Abstract
BACKGROUND:
Blood pressure often rises with aging, but exact mechanisms are still not completely understood. With aging, the level of proinflammatory cytokines increases in T lymphocytes. Prostaglandin D2, a proresolution mediator, suppresses Type 1 T helper (Th1) cytokines through D-prostanoid receptor 1 (DP1). In this study, we aimed to investigate the role of the prostaglandin D2/DP1 axis in T cells on age-related hypertension.
METHODS:
To clarify the physiological and pathophysiological roles of DP1 in T cells with aging, peripheral blood samples were collected from young and older male participants, and CD4+ T cells were sorted for gene expression, prostaglandin production, and Western blot assays. Mice blood pressure was quantified by invasive telemetric monitor.
RESULTS:
The prostaglandin D2/DP1 axis was downregulated in CD4+ T cells from older humans and aged mice. DP1 deletion in CD4+ T cells augmented age-related hypertension in aged male mice by enhancing Th1 cytokine secretion, vascular remodeling, CD4+ T cells infiltration, and superoxide production in vasculature and kidneys. Conversely, forced expression of exogenous DP1 in T cells retarded age-associated hypertension in mice by reducing Th1 cytokine secretion. Tumor necrosis factor α neutralization or interferon γ deletion ameliorated the age-related hypertension in DP1 deletion in CD4+ T cells mice. Mechanistically, DP1 inhibited Th1 activity via the PKA (protein kinase A)/p-Sp1 (phosphorylated specificity protein 1)/neural precursor cell expressed developmentally downregulated 4-like (NEDD4L) pathway–mediated T-box-expressed-in-T-cells (T-bet) ubiquitination. T-bet deletion or forced NEDD4L expression in CD4+ T cells attenuated age-related hypertension in CD4+ T cell–specific DP1-deficient mice. DP1 receptor activation by BW245C prevented age-associated blood pressure elevation and reduced vascular/renal superoxide production in male mice.
CONCLUSIONS:
The prostaglandin D2/DP1 axis suppresses age-related Th1 activation and subsequent hypertensive response in male mice through increase of NEDD4L–mediated T-bet degradation by ubiquitination. Therefore, the T cell DP1 receptor may be an attractive therapeutic target for age-related hypertension.
Keywords: aging, D-prostanoid receptor 1, hypertension, lymphocyte, prostaglandin (PG) D2
Aging is associated with time-dependent decline in physiological functions that are necessary for survival and fertility. It is the main risk factor for cardiovascular diseases, including hypertension; more than 70% of older adults (>60 years) have hypertension, compared with only 30% for adults 40 to 59 years of age.1 Despite medical treatment for patients with hypertension, such as the use of diuretics and renin-angiotensin system blockers that reduce cardiovascular morbidity and mortality in older adults,2 treatment of age-related hypertension remains challenging because of differences in drug metabolism, use of multiple concomitant medications, and enhanced blood pressure (BP) variability in older adults.3,4 Therefore, a specific curative treatment for age-related hypertension is urgently required.
Aging is accompanied by chronic low-grade inflammation, a phenomenon also termed as “inflammaging.”5 The levels of circulating inflammation biomarkers, such as interleukin-6, tumor necrosis factor alpha (TNFα), interferon γ (IFNγ), interleukin-1α, and C-reactive protein, are elevated in healthy elderly individuals.6 The increase in the levels of proinflammatory cytokines is associated with morbidity and mortality of age-related diseases such as diabetes mellitus, hypertension, vascular disease, and cancer.1 The immune response is influenced by the aging process to some extent, and T cells are the most altered among the affected immune cells.7 T cells are also involved in sex differences in development of hypertension.8,9 Accumulated evidence show that the T cells of the elderly secrete large amounts of IFNγ10,11 and TNFα12,13 after activation, suggesting that aging is associated with chronic Th1 type inflammation.14 Indeed, genetic ablation or pharmacological inhibition of either IFNγ or TNFα attenuates angiotensin II (AngII)–induced BP elevation in male mice.15–17 However, the role of T cell–derived cytokines in age-related hypertension remains unknown.
Prostaglandin D2 (PGD2), a proresolution mediator,18,19 is generated from sequential enzymatic reaction of cyclooxygenases and PGD2 synthases. It exerts its biological function via two G protein–coupled receptors named D-prostanoid receptor (DP) 1 and 2 (also called chemoattractant receptor-homologous molecule expressed on T-helper type 2 cells, CRTH2). Both DP1 and DP2 are expressed in T cells, and DP2 mediates the directed movement or chemotaxis of Th2 cell.20 In mice, DP1 deletion exaggerates formation of aneurysm and hypertensive response to AngII and promotes atherogenesis, indicating a protective role of DP1 in vascular diseases.21 However, information regarding the involvement of the PGD2/DP1 axis in the development of age-dependent hypertension is limited.
Here, we observed that the PGD2/DP1 axis was downregulated in CD4+ T cells in older human adults and aged mice. Specific deletion of DP1 in CD4+ T cells (TDP1KO) exacerbated age-related hypertension in male mice by increasing Th1 cytokine release. The PGD2/DP1 axis suppressed age-related Th1 activation by promoting neural precursor cell expressed developmentally downregulated 4-like (NEDD4L)-mediated T-box-expressed-in-T-cells (T-bet) degradation. Overexpression or pharmacological activation of the DP1 receptor prevented age-dependent hypertension in male mice. These results demonstrate that DP1 may be a potential therapeutic target for age-related hypertension in males.
METHODS
Data Availability
The data that support these findings of this study are presented in the article and in the online-only Data Supplement, and additional information will be made available from the corresponding author upon reasonable request.
Mice
CD4-specific mouse DP1 transgenic (CD4-DP1-Tg) mice and CD4-specific mouse NEDD4L transgenic (CD4-NEDD4L-Tg) mice were both generated by Shanghai Model Organisms Center Inc. (Shanghai, China) using the pGEM plasmid containing 9.6 kb of the mouse CD4 promotor/enhancer/silencer.22 All animals were of the male C57/BL6 background and used in accordance with the guidelines of the Institutional Animal Care and Use Committee of the Tianjin Medical University. For transgenic animals, groups of young (2 mo) and aged (18 to 26 mo) mice of each genotype were randomized on the basis of the age group.
Collection of Human Plasma and Analysis
Male participants without serious cardiovascular diseases who underwent routine health examinations were recruited in the Outpatient Department of Ruijin Hospital in Shanghai Jiao Tong University School of Medicine. Peripheral blood samples were collected, and sera were used for ELISA. Peripheral CD4+ T cells were sorted using a negative selection kit (17952, Stem cell, Vancouver, Canada) for gene expression, prostaglandin, and Western analyses. The study was reviewed and approved by the Human Ethics Committee of Shanghai Ruijin Hospital, and all participants provided written informed consent.
BP Measurement
BP was measured using invasive radio telemetry. Pressure transmitters (PA-C10, Data Sciences International, New Brighton, MN) were embedded in mice. After 3 days of rest, telemetry recordings for systolic BP, diastolic BP, mean BP, and heart rate were recorded for 3 consecutive days. For the TNFα-neutralizing experiment, etanercept (8 mg/kg/injection) was administered intraperitoneally every 2 days until the seventh day, and BP was then recorded for the next 3 consecutive days. For BW245C treatment, BW245C (1 mg/kg) was administrated intraperitoneally twice per day until the seventh day and BP was then recorded for another 18 consecutive days. The animals were maintained in a sterile environment and were regularly screened for infections.
Statistical Analysis
All data were expressed as means ± SEM. Detailed information is indicated in each figure legend regarding sample size and data collection. Data were analyzed either by the 2-tailed unpaired Student t test or nonparametric Mann-Whitney U test to compare means between two experimental groups, and by 1- or 2-way ANOVA measurements followed by Bonferroni post hoc test comparing means among multiple groups where appropriate. Pearson’s correlation coefficient was used for correlation analysis. Data were analyzed using GraphPad Prism 5 (GraphPad Software, Inc, San Diego, CA). P<0.05 was considered statistically significant.
RESULTS
PGD2/DP1 Axis Is Downregulated in T Cells in Aged Mice and Older Human Subjects
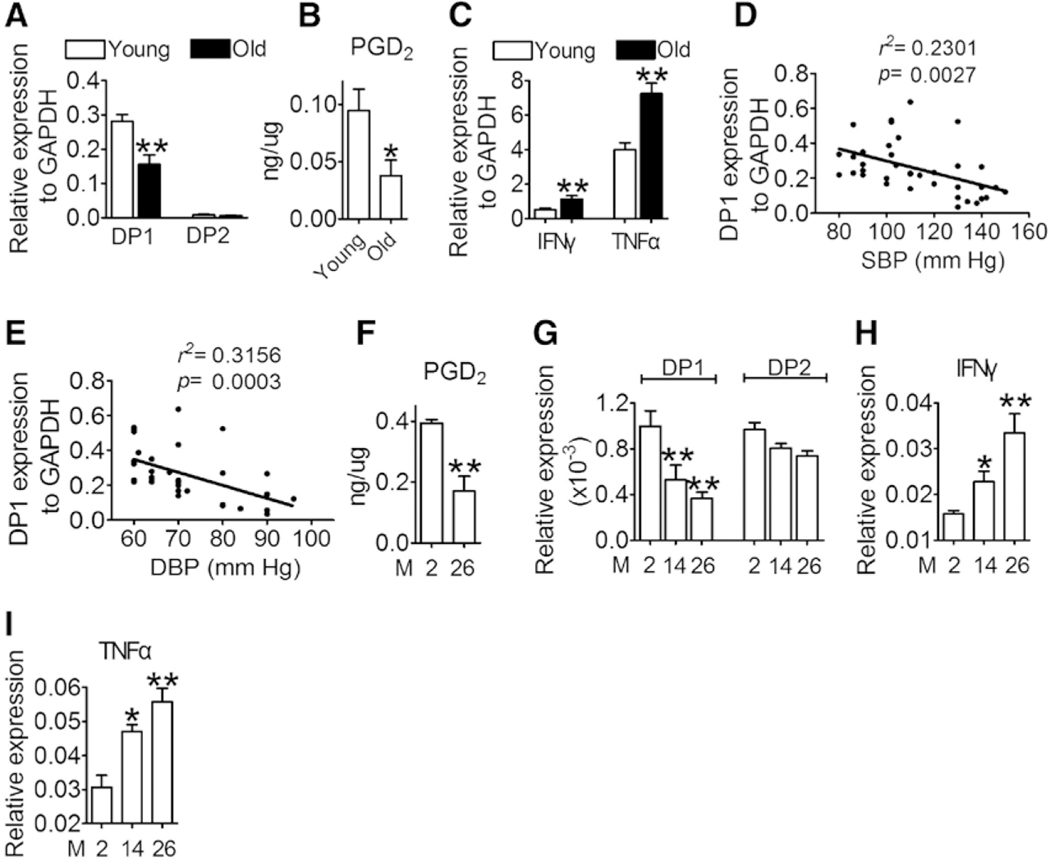
Aging is associated with persistent low-grade chronic inflammation and hypertension,5 and T cell dsyregulation is critical for the development of hypertension,23 especially for sex differences in hypertension.8,9 To investigate whether PGD2 is involved in the regulation of T cell function during aging, we first investigated the alterations in PGD2-mediated signaling and serum Th1 cytokine levels in male older human subjects (age >60 years). As anticipated, the older volunteers had higher BP and levels of serum cholesterol, low-density protein cholesterol, triglycerides, blood glucose, urea, and Th1 cytokines (IFNγ and TNFα) than the young volunteers (Table I in the online-only Data Supplement). The number of CD8+ T cells in peripheral blood was reduced, whereas that of the CD4+ T cells was elevated in older subjects (Table I in the online-only Data Supplement). DP1 expression and PGD2 production in CD4+ T cells decreased dramatically in older subjects (Figure 1A and 1B), whereas the expression of Th1 cytokines (IFNγ and TNFα) increased with aging (Figure 1C). It is interesting to note that DP1 expression levels in T cells correlated negatively with systolic BP and diastolic BP (Figure 1D and 1E). Similarly, both PGD2 production and DP1 expression were markedly suppressed in CD4+ T cells in aged mice (Figure 1F and 1G). In contrast, the serum levels of Th1 cytokines (IFNγ and TNFα), but not Th2 cytokines, increased with aging in mice (Figure 1H and 1I, and Figure I in the online-only Data Supplement). Taken together, the PGD2/DP1 axis was downregulated in CD4+ T cells in an age-dependent manner, suggesting that dysregulation of the PGD2/DP1 axis in T cells may be involved in age-related hypertension.
Figure 1. PGD2/DP1 axis is downregulated with aging in primary CD4+ T cells.
A, mRNA expression of DP1 and DP2 in sorted CD3+CD4+ cells in young (20–30-y-old) and older (>60-y-old) adults (n=36–40). **P<0.01 compared with young. B, Mass spectrometric analysis of PGD2 from sorted CD3+CD4+ cells in young and older adults (n=9–10). *P<0.05 compared with young. C, IFNγ and TNFα mRNA levels in sorted CD3+CD4+ cells from young and older adults (n=36–40). **P<0.01 compared with young. D and E, Correlation analysis of DP1 expression with blood pressure (BP; systolic and diastolic) in young and old people by using Pearson correction coefficient (n=36–40). F, Mass spectrometric analysis of PGD2 from sorted CD3+CD4+ cells in young (2 mo) and aged (26 mo mice) (n=4–5). **P<0.01 compared with 2 months. G through I, mRNA levels of DP1, DP2, IFNγ, and TNFα in sorted CD3+CD4+ cells in young and aged mice (n=6). *P<0.05, **P<0.01 compared with 2 months. Data are expressed as mean ± SEM. Statistical analysis was performed using the unpaired Student t test (A through C and F) and 1-way ANOVA followed by Bonferroni post hoc analysis (G through I). DBP indicates diastolic blood pressure; DP1, D-prostanoid receptor 1; DP2, D-prostanoid receptor 2; IFNγ, interferon γ; M, month; PGD2, prostaglandin D2; SBP, systolic blood pressure; and TNFα, tumor necrosis factor α.
DP1 Deficiency in T Cells Accelerates Age-Dependent Hypertension in Mice by Increasing Th1 Cytokines
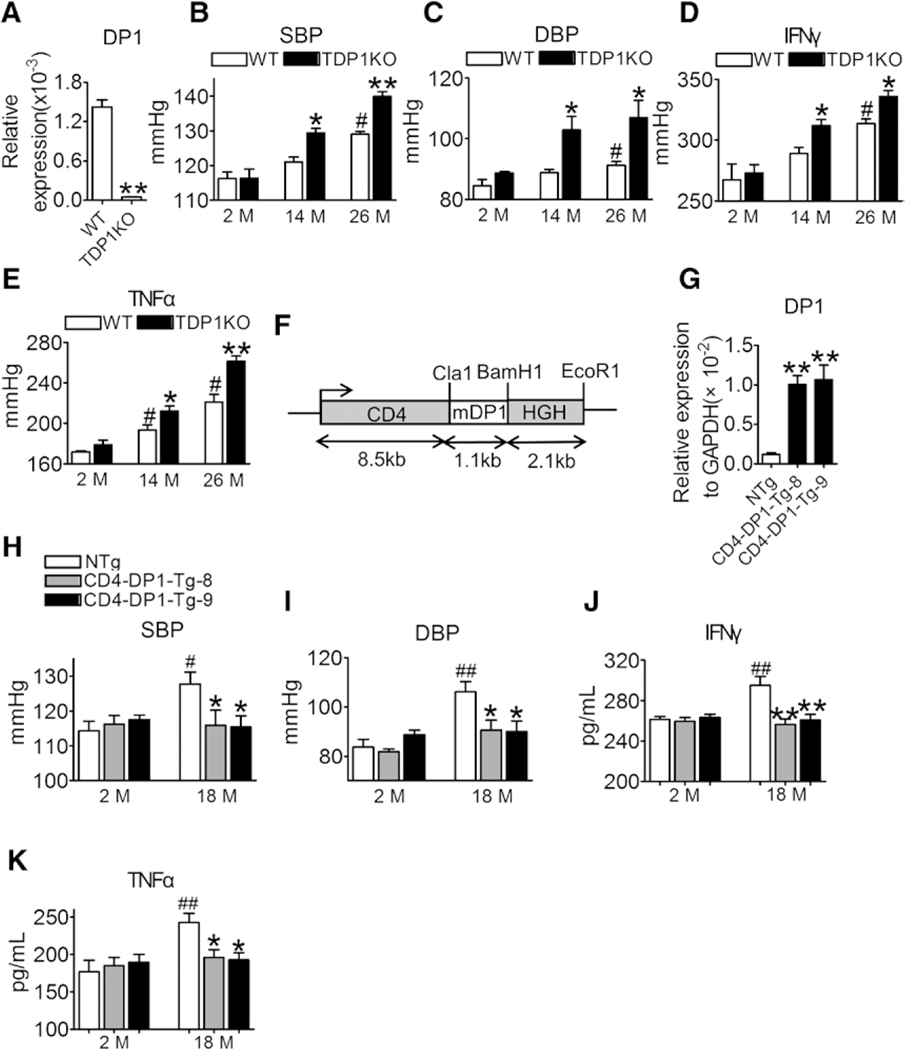
To further investigate the role of the PGD2/DP1 axis in age-related hypertension, CD4+ T cell–specific DP1-deficient mice (Figure 2A) were generated by crossing DP1F/F mice with CD4Cre transgenic mice (called TDP1KO). The DP1 gene was specifically excised in both CD4+ and CD8+ T cells (Figure IIA in the online-only Data Supplement), with much higher efficiency in CD4+ T cells (Figure IIB in the online-only Data Supplement). DP1 expression was not significantly altered in CD8+ T cells from aged mice (Figure IIC in the online-only Data Supplement), and DP1 deletion upregulated IFNγ and TNFα expression in CD4+ T cells from aged mice (Figure IID and IIE in the online-only Data Supplement) but had no significant influence on cytokine expression in CD8+ T cells (Figure IIF through IIH in the online-only Data Supplement). As expected, the BP (including systolic BP and diastolic BP) of floxed control mice increased with aging (Figure 2B and 2C), whereas DP1 deficiency in T cells significantly augmented the age-dependent BP elevation in mice (Figure 2B and 2C). DP1 ablation markedly increased Th1 cytokine release (Figure 2D and 2E), CD4+ T cell infiltration in the aortas and kidneys with elevated CD4+/CD8+ ratio (Figure IIIA through IIIE in the online-only Data Supplement), vascular remodeling (thickness and fibrosis), superoxide production in vasculature and kidneys, and impaired endothelium-dependent relaxation in aged mice (Figure IVA through IVH in the online-only Data Supplement).
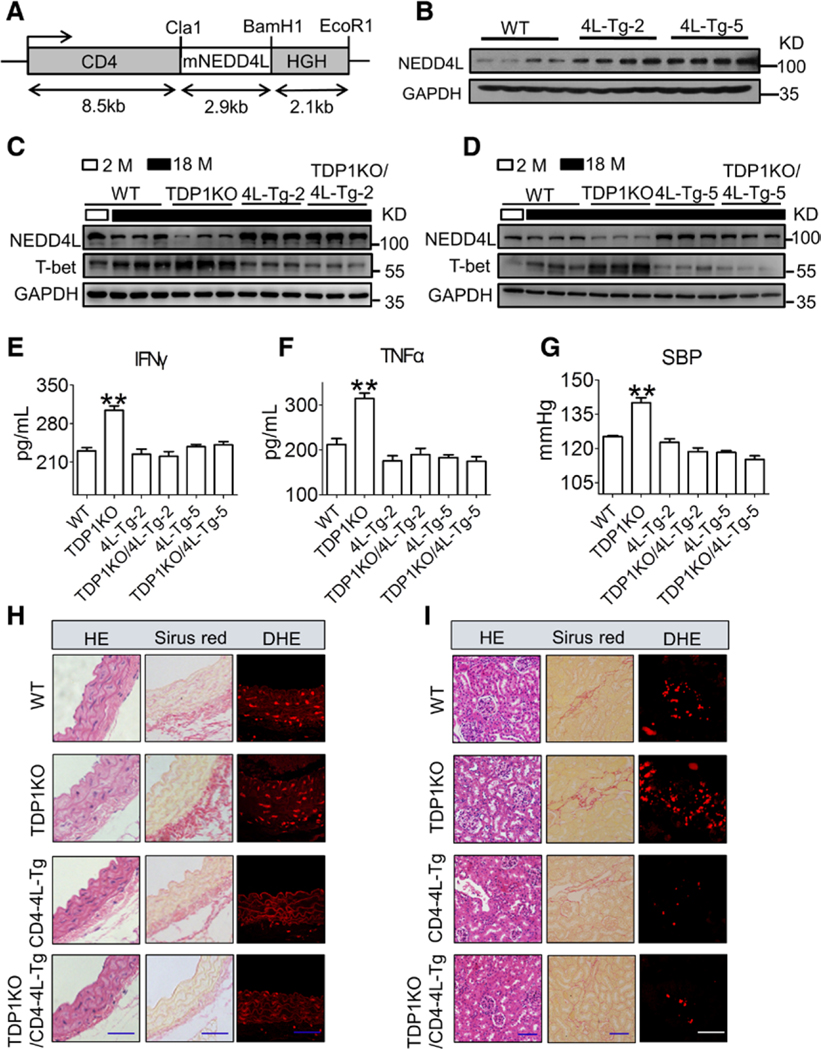
Figure 2. DP1 deficiency in T cells accelerates age-dependent hypertension in mice by increasing the levels of Th1 cytokines.
A, DP1 mRNA levels in CD3+CD4+ T cells from WT and TDP1KO mice. **P<0.01 compared with WT (n=5). B and C, Effect of DP1 deletion in T cells on systolic blood pressure (SBP) and diastolic blood pressure (DBP) in mice at different ages. *P<0.05, **P<0.01 compared with same month’s WT (n=5–8). #P<0.05 compared with 2 months WT. D and E, Effect of DP1 deletion in T cells on serum Th1 cytokine levels in mice at different ages. *P<0.05, **P<0.01 compared with same month’s WT (n=4). #P<0.05 compared with 2 months WT. F, Construct for the generation of CD4 promoter-driven mouse DP1 transgenic mice (CD4-DP1-Tg). Colored boxes show the CD4 promoter/enhancer/silencer and human growth hormone (HGH) polyadenylation as indicated. G, DP1 mRNA levels in CD4+ T lymphocytes in CD4-DP1-Tg mice (n=8). **P<0.01 compared with NTg. H through K, Effect of DP1 overexpression on SBP (H), DBP (I), serum levels of IFNγ (J), and TNFα (K) in mice. *P<0.05, **P<0.01 compared with 18 months NTg. #P<0.05, ##P<0.01 compared with 2 months NTg (n=4–8). Data are expressed as mean ± SEM. Data in A were analyzed by unpaired Student t test. Data in B through E and H through K were analyzed using 2-way ANOVA followed by Bonferroni post hoc analysis. Data in G were analyzed using 1-way ANOVA followed by Bonferroni post hoc analysis. DBP indicates diastolic blood pressure; DP1, D-prostanoid receptor 1; IFNγ, interferon γ; M, month; NTg, not transgenic; SBP, systolic blood pressure; TDP1KO, CD4+ T cell-specific DP1-deficient; Tg, transgenic; TNFα, tumor necrosis factor α; and WT, littermate control.
As DP1 was downregulated in T cells with aging, we investigated whether forced expression of exogenous DP1 in T cells retarded age-associated hypertension by creating CD4+ cell–DP1 transgenic mice (CD4-DP1-Tg, Figure 2F). Multiple transgenic lines with robust DP1 expression in T cells were identified (Figure 2G). Conversely, forced expression of exogenous DP1 expression attenuated BP elevation (Figure 2H and 2I), increased Th1 cytokine secretion (Figure 2J and 2K), and age-associated increase in arterial thickness, fibrosis, and superoxide production in the vasculature and kidneys, and improved vascular endothelial function in aged mice (18 mo old, Figure VA through VD in the online-only Data Supplement). In addition, IFNγ deletion (Figure VIA through VID in the online-only Data Supplement or TNFα neutralization antibody etanercept (Figure VIE through VIH in the online-only Data Supplement) abolished the exacerbated Th1 activation and elevated BP, and vascular and renal superoxide production in aged TDP1KO mice. Taken together, T cell DP1 deficiency promoted age-related BP elevation via increase in Th1 cytokine secretion.
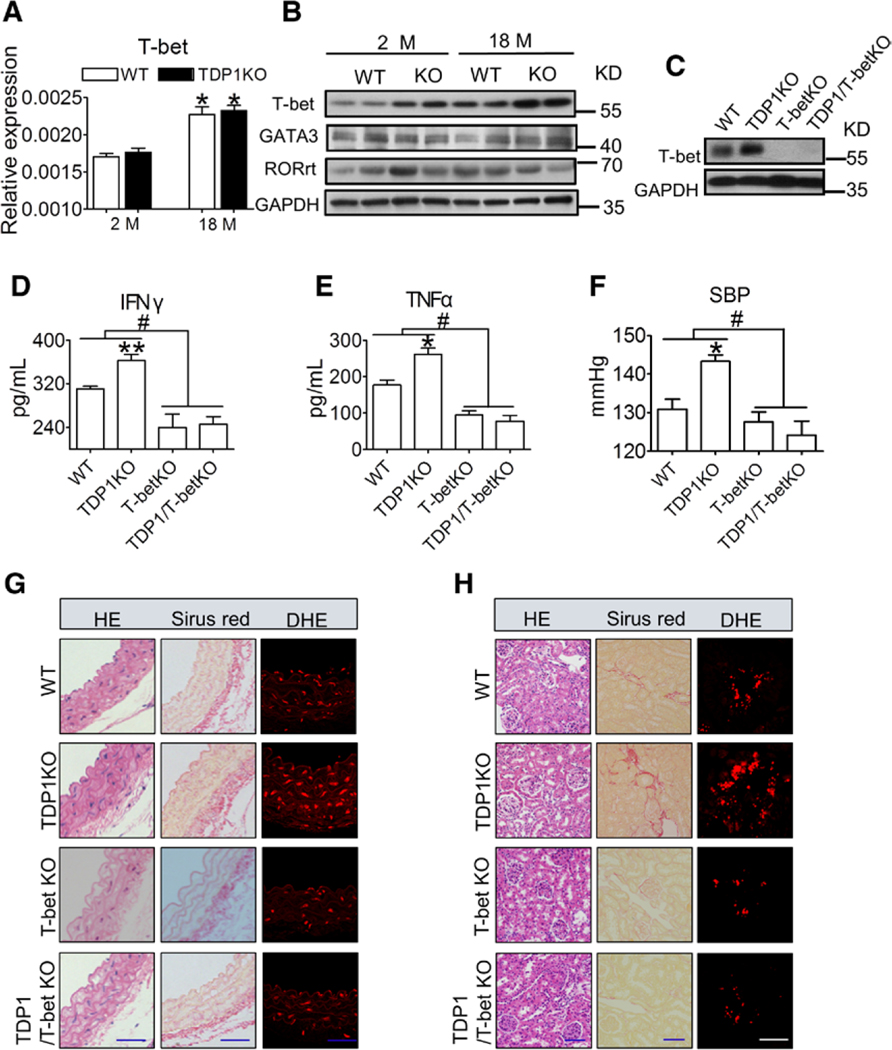
T-bet is the key transcription factor involved in the regulation of Th1 development and function. DP1 deficiency resulted in remarkable increase in T-bet protein expression in T cells, without affecting its mRNA expression (Figure 3A and 3B). Similarly, T-bet ablation (Figure 3C) attenuated the augmented secretion of IFNγ and TNFα (Figure 3D and 3E), the exacerbated age-related BP elevation (Figure 3F) and age-associated increase in arterial thickness, fibrosis, and superoxide production in the vasculature and kidneys (Figure 3G and 3H) in aged TDP1KO mice (18 mo old). Therefore, T-bet–mediated Th1 activation is required for age-related hypertension in aged TDP1KO mice.
Figure 3. T-bet deletion attenuates DP1 deficiency–exacerbated aging-related hypertension in mice.
A, T-bet mRNA levels in CD4+ T cells from TDP1-KO mice of different ages. *P<0.05 compared with 2 months. (n=6). B, Protein levels of master transcription factors in spleen CD4+ T cells from WT and TDP1KO mice of different ages. C, Efficiency of T-bet deletion in spleen CD4+ T cells in TDP1 and T-bet double KO (TDP1/T-bet KO) mice. D and E, Effect of T-bet ablation on serum levels of IFNγ and TNFα in TDP1/T-bet KO mice (18 mo old). Sera were collected for ELISA. *P<0.05, **P<0.01 compared with WT; #P<0.05 compared with as indicated (n=4–7). F, Effect of T-bet ablation on SBP in TDP1/T-bet KO mice. *P<0.05 compared with WT, #P<0.05 compared with as indicated (n=4–8). G and H, Representative HE, Sirius Red, and DHE staining of aortic and kidney sections from TDP1/T-bet KO mice. Blue scale bars, 50 μm; white scale bar, 10 μm. Data are expressed as mean ± SEM. Data in A and D through F were analyzed using 2-way ANOVA followed by Bonferroni post hoc analysis. DBP indicates diastolic blood pressure; DHE, dihydroethidium; GATA3, GATA binding protein 3; HE, hematoxylin and eosin; IFNγ, interferon γ; KO, knockout; M, month; RORrt, RAR-related orphan receptor γ isoform 2; SBP, systolic blood pressure; T-bet, T-box-expressed-in-T-cells; TDP1KO, CD4+ T cell-specific DP1-deficient; TNFα, tumor necrosis factor alpha; and WT, littermate control.
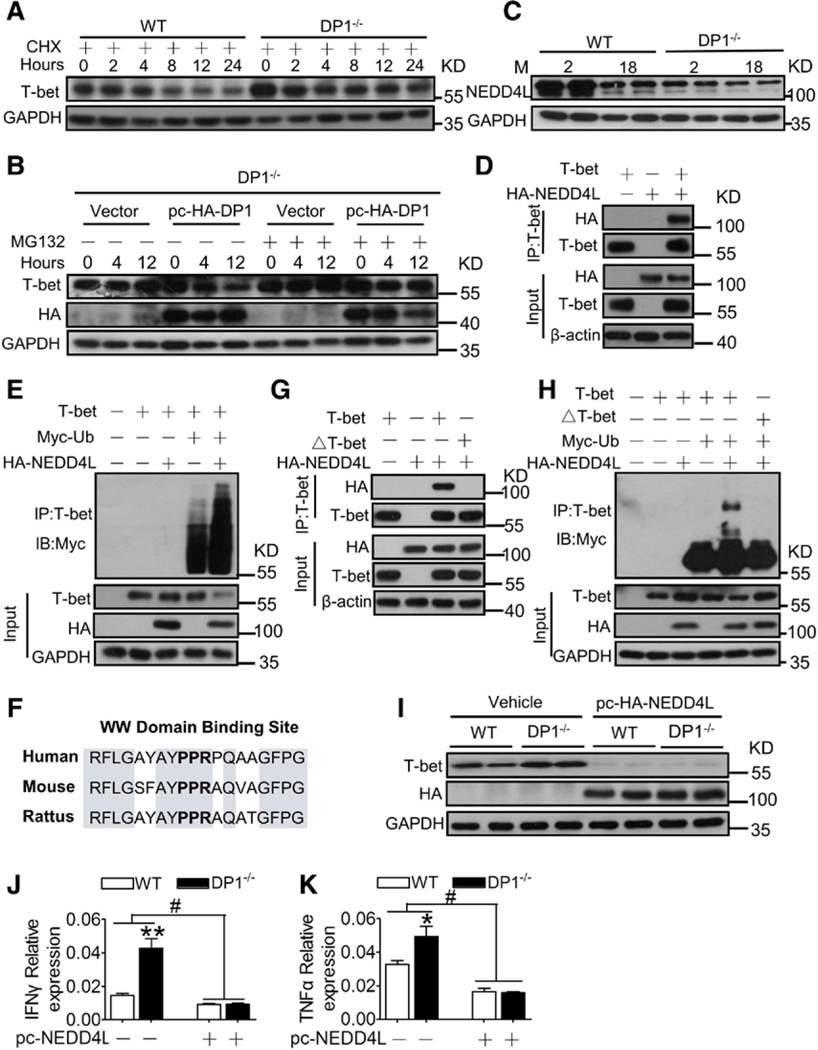
DP1 Promotes T-Bet Protein Degradation in T Cells Via NEDD4L
The level of T-bet protein, but not mRNA, increased in DP1-deficient T cells (Figure 3A and 3B), indicating that DP1 is involved in posttranscriptional regulation of T-bet. Cycloheximide, a eukaryotic protein synthesis inhibitor, did not affect T-bet accumulation in DP1-deficient T cells (Figure 4A). DP1 reconstitution decreased T-bet protein expression in DP1−/− T cells, which was restored by the proteasome inhibitor MG132 (Figure 4B), indicating that DP1 suppresses T-bet protein production via ubiquitin-mediated degradation. Both E3 ubiquitin ligases, which promote protein ubiquitination, and deubiquitinating enzymes, which block degradation, are involved in the regulation of intracellular protein homeostasis. We observed that multiple E3 ubiquitin ligases, but not deubiquitinating enzymes, were downregulated in CD4+ T cells from aged mice (Figure VIIA and VIIB in the online-only Data Supplement). However, DP1 deficiency markedly suppressed NEDD4L expression in aged mice (Figure 4C and Figure VIIC in the online-only Data Supplement), suggesting that the reduced NEDD4L expression may lead to T-bet accumulation in DP1-deficient T cells. Indeed, coimmunoprecipitation experiments verified an interaction between NEDD4L and T-bet (Figure 4D). Overexpression of NEDD4L facilitated T-bet ubiquitination in vitro (Figure 4E). NEDD4L targets substrates via binding to polyproline (or similar) motifs.24 We observed that the PPR domain24 of T-bet was shared across different species (Figure 4F). Mutation of the PPR motif to AAA in T-bet protein disrupted the direct binding of NEDD4L to T-bet (Figure 4G), resulting in resistance of the T-bet protein to NEDD4L-mediated ubiquitination (Figure 4H). Accordingly, forced expression of NEDD4L strikingly diminished the accumulation of T-bet in DP1-deficient T cells (Figure 4I), and subsequently suppressed its downstream cytokine expression in DP1−/− T cells (Figure 4J and 4K).
Figure 4. DP1 activation accelerates T-bet ubiquitin-mediated degradation in CD4+ T cells by promoting NEDD4L transcription.
A, Effect of cycloheximide (CHX) on T-bet protein expression in DP1−/− CD4+ T cells. B, Effect of MG132 on T-bet protein level in DP1-reconsistuted DP1−/− CD4+ T cells. C, NEDD4L protein level in spleen CD4+ T cells from TDP1KO mice of different ages. D, Interaction between T-bet and NEDD4L in HEK293T cells. E, NEDD4L mediates ubiquitination of T-bet in HEK-293T cells. The top panel shows ubiquitination of T-bet, and the bottom 3 panels show the level of each protein, as indicated. Ub, ubiqutin. F, WW domain binding site (PPR) of T-bet protein sequence in different species. G and H, The effect of PPR mutation to AAA (△T-bet) on interaction between T-bet and NEDD4L (G) and NEDD4L mediated T-bet ubiquitination (H) in HEK-293T cells. I through K, Effect of NEDD4L overexpression on T-bet protein expression (I), mRNA levels of IFNγ (J), and TNFα (K) in DP1−/− CD4+ T cells. *P<0.05, **P<0.01 compared with WT, #P<0.05 compared with as indicated (n=6). Data are expressed as mean ± SEM. Data in J and K were analyzed using 2-way ANOVA followed by Bonferroni post hoc analysis. DP1 indicates D-prostanoid receptor 1; HA, hemagglutinin; IB, immunoblotting; IFNγ, interferon γ; IP, immunoprecipitation; M, month; NEDD4L, neural precursor cell expressed developmentally downregulated 4-like; pc, pcDNA-3.1; T-bet, T-box expressed in T cells; TDP1KO, CD4+ T cell-specific DP1-deficient; TNFα, tumor necrosis factor α; and WT, littermate control.
Overexpression of NEDD4L in CD4+ T Cells Attenuates the Exacerbated Age-Dependent Hypertension in TDP1KO Mice
We next investigated whether increase in NEDD4L expression restored hypertensive response in aged TDP1KO mice. NEDD4L transgenic mice were generated under the control of CD4 regulatory elements22 (CD4-NEDD4L-Tg; Figure 5A). As expected, forced expression of NEDD4L E3 ligase (4L-Tg-2 and 4L-Tg-5; Figure 5B) decreased the level of the accumulated T-bet protein (Figure 5C and 5D), and suppressed Th1 cytokine secretion in DP1−/− CD4+ T cells tested in 2 aged transgenic strains (Figure 5E and 5F). It is important to note that NEDD4L overexpression suppressed BP elevation in aged TDP1KO mice (Figure 5G) by reversing the increase in arterial thickness, perivascular fibrosis, and superoxide production in the vasculature and kidneys (Figure 5H and 5I).
Figure 5. CD4-specific NEDD4L overexpression blunts age-related hypertension in TDP1KO mice.
A, Schematic diagram of the construct used for the generation of CD4-specific mouse NEDD4L overexpression mice (CD4-NEDD4L-Tg). B, CD4-specific NEDD4L expression in CD4+ T lymphocytes from CD4-NEDD4L-Tg mice (2 mo old). C and D, The effect of CD4-specific NEDD4L overexpression on T-bet protein levels in aged CD4+ T cells tested in 2 different mouse strains (4L-Tg-2 and 4L-Tg-5). E and F, The effect of CD4-specific NEDD4L overexpression on serum levels of IFNγ (E) and TNFα (F) in aged mice (18 mo). **P<0.01 compared with WT (n=4–8). G, The effect of CD4-specific NEDD4L overexpression on SBP in aged TDP1KO mice (18 mo old). **P<0.01 compared with WT (n=6–8). H and I, Representative hematoxylin and eosin (HE), Sirius Red, and dihydroethidium (DHE) staining of aortic and kidney sections. Blue scale bars, 50 μm; white scale bar, 10 μm. Data are expressed as mean ± SEM. Data in E through G were analyzed by 2-way ANOVA followed by Bonferroni post hoc analysis. 4L-Tg indicates NEDD4L transgenic; DBP, diastolic blood pressure; DHE, dihydroethidium; HE, hematoxylin and eosin; HGH, human growth hormone; IFNγ, interferon γ; M, month; mNEDD4L, mouse NEDD4L mRNA; NEDD4L, neural precursor cell expressed developmentally downregulated 4-like; SBP, systolic blood pressure; T-bet, T-box-expressed-in-T-cells; TDP1KO, CD4+ T cell-specific DP1-deficient; TNFα, tumor necrosis factor α; and WT, littermate control.
DP1 Promoted NEDD4L Expression in T Cells Via the PKA/Sp1 Pathway
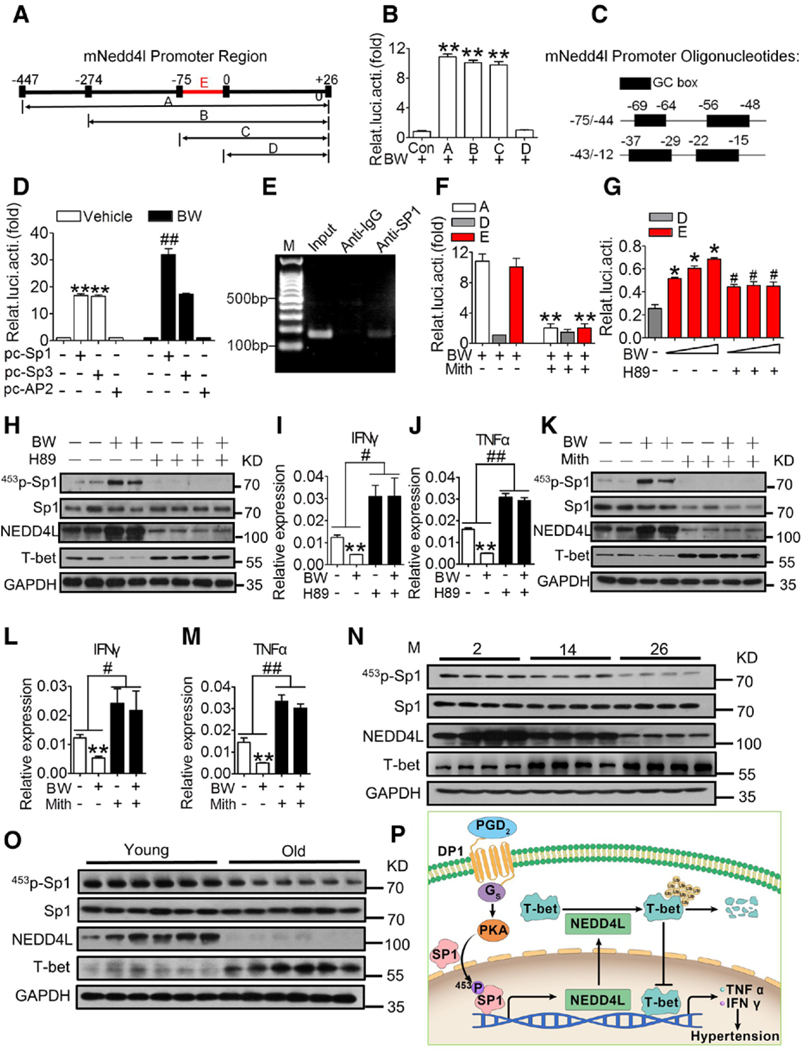
To investigate the mechanism via which DP1 regulates NEDD4L gene transcription, we cloned the truncated mouse NEDD4L promoters for luciferase assay in lymphoma cell line EL4 (Figure 6A). Treatment with the DP1 agonist BW245C increased NEDD4L transcription, and serial deletion of NEDD4L indicated that the 75-bp fragment near exon 1 is the key promoter region responsive to the DP1 agonist (Figure 6B). Bioinformatics analysis showed that the 75-bp promoter is Guanine-Cytosine (GC) box-rich (Figure 6C), and can be activated by specificity proteins (Sp) 1 and 3.25 The DP1 agonist boosted NEDD4L transcription in Sp1-, but not Sp3-, overexpressing cells, although both transcription factors triggered NEDD4L expression (Figure 6D). Chromatin immunoprecipitation assay confirmed Sp1 binding to the NEDD4L promoter region (Figure 6E). BW245C-induced NEDD4L transcription was blocked by the Sp1 selective inhibitor mithramycin A (Figure 6F) and the PKA (protein kinase A) inhibitor H89 (Figure 6G) in cultured primary CD4+ T cells. Similarly, we observed the conserved GC box (Figure VIIIA in the online-only Data Supplement) and similar transcriptional activity in the human NEDD4L promoter region (Figure VIIIB in the online-only Data Supplement).
Figure 6. DP1 activation promotes mNEDD4L expression via PKA-mediated Sp1 phosphorylation.
A, Schematic illustration of luciferase reporter constructs containing truncated mouse NEDD4L promoter region. B, Luciferase reporter assay of BW245C (BW)-treated EL4 cells transiently transfected with truncated reporter plasmids or control plasmid (pGL3-basic). **P<0.01 compared with control (n=6). C, Illustration of the predicted fragment of the NEDD4L promoter region. D, Effect of Sp1, Sp3, and AP2 overexpression on mouse NEDD4L promoter fragment-mediated luciferase activity in BW254C-treated EL4 cells (n=4). E, Gel electrophoresis of polymerase chain reaction-amplified fragments in the promoter of mouse NEDD4L using anti-Sp1 immunoprecipitation. F, Effect of the Sp1 inhibitor (Mith, 0.1 mM) on the NEDD4L promoter fragment-mediated luciferase activity in EL4 cells. **P<0.01 compared with without Mith (n=3). G, Effect of BW and protein kinase A (PKA) inhibitor H89 on the NEDD4L promoter fragment-mediated luciferase activity in sorted primary CD4+ T cells. *P<0.05 compared with D fragment. #P<0.05 compared with without BW (n=3). H through J, Effect of H89 on Sp1 phosphorylation, NEDD4L and T-bet expression, and IFNγ (I) and TNFα (J) mRNA levels in BW-treated CD4+ T cells. **P<0.01 compared with untreated, #P<0.05, ##P<0.01 compared with as indicated (n=6–8). K through M, Effect of the Sp1 inhibitor Mith on Sp1 phosphorylation, NEDD4L and T-bet expression, and IFNγ (L) and TNFα (M) mRNA expression in BW-treated CD4+ T cells. **P<0.01 compared with untreated, #P<0.05, ##P<0.01 compared with as indicated (n=6–8). N, Alterations in Sp1/NEDD4L/T-bet signal pathway in CD4+ cells in aged mice (26 mo) compared with young ones (2 mo). O, Alterations in Sp1/NEDD4L/T-bet signaling pathway in CD4+ cells in old individuals (> 60 y old) compared with young ones (20−30 y old). P, Schematic of PGD2/DP1/Sp1/NEDD4L/T-bet pathway–mediated Th1 activation in T cells. Data are expressed as mean ± SEM. Data analyses in B, D, F, and G were performed using 1-way ANOVA followed by Bonferroni post hoc analysis. Data in I, J, L, and M were analyzed using 2-way ANOVA followed by Bonferroni post hoc analysis. BW indicates BW245C, DP1 agonist; Con, control; DP1, D-prostanoid receptor 1; GC, Guanine-Cytosine; IFNγ, interferon γ; M, month; Mith, mithramycin A, the Sp1 selective inhibitor; mNedd4l, mouse NEDD4L; NEDD4L, neural precursor cell expressed developmentally downregulated 4-like; pc, pcDNA3.1; PGD2, prostaglandin D2; p-Sp1, phosphorylated Sp1; Relat.luci.acti, relative luciferase activity; Sp1, specificity protein 1; T-bet, T-box-expressed-in-T-cells; and TNFα, tumor necrosis factor α.
The DP1 receptor couples to the stimulatory G protein alpha-subunit to trigger PKA-dependent cascades. Sp1 can be activated by PKA-mediated phosphorylation.26 Forskolin-mediated activation of PKA induced Sp1 phosphorylation at threonine (T), but not serine (S), in T cells (Figure IXA in the online-only Data Supplement). In a screen using mutated Sp1-transfected cells, we identified T453 to be the direct phosphorylation site of PKA in Sp1 (Figure IXB in the online-only Data Supplement), and observed that DP1 activation promoted T453 phosphorylation of Sp1 (T452 in mouse sequence, Figure IXC and IXD in the online-only Data Supplement), facilitated NEDD4L expression and NEDD4L-mediated ubiquitination of T-bet in T cells (Figure 6H), and suppressed Th1-derived cytokines in culture via PKA (Figure 6I and 6J). As anticipated, the Sp1 inhibitor mithramycin A repressed DP1-mediated Sp1 activation and NEDD4L induction, and diminished T-bet ubiquitination in T cells, thereby reversing the expression of Th1 cytokines in BW245C-treated T cells (Figure 6K through 6M). Consistent with DP1 downregulation, phosphorylation of Sp1 at T453 and NEDD4L expression were gradually reduced, whereas T-bet protein expression was elevated in T cells from both aged mice and old human subjects (Figure 6N and 6O). Thus, our results suggested that the PGD2/DP1 axis suppressed Th1 activity via the PKA/p-Sp1/NEDD4L/T-bet pathway (Figure 6P).
DP1 Activation Attenuates Age-Related BP Elevation in Mice
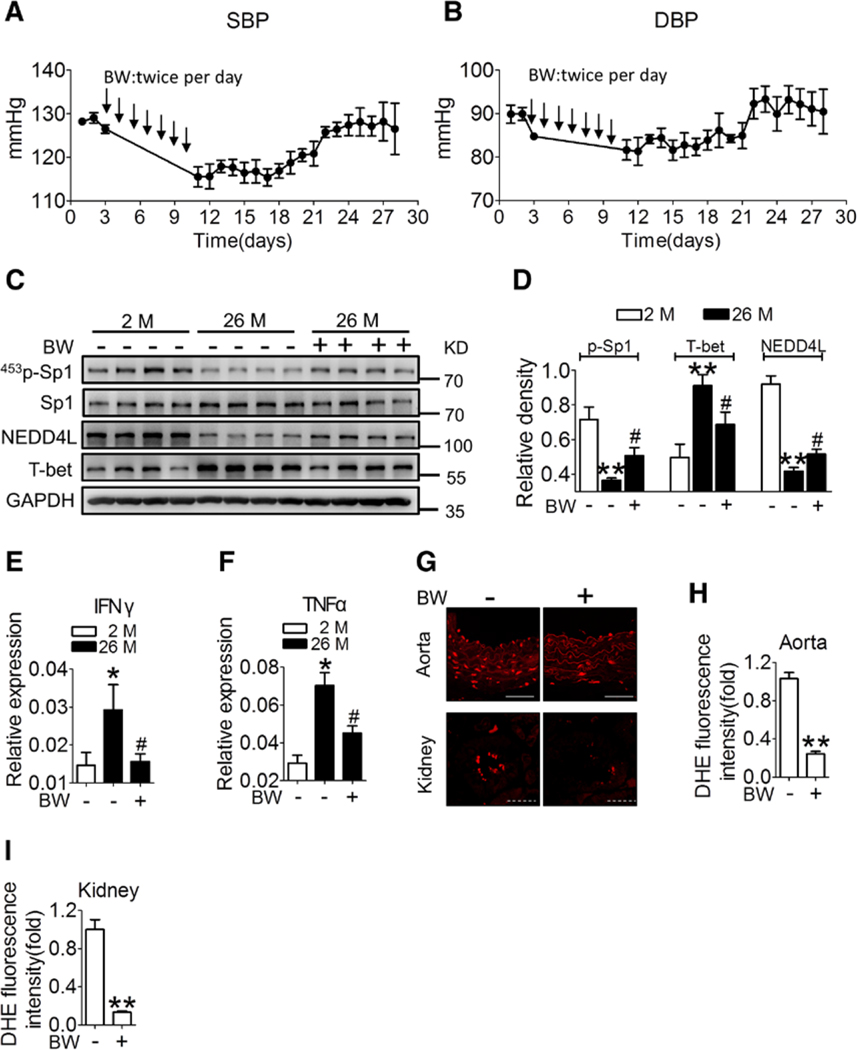
Last, we investigated whether DP1 activation is effective in reversing age-related hypertension. We injected the DP1 agonist BW245C via intraperitoneal route in 26-month-old mice (1 mg/kg) at 12-hour intervals for 7 consecutive days. As shown in Figure 7A and 7B, the systolic BP and diastolic BP in BW245C-treated aged mice were significantly decreased to the comparable level seen in young mice. BP remained low for at least 11 consecutive days (Figure 7A and 7B), demonstrating the prolonged efficacy of the DP1 agonist in maintaining low BP in these mice. We also observed that the dysregulated Sp1/NEDD4L/T-bet signaling pathway in aged CD4+ T cells was reversed in BW245C-treated mice (Figure 7C and 7D). BW245C treatment also markedly reduced Th1 cytokine levels and tissue superoxide production in the aged mice (Figure 7E through 7I).
Figure 7. DP1 agonist BW245C treatment attenuates age-related BP elevation in mice.
A and B, Twenty-four-hour continuous radio telemetry recordings of SBP and DBP in 26-month-old WT mice injected with BW245C (BW, 1 mg/kg) with 12-h intervals for 7 days. Means ± SEM of BP values were plotted at 24-h intervals over 28 days (n=3–5). C through F, Effect of BW on Sp1 phosphorylation, protein levels of NEDD4L and T-bet (C and D) and Th1 cytokine mRNA levels (E and F) in sorted CD4+ cells from aged mice (26-month-old). *P<0.05 **P<0.01 compared with 2 months, #P<0.05 compared with untreated 26 months. G through I, Effect of BW treatment on superoxide production in aorta and kidneys (n=4). **P<0.01 compared with vehicle. Scale bar (straight line), 50 μm; scale bar (dotted line), 10 μm. Data are expressed as mean ± SEM. Statistical analysis in D through F were performed using one-way ANOVA followed by Bonferroni post hoc analysis. Statistical analysis in H and I was performed using unpaired Student’s t-test. BP indicates blood pressure; DBP, diastolic blood pressure; DHE, dihydroethidium; DP1, prostaglandin D2 receptor subtype 1; IFNγ, interferon γ; M, month; NEDD4L, neural precursor cell expressed developmentally downregulated 4-like; p-Sp1, phosphorylated Sp1; SBP, systolic blood pressure; Sp1, specificity protein 1; T-bet, T-box-expressed-in-T-cells; and TNFα, tumor necrosis factor α.
DISCUSSION
T lymphocyte dysregulation contributes to AngII-induced and age-related hypertension,15,27 although the underlying mechanism is not clear. In this study, we observed that PGD2 production and DP1 expression were markedly reduced in CD4+ T cells from old human subjects and aged mice. DP1 deletion in CD4+ T cells exaggerated aging-dependent BP elevation in male mice by increasing TNFα and IFNγ secretion, whereas DP1 overexpression showed the opposite effect. DP1 suppressed Th1 cytokines via the PKA/p-Sp1/ NEDD4L/T-bet pathway. Furthermore, treatment with the DP1 agonist reduced the elevated BP in aged male mice. Therefore, DP1 and its downstream pathway may constitute an attractive immunotherapeutic target for age-dependent hypertension in males.
Aging is a progressive process that leads to reduced physiological functions across different organ systems. The resulting physiological decline increases the risk for various age-related diseases, such as hypertension. Despite the complexity and diverse etiologies, accumulated evidence demonstrates that two interconnected mechanisms may primarily contribute toward pathogenesis of age related-diseases, low-grade inflammation, and augmented cellular oxidative stress.1,28 Both the innate and adaptive immune systems are impaired with aging. The most remarkable changes are observed in T cell immunity, which include imbalances in T cell populations and senescent T cell expansion.27 The dysregulated T cells produce excessive inflammatory cytokines and cytotoxic mediators.29 Inflammatory reactions, in turn, also increase tissue oxidative stress via production of reactive oxygen species by infiltrated neutrophils and macrophages.30 Indeed, we and others31 have observed elevated levels of proinflammatory cytokines (TNFα, IFNγ) in circulation, tissue inflammation, and increased reactive oxygen species levels in targeted organs in aged adults and mice. We observed that the PGD2/DP1 axis was downregulated in CD4+ T cells from older individuals and aged mice. Ectopic expression of DP1 or activation of the endogenous DP1 receptor in CD4+ T cells reduced proinflammatory cytokine secretion and reactive oxygen species formation in vasculatures, and subsequently delayed aged-related hypertension. Thus, the reduced DP1-mediated signaling may contribute to T cell inflammation and age-related hypertension in older individuals.
Hypertension is well-known to differ by sex in prevalence. The differences of the renin-angiotensin system in vasculature and kidney contribute to this gender distinction in BP,9 and sexual hormonal milieu and sex chromosomes also play an essential role in and of themselves.8,9 It is interesting to note that sex-specific effects of T cells were recently observed to determine the magnitude of AngII-induced hypertension in mice.8 These sex differences in adaptive immune regulation of BP maybe associated with gender-dependent T cell infiltration capacity,32,33Tregs proportion,34,35 and balance of proinflammatory with anti-inflammatory cytokines in end organs.9 Despite that DP1-mediated signaling pathway downregulated in T cells from aged mice and old human subjects, further investigation is warranted to evaluate sex differences of PGD2/DP1 involvement in age-related hypertension.
As a vasodilator, PGD2 exerts multiple cardiovascular benefits via the DP1 receptor. Experimental animal studies showed that DP1 activation protects mice against acute excitotoxicity and cerebra ischemia.36,37 The absence of the DP1 receptor augments AngII-induced hypertension and aneurism, and high-fat diet–induced atherosclerosis.21 DP1 mediates PGD2-triggered resolution of inflammation by promoting macrophage polarization toward M2 status, thereby facilitating cardioprotection against ischemia.18,38 Furthermore, glucocorticoids confer cardiac protection against ischemia/reperfusion via activation of the PGD2/DP1 axis,39 perhaps via upregulation of Nrf2.40 In humans, PGD2 inhibits platelet activation via DP1.21 We observed that DP1 activation in T cells prevented age-related hypertension by suppressing TNFα and IFNγ. Consistent with our observations, PGD2/DP1 activation also inhibits IFNγ production in natural killer T cells and dendritic cells via PKA.41 Therefore, the DP1 receptor may be an attractive therapeutic target for inflammation, especially for cardiovascular inflammation.
NEDD4L is an E3 ubiquitin protein ligase, which contains 4 WW domains (protein–protein interaction domains) that specifically recognize targeting substrates containing “PPXY,” “LPXY,” or “PPR” motifs.24 Genetic studies showed that multiple single-nucleotide polymorphisms in NEDD4L are associated with hypertension in different ethnic populations.42 The epithelial Na+ channel, involved in salt reabsorption and BP regulation, is a NEDD4L substrate in the kidney.43 Loss of NEDD4L results in sodium-sensitive hypertension in mice due to elevated epithelial Na+ channel activity in the kidney.44 There are 2 splicing isoforms of NEDD4L (a short isoform with 110 kDa molecular weight and a long isoform of 130 kDa). Both isoforms contain the substrate-targeting WW domains, but the short isoform lacks the N-terminal C2 domain.45 We observed that the long isoform of NEDD4L was dominantly expressed in T cells, and that NEDD4L regulated Th1 cytokine expression by targeting the T-bet transcription factor. It is interesting to note that along with DP1 downregulation and Th1 activation, NEDD4L expression was low in CD4+ T cells from older individuals. Forced expression of NEDD4L prevented age-related hypertension in mice. Thus, NEDD4L may be implicated in BP regulation in both inflammatory and renal tubule cells.
T-bet, a Th1 transcription factor, drives the expression of proinflammatory cytokines such as IFNγ and TNFα.46–48 T-bet deletion tends to have a less hypertensive response to AngII and reduces Th1 immune reaction-mediated kidney damage in mice.16 Furthermore, deficiency of Th1 cytokines TNFα or IFNγ blunts AngII-induced hypertension in mice.16,17 We observed that expression of T-bet protein was induced in T cells from aged subjects, and deletion of T-bet in CD4+ T cells reduced age-related BP elevation in mice. During Th cell differentiation, T-bet undergoes various posttranslational modifications, including phosphorylation and ubiquitination at lysine residues.47 For example, lysine 313 of T-bet mediates proteasome degradation via ubiquitination, which fine-tunes Th1 cytokine production.49 We observed accumulation of T-bet protein in T cells caused by impairment of ubiquitination during aging, and further identified NEDD4L as one of the key E3 ligases for T-bet in T cells, which is positively regulated by PGD2/DP1 signaling. In agreement with our observations, T-bet protein is also expressed in influenza virus-specific CD8+ T cells from aged subjects.50
In summary, PGD2/DP1 activation in CD4+ T cells delayed age-related hypertension via the PKA/Sp1/NEDD4L axis–mediated T-bet ubiquitination. Our results indicate that the DP1 receptor maybe an attractive immunotherapeutic target for age-related diseases, such as hypertension.
Supplementary Material
Clinical Perspective.
What Is New?
Prostaglandin D2 biosynthesis and D-prostanoid receptor 1 (DP1) expression are markedly declined in CD4+ T cells from older humans and aged mice.
DP1 deletion in CD4+ T cells exaggerates aging-dependent BP elevation in mice by increasing tumor necrosis factor α and interferon γ secretion, whereas DP1 overexpression shows the opposite effect.
DP1 activation suppresses Type 1 T helper cell cytokines via the protein kinase A/phosphorylated specificity protein 1/neural precursor cell expressed developmentally downregulated 4-like/T-box-expressed-in-T-cells pathway.
What Are the Clinical Implications?
Treatment with DP1 agonist BW245C reverses age-related hypertension in male aged mice.
Our results indicate DP1 and its downstream pathway may serve as an attractive immunotherapeutic target for age-dependent hypertension.
Acknowledgments
The authors thank Professor Limin Lu (Department of Physiology and Patho-physiology, School of Basic Medicine Science, Fudan University, Shanghai, China) for providing technical assistance. D.K. and Q.W. performed data analysis; S.D., B.Z., A.L., M.L., R.M.B., F.G., and Y.Y. handled funding and supervision; D.K., Q.W., L.L., S.Z., G.L., Q.L., C.W., L.W., and P.B. acquired the data; D.K., Q.W., and Y.Y. conceived and designed the research; D.K., Q.W., and Y.Y. drafted the article; and Y.Y. critically revised the article for key intellectual content.
Sources of Funding
This work was supported by the National Natural Science Foundation of China (grants 81790623, 81525004, 81700369, and 91639302), the Chinese Ministry of Science and Technology (2017YFC1307404 and 2017YFC1307402), the Tianjin Natural Science Foundation (17JCYBJC40700), and the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (2017M621088). Dr Yu is a fellow at the Jiangsu Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Footnotes
Disclosures
None.
REFERENCES
- 1.Buford TW. Hypertension and aging. Ageing Res Rev. 2016;26:96–111. doi: 10.1016/j.arr.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pimenta E, Oparil S. Management of hypertension in the elderly. Nat Rev Cardiol. 2012;9:286–296. doi: 10.1038/nrcardio.2012.27 [DOI] [PubMed] [Google Scholar]
- 3.Sera LC, McPherson ML. Pharmacokinetics and pharmacodynamic changes associated with aging and implications for drug therapy. Clin Geriatr Med. 2012;28:273–286. doi: 10.1016/j.cger.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 4.Benetos A, Labat C, Rossignol P, Fay R, Rolland Y, Valbusa F, Salvi P, Zamboni M, Manckoundia P, Hanon O, et al. Treatment with multiple blood pressure medications, achieved blood pressure, and mortality in older nursing home residents: the PARTAGE Study. JAMA Intern Med. 2015;175:989–995. doi: 10.1001/jamainternmed.2014.8012 [DOI] [PubMed] [Google Scholar]
- 5.Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci. 2014;69 (suppl 1):S4–S9. doi: 10.1093/gerona/glu057 [DOI] [PubMed] [Google Scholar]
- 6.Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD, Ross OA. Age and age-related diseases: role of inflammation triggers and cytokines. Front Immunol. 2018;9:586. doi: 10.3389/fimmu.2018.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fülöp T, Larbi A, Pawelec G. Human T cell aging and the impact of persistent viral infections. Front Immunol. 2013;4:271. doi: 10.3389/fimmu.2013.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sandberg K, Ji H, Hay M. Sex-specific immune modulation of primary hypertension. Cell Immunol. 2015;294:95–101. doi: 10.1016/j.cellimm.2014.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramirez LA, Sullivan JC. Sex differences in hypertension: where we have been and where we are going. Am J Hypertens. 2018;31:1247–1254. doi: 10.1093/ajh/hpy148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bandrés E, Merino J, Vázquez B, Inogés S, Moreno C, Subirá ML, Sánchez-Ibarrola A. The increase of IFN-gamma production through aging correlates with the expanded CD8(+high)CD28(−)CD57(+) subpopulation. Clin Immunol. 2000;96:230–235. doi: 10.1006/clim.2000.4894 [DOI] [PubMed] [Google Scholar]
- 11.Yen CJ, Lin SL, Huang KT, Lin RH. Age-associated changes in interferon-gamma and interleukin-4 secretion by purified human CD4+ and CD8+ T cells. J Biomed Sci. 2000;7:317–321. doi: 10.1007/bf02253251 [DOI] [PubMed] [Google Scholar]
- 12.Bauernfeind F, Niepmann S, Knolle PA, Hornung V. Aging-associated TNF production primes inflammasome activation and NLRP3-related metabolic disturbances. J Immunol. 2016;197:2900–2908. doi: 10.4049/jimmunol.1501336 [DOI] [PubMed] [Google Scholar]
- 13.Bruunsgaard H, Skinhøj P, Pedersen AN, Schroll M, Pedersen BK. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin Exp Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Interferon-gamma Oxenkrug G. - inducible inflammation: contribution to aging and aging-associated psychiatric disorders. Aging Dis. 2011;2:474–486. [PMC free article] [PubMed] [Google Scholar]
- 15.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Patel MB, Griffiths R, Mao A, Song YS, Karlovich NS, Sparks MA, Jin H, Wu M, Lin EE, et al. Tumor necrosis factor-α produced in the kidney contributes to angiotensin II-dependent hypertension. Hypertension. 2014;64:1275–1281. doi: 10.1161/HYPERTENSIONAHA.114.03863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saleh MA, McMaster WG, Wu J, Norlander AE, Funt SA, Thabet SR, Kirabo A, Xiao L, Chen W, Itani HA, et al. Lymphocyte adaptor protein LNK deficiency exacerbates hypertension and end-organ inflammation. J Clin Invest. 2015;125:1189–1202. doi: 10.1172/JCI76327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kong D, Shen Y, Liu G, Zuo S, Ji Y, Lu A, Nakamura M, Lazarus M, Stratakis CA, Breyer RM, et al. PKA regulatory IIα subunit is essential for PGD2-mediated resolution of inflammation. J Exp Med. 2016;213:2209–2226. doi: 10.1084/jem.20160459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Kong D, Wang Q, Wu W, Tang Y, Bai T, Guo L, Wei L, Zhang Q, Yu Y, et al. Niacin ameliorates ulcerative colitis via prostaglandin D2-mediated D prostanoid receptor 1 activation. EMBO Mol Med. 2017;9:571–588. doi: 10.15252/emmm.201606987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marone G, Galdiero MR, Pecoraro A, Pucino V, Criscuolo G, Triassi M, Varricchi G. Prostaglandin D2 receptor antagonists in allergic disorders: safety, efficacy, and future perspectives. Expert Opin Investig Drugs. 2019;28:73–84. doi: 10.1080/13543784.2019.1555237 [DOI] [PubMed] [Google Scholar]
- 21.Song WL, Stubbe J, Ricciotti E, Alamuddin N, Ibrahim S, Crichton I, Prempeh M, Lawson JA, Wilensky RL, Rasmussen LM, et al. Niacin and biosynthesis of PGD₂ by platelet COX-1 in mice and humans. J Clin Invest. 2012;122:1459–1468. doi: 10.1172/JCI59262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghajani K, Keerthivasan S, Yu Y, Gounari F. Generation of CD4CreER(T2) transgenic mice to study development of peripheral CD4-T-cells. Genesis. 2012;50:908–913. doi: 10.1002/dvg.22052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norlander AE, Madhur MS, Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. doi: 10.1084/jem.20171773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sudol M, Hunter T. NeW wrinkles for an old domain. Cell. 2000;103:1001–1004. doi: 10.1016/s0092-8674(00)00203-8 [DOI] [PubMed] [Google Scholar]
- 25.Su S, Omiecinski CJ. Sp1 and Sp3 transcription factors regulate the basal expression of human microsomal epoxide hydrolase (EPHX1) through interaction with the E1b far upstream promoter. Gene. 2014;536:135–144. doi: 10.1016/j.gene.2013.11.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohlff C, Ahmad S, Borellini F, Lei J, Glazer RI. Modulation of transcription factor Sp1 by cAMP-dependent protein kinase. J Biol Chem. 1997;272:21137–21141. doi: 10.1074/jbc.272.34.21137 [DOI] [PubMed] [Google Scholar]
- 27.Yu HT, Park S, Shin EC, Lee WW. T cell senescence and cardiovascular diseases. Clin Exp Med. 2016;16:257–263. doi: 10.1007/s10238-015-0376-z [DOI] [PubMed] [Google Scholar]
- 28.Ungvari Z, Tarantini S, Donato AJ, Galvan V, Csiszar A. Mechanisms of vascular aging. Circ Res. 2018;123:849–867. doi: 10.1161/CIRCRESAHA.118.311378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn JC, Yu HT, Lim BJ, Koh MJ, Lee J, Chang DY, Choi YS, Lee SH, Kang SM, Jang Y, et al. Immunosenescent CD8+ T cells and C-X-C chemokine receptor type 3 chemokines are increased in human hypertension. Hypertension. 2013;62:126–133. doi: 10.1161/HYPERTENSIONAHA.113.00689 [DOI] [PubMed] [Google Scholar]
- 30.Crowley SD. The cooperative roles of inflammation and oxidative stress in the pathogenesis of hypertension. Antioxid Redox Signal. 2014;20:102–120. doi: 10.1089/ars.2013.5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh T, Newman AB. Inflammatory markers in population studies of aging. Ageing Res Rev. 2011;10:319–329. doi: 10.1016/j.arr.2010.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pollow DP, Uhrlaub J, Romero-Aleshire M, Sandberg K, Nikolich-Zugich J, Brooks HL, Hay M. Sex differences in T-lymphocyte tissue infiltration and development of angiotensin II hypertension. Hypertension. 2014;64:384–390. doi: 10.1161/HYPERTENSIONAHA.114.03581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji H, Zheng W, Li X, Liu J, Wu X, Zhang MA, Umans JG, Hay M, Speth RC, Dunn SE, et al. Sex-specific T-cell regulation of angiotensin II-dependent hypertension. Hypertension. 2014;64:573–582. doi: 10.1161/HYPERTENSIONAHA.114.03663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have greater renal anti-inflammatory T lymphocyte infiltration than males. Am J Physiol Regul Integr Comp Physiol. 2012;303:R359–R367. doi: 10.1152/ajpregu.00246.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tipton AJ, Baban B, Sullivan JC. Female spontaneously hypertensive rats have a compensatory increase in renal regulatory T cells in response to elevations in blood pressure. Hypertension. 2014;64:557–564. doi: 10.1161/HYPERTENSIONAHA.114.03512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ahmad AS, Ahmad M, Maruyama T, Narumiya S, Doré S. Prostaglandin D2 DP1 receptor is beneficial in ischemic stroke and in acute exicitotoxicity in young and old mice. Age (Dordr). 2010;32:271–282. doi: 10.1007/s11357-010-9135-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saleem S, Zhuang H, de Brum-Fernandes AJ, Maruyama T, Narumiya S, Doré S. PGD(2) DP1 receptor protects brain from ischemia-reperfusion injury. Eur J Neurosci. 2007;26:73–78. doi: 10.1111/j.1460-9568.2007.05627.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong D, Li J, Shen Y, Liu G, Zuo S, Tao B, Ji Y, Lu A, Lazarus M, Breyer RM, et al. Niacin promotes cardiac healing after myocardial infarction through activation of the myeloid prostaglandin D2 receptor subtype 1. J Pharmacol Exp Ther. 2017;360:435–444. doi: 10.1124/jpet.116.238261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tokudome S, Sano M, Shinmura K, Matsuhashi T, Morizane S, Moriyama H, Tamaki K, Hayashida K, Nakanishi H, Yoshikawa N, et al. Glucocorticoid protects rodent hearts from ischemia/reperfusion injury by activating lipocalin-type prostaglandin D synthase-derived PGD2 biosynthesis. J Clin Invest. 2009;119:1477–1488. doi: 10.1172/JCI37413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katsumata Y, Shinmura K, Sugiura Y, Tohyama S, Matsuhashi T, Ito H, Yan X, Ito K, Yuasa S, Ieda M, et al. Endogenous prostaglandin D2 and its metabolites protect the heart against ischemia-reperfusion injury by activating Nrf2. Hypertension. 2014;63:80–87. doi: 10.1161/HYPERTENSIONAHA.113.01639 [DOI] [PubMed] [Google Scholar]
- 41.Torres D, Paget C, Fontaine J, Mallevaey T, Matsuoka T, Maruyama T, Narumiya S, Capron M, Gosset P, Faveeuw C, et al. Prostaglandin D2 inhibits the production of IFN-gamma by invariant NK T cells: consequences in the control of B16 melanoma. J Immunol. 2008;180:783–792. doi: 10.4049/jimmunol.180.2.783 [DOI] [PubMed] [Google Scholar]
- 42.Svensson-Färbom P, Wahlstrand B, Almgren P, Dahlberg J, Fava C, Kjeldsen S, Hedner T, Melander O. A functional variant of the NEDD4L gene is associated with beneficial treatment response with β-blockers and diuretics in hypertensive patients. J Hypertens. 2011;29:388–395. doi: 10.1097/HJH.0b013e3283410390 [DOI] [PubMed] [Google Scholar]
- 43.Goel P, Manning JA, Kumar S. NEDD4–2 (NEDD4L): the ubiquitin ligase for multiple membrane proteins. Gene. 2015;557:1–10. doi: 10.1016/j.gene.2014.11.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shi PP, Cao XR, Sweezer EM, Kinney TS, Williams NR, Husted RF, Nair R, Weiss RM, Williamson RA, Sigmund CD, et al. Salt-sensitive hypertension and cardiac hypertrophy in mice deficient in the ubiquitin ligase Nedd4–2. Am J Physiol Renal Physiol. 2008;295:F462–F470. doi: 10.1152/ajprenal.90300.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Itani OA, Campbell JR, Herrero J, Snyder PM, Thomas CP. Alternate promoters and variable splicing lead to hNedd4–2 isoforms with a C2 domain and varying number of WW domains. Am J Physiol Renal Physiol. 2003;285:F916–F929. doi: 10.1152/ajprenal.00203.2003 [DOI] [PubMed] [Google Scholar]
- 46.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3 [DOI] [PubMed] [Google Scholar]
- 47.Oh S, Hwang ES. The role of protein modifications of T-bet in cytokine production and differentiation of T helper cells. J Immunol Res. 2014;2014:589672. doi: 10.1155/2014/589672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singer CA. T-bet is induced by interferon-γ to mediate chemokine secretion and migration in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L633–L641. doi: 10.1152/ajplung.00163.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jang EJ, Park HR, Hong JH, Hwang ES. Lysine 313 of T-box is crucial for modulation of protein stability, DNA binding, and threonine phosphorylation of T-bet. J Immunol. 2013;190:5764–5770. doi: 10.4049/jimmunol.1203403 [DOI] [PubMed] [Google Scholar]
- 50.Dolfi DV, Mansfield KD, Polley AM, Doyle SA, Freeman GJ, Pircher H, Schmader KE, Wherry EJ. Increased T-bet is associated with senescence of influenza virus-specific CD8 T cells in aged humans. J Leukoc Biol. 2013;93:825–836. doi: 10.1189/jlb.0912438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support these findings of this study are presented in the article and in the online-only Data Supplement, and additional information will be made available from the corresponding author upon reasonable request.