC%. MIBG, meta-iodobenzylguanidine; NBL, neuroblastoma; NGS, next-generation sequencing; SUV, standard uptake value; TC%, tumor cell percent.
Biopsy yield was calculated as the fraction of MIBG- or FDG-avid lesions demonstrating neuroblastoma-positive tissue on biopsy. Comparison of proportions, as in yield between bone and soft-tissue lesions, was performed by the chi-squared test. Assessment of factors (TC%, needle passes, and tissue volume) influencing sample NGS adequacy was carried out using two-tailed t-tests. Statistical significance for the various tests was achieved if the probability of type 1 error (P) < .05. Linear regression was used to detect correlation between MIBG uptake and TC%.
RESULTS
Patient Characteristics
A total of 66 patients (30 female) with a median age of 8.7 years (range, 0.9-49 years)—who underwent 95 total biopsies—were included in the study, 55 with relapsed disease (75 biopsies) and 11 with refractory disease (20 biopsies). Of these 95 biopsies, 55 were at bony sites and 40 were at soft-tissue sites, with a trend toward relapse patients more frequently having a bony site biopsied (P = .08).
Safety
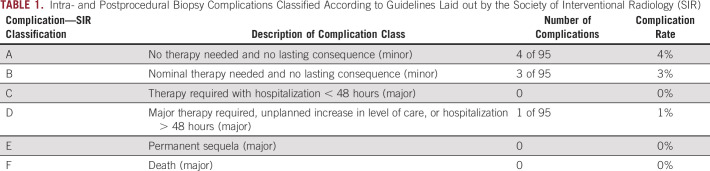
Of 95 procedures, there were seven minor complications (7%), of which four were class A and three class B (Table 1). The class A complications involved the self-resolved production of blood-tinged sputum after thoracic biopsy and three small hematomas near biopsy sites. All three class B complications involved persistent pain at the biopsy site for > 3 days and were managed effectively with oral analgesics. There was one major, class D complication: a ureteral perforation during CT-guided biopsy of a retroperitoneal mass encasing the ureter. The complication was immediately recognized, and the patient was transferred to the operating room under the same anesthesia, where urology placed a double-J stent. This was removed after 6 weeks without further complications. There was no difference between the mean number of needle passes in complicated and uncomplicated cases (six passes v six passes).
TABLE 1.
Intra- and Postprocedural Biopsy Complications Classified According to Guidelines Laid out by the Society of Interventional Radiology (SIR)
Biopsy Yield
The technical success rate was 100%. Eighty-six of 95 lesions were MIBG-avid (91%). The yield for obtaining neuroblastic tissue from MIBG-avid or FDG-avid lesions was 87% (79 of 91). Non-MIBG-avid lesions showing 18F-FDG avidity (n = 5) were neuroblastoma-positive in two cases. Four lesions were neither MIBG- nor FDG-avid; all four were neuroblastoma-negative. Of the neuroblastoma-positive masses, 71 of 79 (90%) were active disease, whereas eight of 79 (10%) were post-treatment neuroblastic tissues and/or mature ganglionic disease. Bone biopsies were not more likely to yield neuroblastic tissue (45 of 54) compared with soft-tissue biopsies (34 of 37) (85% v 94%, P = .21). Seventy-seven percent of relapse patients had biopsies showing viable neuroblastoma, compared with 60% of patients with refractory disease (P = .13). Yield in radiographically occult lesions is discussed in the Appendix.
Adequacy for NGS Analysis
Fifty of 73 samples intended for NGS were adequate for analysis (69%); bone sample adequacy (27 of 42) did not differ significantly from soft tissue (23 of 31, 64% v 74%, P = .37). Among active neuroblastoma-positive biopsies, adequacy for NGS was 88% (82% in bone v 96% in soft tissue, P = .11). Adequacy in relapsed versus refractory cases (for samples with viable neuroblastoma recovered) was seen in 42 of 46 cases (91%) versus eight of 11 (73%) (P = .11). NGS-adequate biopsies had a greater mean TC% than in-lab NGS failures (51% v 18%, P = .03). Biopsies adequate for NGS had a TC% range of 5% to 100%, whereas in-lab failures (n = 4) ranged from 10% to 30% TC. NGS-adequate biopsies had a higher mean number of needle passes than inadequates (7.5 v 3.4, P = .0002). The median number of passes in bone was four (range, 2-25) versus 8 (range, 4-20) in soft tissue. The mean sample tissue volume for NGS-adequate soft-tissue biopsy was 0.16 cm3 ± 0.12 (range, 0.02-0.45 cm3). When adjusting for TC% therein, NGS-adequate soft-tissue samples had a mean TC volume of 0.10 ± 0.09 cm3 (range, 0.01-0.39 cm3). Mean TC volume for soft-tissue NGS failures was not available because there were no in-lab failures for soft-tissue biopsy samples.
MIBG Uptake and TC%
There was a positive correlation between the lesion:liver MIBG SUV ratio and TC% (r = 0.74 and n = 14) (Fig 4A). With the right psoas muscle as internal control, a similarly positive correlation was found (r = 0.72 and n = 14) (Fig 4B). Liver SUVs (average within region of interest) had a mean of 1.7 ± 1.0 (n = 14; range, 0.4-4.28), whereas right psoas SUVs had a mean of 0.43 ± 0.17 (n = 14; range, 0.2-0.76; P = 4.0e−7 for standard deviations). Using linear regression, a TC% of 50% (the mean TC% in NGS-adequate samples) corresponded to a lesion:liver SUV ratio of 2.9 and a lesion:psoas ratio of 9.0 (Figs 4A and 4B).
FIG 4.
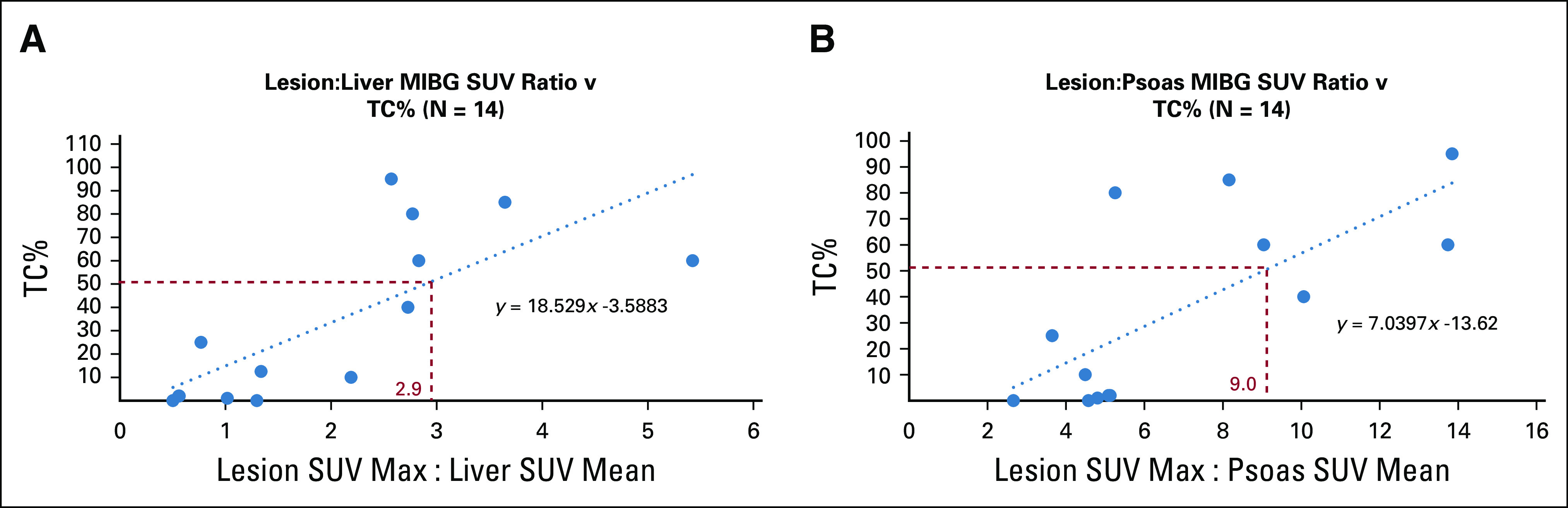
(A) Linear regression analysis demonstrating the relation between the ratio lesion maximum SUV and right lobe of liver mean SUV and lesion TC%. The red dotted lines mark the SUV ratio corresponding to a TC% of 50%, assuming a linear relationship. (B) Linear regression analysis demonstrating the relation between the ratio lesion maximum SUV and right psoas mean SUV. The red dotted lines mark the SUV ratio corresponding to a TC% of 50%, assuming a linear relationship. MIBG, meta-iodobenzylguanidine; SUV, standard uptake value; TC%, tumor cell percent.
NGS Findings
One or more DNA variants of potential clinical significance (reported as potential therapeutic interventions by Foundation Medicine or as a tier 1 or tier 2 variant by Children's Hospital of Philadelphia, excluding variants of unknown significance) were reported in 39 of 43 subjects (91%) (Fig 5). ALK hotspot mutations or gene amplification events were found in 30% of cases.
FIG 5.
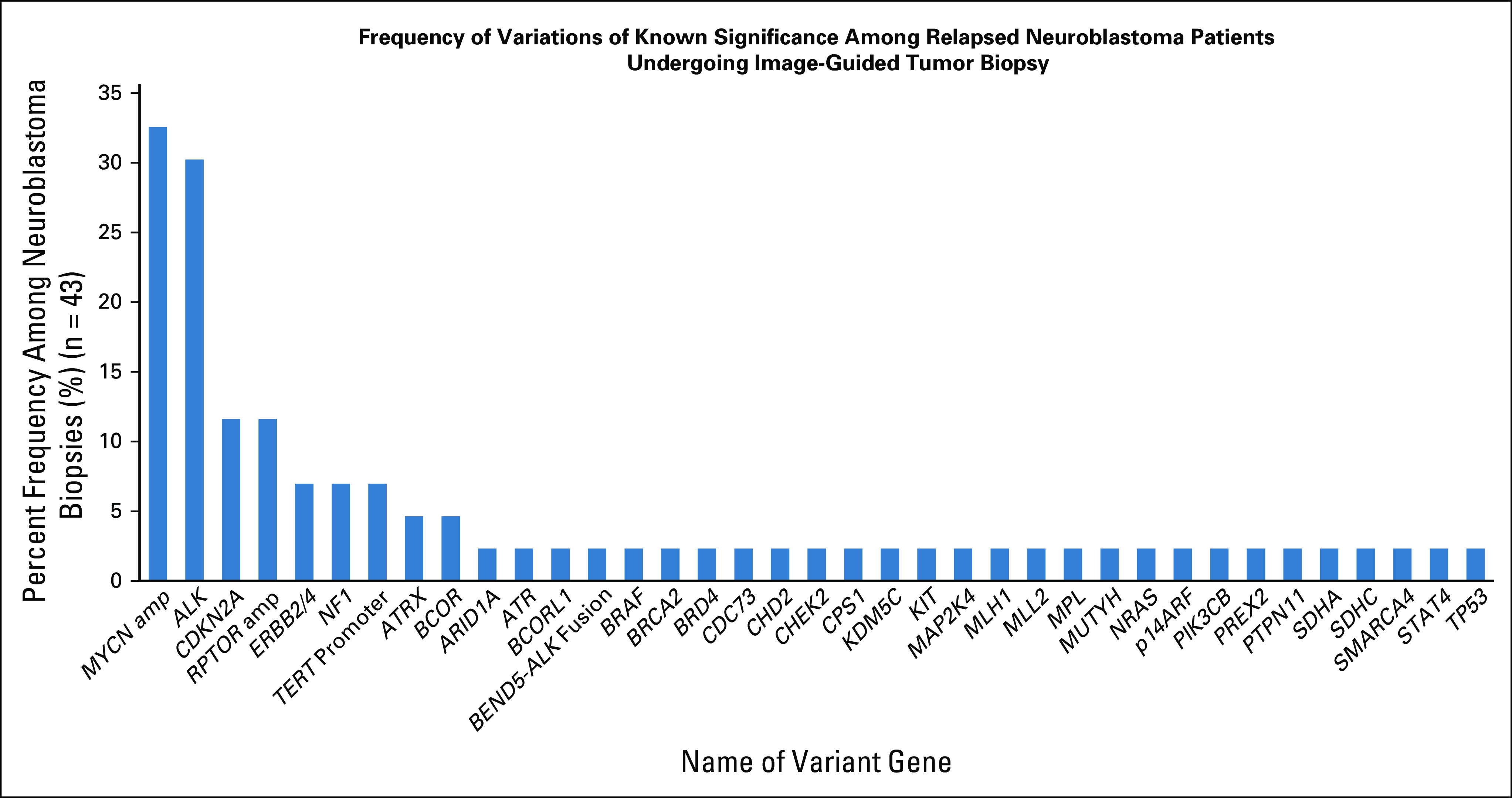
Graphical representation of the percent frequency of selected genetic variants (defined on the Foundation Medicine next-generation sequencing [NGS] report as variants of known significance or tier 1 and tier 2 variants as determined by the Children's Hospital of Philadelphia Division of Genomic Diagnostics) found within 43 patient cases that underwent NGS analysis. This analysis includes only initial biopsies of relapsed tumors and not any subsequent biopsies within the same patient, to avoid double-counting mutations or copy number variations that were already present in the initial biopsy (hence n = 43 v 50 NGS-adequate biospy procedures).
DISCUSSION
Biopsy of primary neuroblastoma at North American children's hospitals is typically performed via open or laparoscopic biopsy; this is partly due to the current recommendation that 1-2 g of tissue is procured for diagnosis, histology, and genomic analysis.18 Core needle biopsy provides a less-invasive alternative to open biopsy. Tissue volume obtained in needle biopsy is significantly smaller than that in open biopsy; consider the mean volume of tissue procured from NGS-adequate soft-tissue masses of 0.16 cm3. Approximating to water density, this corresponds to 0.16 g versus 1-2 g obtained in open biopsy. Even the largest-volume soft-tissue biopsy in this study amounted to 0.45 cm3. This wide gap in tissue mass between open and needle biopsy is one of the primary motivations behind this study.
Six studies between 2006 and 2017 investigated image-guided core needle biopsy for primary neuroblastoma. Reported biopsy yields ranged from 90% to 100%, with a weighted mean of 96.5%. Minor complication rates ranged from 0% to 32% with a weighted mean of 4.4%, and major complication rates ranged from 0% to 36% with a weighted mean of 4.1%. Adequacy for cytogenetic analysis (MYCN amplification, chromosomal abnormalities, and tumor ploidy) ranged from 36% to 96% (weighted mean = 86%), with one study reporting adequacy for either fluorescence in situ hybridization or single nucleotide polymorphism array analysis (but not NGS) of 96%.22 Note that the sample sizes among these studies varied widely (n ranging from 11 to 86 subjects), explaining the wide variation in complication rates and adequacy for cytogenetics.19-24 The rate of complications in our study was consistent with the weighted means calculated from these prior studies focused on newly diagnosed patients.
Our study expands the extant literature to relapsed and refractory high-risk neuroblastoma. Additionally, the above studies did not investigate adequacy for NGS, which is now commonly employed in precision therapy selection. To our knowledge, this is the first study focusing on patients with relapsed or refractory disease being evaluated for investigational studies, with a large number of biopsies performed in sites of osseous metastases. Furthermore, to our knowledge, this is the first study examining imaging and procedural correlates of biopsy adequacy for genetic analysis. The demonstration of safety and diagnostic yield in this study should encourage broader adoption of image-guided biopsies in the relapse setting as we now have a growing armamentarium of precision therapies.9,25,26
Here, we have also expanded the use of quantitative MIBG SPECT/CT from diagnostic staging and tracking tumor response12 to predicting appropriate biopsy sites. Quantitative calculations of lesion:liver and lesion:psoas uptake ratios allowed the demonstration of a positive correlation between MIBG uptake and TC%. Also important was the comparison of right psoas with right lobe of liver as the internal SUV control. This study suggests that psoas has a narrower SUV range and smaller standard deviation than right lobe of liver while producing a lesion:psoas ratio that correlates well with TC%. Although using liver as an internal SUV control is the standard semiquantitative method used in nuclear medicine for assessment of background SUV, we found that the psoas exhibited less interpatient background MIBG variability. In contrast to 18F-FDG positron emission tomography with CT imaging, standard practice for 123I-MIBG SPECT/CT is to limit the CT imaging component to the area of interest, sometimes causing liver to be out of view (particularly with lower extremity lesions). In our study, the psoas was in view on all SPECT/CT images, leading to our consideration of using psoas for background activity assessment. This practice deserves prospective investigation to provide further validity of its use. We recommend psoas as an internal control particularly in cases where SPECT/CT of lower extremities excludes liver but leaves psoas visible.27,28
Beyond demonstrating safety, high yield, and a reasonable rate of NGS adequacy, the results presented here suggest that an interventional radiologist should attempt to obtain at least 0.16 cm3 (calculated as described in methods) of soft-tissue tumor to maximize NGS adequacy. Correction for the TC% contained therein yielded a mean TC volume of 0.10 cm3 in NGS-adequate soft-tissue biopsies. The ability to predict a lesion's TC% based on MIBG uptake would enhance a clinician's ability to maximize sample adequacy. Although calculations of bone sample volume were not performed, the recommendations for a minimum TC volume (0.10 cm3) may still be useful for minimally invasive proceduralists. Generally, we recommend pursuing lesions with a lesion:liver MIBG SUV ratio of at least 3.0 or a lesion:psoas SUV ratio of 9.0. This corresponded to a TC% of 50—more than adequate for molecular genetic analyses, including assays not yet routinely deployed such as RNA sequencing and proteomics. Also note that the minimum TC% compatible with NGS adequacy was 5% in both bone and soft-tissue masses.
A limitation of this study is the retrospective evaluation of an ultrarare condition. Analysis of MIBG SUV to guide biopsy site choice was limited by the sample size of 14, because of late adoption of SUV quantification software. This sample size also prevented SUV comparison between soft-tissue and bone tumors. Also note that correlation of SUV with TC% was made assuming a linear relationship. As more biopsies are performed using MIBG SUV quantification, the sample size–related limitations will diminish, increasing the validity of TC% predictions. More subjects would also enable scrutiny of tumor samples with NGS success despite low TC% and vice versa. Finally, changes in specimen processing over the last decade might have also affected adequacy in ways that are impossible to evaluate.
It is clear that cancers evolve dramatically over the course of therapy and that the genetic landscape of relapsed disease is different from the time of diagnosis.6-10 As more precision therapies are tested in children, there is an urgent need to expand on this experience to access disease at any point in the therapy continuum to guide rational therapeutic decisions. Given the relapsed or refractory nature of these patients' disease, a nonsurgical approach is valuable in determining appropriate therapies. Prospective multi-institutional collaboration will be required to develop widely accepted standard operating procedures for genomic analysis of progressing childhood cancers. We recommend multidisciplinary collaboration between oncologists and interventional and nuclear medicine radiologists for image-guided biopsies in patients with relapsed neuroblastoma to select the lesion(s) that can be approached safely with a high yield for NGS results.
Acknowledgments
ACKNOWLEDGMENT
The authors would like to thank Sandy Mccord for collection and assembly of nuclear medicine imaging data.
APPENDIX 1. Data and Methods Supplement
Criteria for Inclusion in Analysis of Next-Generation Sequencing Adequacy
There were four samples with tumor cell percent (TC%) of 5%, 5%, 5%, and 7.5% that were not submitted for sequencing and thus were removed from the next-generation sequencing (NGS) adequacy portion of the study. Also removed from the NGS adequacy portion of the study were biopsies done before adoption of NGS as the standard at this institution and with TC ≥ 5% (n = 8); biopsies not sent for NGS because another concurrent sample from the same patient was being sent instead (n = 2); and mature neuroblastic tumors, which were not considered failed image-guided biopsies but which could also not be considered NGS-adequate as they were not sent out (n = 8). With these cases excluded (n = 22), 73 biopsies remained for analysis of NGS adequacy. This is illustrated in Figure 3.
Source of TC% Estimates
In 27 cases, estimated TC% was included in the pathology report. In the remaining 46 neuroblastoma-positive cases, a single pathologist retrospectively reviewed existing hematoxylin- and eosin-stained slides to provide an estimate.
Tissue Processing and Sample Integrity
Both decalcifying agents and prolonged formalin fixation can alter DNA integrity, and although both these are avoided in our current pathology lab practice, it is impossible to determine their effects in older samples.
Neuroblastoma Positivity of Radiographically Occult Bone Lesions
Neuroblastoma positivity of radiographically occult bone lesions was investigated. Forty lesions were not visible on computed tomography (CT) and had no concurrent magnetic resonance imaging (MRI), whereas 39 lesions were visible on CT and/or MRI. Neuroblastoma positivity in the nonvisible on CT plus no concurrent MRI group was 11 of 14 (79%) versus 35 of 39 (90%) in the visible on CT and/or MRI group, although this was not statistically significant (P = .30).
FIG A1.
(A) Individual biopsy sites are denoted by a red x in the figure, with soft-tissue sites shown at left and bone sites shown at right. Please note that these are approximations of biopsy sites, not precise locations. (B) General anatomic locations of bone biopsies and number of biopsies in each location. (C) General anatomic locations of soft-tissue biopsies and number of biopsies in each location.
SUPPORT
Supported by NIH grants R35 CA220500 (J.M.M.) and R01 CA140198 (Y.P.M.) and grants from the Alex's Lemonade Stand Foundation (Y.P.M. and J.M.M.), the Band of Parents Foundation (Y.P.M. and J.M.M.), the Solving Kid's Cancer Foundation (Y.P.M. and J.M.M.), and the Open Hands Overflowing Hearts Foundation (Y.P.M.).
AUTHOR CONTRIBUTIONS
Conception and design: Andrew Samoyedny, Abhay Srinivasan, Lisa States, Yael P. Mosse, Michael Acord, John M. Maris, Anne Marie Cahill
Financial support: John M. Maris
Administrative support: John M. Maris
Provision of study materials or patients: Lisa States, Seth Vatsky, John M. Maris, Anne Marie Cahill
Collection and assembly of data: Lisa States, Yael P. Mosse, Emma Alai, Bruce Pawel, Jennifer Pogoriler, Sphoorti Shellikeri, Seth Vatsky
Data analysis and interpretation: Andrew Samoyedny, Abhay Srinivasan, Lisa States, Yael P. Mosse, Emma Alai, Sphoorti Shellikeri, Fernando Escobar, J. Christopher Edgar, John M. Maris
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Yael P. Mosse
Consulting or Advisory Role: TAPUR DSMB
Research Funding: Pfizer
Jennifer Pogoriler
Employment: Bristol-Myers Squibb
Stock and Other Ownership Interests: Bristol-Myers Squibb
John M. Maris
Consulting or Advisory Role: Auron Therapeutics, Illumina Radiopharmaceuticals
Patents, Royalties, Other Intellectual Property: GPC2 binders and CARs, Neuroblastoma antigens
No other potential conflicts of interest were reported.
REFERENCES
- 1.Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 2.Smith V, Foster J. High-risk neuroblastoma treatment review. Children. 2018;5:114. doi: 10.3390/children5090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maris JM.Recent advances in neuroblastoma N Engl J Med 3622202–22112010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davidoff AM.Neuroblastoma Ashcrafts Pediatr Surg 872–8942010 [Google Scholar]
- 5.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma J Clin Oncol 333008–30172015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma X, Liu Y, Liu Y, et al. Pan-cancer genome and transcriptome analyses of 1,699 paediatric leukaemias and solid tumours Nature 555371–3762018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grobner SN, Worst BC, Weischenfeldt J, et al. The landscape of genomic alterations across childhood cancers Nature 555321–3272018 [DOI] [PubMed] [Google Scholar]
- 8.Morrissy AS, Garzia L, Shih DJ, et al. Divergent clonal selection dominates medulloblastoma at recurrence Nature 529351–3572016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eleveld TF, Oldridge DA, Bernard V, et al. Relapsed neuroblastomas show frequent RAS-MAPK pathway mutations Nat Genet 47864–8712015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Padovan-Merhar OM, Raman P, Ostrovnaya I, et al. Enrichment of targetable mutations in the relapsed neuroblastoma genome. PLoS Genet. 2016;12:e1006501. doi: 10.1371/journal.pgen.1006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mosse YP. Next generation personalized neuroblastoma therapy (NEPENTHE) 2016 Clinicatrials.gov Identifier: NCT02780128. https://clinicaltrials.gov/ct2/show/NCT02780128.
- 12.Brady SL, Shulkin BL. Analysis of quantitative [I-123] mIBG SPECT/CT in a phantom and in patients with neuroblastoma. EJNMMI Phys. 2019;6:31. doi: 10.1186/s40658-019-0267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shellikeri S, Setser RM, Hwang TJ, et al. Real-time fluoroscopic needle guidance in the interventional radiology suite using navigational software for percutaneous bone biopsies in children Pediatr Radiol 47963–9732017 [DOI] [PubMed] [Google Scholar]
- 14.Shellikeri S, Setser RM, Vatsky S, et al. Prospective evaluation of MR overlay on real-time fluoroscopy for percutaneous extremity biopsies of bone lesions visible on MRI but not on CT in children in the interventional radiology suite Pediatr Radiol 48270–2782017 [DOI] [PubMed] [Google Scholar]
- 15.Khalilzadeh O, Baerlocher M, Katsarelis D, et al. Proposal of a new adverse event classification system by the SIR Standards of Practice Committee J Vasc Interv Radiol 281432–1437.e32017 [DOI] [PubMed] [Google Scholar]
- 16.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing Nat Biotechnol 311023–10312013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Surrey LF, MacFarland SP, Chang F, et al. Clinical utility of custom-designed NGS panel testing in pediatric tumors. Genome Med. 2019;11:32. doi: 10.1186/s13073-019-0644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ambros PF, Ambros IM, Brodeur GM, et al. International consensus for neuroblastoma molecular diagnostics: Report from the International Neuroblastoma Risk Group (INRG) Biology Committee Br J Cancer100:1471-1482, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hassan SF, Mathur S, Magliaro TJ, et al. Needle core vs open biopsy for diagnosis of intermediate- and high-risk neuroblastoma in children J Pediatr Surg 471261–12662012 [DOI] [PubMed] [Google Scholar]
- 20.Mullassery D, Sharma V, Salim A, et al. Open versus needle biopsy in diagnosing neuroblastoma J Pediatr Surg 491505–15072014 [DOI] [PubMed] [Google Scholar]
- 21.Gupta A, Kumar A, Walters S, et al. Analysis of needle versus open biopsy for the diagnosis of advanced stage pediatric neuroblastoma Pediatr Blood Cancer 47875–8792006 [DOI] [PubMed] [Google Scholar]
- 22.Georgantzi K, Sköldenberg E, Janson ET, et al. Diagnostic ultrasound-guided cutting needle biopsies in neuroblastoma: A safe and efficient procedure J Pediatr Surg 541253–12562019 [DOI] [PubMed] [Google Scholar]
- 23.Campagna G, Rosenfeld E, Foster J, et al. Evolving biopsy techniques for the diagnosis of neuroblastoma in children J Pediatr Surg 532235–22392018 [DOI] [PubMed] [Google Scholar]
- 24.Zhao L, Mu J, Du P, et al. Ultrasound-guided core needle biopsy in the diagnosis of neuroblastic tumors in children: A retrospective study on 83 cases Pediatr Surg Int 33347–3532016 [DOI] [PubMed] [Google Scholar]
- 25.Irwin MS, Park JR.Neuroblastoma: Paradigm for precision medicine Pediatr Clin North Am 62225–2562015 [DOI] [PubMed] [Google Scholar]
- 26.Mosse YP.Anaplastic lymphoma kinase as a cancer target in pediatric malignancies Clin Cancer Res 22546–5522016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perry K, Tann M, Miller M. Which reference tissue is best for semiquantitative determination of FDG activity? J Nucl Med. 2008;49:425. [Google Scholar]
- 28.Blautzik J, Grelich L, Schramm N, et al. What and how should we measure in pediatric oncology FDG PET/CT? Comparison of commonly used SUV metrics for differentiation between pediatric tumors. EJNMMI Res. 2019;9:15. doi: 10.1186/s13550-019-0577-7. [DOI] [PMC free article] [PubMed] [Google Scholar]