Summary
Background
Circadian disturbances are commonly seen in people with Alzheimer’s disease and have been reported in individuals without symptoms of dementia but with Alzheimer’s pathology. We aimed to assess the temporal relationship between circadian disturbances and Alzheimer’s progression.
Methods
We did a prospective cohort study of 1401 healthy older adults (aged >59 years) enrolled in the Rush Memory and Aging Project (Rush University Medical Center, Chicago, IL, USA) who had been followed up for up to 15 years. Participants underwent annual assessments of cognition (with a battery of 21 cognitive performance tests) and motor activities (with actigraphy). Four measures were extracted from actigraphy to quantify daily and circadian rhythmicity, which were amplitude of 24-h activity rhythm, acrophase (representing peak activity time), interdaily stability of 24-h activity rhythm, and intradaily variability for hourly fragmentation of activity rhythm. We used Cox proportional hazards models and logistic regressions to assess whether circadian disturbances predict an increased risk of incident Alzheimer’s dementia and conversion of mild cognitive impairment to Alzheimer’s dementia. We used linear mixed-effects models to investigate how circadian rhythms changed longitudinally and how the change integrated to Alzheimer’s progression.
Findings
Participants had a median age of 81·8 (IQR 76·3–85·7) years. Risk of developing Alzheimer’s dementia was increased with lower amplitude (1 SD decrease, hazard ratio [HR] 1·39, 95% CI 1·19–1·62) and higher intradaily variability (1 SD increase, 1·22, 1·04–1·43). In participants with mild cognitive impairment, increased risk of Alzheimer’s dementia was predicted by lower amplitude (1 SD decrease, HR 1·46, 95% CI 1·24–1·72), higher intradaily variability (1 SD increase, 1·36, 1·15–1·60), and lower interdaily stability (1 SD decrease, 1·21, 1·02–1·44). A faster transition to Alzheimer’s dementia in participants with mild cognitive impairment was predicted by lower amplitude (1 SD decrease, odds ratio [OR] 2·08, 95% CI 1·53–2·93), increased intradaily variability (1 SD increase, 1·97, 1·43–2·79), and decreased interdaily stability (1 SD decrease, 1·35, 1·01–1·84). Circadian amplitude, acrophase, and interdaily stability progressively decreased over time, and intradaily variability progressively increased over time. Alzheimer’s progression accelerated these aging effects by doubling or more than doubling the annual changes in these measures after the diagnosis of mild cognitive impairment, and further doubled them after the diagnosis of Alzheimer’s dementia. The longitudinal change of global cognition positively correlated with the longitudinal changes in amplitude and interdaily stability and negatively correlated with the longitudinal change in intradaily variability.
Interpretation
Our results indicate a link between circadian dysregulation and Alzheimer’s progression, implying either a bidirectional relation or shared common underlying pathophysiological mechanisms.
Funding
National Institutes of Health, and the BrightFocus Foundation.
Introduction
Physiological processes such as sleep and motor activity show rhythms that are generated and orchestrated by the circadian system in synchrony with the 24-h daily cycle. Findings of laboratory studies have shown that disrupted circadian regulation underlies many of the adverse health outcomes of shift work, including cognitive decline.1 In cross-sectional studies, researchers suggest that the circadian system deteriorates with age, leading to changes in the circadian or daily rhythms of behaviour and physiology, including suppressed daily rhythms of body temperature and melatonin and advanced phases of daily behavioural cycles (eg, earlier sleep or wake times).2
Circadian dysfunction has been linked to neurodegenerative diseases, including Alzheimer’s disease.3 Individuals with Alzheimer’s disease have more disruptions in circadian rhythms4 and neurofunctional changes in the central pacemaker of the circadian network (ie, the suprachiasmatic nucleus)5 than do aged matched healthy individuals. Altered circadian or daily rhythms of motor activity have been linked to amyloid pathology, even in people who are cognitively intact.6 No prospective cohort studies have directly tested whether circadian dysfunction is an early sign of or a risk factor for Alzheimer’s disease, or how clinical manifestation and progression of Alzheimer’s disease affects the aging process of circadian regulation.
To better understand the interaction between aging of circadian regulation and Alzheimer’s progression, we examined longitudinal changes in circadian regulation of community-based older adults (aged >59 years) participating in the Rush Memory and Aging Project (Rush University Medical Center, Chicago, IL, USA). We aimed to test two hypotheses. First, we tested whether participants with more perturbed daily activity rhythms at baseline were at increased risk for developing Alzheimer’s dementia. Second, we tested whether aging led to perturbations in daily activity rhythms and whether the aging process was accelerated after onset of mild cognitive impairment and further accelerated after diagnosis of Alzheimer’s dementia.
Methods
Participants
The Rush Memory and Aging Project7 is a community-based study of older adults (aged >59 years) without dementia from about 40 residential facilities, senior and subsidised housing, church groups, and social service agencies in northeastern Illinois, USA.
The protocol of the Rush Memory and Aging Project was approved by the Institutional Review Board of Rush University Medical Center. Written informed consent was obtained from all participants, who also signed a repository consent to allow their data to be repurposed. The protocol for this current study was approved by the Institutional Review Board of Mass General Brigham.
Procedures
Motor activity data collection and assessment of circadian function
For annual assessment of daily motor activity, participants wore a wristwatch-like device which monitored their activity (Actical; Philips Respironics, Bend, OR, USA) on their non-dominant wrist for about 10 days. To quantify circadian daily rhythms from actigraphy, we did both parametric cosinor curve fitting8 and non-parametric analyses.9 Specifically, cosinor curve fitting extracted the 24-h component from the raw actigraphy signal. The amplitude and phase of the best-fit cosine curve defined the 24-h oscillation in the signal. Additionally, the amplitude was normalised by the individual SD to enable a fair between-participant comparison. The amplitude represents the difference in magnitude of activity between active and rest phases. The phase, usually named acrophase, represents the peak activity time of the 24-h component. Non-parametric analyses resulted in two additional measures: the interdaily stability that quantified the day-to-day robustness of the rhythm, and the intradaily variability that quantified the fragmentation of the rhythm. Greater interdaily stability indicates more stable rhythms whereas higher intradaily variability represents more fragmented activity rhythm. Since non-parametric analyses depend on data length, the first 7 days of motor activity recordings were used if data length was 7 days or longer, whereas recordings shorter than 7 days were excluded. Data were resampled from 15-s intervals to hourly intervals for non-parametric analyses to alleviate the effect of sampling interval. Algorithm details for these analyses are summarised in the appendix (p 1).
Annual assessment of cognition and clinical diagnoses
Cognitive function was assessed annually with 21 neuropsychological tests,10 19 of which were used to assess cognitive function in different domains. Among the 21 tests, the Mini-Mental State Examination (MMSE) is primarily used to describe the cohort. MMSE is a 30-point test with lower values indicating more impaired cognition. Individual scores on the tests within each domain were first converted to z scores using the mean and SD from the baseline assessment of all participants; z scores were then averaged to yield a summary measure of overall cognitive function—ie, global cognition. This composite score helps minimise floor and ceiling effects and other sources of random variability11 and it has been validated.12 For this score, zero represents the mean and one represents 1 SD of the baseline score of all participants in the Rush Memory and Aging Project. A negative z score means that someone has an overall score that is lower than the average of the entire cohort at baseline. Positive scores indicate better cognitive performance. A diagnosis of Alzheimer’s dementia was based on standard criteria.13 A diagnosis of mild cognitive impairment was made for participants who had cognitive impairment but who did not meet criteria for dementia.
Assessment of covariates
In addition to the covariates age, sex, and years of education, we grouped covariates into six categories assessing sleep (total night-time sleep duration and sleep fragmentation),14 physical activity (total daily activity),15 depression,16 comorbidities (body-mass index, vascular diseases, and vascular risk factors),17 cognition, and APOE ε4 genotype. Total night-time sleep duration (in h) was estimated between 2100 h and 0700 h using a published actigraphy-based sleep scoring system that is about 88–97% in agreement with polysomnography-based sleep scoring.18 Sleep fragmentation was estimated by an actigraphy index that represents the probability (as %) of rousing (eg, a non-zero activity count) after a long (about 5 min) period of rest or sleep.19 A greater value means more fragmented sleep and vice versa. Total daily activity was also calculated from actigraphy as the average sum of all daily activity counts.20 Depressive symptoms were measured with a modified ten-item version of the Center for Epidemiologic Studies Depression scale (CES-D).16 Scores ranged from 0 to 10, with a score of 10 indicating participants had reported all ten depressive symptoms in the past week and a score of 0 indicating that no depressive symptoms were reported in the past week. The CES-D score was square-root-transformed to correct right-skewness. A composite score for vascular disease burden was calculated using self-report questions for claudication, stroke, heart conditions, and congestive heart failure (ie, each item was given a value of 0 or 1); the composite score was the mean of the four individual scores multiplied by four. Thus, the composite score for vascular disease burden ranged from 0 to 4, with higher scores indicating greater vascular disease burden. Similarly, a composite score for vascular disease risk factors was calculated using self-reported questions on hypertension, diabetes, and smoking history. The score ranged from 0 to 3, with higher scores indicating more risk factors. APOE ε4 genotype was dichotomised to carrier (one or two alleles) versus non-carrier.
Statistical analysis
To test our first hypothesis relating to associations of baseline circadian measures with incident Alzheimer’s dementia, we used a series of Cox proportional hazards models. The core models included amplitude, acrophase, interdaily stability, and intradaily variability separately as a predictor. Adjusted models were subsequently used to control for each of the six categories of covariates (sleep, physical activity, depression, comorbidities, cognition, and APOE ε4 genotype), excluding participants who had not had follow-up clinical assessments or had been diagnosed with Alzheimer’s dementia at baseline. As secondary analyses, we examined with Cox models the associations between circadian measures and incident mild cognitive impairment (excluding participants who had mild cognitive impairment at baseline) and between circadian measures and incident Alzheimer’s dementia within the subgroup of participants who had mild cognitive impairment at baseline or during follow-up (note that onset of mild cognitive impairment was used as the analytical baseline for this analysis). We used logistic regression models to assess the associations between circadian measures and odds of conversion to Alzheimer’s dementia from mild cognitive impairment within 3 years. Age, sex, education, and interactions between circadian measures and these demographic characteristics were included in all models. Time on study was used as the scale for time, since this approach is more common and easier to implement than is modelling with age as a time scale, which needs to address the issue of left-truncation and healthy cohort effects. These analyses were done using JMP Pro version 14.
To investigate longitudinal changes in circadian function during Alzheimer’s progression, we used linear mixed-effects models with two change points anchored at the diagnoses of mild cognitive impairment and Alzheimer’s dementia. The circadian measures were included separately as a longitudinal outcome, time in years since baseline was a predictor, time in years since diagnosis of mild cognitive impairment was included to estimate additional changes after mild cognitive impairment diagnosis, and time in years since diagnosis of Alzheimer’s dementia was included to estimate additional changes after the diagnosis of Alzheimer’s dementia. Participant-specific random intercepts and slopes were considered. As secondary analyses, we also assessed longitudinal changes of circadian measures and the longitudinal change in global cognition simultaneously using bivariate linear mixed-effects models, for which the covariance structure of the individual-specific random slopes captured correlations between the changes.21 The corresponding two residuals were also allowed to covary with each other to allow better model fit. Positive or negative covariance between the individual-specific slopes of circadian metrics and cognition would indicate that they changed in the same or opposite direction over time. All models were adjusted for baseline age, sex, and education. We excluded participants without follow-up motor activity assessment. 95% CIs for correlations between random effects were estimated using a non-parametric bootstrap approach with 1000 bootstrapped samples. These analyses were done using MATLAB version R2019a.
Role of the funding source
The funder had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Results
The Rush Memory and Aging Project began in 1997 and started collecting motor activity data from 2005. For this study, datasets were frozen on Jan 24, 2020. 1401 participants in the Rush Memory and Aging Project, who were followed up for up to 15 years, had motor activity recordings and were included in our study (figure 1).
Figure 1: Flow of participants through the study.
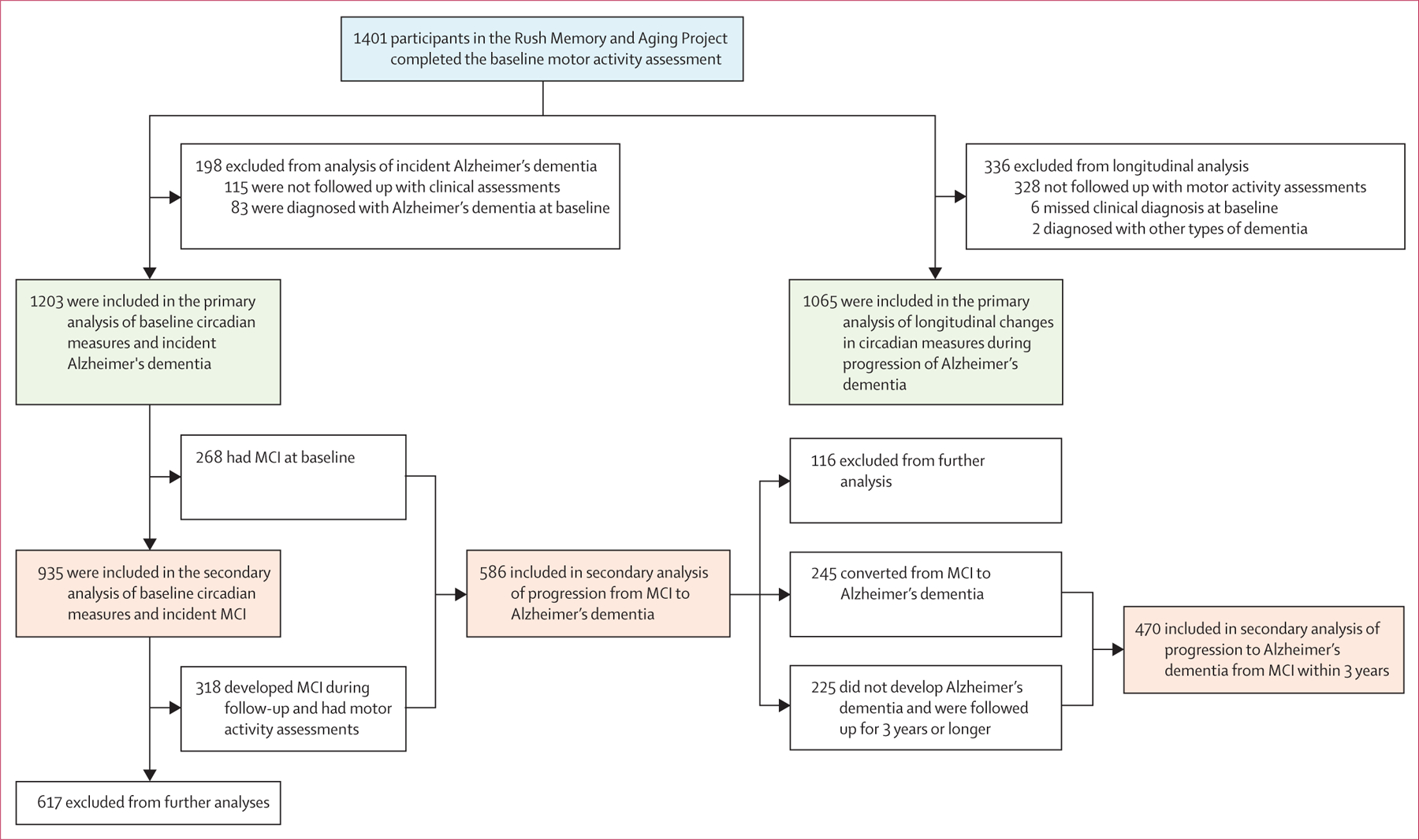
MCI=mild cognitive impairment.
Relations of the four circadian measures at baseline with demographics are summarised in the appendix (pp 1–3). Demographic and clinical characteristics of 1203 participants who did not have Alzheimer’s dementia at baseline are presented in table 1. In the primary analysis of baseline circadian measures and incident Alzheimer’s dementia in this population, 276 (23%) of 1203 participants developed Alzheimer’s dementia a mean 5·8 years (SD 3·4) after baseline. Lower amplitude was associated with higher hazard of Alzheimer’s dementia (per 1 SD decrease, hazard ratio [HR] 1·39, 95% CI 1·19–1·62; p<0·0001; table 2; appendix p 7). A participant with low amplitude at the 10th percentile of this cohort (figure 2A; red line) would be expected to have 2·4 times increased risk for developing Alzheimer’s dementia compared with an individual with high amplitude at the 90th percentile (figure 2A; blue line). This association seemed to be stronger in men than in women (pinteraction=0·0087, which was supported by sex-stratified analyses appendix pp 7, 9). The association remained significant after further adjustments for covariates at baseline including sleep, physical activity, depression, medical comorbidities, cognition, and APOE ε4 genotype (appendix p 7). Increased intradaily variability was also associated with higher risk of Alzheimer’s dementia (per 1 SD increase, HR 1·22, 95% CI 1·04–1·42; p=0·017; table 2, figure 2B; appendix p 8). This association remained significant after further adjustments for sleep, depression, medical comorbidities, and cognition but became not significant after adjustment for physical activity level and APOE ε4 genotype (appendix p 8). Neither acrophase nor interdaily stability was associated with incident Alzheimer’s dementia (table 2). Results from core models without interaction items supported these findings (appendix p 10).
Table 1:
Demographic and clinical characteristics of participants
| Participants (n=1203) | |
|---|---|
| Sex | |
| Female | 929 (77%) |
| Male | 274 (23%) |
| Age, years | 81·8 (76·3–85·7) |
| Education, years | 15 (12–17) |
| Circadian rhythmicity characteristics | |
| Amplitude (normalised units) | 0·32 (0·11) |
| Acrophase, h | 13·20 (1·78) |
| Interdaily stability (arbitrary units) | 0·52 (0·13) |
| Intradaily variability (arbitrary units) | 1·17 (0·27) |
| Sleep | |
| Total night-time sleep, h | 5·61 (1·45) |
| Sleep fragmentation index, % | 0·03 (0·01) |
| Physical activity | |
| Total daily activity count (× 105) | 2·66 (1·54) |
| Depression | |
| CES-D | 0 (0–1) |
| Comorbidities | |
| Body-mass index, kg/m2 | 27·4 (5·4) |
| Vascular diseases (composite score) | 0 (0–1) |
| Vascular risk factors (composite score) | 1 (1–2) |
| Cognition | |
| MMSE | 28·0 (2·0) |
| Global cognition (composite score) | 0·14 (0·53) |
| Genetic risk | |
| Carrier of APOE ε4 genotype | 247 (21%) |
Data are n (%), median (IQR), or mean (SD). CES-D=Center for Epidemiologic Studies Depression Scale. MMSE=Mini-Mental State Examination.
Table 2:
Association of circadian regulation and incident Alzheimer’s dementia after adjustment for demographics
| Amplitude |
Acrophase |
Interdaily stability |
Intradaily variability |
|||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age, 1-unit increase | 1·11 (1·09–1·14) | <0·0001 | 1·12 (1·10–1·14) | <0·0001 | 1·12 (1·10–1·14) | <0·0001 | 1·11 (1·09–1·14) | <0·0001 |
| Female sex | 1·12 (0·81–1·56) | 0·51 | 0·92 (0·70–1·24) | 0·58 | 0·97 (0·72–1·31) | 0·82 | 1·06 (0·78–1·47) | 0·72 |
| Education, 1-unit decrease | 1·05 (1·00–1·09) | 0·030 | 1·04 (1·00–1·09) | 0·048 | 1·05 (1·00–1·09) | 0·039 | 1·04 (1·00–1·09) | 0·053 |
| Amplitude, 1 SD decrease | 1·39 (1·19–1·62) | <0·0001 | .. | .. | .. | .. | .. | .. |
| Acrophase, 1-unit increase | .. | .. | 1·02 (0·94–1·11) | 0·61 | .. | .. | .. | .. |
| Interdaily stability, 1 SD decrease | .. | .. | .. | .. | 1·10 (0·94–1·27) | 0·24 | .. | .. |
| Intradaily variability, 1 SD increase | .. | .. | .. | .. | .. | .. | 1·22 (1·04–1·42) | 0·017 |
Figure 2: Predicted cumulative hazard for developing Alzheimer’s dementia.
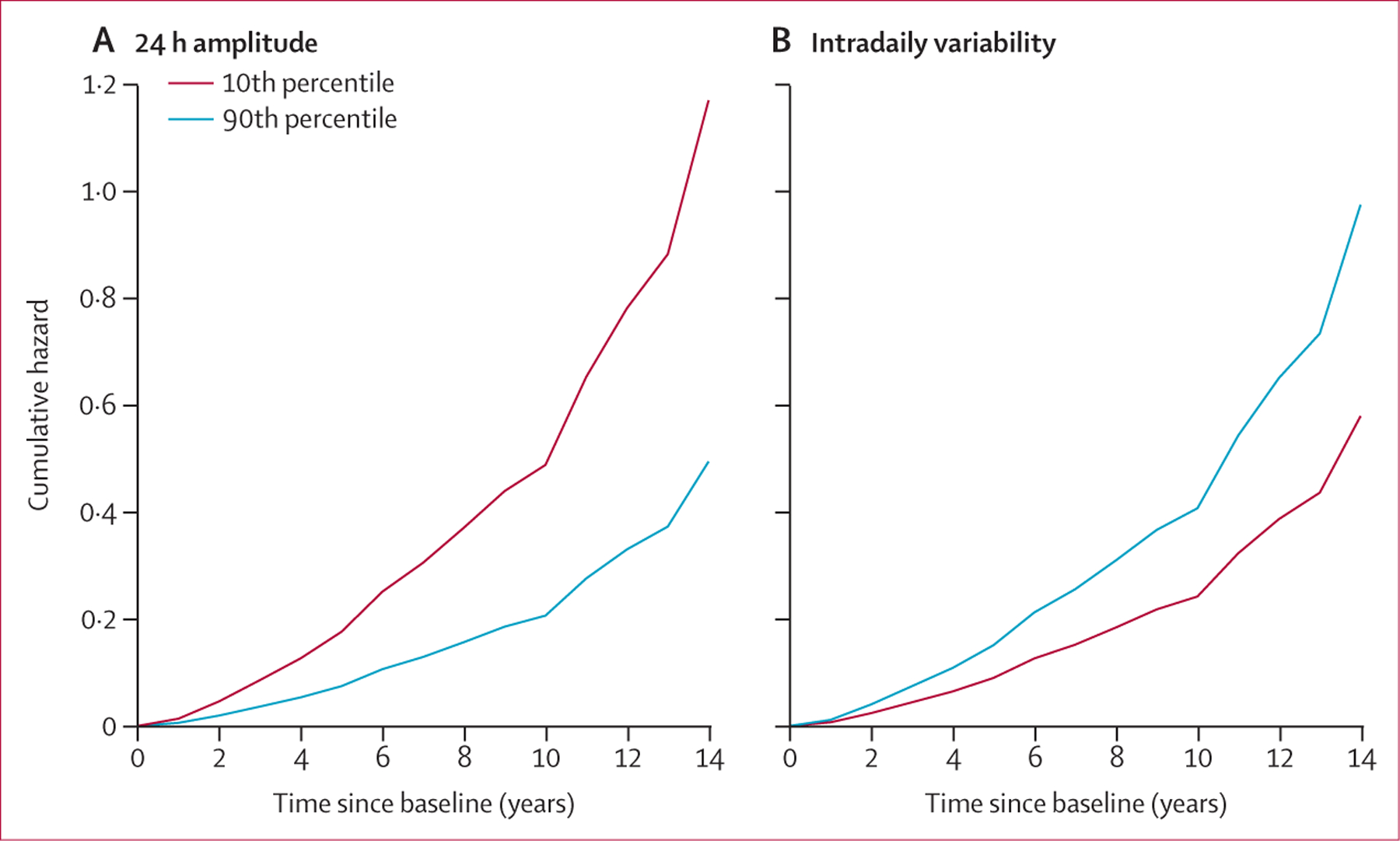
Plots show cumulative hazard functions for amplitude (A) or intradaily variability (B) for two representative individuals.
In a secondary analysis of the association of circadian measures with risk of mild cognitive impairment, 415 (44%) of 935 participants without mild cognitive impairment at baseline developed mild cognitive impairment a mean 4·3 years (SD 3·1) after baseline. None of the four circadian measures were associated with incident mild cognitive impairment (table 3). A further secondary analysis of the association between circadian measures and incident Alzheimer’s dementia among participants with mild cognitive impairment at baseline showed that 245 (42%) of 586 individuals with mild cognitive impairment (either at baseline or developed during follow-up) went on to develop Alzheimer’s dementia. Lower circadian amplitude, lower interdaily stability, and higher intradaily variability were significantly associated with higher risk of incident Alzheimer’s dementia in this population (table 3). An additional secondary analysis considered the odds of converting from mild cognitive impairment to Alzheimer’s dementia within 3 years. Among 470 participants with mild cognitive impairment, 108 (23%) converted to Alzheimer’s dementia within 3 years, 137 (29%) converted to Alzheimer’s dementia after 3 years or more, and 225 (48%) were never diagnosed with Alzheimer’s dementia during follow-up of longer than 3 years. Lower amplitude, increased intradaily variability, and decreased interdaily stability were associated with increased odds of conversion from mild cognitive impairment to Alzheimer’s dementia within 3 years (table 3).
Table 3:
Secondary analyses of associations of circadian regulation with MCI and Alzheimer’s dementia within participants with MCI, and risk of conversion from MCI to Alzheimer’s dementia within 3 years
| Amplitude |
Acrophase |
Interdaily stability |
Intradaily variability |
|||||
|---|---|---|---|---|---|---|---|---|
| Risk ratio (95% CI) | p value | Risk ratio (95% CI) | p value | Risk ratio (95% CI) | p value | Risk ratio (95% CI) | p value | |
| Circadian regulation and incident MCI within participants who were cognitively intact at baseline (n=935)* | ||||||||
| Age, 1-unit increase | 1·08 (1·06–1·10) | <0·0001 | 1·08 (1·07–1·10) | <0·0001 | 1·08 (1·07–1·10) | <0·0001 | 1·08 (1·06–1·10) | <0·0001 |
| Female sex | 1·24 (0·75–1·99) | 0·39 | 1·34 (0·84–2·14) | 0·21 | 1·25 (0·76–2·01) | 0·37 | 1·23 (0·75–2·03) | 0·41 |
| Education, 1-unit decrease | 1·00 (0·97–1·04) | 0·89 | 1·00 (0·97–1·04) | 0·95 | 1·00 (0·96–1·03) | 0·94 | 1·00 (0·96–1·03) | 0·78 |
| Amplitude, 1 SD decrease | 1·07 (0·96–1·19) | 0·21 | .. | .. | .. | .. | .. | .. |
| Acrophase, 1-unit increase | .. | .. | 1·01 (0·94–1·07) | 0·87 | .. | .. | .. | .. |
| Interdaily stability, 1 SD decrease | .. | .. | .. | .. | 1·01 (0·91–1·12) | 0·83 | .. | .. |
| Intradaily variability, 1 SD increase | .. | .. | .. | .. | .. | .. | 1·02 (0·91–1·14) | 0·70 |
| Circadian regulation and incident Alzheimer’s dementia within participants with MCI (who had MCI at baseline or developed MCI during follow-up; n=586)* | ||||||||
| Age, 1-unit increase | 1·07 (1·07–1·09) | <0·0001 | 1·08 (1·06–1·11) | <0·0001 | 1·08 (1·06–1·11) | <0·0001 | 1·07 (1·05–1·10) | <0·0001 |
| Female sex | 1·27 (0·90–1·80) | 0·17 | 1·05 (0·78–1·44) | 0·76 | 1·19 (0·86–1·69) | 0·30 | 1·28 (0·90–1·82) | 0·15 |
| Education, 1-unit decrease | 1·06 (1·01–1·11) | 0·019 | 1·05 (1·00–1·09) | 0·050 | 1·05 (1·01–1·10) | 0·022 | 1·05 (1·01–1·15) | 0·025 |
| Amplitude, 1 SD decrease | 1·45 (1·24–1·72) | <0·0001 | .. | .. | .. | .. | .. | .. |
| Acrophase, 1-unit increase | .. | .. | 0·99 (0·91–1·09) | 0·89 | .. | .. | .. | .. |
| Interdaily stability, 1 SD decrease | .. | .. | .. | .. | 1·21 (1·02–1·44) | 0·028 | .. | .. |
| Intradaily variability, 1 SD increase | .. | .. | .. | .. | .. | .. | 1·36 (1·15–1·60) | 0·0003 |
| Circadian regulation and odds of conversion from MCI to Alzheimer’s dementia within 3 years (n=470)† | ||||||||
| Age, 1-unit increase | 1·05 (1·01–1·10) | 0·0089 | 1·09 (1·06–1·14) | <0·0001 | 1·10 (1·06–1·14) | <0·0001 | 1·06 (1·02–1·10) | 0·0069 |
| Female sex | 1·40 (0·73–2·97) | 0·34 | 0·94 (0·55–1·63) | 0·82 | 1·07 (0·60–1·99) | 0·82 | 1·40 (0·73–2·89) | 0·34 |
| Education, 1-unit decrease | 1·10 (1·01–1·20) | 0·039 | 1·08 (0·99–1·17) | 0·071 | 1·11 (1·02–1·21) | 0·012 | 1·13 (1·04–1·24) | 0·0070 |
| Amplitude, 1 SD decrease | 2·08 (1·53–2·93) | <0·0001 | .. | .. | .. | .. | .. | .. |
| Acrophase, 1-unit increase | .. | .. | 1·01 (0·87–1·17) | 0·89 | .. | .. | .. | .. |
| Interdaily stability, 1 SD decrease | .. | .. | .. | .. | 1·35 (1·01–1·84) | 0·046 | .. | .. |
| Intradaily variability, 1 SD increase | .. | .. | .. | .. | .. | .. | 1·97 (1·43–2·79) | <0·0001 |
MCI=mild cognitive impairment.
Data are hazard ratio (95% CI).
Data are odds ratio (95% CI).
Data from 1065 participants were used in the models of longitudinal changes in circadian function during progression of Alzheimer’s dementia (figure 1). Of these individuals, 812 (76%) had no cognitive impairment at baseline, 209 (19%) had mild cognitive impairment at baseline, and 44 (4%) were diagnosed with Alzheimer’s dementia at baseline. Of 812 participants with no cognitive impairment at baseline, 378 developed mild cognitive impairment and 141 progressed to Alzheimer’s dementia during follow-up. Of 209 with mild cognitive impairment at baseline, 99 converted to Alzheimer’s dementia during follow-up. During the no cognitive impairment phase, amplitude decreased over time, with a mean annual decline of 0·010 (SE 0·001; p<0·0001; figure 3A; appendix p 4). The decline rate doubled after the diagnosis of mild cognitive impairment (0·020 [SE 0·001]; p<0·0001) and doubled again after the diagnosis of Alzheimer’s dementia (0·044 [0·003]; p<0·0001). A weak augmented effect of age on the decrease in amplitude was also shown (ie, 1 year older baseline age corresponded to 4% increase in rate of decline; appendix p 11). Thus, the effects of mild cognitive impairment and Alzheimer’s dementia are equivalent to the effect of being a couple of decades older. Sex did not affect the change rates of amplitude, although male participants had lower amplitude at baseline (figure 3A; appendix p 11).
Figure 3: Interaction of circadian disturbance with Alzheimer’s disease progression.
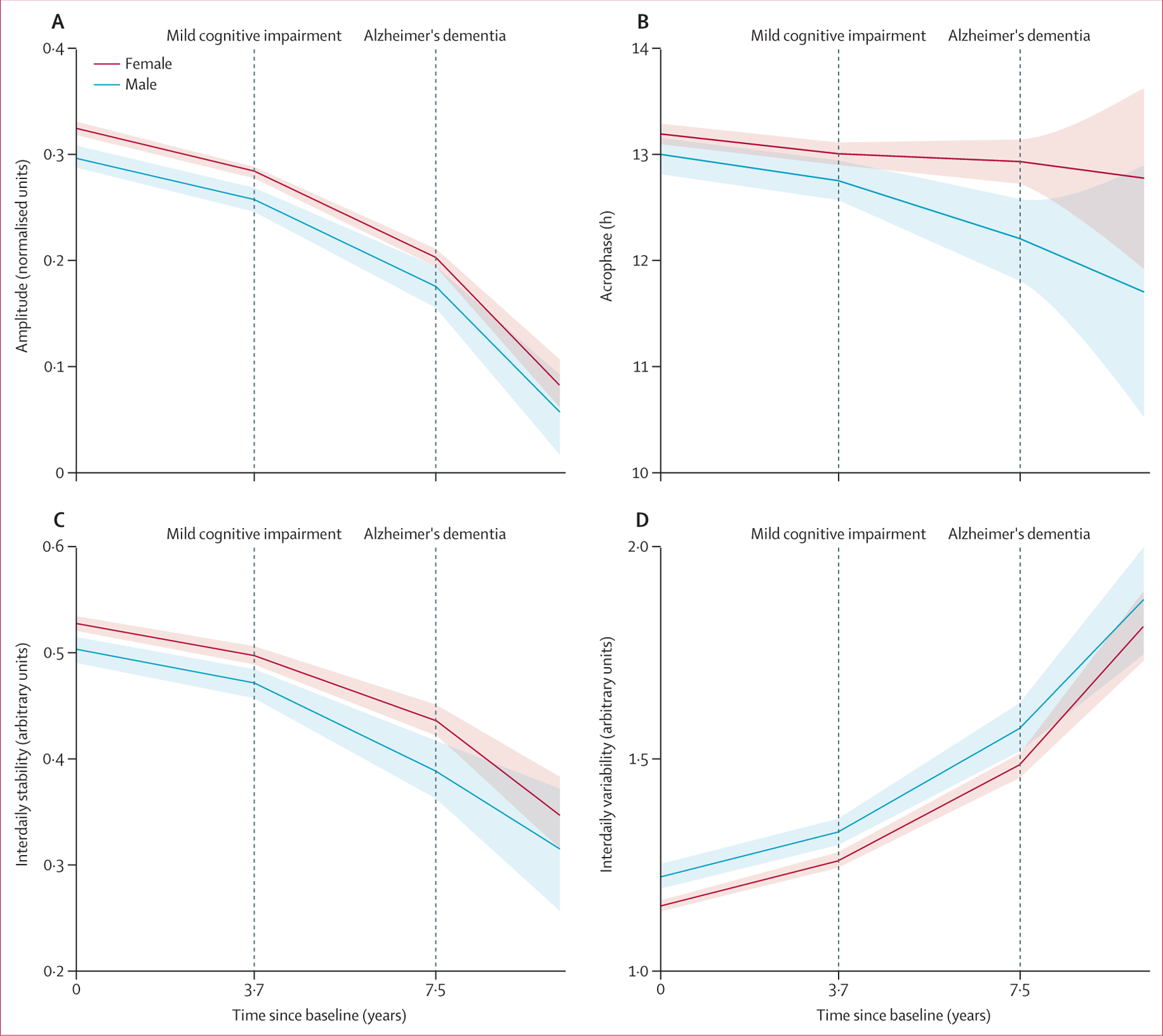
Plots show predicted mean levels of amplitude (A), acrophase (B), interdaily stability (C), and intradaily variability (D) based on mixed models for hypothetical individuals with a mean age of 81 years (mean age of the whole cohort) who developed mild cognitive impairment at 3·7 years after baseline and Alzheimer’s dementia at 7·5 years after baseline. Predicted 95% CIs are shown as shaded regions.
Further, during the no cognitive impairment phase, acrophase advanced over time by mean 0·038 h per year (SE 0·012; p=0·0024). The transition to mild cognitive impairment did not accelerate this advance (p=0·38) whereas the transition to Alzheimer’s dementia did (rate of advance increased by 0·130 h per year [SE 0·055], or 3·4 times the rate with no cognitive impairment; p=0·018; figure 3B; appendix p 4). Baseline age and sex had no effect on the annual change of acrophase (figure 3B; appendix p 11).
Moreover, during the no cognitive impairment phase, interdaily stability progressively decreased over time, by mean 0·008 per year (SE 0·001; p<0·0001; figure 3C), whereas intradaily variability increased, by mean 0·029 per year (0·002; p<0·0001; figure 3D). Both changes were accelerated after diagnosis of mild cognitive impairment, with interdaily stability of mean 0·016 per year (SE 0·001; p<0·0001) and intradaily variability of mean 0·059 per year (0·005; p<0·0001). Changes were accelerated further after diagnosis of Alzheimer’s dementia, with interdaily stability of mean 0·035 per year (SE 0·006; p=0·0034) and intradaily variability of mean 0·128 per year (0·016; p<0·0001). Baseline age did not affect the annual decrease of interdaily stability, whereas it did accelerate the increase of intradaily variability, by mean 0·0008 per year (SE 0·0002; p=0·0008), a 3% increase. Thus, the effects of mild cognitive impairment and Alzheimer’s dementia diagnosis on intradaily variability increase are equivalent to being decades older in age (appendix p 11). Sex did not affect the changes of either variable (appendix p 11).
In secondary analyses using data from 1065 participants, bivariate linear mixed models confirmed gradual decreases in amplitude, acrophase, and interdaily stability, the gradual increase in intradaily variability, and gradual cognitive decline. Consistently, the individual-specific longitudinal change in global cognition was positively correlated with individual-specific longitudinal changes in amplitude (r=0·57), interdaily stability (r=0·31), and negatively correlated with that of intradaily variability (r=–0·52), with all correlations significant at an α of 0·05 (appendix p 12).
Discussion
To our knowledge, we report the first, large, prospective cohort study showing an integral or a bidirectional relation between circadian aging and Alzheimer’s progression. More suppressed, less stable, and greater fragmented daily activity rhythms were associated with higher risk of developing Alzheimer’s dementia in older people with normal cognition or mild cognitive impairment, and with increased risk for conversion from mild cognitive impairment to Alzheimer’s dementia. Our results also showed simultaneous progression of circadian dysregulation and cognitive impairment; specifically, the aging process progressively worsened circadian regulation, whereas Alzheimer’s progression substantially accelerated this age-related decline.
Accumulating evidence suggests dysregulation of circadian rhythm is a common symptom or outcome of neurodegenerative processes.22,23 For example, findings of both animal and human studies indicate that disturbed circadian function is a result of Alzheimer’s disease pathology or dementia,24,25 and it could have a potential causative role. In an in vivo human pathological study,6 disturbances in circadian rhythmicity were associated with increased burden of Alzheimer’s pathology in individuals with preclinical Alzheimer’s disease. Two landmark studies in women26 and men27 also showed an association of reduced circadian amplitude and increased fragmentation with future incidence of cognitive decline or dementia. Besides, they also reported that phase delay was associated with greater cognitive decline in women whereas phase advance was associated with greater cognitive decline in men. We did not note any relevant interaction between acrophase and sex. Unfortunately, comparisons between studies are challenging because of different populations and definitions—eg, phase was categorised in the two studies26,27 using different criteria based on population mean and age distributions. However, this difference highlights several important points for discussion.
First, why are amplitude and fragmentation (but not acrophase) of daily activity rhythm at baseline associated with incident Alzheimer’s dementia? It is possible that circadian amplitude and fragmentation changed at earlier stages of Alzheimer’s disease than did acrophase. For instance, Alzheimer’s disease-related neurodegeneration within specific neural nodes in the circadian network (eg, the suprachiasmatic nucleus) might cause neuron loss, perturb phase synchronisation between individual neurons (ie, the peak of the neural activity appears at different times for different neurons), or both,28 leading to suppressed and more fragmented rhythms in the overall output of the neural node, whereas the interaction between the circadian system and the 24-h cycle of environmental changes (eg, the light–dark cycle, which ascertains the alignment between intrinsic circadian rhythm and rest–activity cycle [thus, acrophase]) is altered later. Second, the absence of an association between acrophase and incident Alzheimer’s dementia might point to scant reliability in acrophase estimation, which can be affected or masked more by the scheduled daily behavioural cycle than by other measures. Finally, multiple peaks probably occur in perturbed daily activity rhythms in people with Alzheimer’s disease; thus, it is hard to robustly estimate acrophase of such a non-sinusoidal oscillation.
More fragmented circadian rhythm was previously reported in cognitively normal individuals with positive amyloid β deposition than in negative controls.6 Our observed association between circadian dysfunction at baseline (when participants were cognitively normal) and incident Alzheimer’s dementia was consistent with this finding. However, none of the four circadian measures at baseline were associated with incident mild cognitive impairment. It is important to note that other age-related changes (different from Alzheimer’s disease pathology) might also cause mild cognitive impairment, and mild cognitive impairment does not always lead to dementia. Thus, a predictor of incident Alzheimer’s dementia does not necessarily have to be associated with incident mild cognitive impairment. Nevertheless, our observation that circadian measures in participants with mild cognitive impairment were associated with risk of conversion from mild cognitive impairment to Alzheimer’s disease lends additional support to the possibility that circadian dysfunction is a potential risk factor for Alzheimer’s disease. Further mechanistic understanding of circadian regulation along the path of Alzheimer’s disease development is warranted.
Our longitudinal data showed that aging was associated with decreased amplitude, advanced acrophase, and increased intradaily variability of daily activity rhythms, findings that are consistent with those of cross-sectional studies.29,30 We also reported that interdaily stability progressively decreased over time. By contrast, our baseline cross-sectional analysis suggested a positive correlation between interdaily stability and age; previous studies also reported either unchanged or higher interdaily stability in older adults.6,29,31 We believe our longitudinal within-participant design was better suited to capture age-related changes. The potential relation between interdaily stability and Alzheimer’s progression is also further supported by the observation that decreased interdaily stability predicted higher risk within participants with mild cognitive impairment and a higher chance of conversion from mild cognitive impairment to Alzheimer’s dementia. Furthermore, our participants were retired from employment and were moderately older than those in other studies. A potential advantage of retirement from employment for interpretation of spontaneous activities is that daily rhythms are potentially closer aligned to intrinsic circadian regulation—ie, being less masked by daily work-related or study-related schedules in younger populations. However, retirement does not necessarily free interdaily stability (and other circadian measures as well) from masking effects, particularly in institutionalised individuals. More robust biomarkers for intrinsic circadian regulation and controlled environmental conditions are needed to address this concern.
In addition to the prospective associations and parallel progression, we also showed that Alzheimer’s progression further sped up the longitudinal decline of circadian regulation. Altogether, circadian function might serve not only as a biomarker for future risk of Alzheimer’s disease but also as one that monitors disease progression. Whether interventions to optimise or restore circadian rhythms can help prevent or slow the progression of Alzheimer’s dementia or mitigate its related symptoms remains unknown. Targeted clinical trials with ambulatory-feasible tools (such as lifestyle modifications and light treatment) are needed to test this hypothesis. The actigraphy-based approach we used is unobtrusive and possesses long-term monitoring capabilities that are scalable to large populations; this method can easily be translated to other observational cohorts to assist in the assessment of Alzheimer’s treatments, as well as identifying individuals at increased risk of Alzheimer’s disease.
Our study has some limitations. First, we did not do an in vivo assessment of Alzheimer’s disease pathology, to examine how circadian function and Alzheimer’s pathology interact during different clinical stages of Alzheimer’s dementia. However, all participants agreed to a post-mortem brain autopsy. Future work should investigate how circadian disturbances link to late-life brain pathological features and how preclinical Alzheimer’s disease biomarkers relate to changes in circadian rhythms, particularly in people with no or mild cognitive impairment. Second, a complex interplay might exist between circadian function, sleep homoeostasis, and Alzheimer’s disease (appendix pp 1–2). We did not do a comprehensive sleep assessment. Halfway through follow-up, questionnaire and wearable-based screenings for sleep and sleep disorders were added to the Rush Memory and Aging Project (eg, sleep apnoea screening was added in 2018), which will address this limitation in future work. Finally, our older cohort had a mean age at baseline of more than 80 years. Caution should be taken to translate our findings to younger populations. Further studies on how circadian function in earlier life relates to cognition and dementia are warranted.
Supplementary Material
Research in context.
Evidence before this study
We searched PubMed for journal articles published between Jan 1, 1990, and May 20, 2020, with the terms “circadian rhythm” AND “aging” AND (“Alzheimer’s disease” OR “cognitive function” OR “cognitive decline” OR “dementia”). We further restricted our results to participants aged 36–55 years and those older than 55 years. We did not restrict our search by language. We identified 27 peer-reviewed journal articles. Four cross-sectional studies reported circadian rhythmicity changes with advanced age; 18 cross-sectional studies reported circadian changes in Alzheimer’s disease and related dementias; and five longitudinal studies reported circadian rhythmicity changes before incident cognitive impairment. Previous evidence indicated that circadian regulation deteriorates with aging, leading to suppressed circadian amplitude and advanced phase of daily behavioural cycle. Moreover, circadian rhythm disturbances were noted in individuals not only with Alzheimer’s dementia but also at the preclinical stage, predicting faster cognitive decline and potentially reflecting a profound aging effect. No studies were identified that specifically examined whether circadian disturbances link to future risk of incident Alzheimer’s dementia, and how Alzheimer’s progression affects the changes of circadian rhythmicity with time.
Added value of this study
Our longitudinal study ascertained within-person changes in circadian rhythmicity of spontaneous motor activity in more than 1000 adults (mean age 81 years) covering different clinical stages of Alzheimer’s dementia with up to 15 years of follow-up. The relation between circadian rhythmicity at baseline and future development of incident Alzheimer’s dementia was also investigated.
Implications of all the available evidence
A bidirectional relation seems to exist between circadian aging and Alzheimer’s progression, or else pathophysiological processes are shared. The findings that circadian disturbances increase future risk of incident Alzheimer’s dementia, and that Alzheimer’s progression accelerates circadian disturbances, support the hypothesis that interventions to optimise or restore circadian rhythms might provide some benefit to prevent or slow the progression of Alzheimer’s disease and mitigate downstream symptoms. Targeted clinical trials during different stages of Alzheimer’s disease to optimise circadian rhythmicity via behavioural or lifestyle modifications are warranted to test this hypothesis. The actigraphy-based approach used in our study is unobtrusive and has long-term monitoring capabilities that are scalable to large populations. This method can easily be translated to other observational cohorts to assist in the evaluation of Alzheimer’s treatments and in identifying individuals at increased risk of Alzheimer’s disease.
Acknowledgments
This study was fully supported by the National Institutes of Health (NIH; grant nos RF1AG064312, RF1AG059867, R01AG17917, and R01AG56352). PL is also supported by the Alzheimer’s Disease Research Program of the BrightFocus Foundation (grant no A2020886S). LG is supported by the NIH (grant no T32GM007592). We thank participants and staff of the Rush Memory and Aging Project and the Rush Alzheimer’s Disease Center.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
Data are available on request through the Rush Alzheimer’s Disease Center Research Resource Sharing Hub.
Contributor Information
Peng Li, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
Lei Gao, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Department of Anesthesia, Critical Care and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Arlen Gaba, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Lei Yu, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Longchang Cui, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Wenqing Fan, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Andrew S P Lim, Division of Neurology, Department of Medicine, Sunnybrook Health Sciences Centre, University of Toronto, Toronto, ON, Canada.
David A Bennett, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Aron S Buchman, Rush Alzheimer’s Disease Center, Rush University Medical Center, Chicago, IL, USA.
Kun Hu, Medical Biodynamics Program, Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA; Division of Sleep Medicine, Harvard Medical School, Boston, MA, USA.
References
- 1.Chellappa SL, Morris CJ, Scheer FAJL. Effects of circadian misalignment on cognition in chronic shift workers. Sci Rep 2019; 9: 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duffy JF, Zitting K-M, Chinoy ED. Aging and circadian rhythms. Sleep Med Clin 2015; 10: 423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K. Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 2019; 18: 307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Homolak J, Mudrovčić M, Vukić B, Toljan K. Circadian rhythm and Alzheimer’s disease. Med Sci 2018; 6: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Erum J, Van Dam D, De Deyn PP. Sleep and Alzheimer’s disease: a pivotal role for the suprachiasmatic nucleus. Sleep Med Rev 2018; 40: 17–27. [DOI] [PubMed] [Google Scholar]
- 6.Musiek ES, Bhimasani M, Zangrilli MA, Morris JC, Holtzman DM, Ju Y-ES. Circadian rest-activity pattern changes in aging and preclinical Alzheimer disease. JAMA Neurol 2018; 75: 582–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the Rush Memory and Aging Project. Curr Alzheimer Res 2012; 9: 646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naitoh P, Englund CE, Ryman DH. Circadian rhythms determined by cosine curve fitting: analysis of continuous work and sleep-loss data. Behav Res Methods Instrum Comput 1985; 17: 630–41. [Google Scholar]
- 9.Gonçalves BSB, Cavalcanti PRA, Tavares GR, Campos TF, Araujo JF. Nonparametric methods in actigraphy: an update. Sleep Sci 2014; 7: 158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Yu L, Lim ASP, et al. Fractal regulation and incident Alzheimer’s disease in elderly individuals. Alzheimers Dement 2018; 14: 1114–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA. Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 2012; 72: 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology 2015; 85: 984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984; 34: 939–44. [DOI] [PubMed] [Google Scholar]
- 14.Lim ASP, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep 2013; 36: 1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012; 78: 1323–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson RS, Barnes LL, Mendes de Leon CF, et al. Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002; 59: 364–70. [DOI] [PubMed] [Google Scholar]
- 17.Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol 2009; 66: 1339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jean-Louis G, Kripke DF, Mason WJ, Elliott JA, Youngstedt SD. Sleep estimation from wrist movement quantified by different actigraphic modalities. J Neurosci Methods 2001; 105: 185–91. [DOI] [PubMed] [Google Scholar]
- 19.Lim ASP, Yu L, Costa MD, et al. Quantification of the fragmentation of rest-activity patterns in elderly individuals using a state transition analysis. Sleep 2011; 34: 1569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buchman AS, Wilson RS, Bennett DA. Total daily activity is associated with cognition in older persons. Am J Geriatr Psychiatry 2008; 16: 697–701. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Yu L, Yang J, et al. Interaction between the progression of Alzheimer’s disease and fractal degradation. Neurobiol Aging 2019; 83: 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coogan AN, Schutová B, Husung S, et al. The circadian system in Alzheimer’s disease: disturbances, mechanisms, and opportunities. Biol Psychiatry 2013; 74: 333–39. [DOI] [PubMed] [Google Scholar]
- 23.Musiek ES, Xiong DD, Holtzman DM. Sleep, circadian rhythms, and the pathogenesis of Alzheimer disease. Exp Mol Med 2015; 47: e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou JN, Hofman MA, Swaab DF. VIP neurons in the human SCN in relation to sex, age, and Alzheimer’s disease. Neurobiol Aging 1995; 16: 571–76. [DOI] [PubMed] [Google Scholar]
- 25.Long DM, Blake MR, Dutta S, et al. Relationships between the circadian system and Alzheimer’s disease-like symptoms in drosophila. PLoS One 2014; 9: e106068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tranah GJ, Blackwell T, Stone KL, et al. Circadian activity rhythms and risk of incident dementia and mild cognitive impairment in older women. Ann Neurol 2011; 70: 722–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers-Soeder TS, Blackwell T, Yaffe K, et al. Rest-activity rhythms and cognitive decline in older men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc 2018; 66: 2136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang JL, Lim AS, Chiang W-Y, et al. Suprachiasmatic neuron numbers and rest-activity circadian rhythms in older humans. Ann Neurol 2015; 78: 317–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell JA, Quante M, Godbole S, et al. Variation in actigraphy-estimated rest-activity patterns by demographic factors. Chronobiol Int 2017; 34: 1042–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Feijter M, Lysen TS, Luik AI. 24-h activity rhythms and health in older adults. Curr Sleep Med Rep 2020; 6: 76–83. [Google Scholar]
- 31.Luik AI, Zuurbier LA, Hofman A, Van Someren EJW, Tiemeier H. Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiol Int 2013; 30: 1223–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


