Abstract
We generated self-adjuvanted protein nanoparticles of conserved influenza antigens and immunized mice via skin vaccination with dissolvable microneedle patches (MNPs) to increase the strength and breadth of immune responses. We produced M2e nanoparticles via ethanol desolvation, and double-layered NA1/M2e (shell/core), NA1-FliC/M2e, NA2/M2e, and NA2-FliC/M2e protein nanoparticles by chemically crosslinking influenza NA and flagellin (FliC) onto the surfaces of the M2e nanoparticles. The resulting nanoparticles retained FliC TLR5 innate signaling activity and significantly increased antigen-uptake and dendritic cell maturation in vitro. We incorporated the nanoparticles into MNPs for skin vaccination in mice. The nanoparticle MNPs significantly increased M2e and NA-specific antibody levels, the numbers of germinal center B cells, and IL-4 positive splenocytes. Double-layered nanoparticle MNP skin vaccination protected mice against homologous and heterosubtypic influenza viruses. Our results demonstrated that MNP skin vaccination of NA-FliC/M2e nanoparticles could be developed into a standalone or synergistic component of a universal influenza vaccine strategy.
Keywords: Skin vaccination, Dissolvable microneedle patch, Influenza NA protein nanoparticle, Flagellin adjuvant, Universal influenza vaccine
Graphical Abstrat

Introduction
Despite the worldwide application of seasonal influenza vaccines, influenza continues to be a severe threat to public health. The seasonal influenza vaccine requires yearly updates due to the antigenic drift of the most critical influenza antigen, hemagglutinin (HA). To compensate for the weaknesses of the seasonal vaccines and achieve a more broadly protective influenza vaccine, we have studied protein nanoparticles comprised of conserved influenza antigens, such as the membrane-proximal region of HA (headless HA stalk domains) and the matrix protein 2 ectodomain (M2e)1.
Neuraminidase (NA) is another conserved antigen compared to the much variable HA from influenza viruses2. Previously, we fabricated protein nanoparticles coated with a layer of M2e-NA fusion proteins. Vaccination with these nanoparticles provided much higher and broader cross-protection against various influenza strains than soluble proteins3. However, the M2e-NA nanoparticles still showed limited protection to heterosubtypic influenza viruses. To improve the protection, we modified our previous nanoparticles by incorporating flagellin (FliC) as an adjuvant to enhance immune responses to NA and M2e. We also encapsulated our nanoparticles into dissolvable microneedle patches (MNPs) to take advantage of skin vaccination’s immunological benefits.
FliC is the major structural protein of Gram-negative bacteria flagella and a potent adjuvant4. Toll-Like Receptor 5 (TLR-5) recognizes FliC and initiates an innate signaling cascade. The consequence includes the generation of transcription factor NF-κB and subsequent gene expression, such as TNF-α. Previous studies have added FliC to different antigen combinations to increase dendritic cell (DC) cytokine and chemokine production, maturation, and antigen-processing/presentation. FliC could also enhance the protection breadth of the influenza vaccines. For example, while H1/M1 VLPs conferred no protection to H3N2, FliC-adjuvanted H1/M1 VLPs effectively improved heterosubtypic protection against H3N25. We have expressed 4M2e-tFliC and 4M2e-HA stalk fusion proteins as standalone vaccines or as supplements to improve the immunity breadth of inactivated vaccines6.
However, a high dose of a flagellin-M2e fusion protein (STF2.4×M2e) vaccination generated an overproduction of inflammatory molecules in a clinical trial7. We found that a lower dose (0.5 μg) of FliC-adjuvanted HIV VLPs induced significantly higher levels of neutralizing antibody responses than non-adjuvanted VLPs without abnormal symptoms8. In the present work, we are using a flexible and controllable DTSSP crosslinking method to introduce FliC onto the surface of the M2e core nanoparticles to enhance immune responses while minimizing the side effects of FliC.
The advantages of dissolvable microneedle patch-based skin administration over traditional vaccination routes are simplified administration, lack of pain, no biohazardous sharps waste, and improved immune responses due to antigen delivery to the skin. Abundant principal phagocytes like DCs, macrophages, neutrophilic granulocytes, and adaptive immune cells like CD4+ and CD8+ T cells reside in different skin layers, providing targets for dissolvable MNPs9. Researchers have combined MNPs and nanoparticles to selectively target DCs within the dermis to improve vaccine immunogenicity10.
Our laboratory has investigated MNP skin vaccination with an M2e-FliC/inactivated influenza vaccine combination and M2e/NP nanoparticles. We found that the MNP skin immunization induced enhanced protection against high lethal doses of homologous and heterosubtypic influenza viruses1a, 11. Skin vaccination with influenza antigens nanoparticles has shown promising results in our research.
In this study, we generated double-layered NA1/M2e (shell/core, designated MN1), NA1-FliC/M2e (designated MFN1), NA2/M2e (designated MN2), and NA2-FliC/M2e (designated MFN2) protein nanoparticles by coating NA and FliC onto M2e nanoparticle cores. We characterized the physicochemical properties of the resulting nanoparticles and compared the formulation with or without FliC in the same vaccination route or same nanoparticle formulation by different vaccination routes. Skin vaccination with the FliC-adjuvanted nanoparticles by MNPs contributed to the generation of germinal centers, maturation of B cells, and induced robust humoral and cellular immune responses specific to M2e and NA. These enhanced immune responses translated into broad cross-protection against the influenza viruses of homologous and heterosubtypic NA.
Method and Materials
Ethics statement and statistical analysis methods:
Animal studies were pre-approved by the Georgia State University Institutional Animal Care and Use Committee (IACUC, protocol # A19025). Data were presented by mean +/− SEM. One-way ANOVA and Tukey’s multiple comparison post-test were used for statistical significance analysis. A p-value less than 0.05 (p < 0.05) was recognized as significant. The survival rates among groups were analyzed using the Log-rank test.
Design, expression, and characterization of recombinant proteins:
The construction of the tetrameric M2e protein-encoding gene (m2e) and NA fusion protein-encoding gene was done as previously described3. Briefly, a honeybee melittin signal peptide (melittin), a hexahistidine-tag (his-tag), a tetrameric motif tetrabrachion (tetra-), and four tandem copies of different M2e or NA fusion protein-encoding genes (na1 and na2) were fused in frame and subcloned into the transfer vector pFastBac for recombinant baculovirus (rBV) generation.
FliC was purified from E. coli protein expression as described previously12. All other recombinant proteins were purified from rBV-based insect cell cultures. Ni-NTA resin was used to purify the above recombinant proteins from insect cell culture supernatants or E.coli cell lysates, as described previously3.
Fabrication of protein nanoparticles:
We fabricated and characterized double-layered MFN1, MFN2, MN1, and MN2 protein nanoparticles, as described previously1b. Briefly, NA and FliC were crosslinked onto the desolvated M2e nanoparticle surfaces using a DTSSP (3,3’-dithiobis(sulfosuccinimidyl propionate)) crosslinking reaction. The nanoparticles were pelleted, and the pellets were collected after 1.5 hours of crosslinking reaction.
The size and ζ-potentials of the MFN (both NFN1 and NFN2) and MN (both MN1 and MN2) nanoparticles were measured by dynamic light scattering (DLS) analysis with a Malvern Zetasizer Nano ZS. The recombinant NA polymeric states were examined by using Bis [sulfosuccinimidyl] (BS3) crosslinking3. MN and MFN nanoparticle compositions were confirmed by using SDS-PAGE followed by Coomassie blue staining. The ratio of NA or FliC to M2e was measured using GelQuantNET software after Coomassie blue staining. Anti-flagellin antibody (Abcam, ab93713) or monoclonal antibody 14C2 were used for detecting the FliC or M2e, respectively, in the MFN nanoparticles in the Western blotting analysis.
Microneedle patch fabrication:
Microneedle patch fabrication has been previously described1a. Briefly, nanoparticles (5 μg) were cast onto a polydimethylsiloxane microneedle mold with casting solution (1% polyvinyl alcohol and 10% sucrose). A second solution (18% sucrose and 18% polyvinyl alcohol) was added to the mold. The MNP backing was formed after the drying process. The intact MNP was then removed from the mold and stored until use.
Immunization and influenza infection:
BALB/c mice (aged 6–8 weeks) received the primary and boost immunizations on day 0 and day 28, respectively, by intramuscular injection or skin vaccination via microneedle patches. There were five mice groups for each NA1 or NA2 set: (1) FMN nanoparticles by microneedle patch (NA, 2.8 μg; FliC, 0.5 μg; core M2e, 1.7 μg; designated MFN MNP); (2) FMN nanoparticles by intramuscular injection (same protein composition as in MFN MNP; designated FMN IM); (3) MN nanoparticles by microneedle patch (NA, 3 μg; core M2e, 2 μg; designated MN MNP); (4) soluble M2e, NA, and FliC protein mixture by intramuscular injection (same protein composition as in MFN MNP; designated sMFN IM) (5) PBS by intramuscular injection. Twenty-one days after the boost immunization, immune sera from the different groups were collected.
Mice were challenged by different influenza viruses (50 μL in saline solution) 28 days after the boost immunization. For the NA1 group cluster, 5×LD50 reassortant Viet (rViet, H5N1) or A/Aichi/2/1968 (Aichi, H3N2) was used. For the NA2 group cluster, 5×LD50 of Aichi (H3N2) or A/PR8/1934 (H1N1) was used. Mouse body weight losses and final survival rates were recorded for 14 days post-infection.
Humoral and cellular immune response assays:
IgG levels specific to NA1, NA2, or M2e were examined using the ELISA method, as described previously11. TMB and 1 M sulfuric acid were used for developing the colors.
The ELISpot method was used to evaluate the number of M2e, NA1, or NA2-specific IL-4-secreting cells after the boost immunization11. 3 × 105 splenocyte single-cell suspensions were seeded onto 96-well filtration plates. A final concentration of 2 μg/mL M2e, NA1, or NA2 peptide mixture was used for stimulating the cells.
Fluorescence imaging of antigen cellular uptake:
We prepared nanoparticle samples with fluorescence using previously described methods13. Briefly, Alexa Fluor 488 was crosslinked to M2e. Then the regular M2e and fluorescent M2e were mixed at a ratio of 3:1 for the generation of M2e desolvation nanoparticles. The desolvated M2e particle cores were crosslinked with NA1 or both NA1 and FliC to generate double-layered protein nanoparticles. JAWS II cells were seeded on 24-well plates and treated with fluorescent MFN1, fluorescent MN1 particles, or a soluble mixture of M2e, FliC, and NA1. 2 μg/mL M2e, 0.5 μg/mL FliC, and 3 μg/mL NA1 were applied in the nanoparticle or protein mixture treatments. The plates were triple washed with PBS to remove excess antigens. The cells were then fixed, permeabilized, and stained with DAPI. We used a Keyence BZ-X710 microscope to image the fluorescent signal.
Flow cytometry:
For germinal center B cell detection, the inguinal lymph nodes (iLNs) from different groups were collected 7 days post the primary immunization. The homogenized lymphocytes were stained with B220-FITC, CD95-PE, and GL7-Pacific Blue (Biolegend). CD95 and GL7 positive cells were selected by sub-gating B220 positive cells.
Spleens were collected from different groups of infected mice for CD4 and IFN-γ positive splenocytes detection. A NA2 or M2e peptide mixture (at a final concentration of 4 μg/mL) was added to homogenized splenocytes for stimulation overnight. The splenocytes were stained by surface marker CD4-PE-Cy7 and intracellular cytokine IFN-γ-BV711. BD LSRFortessa™ was used for examining stained cells. Data ware analyzed with the FlowJo software application.
Histological analysis and lung virus titration:
The immunized mice from the NA1 and NA2 sets were infected by 1× LD50 rViet or 1× LD50 Aichi virus, respectively. On day 5 post-infection, murine lungs were harvested, dehydrated, fixed, and embedded in paraffin. Lung sample paraffin sections were stained with hematoxylin and eosin. The degrees of leukocyte infiltration were scored on a scale of 0 to 4: absent, 0; subtle, 1; mild, 2; moderate, 3; and severe, 4.
For lung virus titration, lung samples were ground on a cell strainer and separated by Percoll gradient centrifugation, as described previously3. 100 μL of serially 10-fold diluted lung supernatants from infected mice were added to MDCK cells coated 96-well plates. Five days post-incubation, 50 μL of MDCK supernatants were transferred to V-shape 96-well plates and mixed with the same volume of 0.5% turkey red blood cells. The Reed-München method was used for the calculation of hemagglutinin activity titers.
Neuraminidase inhibition test:
For the neuraminidase-inhibition (NAI) test, 50 μL serially 2-fold-diluted, heat-inactivated sera from different groups were mixed with 50 μL of diluted H5N1 or H3N2 influenza virus solution at 37°C for 30 minutes. Then the serum-virus mixtures were incubated in Fetuin-coated plates at 37°C for 2 hours. An HRP-conjugated lectin was added and then incubated for 2 hours. The color development was facilitated by adding TMB and stopped with 1M sulfuric acid.
TLR5 signaling activity:
We measured the TLR5 signaling activity as previously described13. Briefly, HEK 293T cells were transfected with pUNO1-hTLR5 and reporter pGL4.32 plasmids using Lipofectamine® 3000. We treated the transfected cells with MFN1, MFN2, or soluble FliC for 5 hours. Steady-Glo luciferase assay reagent (100 μL) was added to each well and luciferase activity was read on the Promega GloMax 96 luminometer.
Protein nanoparticle TEM observation:
We carried out the TEM observation as described previously3. Fine forceps were used to hold a formvar/carbon-coated TEM grid while nanoparticle solution (5 μL) was applied. A drop (5 μL) of 1% phosphotungstic acid (PTA, pH 7.4) was immediately applied to the nanoparticle solution on the TEM grid. The nanoparticle/PTA solution was incubated on the TEM grid for 1 min. After the incubation, the nanoparticle/PTA solution was wicked from the side of the TEM grid with blot paper. The grid was air dried at room temperature and stored for TEM imaging on a Jeol JEM‐100CX II at 100 kV. Digital images were acquired with an Apogee Imaging Systems CCD camera system and software.
Results
Fabrication and characterization of double-layered NA/M2e and NA-FliC/M2e protein nanoparticles
We generated recombinant baculoviruses (rBVs) encoding the extracellular domain of M2 (M2e), neuraminidase1 (NA1), and neuraminidase2 (NA2) (Figure S1). We purified FliC from a bacterial protein expression as previously described. The purified NA1, NA2, and FliC proteins showed major bands in the Coomassie Blue gel staining and Western blotting results, respectively (Figure 1A, Figure S2). After the BS3 crosslinking to fix the polymeric states of the purified proteins, M2e, NA1, and NA2 showed a four-fold higher molecular weight than non-cross-linked proteins (Figure S3)3.
Figure 1. The characterization of double-layered NA-FliC/M2e and NA/M2e protein nanoparticles.

A. Coomassie blue staining of purified NA1 (N1), NA2 (N2), and FliC recombinant proteins. B. Coomassie blue staining and Western Blot analysis of the fabricated MFN1 and MFN2 nanoparticles. C. Left panel, The size range of protein nanoparticles. Right panel, TEM images of MFN1 and MFN2. Bar scale: 200 nm. D. TLR5-specific bioactivity of MFN1, MFN2, and soluble FliC.
We fabricated double-layered MFN1 and MFN2 nanoparticles by using DTSSP crosslinking method as diagramed in Figure S5. The Coomassie blue staining confirmed the M2e, NA, and FliC composition of the layered protein nanoparticles (Figure 1B, MFN1, MFN2). Protein components in double-layered nanoparticles were confirmed by anti-His, FliC, or M2e antibodies (Figure 1B).
The resulting double-layered protein nanoparticles had particle sizes in a range of 150–250 nm (MN1, 195.8 +/− 26.2 nm; MN2, 213.4 +/− 27.6 nm; MFN1, 245.6 +/−20.5 nm; and MFN2, 223.5 +/− 15.6 nm. Figure 1C). The polydispersity index (PDI) of four nanoparticles is less than 0.2. All layered protein nanoparticles showed negative ζ-potentials: MN1, −27.76 +/−1.19 mV; MN2, −21.26 +/− 0.98 mV; MFN1, −26.5+/− 1.01 mV; MFN2, −22.36 +/− 0.43 mV (Figure S4). Transmission electron microscopy (TEM) observation revealed the roughly spherical shape of the layered protein nanoparticles (Figure 1C, Figure S7).
MFN1, MFN2, or soluble FliC protein, stimulated 293T cells (with transfection of plasmids containing TLR5 and NF-ĸB-luciferase reporter genes) to produce comparable levels of TLR5 innate signal as demonstrated by their similar RLUs13 (Figure 1D).
Cytokine secretion after nanoparticle stimulation of dendritic cells
The skin is the largest immune organ with high populations of dermal dendritic cells (DCs)14. Our nanoparticles target skin dendritic cells for optimal DC maturation by MNP skin vaccination. We observed an increased fluorescence intensity in JAWS II cells - a dendritic cell line originated from mouse bone marrow - incubated with AF488-labeled MFN1 nanoparticles compared with a soluble protein mixture of AF488-labeled M2e, NA1, and FliC (designated sMFN1) (Figure 2A, MFN1 vs. sMFN1). MFN1 nanoparticles significantly increased the fluorescence intensity 1 hour after incubating with JAWS II cells. Meanwhile, the soluble protein-treated group showed substantially fewer cells with positive AF488 signals (Figure 2A). These results demonstrated that DCs can effectively internalize double-layered protein nanoparticles.
Figure 2. In-vitro internalization of MFN1 nanoparticles by dendritic cells.

A. Fluorescence images of JAWS II cells incubated with Alexa Fluor 488-labeled soluble M2e and NA1 or MN1 or MFN1. The fluorescence intensity of AF488 was counted using mean fluorescence intensity (MFI). Bar scale, 50 μm. B, C. Cytokines (IL-6 and TNF-α) secretion in JAWS II cells or BMDCs treated with soluble M2e and NA1 (sMN1), soluble M2e, NA1, and FliC (sMFN1), MN1, and MFN1 at a concentration of 5 μg/mL. (n = 5; **p < 0.01; ***p < 0.001; ns, p > 0.05)
We further evaluated whether protein nanoparticles induced DC maturation. Because IL-6 and TNF-α cytokines can trigger DC maturation via an autocrine fashion15, we measured the two cytokines’ secretion levels by JAWS II cell culture or primary bone marrow dendritic cells (BMDC) after treatments with different antigens. FliC significantly elevated the IL-6 and TNF-α production by JAWS II cells (Figure 2B, C, sMN1 vs. sMFN1).
The nanoparticle groups induced substantially higher IL-6 and TNF-α levels than the soluble protein mixture groups. The nanoparticle group without FliC triggered comparable cytokine production to soluble FliC, demonstrating the adjuvant efficacy of the protein nanoparticles themselves. The FliC-containing nanoparticle group produced the highest cytokines (Figure 2B, C, JAWS II cells, sMFN1 vs. MN1 group). In BMDCs, both MN1 and MFN1 nanoparticles induced significantly higher levels of IL-6 and TNF-α than the soluble protein mixture groups (Figure 2B, C, MN1 and MFN1 vs. sMFN1). These data demonstrated that nanoparticles induced higher expression of IL-6 and TNF- α by DCs.
Vaccine-delivery efficacy of MNPs and germinal center immune responses
Four types of MNPs encapsulating different nanoparticles (MN1, MN2, MFN1, and MFN2) were fabricated as described previously11. After MNP administration to mice, Western blotting results showed that less than 30% of M2e and 20% of NA were retained in the microneedle bases (Figure S6). These results indicated that MNP skin vaccination delivered 70%−80% of the MNP-loaded nanoparticles.
High-affinity antibody production is closely related to antigen persistence, B cell receptor somatic hypermutation, and B cell affinity maturation in germinal centers (GCs)16. CD95 and GL7 double-positive GC B cell populations were determined in ILNs at day 7 after one immunization. As shown in Figure 3A and B and Figure S8, after MFN2 MNP immunization, there were significantly increased numbers of B220+CD95+GL7+ GC B cells in ILNs compared with the other groups. The MN2 MNP-immunized group also showed a higher B220+CD95+GL7+ population than the sMFN2 or PBS group. Therefore, MNP skin vaccination induced enhanced germinal center reactions.
Figure 3. Germinal center B cell responses in vaccinated mice.
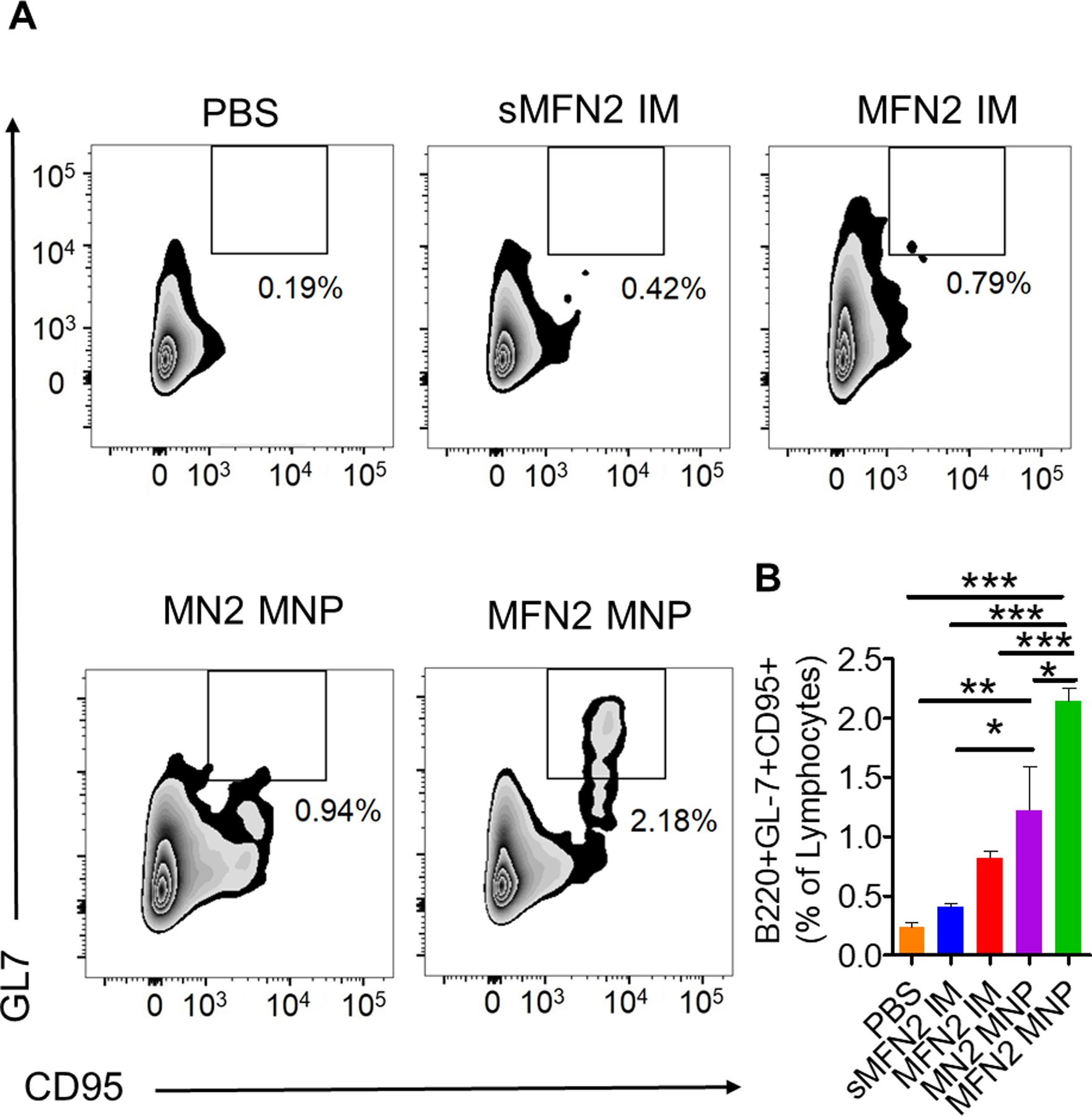
A, B. The percentage of CD95 and GL7 positive cells from inguinal lymph nodes 7 days after the primary immunization-. (n = 3; * p<0.05, **p < 0.01; ***p < 0.001; ns, p > 0.05)
Humoral and cellular immune responses in vaccinated mice
We designed the different vaccination groups to consider IM vs. MNP delivery, soluble protein vs. nanoparticle antigens, and FliC vs. no FliC. As shown in Figure 4A, B, the MFN1 MNP immunization induced the highest levels of M2e and NA1-specific antibody responses among the five groups in the M2e-NA1 group set. In contrast, MN2 and MFN2 MNP immunizations induced similarly high levels of M2e and NA2-specific antibody responses in the M2e-NA2 group set.
Figure 4. Humoral and cellular immune responses in vaccinated mice.

A. M2e-specific antibody responses. B. NA-specific antibody responses. C. NA inhibition test against H5N1 or H3N2. D, E. Enumeration of M2e or NA activated IL-4 secreting splenocytes by ELIspot. (n = 5; * p<0.05; **p < 0.01; ***p < 0.001; ns, p > 0.05)
Neuraminidase inhibition (NAI) of immune sera correlates to NA-mediated immune protection17. Our NAI assay results showed that immune sera from MN1 and MN2 groups possessed potent neutralization to homologous NA influenza viruses. MFN1and MN1 MNP and MN1 IM immune sera showed a significant decrease in OD450 (indicating an elevated NAI) compared with sMFN1 immune sera or untreated NA1 virus (Figure 4C, H5N1) up to a 20,480-fold dilution. MFN2 and MN2 MNP immune sera showed a similar inhibition, at a 20,480-fold dilution, in OD450 compared with untreated NA2 virus (Figure 4C, H3N2).
IL-4 mediates the differentiation of naïve T cells into Th2 cells and the differentiation and proliferation of B cells, driving immunoglobulin(Ig) class switching to IgG1 and memory B cell production18. We determined the IL-4 secreting cell population at week three after boosting immunization. The MFN1 and MFN2 MNP immunizations significantly increased the numbers of M2e and NA-specific IL-4 secreting splenocytes in each group (Figure 4D, E).
MFN MNP induced immune protection against homologous and heterosubtypic NA viral challenges
To determine immune protection against homologous viral infection, mice in M2e-NA1 group set were challenged by 5× LD50 of the reassortant A/Vietnam/1203/2004 (rViet, H5N1) and mice in M2e-NA2 group set with were challenged by 5× LD50 of Aichi (H3N2) (Figure 5). Mice receiving the MN1-MNP, MFN1-MNP, MFN1-IM or MN2-MNP, MFN2-MNP, MFN2-IM immunizations completely survived their corresponding NA virus challenges (Figure 5A and C). Mice receiving the soluble sMFN2 immunization showed a 50% survival rate against the rViet challenge and partial protection (40% survival rate) against the homologous viral (Aichi) challenge (Figure 5A and C).
Figure 5. Immune protection against influenza viruses with homologous and heterosubtypic NA.

A, B. Bodyweight monitoring and survival rate of NA1-Flic/M2e group set. Challenge dose: A. 5×LD50 rViet (H5N1); B. 5×LD50 Aichi (H3N2). C, D Bodyweight monitoring and survival rate of NA2-Flic/M2e group set. Challenge dose: C. 5×LD50 Aichi (H3N2) challenge; D. 5×LD50 PR8 (H1N1). (* p<0.05, **p < 0.01; n = 5).
To determine if the immunity protected mice against heterosubtypic NA virus, mice in M2e-NA2 group cluster were challenged by 5× LD50 PR8 (H1N1) and mice in M2e-NA1 group cluster were challenged by 5× LD50 of Aichi (H3N2). Mice receiving skin MNP vaccination (MN1, MFN1, MN2, and MFN2 MNP groups) showed 100% survival. Mice receiving MFN1 or MFN2 IM vaccination resulted in an 80% survival rate to H3N2 or a 50% survival rate to the challenge of H1N1, respectively. Mice immunized with sMFN1 or sMFN2 showed a 50% survival rate to the challenge of H3N2 and H1N1, respectively (Figure 5B and D). The FliC-adjuvanted group (MFN MNP) displayed a quicker body weight recovery compared with the non-adjuvanted group (MN MNP) and an increased survival rate compared with the MFN IM group in the heterosubtypic NA influenza challenge.
Lung tissues from all the challenged mice were histologically analyzed. The MNP and IM nanoparticle groups showed less alveolar inflammation and less immune cell infiltration than the naïve infection or soluble protein mixture groups (Figure 6A, B). Consistent with the decreased injury scores in the layered protein nanoparticle immunized groups, all the MNP skin immunization groups showed significantly reduced viral titers in the lungs compared with the corresponding soluble protein mixture groups (Figure 6C).
Figure 6. Histology examination and lung viral titers.

A. Histology examination by using H&E staining. Bar scale, 100 μm. B. Lung injury scores of H5N1 and H3N2 infected groups. C. Viral titers of lungs from different immunization groups. (n = 5; * p<0.05; **p < 0.01; ***p < 0.001)
Interferon-gamma (IFN-γ) is mainly produced by CD4 T cells and acts as a critical mediator in cellular immunity. IFN-γ is required for effector CD4 T cells to mediate lung protection and necessary for host survival19. Antigen-specific IFN-γ-secreting CD4 T cells were analyzed after viral challenges. Compared with the MN2-MNP, MFN2-IM, or sMFN2-IM groups, the MFN2-MNP immunization significantly increased the percentage of M2e and NA2-specific IFN-γ-secreting CD4 T cells in lungs five days post Aichi (H3N2) infection. However, only a moderate increase of NA2-specific IFN-γ-secreting populations was observed after MN2 MNP immunization. The results indicate that FliC in nanoparticle MNP skin vaccination plays a vital role in inducing antigen-specific IFN-γ secretion (Figure 7A, B).
Figure 7. IFN-γ secreting CD4 T cell responses post-infection.

A. B. The percentage of IFN-γ secreting CD4 T cells from lungs of mice infected by 1× LD50 of H3N2. (n = 3; * p<0.05; **p < 0.01; ***p < 0.001; ns, p > 0.05)
Discussion
The seasonal influenza vaccine efficacy depends on how closely the vaccine strains match the circulating influenza viruses. Mismatches often occur because of the rapid accumulation of mutations in circulating viruses20. The seasonal vaccine strategy is ineffective in protecting against a possible emerging influenza pandemic. A universal influenza vaccine covering antigenically drifting viruses and emerging influenza pandemics is a real need21.
An ideal vaccine should induce broadly protective immune responses to conserved influenza antigens and avoid immunodominant responses to strain-specific structures. Highly immunogenic vaccine nanoparticle platforms and delivery techniques with targeting and controlled release features are attracting increasing attention in universal influenza vaccine. In this study, we focused on double-layered protein nanoparticles because they displayed multiple features attractive to vaccine development, including appropriate size, high antigen-load (almost pure antigenic protein with minor amount crosslinkers) and high safety, good stability, and controlled disassembly1a, 3, 22. The nanoparticle with 20–200 nm could diffuse through lymphatic endothelial cell junctions efficiently, while the nanoparticle above 500 nm is hard to be transported into the lymph vessels23. Our nanoparticles were around 200 nm with narrow distribution (PDI less than 0.2), facilitating antigen trafficking. We also analyzed the benefits of dissolvable microneedle patch delivery of vaccines versus intramuscular injection. We combined protein nanoparticle and dissolvable microneedle patch technology with an immunogen portfolio we have developed for a universal influenza vaccine candidate.
We developed the FMN nanoparticles with M2e nanoparticles as inner cores and NA (NA1 or NA2) and FliC proteins as the outer layers. The DTSSP crosslinking method enabled a new strategy to precisely and adjustably utilize FliC as an innate signal-starter to boost the immune responses, while avoiding excessive reactogenicity of FliC. Unlike the non-reducible crosslinker, DTSSP is a cleavable crosslinker which contains intervening disulfide structure and allows separation of crosslinked products under reducing conditions24. Reducing nanoparticles could quickly release loaded drug or antigens in the early endosome25. In our nanoparticle constructs, the layer proteins, Flic and NA, could be separated from the M2e core by the break-down of the DTSSP under the reducing intracellular environment. Thus, elevated antibody levels were induced not only against outside NA, but also against inner M2e in double layered MFNA groups.
Previous studies have indicated that NA specific antibodies inhibited influenza NA activities within the same NA phylogenetic group but not NA activities across different NA groups3, 26. In this study we observed this again: our NA antigens predominately induced high NAI titers within the same NA group. The low heterosubtypic NA immunity was compensated by the cross-protection provided by M2e in the nanoparticles and the incorporation of FliC as an adjuvant. Our results indicated that it is critical to include different conserved immune determinants and adjuvants in a universal influenza vaccine formulation to induce a comprehensive immunity conferring cross-protection.
In a previous study, 100 or 200 μg of flagellin induced gross lesion and large area of hepatocellular necrosis 12 h after intraperitoneal administration. Dose below 50 μg did not overly stimulate inflammatory cytokines or liver injury27. In our current study, we used a low dose of FliC (0.5 μg), which is far below 50 μg. The FliC adjuvanted mice did not show hunchback, ruffled fur, and apparent bodyweight loss post immunization. Our data also indicated that the FliC-containing nanoparticles retained TLR-5 signaling activities, demonstrated by triggering several cytokines’ output and improving DC maturation. The incorporation of FliC synergistically enhanced the cross-protection against heterosubtypic influenza viruses, creating a more effective nanoparticle than M2e-NA. These results were consistent with our previous studies: membrane-anchored FliC enhanced the immunogenicity of virus-like particle (VLP) expressing H1 HA and significantly increased the mice survival rate to the challenge of heterosubtypic H3N2 influenza virus.
The skin vaccination strategy with MNP has been applied to improve vaccine delivery and immunogenicity. Abundant DCs and macrophages resident within the skin’s dermis that can be targeted by MNP administration28. The microneedle length in this study was 650 μm, which can fully cross the stratum corneum to deposit antigens into the viable epidermis and superficial dermal layer11. Dissolved MNP skin vaccination prolonged the in vivo kinetics of antigen release, enabling the observation of nanoparticles in the application site up to 14 days after immunization29. The intermediately sized nanoparticles (around 100–200nm) diffused slowly from the MNP insertion site and were transported within the more permeable regions of the extracellular matrix30, which allowed more time for antigen-presenting cells capture. It has been well established that prolonged antigen availability contributes to the formation of GCs, leading to enhanced antibody responses29. During our study, the combined microneedle patch and nanoparticle technologies significantly extended antigen persistence and promoted GC B cell responses in ILNs, leading to the enhanced immune responses.
In our study, MFN MNP showed the best protection in terms of reduced viral titers and quicker weight loss recovery in mice. We found higher levels of NA and M2e-specific antibodies, along with enhanced recruitment of IFN-γ secreting CD4 T cells post infection in the MFN MNP groups. Our data shows that the combination of nanoparticles and MNPs is a practical approach to generating broad protection against influenza virus infection.
Conclusions
In summary, we have successfully fabricated layered protein nanoparticles made of relatively conserved influenza NA and M2e and a molecular adjuvant FliC. The introduction of FliC increased the antigen capture, cytokine secretion and maturation of dendritic cells. Compared with traditional intramuscular immunization, the MNP skin vaccination efficiently delivered antigens to antigen-presenting cells, thus eliciting B cells in geminal center and inducing robust NA- and M2e-specific antibody or cellular responses. Therefore, the combination of MNP skin vaccination with FMN protein nanoparticles conferred protection against homologous and heterosubtypic NA viral infection. The self-adjuvanted FMN protein nanoparticles administered to the skin by MNP have the potential to be further developed into a standalone universal influenza vaccine or as part of a synergistic, multi-component vaccine for broad protection against influenza.
Supplementary Material
Acknowledgments
The research was supported by the US National Institutes of Health (NIH) under grants R01AI101047, R01AI116835, and R01AI143844 (B.-Z.W). The electron microscopy study was performed in part at Georgia Institute of Technology for Electronics and Nanotechnology, a member of the National Nanotechnology Coordinated Infrastructure (NNCI) supported by the National Science Foundation (Grant ECCS-1542174). The study is solely the responsibility of the authors and does not necessarily represent the official views of the funders. We are thankful to Mr. Gilbert X. Gonzalez for his generous help in experimental preparation and the electron microscopy study.
Footnotes
Supporting Information
Diagrams of M2e, NA, and Flagellin fusion proteins and their coding sequence compositions; Western blotting analysis of NA1 and NA2 proteins using an anti-Histidine antibody; Western Blotting analysis of BS3 crosslinked NA1 and NA2 using an anti-Histidine antibody; zeta-potential of all four nanoparticles; Diagrams of layered MFN generation; a magnified view of the structure and layout of the MNP before and after attaching to the skin; the magnified TEM images of MFN1; and the percentage of B220 positive cells from inguinal lymph nodes 7 days after the primary immunization of different groups.
Declarations of interest: none
References
- 1.(a) Deng L; Chang TZ; Wang Y; Li S; Wang S; Matsuyama S; Yu G; Compans RW; Li JD; Prausnitz MR; Champion JA; Wang BZ, Heterosubtypic influenza protection elicited by double-layered polypeptide nanoparticles in mice. Proc Natl Acad Sci U S A 2018, 115 (33), E7758–E7767; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Deng L; Mohan T; Chang TZ; Gonzalez GX; Wang Y; Kwon YM; Kang SM; Compans RW; Champion JA; Wang BZ, Double-layered protein nanoparticles induce broad protection against divergent influenza A viruses. Nat Commun 2018, 9 (1), 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eichelberger MC; Monto AS, Neuraminidase, the Forgotten Surface Antigen, Emerges as an Influenza Vaccine Target for Broadened Protection. The Journal of infectious diseases 2019, 219 (Suppl_1), S75–S80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y; Deng L; Gonzalez GX; Luthra L; Dong C; Ma Y; Zou J; Kang SM; Wang BZ, Double-Layered M2e-NA Protein Nanoparticle Immunization Induces Broad Cross-Protection against Different Influenza Viruses in Mice. Adv Healthc Mater 2020, 9 (2), e1901176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizel SB; Bates JT, Flagellin as an adjuvant: cellular mechanisms and potential. Journal of immunology 2010, 185 (10), 5677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Wang BZ; Xu R; Quan FS; Kang SM; Wang L; Compans RW, Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS One 2010, 5 (11), e13972; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang BZ; Quan FS; Kang SM; Bozja J; Skountzou I; Compans RW, Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol 2008, 82 (23), 11813–23; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hayashi F; Smith KD; Ozinsky A; Hawn TR; Yi EC; Goodlett DR; Eng JK; Akira S; Underhill DM; Aderem A, The innate immune response to bacterial flagellin is mediated by Toll-like receptor 5. Nature 2001, 410 (6832), 1099–103. [DOI] [PubMed] [Google Scholar]
- 6.Deng L; Kim JR; Chang TZ; Zhang H; Mohan T; Champion JA; Wang BZ, Protein nanoparticle vaccine based on flagellin carrier fused to influenza conserved epitopes confers full protection against influenza A virus challenge. Virology 2017, 509, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Turley CB; Rupp RE; Johnson C; Taylor DN; Wolfson J; Tussey L; Kavita U; Stanberry L; Shaw A, Safety and immunogenicity of a recombinant M2e-flagellin influenza vaccine (STF2.4xM2e) in healthy adults. Vaccine 2011, 29 (32), 5145–52. [DOI] [PubMed] [Google Scholar]
- 8.Vassilieva EV; Wang BZ; Vzorov AN; Wang L; Wang YC; Bozja J; Xu R; Compans RW, Enhanced mucosal immune responses to HIV virus-like particles containing a membrane-anchored adjuvant. mBio 2011, 2 (1), e00328–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al-Zahrani S; Zaric M; McCrudden C; Scott C; Kissenpfennig A; Donnelly RF, Microneedle-mediated vaccine delivery: harnessing cutaneous immunobiology to improve efficacy. Expert opinion on drug delivery 2012, 9 (5), 541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.(a) Yang HW; Ye L; Guo XD; Yang C; Compans RW; Prausnitz MR, Ebola Vaccination Using a DNA Vaccine Coated on PLGA-PLL/gammaPGA Nanoparticles Administered Using a Microneedle Patch. Advanced healthcare materials 2017, 6 (1); [DOI] [PubMed] [Google Scholar]; (b) Zaric M; Lyubomska O; Touzelet O; Poux C; Al-Zahrani S; Fay F; Wallace L; Terhorst D; Malissen B; Henri S; Power UF; Scott CJ; Donnelly RF; Kissenpfennig A, Skin dendritic cell targeting via microneedle arrays laden with antigen-encapsulated poly-D,L-lactide-co-glycolide nanoparticles induces efficient antitumor and antiviral immune responses. ACS nano 2013, 7 (3), 2042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu W; Pewin W; Wang C; Luo Y; Gonzalez GX; Mohan T; Prausnitz MR; Wang BZ, A boosting skin vaccination with dissolving microneedle patch encapsulating M2e vaccine broadens the protective efficacy of conventional influenza vaccines. J Control Release 2017, 261, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skountzou I; Martin Mdel P; Wang B; Ye L; Koutsonanos D; Weldon W; Jacob J; Compans RW, Salmonella flagellins are potent adjuvants for intranasally administered whole inactivated influenza vaccine. Vaccine 2010, 28 (24), 4103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C; Zhu W; Wang BZ, Dual-linker gold nanoparticles as adjuvanting carriers for multivalent display of recombinant influenza hemagglutinin trimers and flagellin improve the immunological responses in vivo and in vitro. International journal of nanomedicine 2017, 12, 4747–4762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.(a) Gill HS; Kang SM; Quan FS; Compans RW, Cutaneous immunization: an evolving paradigm in influenza vaccines. Expert Opin Drug Deliv 2014, 11 (4), 615–27; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rodgers AM; Cordeiro AS; Donnelly RF, Technology update: dissolvable microneedle patches for vaccine delivery. Med Devices (Auckl) 2019, 12, 379–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.(a) Verboogen DRJ; Revelo NH; Ter Beest M; van den Bogaart G, Interleukin-6 secretion is limited by self-signaling in endosomes. Journal of molecular cell biology 2019, 11 (2), 144–157; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Blanco P; Palucka AK; Pascual V; Banchereau J, Dendritic cells and cytokines in human inflammatory and autoimmune diseases. Cytokine & growth factor reviews 2008, 19 (1), 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stebegg M; Kumar SD; Silva-Cayetano A; Fonseca VR; Linterman MA; Graca L, Regulation of the Germinal Center Response. Frontiers in immunology 2018, 9, 2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Monto AS; Petrie JG; Cross RT; Johnson E; Liu M; Zhong W; Levine M; Katz JM; Ohmit SE, Antibody to Influenza Virus Neuraminidase: An Independent Correlate of Protection. The Journal of infectious diseases 2015, 212 (8), 1191–9. [DOI] [PubMed] [Google Scholar]
- 18.(a) Chen L; Grabowski KA; Xin JP; Coleman J; Huang Z; Espiritu B; Alkan S; Xie HB; Zhu Y; White FA; Clancy J Jr.; Huang H, IL-4 induces differentiation and expansion of Th2 cytokine-producing eosinophils. Journal of immunology 2004, 172 (4), 2059–66; [DOI] [PubMed] [Google Scholar]; (b) Saito T; Kitayama D; Sakamoto A; Tsuruoka N; Arima M; Hatano M; Miyazaki M; Tokuhisa T, Effective collaboration between IL-4 and IL-21 on B cell activation. Immunobiology 2008, 213 (7), 545–55. [DOI] [PubMed] [Google Scholar]
- 19.(a) Doherty PC; Topham DJ; Tripp RA; Cardin RD; Brooks JW; Stevenson PG, Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunological reviews 1997, 159, 105–17; [DOI] [PubMed] [Google Scholar]; (b) Brown DM; Lee S; Garcia-Hernandez Mde L; Swain SL, Multifunctional CD4 cells expressing gamma interferon and perforin mediate protection against lethal influenza virus infection. Journal of virology 2012, 86 (12), 6792–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Belongia EA; Simpson MD; King JP; Sundaram ME; Kelley NS; Osterholm MT; McLean HQ, Variable influenza vaccine effectiveness by subtype: a systematic review and meta-analysis of test-negative design studies. The Lancet. Infectious diseases 2016, 16 (8), 942–51; [DOI] [PubMed] [Google Scholar]; (b) CDC, CDC Seasonal Flu Vaccine Effectiveness Studies. 2020.
- 21.Zhang H; Wang L; Compans RW; Wang BZ, Universal influenza vaccines, a dream to be realized soon. Viruses 2014, 6 (5), 1974–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang TZ; Deng L; Wang BZ; Champion JA, H7 Hemagglutinin nanoparticles retain immunogenicity after >3 months of 25 degrees C storage. PLoS One 2018, 13 (8), e0202300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachmann MF; Jennings GT, Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature reviews. Immunology 2010, 10 (11), 787–96. [DOI] [PubMed] [Google Scholar]
- 24.Taguchi K; Chuang VT; Yamasaki K; Urata Y; Tanaka R; Anraku M; Seo H; Kawai K; Maruyama T; Komatsu T; Otagiri M, Cross-linked human serum albumin dimer has the potential for use as a plasma-retaining agent for the fatty acid-conjugated antidiabetic drugs. The Journal of pharmacy and pharmacology 2015, 67 (2), 255–63. [DOI] [PubMed] [Google Scholar]
- 25.Cerritelli S; Velluto D; Hubbell JA, PEG-SS-PPS: reduction-sensitive disulfide block copolymer vesicles for intracellular drug delivery. Biomacromolecules 2007, 8 (6), 1966–72. [DOI] [PubMed] [Google Scholar]
- 26.(a) Wu CY; Yeh YC; Chan JT; Yang YC; Yang JR; Liu MT; Wu HS; Hsiao PW, A VLP vaccine induces broad-spectrum cross-protective antibody immunity against H5N1 and H1N1 subtypes of influenza A virus. PloS one 2012, 7 (8), e42363; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wohlbold TJ; Nachbagauer R; Xu H; Tan GS; Hirsh A; Brokstad KA; Cox RJ; Palese P; Krammer F, Vaccination with adjuvanted recombinant neuraminidase induces broad heterologous, but not heterosubtypic, cross-protection against influenza virus infection in mice. mBio 2015, 6 (2), e02556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xiao Y; Liu F; Yang J; Zhong M; Zhang E; Li Y; Zhou D; Cao Y; Li W; Yu J; Yang Y; Yan H, Over-activation of TLR5 signaling by high-dose flagellin induces liver injury in mice. Cellular & molecular immunology 2015, 12 (6), 729–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malissen B; Tamoutounour S; Henri S, The origins and functions of dendritic cells and macrophages in the skin. Nature reviews. Immunology 2014, 14 (6), 417–28. [DOI] [PubMed] [Google Scholar]
- 29.Boopathy AV; Mandal A; Kulp DW; Menis S; Bennett NR; Watkins HC; Wang W; Martin JT; Thai NT; He Y; Schief WR; Hammond PT; Irvine DJ, Enhancing humoral immunity via sustained-release implantable microneedle patch vaccination. Proceedings of the National Academy of Sciences of the United States of America 2019, 116 (33), 16473–16478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Irvine DJ; Swartz MA; Szeto GL, Engineering synthetic vaccines using cues from natural immunity. Nature materials 2013, 12 (11), 978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


