PURPOSE
This study examines oncologist-reported reasons for not using multimarker tumor panel testing and the association between these reasons and oncologist-level, facility-level, and patient-mix characteristics.
METHODS
We used data collected from a nationally representative sample (N = 1,281) of medical oncologists participating in the National Cancer Institute's National Survey of Precision Medicine in Cancer Treatment.
RESULTS
In addition to testing not being seen as relevant (87%) and no evidence of test utility (77%), the most frequently reported reasons for not ordering a multimarker tumor panel test was difficulty in obtaining sufficient tissue (57%) and using individual gene tests (72%). These reasons were more likely to be reported by oncologists practicing in rural clinics and less likely to be reported by oncologists with an academic affiliation or with access to genetic services such as on-site genetic counselors and internal genetic testing policies.
CONCLUSION
Modifiable, organizational factors were associated with ordering multimarker tumor panels. Receipt of genomics training and organizational policies related to the use of genomics were associated with lower reporting of barriers to ordering multimarker tumor panels, pointing to potential targets for future studies aimed at increasing appropriate multimarker tumor panel testing in cancer treatment management.
INTRODUCTION
The use of tumor genomics to inform treatment choice is the cornerstone of precision oncology. Increasingly, multimarker tumor panels are used in cancer care and include DNA and RNA analyses through next-generation sequencing (NGS), targeted gene expression profiling tests to estimate prognosis and/or the risk of recurrence, and custom panels that profile tumor characteristics to guide the selection of targeted therapies. For example, 76% of oncologists use NGS tests to guide treatment decisions.1 However, little is known about clinic and organizational factors related to multimarker tumor panel testing. More broadly, a combination of system, provider, and patient factors has been shown to influence the implementation of healthcare innovations; thus, a multilevel perspective may be important for understanding genomic test use to guide patient care.2
CONTEXT
Key Objective
What provider- and organizational-level characteristics are associated with oncologist-reported reasons for not using multimarker tumor panel testing?
Knowledge Generated
Testing not being relevant, no evidence of test utility, difficulty in obtaining sufficient tissue, and using individual gene tests were the most frequently reported reasons for not ordering a multimarker tumor panel test. These reasons were more likely to be reported by oncologists practicing in rural clinics and less likely to be reported by oncologists with an academic affiliation or with access to genetic services such as on-site genetic counselors and internal genetic testing policies.
Relevance
Modifiable, organizational factors were associated with ordering multimarker tumor panels. Pending future research, receipt of genomics training, and organizational policies related to the use of genomics may be targets for increasing appropriate multimarker tumor panel testing in cancer treatment management.
Previous work suggests that provider factors such as younger age, holding a faculty appointment, and genomics training are associated with genomic test use. System factors such as larger patient volume and access to a molecular tumor board are also positively associated with oncologists' use of genomic testing.1 Although understudied, other clinic and organizational factors are likely related to multimarker tumor panel testing. A recent systematic review demonstrated that system factors, including electronic medical record systems and supports, policies and guidelines for integrating genomics into practice, access to genetics services, costs, and a trained workforce, are all determinants for the implementation of genomic medicine.3
Although some studies have identified factors associated with multimarker tumor panel testing, few have described specific reasons why oncologists might choose not to use this testing. This information is important to fully understand the testing landscape and to identify factors that should be addressed to foster appropriate use of multimarker tumor panel testing in practice. This study examines reasons—related to clinic processes, resources, and perceived relevance of testing—that oncologists report for not ordering multimarker tumor panels for a patient. We also examined the association between these reasons and oncologists' provider-, facility-, and patient-mix characteristics.
METHODS
The National Survey of Precision Medicine in Cancer Treatment, conducted by the National Cancer Institute, includes data collected from a nationally representative sample of medical oncologists in 2017. This survey included information on participants' demographics, practice characteristics, and availability of genomic testing resources. Participants were identified through the American Medical Association Physician Masterfile. Oncologists were eligible to participate if they were currently practicing, not in training, and < 75 years of age. Thirty-eight percent of eligible oncologists participated (1,281 of 3,378). Additional information on data collection methods has been published elsewhere.4
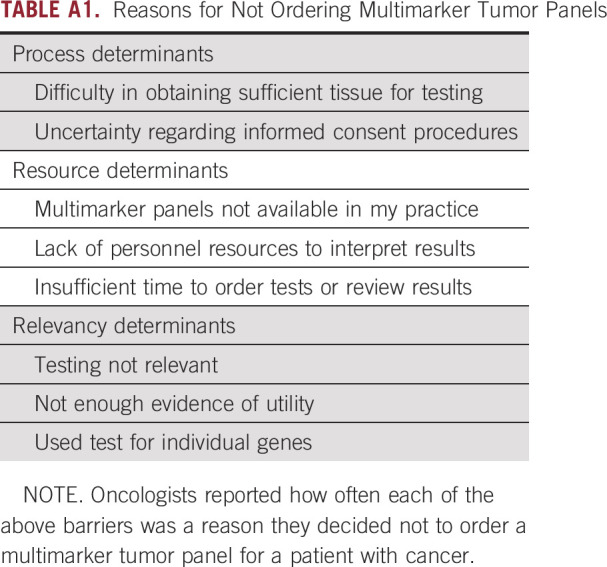
Dependent variables included responses to the following question: “The next question is about the times during the past 12 months when you decided NOT to order a multimarker tumor panel for a cancer patient. When this occurred, how often was it for the following reasons?” Respondents were then asked specifically about issues related to clinic processes: uncertainty regarding informed consent procedures and difficulty in obtaining sufficient tissue for testing; resources: multimarker panels were not available in my practice, lack of personnel or resources to interpret test results, and insufficient time to order tests or review results; and relevance: testing was not relevant, not enough evidence of utility, and used tests for individual genes, rather than multimarker tumor panels (Appendix Table A1). We report the frequency with which these reasons were cited (sometimes or often versus rarely or never).
We used modified Poisson regressions to estimate prevalence ratios (PRs) and 95% CIs to evaluate bivariate associations between these reasons and provider- (primary specialty; practice at or affiliated with an academic center; and formal genomic testing training), facility- (practice type, location, and available genomic services), and patient-mix (number of patients with cancer and patients with metastatic cancer per month; percent of Medicaid patients) characteristics among our full sample (see Table 1 for a full description of included variables). In other words, we examined the provider and organizational factors associated with specific reasons that oncologists did not order a multimarker tumor panel (dependent variables). We employed modified Poisson regressions given that our outcomes occurred in more than 10% of the study population; using logistic regression to estimate odds ratios may have upwardly biased our results. Furthermore, as a sensitivity analysis, we also examined these associations in samples of oncologists who (1) only reported NGS test use and (2) among oncologists who used multimarker tumor panels more broadly, given that participants might have responded to these questions with particular types of multimarker tumor panels in mind.
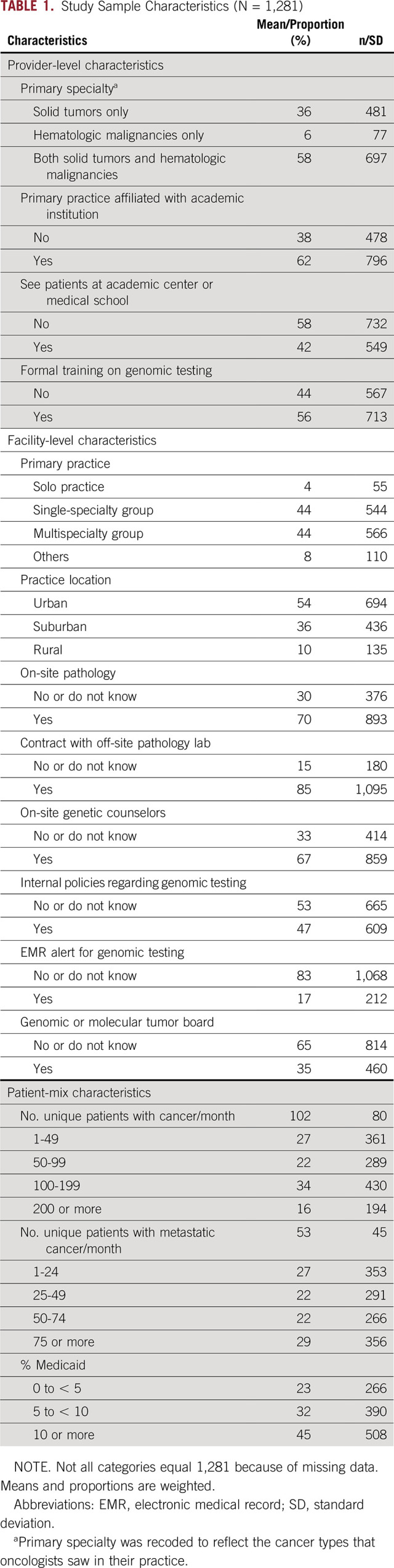
TABLE 1.
Study Sample Characteristics (N = 1,281)
Analyses included survey weights, which were calculated based on oncologist age, sex, and location and also adjusted for complex survey design and accounted for the probability of selection, noncontact, and noncooperation. All analyses were conducted in STATA version 13.0 software (STATA Corp, College Station, TX).
RESULTS
Sample Characteristics
On average, oncologists cared for 102 patients with cancer/month, 53 of whom had metastatic cancer (Table 1). More than half (58%) saw patients with both solid and hematologic malignancies, and about a third (36%) saw only patients with solid tumors. Most were affiliated with academic institutions (62%), and about half (54%) practiced in urban areas versus 10% in rural locations. Oncologists were part of single-specialty (44%) or multispecialty (44%) groups. Most had some formal genomic training (56%). The availability of genomic services varied; most oncologists reported having on-site pathology services (70%), contracts with off-site pathology services (85%), on-site genetic counselors (67%), and internal policies regarding genomic testing (53%). In contrast, only 17% had electronic medical record (EMR) alerts for genomic testing and 35% had genomic and/or molecular tumor boards (35%).
Reasons for Not Ordering Multimarker Tumor Panels
Oncologists' reasons for not ordering multimarker tumor panels varied widely. Testing not being relevant was most frequently reported as sometimes or often a reason for not ordering multimarker tumor panels (87%), followed by lack of evidence of the tests' utility (77%) (Fig 1). More than half cited difficulty in obtaining sufficient tissue for testing (57%) and using tests for individual genes (72%) as sometimes or often a reason for not using multimarker tumor panel testing. In contrast, a minority of oncologists reported that uncertainty regarding informed consent procedures (8%), unavailability of multimarker tumor panels in their practice (17%), not having the personal resources to interpret results (12%), and insufficient time to order tests or review results (25%) were sometimes or often a reason for not using multimarker tumor panels.
FIG 1.
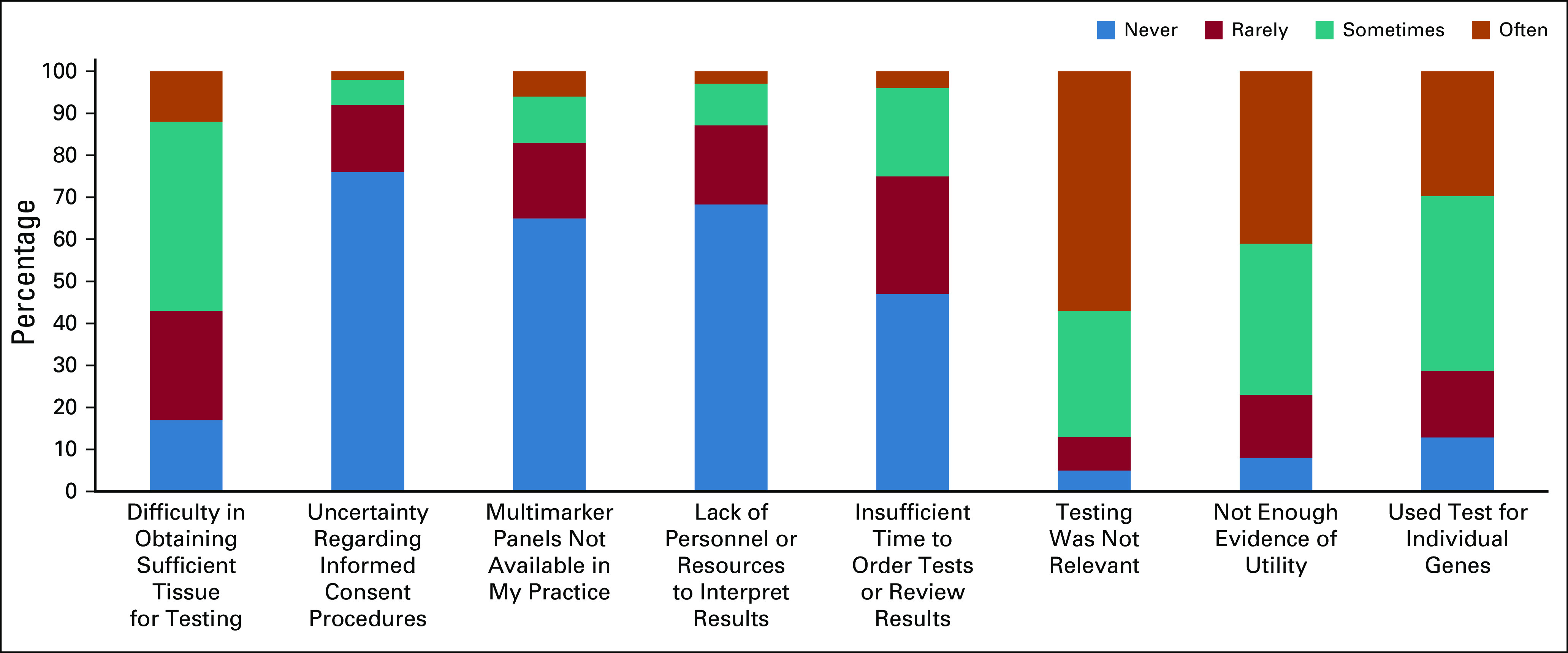
Reasons during the past 12 months oncologists decided not to order a multimarker tumor panel for a patient with cancer.
Provider and Organizational Factors Associated With Reasons for Not Ordering Multimarker Tumor Panels
Processes
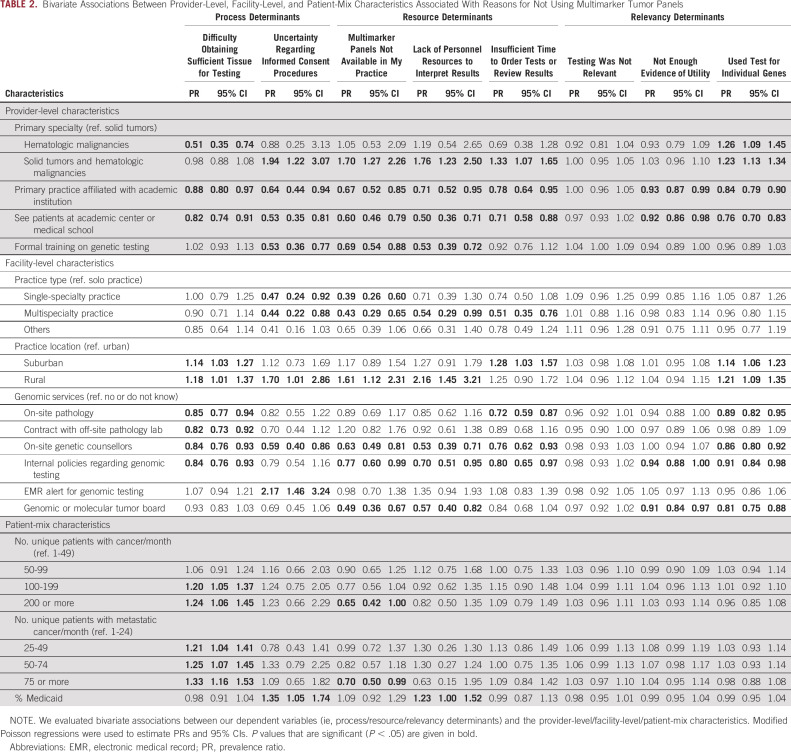
Oncologists affiliated with or practicing at academic centers or at facilities with on-site genetic counselors were less likely to report difficulty in obtaining sufficient tissue for testing or uncertainty regarding informed consent procedures as reasons for not ordering multimarker tumor panels (Table 2). Additionally, oncologists at facilities with on-site pathology, internal genomic testing policies, and on-site genetic counselors were less likely to report difficulty in obtaining sufficient tissue for testing as a reason for not ordering multimarker tumor panels. Having EMR alerts for genomic testing was associated with reporting uncertainty regarding informed consent procedures as a reason for not ordering multimarker tumor panels.
TABLE 2.
Bivariate Associations Between Provider-Level, Facility-Level, and Patient-Mix Characteristics Associated With Reasons for Not Using Multimarker Tumor Panels
Resources
Oncologists who were affiliated with or seeing patients at academic medical centers, were working in a multispecialty practice, had on-site genetic counselors, and had internal genetic testing policies were less likely to report:(1) not having panels available in their practice and (2) inadequate time and personnel to review and interpret results as reasons for not ordering multimarker tumor panels. Additionally, oncologists formally trained in genetic testing were less likely to report lack of personnel resources to interpret results and multimarker tumor panels not being available in their practice as reasons for not ordering testing. In contrast, rural oncologists were more likely to report not having multimarker tumor panel tests in their practice and inadequate personnel resources to interpret them as reasons for not ordering multimarker tumor panels.
Relevance
Oncologists who were affiliated with or saw patients at an academic center or practiced at facilities with internal policies regarding testing or a genomic tumor board were less likely to report not having enough evidence of a panel test's utility or using individual gene tests instead as reasons for not using panel testing. In contrast, oncologists practicing in rural and suburban areas were more likely to report using individual gene tests as reasons for not using multimarker tumor panels compared with urban oncologists. No provider-level, facility-level, or patient-mix characteristics were associated with testing not being relevant as a reason for not using multimarker tumor panel testing.
Our sensitivity analyses demonstrated similar results (results not shown), suggesting that findings were similar for NGS users only and those using different types of multimarker tumor panel tests.
DISCUSSION
Oncologists report multiple reasons for not ordering a multimarker tumor panel. In particular, perceived relevance and clinical utility were the most important drivers of testing. However, using single-gene tests and difficulty in obtaining sufficient tissue for testing also appear to drive the use of multimarker tumor panel testing. Of interest, seeing patients in an academic center and having internal policies regarding genomic testing were associated with a lower likelihood of reporting that these tests often or sometimes do not have enough evidence of utility, that they used test for individual genes instead, and that there was difficulty in obtaining sufficient tissue for testing. These oncologists have greater access to support infrastructure for multimarker tumor panel testing, including genomic services.
Other reasons for not using multimarker tumor panels, such as lack of availability of multimarker panels and a lack of personnel resources, were less likely to be endorsed as reasons for not ordering tests by oncologists who reported receiving genomics training. Indeed, a recent review identified studies showing that genetics and/or genomics training for nongenetics providers can improve outcomes including knowledge, self-efficacy, and skills related to the use of genomics in clinical practice.5 In addition, lack of resources and clinical processes around informed consent were more likely to be reported often or sometimes among oncologists who did not have an academic affiliation.
In addition to provider characteristics, organizational and community factors were associated with reasons for not ordering tests. Oncologists practicing in rural communities were more likely to report a lack of resources and genomic testing process concerns. In addition, process concerns and lacking time to perform panels were reported particularly among oncologists practicing in a solo practice. Taken together, these findings suggest that future work should support the appropriate implementation of multimarker tumor panel use among oncologists without genomics training and academic affiliations, as well as those who practice within solo practices and rural communities. Future research should explore the acceptability and feasibility of employing virtual tumor boards, electronic health record ordering assistance, and tailored decision aids to address these reasons for not using multimarker tumor panel testing.6-8
Other research on the implementation of precision medicine highlighted the importance of dedicated pathologists and laboratory staff as well as bioinformaticians for the sustained implementation of their precision medicine program.9 Similarly, a recent paper on the implementation of a pharmacogenomics program noted the importance of clinical decision support and integration of laboratory results into the electronic health record and the key role that dedicated IT and laboratory staff play in making genomic tests available to providers and their patients.10 Future work should include these key stakeholders' perspectives on the use of multimarker tumor panel tests in practice and examine whether organizational-level strategies to improve access to genomic services may improve oncologists' ability to appropriately order multimarker tumor panel testing.
Oncologists who participated in this survey may differ from those who did not participate. However, we used statistical adjustment for nonresponse such that respondents are representative of the population of practicing oncologists in the United States in terms of age, sex, and geographic location.1 Additionally, our participation rate was 38%; however, we accounted for nonresponse bias by including weights calculated using data from the survey's sample frame. Third, we examined barriers to testing from oncologists' perspectives. Thus, the perspectives of nononcologist providers and clinic administrators are not represented. Studying the views across stakeholders will provide a more holistic view of the implementation of multimarker panel use. Finally, we were limited to exploring the reasons for not ordering multimarker tumor panels that were provided in the survey; future research might employ qualitative methods to further explore other potential reasons that oncologists do not order these panels.
In conclusion, the use of multimarker tumor panels is largely driven by clinical utility and relevance. However, we also identified modifiable reasons for not using multimarker tumor panel tests on the provider and organizational levels that can be targets of multilevel interventions. Organizations without certain genomic services, as well as oncologists without genomics training or who practice in rural and community settings, may be important targets for future studies aimed at increasing appropriate uptake of these tests.
Appendix
TABLE A1.
Reasons for Not Ordering Multimarker Tumor Panels
SUPPORT
Supported in part by the National Institutes of Health and American Cancer Society (contract Nos. HHSN261201400011) to Scientific Consulting Group and HHSN261201000086I to RTI International. L.P.S. was supported by a Cancer Care Quality Postdoctoral Traineeship, University of North Carolina at Chapel Hill, Grant No. T32-CA-116339. M.C.R. was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2TR002490. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the US Department of Health and Human Services. The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report.
AUTHOR CONTRIBUTIONS
Conception and design: Megan C. Roberts, Lisa P. Spees, Andrew N. Freedman, Janet S. de Moor
Administrative support: Lisa P. Spees
Provision of study materials or patients: Andrew N. Freedman, Janet S. de Moor
Collection and assembly of data: Andrew N. Freedman, Janet S. de Moor
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Megan C. Roberts
Stock and Other Ownership Interests: Merck (I)
Janet S. de Moor
Employment: Biogen
Stock and Other Ownership Interests: Biogen
Travel, Accommodations, Expenses: Biogen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Freedman AN, Klabunde CN, Wiant K, et al. : Use of next-generation sequencing tests to guide cancer treatment: Results from a nationally representative survey of oncologists in the United States. JCO Precis Oncol:1-13, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Damschroder LJ, Aron DC, Keith RE, et al. : Fostering implementation of health services research findings into practice: A consolidated framework for advancing implementation science. Implement Sci 4:50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pearce C, Goettke E, Hallowell N, et al. : Delivering genomic medicine in the United Kingdom National Health Service: A systematic review and narrative synthesis. Genet Med 21:2667-2675, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Wiant K, Geisen E, Creel D, et al. : Risks and rewards of using prepaid vs. postpaid incentive checks on a survey of physicians. BMC Med Res Methodol 18:104, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Talwar D, Tseng TS, Foster M, et al. : Genetics/genomics education for nongenetic health professionals: A systematic literature review. Genet Med 19:725-732, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Rao S, Pitel B, Wagner AH, et al. : Collaborative, multidisciplinary evaluation of cancer variants through virtual molecular tumor boards informs local clinical practices. JCO Clin Cancer Inform 4:602-613, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen SA, Bradbury A, Henderson V, et al. : Genetic counseling and testing in a community setting: Quality, access, and efficiency. Am Soc Clin Oncol Educ Book 39:e34-e44, 2019 [DOI] [PubMed] [Google Scholar]
- 8.Wakefield CE, Meiser B, Homewood J, et al. : A randomized controlled trial of a decision aid for women considering genetic testing for breast and ovarian cancer risk. Breast Cancer Res Treat 107:289-301, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Meric-Bernstam F, Farhangfar C, Mendelsohn J, et al. : Building a personalized medicine infrastructure at a major cancer center. J Clin Oncol 31:1849-1857, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petry N, Baye J, Aifaoui A, et al. : Implementation of wide-scale pharmacogenetic testing in primary care. Pharmacogenomics 20:903-913, 2019 [DOI] [PubMed] [Google Scholar]