PURPOSE
Advances in precision oncology, including RAS testing to predict response to epidermal growth factor receptor monoclonal antibodies (EGFR mAbs) in colorectal cancer (CRC), can extend patients’ lives. We evaluated uptake and clinical use of KRAS molecular testing, guideline recommended since 2010, in the Veterans Affairs Healthcare System (VA).
MATERIALS AND METHODS
We conducted a retrospective cohort study of patients with stage IV CRC diagnosed in the VA 2006-2015. We gathered clinical, demographic, molecular, and treatment data from the VA Corporate Data Warehouse and 29 commercial laboratories. We performed multivariable analyses of associations between patient characteristics, KRAS testing, and EGFR mAb treatment.
RESULTS
Among 5,943 patients diagnosed with stage IV CRC, only 1,053 (17.7%) had KRAS testing. Testing rates increased from 2.3% in 2006 to 28.4% in 2013. In multivariable regression, older patients (odds ratio, 0.17; 95% CI, 0.09 to 0.32 for ≥ age 85 v < 45 years) and those treated in the Northeast and South regions were less likely, and those treated at high-volume CRC centers were more likely to have KRAS testing (odds ratio, 2.32; 95% CI, 1.48 to 3.63). Rates of potentially guideline discordant care were high: 64.3% (321/499) of KRAS wild-type (WT) went untreated with EGFR mAb and 8.8% (401/4,570) with no KRAS testing received EGFR mAb. Among KRAS-WT patients, survival was better for patients who received EGFR mAb treatment (29.6 v 18.8 months; P < .001).
CONCLUSION
We found underuse of KRAS testing in advanced CRC, especially among older patients and those treated at lower-volume CRC centers. We found high rates of potentially guideline discordant underuse of EGFR mAb in patients with KRAS-WT tumors. Efforts to understand barriers to precision oncology are needed to maximize patient benefit.
BACKGROUND
Colorectal cancer (CRC) is among the most frequently diagnosed cancers for both men and women, and one of the leading causes of cancer-related deaths.1 An estimated 145,600 new cases of CRC will be diagnosed in the United States in 2019 and an estimated 51,020 will die from the disease.2 The 5-year survival rate of metastatic CRC has doubled in the past few decades, thanks in part to advances in targeted and personalized therapies.3 Cetuximab and panitumumab, both monoclonal antibodies (mAb) targeting the epidermal growth factor receptor (EGFR), extend survival in patients whose tumors lack RAS gene mutations but are ineffective in those with KRAS and NRAS mutations.4,5 To assure appropriate selection of therapy, RAS mutation testing is the standard of care and has been included in both ASCO and National Comprehensive Cancer Network recommendations since 2009.6-9 Testing for RAS mutations ensures that patients with mutations will neither waste precious time on EGFR antibody therapy nor be exposed to toxicities from medications unlikely to help them.
CONTEXT
Key Objective
Did Veterans Affairs Medical Centers effectively integrate KRAS molecular testing and anti–epidermal growth factor receptor monoclonal antibody (EGFR mAb) therapy into clinical care for patients with stage IV colorectal cancers between 2006 and 2015? Few studies have used large, comprehensive patient databases to evaluate the uptake and application of cancer molecular testing, the cornerstone of precision oncology.
Knowledge Generated
Although molecular testing increased over the years studied, KRAS testing peaked at only 28% of patients diagnosed with stage IV colorectal cancer in 2013. Among those molecularly selected for EGFR mAb use by KRAS testing, only 35.7% received EGFR mAb therapy.
Relevance
Efforts to understand barriers and to increase rates of molecular testing and targeted therapy are urgently needed.
Emerging studies suggest that many patients with advanced cancer are not receiving guideline concordant molecular testing. Our previous work found underuse of EGFR testing, also essential for selection of targeted therapy, in patients with advanced lung cancer in both Veteran Health Administration (VA) and Medicare populations.10,11 A recent analysis of the SEER database demonstrated that only 30% of patients with stage IV CRC had KRAS testing between 2010 and 2013.12 Other studies, conducted on different patient populations, however, report more extensive uptake of molecular testing.13 The true rates of molecular testing are unknown and may vary by patient population, care setting, and disease-related issues. The limited study of predictors of KRAS testing in the Medicare population suggests that patients with Medicaid insurance are less likely to be tested, adding a concerning socioeconomic dimension to the use of molecular testing.12 Molecular testing can also be costly, which may further complicate attempts to use molecular testing in resource-limited settings.
Following the 2009 ASCO and National Comprehensive Cancer Network recommendations for KRAS testing, in 2010, the VA recommended KRAS testing in patients with advanced CRC to identify those who may benefit from EGFR-directed therapies.9,14 We sought to study the uptake of the recommendation for KRAS molecular testing in the VA nationwide, the predictors of molecular testing, and the use of molecular testing to direct therapy. The VA is an attractive resource for the study of KRAS mutational analysis as the VA maintains comprehensive standardized electronic medical records on more than 9 million enrolled Veterans and eliminates many of the insurance-related challenges to expensive care.
MATERIALS AND METHODS
We conducted a retrospective cohort study of patients with stage IV CRC treated at the VA. We identified patients diagnosed with CRC in the VA between the years 2006 and 2015 using data from the Oncology Raw data set in the VA Corporate Data Warehouse (CDW). Patient demographics, clinical characteristics, and treatment were collected from the CDW data. We aggregated location of diagnosis into one of four census regions: (1) Northeast, (2) Midwest, (3) South, and (4) West. We categorized centers by volume of colon cancer care, based on the number of analytic CRC cases in the cancer registry, and further divided the sites into colon cancer volume quintiles. The five quintiles were defined by the following number of analytic cases/year, averaged over the 10-year period: < 137; 137-208; 209-302; 303-401; and > 402. We calculated the Charlson Comorbidity Index (CCI) for each subject, and divided the CCI into a binary variable with values above and below the mean. We used the percentage of Black patients with CRC treated at the diagnosis facility as a continuous independent variable.
Patient-level KRAS tests from 2006 to 2015 were obtained from 29 commercial sendout laboratories that contract with the VA. We further conducted a search of KRAS testing by Current Procedural Terminology codes and keywords in the CDW data fields for laboratory test name and in the laboratory result comment.
We calculated descriptive statistics of our cohort. Chi-square or Fisher’s exact test and Student’s t test were used to calculate univariate associations between clinical or demographic characteristics and KRAS testing, KRAS mutational status, and treatment with EGFR-directed therapy. Multivariable logistic regression was used to calculate adjusted associations between demographic or clinical characteristics and KRAS testing, KRAS mutations, and EGFR antibody therapy. Kaplan-Meier survival curves were computed for relevant patient groups and compared by logrank tests. Statistical analyses were performed with SAS V 9.4 (SAS Institute, Cary, NC). All work was reviewed and approved by the VA-NYHHS IRB under protocol number 1586.
RESULTS
Likelihood of Testing
Among the 5,943 patients diagnosed with stage IV CRC from 2006 to 2015, 1,053 (17.7%) had KRAS testing. 5,546 had complete data for analyses and constitute our study cohort. Among them, 976 (17.6%) had KRAS testing (Table 1). The population had a median age of 66.7 years (not tested) and 63.8 years of age (tested) at diagnosis, and represented all four national census regions, with 42.9% of the patients from the South. The regional distribution of Veterans with CRC is similar to the regional distribution of all Veterans. The Veterans were predominantly male (97.7%), 68.4% White, 20.3% Black, and 11.2% other races, similar to the general VA patient population. The majority of patients were treated in centers in the two highest quintiles of colon cancer volume.
TABLE 1.
Demographics KRAS Tested and Not Tested
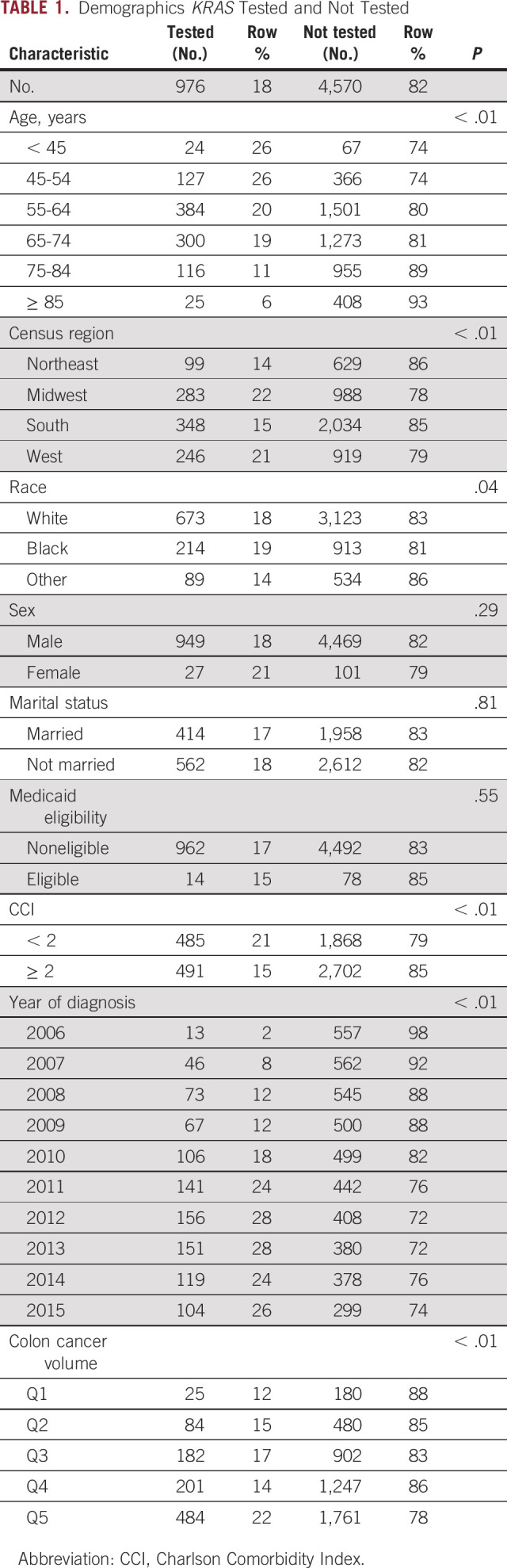
Of the 3,183 patients diagnosed after the 2010 recommendation for testing was instituted, 777 tests were performed (24.4%). Testing rates increased substantially from 2.3% among those diagnosed in 2006 to a peak of 28.4% of patients diagnosed in 2013. In univariate analysis, we found that KRAS testing was significantly associated with patient age, census region, race, CCI, year of diagnosis, and institutional colon cancer volume (Table 1).
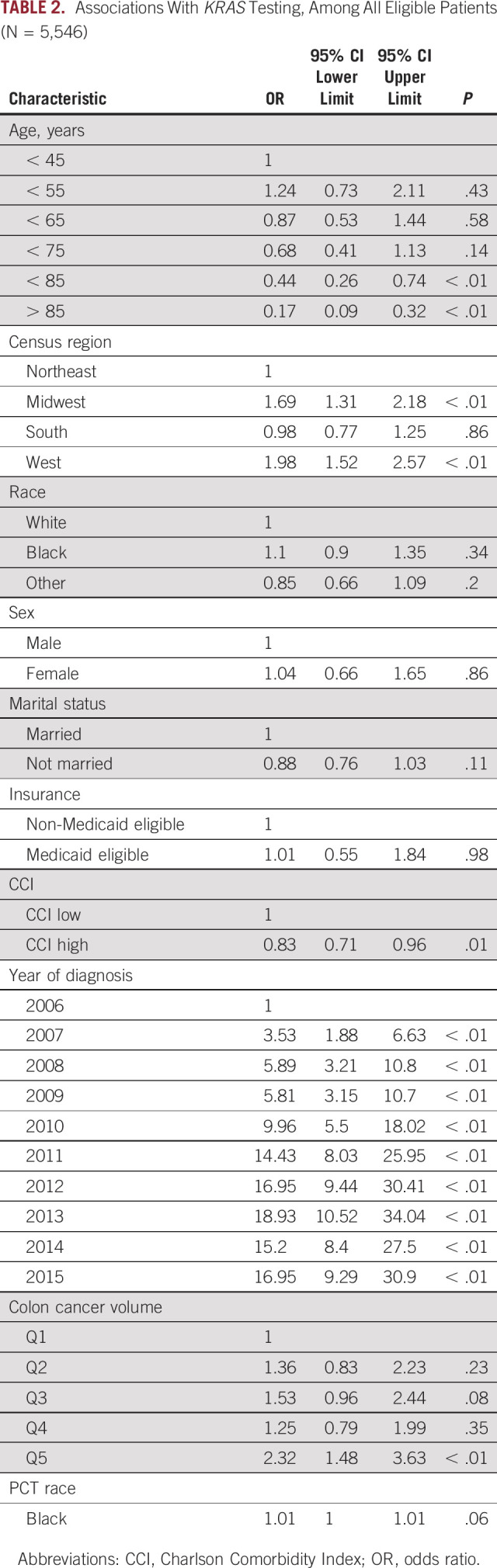
In multivariable logistic regression, we found that after controlling for all of the demographic or clinical covariates, older patients were less likely to have KRAS testing, odds ratio (OR) 0.17 (0.09 to 0.32), for those 85 years of age or older (Table 2). Testing rates varied by census regions, with the lowest rates in the Northeast region and the highest rates in the West region. Marital status, sex, and CCI were not significantly associated with KRAS testing. Patient race was not associated with KRAS testing, OR 1.10 (0.90 to 1.35), for Black patients relative to White patients, but centers that treated more Black patients with CRC were more likely perform KRAS testing, OR 1.01 (1.00 to 1.01). Medicaid eligibility was not associated with KRAS testing, OR 1.01 (0.55 to 1.54). We found that the odds of testing increased in more recent years, with ORs ranging from 9.96 in 2010 to 18.93 in 2013. High-volume centers were more likely to perform testing, OR 2.32 (1.48 to 3.63), relative to centers treating the lowest volume of CRC patients.
TABLE 2.
Associations With KRAS Testing, Among All Eligible Patients (N = 5,546)
Timing of KRAS Testing
We examined days to testing from diagnosis in two strata: those diagnosed from 2006 to 2010, and those diagnosed from 2011 to 2016. We found that the median time to testing in the 305 tested patients diagnosed during 2006-2010 was 295 days, whereas the median time to testing in the 671 tested patients diagnosed during 2011-2015 was 49 days. We further evaluated percentage of testing complete at 180 days and 360 days to give a rough estimate of testing during first-line and second-line therapy in this real-world population. At 180 days, among those diagnosed during 2006-2010, 41% of all tests were complete, whereas among those diagnosed during 2011-2015, 77% of all tests were complete. At 360 days, among those diagnosed during 2006-2010, 55% of all tests were complete, whereas among those diagnosed during 2011-2015, 86% of all tests were complete.
KRAS Mutational Status and Receipt of EGFR mAb
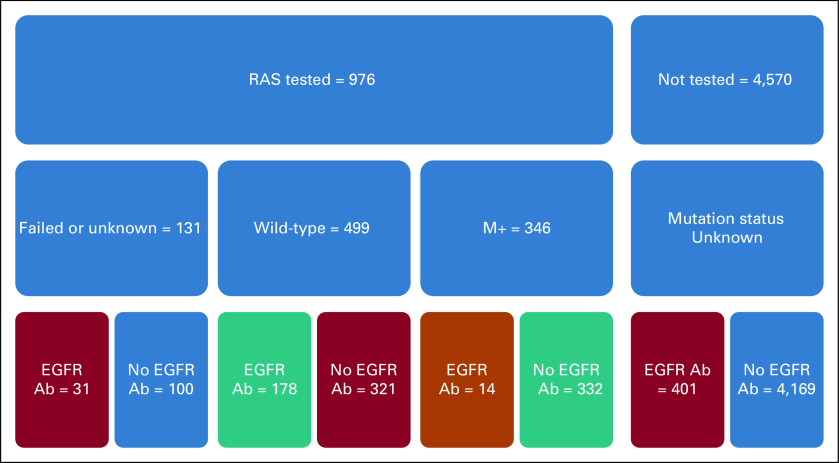
Of 976 KRAS-tested patients, 346 (35.4%) had KRAS mutations. Among those 976 patients, 223 (22.8%) received EGFR mAbs. Of those with KRAS mutations, 14/346 (4.0%) received EGFR mAbs, guideline-discordant care (Fig 1). Among 499 of patients with KRAS wild-type (WT) results, 178 (35.7%) received EGFR mAbs, guideline-concordant care, and 321 (64.3%) did not receive EGFR mAbs, potentially discordant care. Among 4,570 patients who did not have documented KRAS testing, 401 (8.8%) received an EGFR mAb, potentially guideline-discordant care.
FIG 1.
Guideline concordance of care.
Of the 976 KRAS-tested patients included in the multivariable model, Black patients were no more likely than White patients to have tumors harboring KRAS mutations, OR 1.18 (95% CI 0.83 to 1.69). No other demographic or clinical characteristics were significantly associated with KRAS mutation status.
Two hundred twenty-three of 976 (22.8%) tested patients in the multivariable model received an EGFR mAb. Three clinical demographic characteristics were associated with receipt of EGFR mAbs: unmarried patients were less likely to receive an EGFR Ab, OR 0.49 (0.34 to 0.69), diagnosis years 2014 and 2015 were less likely to receive EGFR Abs (OR 0.16 and 0.13 respectively), and patients with KRAS mutant tumors, OR 0.08 (0.04 to 0.13).
One hundred seventy eight of 499 KRAS WT patients received EGFR mAb. In multivariable logistic regression among these 499 patients, we found that nonmarried status, OR 0.45 (0.29 to 0.69), and treatment at a site with higher percentage of Black patients, OR 1.02 (1.00 to 1.04) (a surrogate for urban treatment center), were associated with receipt of EGFR mAb.
Outcomes of Patients Receiving EGFR mAb
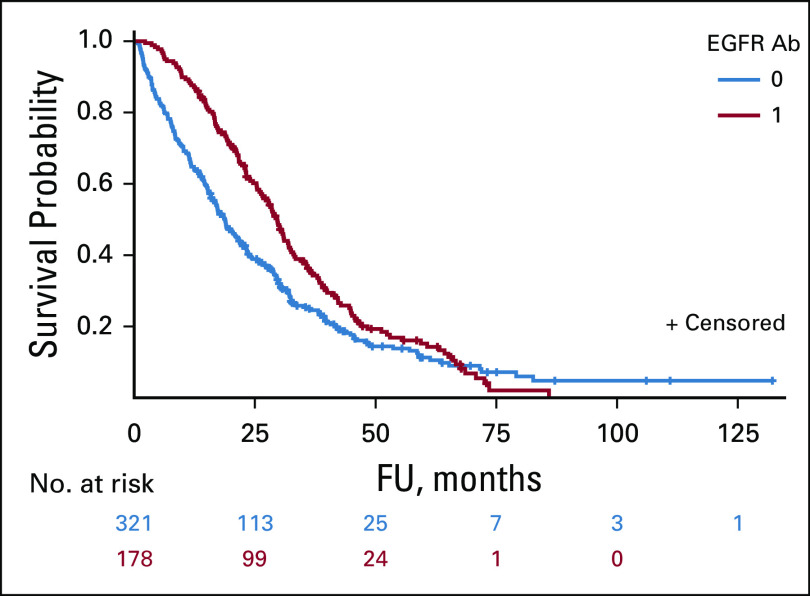
In a multivariable Cox proportional hazards model, patients who were tested for KRAS mutations had significantly better survival than those who were not tested, hazard ratio 0.63 (0.59 to 0.68; P < .001). The model controlled for patient age, location, race, year of diagnosis, insurance, comorbidities, colon cancer site volume, and percentage of Black patients (which serves as a surrogate for urban or rural). Among those who were KRAS WT, survival was better for patients who received EGFR mAbs than for those who did not, with median overall survival of 18.8 months (95% CI, 16.4 to 21.7) and 29.6 months (95% CI, 25.9 to 32.0), respectively (logrank P = .0016; Fig 2).
FIG 2.
Overall survival among KRAS tested and WT by receipt of EGFR mAb. EGFR, epidermal growth factor receptor; FU, follow-up time; mAb, monoclonal antibody; WT, wild-type.
DISCUSSION
In our analysis, we found substantial undertesting of KRAS for patients with stage IV CRC in the VA hospital system, with only 17.7% of eligible patients diagnosed between 2006 and 2015 receiving testing. Among those diagnosed in the year 2010 or later, only 24.4% of stage IV CRC patients had KRAS testing. KRAS testing rates increased substantially from 2.3% among those diagnosed in 2006 to 28.4% for those diagnosed in 2013, but testing rates remained low. We found that high-volume CRC centers were more likely to perform KRAS testing. There were no differences in KRAS testing rates by race or Medicaid eligibility. Older patients were less likely to be tested for KRAS and there was significant regional variability in testing rates. We found that in 2011-2015 KRAS testing was performed sooner after diagnosis, than had been done in 2006-2010. We also found underuse of EGFR mAbs, and substantial use among patients with no documentation of KRAS testing.
Underuse of KRAS testing in the VA population is consistent with previous reports. Charlton et al12 reported a SEER database analysis, covering approximately 26% of the US population, in which only 30% of more than 22,000 stage IV CRC cases diagnosed between 2010 and 2013 had KRAS testing. Some patients are ineligible for antineoplastic therapy because of comorbidities or performance status, and KRAS testing would be inappropriate for these patients. The low use of KRAS testing reported in both the VA and SEER, however, suggest that many patients who are eligible for EGFR mAb therapy are not being tested and therefore denied the precision oncology advances dependent upon RAS testing results. Carter et al15 reported higher rate of testing (overall 47.5%) in a cohort of 1,363 patients with stage IV CRC treated in a community-based oncology practice. It is possible that practice patterns vary between the VA, SEER, and community-based practices, and these investigators nonetheless conclude that testing is underused. Uptake of testing may have been better outside of the United States. Ciardiello et al reported a chart review of approximately 3,800 patients being treated for colon cancer in 2010 across 14 countries in Europe, Asia, and Latin America. These authors report that 69% of all patients had KRAS testing in 2010. Only limited details of chart selection were provided, so it is unclear if the sampled population is similar to patients studied in the United States. Nonetheless, it may be that more effective systems are in place outside of the United States to increase the uptake of proven molecular testing. Overall, the two largest US analyses, from our VA database and from SEER, conclude that there is substantial underuse of molecular testing in patients with CRC who could benefit from molecular selection of targeted therapy.
Our study found that centers in the highest quintile of CRC volume were 2.32 times more likely than those in the lowest quintile to perform KRAS testing on patients with stage IV colon cancer. The relationship between volume of colon cancer surgery and outcomes, including 30-day mortality and 5-year mortality, is well established in both the VA population and the United States at large.16,17 Few studies, however, have addressed the volume-outcome relationship in regards to medical care for CRCs. And, although multiple studies have sought causes for disparities in medical care for CRC among Veterans,18-21 none have controlled for the volume of colon cancer treated at each site. It is interesting to note that our data do not suggest a clear dose-response relationship between colon cancer volume and molecular testing. Patients diagnosed at the highest-volume colon cancer centers are significantly more likely to have KRAS testing than those diagnosed at the lowest volume centers, but it is not clear that each quintile above the lowest does more KRAS testing than the previous. The data suggest the possibility that above a certain threshold, KRAS testing is performed more regularly. It may be that centers with sufficient colon cancer volume develop more effective systems to perform KRAS testing.
Older patients in both the VA and SEER were less likely to have KRAS testing. Multiple previous studies have confirmed that older patients are less likely to receive chemotherapy for colon cancer than younger counterparts.20 Older patients are more likely than younger patients to have comorbid conditions that may increase the toxicity of chemotherapy, but that relationship may not hold with targeted therapy such as EGFR mAbs.22,23 Jehn et al reported findings that grade III or IV cetuximab toxicities were limited to approximately 30% of patients, with no difference between patients who were older than or younger than 65 years of age.24 Although it is difficult to say exactly what percentage of older patients with stage IV CRC are EGFR mAb eligible and therefore should have KRAS testing, our data suggest that KRAS testing is underused in the elderly for whom targeted therapy may have advantages over conventional chemotherapy.
In an effort to examine racial, socioeconomic, and location-related disparities in the use of KRAS testing, we included race, Medicaid eligibility, and percentage of Black patients treated at each site in the multivariable models. As with many previous reports on racial disparities in VA care,25 we found no racial disparities in KRAS testing. Outside of the VA, racial disparities in CRC care are prevalent.26,27 We did, however, find that medical centers with more Black patients were more likely to perform KRAS testing. We do not suggest that this is a causal association, and suspect that percentage of Black patients is a proxy for other factors that are related to KRAS testing, such as urban medical centers, or university affiliation. We also noted that within the VA, Medicaid eligibility is not associated with KRAS testing, which is different from findings in the SEER database.12 This finding is consistent with previous data that the VA single-payer system can decrease disparities in care. It is encouraging to note the VA system minimizes socioeconomic disparities for even the most technologically advanced tests offered.
In our exploration of guideline concordance of care, we found little EGFR mAb overuse in patients with KRAS mutations (14/346, 4%; Fig 1). We found potentially large underuse of EGFR mAb in the KRAS WT population (321/499, 64.3%). We also found that the majority of patients who received EGFR mAb received them without documented KRAS testing (489/957, 51.1%), potentially guideline-discordant care. Few studies have reviewed the clinical impact of KRAS testing on treatment choice. Rico et al28 reviewed data from 10 state population-based cancer registries and found that only 7% of KRAS WT patients in 2011 received EGFR mAb. Follow-up in this study was short but the findings are consistent with ours in that EGFR mAb may be underused in practice even among patients with KRAS testing.
Our study has several potential limitations. It is possible that not all molecular testing was recorded in the VA CDW. Some VA Medical Centers may have conducted molecular testing through a laboratory for which no VA procedure code was captured. We suspect, however, that these tests likely represent a minority of cases as the national VA contracts with the 29 source laboratories that were supported by the VA central office of laboratory medicine and came with no additional cost to the medical center department of pathology. Second, cetuximab and panitumumab were initially approved in the third-line setting without reference to KRAS mutation status. Data suggesting that KRAS mutation may affect mAb efficacy were available as early as 2008, but some of the use that we characterized as likely inappropriate may have been before the availability of that information. Third, KRAS testing can reasonably be performed after first-line therapy, so rates of testing of those diagnosed in 2014 and 2015 likely underrepresent the testing rate for those patients with continued follow-up.
In conclusion, we found underuse of KRAS molecular testing among VA patients with stage IV CRC diagnosed between 2010 and 2015. Older patients and those treated at low-volume colon cancer centers were less likely to have KRAS testing, but race and Medicaid eligibility were not associated with KRAS testing. KRAS testing appears to have been appropriately used to exclude patients from EGFR mAb use, but we found low rates of EGFR mAb use even among KRAS WT patients who were molecularly appropriate for therapy. We also found better survival for KRAS WT patients treated with EGFR mAbs, consistent with the benefit previously shown in clinical trials. Additional research is needed to understand barriers to the complete implementation of precision oncology in colorectal and other cancers. The VA has a unified national electronic medical record and a centrally organized Precision Oncology Program, which was instituted after 2015 to help implement molecular tumor testing, but data on its efficacy remain limited.29 These and other effective systems are urgently needed to help clinicians apply expanding knowledge of molecularly targeted therapy to everyday practice and to improve patient care.
PRIOR PRESENTATION
Presented at ASCO Annual Meeting, Chicago, IL, June 1-5, 2018 (abstract e18844).
SUPPORT
Supported in part by the United States Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (No. CDA HX 002808 to D.J.B. Supported in part by United States Department of Veterans Affairs, Veterans Health Administration, Health Services Research and Development Service (VA IIR 15-356); National Institute of Health (1R01MD012243-01A1); The Prostate Cancer Foundation (PCF); The John and Daria Barry Precision Oncology Center of Excellence of the VANYHHS; Prostate Cancer Foundation Young Investigator awardee; and The Edward Blank and Sharon Colsloy Blank Family Foundation to D.V.M.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel J. Becker, Steve Y. Lee, Scott E. Sherman, Michael J. Kelley, Danil V. Makarov, Julie A. Lynch
Administrative support: Daniel J. Becker, Scott E. Sherman, Danil V. Makarov, Julie A. Lynch
Financial support: Daniel J. Becker, Julie A. Lynch
Provision of study materials or patients: Daniel J. Becker, Kyung M. Lee, Kristine E. Lynch, Julie A. Lynch
Collection and assembly of data: Daniel J. Becker, Kyung M. Lee, Steve Y. Lee, Kristine E. Lynch, Christy D. Morrissey, Julie A. Lynch
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Steve Y. Lee
Honoraria: Novocure
Travel, Accommodations, Expenses: Galera Therapeutics
Danil V. Makarov
Employment: New Hope Fertility
Michael J. Kelley
Consulting or Advisory Role: IBM
Research Funding: Novartis, AstraZeneca, Bristol-Myers Squibb, Regeneron, Genentech
Other Relationship: IBM
Open Payments Link: https://openpaymentsdata.cms.gov/physician/827136
Julie A. Lynch
Research Funding: Genomic Health, AstraZeneca, Myriad Genetics, Boehringer Ingelheim, Astellas Pharma, CardioDx, Janssen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2019. CA Cancer J Clin 69:7-34, 2019 [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute : Surveillance, Epidemiology, and End Results (SEER) Program. https://seer.cancer.gov/ [Google Scholar]
- 3.Dekker E, Tanis PJ, Vleugels JLA, et al. : Colorectal cancer. Lancet 394:1467-1480, 2019 [DOI] [PubMed] [Google Scholar]
- 4.Lievre A, Bachet JB, Le Corre D, et al. : KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res 66:3992-3995, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Benvenuti S, Sartore-Bianchi A, Di Nicolantonio F, et al. : Oncogenic activation of the RAS/RAF signaling pathway impairs the response of metastatic colorectal cancers to anti-epidermal growth factor receptor antibody therapies. Cancer Res 67:2643-2648, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Douillard J-Y, Oliner KS, Siena S, et al. : Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 369:1023-1034, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Bokemeyer C, Köhne C-H, Ciardiello F, et al. : FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer 51:1243-1252, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Lenz HJ, Kohne CH, et al. : Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 33:692-700, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Engstrom PF, Arnoletti JP, Benson AB III, et al. : NCCN clinical practice guidelines in oncology: Colon cancer. J Natl Compr Canc Netw 7:778-831, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Lynch JA, Berse B, Chun D, et al. : Epidermal growth factor receptor mutational testing and erlotinib treatment among veterans diagnosed with lung cancer in the United States Department of Veterans Affairs. Clin Lung Cancer 18:401-409, 2017 [DOI] [PubMed] [Google Scholar]
- 11.Lynch JA, Berse B, Rabb M, et al. : Underutilization and disparities in access to EGFR testing among Medicare patients with lung cancer from 2010-2013. BMC Cancer 18:306, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charlton ME, Kahl AR, Greenbaum AA, et al. : KRAS testing, tumor location, and survival in patients with stage IV colorectal cancer: SEER 2010-2013. J Natl Compr Canc Netw 15:1484-1493, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciardiello F, Tejpar S, Normanno N, et al. : Uptake of KRAS mutation testing in patients with metastatic colorectal cancer in Europe, Latin America and Asia. Target Oncol 6:133, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Allegra CJ, Jessup JM, Somerfield MR, et al. : American Society of Clinical Oncology provisional clinical opinion: Testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol 27:2091-2096, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Carter GC, Landsman-Blumberg PB, Johnson BH, et al. : KRAS testing of patients with metastatic colorectal cancer in a community-based oncology setting: A retrospective database analysis. J Exp Clin Cancer Res 34:29, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrag D, Cramer LD, Bach PB, et al. : Influence of hospital procedure volume on outcomes following surgery for colon cancer. JAMA 284:3028-3035, 2000 [DOI] [PubMed] [Google Scholar]
- 17.Rabeneck L, Davila JA, Thompson M, et al. : Surgical volume and long-term survival following surgery for colorectal cancer in the Veterans Affairs Health-Care System. Am J Gastroenterol 99:668-675, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Zullig LL, Carpenter WR, Provenzale D, et al. : Examining potential colorectal cancer care disparities in the Veterans Affairs Health Care System. J Clin Oncol 31:3579-3584, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sabounchi S, Keihanian S, Anand BS: Impact of race on colorectal cancer. Clin Colorectal Cancer 11:66-70, 2012 [DOI] [PubMed] [Google Scholar]
- 20.Zeber JE, Copeland LA, Hosek BJ, et al. : Cancer rates, medical comorbidities, and treatment modalities in the oldest patients. Crit Rev Oncol Hematol 67:237-242, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Zullig LL, Williams CD, Fortune-Britt AG: Lung and colorectal cancer treatment and outcomes in the Veterans Affairs Health Care System. Cancer Manag Res 7:19-35, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Repetto L: Greater risks of chemotherapy toxicity in elderly patients with cancer. J Support Oncol 1:18-24, 2003 [PubMed] [Google Scholar]
- 23.Shahrokni A, Wu AJ, Carter J, et al. : Long-term toxicity of cancer treatment in older patients. Clin Geriatr Med 32:63-80, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jehn CF, Böning L, Kröning H, et al. : Cetuximab-based therapy in elderly comorbid patients with metastatic colorectal cancer. Br J Cancer 106:274-278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peterson K, Anderson J, Boundy E, et al. : Mortality disparities in racial/ethnic minority groups in the Veterans Health Administration: An evidence review and map. Am J Public Health 108:e1-e11, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tehranifar P, Neugut AI, Phelan JC, et al. : Medical advances and racial/ethnic disparities in cancer survival. Cancer Epidemiol Biomarkers Prev 18:2701-2708, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breslin TM, Morris AM, Gu N, et al. : Hospital factors and racial disparities in mortality after surgery for breast and colon cancer. J Clin Oncol 27:3945-3950, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rico A, La P, Thompson TD, et al. : KRAS testing and first-line treatment among patients diagnosed with metastatic colorectal cancer using population data from ten National Program of Cancer Registries in the United States. J Cancer Res Ther 5:7-13, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poonnen PJ, Duffy JE, Hintze B, et al. : Genomic analysis of metastatic solid tumors in veterans: Findings from the VHA National Precision Oncology Program. J Clin Oncol 37, 2019. (suppl; abstr 3074) [DOI] [PMC free article] [PubMed] [Google Scholar]