Abstract
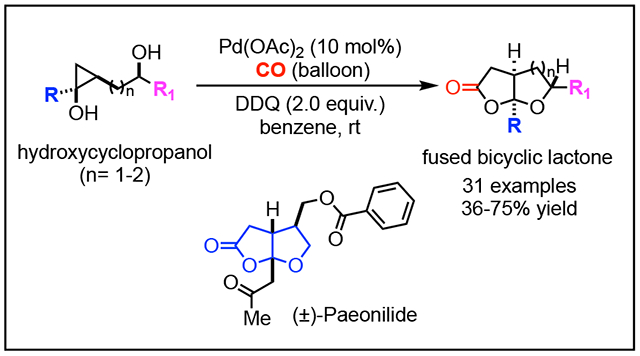
A novel palladium-catalyzed ring opening carbonylative lactonization of readily available hydroxycyclopropanols was developed to efficiently synthesize tetrahydrofuran (THF) or tetrahydropyran (THP)-fused bicyclic γ-lactones, two privileged scaffolds often found in natural products. The reaction features mild reaction conditions, good functional group tolerability, and scalability. Its application was demonstrated in a short total synthesis of (±)-paeonilide. The fused bicyclic γ-lactone products can be easily diversified to other medicinally important scaffolds, which further broadens the application of this new carbonylation method.
Graphical Abstract
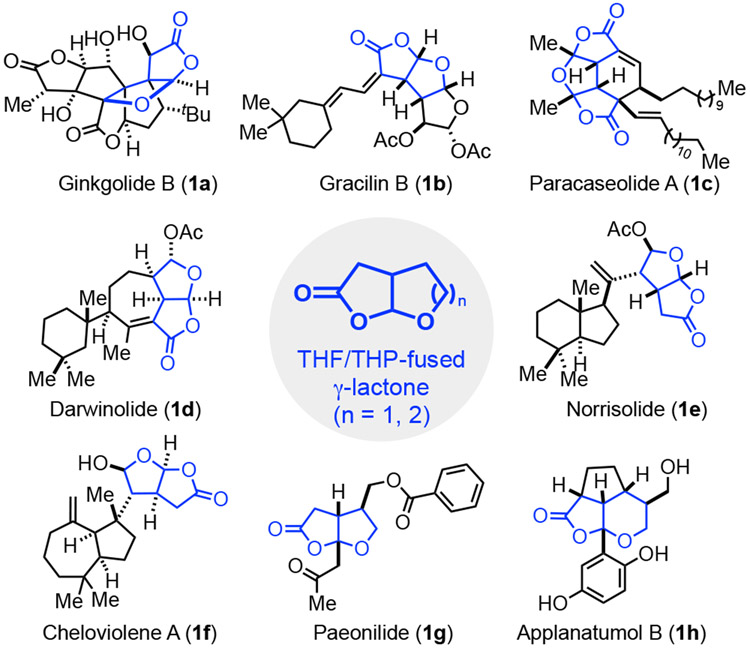
Nature has been constantly creating natural products with novel, diverse, and complex structures. Many of these natural products or their derivatives have been used as medicines to treat human diseases or are lead compounds for drug discovery.1 While various biosynthetic pathways and different organisms are involved in natural product production, privileged structural scaffolds shared by a large number of natural products can often be identified.1c Cis-2,8-dioxabicyclo[3.3.0]octan-3-one (a THF-fused bicyclic γ-lactone, also called cis-tetrahydro[2,3-b]furan-2(3H)-one) is one such privileged structural scaffold, which has been found in over 100 natural products from various origins (Figure 1).2 In these molecules, the THF-fused γ-lactone forges diverse connections with the rest of the molecular structures. Part of them such as ginkgolide B (1a),3 gracilin B (1b),4 paracaseolide A (1c),5 and darwinolide (1d),6 have the bicyclic γ-lactone moiety embedded in a large polycyclic ring system. Another portion of them including norrisolide (1e)7 and cheloviolene A (1f)8 have the bicyclic γ-lactone connected with a second (poly)cyclic ring system via a C-C single bond. Why and how nature produces such fused bicyclic skeletons are yet to be understood. Natural products with such a bicyclic γ-lactone motif have demonstrated a wide range of biological activity and possess great potential in novel therapeutic development. For example, the ginkgolides can act as anti-platelet-activating factors; gracilins exhibit immunosuppressive and neuroprotective properties; paracaseolide A inhibits phosphatase CDC25B, an emerging and important target for anticancer drug discovery; the marine spongian diterpenoid norrisolide and its analogs demonstrated unique Golgi-modifying activity; darwinolide is a biofilm-penetrating anti-MRSA agent. Additionally, the related cis-2,9-dioxabicyclo[4.3.0]nonan-8-one (a THP-fused bicyclic γ-lactone, also called cis-tetrahydro-4H-furo[2,3-b]pyran-2(3H)-one) has been found in natural products,9 including applanatumol B (1h),9a which showed activity against renal fibrosis.
Figure 1.
Selected natural products with a THF/THP-fused bicyclic γ-lactone
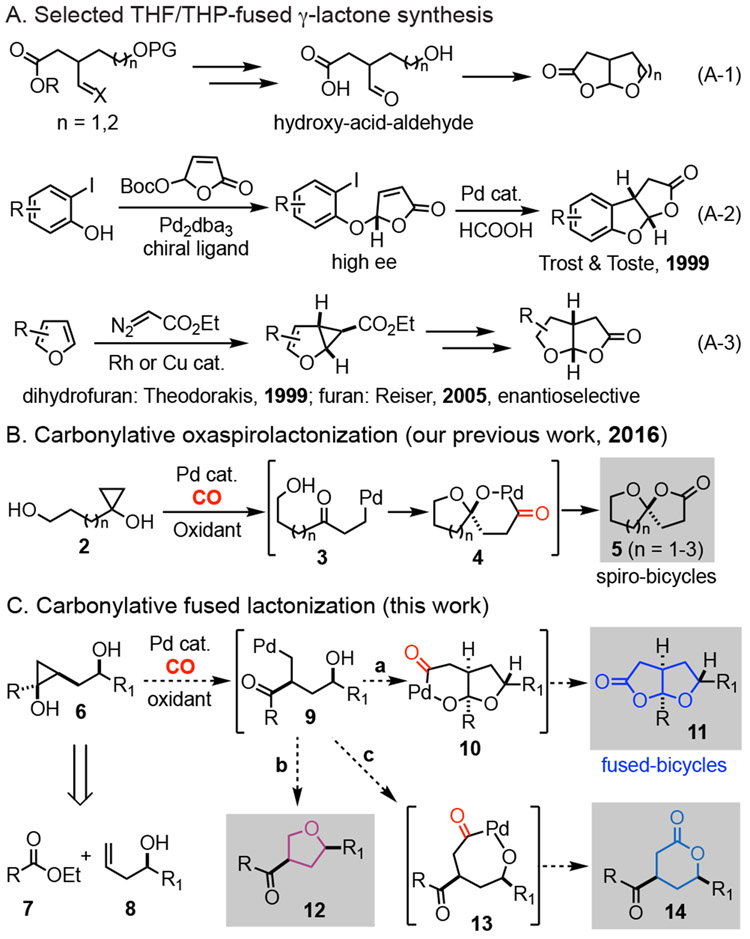
Due to their unique and challenging chemical structures as well as their diverse biological applications, natural products with a THF/THP-fused bicyclic γ-lactone moiety have been popular targets in total synthesis practices.10 Notably, the Overman group recently accomplished elegant total syntheses of several marine spongian diterpenoids including cheloviolene A (1f, Figure 1) with a THF-fused γ-lactone.11 So far, most of these total syntheses utilized the hydroxy-acid-aldehyde (or their masked forms) condensation reactions to construct the desired THF/THP-fused γ-lactone moiety in the target molecules (eq. A-1, Figure 2A). In these processes, tedious functional group manipulations, protections, and deprotections are often involved, which added extra steps to the entire synthesis. Surprisingly, synthetic methodology development in this area is extremely barren.12 Notably, Trost and Toste developed a Pd-catalyzed asymmetric nucleophilic substitution of 5-aceloxy-2-(5H)-furanone followed by reductive intramolecular Heck reaction to build THF-fused γ-lactones,12a but this method is limited to benzo-fused tricyclic systems (eq. A-2). Theodorakis12b and Reiser12c independently reported elegant activated cyclopropane rearrangements to THF-fused γ-lactones (eq. A-3). In the Reiser’s case, the activated cyclopropanes were generated via Rh-catalyzed enantioselective cyclopropanation of furans. In general, these prior arts are limited to 5,5-fused γ-lactones and hydrogen atom substitution at the ring junction carbons. Thus, versatile, modular, and catalytic methods are highly desirable.
Figure 2.
Synthetic methodology design
We recently developed a Pd-catalyzed hydroxycyclopropanol ring opening carbonylative lactonization to synthesize oxaspirolactones, another privileged scaffold frequently appears in natural products (2→5, Figure 2B).13 This new synthetic capability significantly facilitated our total syntheses of C12 oxygenated diterpenes and Stemona alkaloids.14 With this success, we wondered the possibility of developing a catalytic carbonylation chemistry to prepare THF/THP-fused γ-lactones with various substitution patterns (Figure 2C). To achieve this goal, cyclopropanols such as 6 would be required. They can be convergently assembled from readily available ester 7 and homoallylic alcohol 8 using the cyclopropanol synthesis15 protocol developed by Cha and co-workers.16 We envisioned a process involving Pd-catalyzed β-carbon elimination to generate Pd-homoenolate 9. Ideally, we hoped for an acetal formation followed by carbonylative lactonization to convert 9 to desired fused bicyclic γ-lactone 11, presumably via an intermediate like 10. In principle, several processes could compete with the desired one. For instance, β-hydride elimination would decompose 9 to an enone; the tethered secondary alcohol could attack the Pd center and a following C-O reductive elimination would give THF product 12. Additionally, carbonylative lactonization with the same tethered alcohol would give δ-lac-tone 14 (9→13→14). At the planning stage, we didn’t know which pathway would dominate, but it would be ideal to develop conditions to access 11, 12, or 14 at wish. We were particularly attracted by the conversion of hydroxycyclopropanol 6 to fused lactone 11. The established modular assembly of 6 would enable the introduction of various substitutions in product 11, especially at the ring junction carbon where the R group resides. Meanwhile, we were hoping that the use of bishomoallylic alcohols may eventually result in THP-fused γ-lactones. Given the prevalence of THF/THP-fused γ-lactones in biologically active natural products, this chemistry is expected to find broad application in facilitating the chemical syntheses of those natural products and their analogs. Herein, we report such a Pd-catalyzed hydroxycyclopropanol ring opening carbonylative lactonization to the aforementioned bicyclic γ-lactones and its application in a short total synthesis of (±)-paeonilide (1g) and other medicinally important bicyclic and polycyclic scaffolds.
Our investigation started with known hydroxycyclopropanol 15a (Table 1).16 When it was subjected to the carbonylative spirolactonization conditions we developed before with ([Pd(neoc)(OAc)]2(OTf)2 as catalyst and 1,4-benzoquinone (BQ) as oxidant,13 the formation of 16a was not observed (entry 1). Switching [Pd(neoc)(OAc)]2(OTf)2 to palladium(II) trifluoroacetate (Pd(TFA)2) was not fruitful either (entry 2). The break-through came by using Cu(OTf)2 to replace BQ as the oxidant, which resulted in the formation of 16a in 6% yield (entry 3). The structure of 16a was unambiguously confirmed by X-ray crystallographic analysis.17 The use of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) as oxidant increased the yield to 18% (entry 4). Further investigation of palladium catalysts led to the identification of Pd(OAc)2 as an optimal choice (entry 5-6). Other 1,4-benzoquinone derivatives including 2,5-DMBQ and 2,6-DMBQ were not effective (entry 8, 9). THF (entry 7), toluene (entry 10), and benzene (entry 14) were suitable solvents with benzene giving slightly better yield (also see 16e and 16n, Table 2). Elevating reaction temperature (40 °C, entry 11) or increasing the amount of DDQ (entry 12) led to reduced reaction yield. Finally, reducing the reaction concentration from 0.03 M to 0.01 M was beneficial and product 16a was obtained in 74% yield (entry 14). The reaction is also scalable. Scaling up the reaction from 0.2 mmol to 2.92 mmol led to the formation of 16a in 65% yield. Moreover, when enantioenriched homoallylic alcohol (1-phenylbut-3-en-1-ol, 92% ee) was used, 16a was obtained in 92% ee (73% yield) after a sequence of cyclopropanol formation and carbonylative lactonization. Given the ready availability of enantioenriched homoallylic alcohols, our method offers an efficient asymmetric alternative to THF-fused γ-lactones.
Table 1.
Reaction condition optimizationa
 | ||
|---|---|---|
| entry | reaction conditions | yieldb |
| 1 | [Pd(neoc)(OAc)]2(OTf)2, BQ, DCE (0.03), rt | 0% |
| 2 | Pd(TFA)2, BQ, DCE (0.03), rt | 0% |
| 3 | Pd(TFA)2, Cu(OTf)2, DCE (0.03), rt | 6% |
| 4 | Pd(TFA)2, DDQ, DCE (0.03), rt | 18% |
| 5 | PdCl2, DDQ, DCE (0.03), rt | 0% |
| 6 | Pd(dppf)Cl2, DDQ, DCE (0.03), rt | trace |
| 7 | Pd(OAc)2, DDQ, THF (0.03), rt | 58% |
| 8 | Pd(OAc)2, 2,5-DMBQ, THF (0.03), rt | trace |
| 9 | Pd(OAc)2, 2,6-DMBQ, THF (0.03), rt | trace |
| 10 | Pd(OAc)2, DDQ, toluene (0.03), rt | 65% |
| 11 | Pd(OAc)2, DDQ, toluene (0.03), 40 °C | 51% |
| 12 | Pd(OAc)2, DDQ (3.0 equiv.), toluene (0.03), rt | 58% |
| 13 | Pd(OAc)2, DDQ, toluene (0.01), rt | 70% |
| 14 | Pd(OAc)2, DDQ, benzene (0.01), rt | 74%c,d |
General reaction conditions: To a stirred solution of 15a (1.0 equiv., 0.2 mmol) and DDQ (2.0 equiv.) in benzene (0.01 M) under carbon monoxide (balloon) was added Pd(OAc)2 (0.1 equiv.) in one portion. The resulting solution was stirred at room temperature until no more starting material left. The reaction process was monitored by thin-layer chromatography
Isolated yield
65% for 0.56-gram scale (2.92 mmol)
73% yield and 92% ee when 15a was prepared from the corresponding enantioenriched homoallylic alcohol with 92% ee; 2,5-DMBQ: 2,5-dimethylbenzoquinone; 2,6-DMBQ: 2,6-dimethylbenzoquinone.
Table 2.
Substrate scope
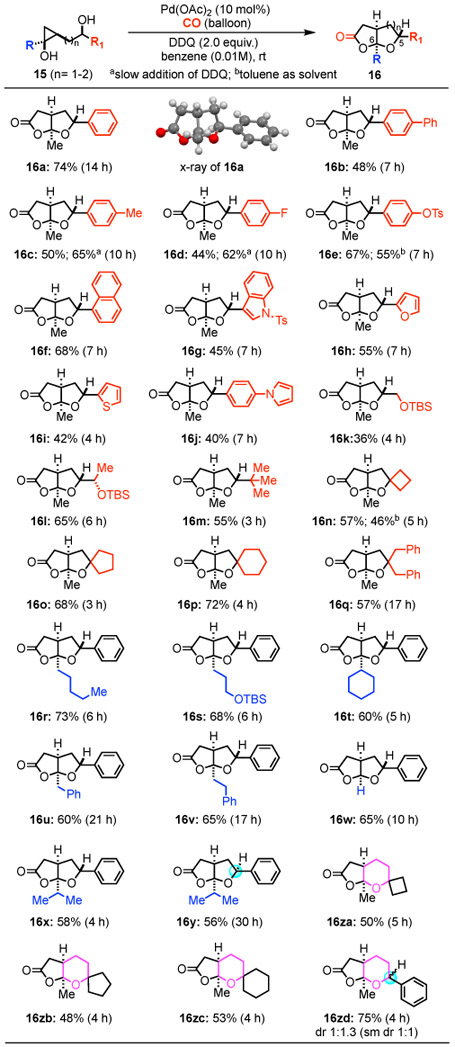 |
We then evaluated the scope and generality of this new Pd-catalyzed hydroxycyclopropanol ring opening carbonylative lactonization. The reaction proved to be very robust. A variety of hydroxycyclopropanol substrates could be transformed into the corresponding cis-fused bicyclic γ-lactones. THF-fused γ-lactone products with either an aryl or an alkyl group at the C5-position could be obtained. Notably, tosylate (16e), TBS-ether group (16k, 16l, 16s), and heteroaromatics including indole (16g), furan (16h), thiophene (16i), and pyrrole (16j) are all tolerated under the mild reaction conditions. Substrates containing sterically hindered secondary (16m) and tertiary alcohols (16n, 16o, 16p, 16q) were effective as well. Most of the previously reported methods are limited to have hydrogen atom at C6 of the ring junction. In our case, the substituent at this position can be altered from a methyl group to a hydrogen (16w) or other bulkier alkyl groups including cyclohexyl and isopropyl groups (16r, 16s, 16t, 16u, 16v, 16x, and 16y). Variation of the stereochemistry of the secondary alcohol on the side chain was tolerated as well (cf. 16x vs 16y). Similar reaction yields were obtained, but the reaction of 16y took longer time. Furthermore, THP-fused γ-lactones could be produced in modest to good yield (16za, 16zb, 16zc, and 16zd). Additionally, slow addition of DDQ to the reaction mixture could be beneficial to improve the reaction yield (cf. 16c and 16d), but was not employed to all the substrates due to the operational inconvenience.
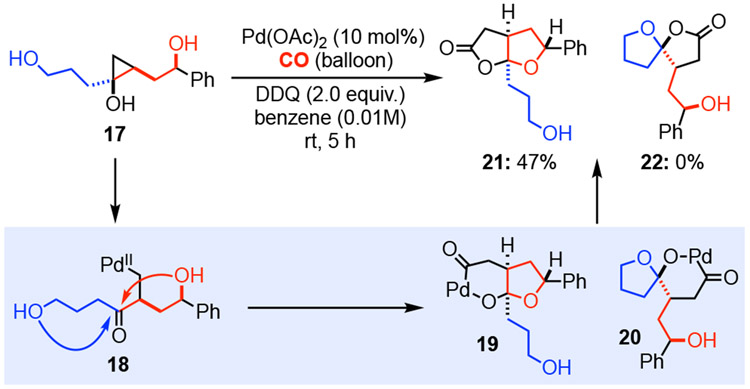
We also evaluated hydroxycyclopropanol 17, which could undergo ring opening carbonylation to form either fused γ-lactone 21 or spirolactone 22 presumably via intermediate 19 or 20, respectively (Scheme 1). Both 19 and 20 could be derived from the same palladium-homoenolate 18. When 17 was subjected to the optimized carbonylative conditions (entry 14, Table 1), fused γ-lactone 21 was obtained in 47% yield without any spirolactone 22 formation. Interestingly, even when the carbonylative spirolactonization conditions were used ([Pd(neoc)(OAc)]2(OTf)2 and BQ),13 fused lactone was still the dominant product, but in lower yield.
Scheme 1.
A “competition” case
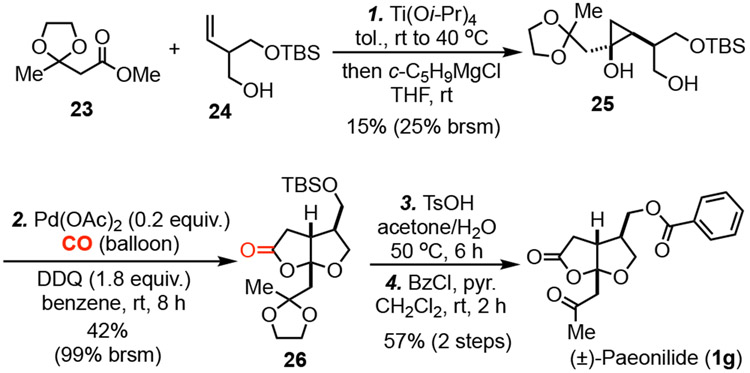
We next decided to test this new carbonylative lactonization method in natural product total synthesis and chose paeonilide (1g) as our initial target. Paeonilide is a monoterpenoid isolated from Paeonia delavayi by Liu and co-workers.18 It selectively inhibits platelet aggregation induced by platelet activating factor and has been previously synthesized by the groups of Zhang (chiral pool synthesis starting from (R)-(-)-carvone, 14 LLS steps;19a racemic, 17 LLS steps19b), Du (racemic, 6 LLS steps),19c Reiser (enantioselective, 12 LLS steps),19d and Argade (racemic, 11 LLS steps)19e from commercially available starting materials. Our synthesis started with known compounds 2320 and 24,21 which were prepared in one and three steps, respectively, from commercially available starting materials. They were united via a Kulinkovich reaction to give desired hydroxycyclopropanol 25, albeit in low yield due to the existence of the ketal, free alcohol, and primary TBS ether. When 25 was subjected to the carbonylative lactonization conditions, bicyclic γ-lactone 26 was produced in 42% yield (99% yield brsm). Removal of the TBS and ketal protecting groups under acidic conditions followed by benzoate formation completed the total synthesis (±)-paeonilide in 4 LLS steps in 3.6% yield from known compounds 23 and 24 or 7 LLS steps from commercially available starting materials.
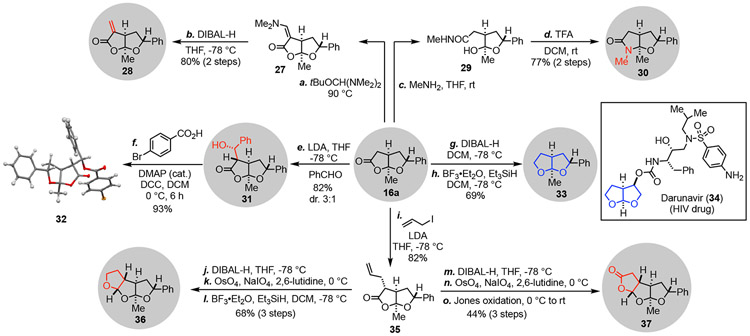
Additionally, the fused bicyclic lactone product can be diversified to other medicinally important structural scaffolds (Scheme 3). α-Methylene γ-butyrolactone is another privileged scaffold frequently found in natural products22 Treatment of 16a with the Bredereck’s reagent followed by DIBAL-H reduction23 could convert 16a to α-methylene γ-butyrolactone containing bicyclic product 28 in high yield. The lactone moiety of 16a could also be converted to a lactam (cf. 30) via the treatment of MeNH2 followed by acid-promoted cyclization. Aldol reaction of 16a with benzaldehyde gave a 3:1 mixture of diastereomers in 82% yield. The relative stereochemistry of the major product 31 was established by X-ray crystallographic analysis of its p-bromobenzoate derivative 32.17 Hexahydrofuro[2,3-b]furan is an important scaffold in protease inhibitors, including the FDA-approved anti-HIV drug Darunavir (34).24 Bicyclic lactone 16a could be readily converted to hexahydrofuro[2,3-b]furan-containing product 33 via a sequential DIBAL-H and triethylsilane reduction process. Furthermore, 16a could also be elaborated to tricyclic products 36 and 37 via a series of standard functional group manipulations.25 These two tricyclic scaffolds have been found in natural products such as gracilin B (1b) and potent protease inhibitors including GRL-0519A.26 These diversification pathways demonstrate the potential broad application of the newly developed carbonylative lactonization method.
Scheme 3.
Synthetic diversifications.
In summary, we have developed a novel Pd-catalyzed hydroxycyclopropanol ring opening carbonylative lactonization to synthesize THF/THP-fused bicyclic γ-lactones. This method features mild reaction conditions, good functional group tolerability, and scalability. Its application was demonstrated in a short total synthesis of racemic paeonilide. The bicyclic lactone products could be further elaborated to other valuable structures.
Supplementary Material
Scheme 2.
Total synthesis of (±)-paeonilide
ACKNOWLEDGMENT
This work was financially supported by NSF CAREER Award 1553820. The NIH P30 CA023168 is acknowledged for supporting shared NMR resources to Purdue Center for Cancer Research. The XRD data is collected on a new single crystal X-ray diffractometer supported by the NSF through the Major Research Instrumentation Program under Grant No. CHE 1625543.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental procedures and spectra data (PDF file)
Cystallographic data for 16a and 32 (cif file)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Wilson RM; Danishefsky SJ Small molecule natural products in the discovery of therapeutic agents: the synthesis connection. J. Org. Chem 2006, 71, 8329–8351. [DOI] [PubMed] [Google Scholar]; (b) Newman DJ; Cragg GM Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod 2012. 75, 311–335. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Davison EK; Brimble MA Natural Product Derived Privileged Scaffolds in Drug Discovery. Curr. Opin. in Chem. Biol 2019, 52, 1–8. [DOI] [PubMed] [Google Scholar]; (d) Rodrigues T; Reker D; Schneider P; Schneider G Counting on natural products for drug design. Nat. Chem 2016, 8, 531–541. [DOI] [PubMed] [Google Scholar]
- (2).Garnsey MR; Slutskyy Y; Jamison CR; Zhao P; Lee J; Rhee YH; Overman LE Short Enantioselective Total Syntheses of Cheloviolenes A and B and Dendrillolide C via Convergent Fragment Coupling Using a Tertiary Carbon Radical. J. Org. Chem 2018, 83, 6958–6976. [DOI] [PubMed] [Google Scholar]
- (3).Sakabe N; Takada S; Okabe K The structure of ginkgolide A, a novel diterpenoid trilactone. J. Chem. Soc., Chem. Commun 1967, 259–261. [Google Scholar]
- (4).Mayol L; Piccialli V; Sica D Minor Bisnorditerpenes from the Marine Sponge Spongionella gracilis and Revision of the Δ6 Configuration of Gracilin B. J. Nat. Prod 1986, 49, 823–828. [Google Scholar]
- (5).Chen X-L; Liu H-L; Li J; Xin G-R; Guo Y-W Paracaseolide A, First α-Alkylbutenolide Dimer with an Unusual Tetraquinane Oxa-Cage Bislactone Skeleton from Chinese Mangrove Sonneratia Paracaseolaris. Org. Lett 2011, 13, 5032–5035. [DOI] [PubMed] [Google Scholar]
- (6).von Salm JL; Witowski CG; Fleeman RM; McClintock JB; Amsler CD; Shaw LN; Baker BJ Darwinolide, a New Diterpene Scaffold That Inhibits MethicillinResistant Staphylococcus aureus Biofilm from the Antarctic Sponge Dendrilla membranosa. Org. Lett 2016, 18, 2596–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).(a) Hochlowski J; Faulkner DJ; Matsumoto G; Clardy J Synthetic Studies on Norrisolide: Enantioselective Synthesis of the Norrisane Side Chain. J. Org. Chem 1983, 48, 1141–1142. [Google Scholar]; (b) Guizzunti G; Brady TP; Fischer D; Malhotra V; Theodorakis EA Chemical biology studies on norrisolide. Bioorg. Med. Chem 2010, 18, 2115–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Buckleton JS; Cambie RC; Clark GR Structures of cheloviolene A from the sponge Chelonaplysilla violacea. Acta Crystallogr., Sect. C: Cryst. Struct. Commun 1991, 47, 1438–1440. [Google Scholar]
- (9).(a) Luo Q; Di L; Yang XH; Cheng YX Applanatumols A and B, meroterpenoids with unprecedented skeletons from Ganoderma applanatum. RSC Adv. 2016, 6, 45963–45967. [Google Scholar]; (b) Qi B; Liu X; Mo T; Zhu Z; Li J; Wang J; Shi X; Zeng K; Wang X; Tu P; Abe I; Shi S 3,5-Dimethylorsellinic Acid Derived Meroterpenoids from Penicillium chrysogenum MT-12, an Endophytic Fungus Isolated from Huperzia serrata. J. Nat. Prod 2017, 80, 2699–2707. [DOI] [PubMed] [Google Scholar]; (c) Tan S-J; Lim J-L; Low Y-Y; Sim K-S; Lim S-H; Kam T-S Oxidized Derivatives of Macroline, Sarpagine, and Pleiocarpamine Alkaloids from Alstonia angustifolia. J. Nat. Prod 2014, 77, 2068–2080. [DOI] [PubMed] [Google Scholar]; (d) Li TX; Liu RH; Wang XB; Luo J, Luo JG, Kong LY; Yang MH Hypoxia-protective azaphilone adducts from Peyronellaea glomerata. J. Nat. Prod 2018, 81, 1148–1153. [DOI] [PubMed] [Google Scholar]; (e) Aziz AN; Ismail NH; Halim SNA; Looi CY; Anouar EH; Langat MK; Mulholland D; Awang K Laevifins A─G, clerodane diterpenoids from the Bark of Croton oblongus Burm.f.. Phytochemistry 2018, 156, 193–200. [DOI] [PubMed] [Google Scholar]
- (10).(a) Corey EJ; Kang MC; Desai MC; Ghosh AK; Houpis IN Total synthesis of (.+−.)-ginkgolide B. J. Am. Chem. Soc 1988, 110, 649–651. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Corey EJ; Letavic MA Enantioselective Total Synthesis of Gracilins B and C Using Catalytic Asymmetric Diels-Alder Methodology. J. Am. Chem. Soc 1995, 117, 9616–9617. [Google Scholar]; (c) Crimmins MT; Pace JM; Nantermet PG; Kim-Meade AS; Thomas JB; Watterson SH; Wagman AS The Total Synthesis of (±)-Ginkgolide B. J. Am. Chem. Soc 2000, 122, 8453–8463. [Google Scholar]; (d) Brady TP; Kim SH; Wen K; Theodorakis EA Stereoselective Total Synthesis of (+)-Norrisolide. Angew. Chem., Int. Ed 2004, 43, 739–742 [DOI] [PubMed] [Google Scholar]; (e) Shi H; Fang L; Tan C; Shi L; Zhang W; Li CC; Luo T; Yang Z Total Syntheses of Drimane-Type Sesquiterpenoids Enabled by a Gold-Catalyzed Tandem Reaction. J. Am. Chem. Soc 2011, 133, 14944–14947. [DOI] [PubMed] [Google Scholar]; (f) Guney T; Kraus GA Total Synthesis of Paracaseolide A. Org. Lett 2013, 15, 613–615. [DOI] [PubMed] [Google Scholar]; (g) Wang L; Wang H; Li Y; Tang P Total Synthesis of Schilancitrilactones B and C. Angew. Chem., Int. Ed 2015, 54, 5732–5735. [DOI] [PubMed] [Google Scholar]; (h) Yuan Z; Hu X; Zhang H; Liu L; Chen P; He M; Xie X; Wang X; She X Total synthesis of conosilane A via a site-selective C─H functionalization strategy. Chem. Commun, 2018. 54, 912–915. [DOI] [PubMed] [Google Scholar]; (i) Okamura H; Mori N; Watanabe H; Takikawa H Synthesis of both enantiomers of conosilane A. Tetrahedron Lett. 2018, 59, 4397–4400. [Google Scholar]; (j) Siemon T; Steinhauer S; Christmann M Synthesis of (+) - Darwinolide, a Biofilm - Penetrating Anti - MRSA Agent. Angew. Chem., Int. Ed 2019, 58, 1120–1122. [DOI] [PubMed] [Google Scholar]; (k) Li C; Quan T; Xue Y; Cao Y; Chen SC; Luo T Synthesis of 17-Deacetoxyl Chromodorolide B Based on a Gold-Catalyzed Alkoxycyclization Reaction. Org. Lett, 2020, 22, 1655–1658. [DOI] [PubMed] [Google Scholar]; (l) Uchida K; Kawamoto Y; Kobayashi T; Ito H (+/−)-Lucidumone, a COX-2 Inhibitory Caged Fungal Meroterpenoid from Ganoderma lucidum. Org. Lett 2019, 21, 6199–6201. [DOI] [PubMed] [Google Scholar]; (m) Li Y; Zhang Q; Wang H; Cheng B; Zhai H Bioinspired Total Synthesis of (±)-Chaetophenol C Enabled by a Pd-Catalyzed Cascade Cyclization. Org. Lett 2017, 19, 4387–4390. [DOI] [PubMed] [Google Scholar]
- (11).(a) Allred TK; Dieskau AP; Zhao P; Lackner GL; Overman LE Enantioselective Total Synthesis of Macfarlandin C, a Spongian Diterpenoid Harboring a Concave - Substituted cis - Dioxabicyclo [3.3.0] octanone Fragment. Angew. Chem., Int. Ed 2020, 59, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tao DJ; Slutskyy Y; Muuronen M; Le A; Kohler P; Overman LE Total synthesis of (−)-chromodorolide B by a computationally guided radical addition/cyclization/fragmentation cascade. J. Am. Chem. Soc 2018, 140, 3091–3102. [DOI] [PubMed] [Google Scholar]; (c) Slutskyy Y; Jamison CR; Zhao P; Lee J; Rhee YH; Overman LE Versatile Construction of 6-Substituted Cis-2,8-Dioxabicyclo[3.3.0]Octan-3-Ones: Short Enantioselective Total Syntheses of Cheloviolenes A and B and Dendrillolide C. J. Am. Chem. Soc 2017, 139, 7192–7195. [DOI] [PubMed] [Google Scholar]; (d) Tao DJ; Slutskyy Y; Overman LE Total synthesis of (−)-chromodorolide B. J. Am. Chem. Soc 2016, 138, 2186–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).(a) Trost BM; Toste FD Palladium-Catalyzed Kinetic and Dynamic Kinetic Asymmetric Transformation of 5-Acyloxy-2-(5H)-furanone. Enantioselective Synthesis of (−)-Aflatoxin B Lactone. J. Am. Chem. Soc 1999, 121, 3543–3544. [DOI] [PubMed] [Google Scholar]; (b) Kim C; Hoang R; Theodorakis EA Synthetic studies on norrisolide: enantioselective synthesis of the norrisane side chain. Org. Lett 1999, 1, 1295–1297. [Google Scholar]; (c) Weisser R; Yue W; Reiser O Enantioselective Synthesis of Furo[2,3b ]furans, a Spongiane Diterpenoid Substructure. Org. Lett 2005, 7, 5353–5356. [DOI] [PubMed] [Google Scholar]; (d) Schnermann MJ; Beaudry CM; Genung NE; Canham SM; Untiedt NL; Karanikolas BDW; Sutterlin C; Overman LE Divergent Synthesis and Chemical Reactivity of Bicyclic Lactone Fragments of Complex Rearranged Spongian Diterpenes. J. Am. Chem. Soc 2011, 133, 17494–17503. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Yousuf SK; Taneja SC; Mukherjee D Highly Regio- and Stereoselective One-Pot Synthesis of CarbohydrateBased Butyrolactones. Org. Lett 2011, 13, 576–579. [DOI] [PubMed] [Google Scholar]; (f) Zhao WW; Liu Y-K Enantio- and diastereoselective synthesis of tetrahydrofuro[2,3-b]furan-2(3H)-one derivatives and related oxygen heterocycles via an asymmetric organocatalytic cascade process. Org. Chem. Front 2017, 4, 2358–2363. [Google Scholar]; (g) Thorat SS; Kataria P; Kontham R Synthesis of Furo[2,3-b]pyran-2-ones through Ag(I)- or Ag(I)–Au(I)-Catalyzed Cascade Annulation of Alkynols and α-Ketoesters. Org. Lett 2018, 20, 872–875. [DOI] [PubMed] [Google Scholar]; (h) Zhang T; Shimizu Y; Fukaya S; Sawa T; Maekawa H Construction of a CF3-Containing Benzofurofuranone Skeleton from Coumarins via Reductive Coupling and Acid-Mediated Ring Contraction. J. Org. Chem 2019, 84, 12165–12171. [DOI] [PubMed] [Google Scholar]
- (13).Davis DC; Walker KL; Hu C; Zare RN; Waymouth RM; Dai MJ Catalytic Carbonylative Spirolactonization of Hydroxycyclopropanols. J. Am. Chem. Soc 2016, 138, 10693–10699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).(a) Ma K; Yin X; Dai MJ Total Syntheses of Bisdehydroneostemoninine and Bisdehydrostemoninine by Catalytic Carbonylative Spirolactonization. Angew. Chem., Int. Ed 2018, 57, 15209–15212. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yin X; Ma K; Dong Y; Dai MJ Pyrrole Strategy for the γ-Lactam-Containing Stemona Alkaloids: (±)Stemoamide, (±)Tuberostemoamide, and (±)Sessilifoliamide A. Org. Lett 2020, 22, 5001–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).For reviews: (a) Kulinkovich OG The Chemistry of Cyclopropanols. Chem. Rev 2003, 103, 2597–2632. [DOI] [PubMed] [Google Scholar]; (b) Mack DJ; Njardarson JT Recent Advances in the Metal-Catalyzed Ring Expansions of Three and Four-Membered Rings. ACS Catal. 2013, 3, 272–286. [Google Scholar]; (c) Nikolaev A; Orellana A Transition-Metal-Catalyzed C-C and C-X Bond-Forming Reactions Using Cyclopropanols. Synthesis 2016, 48, 1741–1768. [Google Scholar]; (d) Mills LR; Rousseaux SAL Modern Developments in the Chemistry of Homoenolates. Eur. J. Org. Chem 2019, 8–26. [Google Scholar]; (e) Cai X; Liang W; Dai M Total Syntheses via Cyclopropanols. Tetrahedron 2019, 75, 193–208. [Google Scholar]
- (16).(a) Lee J; Kim H; Cha JK A New Variant of Kulinkovich Hydroxycyclopropanation. Reductive Coupling of Carboxylic Esters with Terminal Olefins. J. Am. Chem. Soc 1996, 118, 4198–4199. [Google Scholar]; (b) Quan LG; Kim S-H; Lee JC; Cha JK Diastereoselective Synthesis of trans-1,2-Dialkylcyclopropanols by the Kulinkovich Hydroxycyclopropanation of Homoallylic Alcohols. Angew. Chem. Int. Ed 2002, 41, 2160–2162. [PubMed] [Google Scholar]
- (17).CCDC 1997763 and 2016932 contain the supplementary crystallographic data for 16a and 32.
- (18).Liu JK; Ma YB; Wu DG; Lu Y; Shen ZQ; Zheng QT; Chen ZH Paeonilide, a Novel Anti-PAF-active Monoterpenoid-derived Metabolite from Paeonia delavayi. Biosci. Biotechnol. Biochem 2000, 64, 1511–1514. [DOI] [PubMed] [Google Scholar]
- (19).(a) Wang C; Zhang H; Liu J; Ji Y; Shao Z; Li L Stereoselective Synthesis of (+)-Paeonilide and Confirmation of its Absolute Configuration. Synlett 2006, 1051–1054. [Google Scholar]; (b) Wang C; Liu J; Ji Y; Zhao J; Li L; Zhang H Total Synthesis of (±)-Paeonilide. Org. Lett 2006, 8, 2479–2481. [DOI] [PubMed] [Google Scholar]; (c) Du Y; Liu J; Linhardt RJ Facile Synthesis of (±)-Paeonilide. J. Org. Chem 2007, 72, 3952–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Harrar K; Reiser O Enantioselective synthesis of (−)-paeonilide. Chem. Commun 2012, 48, 3457–3759. [DOI] [PubMed] [Google Scholar]; (e) Deore PS; Argade NP Reactivity Umpolung in Intramolecular Ring Closure of 3,4-Disubstituted Butenolides: Diastereoselective Total Synthesis of Paeonilide. Org. Lett 2013, 15, 5826–5829. [DOI] [PubMed] [Google Scholar]
- (20).Ueda Y; Abe H; Iguchi K; Ito H Synthetic Study of Yonarolide: Stereoselective Construction of the Tricyclic Core. Tetrahedron Lett. 2011, 52, 3379–3381. [Google Scholar]
- (21).Lohse-Fraefel N; Carreira EM Polyketide Building Blocks via Diastereoselective Nitrile Oxide Cycloadditions with Homoallylic Alcohols and Monoprotected Homoallylic Diols. Chem. Eur. J 2009, 15, 12065–12081. [DOI] [PubMed] [Google Scholar]
- (22).Jackson PA; Widen JC; Harki DA; Brummond KM Covalent Modifiers: A Chemical Perspective on the Reactivity of α,β-Unsaturated Carbonyls with Thiols via Hetero-Michael Addition Reactions. J. Med. Chem 2017, 60, 839–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Barthel A; Kaden F; Jager A; Metz P Enantioselective Synthesis of Guaianolides in the Osmitopsin Family by Domino Metathesis. Org. Lett 2016, 18, 3298–3301. [DOI] [PubMed] [Google Scholar]
- (24).Ghosh AK; Chapsal BD; Weber IT; Mitsuya H Design of HIV Protease Inhibitors Targeting Protein Backbone: An Effective Strategy for Combating Drug Resistance. Acc. Chem. Res 2008, 41, 78–86. [DOI] [PubMed] [Google Scholar]
- (25).Rashid S; Bhat BA; Mehta G Regenerative γ-Lactone Annulations: A Modular, Iterative Approach to Oligo-tetrahydrofuran Molecular Stairs and Related Frameworks. Org. Lett 2015, 17, 3604–3607. [DOI] [PubMed] [Google Scholar]
- (26).Amano M; Koh Y; Das D; Li J; Leschenko S; Wang YF; Boross PI; Weber IT; Ghosh AK; Mitsuya H A novel bistetrahydrofuranylurethane-containing nonpeptidic protease inhibitor (PI), GRL-98065, is potent against multiple-PI-resistant human immunodeficiency virus in vitro. Antimicrob. Agents Chemother 2007, 51, 2143–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.