PURPOSE
The objectives of the study were to characterize the tumor burden dynamics on serial computed tomography scans in patients with advanced non–small-cell lung cancer treated with first-line pembrolizumab and to identify imaging markers for prolonged overall survival (OS).
MATERIALS AND METHODS
Eighty-eight patients treated with first-line pembrolizumab monotherapy were evaluated on serial computed tomography scans to characterize their quantitative tumor burden during therapy. Tumor burden dynamics were studied for the association with OS.
RESULTS
The overall response rate was 42% (37/88), with the median tumor burden changes at the best overall response of −18.3% (range, −100.0% to +103.6%). Response rates were higher in men than in women (P = .05) and in patients with higher programmed cell death ligand-1 expression levels (P = .02). Tumor burden stayed below the baseline burden throughout therapy in 55 patients (63%). In an 8-week landmark analysis, patients with tumor burden below the baseline burden during the first 8 weeks of therapy had longer OS compared with patients who had ≥ 0% increase (median OS, 30.7 v 16.2 months; hazard ratio [HR] = 0.44; P = .01). In the extended Cox models, patients whose tumor burden stayed below the baseline burden throughout therapy had significantly reduced hazards of death (HR = 0.41, P = .003, univariate; HR = 0.35, P = .02, multivariate). Only one patient (1.1%) experienced pseudoprogression with initial tumor increase and subsequent tumor regression.
CONCLUSION
In patients with advanced non–small-cell lung cancer treated with first-line single-agent pembrolizumab, tumor burden reduction below the baseline burden during therapy was an independent marker for prolonged OS, which may serve as a practical guide for treatment decisions.
INTRODUCTION
With the recent remarkable advances in clinical applications of immune-checkpoint blockade for advanced cancer, programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) inhibitors have become a major treatment option for patients with advanced non–small-cell lung cancer (NSCLC).1-7 Among the immune-checkpoint inhibitors (ICIs) for advanced NSCLC, pembrolizumab (PD-1 inhibitor) was the first agent that received approval as the first-line monotherapy for NSCLC in 2016, for patients whose tumors demonstrate ≥ 50% PD-L1 expression.7 Pembrolizumab was later approved as the first-line therapy in combination with platinum doublet chemotherapy for advanced NSCLC, regardless of PD-L1 expression levels.8,9 The indication of the first-line pembrolizumab monotherapy was further expanded in 2019 and now includes unresectable stage III or metastatic NSCLC with ≥ 1% PD-L1 expression but without EGFR or ALK genomic aberrations.10 With these recent developments, first-line pembrolizumab therapy has clearly become one of the mainstays of systemic therapy for patients with advanced NSCLC.
CONTEXT
Key Objective
Approval of first-line pembrolizumab has brought another paradigm shift in treatment approaches for advanced non–small-cell lung cancer (NSCLC). The present study characterized quantitative tumor burden dynamics in patients with advanced NSCLC treated with first-line pembrolizumab monotherapy using serial computed tomography scans.
Knowledge Generated
Tumor burden staying below the baseline burden throughout therapy, noted in 55 patients (63%), was significantly associated with prolonged overall survival and thus may serve as an objective marker for treatment benefit that can guide therapeutic decisions. Pseudoprogression was noted in one patient (1.1%), confirming the rarity of the phenomenon when using programmed cell death-1 inhibitor monotherapy for advanced NSCLC.
Relevance
The study provided a unique threshold on the basis of tumor burden dynamics for monitoring of first-line pembrolizumab monotherapy in patients with advanced NSCLC, which can be further validated in prospective cohorts as a practical imaging marker for treatment benefit and survival.
Objective response evaluations using serial computed tomography (CT) imaging have been a topic of interest in patients treated with ICI because of unique mechanism of action of these agents. Several characteristic patterns of immune-related response and tumor burden dynamics have been described, including pseudoprogression, hyperprogression, and durable stability of tumor burden.2,11-19 Although PD-L1 expression on tumor cells by immunohistochemistry is used as a baseline biomarker to predict tumor response to PD-1 and PD-L1 inhibitor monotherapy in patients with advanced NSCLC, it has limitations particularly as a marker to guide treatment modifications during therapy. Practically, the decision to change therapy is based on the assessment of tumor burden changes on serial imaging, referring to the criteria defined in RECIST guidelines.20-22 However, RECIST has a limited value to guide treatment decisions at tumor progression, especially in patients treated with novel therapies including molecular-targeting agents and ICI who may be having therapeutic benefit beyond RECIST progression.18,23-25
To supplement limitations of current strategy and better guide treatment decisions during ICI therapy, longitudinal tumor burden dynamics on serial CT scans during ICI therapy have been previously studied in patients with advanced melanoma treated with pembrolizumab and in advanced NSCLC who received PD-1 inhibitors after previous treatment and has shown to provide quantitative markers for treatment benefit and prolonged survival.14,15,26 The purpose of the present study is to characterize the tumor burden dynamics on serial CT scans during the first-line pembrolizumab monotherapy for patients with advanced NSCLC and to identify markers for prolonged survival that can provide objective guides for treating physicians.
MATERIALS AND METHODS
Patients
The study cohort consisted of 88 patients with advanced NSCLC treated with the first-line pembrolizumab monotherapy at our institution, who had baseline CT scans before the initiation of therapy, demonstrating at least one measurable lesion by RECIST1.1, and had at least one follow-up CT scan during therapy available for review. The medical records and imaging studies were retrospectively reviewed in these patients who had consented to a correlative research study approved by the institutional review board.
Tumor Measurements and Response Evaluations
Baseline and all follow-up CT scans during therapy were retrospectively reviewed by a board-certified radiologist (M.N.) to quantify tumor burden changes using RECIST1.1.20-22 Other imaging studies such as brain magnetic resonance imaging and positron emission tomography-CT scans were also reviewed to identify new lesions and assess nontarget lesions.27 One observer performed the serial measurements because high interobserver agreements of the RECIST1.1 measurements have been previously reported.28,29 Response assessment categories were assigned at each follow-up scan, and the best overall response (BOR) was assigned according to RECIST1.1, using the BOR at or before RECIST-PD. Maximal tumor shrinkage at BOR was obtained in reference to the baseline tumor burden. RECIST1.1 was used because it has been the primary response criteria used for the published trials of PD-1 inhibitors.2,7,30 However, to capture a minority of patients with atypical responses, tumor measurements using RECIST1.1 were continued on serial scans beyond RECIST-PD while the patients are on therapy.14,16,18
Statistical Analysis
Comparison across groups was performed using a Fisher’s exact test and chi-square test for categorical variables, chi-square trend test for ordinal outcome, and a Kruskal-Wallis test for continuous variables. Spider plots of the tumor burden changes throughout therapy for all patients were generated to visually demonstrate tumor burden dynamics during therapy and evaluate the characteristics in this cohort.14,15 Overall survival (OS) was estimated using the method of Kaplan-Meier.
The 8-week conditional landmark analyses were performed to assess relationships between OS and tumor burden dynamics during the first 8 weeks of therapy. Extended Cox models with time-dependent covariates were used to evaluate associations between OS and tumor burden dynamics throughout therapy. Multivariable Cox models were used to adjust for clinical variables and potential confounders. All P values are based on a two-sided hypothesis. A P value of < .05 was considered to be significant.
RESULTS
Tumor Response Characteristics in First-Line Pembrolizumab Therapy
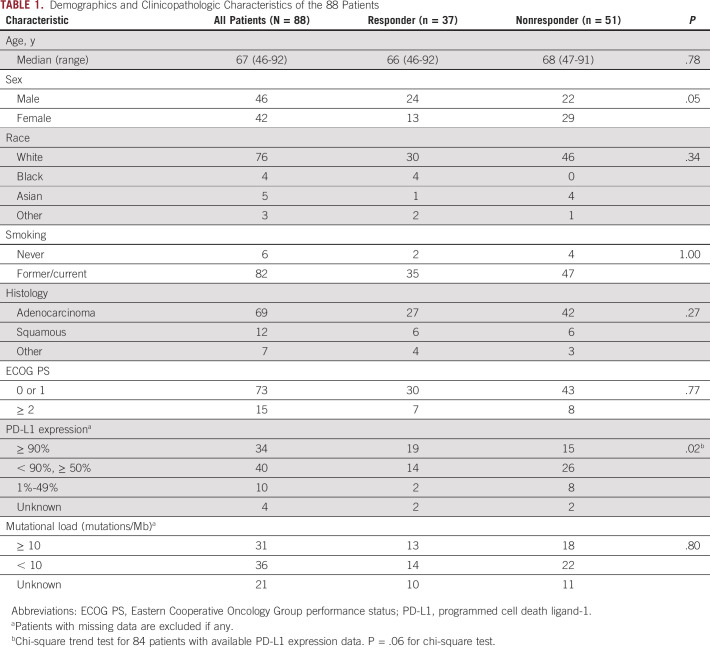
Table 1 summarizes the demographics and clinicopathologic characteristics of 88 patients with advanced NSCLC treated with first-line single-agent pembrolizumab. Among the 88 patients, the median age was 67 years, 46 patients (52%) were male, 82 (93%) were current or former smokers, and 69 (78%) had adenocarcinoma. A median follow-up time was 26.4 months for the study patient population.
TABLE 1.
Demographics and Clinicopathologic Characteristics of the 88 Patients
Median tumor burden change in reference to baseline at the BOR as −18.3% (range, −100.0% to +103.6%) (Fig 1). Response rate was 42% (37/88), and all responders had partial response (PR) as their BOR. One patient had −100% tumor shrinkage for the target lesions but had persistent nontarget lesions, resulting in PR as BOR. BOR in the remaining patients was stable disease in 33 patients (37.5%) and was progressive disease (PD) in 18 patients (20.5%).
FIG 1.
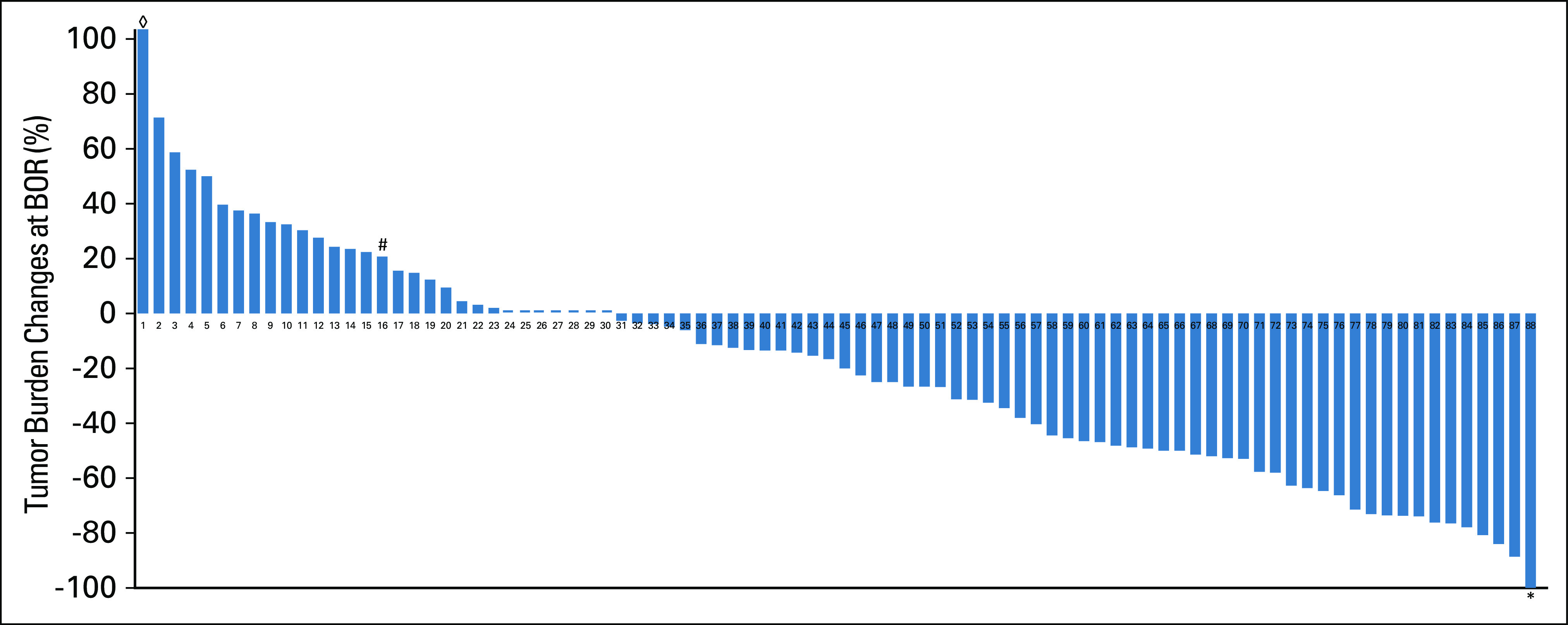
Waterfall plot of the maximal tumor burden changes relative to the baseline burden. One patient (#) experienced an initial tumor increase meeting the criteria for PD followed by tumor shrinkage of ≥ 30% decrease compared with the baseline (pseudoprogression). One patient achieved a 100% decrease of the target lesions (*); however, the nontarget lesions did not disappear completely and thus had partial response as the best overall response. One patient had +103.6% increase of tumor burden at best overall response (◊) that is beyond the maximum value of the y axis in the graph. BOR, best overall response.
Clinical characteristics of 37 responders and 51 nonresponders are also shown in Table 1. Male patients were more likely to respond to therapy than female patients (response rate, 52% [24/46] v 31% (13/42), respectively; P = .05). Other clinical characteristics including age, race, smoking history, histology, and performance status showed no significant differences between responders and nonresponders. PD-L1 expression levels were available in 84 patients. In these patients, higher levels of PD-L1 expression were associated with higher response rates (56% [19/34] in ≥ 90% group, 35% [14/40] in 50%-90% group, and 20% (2/10) in < 50% group; P = .02). The tumor mutational load (number of mutations/Mb) was available in 67 patients, with the median mutational load of 9.89. In the 67 patients, the mutational load was not associated with response rates either as a continuous variable (P = .19) or in groups dichotomized at the mutation load of 10 on the basis of the median value in this cohort (P = .80).
Tumor Burden Dynamics and the Association With OS
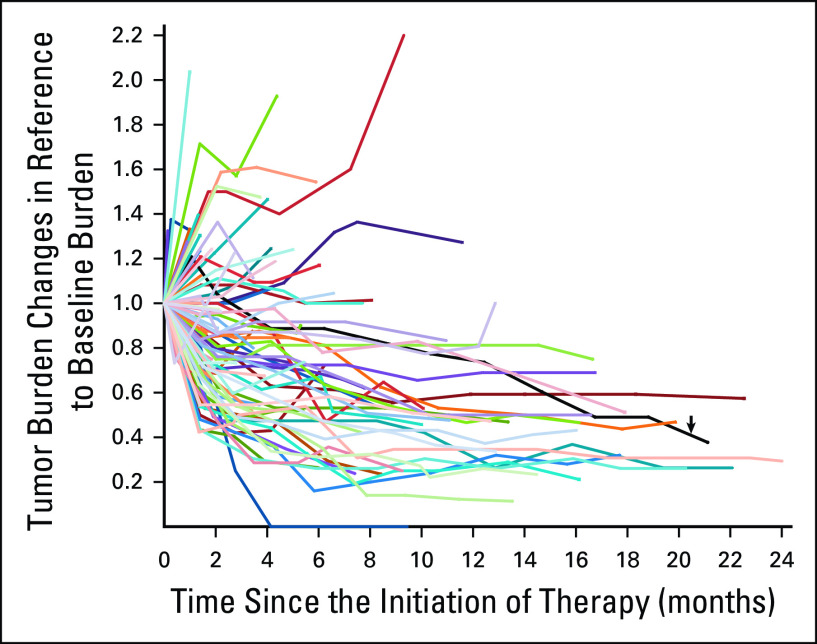
Tumor burden dynamics during the first-line therapy using single-agent pembrolizumab are demonstrated in a spider plot (Fig 2). Tumor burden stayed below the baseline burden throughout therapy in 55 patients (63%). Twelve of the 55 patients experienced PD by RECIST1.1 during therapy though their tumor burden stayed below the baseline burden because RECIST-PD can be reached with ≥ 20% and ≥ 5 mm increase compared with the nadir (the smallest tumor burden since therapy initiation) even if the tumor burden remains below the baseline burden.
FIG 2.
Spider plot of tumor burden changes during the first-line pembrolizumab monotherapy. The spider plot visually presents the tumor burden dynamics in 88 patients treated with the first-line pembrolizumab monotherapy. Fifty-five patients whose tumor burden stayed below the baseline burden (< 1.0 or < 0% increase) had longer survival compared with others who had ≥ 0% increase in tumor burden in the subsequent analyses. One patient demonstrated an initial tumor burden increase meeting the criteria for RECIST progression followed by tumor shrinkage, representing pseudoprogression with subsequent tumor response (black line indicated by a black arrow).
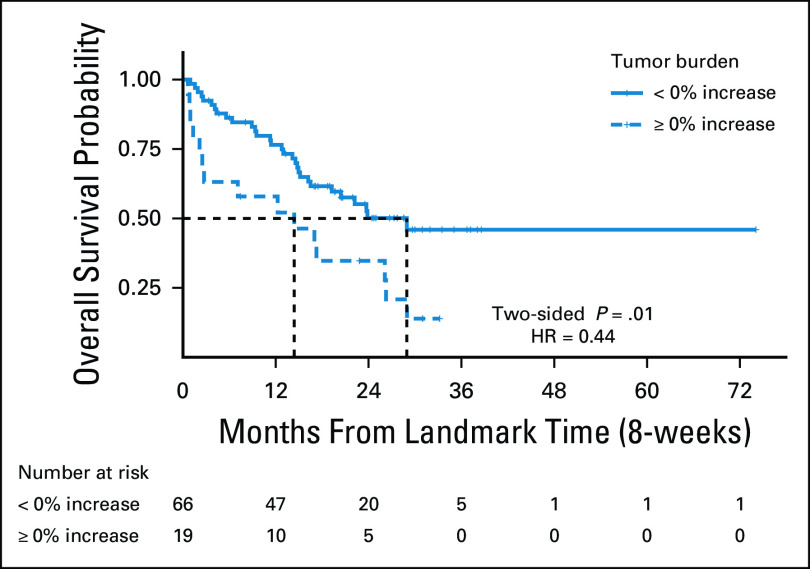
On the basis of the visual inspection of the spider plot, tumor burden below the baseline burden (< 0% increase) was further studied for the association with prolonged OS, using an 8-week landmark analysis and extended Cox models, as previously published.14,15 The 8-week landmark time point was chosen because it has been previously used as the landmark time point for survival markers in advanced NSCLC patients treated with PD-1 inhibitors and with other molecular targeting agents.14,31-33 After excluding three patients with survival time shorter than 8 weeks, 85 patients were studied in the landmark analysis. Among them, 34 patients had first disease assessment after 8 weeks. First analysis including these 34 patients in the group whose tumor burden stayed below the baseline burden during the first 8 weeks of therapy, which was shown to have longer OS compared with 19 patients who had ≥ 0% increase in tumor burden by 8 weeks of therapy (median OS, 30.7 v 16.2 months; hazard ratio [HR] = 0.44; P = .01) (Fig 3). Second analysis, excluding the 34 patients without response assessment in the first 8 weeks and thus including a subset of 54 patients who had first tumor measurement within 8 weeks, provided a similar result (median OS not reached v 16.2 months, HR = 0.37, P = .01).
FIG 3.
Kaplan-Meier estimates of OS of patients dichotomized by tumor burden changes at the 8 weeks as the landmark time point. Of 85 patients who were eligible for the 8-week landmark analysis, 66 patients whose tumor burden stayed below the baseline burden (< 0% increase) during the first 8 weeks of therapy had longer OS compared with 19 patients who had ≥ 0% increase in tumor burden by 8 weeks of therapy (median OS: 30.7 v 16.2 months, HR = 0.44, P = .01). HR, hazard ratio; OS, overall survival.
The extended Cox models with time-dependent covariates included all 88 patients, who were initially classified in the < 0% increase group. Any patient who experienced ≥ 0% increase in tumor burden was reclassified into the ≥ 0% increase group at that time during therapy. Patients whose tumor burden stayed below the baseline burden (< 0% increase) throughout therapy had significantly reduced hazards of death compared with those who experienced tumor burden increase ≥ 0% from baseline burden at any time point during therapy (HR = 0.41, P = .003) for the univariate analyses. Multivariate analyses were performed accounting for other significant variables in univariate analyses, including age, Eastern Cooperative Oncology Group performance status, and PD-L1 expression levels. The tumor burden staying below baseline (< 0% increase) during therapy remained as a significant predictor of prolonged OS (HR = 0.35, P = .02), after adjusting for these variables (age > 70, HR = 1.95, P = .05, Eastern Cooperative Oncology Group performance status ≥ 2, HR = 1.87, P = .12, PD-L1 expression levels, 50%-90%, HR = 0.94, P = .88 ≥ .90%, HR = 0.49, P = .13, in reference to < 50%), indicating that tumor burden staying below the baseline burden is an independent predictor of prolonged OS during the first-line pembrolizumab therapy for patients with advanced NSCLC.
Incidence of Pseudoprogression
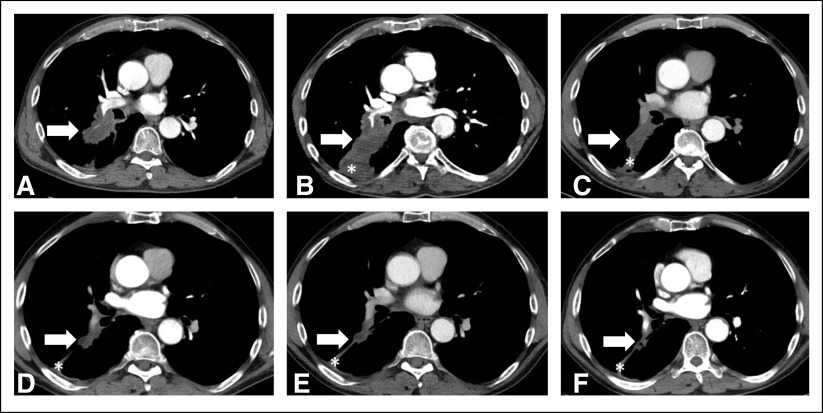
Of the 88 patients, one patient experienced an initial increase of tumor burden ≥ 20% and ≥ 5 mm from the baseline meeting the criteria for PD, which was followed by tumor burden decrease below the threshold for RECIST-PR (≥ 30% decrease compared with the baseline) noted on more than two consecutive scans, demonstrating pseudoprogression (Fig 4).
FIG 4.
A 62-year-old man with lung adenocarcinoma treated with first-line pembrolizumab. Baseline computed tomography before starting pembrolizumab showed a right lower lobe lung mass contiguous from the hilum, measuring 5.3 cm (A, arrow). On the initial follow-up scan at 1.5 months of therapy, the tumor measured 6.4 cm (B, arrow), demonstrating 21% increase compared with the baseline and meeting the criteria for progressive disease by RECIST1.1. However, the subsequent scans demonstrated tumor shrinkage (C at 4.5 months and D at 12 months as representative scans), later achieving ≥ 30% decrease compared with the baseline at 14 months of therapy (E) when it measured 3.2 cm (40% decrease since the baseline). Further decrease was noted on the consecutive follow-up scans at 16 months (F), confirming response. The tumor burden continued to remain small on the subsequent follow-up scans, and the patient remained progression-free at 21 months of therapy. Asterisks demonstrate postobstructive atelectasis that is not included in the tumor burden measurement.
DISCUSSION
Tumor burden dynamics in advanced NSCLC patients treated with the first-line pembrolizumab monotherapy were characterized in the present study using the quantitative assessments on serial CT scans. Tumor burden staying below the baseline burden (< 0% increase) during therapy was associated with longer OS, indicating that it can serve as a marker for treatment benefit and prolonged survival in these patients. The results can help to provide an objective guide when to continue therapy and when to consider alternative or additional therapy in patients undergoing the first-line pembrolizumab monotherapy.
The overall response rate was 42% by RECIST and was similar to the response rate of a phase III trial of the first-line pembrolizumab monotherapy for advanced NSCLC, which showed 44.8% response rate in patients with ≥ 50% PD-L1 expression.7 Most patients (74/88, 84%) in the present cohort had the PD-L1 expression level of ≥ 50%, representing a relatively well-selected cohort using PD-L1 expression level as a biomarker. The median tumor burden change was −18.3% at best response, reflecting the relatively high response rate and confirming that the PD-L1 expression level-based selection can contribute to generate a good degree of tumor shrinkage from pembrolizumab therapy. Men were more likely to have response than women; however, the significance was marginal (P = .05). Smoking history was not associated with response rates, likely because most patients in the cohort were smokers (82/88, 93%).
Higher PD-L1 expression levels were associated with higher response rates, as expected and in concordance with the results in the published trials of first-line pembrolizumab therapy.7,10 On the other hand, the tumor mutational load was not associated with response to therapy; however, the mutational load data were not available in all patients in this retrospective cohort, and thus, the association is tested in a subcohort of patients (n = 67; 76%) who had their tumor mutational load evaluated.
The spider plot was generated on the basis of the quantitative evaluation on serial CT scans to visually demonstrate tumor burden dynamics during therapy as in the previous studies,14,15,26 providing further insights for immune-related response patterns for the cohort. On the basis of the visual inspection of the spider plot, it was hypothesized that the patients whose tumor burden stayed below the baseline burden (< 0% increase), which accounted for about two thirds of the cohort, may experience prolonged treatment benefit and have longer OS. Using the 8-week landmark analysis, tumor burden below the baseline burden (< 0% increase) in the first 8 weeks of therapy was shown to be associated with longer OS (HR = 0.44, P = .01), indicating that the threshold can be used as an early marker for prolonged survival. Moreover, tumor burden remaining below the baseline burden throughout therapy was also associated with longer OS in the extended Cox models with a time varying covariate, using all the CT time point measurements during therapy, in univariate analysis (HR = 0.41, P = .003) as well as in multivariate analyses (HR = 0.35, P = .02) after adjusting for other significant variables and potential confounders. The results propose a practical marker for prolonged OS for patients undergoing first-line pembrolizumab therapy for their advanced NSCLC, indicating that patients whose tumor burden staying below the baseline burden can safely continue therapy with expected survival benefit. Importantly, 12 of the 55 patients whose tumor burden stayed below the baseline throughout therapy met the criteria for RECIST-PD during therapy because RECIST-PD can be reached by ≥ 20% and ≥ 5 mm increase compared with the nadir, regardless of whether the tumor burden is below or above the baseline burden. The results of the present study may contribute to address the limitations of RECIST in terms of guiding treatment options at the time of RECIST-PD.
The threshold of tumor burden staying below the baseline burden (< 0% increase) was based on the inspection of the spider plot of the present first-line cohort and was different from the previous studies that used the similar approach in advanced melanoma patients treated with pembrolizumab and in previously treated advanced NSCLC patients treated with PD-1 inhibitors (nivolumab in 88% and pembrolizumab 12%), which used the threshold of < 20% increase from baseline as a marker for prolonged survival.14,15 Different thresholds are expected in different cohorts of patients in different clinical settings (ie, tumor types, agents, or lines of therapy). For the present cohort treated with first-line pembrolizumab, patients were better selected on the basis of the PD-L1 expression levels and demonstrated more uniform trends of their tumor burden dynamics, with higher proportion of patients receiving therapeutic benefits. The threshold of < 20% increase from baseline was also examined in the present cohort as a comparison, which was not significant in the 8-week landmark analysis (HR, 0.75; P = .48), although significant in the extended Cox model (HR, 0.51; P = .04) for prolonged OS, confirming that the threshold below the baseline burden (< 0% increase) is a better predictive marker for OS in this cohort receiving first-line pembrolizumab.
Pseudoprogression was noted in one of 88 patients (1.1%), who experienced initial tumor burden increase beyond RECIST-PD threshold followed by subsequent tumor burden decrease below the threshold of RECIST-PR (≥ 30% decrease compared with the baseline) documented on multiple follow-up scans. The result further confirms that pseudoprogression is a very rare event, especially in advanced NSCLC patients treated with PD-1 inhibitor monotherapy, including the first-line pembrolizumb.14-17,30,34 The observation also support the choice of RECIST1.1 as the method to characterize tumor burden for this cohort rather than other modified criteria proposed for immune-related response evaluations11,26,28,35-37 because the growing evidence of extremely low incidence of pseudoprogression indicates the minimal impact of modified assessment for the setting of PD-1 inhibitor monotherapy for NSCLC.
The limitation of this study includes a retrospective design with patients treated at a single institution. Tumor mutational load is an important marker but was not available in all patients in this cohort and thus could not be included as a variable in the Cox model. However, other variables including the PD-L1 expression levels were tested in the model, which represent the practical markers routinely used in the clinical settings. Imaging markers for survival benefit described in this study need to be validated in larger, multicenter prospective cohorts. Further evaluation using the similar approach can be performed in different subsets of patients, such as those who are treated with first-line pembrolizumab in combination with cytotoxic chemotherapy, to characterize the tumor burden dynamics and determine the optimal threshold for a prognostic maker on serial scans in specific subsets of ICI therapy for advanced NSCLC.
In conclusion, tumor burden staying below the baseline burden during first-line pembrolizumab monotherapy was an independent marker for prolonged OS in patients with advanced NSCLC and may serve as a practical marker for treatment guidance in these patients, if validated in prospective cohorts. Pseudoprogression was noted in only one patient (1.1%), confirming the exceedingly low rate of the phenomenon in NSCLC patients treated with PD-1 inhibitor.
SUPPORT
The investigator, M.N., was supported by R01CA203636 (NCI).
AUTHOR CONTRIBUTIONS
Conception and design: Mizuki Nishino, Mark M. Awad
Financial support: Mark M. Awad
Provision of study materials or patients: Mark M. Awad
Collection and assembly of data: Mizuki Nishino, Biagio Ricciuti, Mark M. Awad
Data analysis and interpretation: Fangxin Hong, Hiroto Hatabu, Mark M. Awad
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Mizuki Nishino
Consulting or Advisory Role: Daiichi Sankyo, AstraZeneca
Research Funding: Merck, Toshiba, AstraZeneca, Daiichi Sankyo
Fangxin Hong
Consulting or Advisory Role: Merck Sharp and Dohme
Hiroto Hatabu
Consulting or Advisory Role: Canon Medical System Inc
Research Funding: Konica Minolta, Canon Medical Systems Inc
Mark M. Awad
Consulting or Advisory Role: Genentech, Merck, Pfizer, Boehringer Ingelheim, Abbvie, AstraZeneca/MedImmune, Clovis Oncology, Nektar, Bristol-Myers Squibb, ARIAD, Foundation Medicine, Syndax, Novartis, Blueprint Medicines, Maverick Therapeutics, Achilles Therapeutics, Neon Therapeutics, Hengrui Therapeutics, Gritstone Oncology, Archer, Mirati Therapeutics, NextCure, EMD Serono, Panvaxal
Research Funding: Genentech/Roche, Lilly, AstraZeneca, Bristol-Myers Squibb
No other potential conflicts of interest were reported.
REFERENCES
- 1.Rizvi NA, Mazieres J, Planchard D, et al. : Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol 16:257-265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gettinger SN, Horn L, Gandhi L, et al. : Overall survival and long-term safety of nivolumab (anti-programmed death 1 antibody, BMS-936558, ONO-4538) in patients with previously treated advanced non-small-cell lung cancer. J Clin Oncol 33:2004-2012, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garon EB, Rizvi NA, Hui R, et al. : Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018-2028, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, et al. : Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 373:1627-1639, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herbst RS, Baas P, Kim DW, et al. : Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet 387:1540-1550, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Fehrenbacher L, Spira A, Ballinger M, et al. : Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): A multicentre, open-label, phase 2 randomised controlled trial. Lancet 387:1837-1846, 2016 [DOI] [PubMed] [Google Scholar]
- 7.Reck M, Rodriguez-Abreu D, Robinson AG, et al. : Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 375:1823-1833, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Langer CJ, Gadgeel SM, Borghaei H, et al. : Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: A randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol 17:1497-1508, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paz-Ares L, Luft A, Vicente D, et al. : Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 379:2040-2051, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Mok TSK, Wu YL, Kudaba I, et al. : Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 393:1819-1830, 2019 [DOI] [PubMed] [Google Scholar]
- 11.Wolchok JD, Hoos A, O'Day S, et al. : Guidelines for the evaluation of immune therapy activity in solid tumors: Immune-related response criteria. Clin Cancer Res 15:7412-7420, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Ferrara R, Mezquita L, Texier M, et al. : Hyperprogressive disease in patients with advanced non-small cell lung cancer treated with PD-1/PD-L1 inhibitors or with single-agent chemotherapy. JAMA Oncol 23:1543-1552, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champiat S, Dercle L, Ammari S, et al. : Hyperprogressive disease is a new pattern of progression in cancer patients treated by anti-PD-1/PD-L1. Clin Cancer Res 23:1920-1928, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Nishino M, Dahlberg SE, Adeni AE, et al. : Tumor response dynamics of advanced non-small cell lung cancer patients treated with PD-1 inhibitors: Imaging markers for treatment outcome. Clin Cancer Res 23:5737-5744, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nishino M, Giobbie-Hurder A, Manos MP, et al. : Immune-related tumor response dynamics in melanoma patients treated with pembrolizumab: Identifying markers for clinical outcome and treatment decisions. Clin Cancer Res 23:4671-4679, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino M, Hatabu H, Hodi FS. Imaging of cancer immunotherapy: Current approaches and future directions. Radiology 290:9-22, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishino M, Ramaiya NH, Hatabu H, et al. : Monitoring immune-checkpoint blockade: Response evaluation and biomarker development. Nat Rev Clin Oncol 14:655-668, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishino M, Jagannathan JP, Krajewski KM, et al. : Personalized tumor response assessment in the era of molecular medicine: Cancer-specific and therapy-specific response criteria to complement pitfalls of RECIST. AJR Am J Roentgenol 198:737-745, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Regan KN, Jagannathan JP, Ramaiya N, et al. : Radiologic aspects of immune-related tumor response criteria and patterns of immune-related adverse events in patients undergoing ipilimumab therapy. AJR Am J Roentgenol 197:W241-W246, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Therasse P, Arbuck SG, Eisenhauer EA, et al. : New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Nishino M, Jagannathan JP, Ramaiya NH, et al. : Revised RECIST guideline version 1.1: What oncologists want to know and what radiologists need to know. AJR Am J Roentgenol 195:281-289, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Nishino M, Cardarella S, Dahlberg SE, et al. : Radiographic assessment and therapeutic decisions at RECIST progression in EGFR-mutant NSCLC treated with EGFR tyrosine kinase inhibitors. Lung Cancer 79:283-288, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park K, Yu CJ, Kim SW, et al. : First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: The ASPIRATION study. JAMA Oncol 2:305-312, 2016 [DOI] [PubMed] [Google Scholar]
- 25.Nishino M: Tumor response assessment for precision cancer therapy: Response evaluation criteria in solid tumors and beyond. Am Soc Clin Oncol Educ Book 38:1019-1029, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Nishino M, Ramaiya NH, Chambers ES, et al. : Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer 4:84, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino M, Cardarella S, Jackman DM, et al. : RECIST 1.1 in NSCLC patients with EGFR mutations treated with EGFR tyrosine kinase inhibitors: Comparison with RECIST 1.0. AJR Am J Roentgenol 201:W64-W71, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nishino M, Giobbie-Hurder A, Gargano M, et al. : Developing a common language for tumor response to immunotherapy: Immune-related response criteria using unidimensional measurements. Clin Cancer Res 19:3936-3943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishino M, Jackman DM, Hatabu H, et al. : New response evaluation criteria in solid tumors (RECIST) guidelines for advanced non-small cell lung cancer: Comparison with original RECIST and impact on assessment of tumor response to targeted therapy. AJR Am J Roentgenol 195:W221-W228, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gettinger S, Rizvi NA, Chow LQ, et al. : Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 34:2980-2987, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lara PN Jr, Redman MW, Kelly K, et al. : Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: Results from southwest Oncology group randomized trials. J Clin Oncol 26:463-467, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Gold KA, Kim ES, Lee JJ, et al. : The BATTLE to personalize lung cancer prevention through reverse migration. Cancer Prev Res (Phila) 4:962-972, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishino M, Dahlberg SE, Cardarella S, et al. : Tumor volume decrease at 8 weeks is associated with longer survival in EGFR-mutant advanced non-small-cell lung cancer patients treated with EGFR TKI. J Thorac Oncol 8:1059-1068, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park HJ, Kim KW, Pyo J, et al. : Incidence of pseudoprogression during immune checkpoint inhibitor therapy for solid tumors: A systematic review and meta-analysis. Radiology 297:87-96, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishino M, Giobbie-Hurder A, Ramaiya NH, et al. : Response assessment in metastatic melanoma treated with ipilimumab and bevacizumab: CT tumor size and density as markers for response and outcome. J Immunother Cancer 2:40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hodi FS, Ballinger M, Lyons B, et al. : Immune-modified response evaluation criteria in solid tumors (imRECIST): Refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 36:850-858, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Seymour L, Bogaerts J, Perrone A, et al. : iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18:e143-e152, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]