Abstract
High homocysteine (Hcy) levels, mainly caused by vitamin B12 deficiency, have been reported to induce amyloid-β (Aβ) formation and tau hyperphosphorylation in mouse models of Alzheimer’s disease. However, the relationship between B12 deficiency and Aβ aggregation is poorly understood, as is the associated mechanism. In the current study, we used the transgenic C. elegans strain GMC101, which expresses human Aβ1–42 peptides in muscle cells, to investigate the effects of B12 deficiency on Aβ aggregation–associated paralysis. C. elegans GMC101 was grown on nematode growth medium with or without B12 supplementation or with 2-O-α-D-glucopyranosyl-L-ascorbic acid (AsA-2G) supplementation. The worms were age-synchronized by hypochlorite bleaching and incubated at 20 °C. After the worms reached the young adult stage, the temperature was increased to 25 °C to induce Aβ production. Worms lacking B12 supplementation exhibited paralysis faster and more severely than those that received it. Furthermore, supplementing B12-deficient growth medium with AsA-2G rescued the paralysis phenotype. However, AsA-2G had no effect on the aggregation of Aβ peptides. Our results indicated that B12 supplementation lowered Hcy levels and alleviated Aβ toxicity, suggesting that oxidative stress caused by elevated Hcy levels is an important factor in Aβ toxicity.
Keywords: Alzheimer’s disease, amyloid-β, Caenorhabditis elegans, oxidative stress, reactive oxygen species, vitamin B12
1. Introduction
B12 is a water-soluble vitamin that is well known for its complex chemical structure. The B12 molecule is composed of a corrin ring with a central cobalt atom linked to dimethylbenzimidazole. B12 exists in four different chemical forms, depending on the chemical group bound to the cobalt atom. Most commercially available B12 supplements contain a cyano group and are known as cyanocobalamin. In biological systems, the cyano group can be replaced with adenosyl, or methyl group to form the biologically active form adenosylcobalamin (AdoCbl), or methylcobalamin (MeCbl), respectively [1]. Humans mainly acquire B12 by consuming meat, dairy products, fish, and shellfish, as plants and mushrooms do not contain substantial amounts [2].
B12-protein complexes in food must be digested by pepsin in the stomach to enable absorption. Free B12 is transported by three different B12-binding proteins, haptocorrin, intrinsic factor, and transcobalamin, before being absorbed in the small intestine. Elderly people tend to have a lower rate of B12 absorption due to a high prevalence of atrophic gastritis [3], which decreases the production of pepsin and intrinsic factor, resulting in B12 malabsorption. Therefore, vegetarians and the elderly are the most prone to B12 deficiency.
B12 plays an important role in the production of red blood cells, synthesis of DNA, and protection of the nervous system [1,4]. The active forms, AdoCbl and MeCbl, function as cofactors for the enzymes methylmalonyl-CoA mutase (MCM, EC 5.4.99.2) and methionine synthase (MS, EC 2.1.1.13), respectively. MCM catalyzes the isomerization of L-methylmalonyl-CoA to succinyl-CoA, whereas MS catalyzes the synthesis of methionine from homocysteine (Hcy) [4]. B12 deficiency disrupts the homeostasis of propionate catabolism and the S-adenosyl methionine cycle, leading to the accumulation of Hcy and methylmalonic acid (MMA), a decrease in reduced glutathione levels, and a decrease in immune response control, resulting in oxidative stress [5]. Oxidative stress occurs when there is an imbalance between the production of reactive oxygen species (ROS) and the availability of antioxidants in the body. Furthermore, excessive production of ROS leads to the degradation of macromolecules, resulting in cellular damage that triggers various diseases such as diabetes, cardiovascular diseases, carcinogenesis, and neurodegeneration [5].
Alzheimer’s disease (AD) is the most common cause of dementia, accounting for 60–80% of all cases, and has been estimated to affect approximately 10–30% of people over 65 years old [6,7]. AD is characterized by the extracellular aggregation of Aβ and the accumulation of hyperphosphorylated tau protein in neurons (neurofibrillary tangles) [7]. Aβ peptide found in the brains of patients with AD is generated after consecutive cleavages of amyloid precursor protein (APP) by β- and γ-secretases [8]. Although AD has been studied for more than a century, owing to its complex mechanism, many clinical trials conducted to discover effective therapeutic strategies against AD have failed [9]. Current treatments for AD only treat the symptoms and are unable to prevent progression of the disease [7].
B12 deficiency has been proposed as a risk factor for AD [1]. In addition, elevated plasma Hcy levels, mainly caused by B12 deficiency, have been observed in elderly individuals with mild cognitive impairment [1]. Furthermore, a study using transgenic mouse models of AD reported that hyperhomocysteinemia causes memory deficits, increases Aβ peptide accumulation, and increases tau phosphorylation—the three major pathological features of AD [10]. However, whether a high plasma Hcy level is the primary factor in AD pathology in humans or merely a marker for another underlying condition, such as low folate level, poor lifestyle, or renal failure, is still under debate [11]. In addition, other consequences of B12 deficiency, such as mitochondrial dysfunction and oxidative stress [5], must also be considered to understand the relationship between high plasma Hcy levels and the mechanism of AD.
Caenorhabditis elegans is a powerful genetic model organism that was introduced by Sydney Brenner [12]. This free-living roundworm has been widely used as an animal model in various fields of study, including neurodegenerative disease research. In our previous study, we successfully induced B12 deficiency in C. elegans by growing them in M9 minimal media with Escherichia coli OP50 as a food source [13]. Worms fed a strict B12-restricted diet exhibited elevated Hcy and MMA levels, fertility loss, extended life cycles, and reduced lifespans. In addition, C. elegans grown on nematode growth medium (NGM) with E. coli OP50 as a food source have also been reported to exhibit B12 deficiency [14].
Transgenic C. elegans worms expressing Aβ peptides have been engineered to mimic the pathological features of AD [15]. Multiple strains of transgenic worms have been developed to produce Aβ peptides. In the C. elegans strain, GMC101, the production of Aβ1–42 peptides in muscle cells is induced by temperature upshift [16]. In the current study, we used the GMC101 strain to investigate the effects of B12 deficiency on Aβ peptide toxicity and to gain a better understanding of the relationship between B12 deficiency and AD.
2. Materials and Methods
2.1. C. elegans Strains
Pacdh-1::GFP (VL749) transgenic worms were used as reporters of dietary B12 status [14]. The VL749 and GMC101 strains used in the current study were provided by the Caenorhabditis Genetic Center (CGC), funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). The worms were maintained at 20 °C.
2.2. Preparation of B12-Deficient Worms
Worms grown on NGM (1 mmol/L MgSO4, 1 mmol/L CaCl2, 5 mg/L cholesterol, 25 mmol/L KPO4 (pH 6), 17 g/L agar, 3 g/L NaCl, and 2.5 g/L bacto-peptone) without cyanocobalamin were considered B12 deficient. Worms administered B12 supplementation were grown on NGM with 100 μg/L cyanocobalamin.
2.3. Fluorescence Imaging of Pacdh: GFP Transgenic Worms
Worms were age-synchronized by hypochlorite bleaching and grown at 20 °C on NGM with or without B12 supplementation until they reached the young adult stage. The worms were then mounted on 5% agarose gel, and fluorescence images were captured using an SZX-RFL-2 fluorescence microscope (Olympus Co., Tokyo, Japan).
2.4. Measurement of B12-Related Biomarkers Using Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
The N2 Bristol C. worms were age-synchronized by hypochlorite bleaching and grown at 20 °C on NGM with or without B12 supplementation until they reached the young adult stage. Then, the worms were collected from the plates and washed using M9 buffer. The worm pellets were then homogenized, and the supernatant was used for the measurement of Hcy and MMA levels via LC-MS/MS according to the methods described by Weaving et al. [17] and Mineva et al. [18], respectively.
2.5. Paralysis Assay
The paralysis assay was performed using GMC101 transgenic worms expressing Aβ peptide according to the method described by McColl et al. [16]. Briefly, C. elegans GMC101 was grown on NGM with or without B12 supplementation or with AsA-2G supplementation. The worms were age-synchronized by hypochlorite bleaching and incubated at 20 °C. After the worms reached the young adult stage, the temperature was increased to 25 °C to induce Aβ production. The number of paralyzed worms was counted at 12 h intervals. Worms unable to perform full-body wave propagation were scored as paralyzed.
2.6. Quantitative Reverse Transcription PCR (qRT-PCR)
GMC101 worms were age-synchronized by hypochlorite bleaching and grown at 20 °C on NGM with or without B12 supplementation until they reached the young adult stage. Worms were then transferred to NGM plates containing 75 μmol/L 5-fluoro-2′-deoxyuridine, and the temperature was increased to 25 °C to induce Aβ peptide expression. After 24 h, worms were collected, snap-frozen in liquid nitrogen, and stored at -80 °C until RNA extraction was performed using Sephasol®-RNA1 (Nacalai Tesque, Kyoto, Japan). Total RNA was used for cDNA synthesis using the PrimeScript™ RT Reagent Kit with gDNA Eraser (Takara Bio, Shiga, Japan). The following primers were used for the amplification: actin-1 (T04C12.6) (F) 5′-TCCAAGAGAGGTATCCTTACCC-3′ and (R) 5′-CTCCATATCATCCCAGTTGGTG-3′; Aβ (F) 5′-GCGGATGCAGAATTCCGACATGAC-3′ and (R) 5′-TATGACAACACCGCCCACCATGAG-3′. qPCR was performed on a CFX Connect Real-Time System (Bio-Rad Laboratories, Inc., Berkeley, CA, USA) using GeneAce SYBR® qPCR Mix α (Nippon Gene Co., Ltd., Tokyo, Japan). The mRNA level of Aβ was normalized to that of actin-1.
2.7. In Situ Detection of Intracellular ROS
Intracellular ROS production was detected via 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) assay, as previously described [19]. Briefly, N2 worms were age-synchronized by hypochlorite bleaching and grown at 20 °C on NGM with or without B12 supplementation until they reached the young adult stage. The worms were then collected from the plates and washed with M9 buffer. DCFH-DA was dissolved in dimethyl sulfoxide, diluted to a final concentration of 100 μM using sterilized M9 medium, and used as a staining solution. Worms grown with or without B12 supplementation (labeled B12+ and B12−, respectively; approximately 12 worms per group) were treated with 1 mL of the staining solution for 5 h in the dark. Following the staining, the worms were washed 10 times with sterile water and mounted on 5% agarose gel. Fluorescence images were captured using the BZ-9000 series HS All-in-One fluorescence microscope with an FITC filter (Keyence, Osaka, Japan). The anterior part of the worms was observed. Exposure time was 666 ms. Fluorescence intensity was quantified using ImageJ (ImageJ Software, Bethesda, MD, USA, http://imagej.nih.gov/ij/, accessed on 18 May 2021) for 8 worms per treatment condition.
2.8. Immunoblot Analysis
Immunoblot analysis was performed as previously described [20], with a few modifications. GMC101 worms were weighed and homogenized in 500 µL of phosphate-buffered saline. The homogenates were centrifuged at 800× g for 10 min, and the supernatants were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) and immunoblotting. SDS–PAGE was performed using p-PAGEL slab gels (P–T16.5S; ATTO Corporation, Tokyo, Japan) according to the manufacturer’s instructions.
The gels were then stained with Coomassie Brilliant Blue R-250, and proteins were transferred to polyvinylidene difluoride membranes (Immuno-Blot PVDF; Bio-Rad Laboratories, Hercules, CA, USA) using an electroblotting apparatus (model 200/2.0, Bio-Rad Laboratories) set at 13 V for 30 min. Aβ peptide was detected using a monoclonal anti-Aβ1–16 primary antibody (BioLegend, San Diego, CA, USA) and an anti-mouse IgG–horseradish peroxidase conjugate (Promega Corp. Madison, WI, USA) secondary antibody. Tubulin (the loading control) was detected using a monoclonal anti-tubulin primary antibody (ab6160, Abcam, Cambridge, MA, USA) and an anti-mouse IgG–horseradish peroxidase conjugate (SA00001-15, Proteintech Japan, Tokyo, Japan) secondary antibody. Signals were detected using EzWestBlue (ATTO Corporation) according to the manufacturer’s instructions.
2.9. Statistical Analyses
Statistical differences were assessed using the two-tailed Student’s t-test with parametric unequal variance in Microsoft Excel 2013 (Microsoft, Redmond, WA, USA). Data in graphs are presented as the mean ± standard deviation (SD). Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Worms Grown on NGM without B12 Supplementation Exhibited B12 Deficiency
Although it was possible to induce severe B12 deficiency in worms by growing them on M9 medium without B12 supplementation for five generations, the limited amount of nutrition available in M9 medium may have inhibited Aβ expression. Furthermore, Revtovich et al. [21] reported that worms grown on M9 medium for five generations have lower fecundity, suggesting that M9 medium has insufficient nutrition to support the development of worms. Therefore, since worms grown on NGM with E. coli OP50 as a food source have been reported to exhibit B12 deficiency [14,21], we used NGM with or without B12 supplementation for the paralysis assay.
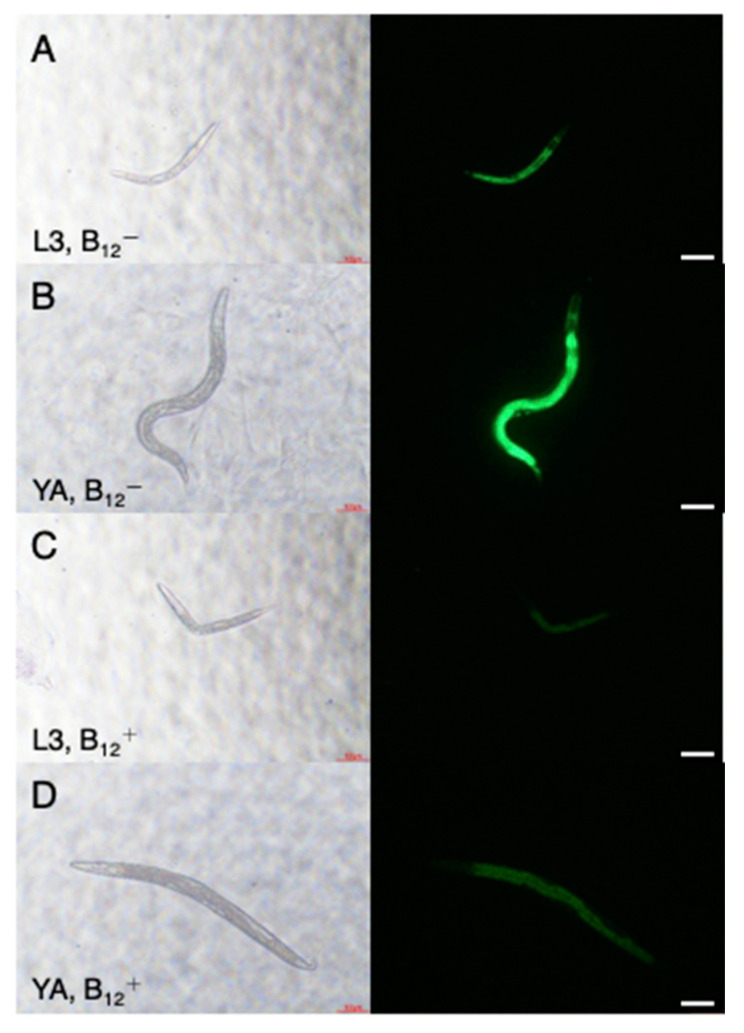
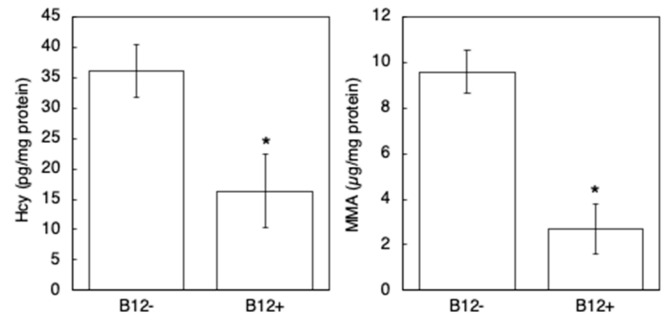
Before performing paralysis assay in this culture condition, first, fluorescence images of Pacdh-1::GFP (VL749) worms were taken to observe dietary B12 status of worms cultivated on NGM with or without B12 supplementation (B12+ worms or B12− worms). The B12− VL749 worms exhibited GFP expression, which increased as the worms developed (Figure 1A,B), whereas no GFP expression was observed in the B12+ VL749 worms (Figure 1C,D). Furthermore, to investigate the degree of B12 deficiency in the B12− worms, Hcy and MMA levels were measured using LC-MS/MS. The results showed a significant accumulation of Hcy and MMA in B12− worms compared to that in B12+ worms (Figure 2); the Hcy and MMA levels in the B12− worms were 2.2- and 3.6-fold higher, respectively, than those in the B12+ worms. These findings clearly indicated the B12 deficiency in the B12− worms.
Figure 1.
Representative fluorescence images of L3 and young adult VL749 B12− and B12+ worms. (A), L3 B12− larva; (B), young adult B12− worm; (C), L3 B12+ larva; (D), young adult B12+ worm. Worms grown with B12 supplementation, B12+; without B12 supplementation, B12−. Scale bars = 100 μm.
Figure 2.
Hcy and MMA levels in B12− and B12+ N2 worms. Hcy and MMA levels were measured as described in the Materials and Methods section. All values represent the mean ± SD of three independent experiments (n = 3). Asterisks indicate significant differences compared to the B12− worms (* p < 0.05).
3.2. B12 Supplementation Reduced Aβ Toxicity in B12-Deficient GMC101 Worms
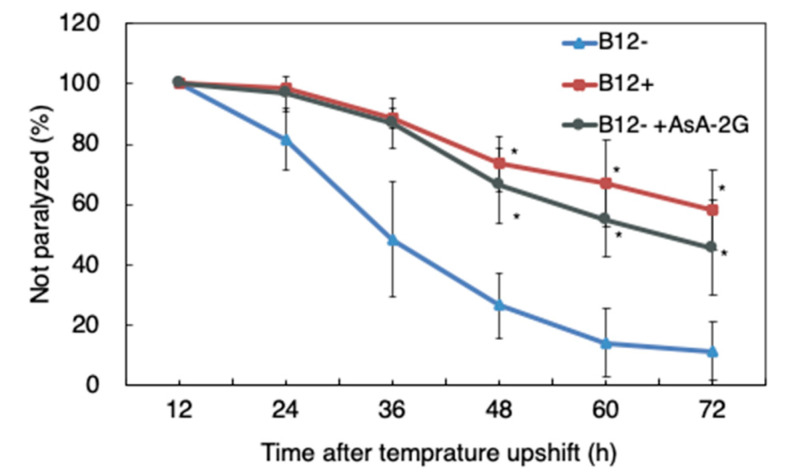
To evaluate the effects of B12 deficiency on the nervous system, we performed a paralysis assay using GMC101 worms grown on NGM with or without B12 supplementation. After 48 h of incubation, approximately 74% of the B12− GMC101 worms were paralyzed, whereas after 72 h, more than 90% of the worms were paralyzed (Figure 3). In contrast, only 26% of the B12+ GMC101 worms were paralyzed after 48 h. The paralysis rate was found to be significantly different between the B12+ and B12− GMC101 worms 48 h after the temperature upshift.
Figure 3.
Paralysis rate of B12−, B12+, and B12− +AsA-2G GMC101 worms. Young adult worms were incubated at 25 °C to induce Aβ production. The mean percentage of unparalyzed worms is plotted against the time post temperature shift (h). All values represent the mean ± SD of three independent experiments (n = 3). Approximately 270 worms were screened for each condition. Asterisks indicate significant differences compared to the B12− worms at same time point (* p < 0.05).
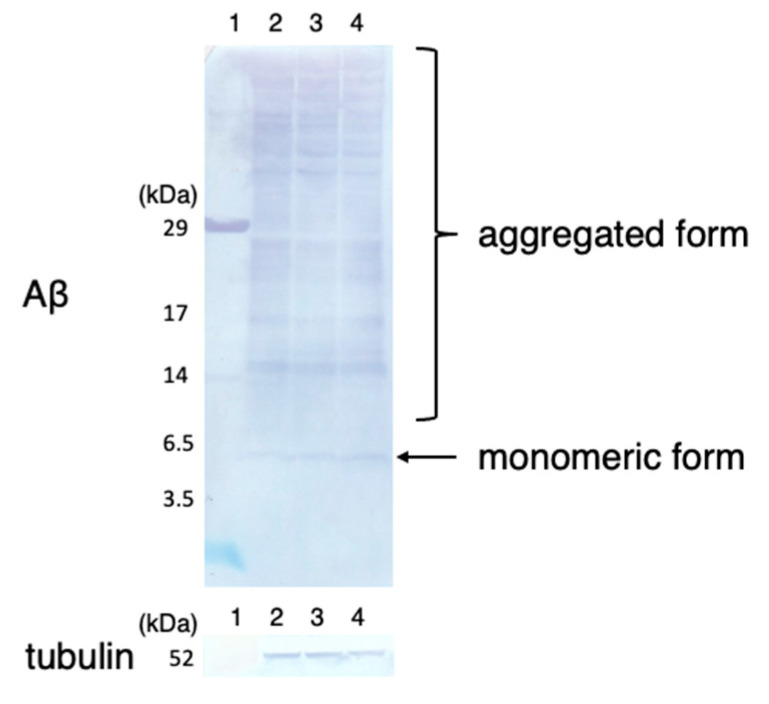
To investigate if B12 supplementation affects the transcription level of Aβ rather than simply reducing its accumulation in muscle cells, qRT-PCR of the B12+ and B12− GMC101 worms was performed 24 h after inducing Aβ expression. The results indicated no significant difference in the Aβ mRNA levels between the B12+ and B12− GMC101 worms (Figure 4). Additionally, immunoblotting indicated no significant difference in the level of the monomeric form and aggregated form of the Aβ peptide (Aβ1–42) between the B12− and B12+ GMC101 worms (Figure 5).
Figure 4.
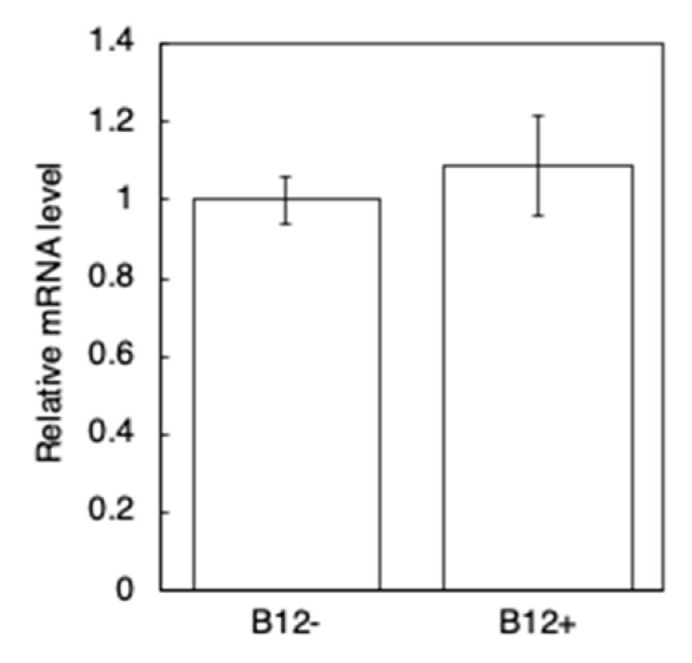
Aβ mRNA levels in B12−, and B12+ GMC101 worms. The relative expression of Aβ mRNA was normalized to that of actin-1. The expression value for B12− worms was set to 1. The data represent the mean ± SD of three independent experiments (n = 3).
Figure 5.
Accumulation of Aβ peptides in B12−, B12+, and B12− +AsA-2G GMC101 worms. The crude extract from B12−, B12+, or B12− +AsA-2G worms (40 µg each) was subjected to SDS-PAGE and immunoblotting using antibodies against anti-β-amyloid(1–16) and anti-tubulin antibodies. Lane 1, molecular marker; lane 2, B12+ worms; lane 3, B12− worms; lane 4, B12− +AsA-2G worms.
3.3. NGM Supplemented with AsA-2G Ameliorated Aβ Toxicity in B12-Deficient GMC101 Worms
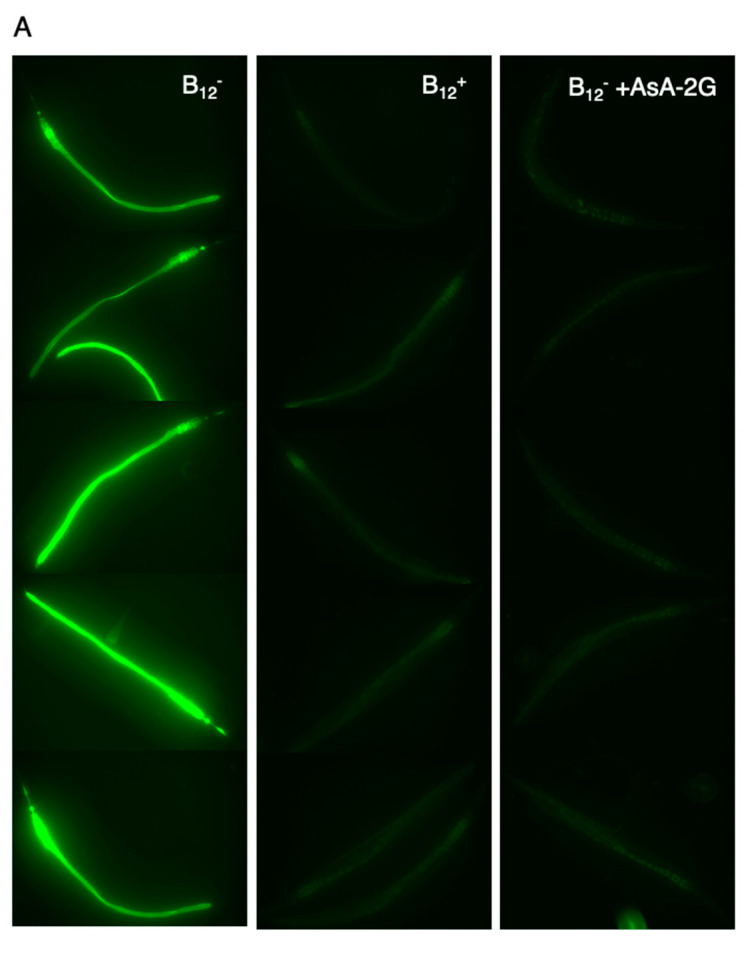
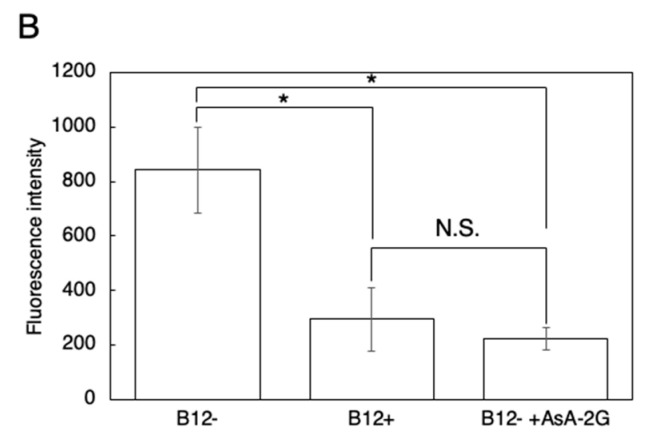
Oxidative stress induced by B12 deficiency may have enhanced the paralysis rate of B12− GMC101 worms [22]. Therefore, we determined the amount of ROS accumulation in B12+ and B12− worms using DCFH-DA, an indicator of ROS. We observed a high level of ROS accumulation in the B12− worms compared to that in B12+ worms (Figure 6), which supported the findings from a recent study [22].
Figure 6.
ROS levels in B12−, B12+, and B12− +AsA-2G N2 worms. (A). Fluorescence images of young adult N2 B12+ or B12− worms. Reactive oxygen species (ROS) accumulation in the worms was assessed via assay of the ROS indicator 2′,7′-dichlorodihydrofluorescein diacetate. (B). Fluorescence intensity. All values represent the mean ± SD of eight worms per treatment condition (n = 8). Asterisks indicate significant differences (* p < 0.05). N.S., not significant.
To investigate the effect of reduced ROS levels in B12− GMC101 worms on paralysis and aggregation of the Aβ peptide, the B12− worms were supplemented with AsA-2G, a stable ascorbic acid. Supplementation with AsA-2G reduced the amount of ROS accumulation in B12− worms to the same level as in B12+ worms (Figure 6). Interestingly, AsA-2G supplementation improved the paralysis phenotype in the B12− GMC101 worms (B12− +AsA-2G) (Figure 3). However, AsA-2G supplementation did not affect to aggregation of the Aβ peptide (Figure 5).
4. Discussion
AD is a progressive neurodegenerative disorder characterized by memory impairment, disorientation, behavioral changes, and impaired communication [6]. A high Hcy level, caused by low B12 or folate levels, is known to be one of the risk factors for AD [11]. In a clinical trial conducted on healthy elderly individuals with high Hcy levels, folate supplementation over three years improved global functioning, memory storage, and information-processing speed [23], suggesting that AD may be prevented by lowering Hcy levels. In the current study, we used a transgenic C. elegans model expressing the Aβ1–42 peptide in muscle cells to investigate the effect of B12 deficiency on Aβ toxicity. Our results showed that the level of Hcy and MMA accumulation was higher in B12− worms than in B12+ worms (Figure 2). In addition, B12− GMC101 worms exhibited a higher paralysis rate (Figure 3) and higher ROS levels (Figure 6) than B12+ worms.
In addition to its primary function as a cofactor, B12 is known to possess antioxidant properties. Chan et al. [24] demonstrated that B12 alleviates cellular oxidative stress by directly scavenging superoxide, in vitro and in vivo. Additionally, the protective properties of B12 include the preservation of glutathione and modulation of cytokines and growth factors that induce immune response–mediated oxidative stress [5]. Furthermore, Hcy is known to produce ROS by autoxidation [25] and activation of NADPH oxidase [26], although the complete mechanism is unclear. In addition, MMA is known to induce oxidative stress [27]. Thus, B12 deficiency induces oxidative stress through various mechanisms.
To investigate the effects of oxidative stress caused by B12 deficiency on Aβ toxicity, B12− GMC101 worms were grown with AsA-2G supplementation. As expected, AsA-2G supplementation alleviated paralysis, and decreased ROS levels in B12− worms (Figure 3 and Figure 6). However, AsA-2G supplementation had no effect on the aggregation of Aβ peptides, suggesting that the enhanced ROS level was not involved in promoting Aβ aggregation.
Yatin et al. [28] reported that Aβ1–42 peptide alone produces free radicals in vitro and that Aβ peptide causes oxidative stress in rat neuron cells and C. elegans. These findings suggest that Aβ toxicity is associated with oxidative stress. Interestingly, oxidative stress and the paralysis phenotype in the transgenic C. elegans preceded the fibrillar deposition of Aβ peptides, suggesting that Aβ toxicity is caused by pre-fibrillar Aβ [29]. Multiple studies have reported that increased oxidative stress plays an important role in the pathogenesis of AD; oxidative stress can accelerate Aβ generation and aggregation, tau hyperphosphorylation and aggregation, and neuronal apoptosis (reviewed by Wu et al. [30]). As tau hyperphosphorylation and aggregation and neuronal apoptosis were not observed in the GMC101 worms, our results suggest that oxidative stress induced by B12 deficiency may be associated with the cellular events leading to paralysis after Aβ expression. In conclusion, oxidative stress caused by elevated Hcy and/or MMA levels may enhance Aβ toxicity in B12-deficient worms.
Transcriptomic analysis has been performed to elucidate the mechanisms associated with paralysis caused by Aβ expression [31]; however, these mechanisms are not completely understood. Enhanced mitochondrial proteostasis has been reported to reduce the amount of Aβ aggregation in cells, GMC101 worms, and transgenic mouse models of AD [32]. Further analyses are required to understand the molecular mechanisms underlying the alleviation of Aβ toxicity by AsA-2G supplementation.
B12 has a relatively low recommended dietary allowance compared to other micronutrients, and our body maintains sufficient storage of B12 for up to several years. Therefore, clinical B12 deficiency is uncommon and is mainly found in patients with hereditary diseases [4]. However, with age, the risk of developing B12 deficiency increases due to several factors, such as malabsorption and depletion of B12 storage [4]. A high Hcy level caused by B12 deficiency is a known risk factor for neurodegenerative diseases; approximately 20% of dementia cases are reported to be strongly related to high plasma Hcy levels [33]. Furthermore, high Hcy and MMA levels have been observed in elderly people with normal or high serum B12 levels [34]. Oxidative stress is known to promote the generation and aggregation of Aβ, which is believed to be a toxic factor linked to AD pathogenesis [30]. Therefore, a higher daily intake of B12, folate, and antioxidants is required to lower Hcy and MMA levels and reduce the incidence of AD.
In conclusion, our study suggests that the oxidative stress induced by B12 deficiency promotes Aβ toxicity. Although the detailed mechanism is unknown, we will investigate the effect of B12 deficiency on mitochondrial proteostasis.
Acknowledgments
VL749 and GMC101 strains and the E. coli OP50 strain were provided by the Caenorhabditis Genetic Center. We thank Tsuyoshi Kawano for the helpful discussions and Maki Taniguchi for the technical assistance.
Author Contributions
A.A. and S.T. performed the experiments and wrote the manuscript. T.B., A.I. and F.W. contributed to the project design and discussed the results. Y.Y. designed the study and wrote the manuscript with inputs from all the co-authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by JSPS KAKENHI, grant number 19K05929 to Y.Y.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the results are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Smith A.D., Warren M.J., Refsum H. Vitamin B12. Adv. Food Nutr. Res. 2018;83:215–279. doi: 10.1016/bs.afnr.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Watanabe F., Bito T. Vitamin B12 sources and microbial interaction. Exp. Biol. Med. 2018;243:148–158. doi: 10.1177/1535370217746612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Asselt D.Z., van den Broek W.J., Lamers C.B., Corstens F.H., Hoefnagels W.H. Free and protein-bound cobalamin absorption in healthy middle-aged and older subjects. J. Am. Geriatr. Soc. 1996;44:949–953. doi: 10.1111/j.1532-5415.1996.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 4.Green R., Allen L.H., Bjørke-Monsen A.L., Brito A., Guéant J.L., Miller J.W., Molloy A.M., Nexo E., Stabler S., Toh B.H., et al. Vitamin B12 deficiency. Nat. Rev. Dis. Prim. 2017;3 doi: 10.1038/nrdp.2017.40. [DOI] [PubMed] [Google Scholar]
- 5.van de Lagemaat E., de Groot L., van den Heuvel E. Vitamin B12 in relation to oxidative stress: A systematic review. Nutrients. 2019;11:482. doi: 10.3390/nu11020482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alzheimer’s Association Alzheimer’s Association 2020 Facts and Figures Report. Alzheimer’s Association. [(accessed on 1 January 2020)]; Available online: https://www.alz.org/
- 7.Masters C.L., Bateman R., Blennow K., Rowe C.C., Sperling R.A., Cummings J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015;1 doi: 10.1038/nrdp.2015.56. [DOI] [PubMed] [Google Scholar]
- 8.Link P. Ph.D. Thesis. University of Heidelberg; Heidelberg, Germany: May 9, 2016. Natural Products against Neurodegenerative Diseases: Effects in the Model Organism Caenorhabditis Elegans. [DOI] [Google Scholar]
- 9.Selkoe D.J., Hardy J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li J.G., Chu J., Barrero C., Merali S., Praticò D. Homocysteine exacerbates β-amyloid pathology, tau pathology, and cognitive deficit in a mouse model of Alzheimer disease with plaques and tangles. Ann. Neurol. 2014;75:851–863. doi: 10.1002/ana.24145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith A.D., Refsum H. Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 2016;36:211–239. doi: 10.1146/annurev-nutr-071715-050947. [DOI] [PubMed] [Google Scholar]
- 12.Corsi A.K., Wightman B., Chalfie M. A transparent window into biology: A primer on Caenorhabditis elegans. Genetics. 2015;200:387–407. doi: 10.1534/genetics.115.176099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bito T., Matsunaga Y., Yabuta Y., Kawano T., Watanabe F. Vitamin B12 deficiency in Caenorhabditis elegans results in loss of fertility, extended life cycle, and reduced lifespan. FEBS Open Bio. 2013;3:112–117. doi: 10.1016/j.fob.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson E., Macneil L.T., Ritter A.D., Yilmaz L.S., Rosebrock A.P., Caudy A.A., Walhout A.J. Interspecies systems biology uncovers metabolites affecting C. elegans gene expression and life history traits. Cell. 2014;156:759–770. doi: 10.1016/j.cell.2014.01.047. Erratum in: Cell2014, 156, 1336–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen P., Yue Y., Zheng J., Park Y. Caenorhabditis elegans: A convenient in vivo model for assessing the impact of food bioactive compounds on obesity, aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018;9:1–22. doi: 10.1146/annurev-food-030117-012709. [DOI] [PubMed] [Google Scholar]
- 16.McColl G., Roberts B.R., Pukala T.L., Kenche V.B., Roberts C.M., Link C.D., Ryan T.M., Masters C.L., Barnham K.J., Bush A.I., et al. Utility of an improved model of amyloid-beta (Aβ1-42) toxicity in Caenorhabditis elegans for drug screening for Alzheimer’s disease. Mol. Neurodegener. 2012;7 doi: 10.1186/1750-1326-7-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weaving G., Rocks B.F., Iversen S.A., Titheradge M.A. Simultaneous quantitation of homocysteine, cysteine and methionine in plasma and urine by liquid chromatography-tandem mass spectrometry. Ann. Clin. Biochem. 2006;43:474–480. doi: 10.1258/000456306778904605. [DOI] [PubMed] [Google Scholar]
- 18.Mineva E.M., Zhang M., Rabinowitz D.J., Phinney K.W., Pfeiffer C.M. An LC-MS/MS method for serum methylmalonic acid suitable for monitoring vitamin B12 status in population surveys. Anal. Bioanal. Chem. 2015;407:2955–2964. doi: 10.1007/s00216-014-8148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon D.S., Lee M.H., Cha D.S. Measurement of Intracellular ROS in Caenorhabditis elegans Using 2′,7′-Dichlorodihydrofluorescein Diacetate. Bio Protoc. 2018;8:e2774. doi: 10.21769/BioProtoc.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yabuta Y., Kamei Y., Bito T., Arima J., Yoneda K., Sakuraba H., Ohshima T., Nakano Y., Watanabe F. Functional and structural characteristics of methylmalonyl-CoA mutase from Pyrococcus horikoshii. Biosci. Biotechnol. Biochem. 2015;79:710–717. doi: 10.1080/09168451.2014.993353. [DOI] [PubMed] [Google Scholar]
- 21.Revtovich A.V., Lee R., Kirienko N.V. Interplay between mitochondria and diet mediates pathogen and stress resistance in caenorhabditis elegans. PLoS Genet. 2019;15:e1008011. doi: 10.1371/journal.pgen.1008011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bito T., Misaki T., Yabuta Y., Ishikawa T., Kawano T., Watanabe F. Vitamin B12deficiency results in severe oxidative stress, leading to memory retention impairment in Caenorhabditis elegans. Redox Biol. 2017;11:21–29. doi: 10.1016/j.redox.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malouf R., Evans J.G. Folic acid with or without vitamin B12 for the prevention and treatment of healthy elderly and demented people. Cochrane. Database Syst. Rev. 2008 doi: 10.1002/14651858.CD004514.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Chan W., Almasieh M., Catrinescu M.M., Levin L.A. Cobalamin-associated superoxide scavenging in neuronal cells is a potential mechanism for vitamin B12-deprivation optic neuropathy. Am. J. Pathol. 2018;188:160–172. doi: 10.1016/j.ajpath.2017.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tyagi N., Sedoris K.C., Steed M., Ovechkin A.V., Moshal K.S., Tyagi S.C. Mechanisms of homocysteine-induced oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2005;289:H2649–H2656. doi: 10.1152/ajpheart.00548.2005. [DOI] [PubMed] [Google Scholar]
- 26.Siow Y.L., Au-Yeung K.K., Woo C.W., Karmin O. Homocysteine stimulates phosphorylation of NADPH oxidase p47phox and p67phox subunits in monocytes via protein kinase Cbeta activation. Biochem. J. 2006;398:73–82. doi: 10.1042/BJ20051810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandes C.G., Borges C.G., Seminotti B., Amaral A.U., Knebel L.A., Eichler P., de Oliveira A.B., Leipnitz G., Wajner M. Experimental evidence that methylmalonic acid provokes oxidative damage and compromises antioxidant defenses in nerve terminal and striatum of young rats. Cell Mol. Neurobiol. 2011;31:775–785. doi: 10.1007/s10571-011-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yatin S.M., Varadarajan S., Link C.D., Butterfield D.A. In vitro and in vivo oxidative stress associated with Alzheimer’s amyloid beta-peptide (1-42) Neurobiol. Aging. 1999;20:325–330. doi: 10.1016/s0197-4580(99)00056-1. [DOI] [PubMed] [Google Scholar]
- 29.Drake J., Link C.D., Butterfield D.A. Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1-42) in a transgenic Caenorhabditis elegans model. Neurobiol. Aging. 2003;24:415–420. doi: 10.1016/S0197-4580(02)00225-7. [DOI] [PubMed] [Google Scholar]
- 30.Wu Y., Xu Q., Song W. Oxidative stress and Alzheimer’s disease. In: Ismail L., editor. Systems Biology of Free Radicals and Antioxidants. Springer; Berlin, Germany: 2014. pp. 2147–2174. [Google Scholar]
- 31.Link C.D., Taft A., Kapulkin V., Duke K., Kim S., Fei Q., Wood D.E., Sahagan B.G. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol. Aging. 2003;24:397–413. doi: 10.1016/S0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- 32.Sorrentino V., Romani M., Mouchiroud L., Beck J.S., Zhang H., D’Amico D., Moullan N., Potenza F., Schmid A.W., Rietsch S., et al. Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017;552:187–193. doi: 10.1038/nature25143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beydoun M.A., Beydoun H.A., Gamaldo A.A., Teel A., Zonderman A.B., Wang Y. Epidemiologic studies of modifiable factors associated with cognition and dementia: Systematic review and meta-analysis. BMC Public Health. 2014;14:643. doi: 10.1186/1471-2458-14-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solomon L.R. Functional cobalamin (vitamin B12) deficiency: Role of advanced age and disorders associated with increased oxidative stress. Eur. J. Clin. Nutr. 2015;69:687–692. doi: 10.1038/ejcn.2014.272. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data supporting the results are available from the corresponding author on reasonable request.