Abstract
PURPOSE
The tyrosine kinase receptor anaplastic lymphoma kinase (ALK) can be abnormally activated in neuroblastoma, and somatic ALK mutations occur in 6%-10% of patients. The differential clinical impact of these mutations has not been clearly elucidated.
METHODS
Data on patients with neuroblastoma harboring ALK mutations were retrospectively analyzed. ALK sequencing was performed by whole-genome sequencing, hybrid-based capture of targeted exomes, or hotspot ALK mutation profiling. The differential impact of ALK mutation site on clinical characteristics, response to treatment, and survival was analyzed. In a subgroup of patients with locoregional neuroblastoma diagnosed after 2014, the impact of all ALK mutations was compared with wild-type ALK.
RESULTS
Of 641 patients with neuroblastoma with ALK status analyzed on at least one tumor sample, 103 (16%) had tumors harboring ALK mutations. Mutations existed across all ages (birth to 67.8 years), stages (30% locoregional and 70% metastatic), and risk groups (20%, 11%, and 69% with low-, intermediate-, and high-risk disease, respectively). Mutation sites included F1174 (51%), R1275 (29%), R1245 (10%), and others (10%). Mutation site was not prognostic for progression-free survival or overall survival in the entire cohort, high-risk subgroup, or locoregional subgroup. Locoregional tumors with any ALK mutation were generally invasive: L2 by International Neuroblastoma Research Group staging in 30/31 patients with a 2-year progression-free survival (59%, 95% CI, 37.4 to 80.5) that was inferior to historical controls. This observation was corroborated in the post-2014 subgroup in which gross total resection was less likely for ALK-mutated tumors.
CONCLUSION
Somatic ALK mutations are present across all stages and risk groups of neuroblastoma. No specific mutation carries differential prognostic significance. Locoregional neuroblastoma has an invasive phenotype when harboring somatic ALK mutations in this population.
INTRODUCTION
The anaplastic lymphoma kinase (ALK) gene, which encodes a tyrosine kinase receptor, can be abnormally activated in cancer by chromosomal translocation, point mutation, or gene amplification. When involved in chromosomal translocations, ALK is an oncogenic driver in several cancers including anaplastic large-cell lymphoma, inflammatory myofibroblastic tumor, and non–small-cell lung cancer.1-5 Approximately 10% of all neuroblastomas harbor somatic ALK mutations at diagnosis (with reports of even higher incidence at relapse), 85% of which are accounted for by single-nucleotide variants at three loci: R1275 (43%-49%), F1174 (30%-35%), and F1245 (12%).2,4,6,7 Although neuroblastoma is typically a sporadically acquired disease, 1%-2% of cases have autosomal dominant inheritance8-12 with germline ALK mutations being the most common genetic aberration.3,11,13
CONTEXT
Key Objective
Although there has been significant work evaluating the prognostic relevance of anaplastic lymphoma kinase (ALK)-mutant (compared with ALK wild type) disease in patients with neuroblastoma, to our knowledge, this article is the first with the primary objective of evaluating the differential impact of various ALK mutation loci in this population.
Knowledge Generated
We did not see an association between ALK mutation loci and clinical outcome (including response to chemotherapy, progression-free survival, and overall survival) in our overall cohort, high-risk subgroup, or locoregional subgroup. However, ALK-mutant locoregional disease was associated with a more invasive clinical phenotype.
Relevance
Further work addressing the prognostic relevance of various ALK mutation loci is needed in the context of a prospective clinical trial evaluating uniformly treated patients. Increased surveillance may be considered for ALK-mutant locoregional neuroblastoma.
In preclinical studies, single-nucleotide variants in the tyrosine kinase domain of ALK activate signaling pathways that can potentiate oncogenesis.1,3,11,14,15 The ALK F1174L mutation is thought to lead to a more aggressive disease phenotype; there are no reports of germline F1174 mutations suggesting potential embryonic lethality, and F1174 mutations are associated with resistance to several ALK inhibitors.16,17 Co-expression of ALK F1174L and MYCN amplification in transgenic mouse models appears to augment tumor aggressiveness through decreased latency to tumor generation and development of large, bulky tumors when compared with models with MYCN amplification without ALK F1174L.17
The clinical impact of ALK mutations requires further study. The largest report on clinical correlates of somatic ALK mutations in neuroblastoma included 126 of 1,596 patients enrolled on the multicenter Children's Oncology Group biology study, ANBL00B1, from 2006 to 2014. ALK mutations were more common with high-risk disease, older age (> 10 years), and MYCN amplification.2 Patients with ALK-mutant tumors had inferior survival compared with those with ALK wild-type (WT) tumors, although treatment was not standardized across all cohorts. A subsequent meta-analysis of 49 patients harboring tumors with ALK mutations did not show significant survival differences in patients with and without ALK-mutant tumors. When patients with ALK-WT or ALK R1275-mutant tumors were compared with those with tumors harboring ALK F1174 mutations, survival was inferior in the latter group, possibly because of the co-inheritance of ALK F1174 mutation and MYCN amplification. Other reports on the clinical impact of ALK mutations include anecdotes or small case series.18-20
Although preclinical studies have demonstrated potential benefit of ALK inhibition in neuroblastoma cell lines and xenografts,2,11,21 significant antitumor effects have not yet been recapitulated in the clinic.22,23 These suboptimal clinical response rates may be partially attributable to ALK 1174L mutations, which have demonstrated inherent treatment resistance to other early-generation ALK inhibitors,22 although newer inhibitors have the potential to overcome this resistance.24,25 Both detection and characterization of mutation type might have therapeutic relevance in neuroblastoma therapy. In this study, we contribute additional analyses comparing the differential clinical impact and prognostic relevance of various ALK mutations.
METHODS
Patients
After obtaining a waiver from the institutional review board of Memorial Sloan Kettering Cancer Center (MSK), medical records of all patients with neuroblastoma who had a tumor sample evaluated for ALK status were retrospectively reviewed. Patients with tumors harboring ALK mutations were included in this analysis. Extent of tumor resection was determined by operative reports. Resection was defined as R1 if no macroscopic residual tumor was detected postoperatively. We included analysis of clinical outcomes by treatment era (pre-2011 and 2011-2019 corresponding roughly to the pre-antibody therapy and current eras, respectively).
ALK Status Analysis
Tumor sequencing was performed by whole-genome sequencing (WGS) (35 × depth),26 hotspot ALK mutation profiling (Sequenom),27,28 or hybridization-based capture of targeted exomes (> 500 × depth) on MSK-IMPACT (Integrated Mutation Profiling of Actionable Cancer Targets) or FoundationOne platforms.29,30 The latter two are Food and Drug Administration–approved and Clinical Laboratory Improvement Amendments–certified sequencing assays, whereas the former two are research platforms. From 2015 onwards, all accessible tumor samples underwent targeted-exome sequencing using MSK-IMPACT or FoundationOne platforms. From 2012 to 2014, tumor samples underwent evaluation using the Sequenom platform, whereas before 2012, samples underwent WGS. MSK-IMPACT was done in conjunction with sequencing blood from each participant to serve as a matched normal control to enhance somatic variants calls. Germline variants, if identified, were only reported to the patient and clinician if the patient was enrolled on a clinical trial that permitted germline analysis (ClinicalTrials.gov identifier: NCT01775072) or if testing was done after appropriate genetic counseling. Samples were sequenced if tumor content met a minimum threshold of ≥ 10%-20%, and variant calls were made by expert opinion.
Statistical Methods
Overall survival (OS) was defined as time from diagnosis to time of death, and progression-free survival (PFS) was defined as time from diagnosis to the earlier time of disease progression or death. Patients were censored at the date of last follow-up. Survival rates were estimated using Kaplan-Meier estimates. Cox proportional hazards models were used to estimate the impact of the studied factors on OS and PFS (R v3.5.0 software). Variables that had P < .20 on univariable analysis were assessed by multivariable analysis. As information on ATRX aberration was unknown for 20% of the patients, it was not entered in the main multivariable analysis. P values were calculated using the Wald test. Independent variables related to ALK mutation included in the univariate analysis were presence versus absence of: (1) ALK F1174 mutation, (2) ALK R1275 mutation, and (3) other ALK mutation sites. All patients with locoregional disease seen at MSK between 2015 and 2019 were analyzed for the impact of any ALK mutation on OS and PFS compared with those with ALK-WT tumors.
RESULTS
Patient Characteristics
Of 641 patients with neuroblastoma who had ALK analyzed on at least one tumor sample, 103 (16%) patients (54 males and 49 females) had tumors with an ALK mutation and had long-term follow-up data available for review (Table 1). At diagnosis, median age was 2.6 years (range, birth to 67.8 years) and, by International Neuroblastoma Risk Group staging,31 30% (n = 31) of patients had locoregional disease, 68% (n = 70) had stage M neuroblastoma, and 2% (n = 2) had stage MS neuroblastoma. By International Neuroblastoma Risk Group risk stratification, 21 (20%), 11 (11%), and 71 (69%) patients had low-, intermediate-, and high-risk disease, respectively.31 Tumor specimens were sequenced at diagnosis, relapse, or both (59%, 31%, or 10%, respectively). Of the relapse specimens (n = 32), 59% (n = 19) were first relapse, 31% (n = 10) were subsequent relapses, and 9% (n = 3) were unknown.
TABLE 1.
Patient Characteristics (All Patients)
Mutation Sites and Frequency
Mutation status was evaluated by WGS (n = 21; 20%), MSK-IMPACT (n = 39; 38%) (Data Supplement), FoundationOne platforms (n = 25; 24%), or hotspot ALK mutation profiling (n = 18; 17%). Identified mutations included F1174 (51%), R1275 (29%), R1245 (10%), and others (10%) (Table 1). The other ALK mutations were interrogated using the St Jude PeCan database and relevant preclinical literature and were hypothesized to be activating with the exception of A876V, D1276E, E192K, and P1445S, which are variants of unknown clinical significance.2,16,32 These were included in the analysis. Germline ALK mutations (G1128A and R1275Q) were detected in 3/54 (6%) patients tested (including one pair of siblings). Coexisting MYCN amplification was noted in tumors of 36/103 (35%) patients and ATRX aberrations in tumors of 16/82 (20%), similar to reported findings.26 F1174 mutations were more commonly associated with MYCN amplification compared with other ALK mutations (46% v 22%, respectively; P = .02). Although MYCN amplification and ATRX aberrations were mutually exclusive, F1174 mutations and ATRX aberrations were not (five tumors harbored both F1174 mutation and ATRX aberrations).
Analysis of Serial Samples
Sequential tumor samples from 11 patients including paired diagnosis and/or relapse samples (six patients) and serial relapse samples (five patients) were studied. Six sets (55%) had concordant results. The five patients with discordant ALK status between tumor samples included three who had ALK-WT at diagnosis or first relapse and ALK T1151M (n = 1) and ALK F1174 (n = 2) in subsequent relapses. One patient had ALK-WT neuroblastoma at diagnosis and first relapse but with both R1275Q and F1245V mutations at third and fourth relapse. Another patient had a tumor harboring F1174L mutation at diagnosis, albeit at low mutant allele frequency (2.7%), and R1275Q mutation (with loss of F1174L mutation) in two subsequent relapses. The R1275Q mutation was present at a high frequency (variant allele frequency of 30.4%) in the latter indicating its pathogenicity.
Clinical Correlates of ALK Mutation Sites
The frequency of specific ALK mutations across age, stage, and risk was evaluated to identify enrichment of particular mutations with clinical correlates (Table 1). Regarding age at diagnosis, the cohort was divided into two groups: age 0-12 (n = 79) and > 12 years (n = 24). Age 12 was chosen as the cutoff because of the distinct biology of neuroblastoma in adolescent and adult patients.33-35 The frequency of F1174, R1275, and other ALK mutations did not differ significantly between the younger and older groups (P = .86). The frequency of F1174 mutations was increased in tumors of patients with high-risk disease (59% v 34%, P = .04). The non–high-risk group consisted of roughly equal numbers of mutations from each subgroup (F1174: 34%, R1275: 34%, and others: 31%; Table 1).
Response to Treatment and Survival
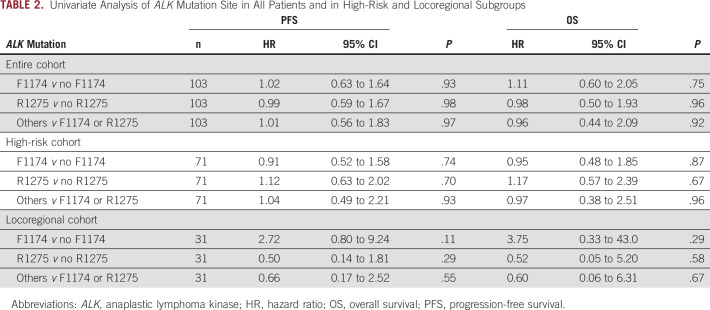
Of the 70 patients with stage M disease, all but one received high-dose chemotherapy (Table 2). This patient had metastases limited to a distant node and was treated with surgical resection and observation alone. Two patients presented with MS disease and were treated with surgery and observation, but both relapsed and were treated with chemotherapy. Of the patients with high-risk neuroblastoma (n = 71) treated with induction chemotherapy, 48% (n = 34) achieved a complete remission. Site of ALK mutation was not associated with complete response to induction chemotherapy in the high-risk group (complete response rates of 51%, 42%, and 50% in F1174, R1275, and other mutations, respectively; P = .79). Median follow-up for the entire cohort was 61.3 months. The 5-year PFS and OS for significant univariate analysis variables and hazard ratios are summarized in the Data Supplement. As expected, for the entire group of patients, those with high-risk, MYCN-amplified, and stage M disease had inferior outcomes. In multivariate analysis, disease stage was the only factor independently prognostic for risk of death. No factors were significantly prognostic for risk of progression. ALK mutation site was not found to be prognostic for PFS, nor OS in the entire cohort, high-risk subgroup, or locoregional subgroup (P > .05 for all groups; Figs 1A and 1B; Table 2). ALK mutation site was also nonprognostic for survival in either of the two treatment eras studied (P > .5), although, as expected, the 5-year PFS (22.6% v 30.7%, P = .61) and 5-year OS (40.6% v 68.2%, P = .008) improved between the two eras. A subgroup analysis evaluating survival differences between patients with MYCN-amplified tumors plus F1174 mutation and those with tumors with MYCN amplification plus all other ALK mutations was performed. There were no statistically significant differences noted in PFS or OS (P > .2 for both; Figs 2A and 2B). When evaluating all patients with F1174 ALK-mutant tumors and stratifying by the presence of MYCN amplification, as expected, there was a statistically significant inferior PFS and OS in the MYCN-amplified cohort (P = .02 and P = .04, respectively). In patients with MYCN-nonamplified stage M disease (Data Supplement), PFS in patients with ALK F1174 tumors compared with all other mutations was slightly superior (median survival of 36.3 months v 20.3 months, P = .04), but no difference was noted in OS (P = .13).
TABLE 2.
Univariate Analysis of ALK Mutation Site in All Patients and in High-Risk and Locoregional Subgroups
FIG 1.

(A) Progression-free survival and (B) overall survival of entire cohort by anaplastic lymphoma kinase mutation site.
FIG 2.

(A) Progression-free survival and (B) overall survival of tumors characterized by ALK F1174 and MYCN amplification compared with all other anaplastic lymphoma kinase mutations and MYCN amplification.
Impact of ALK Mutations on Locoregional Neuroblastoma
Thirty-one patients with ALK-mutant tumors had locoregional disease (diagnostic samples: n = 25, relapse: n = 6; Data Supplement). The site of ALK mutation was not found to affect survival (Table 2). Operative reports were available for review in 28 patients: 16 (57%) had surgery at MSK and 12 (43%) had surgery elsewhere; 85% (n = 24) had incomplete (< R1) resections (n = 2 complete resections each in MSK and outside hospital groups). The presence of segmental chromosomal abnormalities did not affect resectability (Data Supplement); 68% (21/31) of patients relapsed: 15 had local recurrence (n = 8 at MSK and n = 7 in outside hospital referred group) and six had metastatic recurrence, all MYCN-nonamplified (n = 5 referred from outside institutions). Of the patients with MYCN-amplified locoregional tumors, one patient had no progression after initial therapy and two had local recurrence (both ALK F1174L). For patients with ALK-mutant MYCN-nonamplified locoregional tumors (n = 28), 2-year PFS was 59% (95% CI, 37.4 to 80.5) and OS was 89.2% (95% CI, 75 to 100). F1174 mutations were nonprognostic in patients with MYCN-nonamplified locoregional tumors (P = .23 for PFS and OS). To further investigate this apparent poorer PFS compared with historical reports,36,37 we reviewed records of all patients with locoregional neuroblastoma seen at MSK between 2015 and 2019, when sequencing with MSK-IMPACT became routine for all patients. Tumors from 42 patients including the 12 ALK-mutant tumors (all without MYCN amplification) were identified (Data Supplement). Four patients with ALK-WT tumors had MYCN-amplified disease and were excluded from analysis. The 26 patients with ALK-WT, MYCN-nonamplified tumors were compared with the 12 patients with ALK-mutant tumors. There was no significant difference in type of systemic treatment, histology, or DNA index between the two groups (P > .5 for each). However, there was a statistically significant difference in tumor resectability with only 2/12 (17%) R1 resections in the ALK-mutant patients compared with 18/26 (69%) in the ALK-WT cohort (P = .004). All ALK-mutant tumors (12/12) in this group had L2 disease, whereas 18/26 (69%) of the ALK-WT group were classified as L2 (P = .04). Two-year PFS showed a trend toward inferior survival in the ALK-mutant group compared with the ALK-WT group (61.9%, 95% CI, 23.1 to 100 v 90.9, 95% CI, 73.9 to 100; P = .09 via log-rank).
DISCUSSION
To our knowledge, this is the second-largest, clinically annotated publication on patients with ALK-mutant neuroblastoma and the largest single-institution experience. This cohort allowed us to examine the differential impact of various ALK mutations on response to therapy and on survival, although we did not identify an association between ALK mutation site (F1174 v R1275 v others) and PFS or OS in the entire cohort, in patients with high-risk disease, or in patients with locoregional neuroblastoma. Response to chemotherapy in high-risk patients was also not associated with a particular ALK mutation.
However, locoregional ALK-mutant neuroblastoma appears to have a more aggressive phenotype in this cohort. When compared with ALK-WT tumors, ALK-mutant tumors were more likely to have L2 staging, less likely to undergo R1 resection, and had a trend toward lower PFS (P = .09). It is possible that because of the small sample size of patients with ALK-mutant tumors in the MSK-IMPACT era (n = 12 ALK-mutant tumors), the Kaplan-Meier estimate was not adequately powered to show a significant difference in PFS. Resectability and local recurrence were not affected by the site at which surgery was performed (MSK or elsewhere). Additionally, recurrence was noted in a high proportion (68%) of patients and 2-year PFS (59%) was lower than ranges (74%-90%) previously reported by us and large cooperative studies for patients with nonmetastatic disease.36,37 These inferior outcomes were not attributable to coexisting MYCN amplification. However, it is possible that patients with locoregional disease with subsequent distant metastases may represent a selection bias as the majority were referred to MSK from outside centers. Although our single-institution outcomes are not directly generalizable to the broader neuroblastoma population, these data suggest that patients with ALK-mutant locoregional disease may benefit from increased surveillance.
Several studies suggest that ALK F1174 is a particularly pathogenic mutation that can confer resistance to ALK inhibitors,1 is often coexpressed with MYCN amplification, and is potentially associated with inferior event-free survival.2,17 However, our findings only partially confirmed these assertions. We confirmed the finding that ALK F1174 is more commonly coexpressed with MYCN amplification, but there was no difference in survival identified in patients harboring tumors with ALK F1174 plus MYCN amplification versus all other ALK mutations plus MYCN amplification. When comparing the impact of ALK F1174 versus other mutations in metastatic MYCN-nonamplified tumors, our data suggest that F1174 may be associated with a superior PFS (P = .04). Taken together, these results suggest that MYCN amplification is the primary driver of aggressive phenotype rather than ALK F1174, and the contribution of the latter, if any, is modest.
As this study retrospectively evaluated a convenience cohort of a single institution, there are inherent limitations. Before 2015, MSK-IMPACT was done only in select clinical scenarios, often in patients with chemorefractory disease and at times of relapse in hopes of identifying a targetable mutation. This approach led to an overrepresentation of high-risk patients in this cohort (69%) compared with historical experience (42%).2 Because of this selection bias, a higher proportion of tumors tested (16%) harbored an ALK mutation and ALK F1174 mutation was overrepresented in our cohort (51% v historical reports of 30%-35%).2,4,6,7 Furthermore, being a regional referral center, we acknowledge that a referral bias may contribute to an overrepresentation of high-risk patients in this population. Additional limitations with this type of study include variability of completeness of clinical data, exclusion of patients with incomplete follow-up data, and challenges addressing all confounding factors. Last, because of the retrospective nature of this analysis, we did not compare these outcomes with the entire cohort of patients with ALK-WT tumors, nor did we evaluate the impact of ALK amplification. Definitively addressing these critical questions will likely require a prospective study on uniformly treated patients. To validate the results described here (impact of different ALK mutation loci) in a prospective study, assuming a comparison between two groups distributed 1/3 and 2/3 of the patients, 74 events would be required to detect a hazard ratio of 0.50 with 80% power and a two-sided type I error of 0.05.
On the basis of the literature to date and this report of our single-institution experience, the data are mixed regarding the prognostic influence of ALK in patients with neuroblastoma. We did not find a specific ALK mutation site (compared with other loci) prognostic of PFS or OS but did potentially identify a cohort of patients (those with locoregional disease) who may have increased conferred risk because of these aberrations (compared with ALK-WT). Because of poor outcomes of patients with relapsed or refractory neuroblastoma despite chemotherapy and immunotherapy, ALK continues to be an appealing targetable biomarker despite disappointing results to date with early-generation ALK inhibitors. Greater antineuroblastoma activity is anticipated with recently introduced agents such as the third-generation ALK inhibitor, lorlatinib (ClinicalTrials.gov identifier: NCT03107988), but further research is needed to translate these promising therapies into substantial and durable responses in the clinic. A better understanding of enriched responder populations and mechanisms of resistance is necessary to identify the optimal ALK inhibitor and/or combination with other agents to fully exploit this potential therapeutic vulnerability in neuroblastoma.
ACKNOWLEDGMENT
We would like to acknowledge the MSK Kids Pediatric Translational Medicine Program for their support with data curation and Joseph Olechnowicz (Senior Editor, Memorial Sloan Kettering Department of Pediatrics) for editorial assistance.
PRIOR PRESENTATION
Presented in part as a poster presentation at the 51st annual Société Internationale d'Oncologie Pédiatrique (SIOP), Lyon, France, October 23-26, 2019.
SUPPORT
Supported by the Cancer Center Support Grant P30 CA008748. T.O'D is partially supported by the NIH/NCATS Grant no. UL1-TR-002384.
AUTHOR CONTRIBUTIONS
Conception and design: Tara O'Donohue, Stephen Roberts, Shakeel Modak
Administrative support: Shakeel Modak
Provision of study materials or patients: Brian H. Kushner, Nai-Kong Cheung, Shakeel Modak
Collection and assembly of data: Tara O'Donohue, Nitya Gulati, Neerav Shukla, M. Irene Rodriguez-Sanchez, Nancy Bouvier, Ellen Basu, Nai-Kong Cheung, Shakeel Modak
Data analysis and interpretation: Tara O'Donohue, Audrey Mauguen, Brian H. Kushner, Neerav Shukla, Stephen Roberts, Ellen Basu, Nai-Kong Cheung, Shakeel Modak
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Neerav Shukla
Consulting or Advisory Role: Syndax
Nai-Kong Cheung
Stock and Other Ownership Interests: Ymabs Therapeutics Inc, Abpro, Eureka Therapeutics
Consulting or Advisory Role: Abpro, Eureka Therapeutics
Research Funding: Ymabs Therapeutics Inc, Abpro
Patents, Royalties, Other Intellectual Property: scfv constructs of anti-GD2 antibodies, therapy-enhancing glucan, use of mAb 8H9, methods for preparing and using scFv, GD2 peptide mimics, methods for detecting MRD, anti-GD2 antibodies, generation and use of HLA-A2restricted peptide-specific mAbs and CARs, high-affinity anti-GD2 antibodies, multimerization technologies, bispecific HER2 and CD3 binding molecules, affinity matured hu8H9, anti-chondroitin sulfate proteoglycan 4 antibodies and uses thereof, ROR2 antibodies, T cell receptor-like antibody agents specific for EBV latent membrane protein 2A peptide presented by human HLA, Anti-CD33 antibody agents, Anti-KIR3DL1 antibodies, Modular self-assembly disassembly (SADA) technologies, A33 - C825 conjugate for Pretargeted Radioimmunotherapy and application as a theranostic product, Anti-L1-CAM Antibodies and Uses Thereof, Anti-A33 Antibodies and Uses Thereof, DOTA BsAb for new Humanized next generation anti-GPA33 antibodies with Fc-enhanced function or bispecific properties, Herceptin - C825 conjugate for Pretargeted Radioimmunotherapy and application as a theranostic product, Anti-polysialic acid antibodies and uses thereof, Methods of enhancing immunogenicity of poorly immunogenic anti-specific vaccines using oral yeast beta-glucans, Small Molecule Hapten Chelates for Pretargeted Radioimmunotherapy with Anti-DOTA(lanthanide) Bispecific Antibodies (Proteus), A N-acetylgalactosamino dendron-clearing agent for DOTA-pretargeted radioimmunotherapy, 30. Heterodimeric tetravalency and specificity antibody compositions and uses thereof (HDTVS), Multimerization of IL-15/IL-15R alpha complexes to enhance immunotherapy, CD22 antibodies and methods of using the same, CD33 antibodies and methods of using the same to treat cancer, CD19 antibodies and methods of using the same, Anti-CD33 antibodies for treating cancer, Anti-STEAP-1 antibodies and uses thereof, Anti-Glypican 3 antibodies and uses thereof, Multimodal fluorine-Cy3/5/7-DOTA-hapten compositions, diagnostics, fluorescence guided surgery and radioimmunotherapy, Anti-CD3 antibodies and uses thereof40. Anti-CD3 antibodies and uses thereof, Anti-GD2 SADA conjugates and uses thereof, Anti-GD2 antibodies and uses thereof, Dectin-1 (CLEC7A) single nucleotide polymorphism as a biomarker for predicting antibody response when using b-glucan as a vaccine adjuvant
Travel, Accommodations, Expenses: Partners Therapeutics
Shakeel Modak
Consulting or Advisory Role: Ymabs Therapeutics Inc, Illumina RP
Patents, Royalties, Other Intellectual Property: Two patents pending; no financial benefit
No other potential conflicts of interest were reported.
REFERENCES
- 1.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma. Nature. 2008;455:975. doi: 10.1038/nature07397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresler SC, Weiser DA, Huwe PJ, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma Cancer Cell 26682–6942014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janoueix-Lerosey I, Lequin D, Brugieres L, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma Nature 455967–9702008 [DOI] [PubMed] [Google Scholar]
- 4.Hallberg B, Palmer RH.Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology Nat Rev Cancer 13685–7002013 [DOI] [PubMed] [Google Scholar]
- 5.Blume-Jensen P, Hunter T.Oncogenic kinase signalling Nature 411355–3652001 [DOI] [PubMed] [Google Scholar]
- 6.Padovan-Merhar OM, Raman P, Ostrovnaya I, et al. Enrichment of targetable mutations in the relapsed neuroblastoma genome. PLoS Genet. 2016;12:e1006501. doi: 10.1371/journal.pgen.1006501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Brouwer S, De Preter K, Kumps C, et al. Meta-analysis of neuroblastomas reveals a skewed ALK mutation spectrum in tumors with MYCN amplification Clin Cancer Res 164353–43622010 [DOI] [PubMed] [Google Scholar]
- 8.Knudson AG., JrStrong LC: Mutation and cancer: Neuroblastoma and pheochromocytoma Am J Hum Genet 24514–5321972 [PMC free article] [PubMed] [Google Scholar]
- 9.Kushner BH, Gilbert F, Helson L.Familial neuroblastoma—Case reports, literature review, and etiologic considerations Cancer 571887–18931986 [DOI] [PubMed] [Google Scholar]
- 10.Maris JM, Kyemba SM, Rebbeck TR, et al. Molecular genetic analysis of familial neuroblastoma Eur J Cancer 331923–19281997 [DOI] [PubMed] [Google Scholar]
- 11.Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene Nature 455930–9352008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matthay KK, Maris JM, Schleiermacher G, et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2:16078. doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 13.Trochet D, Bourdeaut F, Janoueix-Lerosey I, et al. Germline mutations of the paired-like homeobox 2B (PHOX2B) gene in neuroblastoma Am J Hum Genet 74761–7642004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ardini E, Magnaghi P, Orsini P, et al. Anaplastic lymphoma kinase: Role in specific tumours, and development of small molecule inhibitors for cancer therapy Cancer Lett 29981–942010 [DOI] [PubMed] [Google Scholar]
- 15.Holla VR, Elamin YY, Bailey AM, et al. ALK: A tyrosine kinase target for cancer therapy. Cold Spring Harb Mol Case Stud. 2017;3:a001115. doi: 10.1101/mcs.a001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bresler SC, Wood AC, Haglund EA, et al. Differential inhibitor sensitivity of ALK variants found in neuroblastoma. Sci Transl Med. 2011;3:108ra114. doi: 10.1126/scitranslmed.3002950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berry T, Luther W, Bhatnagar N, et al. The ALK(F1174L) mutation potentiates the oncogenic activity of MYCN in neuroblastoma Cancer Cell 22117–1302012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleiermacher G, Javanmardi N, Bernard V, et al. Emergence of new ALK mutations at relapse of neuroblastoma J Clin Oncol 322727–27342014 [DOI] [PubMed] [Google Scholar]
- 19.Lee JW, Park SH, Kang HJ, et al. ALK protein expression is related to neuroblastoma aggressiveness but is not independent prognostic factor Cancer Res Treat 50495–5052018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Javanmardi N, Fransson S, Djos A, et al. Low frequency ALK hotspots mutations in neuroblastoma tumours detected by ultra-deep sequencing: Implications for ALK inhibitor treatment. Sci Rep. 2019;9:2199. doi: 10.1038/s41598-018-37240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George RE, Sanda T, Hanna M, et al. Activating mutations in ALK provide a therapeutic target in neuroblastoma Nature 455975–9782008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mosse YP, Lim MS, Voss SD, et al. Safety and activity of crizotinib for paediatric patients with refractory solid tumours or anaplastic large-cell lymphoma: A Children's Oncology Group phase I consortium study Lancet Oncol 14472–4802013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geoerger B, Schulte J, Zwaan CM, et al. Phase I study of ceritinib in pediatric patients (Pts) with malignancies harboring a genetic alteration in ALK (ALK+): Safety, pharmacokinetic (PK), and efficacy results. J Clin Oncol. 2015;33:10005. [Google Scholar]
- 24.Guan J, Tucker ER, Wan H, et al. The ALK inhibitor PF-06463922 is effective as a single agent in neuroblastoma driven by expression of ALK and MYCN Dis Model Mech 9941–9522016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cervantes-Madrid D, Szydzik J, Lind DE, et al. Repotrectinib (TPX-0005), effectively reduces growth of ALK driven neuroblastoma cells. Sci Rep. 2019;9:19353. doi: 10.1038/s41598-019-55060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheung NK, Zhang J, Lu C, et al. Association of age at diagnosis and genetic mutations in patients with neuroblastoma JAMA 3071062–10712012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas RK, Baker AC, Debiasi RM, et al. High-throughput oncogene mutation profiling in human cancer Nat Genet 39347–3512007 [DOI] [PubMed] [Google Scholar]
- 28.Shukla N, Ameur N, Yilmaz I, et al. Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways Clin Cancer Res 18748–7572012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng DT, Mitchell TN, Zehir A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology J Mol Diagn 17251–2642015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyman DM, Solit DB, Arcila ME, et al. Precision medicine at Memorial Sloan Kettering Cancer Center: Clinical next-generation sequencing enabling next-generation targeted therapy trials Drug Discov Today 201422–14282015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: An INRG task force report J Clin Oncol 27289–2972009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St. Jude Children's Research Hospital . St. Jude PeCan Data Portal. http://pecan.stjude.cloud [Google Scholar]
- 33.Franks LM, Bollen A, Seeger RC, et al. Neuroblastoma in adults and adolescents: An indolent course with poor survival Cancer 792028–20351997 [DOI] [PubMed] [Google Scholar]
- 34.Gaspar N, Hartmann O, Munzer C, et al. Neuroblastoma in adolescents Cancer 98349–3552003 [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Kushner BH, Kramer K, et al. Treatment and outcome of adult-onset neuroblastoma Int J Cancer 1431249–12582018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker DL, Schmidt ML, Cohn SL, et al. Outcome after reduced chemotherapy for intermediate-risk neuroblastoma N Engl J Med 3631313–13232010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Modak S, Kushner BH, LaQuaglia MP, et al. Management and outcome of stage 3 neuroblastoma Eur J Cancer 4590–982009 [DOI] [PMC free article] [PubMed] [Google Scholar]