PURPOSE
Patients with EGFR-mutant lung cancer have no approved targeted therapies after disease progression on first-line osimertinib, a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI). Preclinical studies suggest that tumors with both EGFR-sensitizing alteration and acquired second-site EGFR resistance alterations after treatment with osimertinib retain sensitivity to second-generation EGFR TKIs. We hypothesized that dacomitinib, a pan-human epidermal growth factor receptor TKI, may be effective in this setting.
METHODS
In this phase II study, patients who had progressed on first-line osimertinib were treated with dacomitinib 45 mg orally daily until disease progression or intolerability. The primary end point was objective response rate.
RESULTS
We enrolled 12 patients. Two partial responses were documented (17% objective response rate; 95% CI, 5 to 45). The median progression-free survival was 1.8 months (95% CI, 1.6 to not reached). One patient with an original sensitizing EGFR G719A mutation and one patient without molecular testing available had partial responses, whereas 0 of the 3 patients with second-site acquired EGFR resistance mutations (two C797S and one G724S) met the response criteria. The patient with EGFR G719A has an ongoing response at 17 months, which exceeds prior time on osimertinib (11 months).
CONCLUSION
In the first trial evaluating a second-generation EGFR TKI after first-line third-generation osimertinib, we found that dacomitinib after disease progression on osimertinib has limited benefit.
INTRODUCTION
Twenty percent of lung adenocarcinomas harbor sensitizing alterations in epidermal growth factor receptor (EGFR).1 When used as initial therapy, the third-generation EGFR tyrosine kinase inhibitor (TKI) osimertinib produces an 80% response rate and median progression-free survival (PFS) of 19 months2 in patients with these lung cancers,1 but the development of resistance is ultimately universal. There are no approved EGFR TKIs after osimertinib although on-target resistance typically with acquisition of a second-site EGFR mutation is a recognized phenomenon.3,4 EGFR C797S is a second-site mutation that confers resistance to osimertinib5-7 and is estimated to occur in 7% of patients treated with first-line osimertinib.3,8 Additional second-site EGFR mutations including G718X and G724X also render lung cancers resistant to osimertinib but may retain sensitivity to earlier-generation EGFR TKIs.9,10
CONTEXT
Key Objective
Patients with advanced epidermal growth factor receptor (EFGR)-mutant lung cancer have no approved targeted therapies after first-line osimertinib, a third-generation EGFR tyrosine kinase inhibitor (TKI). This phase II study investigated whether dacomitinib, an irreversible, second-generation pan-human EGFR TKI, may be effective in patients after disease progression on initial osimertinib.
Knowledge Generated
Among 12 treated patients, the objective response rate was 17% (95% CI, 5 to 45). The median progression-free survival was 1.8 months (95% CI, 1.6 to not reached). One patient with a baseline EGFR G719A alteration had an ongoing partial response lasting over 17 months. Three patients had second-site acquired EGFR alterations, and none of these patients had a response.
Relevance
To our knowledge, this is the first prospective trial investigating a second-generation EGFR TKI after initial osimertinib. Dacomitinib after disease progression on initial osimertinib is not effective as a general treatment strategy.
Dacomitinib is a second-generation, irreversible pan-human epidermal growth factor receptor TKI. First-line treatment with dacomitinib resulted in prolonged PFS11 and overall survival12 compared with treatment with gefitinib. In vitro studies demonstrate that EGFR-mutant cell lines that harbor an EGFR-sensitizing alteration such as L858R or exon 19 deletion (del 19) plus a second-site EGFR alteration such as C797S in the absence of T790M are resistant to osimertinib but retain sensitivity to quinazoline-based EGFR inhibitors such as dacomitinib.13 This finding supports the hypothesis that dacomitinib may be effective after disease progression on osimertinib in patients with acquired second-site EGFR mutations or human epidermal growth factor receptor family–mediated resistance, but this has not been investigated in a prospective study.
To our knowledge, we conducted the first phase II study of dacomitinib in patients with metastatic EGFR-mutant lung cancer with disease progression after initial osimertinib.
METHODS
This trial was a prospective, single-center phase II study in patients with EGFR-mutant lung cancers with disease progression on initial osimertinib. This study is exploratory in nature. The primary end point of the trial was to obtain preliminary estimates of the objective response rate (ORR), defined as partial and complete responses, which could be used to design future phase II studies with a primary efficacy end point. Sample size was selected as one reasonably large enough to estimate the ORR. Secondary objectives included PFS, overall survival, and safety and tolerability of dacomitinib after osimertinib. Correlative analyses included identification of pretreatment somatic alterations associated with response to dacomitinib and identification of mechanisms of resistance to dacomitinib. The trial was conducted after approval of the institutional review board at Memorial Sloan Kettering Cancer Center. The study was registered at ClinicalTrials.gov identifier: NCT03755102.
Patients
Patients had stage IV or recurrent lung cancers with a somatic activating mutation in EGFR. Patients demonstrated radiologic progression during treatment with first-line osimertinib and were required to have had a repeat biopsy after osimertinib progression. No previous treatment with a first-generation or second-generation EGFR TKI or chemotherapy was permitted. Stable brain metastases were allowed. All patients had to have adequate organ function, Karnofsky performance status of 70% of higher, and measurable disease per RECIST 1.1.
Study Design
This was a single-institution, single-arm, open-label phase II study. All patients were treated with dacomitinib 45 mg orally daily until disease progression or intolerability. This study was initially planned to enroll 24 patients, 12 with EGFR second-site mutations such as C797S and 12 without a second-site mutation.
Study Assessment
Treatment cycles were 4 weeks in duration. Patients were assessed every 2 weeks for the first cycle and every 4 weeks subsequently. Patients had history, physical examination, complete blood count, and serum chemistry studies performed at every visit. Toxicity was graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0. Response to therapy was assessed by interval imaging every 8 weeks with response evaluated per RECIST 1.1.
Statistical Analysis
Safety and tolerability were summarized using descriptive statistics. Response rates were calculated using binomial proportions and exact 95% CIs. PFS was estimated using the Kaplan-Meier method and defined as the time from start of dacomitinib treatment until progression or death. Patients who did not experience either event were censored at the date on which they left the study or date of last assessment if they were still receiving dacomitinib.
Next-Generation Sequencing
Patients with available pre- and post-treatment tumor specimens underwent next-generation sequencing with MSK-IMPACT14 or MSK-ACCESS on cell-free DNA collected from peripheral blood. Somatic variants were called as described previously.14
RESULTS
Patients
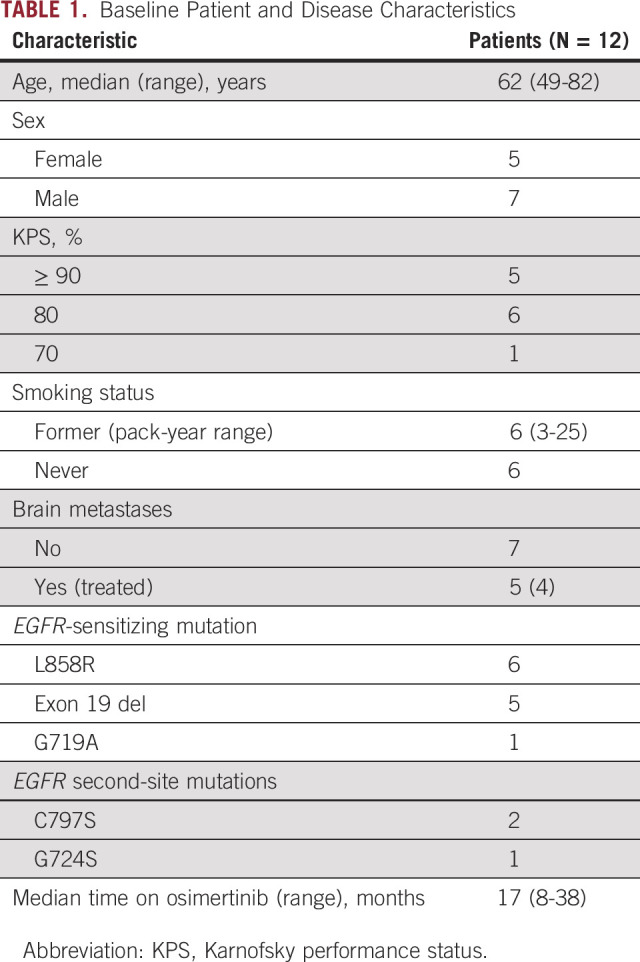
From November 2018 to August 2020, 12 patients were enrolled (Table 1). Ten patients discontinued treatment for disease progression, one withdrew because of toxicity, and one patient continues on study. Given slow accrual and low response rate observed among the first 12 patients enrolled, the Protocol was terminated early.
TABLE 1.
Baseline Patient and Disease Characteristics
Efficacy
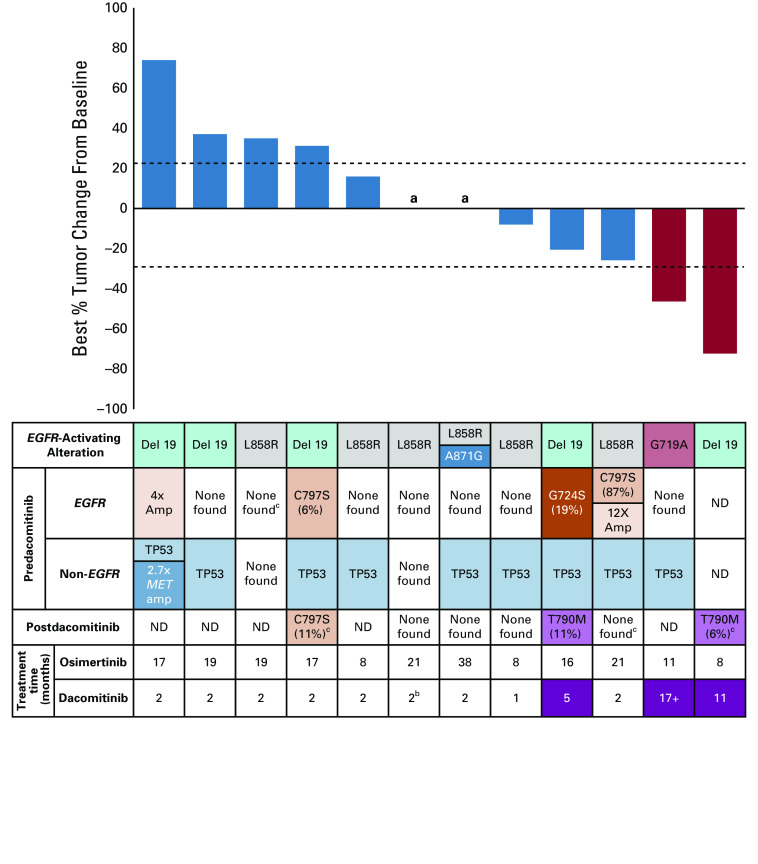
All 12 patients had baseline and on-treatment radiologic assessments. Two patients had confirmed partial responses for an ORR of 17% (95% CI, 5 to 45) (Fig 1). Zero of three patients with acquired second-site EGFR alterations met the response criteria. The median PFS was 1.8 months (95% CI, 1.6 to not reached) with a range of 0.9-17 months. The median survival from time of treatment initiation was not reached, with a median follow-up 13 months.
FIG 1.
Dacomitinib clinical efficacy. Best responses of target lesions (RECIST 1.1). Although samples may have had other alterations found on sequencing, only alterations with known prognostic or therapeutic implications are shown in the non-EGFR and postdacomitinib rows. None found indicates sample underwent sequencing, but no alterations with prognostic or therapeutic implications were found. aIndicates no change in tumor measurements per RECIST criteria, bindicates that the patient withdrew from the study because of toxicity. ND indicates sequencing not done because no sample was available. cIndicates plasma cfDNA sequencing with MSK-ACCESS. All sequencing not marked with “c” was performed on tissue. cfDNA, cell-free DNA.
Tolerability
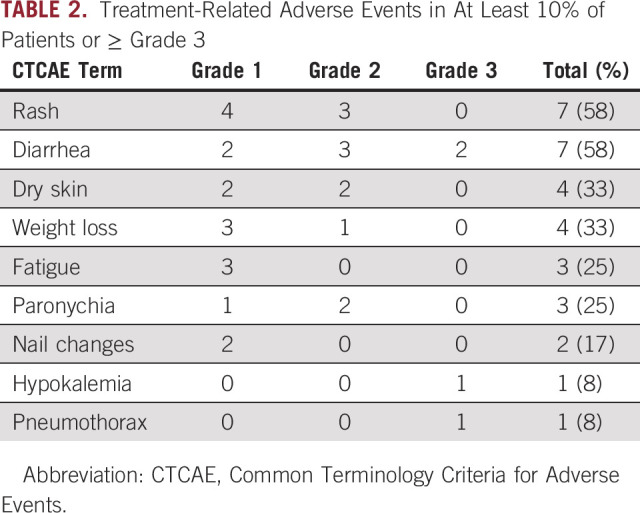
All 12 patients were evaluable for toxicity. The most frequent (≥ 10%) treatment-related adverse events were diarrhea, rash, dry skin, weight loss, fatigue, paronychia, and nail changes (Table 2). There were four grade 3 treatment-related adverse events (two diarrhea, hypokalemia, and pneumothorax), but no grade 4 events and no deaths. One patient underwent dose reduction from 45 to 30 mg for grade 2 maculopapular rash. One patient held study drug for grade 3 diarrhea and later withdrew for grade 2 weight loss and grade 2 rash.
TABLE 2.
Treatment-Related Adverse Events in At Least 10% of Patients or ≥ Grade 3
Pre- and Post-Treatment Genomic Analysis
All patients had a pretreatment biopsy performed within 8 weeks of starting dacomitinib. Ten biopsies were sufficient for targeted exome sequencing with MSK-IMPACT; one patient with an insufficient biopsy sample underwent predacomitinib plasma cfDNA sequencing with MSK-ACCESS. Of the two responders, one harbored an EGFR G719A–sensitizing alteration and the other patient did not have a sufficient pretreatment tissue or plasma sample available but had a known EGFR exon 19 deletion from previous testing. Three patients had acquired second-site EGFR alterations after initial osimertinib: two with C797S (one with concurrent 12-fold EGFR amplification) and one with G724S. Because C797S and G724S are on different exons from the EGFR-sensitizing alterations, we could not determine whether they were cis or trans to the sensitizing alterations. In total, 9 of the 11 (82%) patients had pretreatment TP53 alterations and one patient had 2.7-fold MET amplification (Fig 1). Seven patients had postdacomitinib tissue or plasma cfDNA sequencing. Two of these patients (29%), including one responder, acquired EGFR T790M during treatment with dacomitinib.
Among the three patients with predacomitinib EGFR second-site alterations, two patients had tumor shrinkage not meeting the response criteria. Both cancers with minor tumor shrinkage were notable for higher variant allele frequencies (VAFs) of G724S/C797S before dacomitinib (19% and 87%), which were not detected on post-treatment sampling. The third patient with a low predacomitinib C797S VAF of 6% had primary disease progression on dacomitinib; C797S remained detectable at 11% in plasma after progression on dacomitinib. Of note, pre- and post-treatment VAFs for this patient may be difficult to compare directly because post-treatment sequencing was performed on plasma.
DISCUSSION
Given the lack of approved targeted therapies after disease progression on osimertinib, this trial aimed to ascertain whether dacomitinib, a second-generation EGFR TKI, could be a fruitful strategy for patients with EGFR-mutant lung cancer after disease progression on initial osimertinib. In practice, it is common to attempt sequencing of EGFR inhibitors after first-line osimertinib despite no supporting prospective data. There were two PRs observed among treated patients with an ORR of 17% and median PFS of 1.8 months. The toxicities seen with dacomitinib are consistent with expected toxicities of EGFR inhibition, with diarrhea and rash as the most common treatment-related adverse events.
Molecular characterization of treated patients demonstrated several genomic alterations among treated patients, which may have affected treatment outcomes. Eighty-two percent of pretreatment samples harbored TP53 mutations, which are associated with shortened response to EGFR TKIs and worse prognosis in lung cancer with EGFR alterations.15 MET amplification was also discovered retroactively in one patient’s pretreatment biopsy, likely explaining the patient’s primary disease progression on dacomitinib.
A patient with EGFR G719A, an atypical EGFR exon 18 mutation, achieved a confirmed partial response and has an ongoing response at 17 months, exceeding the 11 months she had been on osimertinib. Previous studies have shown that the second-generation EGFR TKI afatinib can effectively treat patients with atypical EGFR alterations, including G719A.16,17 Clinical evaluation of dacomitinib in rare atypical EGFR alterations is limited. In a phase II trial of dacomitinib after disease progression on erlotinib and chemotherapy, one patient with EGFR G719C had an objective response.18 Further investigation is needed to determine whether upfront treatment with a second-generation TKI such as dacomitinib or afatinib may be superior to osimertinib for patients with atypical EGFR alterations.
EGFR second-site mutations are rare after first-line osimertinib treatment.3 We aimed to compare responses among patients with EGFR second-site alterations with those without, but only two patients with acquired EGFR C797S and one with EGFR G724S were enrolled. None of the patients with EGFR second-site mutations met the response criteria. It is possible that dacomitinib may have differential activity toward the various EGFR second-site alterations because afatinib had enhanced inhibitory activity against C797S compared with G724S in in vitro studies.9 On the basis of the limited number of patients with second-site alterations enrolled, we were not able to investigate whether specific second-site alterations may be more sensitive to dacomitinib and we did not enroll patients with other second-site acquired alterations, such as EGFR L718Q or L844V.
Pre- and postdacomitinib analysis of EGFR second-site alteration VAFs suggests that higher pretreatment VAFs may be associated with greater tumor shrinkage, with low VAFs of the second-site alteration suggesting the second-site alteration is subclonal. In this setting, therapy targeting the second-site alteration is less likely to be effective. A previous study similarly showed that patients with higher pretreatment VAFs of EGFR T790M had great tumor shrinkage when treated with rociletinib, a third-generation EGFR TKI.19
In summary, dacomitinib had limited benefit after disease progression after initial treatment on osimertinib. Accrual to this study was overall lower than expected, likely because of the low frequency of EGFR second-site alterations after first-line osimertinib, estimated to be 7% compared with 30% in patients treated with early-generation EGFR TKIs.3 Given the low ORR observed among the first 12 patients in this study, the study was terminated early. Future studies investigating treatment strategies in patients with acquired second-site EGFR alterations will likely require multi-institutional enrollment. As there is tumor heterogeneity in the lung cancers with EGFR second-site alterations, we hypothesize that combination treatment with dacomitinib and osimertinib may be a more promising strategy. A follow-up trial of dacomitinib plus osimertinib solely in patients with acquired EGFR second-site mutations after osimertinib is ongoing.
SUPPORT
Supported by NIH grant P01CA129243 (PI: M.G.K.), the STARR foundation, and the National Institute of Health P30 CA00874.
AUTHOR CONTRIBUTIONS
Conception and design: Mark G. Kris, Gregory J. Riely, Helena A. Yu
Provision of study materials or patients: Robert M. Daly, Isabel Preeshagul, Afsheen N. Iqbal, Mark G. Kris, Gregory J. Riely, Helena A. Yu
Collection and assembly of data: Noura J. Choudhury, Alex Makhnin, Yosef Y. Tobi, Afsheen N. Iqbal, Linda S. Ahn, Helena A. Yu
Data analysis and interpretation: Noura J. Choudhury, Robert M. Daly, Isabel R. Preeshagul, Sara A. Hayes, Glenn Heller, Mark G. Kris, Gregory J. Riely, Helena A. Yu
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert M. Daly
Leadership: Quadrant Holdings
Stock and Other Ownership Interests: Quadrant Holdings, CVS Health, Walgreens Boots Alliance, Lilly, IBM, Pfizer, Cigna, Baxter, Zoetis
Other Relationship: AstraZeneca
Open Payments Link: https://openpaymentsdata.cms.gov/physician/2785286
Isabel R. Preeshagul
Consulting or Advisory Role: Pfizer, AstraZeneca, Blueprint Medicines, Lilly, Curio Science
Glenn Heller
Research Funding: Janssen Diagnostics
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Pfizer, Daiichi Sankyo, Sanofi/Aventis, Novartis, Genentech/Roche
Travel, Accommodations, Expenses: AstraZeneca, Pfizer, Genentech, Daiichi Sankyo
Other Relationship: Memorial Sloan-Kettering Cancer Center, Genentech/Roche
Open Payments Link: https://openpaymentsdata.cms.gov/physician/markgkris/summary
Gregory J. Riely
Research Funding: Novartis, Roche/Genentech, GlaxoSmithKline, Pfizer, Infinity Pharmaceuticals, Mirati Therapeutics, Merck, Takeda
Patents, Royalties, Other Intellectual Property: Patent application submitted covering pulsatile use of erlotinib to treat or prevent brain metastases
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Other Relationship: Pfizer, Roche/Genentech, Takeda
Helena A. Yu
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen
Research Funding: AstraZeneca, Astellas Pharma, Lilly, Novartis, Pfizer, Daiichi Sankyo, Cullinan Oncology
Travel, Accommodations, Expenses: Lilly
Other Relationship: Astellas Pharma
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cancer Genome Atlas Research Network : Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543-550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soria JC, Ohe Y, Vansteenkiste J, et al. : Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med 378:113-125, 2018 [DOI] [PubMed] [Google Scholar]
- 3.Schoenfeld AJ, Chan JM, Kubota D, et al. : Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin Cancer Res 26:2654-2663, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schoenfeld AJ, Yu HA: The evolving landscape of resistance to osimertinib. J Thorac Oncol 15:18-21, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Thress KS, Paweletz CP, Felip E, et al. : Acquired EGFR C797S mutation mediates resistance to AZD9291 in non-small cell lung cancer harboring EGFR T790M. Nat Med 21:560-562, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chabon JJ, Simmons AD, Lovejoy AF, et al. : Circulating tumour DNA profiling reveals heterogeneity of EGFR inhibitor resistance mechanisms in lung cancer patients. Nat Commun 7:11815, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu HA, Tian SK, Drilon AE, et al. : Acquired resistance of EGFR-mutant lung cancer to a T790M-specific EGFR inhibitor: Emergence of a third mutation (C797S) in the EGFR tyrosine kinase domain. JAMA Oncol 1:982-984, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ramalingam SS, Cheng Y, Zhou C, et al. : Mechanisms of acquired resistance to first-line osimertinib: Preliminary data from the phase III FLAURA study. Ann Oncol 29:viii740, 2018 [Google Scholar]
- 9.Brown BP, Zhang YK, Westover D, et al. : On-target resistance to the mutant-selective EGFR inhibitor osimertinib can develop in an allele-specific manner dependent on the original EGFR-activating mutation. Clin Cancer Res 25:3341-3351, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y, Li Y, Ou Q, et al. : Acquired EGFR L718V mutation mediates resistance to osimertinib in non-small cell lung cancer but retains sensitivity to afatinib. Lung Cancer 118:1-5, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Cheng Y, Zhou X, et al. : Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol 18:1454-1466, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Cheng Y, Zhou X, et al. : Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non–small-cell lung cancer and EGFR-activating mutations. J Clin Oncol 36:2244-2250, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Ercan D, Choi HG, Yun CH, et al. : EGFR mutations and resistance to irreversible pyrimidine-based EGFR inhibitors. Clin Cancer Res 21:3913-3923, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A, et al. : Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagn 17:251-264, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Canale M, Petracci E, Delmonte A, et al. : Impact of TP53 mutations on outcome in EGFR-mutated patients treated with first-line tyrosine kinase inhibitors. Clin Cancer Res 23:2195-2202, 2017 [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Sequist LV, Geater SL, et al. : Clinical activity of afatinib in patients with advanced non-small-cell lung cancer harbouring uncommon EGFR mutations: A combined post-hoc analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol 16:830-838, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Yang JC-H, Schuler M, Popat S, et al. : Afatinib for the treatment of NSCLC harboring uncommon EGFR mutations: A database of 693 cases. J Thorac Oncol 15:803-815, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Reckamp KL, Giaccone G, Camidge DR, et al. : A phase 2 trial of dacomitinib (PF-00299804), an oral, irreversible pan-HER (human epidermal growth factor receptor) inhibitor, in patients with advanced non-small cell lung cancer after failure of prior chemotherapy and erlotinib. Cancer 120:1145-1154, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piotrowska Z, Niederst MJ, Karlovich CA, et al. : Heterogeneity underlies the emergence of EGFRT790 wild-type clones following treatment of T790M-positive cancers with a third-generation EGFR inhibitor. Cancer Discov 5:713-722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]