Abstract
Simple Summary
The pathogenesis of H. pylori-associated gastric MALT lymphoma has been well characterized, but the genetic basis and clinical features of H. pylori negative gastric cases remain elusive. In the present study, we investigated the genetic profiles of a large series of H. pylori negative gastric MALT lymphoma by targeted sequencing for a panel of genes specifically designed for marginal zone lymphoma, together with assessment of common translocations and comprehensive clinical data. Targeted sequencing confirmed that NF-κB activation is a major driver in the pathogenesis of H. pylori negative MALT lymphoma, as shown by frequent TNFAIP3 inactivating mutations and also by translocations of MALT1/IGH. This study adds new insights into the genetic background of H. pylori negative MALT lymphoma and will potentially allow us to more specifically target the underlying molecular pathways in future therapeutic concepts.
Abstract
Background: In Western countries, the prevalence of gastric mucosa-associated lymphoid tissue (MALT) lymphoma has declined over the last three decades. Contemporaneously, H. pylori negative gastric MALT lymphoma is increasingly encountered, and their genetic basis and clinical features remain elusive. Methods: A total of 57 cases of H. pylori negative gastric MALT lymphoma were reviewed and investigated for chromosome translocation by fluorescence in-situ hybridization and for somatic mutations by the targeted sequencing of 93 genes. Results: MALT1 translocation, most likely t(11;18)(q21;q21)/BIRC3-MALT1, was detected in 39% (22/57) cases, and IGH translocation was further seen in 12 MALT1-negative cases, together accounting for 60% of the cohort. Targeted sequencing was successful in 35 cases, and showed frequent mutations in NF-κB signaling pathways (TNFAIP3 = 23%, CARD11 = 9%, MAP3K14 = 9%), together affecting 14 cases (40%). The NF-κB pathway mutations were mutually exclusive from MALT1, albeit not IGH translocation, altogether occurring in 86% of cases. There was no significant correlation between the genetic changes and clinicopathological parameters. The patients showed a median of progression-free survival (PFS) of 66.3 months, and a significant superior PFS when treated with systemic versus antibiotic therapy (p = 0.004). Conclusion: H. pylori negative gastric MALT lymphoma is characterized by highly frequent genetic changes in the NF-κB signaling pathways.
Keywords: extranodal lymphoma 1, MALT lymphoma 2, Helicobacter pylori 3, NF-κB pathway 4
1. Introduction
Extranodal mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) is a distinct type of indolent B-cell lymphoma with an age-adjusted incidence rate of 1.1/100.000 [1]. Histologically, the disease is characterized by a heterogeneous small B-cell infiltrate commonly showing lymphoepithelial lesions or follicular colonization and a typical marginal zone B-cell immunophenotype of CD20+CD5-CD10-cyclinD1- +/− light chain restriction [2,3]. While MALT lymphoma may arise in mucosa-associated tissues throughout the entire body, it is most prominent for its gastric manifestation strongly associated with chronic H. pylori gastritis [4,5]. Chronic H. pylori infection results in an active proinflammatory microenvironment, which facilitates clonal B-cell proliferation triggered by H. pylori specific T-cells through CD40-CD40L interaction and persistent cytokine release [3,6]. The causative role of H. pylori infection in gastric MALT lymphoma development was suggested by a high association between the lymphoma and H. pylori infection in epidemiologic studies, subsequently confirmed by a high efficacy of long-term lymphoma remissions in up to 80% of patients following a single course of antibiotics [7,8,9,10]. However, according to recent reports, there is an increasing trend of H. pylori negative gastric MALT lymphomas, accounting for 10–30% of cases [4,11,12,13]. Pathogenesis of these cases is scarcely understood at the moment, with no standard treatment defined so far [4,11,13].
At a molecular level, activation of the nuclear factor (NF) Kappa (κ) B pathway was considered as a central mechanism for MALT lymphoma, irrespective of their origin as shown by frequent genetic aberrations affecting the NF-kB pathway, including t(11;18)(q21;q21)/BIRC3(API2)-MALT1, t(14;18)(q32;q21)/IGH-MALT1, and t(1;14)(p22;q32)/BCL10-IGH [14]. This is further supported by findings of recurrent somatic mutations in the NF-κB/B-cell receptor (BCR) signaling molecules, including TNFAIP3 (A20), CARD11, CD79B, and MYD88. There is growing evidence suggesting site-specific mutation profiles with variable involvement of the same genetic alterations at different anatomic sites [3,14]. For example, ocular adnexal MALT lymphoma features frequent mutations of TNFAIP3 and MYD88, while those from the thyroid commonly showed TNFRSF14 and TET2 mutations [15]. In addition, whole exome sequencing of salivary gland and thyroid MALT lymphoma has revealed novel recurrent somatic mutations in G-protein coupled receptors (GPR34 and CCR6) [16]. Finally, these NF-κB pathway mutations and MALT1 translocations appeared to be enriched in gastric MALT lymphoma unresponsive to H. pylori eradication and were largely mutually exclusive, albeit based on a small series of cases [17].
Previous studies have shown that t(11;18)(q21;q21) and nuclear BCL10 overexpression/translocation are frequent events in H. pylori negative gastric MALT lymphoma, more common than in H. pylori positive cases [13,18]. However, to the best of our knowledge, there are currently no data on the molecular landscape of H. pylori negative gastric MALT lymphoma. In the present study, we investigated the genetic profiles of a large series of H. pylori negative gastric MALT lymphoma by targeted sequencing for a panel of genes (n = 93) specifically designed for marginal zone lymphoma, together with analysis of the MALT lymphoma associated translocations. In addition, we present comprehensive clinical data of 57 patients with H. pylori negative MALT lymphoma, which is the largest series investigated so far.
2. Materials and Methods
Case selection and materials. Formalin-fixed paraffin embedded (FFPE) diagnostic tissue biopsies and clinical data from a total of 57 patients with H. pylori negative gastric MALT lymphoma were identified from five European lymphoma centers (University Hospital Henry Mondor Créteil, Medical University of Vienna, Hospital del Mar Barcelona, University of Pavia, University Hospital Ramón y Cajal Madrid). All diagnoses were histologically verified according to the recent WHO classification of tumors of hematopoietic and lymphoid tissues [2]. FFPE gastric biopsy specimens at the initial diagnosis prior to anti-lymphoma treatment were available in each case. H. pylori negativity was confirmed by the absence of both H. pylori infection and evidence of H. pylori gastritis on histological examination, and a further negative result by polymerase chain reaction (PCR) for H. pylori DNA using high molecular weight DNA samples or a serological test for H. pylori-IgG, a urea breath test or a H. pylori stool antigen test before initiation of anti-lymphoma treatment. In all patients, clinical data were collected from routine medical records, including basic patient characteristics such as age, performance status and sex, stage of disease according to Lugano staging system, and risk profiles based on the MALT-IPI score [19]. In addition, treatment related data were collected, i.e., type of first line treatment; response based on radiological criteria, classified as complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD); and histological criteria (GELA), classified as CR, probable minimal residual disease (pMRD), responding residual disease (rRD), and no change (NC) [20]; as well as long-term outcome to calculate progression-free survival (PFS) and overall survival (OS).The study was performed in accordance with local ethical guidelines for the research use of tissue materials and clinical data. All further tissue-based investigations and data analysis were performed at the Department of Pathology, University of Cambridge, UK.
DNA extraction and quality assessment. Hematoxylin and eosin (H and E) stained slides were reviewed to identify confluent lymphoma area with >30% tumor cells. Lymphoma enriched areas in each specimen were isolated by crude microdissection on consecutive tissue sections. DNA was extracted using the QIAamp DNA Micro Kit (QIAGEN, Crawly, Manchester, UK), quantified with a Qubit® Fluorometer (Life Technologies, ThermoFisher Scientific, Waltham MA, USA) and assessed for quality by PCR of variably sized genomic fragments using a standardized protocol, as previously described [21].
Targeted sequencing using HaloPlexHS enrichment and Illumina platform. This was carried out for a previously published panel of 93 genes, which were specifically designed for marginal zone lymphoma and included those known to be recurrently mutated, and candidate genes based on our ongoing research and literature search [22]. Briefly, this includes genes involved in the NF-κB pathway, BCR signaling, B-cell development, and NOTCH signaling, but also genes involved in DNA damage/apoptosis/repair, epigenetic or translational regulators, and a broad panel of G-protein coupled receptors (GPCR) genes, including chemokine and adhesion receptors. HaloPlexHS target enrichment was performed according to manufacturer’s instructions for FFPE samples. This essentially included digestion of genomic DNA with restriction enzymes, followed by hybridization to the customized HaloPlexHS probes, ligation and circulation with HS DNA ligase, and purification and PCR-based amplification. The amplified library underwent final purification with AMPure XP beads (Beckman Coulter, Pasadena, CA, USA), was quantified using the 4200 TapeStation (Agilent Technologies, Santa Clara, CA, USA), and pooled. Sequencing was performed with the Illumina HiSeq4000 platform according to the manufacturer’s instructions. As in our previous study, DNA samples that were amenable for PCR of ≥400 bp genomic fragment were investigated in a single replicate, while those amplifiable at 300 bp were analyzed in duplicate [21].
Sequence data analysis. These were carried out using protocols and pipelines established in our recent studies, with single nucleotide variants (SNV) called using UnifiedGenotyper and additionally MuTect2 for variants at low variant allele frequency (VAF), while indels were identified using Pindel v0.2.5. [16,21]. Variants were filtered for read depth (excluded if <50), alternative allele depth (AAD) (excluded if <20), VAF (excluded if <2%), and SNPs with a minor allele frequency ≥ 0.1% and benign changes. Finally, the resulting variants were manually checked using the IGV software (Integrative Genomics Viewer). For samples with adequate DNA quality, variants that passed the above established filtering criteria were considered as a true change, while for those investigated in duplicate, only variants detected in both replicates were accepted.
Florescence in situ hybridization (FISH). FISH analysis was used to investigate translocation at the MALT1 and IGH loci. As t(11;18)(q21;q21)/BIRC3 (API2)-MALT1, t(14;18)(q32;q21)/IGH-MALT1 and t(1;14)(p22;q32)/BCL10-IGH are mutually exclusive, all samples were first investigated with a MALT1 break-apart probe, and only negative cases were further analyzed with an IGH break apart probe (Abbott/Vysis, Abbott Park, IL, USA).
Statistical analysis. IBM Statistics for Mac OS Version 26 (IBM, Armonk, NY, USA) was used for statistical analyses of data. Metric data were reported by median, mean, interquartile range (IQR), and absolute numbers for minimum/maximum. Absolute frequencies and corresponding percentages were presented for categorical variables. Qui-squared and Fisher’s exact test were used to test for associations of categorical variables. PFS and OS estimations were plotted with the Kaplan–Meier method and groups compared by log-rank test. Medians and 95% confidence intervals (CI) are reported; p-values < 0.05 were accepted as significant (two-sided).
3. Results
3.1. Clinical Characteristics
Among the 57 patients with H. pylori negative MALT lymphoma investigated in this study, median age at initial diagnosis was 61.8 years (range 36–84; IQR 53.5–72.4), and there were slightly more female patients (53% female versus 47% male). H. pylori negativity was diagnosed by histological assessment in 100% (57/57) of cases and further confirmed by PCR for H. pylori DNA from gastric biopsies in 56% (32/57), serology in 35% (20/57), breath test in 7% (4/57), and stool antigen test in 4% (2/57). Altogether, H. pylori negativity was confirmed by two or more different methods in 91% (52/57) of cases, with the remaining 5 cases showing no evidence of H. pylori/inflammation by histology. According to initial staging, 60% (34/57) of patients presented with localized MALT lymphoma, i.e., Lugano Stage I, 18% (10/57) had local lymph node involvement, i.e., Lugano stage II, and 23% (13/57) had distant lymph node or further extranodal involvement, i.e., Lugano stage IV. Regarding comorbidities, chronic hepatitis B or C virus was detected in two patients (2/57, 4%) and an autoimmune disorder was detected in two patients (2/57, 4%). Prognostic groups per MALT-IPI factors stratified for low risk in 47% (27/57), intermediate risk in 47% (27/57), and high risk in 5% (3/57) of patients. Table 1 shows a detailed list of clinical characteristics.
Table 1.
Clinical features of 57 patients with H. pylori negative gastric MALT lymphoma.
| Clinical Characteristics | Entire Collective (n = 57) | NGS Collective (n = 35/57) |
|---|---|---|
| Sex (female/ male) | 52.6% (30/57)/47.4% (27/57) | 51.4% (18/35)/48.6% (17/35) |
| Age (median) | 61.8 years (range 36–84) | 65.7 years (range 36–80) |
| H. pylori negativity confirmed by | ||
| Histology | 100% (57/57) | 100% (35/35) |
| PCR for H. pylori | 56.1% (32/57) | 48.6% (17/35) |
| Serology | 35.1% (20/57) | 42.9% (15/35) |
| Breath test | 7% (4/57) | 2.9% (1/35) |
| Stool antigen test | 3.5% (2/57) | - |
| Lugano staging system | ||
| Lugano stage I | 59.6% (34/57) | 62.9% (22/35) |
| Lugano stage II | 17.5% (10/57) | 20% (7/35) |
| Lugano stage IV | 22.8% (13/57) | 17.1% (6/35) |
| MALT IPI status | ||
| Low risk | 47.4% (27/57) | 51.4% (18/35) |
| Intermediate risk | 47.4% (27/57) | 45.7% (16/35) |
| High risk | 5.3% (3/57 | 2.9% (1/57) |
| Further clinical features | ||
| Autoimmune disorder | 3.5% (2/57) | 5.7% (2/35) |
| LDH > upper normal limit | 5.3% (3/57) | 5.7% (2/35) |
| Hepatitis B/C virus | 3.5% (2/57) | none |
| Translocation status | ||
| MALT1 rearrangement | 38.6% (22/57) | 31.4% (11/35) |
| IGH rearrangement | 23.5% (12/51) | 28.1% (9/32) |
| First line treatment | ||
| Chemo-/immunotherapy | 77.2% (44/57) | 71.4% (25/35) |
| Antibiotics (=eradication) | 15.8% (9/57) | 22.9% (8/35) |
| Watch and wait | 3.5% (2/57) | 5.7% (2/35) |
| Radiotherapy | 1.8% (1/57) | - |
| Antiviral therapy hepatitis | 1.8% (1/57 | - |
| Median follow-up time (IQR) | 78.4 months (44.9–112) | 66.5 months (23.9–98.3) |
Abbreviations in chronological order: H. = Helicobacter; PCR = polymerase chain reaction; MALT = mucosa-associated lymphoid tissue; NGS = next generation sequencing, IPI = international prognostic index; LDH = lactate dehydrogenase levels, IQR = interquartile range.
3.2. Chromsomal Translocation
All cases were investigated for MALT1 chromosomal translocation by interphase FISH, and a MALT1 rearrangement was detected in 39% (22/57) of cases. The rearrangement is most likely due to t(11;18)(q21;q21)/BIRC3-MALT1 as t(14;18)/IGH-MALT1 is rarely seen in gastric MALT lymphoma [23]. As translocation in MALT lymphoma is mutually exclusive, additional investigations with IGH break apart probes were performed only in MALT1 negative cases, with the exception of 6 cases lacking sufficient material. IGH rearrangement was detected in a further 12 cases, likely due to t(1;14)(p22;q32)/IGH-BCL10 as this is the most recurrent IGH involving translocation seen in gastric MALT lymphoma [14].
3.3. Somatic Mutations Detected in H. pylori Negative MALT Lymphoma
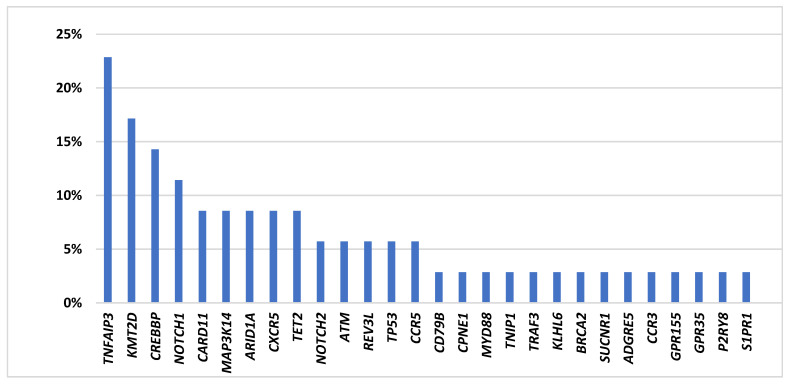
Targeted sequencing results. Of the 57 cases included in the study, 39 had DNA samples meeting the minimal quality requirement for targeted sequencing using HaloPlexHS enrichment. Thirty-five of these cases were successfully investigated, while the remaining four cases failed to yield a sufficient library for sequencing. Among the 35 cases successfully sequenced with the Illumina HiSeq4000 platform, mean coverage was 94% (range 81.3–99.5%), with >90% coverage in 82% (29/35). In total, 67 potentially pathogenic variants were identified in 80% (28/35) of cases (mean number of variants per patient = 1.9; range, 0–6). In 7 cases, no pathogenic mutations were found within the gene panel. Detected variants affected 28 different genes including five genes showing double mutations (Figure 1, Supplementary Table S1).
Figure 1.
Frequencies of mutations detected by targeted sequencing in H. pylori negative gastric MALT lymphoma (n = 35).
TNFAIP3 is the most frequently mutated gene in H. pylori negative gastric MALT lymphoma.TNFAIP3, a negative regulator of NF-κB, was the most frequently affected gene, with mutations detected in 23% (8/35) of cases, including one case carrying two mutations (both frameshift deletions) (Figure 1, Figure 2 and Figure 3). TNFAIP3 mutations included frameshift deletions in four cases, nonsense change in three cases and non-synonymous SNV and frameshift insertion each in one case. Apart from TNFAIP3, mutation was also frequently seen in several other genes that involve NF-κB signaling. These included CARD11 (3/35 = 9%); MAP3K14 (3/35 = 9%); and CD79B, MYD88, TNIP1, CPNE1, and TRAF3 each in one patient (1/35 = 3%), respectively. Together, 40% (14/35) of cases showed one or more mutations in the NF-κB signaling pathways.
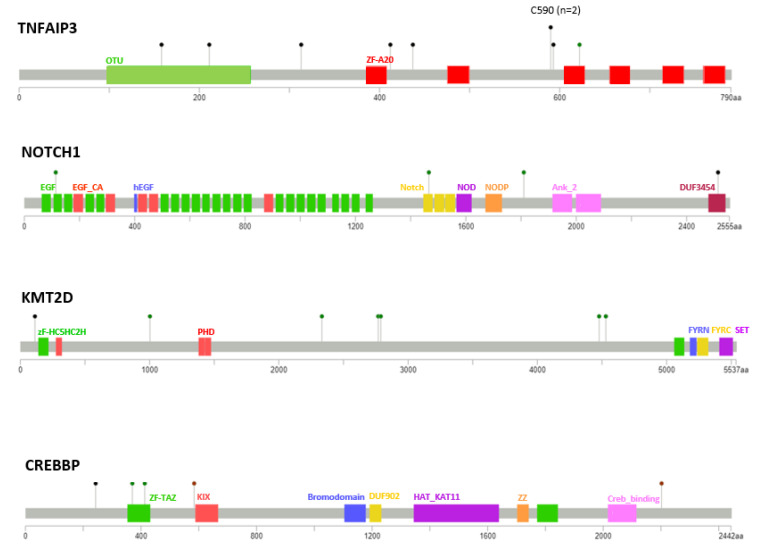
Figure 2.
Distribution and characteristics of TNFAIP3, NOTCH1, KMT2D, and CREBBP mutations seen in H. pylori negative gastric MALT lymphoma. Black dots indicate truncating mutations, green dots missense mutations, and brown dots non-frameshift insertions/deletions. Functional domains are indicated once per gene in the respective color.
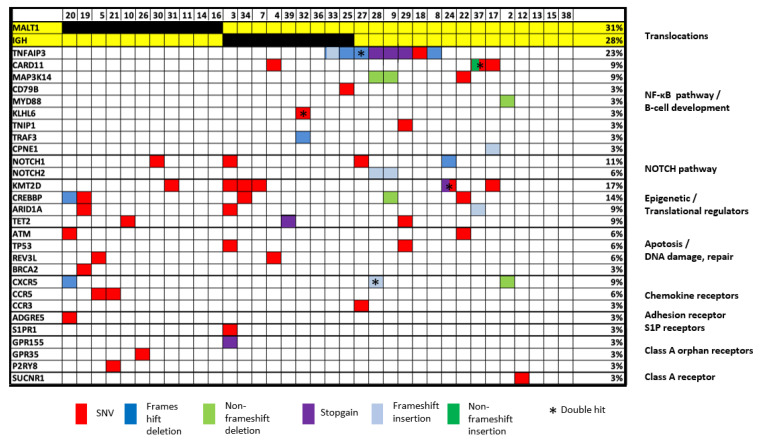
Figure 3.
Heatmap illustration of genetic changes according to molecular pathways. Only cases successfully investigated by targeted sequencing are included in this figure (n = 35).
Previous study of marginal zone lymphoma showed NOTCH signaling alterations, and we detected frequent NOTCH1 (4/35 = 11%) and NOTCH2 mutations (2/35 = 6%). Further pathways frequently altered involved epigenetic/translational regulator genes affecting KMT2D (6/35 = 17%), CREBBP (5/35 = 14%), ARID1A (3/35 = 9%), and TET2 (3/35 = 9%); genes involved in apoptosis/DNA damage including ATM (2/35 = 6%) and TP53 (2/35 = 6%); and chemokine receptor genes including CXCR5 (3/35 = 9%), CCR3 (1/35 = 3%), and CCR5 (2/35 = 6%) (Figure 1, Figure 2 and Figure 3). Most mutations found had been previously described in MALT lymphoma, while the recently detected alterations in G-protein coupled receptors, reported previously by our group, were only detected at low frequency in H. pylori negative gastric MALT lymphoma (GPR 155, n = 1; GPR 35, n = 1) [16].
Correlation among genetic changes. TNFAIP3 mutation was mutually exclusive from MALT1 translocation, and also largely seen in those negative for IGH translocation (Figure 3). In addition, none of the 11 patients with a MALT1 translocation showed any mutation in the NF-κB genes investigated (Figure 3). In contrast, 58% (14/24) of cases lacking MALT1 translocation had one or more mutations in the NF-κB signaling pathways (p = 0.002). Similarly, combined analysis of patients with either MALT1 or IGH rearrangement as one group showed a significant association between MAP3K14 mutation and translocation negative cases (p = 0.04), and also a similar trend for TNFAIP3 mutations (p = 0.07).
Correlation of clinical characteristics and somatic genetic changes. Fisher’s Exact tests for genetic alterations and univariate correlation with predefined clinical factors including disease dissemination status, gender, MALT-IPI scores, LDH, and age (>70 years) were investigated, but there was no apparent association between mutation and clinical characteristics. The only significant correlation was detected for stage of disease and CXCR5, with CXCR5 mutations reported in 3% (1/30) of localized disease versus 40% (2/5) of disseminated disease (p = 0.047). Additionally, a non-significant trend was seen for CREBBP, with CREBBP mutations documented in 10% (3/30) of localized patients versus 40% (2/5) in disseminated patients (p = 0.14). In contrast, TNFAIP3 mutation was exclusively detected in patients with localized disease (8/30, 25%), with no mutation in those with disseminated disease. Nonetheless, this did not reach statistical significance (27% versus 0%, p = 0.32). There was also no correlation between MALT1 and IGH translocation and clinical features, although there was a trend towards a higher frequency of MALT1 translocation in disseminated than localized cases (58% versus 33%, p = 0.18). Finally, we combined all mutations in the NF-κB signaling pathways and correlated with clinical characteristics; again, no significant association was found.
3.4. Response to Treatment and Long-Term Outcome
The median follow-up time for all 57 patients with clinical data available was 78.4 months (IQR 45–112). Regarding the first line treatment, 77% (44/57) of patients received up-front systemic therapy consisting of chemo and/or immunotherapy, 16% (9/57) were treated with H. pylori eradication antibiotics, and one patient each (2%, 1/57) received antiviral treatment for chronic hepatitis and radiotherapy. Rituximab (R) was a part of the systemic treatment in 35 patients (R-chemo-/immunotherapy, n = 22, R-monotherapy, n = 13), and the most frequently applied chemotherapy was chlorambucil +/−R in 20 patients (Table 2). Median time to first line treatment was 4.9 months (IQR 1.2–6.4 months). Two patients with stage I disease (4%, 2/57) had no active therapy but were closely managed by regular surveillance with endoscopies. Overall response (ORR) rate in the actively treated group was 75% (41/55), with 29% (16/41) achieving a CR and 46% (25/55) a PR (including histological responding residual disease and probable minimal residual disease according to GELA criteria), while stable disease was observed in 26% (14/55) and no primary progressive case was reported. The ORR was significantly higher for patients treated with systemic therapy with 84% (37/44) ORR for chemo-/ immunotherapy versus 22% (2/9) ORR in patients receiving antibiotics (p = 0.001). Time to best response was median 6 months (IQR 5.3–11.3 months).
Table 2.
First line treatment and outcome of 57 patients with H. pylori negative MALT lymphoma.
| Treatment/Response | Outcome |
|---|---|
| Entire Collective (n = 57) | |
| Complete remission | 28.1% (16/57) |
| Partial remission | 43.9% (25/57) |
| Stable disease | 28.1% (16/57) |
| PFS (median) months | 66.3 (95%CI 5.4–127.2) |
| Actively treated collective * (n = 55) | |
| Complete remission | 29.1% (16/55) |
| Partial remission | 45.5% (25/55) |
| Stable disease | 25.5% (14/55) |
| PFS (median) months | 66.3 (95%CI 5.4–127.2) |
| Chemo-/immunotherapy (n = 44) ** | |
| Complete remission | 31.8% (14/44) |
| Partial remission | 52.3% (23/44) |
| Stable disease | 15.9% (7/44) |
| PFS (median) months | 82.7 (95%CI 26.5–138.9) |
| Antibiotics (HP eradication) (n = 9) | |
| Complete remission | None |
| Partial remission | 22.2% (2/9) |
| Stable disease | 77.8% (7/9) |
| PFS (median) months | 19.2 (95%CI 6.9–31.5) |
| Watch and wait (n = 2) | |
| Stable disease | 100% (2/2) |
| PFS (absolute) months | 5.1 and 0.9 |
| Radiotherapy (n = 1) | |
| Complete remission | 100% (1/1) |
| PFS (absolute) months | 84.0 |
| Antiviral therapy hepatitis (n = 1) | |
| Complete remission | 100% (1/1) |
| PFS (absolute) months | 105.2 |
* excluding watch and wait, n = 2. ** includes: chlorambucil +/− rituximab (R), n = 20; R-monotherapy, n = 13; lenalidomide +/− R n = 2, R-CHOP, n = 2; R-CVP, n = 2; R-bendamustine, n = 2; ofatumumab, n = 1; R-cyclophosphamide, n = 1; azithromycin, n = 1. Abbreviations in chronological order: H. = Helicobacter; MALT = mucosa-associated lymphoid tissue; PFS = progression-free survival, HP = Helicobacter pylori.
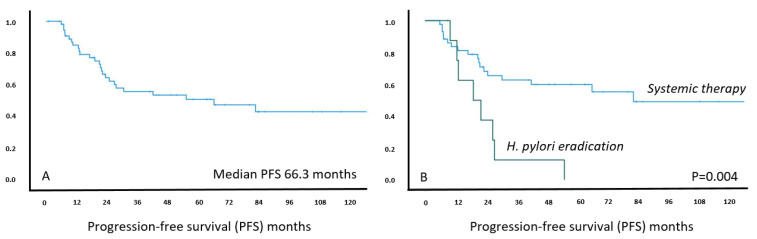
After the first line therapy, 46% (26/57) showed disease progression during follow-up and 40% (23/57) received at least one further treatment. The median PFS for the entire cohort was over five years, being 66.3 months (95%CI 5.4–127.2). The number of cases showing relapses was much lower in the systemic than antibiotics treatment group (43% versus 77%, p = 0.76). In keeping with this, the estimated PFS was significantly longer in patients receiving systemic treatment than those treated with antibiotics (median PFS 82.7 months, 95%CI 26.5–138.9 versus 19.2 months, 95%CI 6.9–31.5) (p = 0.004, Figure 4).
Figure 4.
Kaplan–Meier curve for estimated progression-free survival in H. pylori negative MALT lymphoma patients (n = 57) (left panel A) and treated with systemic treatment versus H. pylori eradication (right panel B), respectively. X-axis: follow-up in months; Y-axis: cumulative progression-free survival.
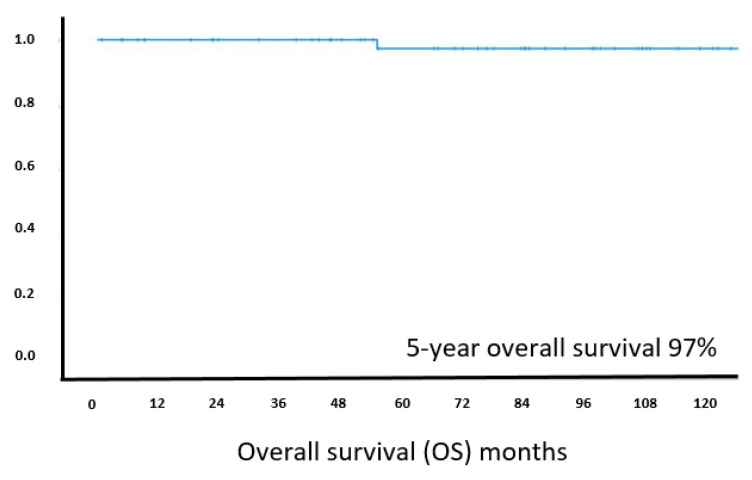
Overall survival was excellent, with 97% of patients reported alive at 5 years follow-up and the median OS clearly not reached (Figure 5). At the last documented follow-up, 68% (39/57) of patients were alive without lymphoma, 30% (17/57) were alive with lymphoma, while one patient had died unrelated to lymphoma. Exploratory analysis of genetic results and long-term outcome revealed that neither a distinct somatic genetic change, nor the combined genetic changes in the NF-κB signaling pathways affect PFS or response to treatment. There was no significant difference in PFS between cases with and without a MALT1 translocation. However, there was a non-significant trend towards a worse PFS in patients with a mutation in KMTD2 in comparison with those without the mutation by log-rank test (p = 0.125) and similarly in patients with a MAP3K14 mutation versus wild type status (p = 0.18).
Figure 5.
Kaplan–Meier curve for estimated overall survival in H. pylori negative MALT lymphoma patients (n = 57). X-axis: follow-up in months, Y-axis: cumulative overall survival.
Finally, we also performed an exploratory analysis of the largest treatment subgroup, i.e., patients receiving systemic therapy, but again no influence of MALT1 translocation or NF-κB signaling mutations regarding ORR or PFS were detected.
4. Discussion
While initially all gastric MALT lymphomas were thought to be associated with H. pylori, there is mounting evidence showing an increase of H. pylori negative gastric MALT lymphoma, reported variably in up to 30% of cases at tertiary referral centers [11,12]. The reason for this remains elusive, but it is of importance to ascertain true H. pylori negativity, as an absence of H. pylori solely based on histological examination does not constitute definitive proof of negativity, because proton pump inhibitor-intake, chronic atrophic gastritis, and intestinal metaplasia may mask occult H. pylori infection [8]. In the current manuscript, we present a large series of well-characterized H. pylori negative MALT lymphomas and their genetic characterization by targeted sequencing of a panel of genes specifically designed for marginal zone lymphoma.
Our study confirmed that the alterations in the NF-κB signaling pathway constitute the central player in MALT lymphoma development, also for H. pylori negative MALT lymphoma [14]. A high proportion (39%) of the H. pylori negative gastric MALT lymphoma had a MALT1 rearrangement, most likely due to t(11;18)(q21;q21)/BIRC3-MALT1. IGH rearrangements, again likely due to t(1;14)(p22;q32)/BLC10-IGH, were documented in 24% of cases investigated. Targeted sequencing revealed mutations in the NF-κB pathway in 40% of cases, exclusively in those lacking MALT1 translocation, with TNFAIP3 mutation being the most frequent change (23%). In addition, potential activating mutations were seen in CARD11, a gene involved in canonical NF-κB activation through formation of the so-called CBM complex (CARD11-BCL10-MALT1), and MAP3K14, which acts via non-canonical NF-κB pathways (both in 9%) [14]. In total, 86% of patients had at least one potentially pathogenic mutation in the NF-κB signaling pathways, including not only canonical but also potentially non-canonical activation [24].
Other molecular pathways affected by mutation include NOTCH signaling (17%), epigenetic and translational regulators (40%), genes involved in apoptosis and DNA damage repair (20%), and chemokine receptors (17%). The frequency of TNFAIP3, TET2, and NOTCH1 mutations in H. pylori negative gastric MALT lymphoma is similar to those seen in unselected gastric MALT lymphomas, whereas the previously reported TNFRSF14 alterations affecting gastric MALT lymphomas were absent in the current series [16]. Interestingly, G-protein coupled receptors, which had previously been detected by our group at high frequency for salivary and thyroid MALT lymphoma, were rare (GPR155, n = 1; GPR 35, n = 1) in H. pylori negative gastric MALT lymphoma, further reinforcing the concept of site-specific mutation profiles as previously suggested [16].
A previous study investigated 19 cases of H. pylori gastric MALT lymphoma unresponsive to antibiotic therapy by sequencing a panel of 425 genes [17]. These authors reported a frequency of TNFAIP3 (16%) and NOTCH1 (16%) mutation, similar to those seen in the present study, and TRAF3 alterations (21%), which were absent in our cases. Similarly to our findings, they reported TNFAIP3 mutations and TRAF3 mutations to be mutually exclusive from MALT1 translocation. In view of this, one might speculate that H. pylori negative MALT lymphoma and H. pylori positive MALT lymphoma that are resistant to eradication may share a common genetic profile.
One limitation of such retrospective analyses is the lack of sufficient material in some cases for targeted sequencing. This is a well-known difficulty in investigating gastric MALT lymphoma with often too little material available due to the endoscopic diagnostic approach and frequently macroscopically occult lesions. Nonetheless, by careful microdissection to enrich tumor cells and assessment of DNA quality, we were able to generate a good quality of sequence data in 61% of cases by targeted sequencing using archival diagnostic tissue specimens with many years of storage [21].
The basic clinical features of our 57 patients correspond well to MALT lymphoma cohorts previously presented in terms of median age, female-to-male ratio, and stage of disease [4]. One feature reported notably low in the current cohort is, however, the presence of autoimmune disorders; while 30–40% of patients had been diagnosed with an additional autoimmune disorder in another series reported in the literature [25], only 2/57 patients had a documented autoimmune condition in our analysis. Whereas the absolute number does not allow for correlation of this feature with somatic mutation data, this suggests that autoimmune disorders might not be involved in H. pylori negative MALT lymphoma.
In terms of treatment-related data including long-term and short-term outcome, the current series is the largest cohort of H. pylori negative gastric MALT lymphoma with stringent diagnostic criteria for H. pylori negativity. There are no established guidelines for treatment of H. pylori negative MALT lymphoma. H. pylori eradication has been shown to be effective at variable frequency; radiotherapy for localized disease or chemo-immunotherapy can be used, with the latter two resulting in high response rates but considerable toxicity in some cases [8,9,26]. In our cohort, 77% of patients received up-front systemic treatment, which was chemotherapy-based in the majority of cases and resulted in an objective response rate of 75% and no primary progressive disease. The PFS of the entire cohort was long, at 66.3 months, but systemic therapy was superior to H. pylori eradication (83 versus 19 months, p = 0.004). Most patients treated with systemic therapy received R +/− chlorambucil, and results were in line with published data for gastric MALT lymphoma; however, no difference in ORR or PFS regarding MALT1 status (as previously suggested) was observed [27,28]. In the literature, responses to H. pylori eradication were reported in up to 30–40% in small series and a recent meta-analysis, and these effects may be partly contributed to the immunomodulatory effects of the macrolide antibiotic clarithromycin commonly used for eradication [29,30,31,32,33]. Effects of antibiotics were particularly observed in patients with localized disease [34,35]. In addition, Helicobacter species other than H. pylori have been suspected to explain the antibiotic treatment responses [36,37]. In the present study, nine H. pylori negative patients were treated with antibiotics (stage I, n = 6; stage II, n = 2; stage IV, n = 1). While the response rate of 22% and the PFS of 19 months for antibiotics only in our cohort appears reasonable, the lower long-term efficacy in comparison with the chemo-/immune collective probably also underlines the “true” negativity of H. pylori in our cases. PFS was comparable if only stage I patients were analyzed (median PFS 22.5 months). One might hypothesize that false negative cases may contribute to eradication responders in some publications, while in our cohort a strict two step algorithm was applied to confirm H. pylori negativity. Nevertheless, none of our patients died due to lymphoma or experienced transformation during follow-up.
5. Conclusions
In the present study, we again confirm that NF-κB activation is a major driver in the pathogenesis of H. pylori negative MALT lymphoma, as shown by highly frequent translocations and TNFAIP3 inactivating mutations. In view of this and the fact that true H. pylori negative MALT lymphoma often appear to be antibiotic non-responders, it would be of interest to further pursue therapeutic strategies of NF-κB inhibition. Inhibition of MALT1 protease activity is a potential treatment approach of interest, and proof of concept data are available for several compounds [38,39,40,41]; however, there are no clinical relevance yet for MALT lymphoma. One already established drug in this context is bortezomib, a proteasome inhibitor dampening NF-κB activities, which resulted in a high response rate of up to 80% for MALT lymphoma but was not further followed due to hemato- and neurotoxicity [42,43]. In view of our data and new advances in development of next generation proteasome inhibitors, further investigation with a focus on H. pylori negative MALT lymphoma might be interesting.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13122993/s1: Table S1: Complete list of mutations detected by next generation sequencing (n = 67).
Author Contributions
Study conception and design: B.K., C.C.-B., M.L. (Michael Levy), A.W., M.R., M.Q.D.; Clinical and laboratory data collection, analysis and histopathological investigations: B.K., C.C.-B., M.L. (Michael Levy), J.D., C.B., L.A., M.P., M.L. (Marco Lucioni), A.B., A.S., C.F.-R., M.A.P., C.R., I.S.-K., A.W., M.R.; Experimental data collection, analysis and interpretation: B.K., F.W., C.B., F.C., R.D., Y.L., Z.C., M.Q.D.; Manuscript drafting and preparation: B.K., F.C., M.R., M.Q.D.; Manuscript com-ment, review/editing, revision and approval of its submission: all authors; Re-search funding: C.C.-B., M.Q.D.; Project coordination: B.K., C.C.-B.; M.R.; M.Q.D. All authors have read and agreed to the published version of the manuscript.
Funding
BK was supported by an EHA Research Mobility Grant awarded by the European Hematology Association. Research in MQD’s lab was supported by grants from Bloodwise (13006, 19010) UK, the Kay Kendall Leukaemia Fund (KKL1141) UK, and also an International Collaborative Award from the Pathological Society of Great Britain and Ireland, UK. In addition, the project was supported by the ARTGIL (Association pour la Recherche Thérapeutique, Génétique et Immunologique dans les hémopathies lymphoïdes). F.W. was supported by a research fellowship from the China Scholarship Council. The Human Research Tissue Bank is supported by the NIHR Cambridge Biomedical Research Centre.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Table S1.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Teras L.R., DeSantis C.E., Cerhan J.R., Morton L.M., Jemal A., Flowers C.R. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J. Clin. 2016;66:443–459. doi: 10.3322/caac.21357. [DOI] [PubMed] [Google Scholar]
- 2.Cook J.R., Chott A., Nakamura S., Müller-Hermelink H.K., Harris N.L., Swerdlow S.H. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) In: Swerdlow S.H., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., editors. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. IARC; Lyon, France: 2017. pp. 259–262. [Google Scholar]
- 3.Troppan K., Wenzl K., Neumeister P., Deutsch A. Molecular Pathogenesis of MALT Lymphoma. Gastroenterol. Res. Pract. 2015;2015:102656. doi: 10.1155/2015/102656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raderer M., Kiesewetter B., Ferreri A.J.M. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) CA Cancer J. Clin. 2016;66:152–171. doi: 10.3322/caac.21330. [DOI] [PubMed] [Google Scholar]
- 5.Wotherspoon A.C., Ortiz-Hidalgo C., Falzon M.R., Isaacson P.G. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–1176. doi: 10.1016/0140-6736(91)92035-Z. [DOI] [PubMed] [Google Scholar]
- 6.Sagaert X., Van Cutsem E., De Hertogh G., Geboes K., Tousseyn T. Gastric MALT lymphoma: A model of chronic inflammation-induced tumor development. Nat. Rev. Gastroenterol. Hepatol. 2010;7:336–346. doi: 10.1038/nrgastro.2010.58. [DOI] [PubMed] [Google Scholar]
- 7.Wündisch T., Thiede C., Morgner A., Dempfle A., Günther A., Liu H., Ye H., Du M.-Q., Kim T.D., Bayerdörffer E., et al. Long-Term Follow-Up of Gastric MALT Lymphoma After Helicobacter Pylori Eradication. J. Clin. Oncol. 2005;23:8018–8024. doi: 10.1200/JCO.2005.02.3903. [DOI] [PubMed] [Google Scholar]
- 8.Ruskoné-Fourmestraux A., Fischbach W., Aleman B.M., Boot H., Du M.Q., Megraud F., Montalban C., Raderer M., Savio A., Wotherspoon A. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747–758. doi: 10.1136/gut.2010.224949. [DOI] [PubMed] [Google Scholar]
- 9.Zucca E., Arcaini L., Buske C., Johnson P.W., Ponzoni M., Raderer M., Ricardi U., Salar A., Stamatopoulos K., Thieblemont C., et al. Marginal zone lymphomas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020;31:17–29. doi: 10.1016/j.annonc.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura S., Sugiyama T., Matsumoto T., Iijima K., Ono S., Tajika M., Tari A., Kitadai Y., Matsumoto H., Nagaya T., et al. Long-term clinical outcome of gastric MALT lymphoma after eradication of Helicobacter pylori: A multicentre cohort follow-up study of 420 patients in Japan. Gut. 2012;61:507. doi: 10.1136/gutjnl-2011-300495. [DOI] [PubMed] [Google Scholar]
- 11.Asano N., Iijima K., Koike T., Imatani A., Shimosegawa T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J. Gastroenterol. 2015;21:8014–8020. doi: 10.3748/wjg.v21.i26.8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiesewetter B., Simonitsch-Klupp I., Dolak W., Mayerhoefer M.E., Raderer M. Depth of Remission Following First-Line Treatment Is an Independent Prognostic Marker for Progression-Free Survival in Gastric Mucosa-Associated Lymphoid Tissue (MALT) Lymphoma. Cancers. 2020;12:492. doi: 10.3390/cancers12020492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamura S., Matsumoto T., Ye H., Nakamura S., Suekane H., Matsumoto H., Yao T., Tsuneyoshi M., Du M.Q., Iida M. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A clinicopathologic and molecular study with reference to antibiotic treatment. Cancer. 2006;107:2770–2778. doi: 10.1002/cncr.22326. [DOI] [PubMed] [Google Scholar]
- 14.Du M.Q. MALT lymphoma: A paradigm of NF-κB dysregulation. Semin. Cancer Biol. 2016;39:49–60. doi: 10.1016/j.semcancer.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Cascione L., Rinaldi A., Bruscaggin A., Tarantelli C., Arribas A.J., Kwee I., Pecciarini L., Mensah A.A., Spina V., Chung E.Y.L., et al. Novel insights into the genetics and epigenetics of MALT lymphoma unveiled by next generation sequencing analyses. Haematologica. 2019;104:e558–e561. doi: 10.3324/haematol.2018.214957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moody S., Thompson J.S., Chuang S.S., Liu H., Raderer M., Vassiliou G., Wlodarska I., Wu F., Cogliatti S., Robson A., et al. Novel GPR34 and CCR6 mutation and distinct genetic profiles in MALT lymphomas of different sites. Haematologica. 2018;103:1329–1336. doi: 10.3324/haematol.2018.191601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hyeon J., Lee B., Shin S.-H., Yoo H.Y., Kim S.J., Kim W.S., Park W.-Y., Ko Y.-H. Targeted deep sequencing of gastric marginal zone lymphoma identified alterations of TRAF3 and TNFAIP3 that were mutually exclusive for MALT1 rearrangement. Mod. Pathol. 2018;31:1418–1428. doi: 10.1038/s41379-018-0064-0. [DOI] [PubMed] [Google Scholar]
- 18.Ye H., Liu H., Raderer M., Chott A., Ruskone-Fourmestraux A., Wotherspoon A., Dyer M.J., Chuang S.S., Dogan A., Isaacson P.G., et al. High incidence of t(11;18)(q21;q21) in Helicobacter pylori-negative gastric MALT lymphoma. Blood. 2003;101:2547–2550. doi: 10.1182/blood-2002-10-3167. [DOI] [PubMed] [Google Scholar]
- 19.Thieblemont C., Cascione L., Conconi A., Kiesewetter B., Raderer M., Gaidano G., Martelli M., Laszlo D., Coiffier B., Lopez Guillermo A., et al. A MALT lymphoma prognostic index. Blood. 2017;130:1409–1417. doi: 10.1182/blood-2017-03-771915. [DOI] [PubMed] [Google Scholar]
- 20.Copie-Bergman C., Wotherspoon A.C., Capella C., Motta T., Pedrinis E., Pileri S.A., Bertoni F., Conconi A., Zucca E., Ponzoni M., et al. Gela histological scoring system for post-treatment biopsies of patients with gastric MALT lymphoma is feasible and reliable in routine practice. Br. J. Haematol. 2013;160:47–52. doi: 10.1111/bjh.12078. [DOI] [PubMed] [Google Scholar]
- 21.Cucco F., Clipson A., Kennedy H., Sneath Thompson J., Wang M., Barrans S., van Hoppe M., Ochoa Ruiz E., Caddy J., Hamid D., et al. Mutation screening using formalin-fixed paraffin-embedded tissues: A stratified approach according to DNA quality. Lab. Investig. 2018;98:1084–1092. doi: 10.1038/s41374-018-0066-z. [DOI] [PubMed] [Google Scholar]
- 22.Wu F., Watanabe N., Tzioni M., Akarca A., Zhang C., Li Y., Chen Z., Cucco F., Carmell N., Noh J.Y., et al. Thyroid MALT lymphoma: Self-harm to gain potential T-cell help. Leukemia. 2021 doi: 10.1038/s41375-021-01289-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye H., Liu H., Attygalle A., Wotherspoon A.C., Nicholson A.G., Charlotte F., Leblond V., Speight P., Goodlad J., Lavergne-Slove A., et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: Significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–1018. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- 24.Rosebeck S., Madden L., Jin X., Gu S., Apel I.J., Appert A., Hamoudi R.A., Noels H., Sagaert X., Van Loo P., et al. Cleavage of NIK by the API2-MALT1 fusion oncoprotein leads to noncanonical NF-kappaB activation. Science. 2011;331:468–472. doi: 10.1126/science.1198946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wöhrer S., Troch M., Streubel B., Zwerina J., Skrabs C., Formanek M., Hauff W., Hoffmann M., Müllauer L., Chott A., et al. MALT lymphoma in patients with autoimmune diseases: A comparative analysis of characteristics and clinical course. Leukemia. 2007;21:1812–1818. doi: 10.1038/sj.leu.2404782. [DOI] [PubMed] [Google Scholar]
- 26.Teckie S., Qi S., Lovie S., Navarrett S., Hsu M., Noy A., Portlock C., Yahalom J. Long-term outcomes and patterns of relapse of early-stage extranodal marginal zone lymphoma treated with radiation therapy with curative intent. Int. J. Radiat. Oncol. Biol. Phys. 2015;92:130–137. doi: 10.1016/j.ijrobp.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 27.Amiot A., Lévy M., Copie-Bergman C., Dupuis J., Szablewski V., Le Baleur Y., Baia M., Belhadj K., Sobhani I., Leroy K., et al. Rituximab, alkylating agents or combination therapy for gastric mucosa-associated lymphoid tissue lymphoma: A monocentric non-randomised observational study. Aliment. Pharm. 2014;39:619–628. doi: 10.1111/apt.12635. [DOI] [PubMed] [Google Scholar]
- 28.Lévy M., Copie-Bergman C., Gameiro C., Chaumette M.-T., Delfau-Larue M.-H., Haioun C., Charachon A., Hemery F., Gaulard P., Leroy K., et al. Prognostic Value of Translocation t(11;18) in Tumoral Response of Low-Grade Gastric Lymphoma of Mucosa-Associated Lymphoid Tissue Type to Oral Chemotherapy. J. Clin. Oncol. 2005;23:5061–5066. doi: 10.1200/JCO.2005.05.660. [DOI] [PubMed] [Google Scholar]
- 29.Jung K., Kim D.H., Seo H.I., Gong E.J., Bang C.S. Efficacy of eradication therapy in Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphoma: A meta-analysis. Helicobacter. 2021;26:e12774. doi: 10.1111/hel.12774. [DOI] [PubMed] [Google Scholar]
- 30.Raderer M., Streubel B., Wöhrer S., Häfner M., Chott A. Successful antibiotic treatment of Helicobacter pylori negative gastric mucosa associated lymphoid tissue lymphomas. Gut. 2006;55:616–618. doi: 10.1136/gut.2005.083022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zullo A., Hassan C., Ridola L., De Francesco V., Rossi L., Tomao S., Vaira D., Genta R.M. Eradication Therapy in Helicobacter pylori-negative, Gastric Low-grade Mucosa–associated Lymphoid Tissue Lymphoma Patients: A Systematic Review. J. Clin. Gastroenterol. 2013;47:824–827. doi: 10.1097/MCG.0b013e318286ff72. [DOI] [PubMed] [Google Scholar]
- 32.Kiesewetter B., Raderer M. Immunomodulatory treatment for mucosa-associated lymphoid tissue lymphoma (MALT lymphoma) Hematol. Oncol. 2020;38:417–424. doi: 10.1002/hon.2754. [DOI] [PubMed] [Google Scholar]
- 33.Ferreri A.J.M., Cecchetti C., Kiesewetter B., Sassone M., Calimeri T., Perrone S., Ponzoni M., Raderer M. Clarithromycin as a “repurposing drug” against MALT lymphoma. Br. J. Haematol. 2018;182:913–915. doi: 10.1111/bjh.14878. [DOI] [PubMed] [Google Scholar]
- 34.Raderer M., Wöhrer S., Kiesewetter B., Dolak W., Lagler H., Wotherspoon A., Muellauer L., Chott A. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: A single center experience with prolonged follow-up. Ann. Hematol. 2015;94:969–973. doi: 10.1007/s00277-014-2298-3. [DOI] [PubMed] [Google Scholar]
- 35.Kuo S.H., Yeh K.H., Wu M.S., Lin C.W., Wei M.F., Liou J.M., Wang H.P., Chen L.T., Cheng A.L. First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma. Sci. Rep. 2017;7:14333. doi: 10.1038/s41598-017-14102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura M., Øverby A., Michimae H., Matsui H., Takahashi S., Mabe K., Shimoyama T., Sasaki M., Terao S., Kamada T., et al. PCR analysis and specific immunohistochemistry revealing a high prevalence of non-Helicobacter pylori Helicobacters in Helicobacter pylori-negative gastric disease patients in Japan: High susceptibility to an Hp eradication regimen. Helicobacter. 2020;25:e12700. doi: 10.1111/hel.12700. [DOI] [PubMed] [Google Scholar]
- 37.Takigawa H., Yuge R., Masaki S., Otani R., Kadota H., Naito T., Hayashi R., Urabe Y., Oka S., Tanaka S., et al. Involvement of non-Helicobacter pylori helicobacter infections in Helicobacter pylori-negative gastric MALT lymphoma pathogenesis and efficacy of eradication therapy. Gastric Cancer. 2021 doi: 10.1007/s10120-021-01172-x. [DOI] [PubMed] [Google Scholar]
- 38.Fontán L., Melnick A. Molecular pathways: Targeting MALT1 paracaspase activity in lymphoma. Clin. Cancer Res. 2013;19:6662–6668. doi: 10.1158/1078-0432.CCR-12-3869. [DOI] [PubMed] [Google Scholar]
- 39.Fontan L., Yang C., Kabaleeswaran V., Volpon L., Osborne M.J., Beltran E., Garcia M., Cerchietti L., Shaknovich R., Yang S.N., et al. MALT1 small molecule inhibitors specifically suppress ABC-DLBCL in vitro and in vivo. Cancer Cell. 2012;22:812–824. doi: 10.1016/j.ccr.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pissot Soldermann C., Simic O., Renatus M., Erbel P., Melkko S., Wartmann M., Bigaud M., Weiss A., McSheehy P., Endres R., et al. Discovery of Potent, Highly Selective, and In Vivo Efficacious, Allosteric MALT1 Inhibitors by Iterative Scaffold Morphing. J. Med. Chem. 2020;63:14576–14593. doi: 10.1021/acs.jmedchem.0c01245. [DOI] [PubMed] [Google Scholar]
- 41.Stephenson R., Singh A. Drug discovery and therapeutic delivery for the treatment of B and T cell tumors. Adv. Drug Deliv. Rev. 2017;114:285–300. doi: 10.1016/j.addr.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conconi A., Martinelli G., Lopez-Guillermo A., Zinzani P.L., Ferreri A.J.M., Rigacci L., Devizzi L., Vitolo U., Luminari S., Cavalli F., et al. Clinical activity of bortezomib in relapsed/refractory MALT lymphomas: Results of a phase II study of the International Extranodal Lymphoma Study Group (IELSG) Ann. Oncol. 2011;22:689–695. doi: 10.1093/annonc/mdq416. [DOI] [PubMed] [Google Scholar]
- 43.Troch M., Jonak C., Müllauer L., Püspök A., Formanek M., Hauff W., Zielinski C.C., Chott A., Raderer M. A phase II study of bortezomib in patients with MALT lymphoma. Haematologica. 2009;94:738–742. doi: 10.3324/haematol.2008.001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Table S1.