Abstract
GB Virus Type C (GBV-C), a blood-borne flavivirus currently infects about one sixth of the world’s population. Its transmission has been reported through parenteral, sexual and vertical routes. Unusually for RNA viruses, it exhibits a high degree of conservation of the polyprotein sequence. The geographical distribution of GBV-C suggests an African origin and a long-term co-evolution in the human population but without any known pathogenicity. The aim of this study was to describe the different sub-types of this virus in Southern Cameroon. We studied the genetic epidemiology of GBV-C among rural populations where many HIV-1 and HCV genotypes have been identified. Plasma samples of 345 subjects with evidence of HCV exposure were tested for GBV-C infection. To detect GBV-C RNA, reverse transcription followed by a nested PCR of 5’UTR were performed. Direct sequencing and phylogenetic studies using PHYLIP, PAUP* and SimPlot were carried out. In total, 31 GBV-C RNA-positive samples were detected giving a prevalence of 9.0% among HCV-exposed individuals. Phylogenetic analysis of the 5’UTR showed two distinct clusters: Genotype 1 and Genotype 2. Twenty-eight isolates (8.0%) clustered with Genotype 1 and 3 (1.0%) with Genotype 2. More than one genotype of GBV-C is prevalent in Cameroon of which GBV-C Genotype 1 is more common, confirming reports in the literature. Studying the near full-length genome sequences of GBV-C isolates from primates in this region may provide clues of viral recombination, evolution and origin.
Keywords: GBV-C, flavivirus, Cameroon, phylogeny, genotype
Introduction
GB Viruses, designated GBV Type A, GBV Type B and GBV Type C have been classified in the Flaviviridae family and genus Pegivirus on the basis of their structure, genomic organization and sequences.1 This flavivirus identified by two different groups, was designated GB Virus Type C or Hepatitis G virus (HGV) with no known pathogenicity,2,3 although its infection is common and persistent in human populations. GBV-C which is closely related to Hepatitis C virus (HCV) (Figure 1),4 has been identified in humans and chimpanzees,2,3,5 and its infection correlates with HCV infection or HCV endemicity.6 Of the six GBV-C Genotypes designated Genotypes 1 through 6 that have been identified,7 three were reported from sub-Saharan Africa. In 1998, GBV-C Genotype 1 and Genotype 2 were reported in Cameroon and Congo in Central Africa,7 after the first report from Ghana.2 International travel, blood transfusion and life style may have contributed to the spread of GBV-C in Cameroon, a region of broad genetic diversity of HCV and HIV-1.
Figure 1.
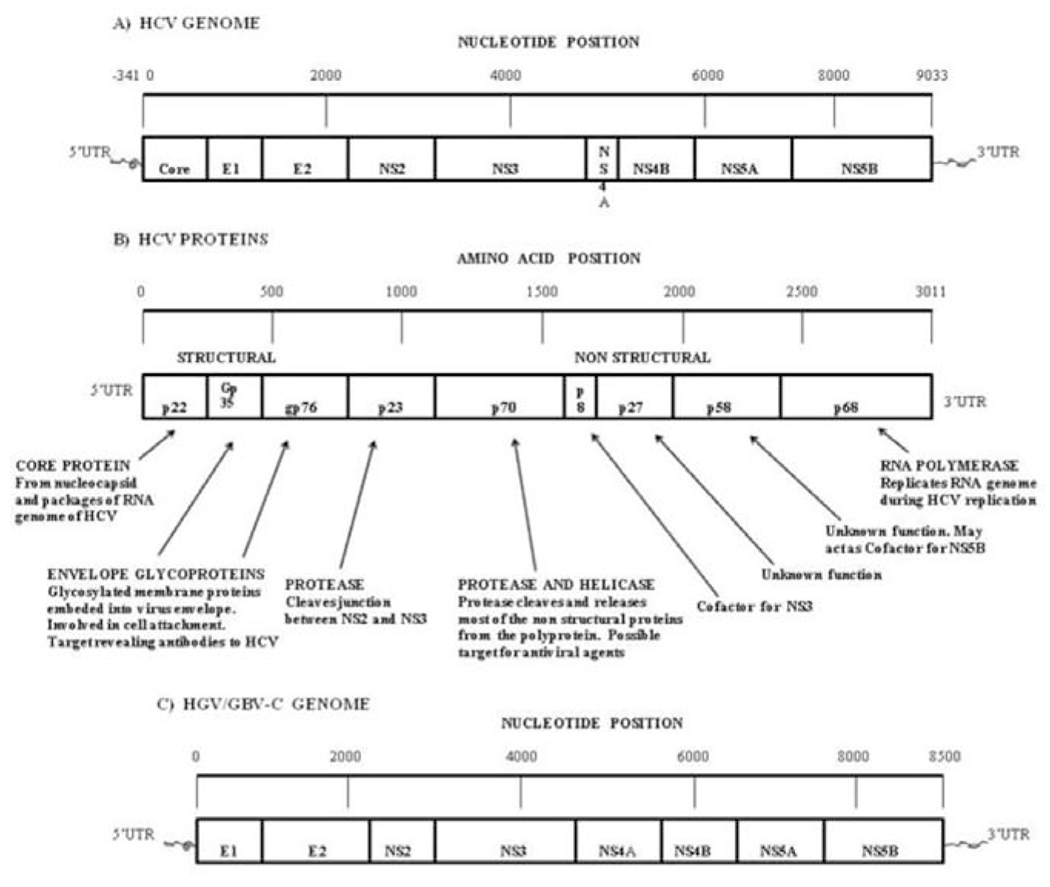
Genome Organization of HCV and HGV/GBV-C. (A) Organization of the HCV genome, showing the 5′ and 3′ untranslated regions, the single open reading frame and its cleavage sites. (B) The relative sizes of the resulting HCV proteins and what is currently understood of their functions. (C) Genome organization of HGV/GBV-C, with genes homologous to those of HCV indicated; note the lack of a protein corresponding to the capsid protein of HCV.
HIV (a lentivirus) and GBV-C (a flavivirus) are two RNA viruses that are transmissible through sexual activity, via blood, blood products, intravenous drug use, from mother-to-child through pregnancy or delivery,8,9 and also show a propensity to recombine in natural populations. During co-infection of these viruses, progression to AIDS is delayed in some individuals,10 mortality rate is reduced and longer survival rates observed once AIDS has developed.11–14 However, other studies did not report these effects probably due to the different stages of AIDS in the study populations.15–17 In addition, these viruses may have an African origin,18 but show different degrees of fitness in primates. The six genotypes of GBV-C show marked geographical differences in distribution.19 Thus far, Genotype 1, which shows greatest overall sequence diversity, is confined in sub-Saharan Africa.7,20 Human isolates of GBV-C show low sequence diversity and are more distantly related to simian isolates. GBV-C Genotype 2b may be associated with better immunological response, i.e. lower HIV-1 RNA viral load and higher CD4+ lymphocyte count.10,21
GBV-C shows no known pathogenicity in humans. The challenges in assessing the possible risks this virus may pose to safety of blood are many. Blood and blood product recipients are vulnerable to emerging and re-emerging infections, such as GBV-C. With several infections, and possibly in the presence of other pathogens and other selective pressures, an isolated epidemic may occur and go unnoticed. A virus closely related to GBV-C co-circulates among chimpanzees and provides evidence of the origin of GBV-C in Africa that pre-dates human migration out of Africa around 100,000 years ago.18,22,23 If recombination plays a role in the genetic diversity pattern of GBV-C, the emerging recombinant strains may show different degrees of fitness, transmissibility and disease induction. In this study, we aimed to identify the genetic diversity of GBV-C among isolated human populations with HCV exposure living in the Cameroon rainforest.
Materials and Methods
Ethical approval for this study was given by the Cameroon Ministry of Public Health and the Johns Hopkins School of Public Health, USA. On obtaining informed consent, blood specimens were collected from inhabitants of 11 rural communities in southern Cameroon (Figure 2).
Figure 2.
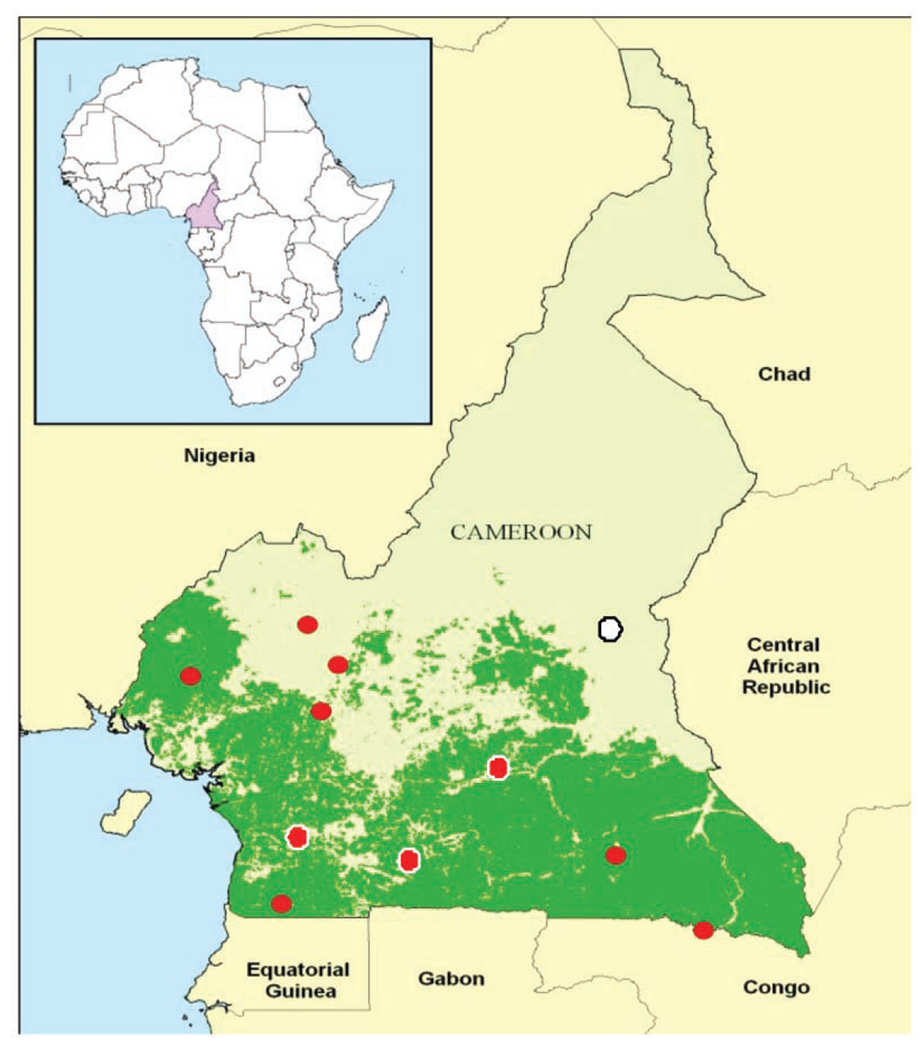
Map of Cameroon showing study sites (red and white dots).
Screening for anti-HCV antibodies in plasma of 345 subjects was performed by ELISA. RNA was extracted from each of the 345 plasma specimens with evidence of HCV exposure for GBV-C typing. Amplification of 5’UTR was performed by nested RT-PCR and direct sequencing of the amplified products to confirm the specificity of amplification as described by Stuart et al.24 The query sequences of the 5’UTR were compared to a GBV-C/HGV reference sequences from Japan, USA, China and Ghana. Phylogenetic analysis was performed by PHYLIP, PAUP* and SIMPLOT.
Results
Thirty-one (9.0%) of the 345 samples were positive for GBV-C RNA. The phylogenetic tree of 5’UTR indicated 2 clusters: Genotype 1 and Genotype 2. Twenty-eight 5’UTR sequences (8.0%) clustered with reference sequences from Ghana and USA as Genotype 1. Within the Genotype 1 cluster, 4 distinct clades were identified (Figure 3). Three of the 31 (1.0%) GBV-C sequences were detected from the same study site (Figure 2, white dot) and they clustered with Genotype 2 sequences from Japan, China, and USA. These 3 isolates formed a distinct clade in the Genotype 2 Cluster.
Figure 3.
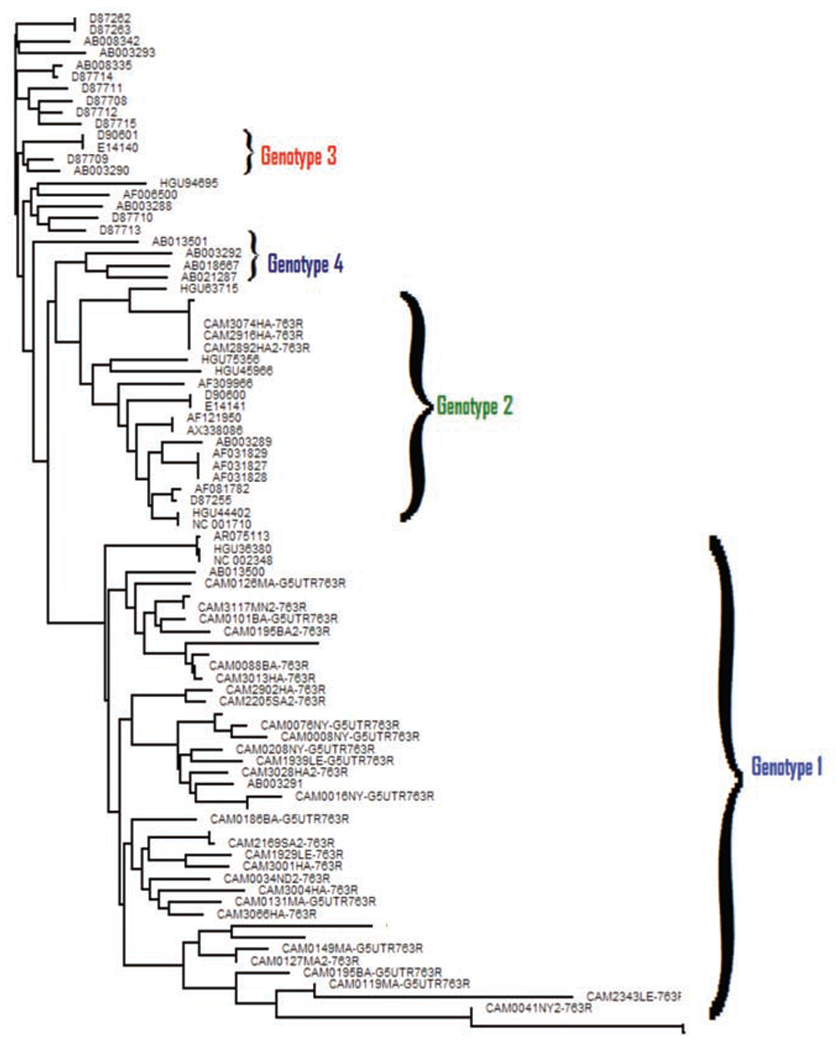
Phylogenetic tree of GBV-C 5’UTR sequences.
Discussion and Conclusions
Three hundred and forty-five HIV-negative individuals exposed to HCV living in rural locations in the rainforest of Cameroon were tested for GBV-C RNA. Our interest in carrying out this study was based on reports from several countries on the epidemiology, genomic heterogeneity and quasi-species composition of this RNA virus in HCV endemic areas. Furthermore, information on co-infection of HIV-1 and GBV-C raises questions on virus influence on HIV disease progression in particular, and correlation with reduction of HIV-1 viral burden while maintaining high CD4+ lymphocyte counts and better immunological response.
In Cameroon, HIV-1 and HCV show a high genetic diversity. Of the six GBV-C Genotypes identified, Genotypes 1 and 2 have been reported in Cameroon. Of the 345 HCV positive individuals studied, 3 (1.0%) were infected with GBV-C Genotype 2 and 28 (9.0%) with Genotype 1. In the absence of any commercially available serological kit for GBV-C, RNA detection is used, a technique that is not widely available because of cost and low access to molecular biology facilities in resource-limited settings such as Cameroon. Although GBV-C infection has not been associated to any liver disease, it is blood-borne and shares routes of transmission.25 Furthermore, its epidemiology should be studied among different target human populations such as blood-donors, HIV-infected individuals, HCV-infected individuals and pregnant women. GBV-C may have an African origin but its prognosis has not been well studied.
GBV-C and HCV are endemic in Cameroon. There have been reports of GBV-C Genotype 1 from Ghana in West Africa,2 of GBV-C Genotype 1 and Genotype 2 from Cameroon and Congo in Central Africa,7 and GBV-C Genotype 1, Genotype 2 and Genotype 5 from South Africa in Southern Africa.19 We identified GBV-C Genotype 2 and Genotype 1 from our study with remarkable predominance of Genotype 1 showing four distinct clades. Ten years after the identification of GBV-C in Ghana, many isolates have been reported from around the world. Our results and those of others, provide evidence of the emergence of new GBV-C in human populations, which may show different biological properties. There is, therefore, a need to set-up prevention measures to reduce the spread of GBV-C. Our results may also provide clues about the development of serological assays for GBV-C E2 protein. If these assays are reliable, then screening of the populations to minimize its spread becomes an important initiative in virus surveillance in the geographical region where there is a broad diversity of other RNA viruses, such as HIV-1 and HCV. It is important to study GBV-C infection in a natural setting of people with monoinfection or co-infection with HIV or HCV in disease progression.
Funding:
this work has been supported by a Fellowship/Grant from the Fogarty International Center/US NIH: Grant # 2 D 43 TW000010-16-AITRP.
Footnotes
Conflict of interests: the authors declare no potential conflict of interests.
References
- 1.Stapleton JT, Foung S, Muerhoff AS, et al. The GB viruses: a review and proposed reclassification as pegiviruses. J Gen Virol 2011;92:233–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons JN, Leary TP, Dawson GJ. Isolation of novel virus-like sequences associated with human hepatitis. Nat Med 1995;1: 564–9. [DOI] [PubMed] [Google Scholar]
- 3.Leary TP, Muerhoff AS, Simons JN, et al. Sequence and genomic organization of GBV-C: a novel member of the flaviviridae associated with human non-a–e hepatitis. J Med Virol 1996;48:60–7. [DOI] [PubMed] [Google Scholar]
- 4.Simmonds P The origin and evolution of hepatitis viruses in humans. J Gen Virol 2001;82:693–712. [DOI] [PubMed] [Google Scholar]
- 5.Birkenmeyer LG, Desai SM, Muerhoff AS, et al. Isolation of a GB Virus-related Genome from a Chimpanzee. J Med Virol 1998;56:44–51. [DOI] [PubMed] [Google Scholar]
- 6.Chang CJ, Chiang JC, Lu SN, Wang JH. Hepatitis delta virus and GBV-C infection in two neighboring hepatitis B virus and hepatitis C virus-endemic villages in Taiwan. Chang Gung Med J 2010;33:137–44. [PubMed] [Google Scholar]
- 7.Muerhoff AS, Smith DB, Leary TP, et al. Identification of GB virus C variants by phylogenetic analysis of 5’-untranslated and coding region sequences. J Virol 1997;71:6501–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sathar MA, York DF, Gouws E, et al. GB virus type C coinfection in HIV-infected African mothers and their infants, KwaZulu Natal, South Africa. Clin Infect Dis 2004;38:405–9. [DOI] [PubMed] [Google Scholar]
- 9.Supapol WB, Remis RS, Raboud J, et al. Reduced mother-to-child transmission of HIV associated with infant but not maternal GB virus C infection. J Infect Dis 2008; 197:1369–77. [DOI] [PubMed] [Google Scholar]
- 10.Alcalde R, Nishiya A, Casseb J, et al. Prevalence and distribution of the GBV-C/HGV among HIV-1-infected patients under anti-retroviral therapy Virus Res 2010;151:148–52. [DOI] [PubMed] [Google Scholar]
- 11.Tillmann HL, Heiken H, Knapik-Botor A, et al. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med 2001;345:715–24. [DOI] [PubMed] [Google Scholar]
- 12.Williams CF, Klinzman D, Yamashita TE, et al. Persistent GB virus C infection and survival in HIV-infected men. N Engl J Med 2004;350:981–90. [DOI] [PubMed] [Google Scholar]
- 13.Xiang J, Wunschmann S, Diekema DJ, et al. Effect of coinfection with GB virus C on survival among patients with HIV infection. N Engl J Med 2001;345:707–14. [DOI] [PubMed] [Google Scholar]
- 14.Heringlake S, Ockenga J, Tillmann HL, et al. GB virus C/hepatitis G virus infection: a favorable prognostic factor in human immunodeficiency virus-infected patients? J Infect Dis 1998;177:1723–6. [DOI] [PubMed] [Google Scholar]
- 15.Birk M, Lindback S, Lidman C. No influence of GB virus C replication on the prognosis in a cohort of HIV-1-infected patients. AIDS 2002;16:2482–5. [DOI] [PubMed] [Google Scholar]
- 16.Bjorkman P, Flamholc L, Naucler A, et al. GB virus C during the natural course of HIV-1 infection: viremia at diagnosis does not predict mortality. AIDS 2004;18:877–86. [DOI] [PubMed] [Google Scholar]
- 17.Brumme ZL, Chan KJ, Dong WW, et al. No association between GB virus-C viremia and virological or immunological failure after starting initial antiretroviral therapy. AIDS 2002;16:1929–33. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka Y, Mizokami M, Orito E, et al. GB virus C/hepatitis G virus infection among Colombian native Indians. Am J Trop Med Hyg 1998;59:462–7. [DOI] [PubMed] [Google Scholar]
- 19.Muerhoff AS, Dawson GJ, Desai SM. A previously unrecognized sixth genotype of GB virus C revealed by analysis of 5 - untranslated region sequences. J Med Virol 2006; 78:105–11. [DOI] [PubMed] [Google Scholar]
- 20.Smith DB, Cuceanu N, Davidson F, et al. Discrimination of hepatitis G virus/GBV-C geographical variants by analysis of the 5’ non-coding region J Gen Virol 1997;78: 1533–42. [DOI] [PubMed] [Google Scholar]
- 21.Dmitriev PN, Tsikina MN, Moiseeva AV, et al. [GBV-C infection in HIV-infected patients in the Russian Federation]. Vopr Virusol 2010;55:23–6. [Article in Russian]. [PubMed] [Google Scholar]
- 22.Adams NJ, Prescott LE, Jarvis LM, et al. Detection in chimpanzees of a novel flavivirus related to GB virus-C/hepatitis G virus. J Gen Virol 1998;79:1871–7. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki Y, Katayama K, Fukushi S, et al. Slow evolutionary rate of GB virus C/hepatitis G virus. J Mol Evol 1999;48: 383–9. [DOI] [PubMed] [Google Scholar]
- 24.Stuart KR, Scott Bowd D. GB virus C: insights into co-infection. J Clin Virol 2005; 33:257–66. [DOI] [PubMed] [Google Scholar]
- 25.Schwarze-Zander C, Neibecker M, Othman S, et al. GB virus C coinfection in advanced HIV type-1 disease is associated with low CCR5 and CXCR4 surface expression on CD4(+) T-cells. Antivir Ther 2010;15:745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


