Abstract
Background
There are no useful biomarkers for the clinical outcome of advanced esophageal squamous cell carcinoma (ESCC). In this study, we aimed to investigate the prognostic value of soluble PD-L1 (sPD-L1) in serum of patients with locally advanced or metastatic ESCC who received cytotoxic chemotherapy as first-line treatment.
Materials and Methods
This study evaluated the expression pattern of PD-L1 by immunohistochemistry and sPD-L1 concentration, and correlation with clinicopathological factors and overall survival (OS) in 190 patients with ESCC.
Results
sPD-L1 concentration was highly expressed in ESCC, especially in female patients. Patients with a high sPD-L1 level (≥0.63 ng/mL) had a shorter OS than those with a low sPD-L1 level (<0.63 ng/mL). In a multivariate analysis, high sPD-L1 concentration remained an independent prognostic factor of OS after adjustment for possible confounders. However, tissue PD-L1 expression level was non-prognostic in this study.
Conclusion
There was no significant correlation between serum sPD-L1 concentration and tissue PD-L1 expression level. sPD-L1 concentration before treatment could be an effective and convenient biomarker of prognosis in patients with locally advanced or metastatic ESCC treated with combination cytotoxic chemotherapy.
Keywords: esophageal squamous cell carcinoma, PD-L1, soluble PD-L1, prognosis, clinical outcome, chemotherapy
Introduction
Esophageal cancer is the sixth most prevalent cancer worldwide. Global burden of esophageal cancer mostly involves esophageal squamous cell carcinoma (ESCC), and the greatest burden of ESCC occurs in China.1,2 Although the incidence and mortality of ESCC gradually declined during the last three decades, the dismal prognosis of locally advanced or metastatic ESCC is still threatening the public health in China.3
ESCC is usually fatal and has a poor 5-year survival (less than 30% in China).4 Such poor survival reflects the fact that ESCC is often unresectable due to advanced stage or metastasis at the time of diagnosis. Systemic chemotherapy has been established as the standard treatment for locally advanced or metastatic ESCC.5 Docetaxel plus nedaplatin-based combination cytotoxic chemotherapy is currently a commonly used first-line regimen.6 However, the outcome varies significantly among individuals with unresectable ESCC who receive first-line chemotherapy. Therefore, clinicians need an effective biomarker of individual prognosis for reliable patient stratification.7
The programmed cell death ligand 1 (PD-L1)/programmed cell death receptor 1 (PD-1) pathway has been considered a promising target for cancer treatment based on its significant role in tumor immunity.8 Several studies have shown PD-L1 expression level as an indicator of outcome in several malignancies.9 It is known that PD-L1 localizes to the cell surface of tumor and immune cells, and recent studies have found the existence of soluble PD-L1 (sPD-L1) released from PD-L1 positive cells in human serum.10,11 Previous research has shown that functional serum sPD-L1 also has PD-1 binding capacity.12 To the best of our knowledge, although sPD-L1 is a promising biomarker, its clinical significance and comparison of the clinical value between the tissue and serum levels have not been reported in patients with ESCC. The purpose of this study was to evaluate the prognostic significance of sPD-L1 in patients with ESCC treated with combination cytotoxic chemotherapy.
Materials and Methods
Study Design and Patient Characteristics
We recruited patients who had histologically proven unresectable, locally advanced or recurrent/metastatic ESCC and had not received prior systemic therapies. Patients with locally advanced or metastatic ESCC who had received at least one cycle of first-line chemotherapy from January 2015 to October 2017 were identified from the computerized patient database of Affiliated Hospital of Shandong University of Traditional Chinese Medicine. The process of selection is shown in Figure 1. The patients were included based on the availability of tissue biopsy specimens and serum samples with complete clinical information. We excluded patients in whom one or more clinicopathological variables of interest were unavailable. Forty-three patients who lost follow-up in the study were also excluded from study. Eventually, 190 patients with locally advanced or metastatic ESCC were enrolled in this study. The following associated data were collected: age at diagnosis, gender, body mass index (BMI), Eastern Cooperative Oncology Group performance score (ECOG performance score), tumor location, radiotherapy, subsequent chemotherapy record, and other initial laboratory values of interest. In addition, we also included 58 healthy volunteers as a control cohort, whose detailed demographic information and laboratory values are described in Supplementary Table 1. All experimental procedures were conducted in accordance with the Declaration of Affiliated Hospital of Shandong University of Traditional Chinese Medicine, and written informed consent was obtained from each patient.
Figure 1.
Flowchart: Selection of the study population.
Measurement of Serum sPD-L1 Levels
Blood samples were collected by venipuncture from healthy volunteers and from ESCC patients within one week before initiation of combination chemotherapy. Samples were centrifuged at 2000 g for 10 min, then divided and stored at −80°C until analysis. Serum sPD-L1 concentrations were determined using enzyme-linked immunosorbent assay kits for human PD-L1 (E-16346; Heguo, Shanghai, China). Serum samples (100 μL) were incubated in duplicate in PD-L1 antibody-coated microtiter plates. After incubation at 37°C for 2 hours, the liquid was removed from each well, and detection reagent A working solution was then added. After incubation at 37°C for 1 hour and subsequent washing, detection reagent B working solution was added, followed by incubation of samples at 37°C for 30 min. The solution was discarded, and after subsequent washing, substrate solution was added. Color development was stopped after 10 min at 37°C in the dark, and the intensity was immediately read at 450 nm. Measurements were performed in duplicate, and mean values were used for data presentation.
Hematologic Markers
We also evaluated the blood parameters at baseline, prior to the first-line chemotherapy. Namely, the absolute neutrophil count, absolute lymphocyte count, platelet count, albumin (ALB) level, C-reactive protein (CRP) level, globulin (GLB) level, and lactate dehydrogenase (LDH) level were collected from electronic medical database. The (neutrophil–lymphocyte ratio) NLR was calculated as the neutrophil count divided by the lymphocyte count, while the (platelet–lymphocyte ratio) PLR was calculated as the platelet count divided by the lymphocyte count.
Staining of Tissue PD-L1
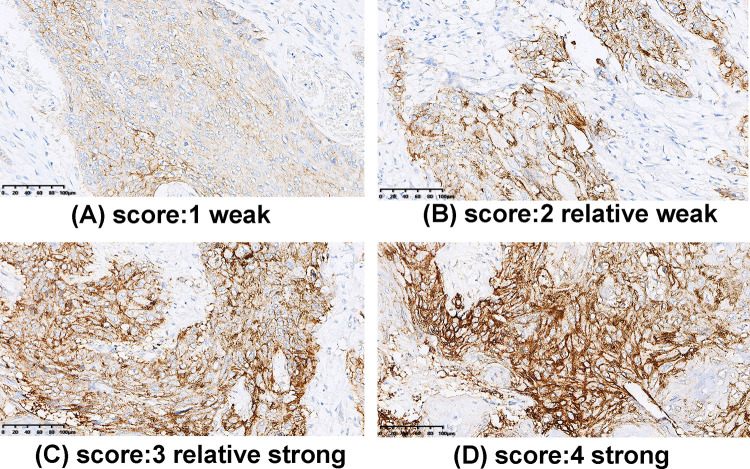
Immunohistochemical (IHC) analysis of PD-L1 on 4 μm paraffin-embedded archived ESCC tissue biopsy specimens was performed in accordance with the standard protocol using commercial antibody. After deparaffinization and dehydration, the sections were placed in 10 mM sodium citrate buffer (pH=6.0) and autoclaved at 121°C for 10 min, and then incubated in normal goat serum (KL-D1418; KALANG, Zhengzhou, China) for 30 min to block non-specific antibody-binding sites. Each section was then incubated for 10 min at room temperature with an anti-PD-L1 rabbit monoclonal antibody (ab228415; Abcam, Shanghai, China) diluted 1:500 in phosphate‐buffered saline containing 1% bovine serum albumin. Phosphate‐buffered saline was used for negative controls. Visualization was performed by Envision Flex kit (DAKO, Glostrup, Denmark). The slides were counterstained with hematoxylin. Each section was observed at magnification of 10× and 20×. Expression levels of PD-L1 were defined as 1 (weak), 2 (relatively weak), 3 (relatively strong), or 4 (strong). All the samples were blindly examined by two pathologists (RZ.L. and L.Y.), and discussion using multi-head microscope was used to resolve discrepancies. The tumor samples were divided into a PD-L1-low group (staining score: 1–2) and PD-L1-high group (staining score: 3–4).
Outcome Assessment
All patients were followed up in 1-month intervals during the first year after first-line chemotherapy, and subsequently at 2-month intervals for the second year, 6-month intervals for the third year, and annually for the fourth year. Overall survival (OS) was defined as the interval between the date of initial treatment and the date of death from any cause or the last follow-up.
Statistical Analysis
Categorical data was analyzed using Fisher’s exact test or the χ2 test. Continuous data were compared between the groups using Mann–Whitney U-test. Box-plot graphics displayed a statistical summary of the median, quartiles, and ranges. The optimal cut-off value of sPD-L1 was determined using X-tile software (http://www.tissuearray.org/rimmlab) and by the minimal P-value approach, as shown in the Supplementary Figure 1. The same method was used for other hematologic markers (NLR, PLR, CRP, ALB, GLB, and LDH). The cut-off values of sPD-L1, NLR, PLR, CRP, ALB, GLB, and LDH in ESCC cohort to separate OS were 0.63 ng/mL, 6.6, 145, 5.21 mg/L, 40.0 g/L, 25.7 g/L, and 212 U/L, respectively. Hazard ratios (HRs) were obtained using the cumulative survival function and were reported with corresponding 95% confidence intervals (95% CI). Univariate Cox regression was constructed and factors with p value <0.1 in univariate survival analysis were introduced into multivariate analysis performed by the Cox proportional-hazards model using the forward procedure based on likelihood ratio for variable selection. A p value <0.05 was considered statistically significant. All analyses were performed using Empower (R) (www.empowerstats.com, XY Solutions, inc. Boston MA)
Results
Serum sPD-L1 is Elevated in Patients with Locally Advanced or Metastatic ESCC
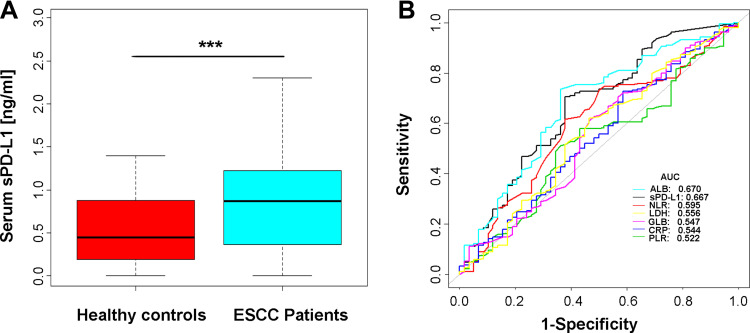
We examined the serum sPD-L1 level in pre-chemotherapy serum samples from 190 patients with ESCC and 58 healthy controls. We showed that sPD-L1 levels in ESCC patients were significantly elevated compared with healthy controls (Figure 2A). The detailed sPD-L1 testing information is shown in Supplementary Table 1. Receiver operating characteristic curve (ROC) analysis for discrimination between patients with locally advanced or metastatic ESCC and healthy volunteers revealed an area under the ROC curve (AUC) of 0.667 for sPD-L1, which was only slightly inferior to ALB (AUCALB=0.670). Other hematologic markers showed an inferior AUC for the discrimination between ESCC patients and healthy controls (AUCNLR=0.595, AUCLDH=0.556, AUCGLB=0.547, AUCCRP=0.544, AUCPLR=0.522) (Figure 2B).
Figure 2.
sPD-L1 concentration in healthy controls and ESCC patients. (A) sPD-L1 concentration was highly elevated in ESCC patients (U-test, p<0.001). (B) ROC analysis of sPD-L1 and other hematologic markers between ESCC patients and healthy controls.
Note: ***p<0.001 was considered significant in U-test.
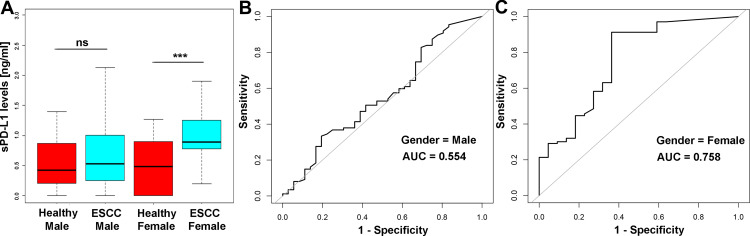
Based on the optimal cut-off level of sPD-L1 (0.63 ng/mL), 190 patients with advanced ESCC were stratified into the low (n=57) and high (n=133) sPD-L1 groups. Patients’ characteristics are summarized in Table 1. Interestingly, sPD-L1 level significantly correlated with gender (p<0.001) and GLB level (p=0.003) in ESCC patients, but not with any other variables examined. Subsequently, we examined the sPD-L1 level in the male and female parts of the cohort. We found no significant difference between patients with unresectable ESCC and healthy controls in the male cohort (p=0.45), but highly significant difference in the female cohort (p<0.001). Beyond that, in the female cohort, sPD-L1 level had a better discrimination ability between ESCC and healthy cohort (AUCsPD-L1-Female=0.758) than in the male cohort (AUCsPD-L1-Male=0.554) (Figure 3).
Table 1.
Clinicopathological and Treatment Characteristics of ESCC Patients, Grouped Based on Serum sPD-L1 Level (Low vs High)
| Factors | Total | Low sPD-L1 Group | High sPD-L1 Group | p | |||
|---|---|---|---|---|---|---|---|
| N = 190 | (%) | N = 57 | (30%) | N = 133 | (70%) | ||
| Age | 0.296 | ||||||
| <68 * | 91 | 47.9 | 24 | 42.1 | 67 | 50.4 | |
| ≥68 | 99 | 52.1 | 33 | 58.9 | 66 | 49.6 | |
| Gender | <0.001 | ||||||
| Male | 87 | 45.8 | 48 | 84.2 | 39 | 29.3 | |
| Female | 103 | 54.2 | 9 | 15.8 | 94 | 70.7 | |
| BMI (Kg/m2) | 0.970 | ||||||
| <25 | 147 | 77.4 | 44 | 77.2 | 103 | 77.4 | |
| ≥25 | 43 | 22.6 | 13 | 22.8 | 30 | 22.6 | |
| Tumor location | 0.454 | ||||||
| Upper | 27 | 14.2 | 5 | 8.8 | 22 | 16.5 | |
| Middle | 83 | 43.7 | 27 | 47.4 | 56 | 42.1 | |
| Lower | 63 | 33.2 | 21 | 36.8 | 42 | 31.6 | |
| Overlapping | 17 | 8.9 | 4 | 7.0 | 13 | 9.8 | |
| ECOG PS | 0.823 | ||||||
| 0–1 | 81 | 42.6 | 25 | 43.7 | 56 | 42.1 | |
| ≥2 | 109 | 57.4 | 32 | 56.1 | 77 | 57.9 | |
| Grade | 0.093 | ||||||
| Well | 6 | 3.2 | 0 | 0 | 6 | 4.5 | |
| Moderately | 92 | 48.4 | 33 | 57.9 | 59 | 44.4 | |
| Poorly | 92 | 48.4 | 24 | 42.1 | 68 | 51.3 | |
| T classification | 0.168 | ||||||
| T2 | 35 | 18.4 | 11 | 19.3 | 24 | 18.0 | |
| T3 | 90 | 47.4 | 32 | 56.1 | 58 | 43.6 | |
| T4 | 65 | 34.2 | 14 | 24.6 | 51 | 38.4 | |
| LN metastasis | 0.457 | ||||||
| Absent | 34 | 17.9 | 12 | 21.0 | 22 | 16.5 | |
| Present | 156 | 82.1 | 45 | 79.0 | 111 | 83.5 | |
| Distant metastasis | 0.336 | ||||||
| Absent (III) | 110 | 57.9 | 36 | 63.2 | 74 | 55.6 | |
| Present (III) | 80 | 42.1 | 21 | 36.8 | 59 | 44.4 | |
| Radiotherapy | 0.747 | ||||||
| Absent | 154 | 81.1 | 47 | 82.5 | 107 | 80.5 | |
| Present | 36 | 18.9 | 10 | 17.5 | 26 | 19.5 | |
| Sub-therapy | 0.061 | ||||||
| Absent | 76 | 40.0 | 17 | 29.8 | 59 | 44.4 | |
| Present | 114 | 60.0 | 40 | 70.2 | 74 | 55.6 | |
| NLR | 0.536 | ||||||
| < 6.60 | 73 | 38.4 | 20 | 35.1 | 53 | 39.8 | |
| ≥6.60 | 117 | 61.6 | 37 | 64.9 | 80 | 60.2 | |
| PLR | 0.758 | ||||||
| < 145 | 29 | 15.3 | 8 | 14.0 | 21 | 15.8 | |
| ≥145 | 161 | 84.7 | 49 | 86.0 | 112 | 84.2 | |
| CRP (mg/L) | 0.089 | ||||||
| < 5.21 | 60 | 31.6 | 23 | 40.3 | 37 | 27.8 | |
| ≥5.21 | 130 | 68.4 | 34 | 59.7 | 96 | 72.2 | |
| ALB (g/L) | 0.776 | ||||||
| < 40.0 | 97 | 51.1 | 30 | 52.6 | 67 | 50.4 | |
| ≥40.0 | 93 | 48.9 | 27 | 47.4 | 66 | 49.6 | |
| GLB (g/L) | 0.003 | ||||||
| < 25.7 | 111 | 58.4 | 24 | 42.1 | 87 | 65.4 | |
| ≥25.7 | 79 | 41.6 | 33 | 57.9 | 46 | 34.6 | |
| LDH (U/L) | 0.203 | ||||||
| < 212 | 126 | 66.3 | 34 | 59.6 | 92 | 69.2 | |
| ≥212 | 64 | 33.7 | 23 | 40.4 | 41 | 30.8 | |
Note: *The median age at diagnosis is 68 years for ESCC patients in this study.
Abbreviations: ESCC, esophageal squamous cell carcinoma; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance score; LN metastasis, lymph node metastasis; Sub-therapy, subsequent second-line chemotherapy; NLR, Neutrophil lymphocyte ratio; PLR, platelet lymphocyte ratio; CRP, C-reactive protein; ALB, albumin; GLB, globulin; LDH, lactate dehydrogenase.
Figure 3.
(A) sPD-L1 concentration was not significantly different between healthy male controls and male ESCC patients (U-test, p=0.448), but it was significantly elevated in the ESCC female patients compared with healthy female controls (U-test, p < 0.001). (B and C) sPD-L1 showed a powerful discrimination ability between ESCC patients and healthy controls in the female cohort (AUC=0.758) than in the male cohort (AUC=0.554).
Note: ***p<0.001 was considered significant in U-test.
PD-L1 Expression in Locally Advanced or Metastatic ESCC
To evaluate the tissue expression pattern of PD-L1 in ESCC, we performed IHC analysis of PD-L1 and quantified the staining as described in the Material and Methods section. Consistent with previous reports for other types of cancers, the IHC staining signal of PD-L1 was mainly distributed in the cellular membrane, but the cytoplasmic and nuclear localization of PD-L1 were also observed in some specimens. The staining intensity of PD-L1 was scored on a scale from 1 to 4 (Figure 4). According to the IHC staining results, 86 (45.3%) ESCC patients were classified into the PD-L1-Low group (score 1 or 2) and 104 (54.7%) ESCC patients into the PD-L1-High group (score 3 or 4). Correlations of tissue PD-L1 expression level with other patients’ characteristics are shown in Supplementary Table 2. We found no variables correlating with tissue PD-L1 expression level (p>0.05).
Figure 4.
Immunohistochemical staining of PD-L1 in ESCC. (A) Score 1: weak; (B) Score 2: relatively weak; (C) Score 3: relatively strong, and (D) Score 4: strong. Scale bar: 100 μm.
Correlation Between Tissue PD-L1 Expression and Serum sPD-L1 Levels
According to the IHC staining and serum Elisa results, we subsequently evaluated the correlation between tissue PD-L1 and serum sPD-L1 levels in locally advanced or metastatic ESCC patients. There was no tendency to elevated sPD-L1 level in ESCC patients with high tissue PD-L1 expression compared with those with low expression level (Figure 5 and Table 2).
Figure 5.
Correlation between tissue PD-L1 expression and serum sPD-L1 levels (U-test, p=0.246).
Table 2.
Correlation Between Tissue PD-L1 Expression and sPD-L1 Levels
| n | sPD-L1 Levels Mean ± SD | p value | |
|---|---|---|---|
| PD-L1 expression | 0.246 | ||
| Low | 86 | 0.87±0.58 | |
| High | 104 | 0.88±0.54 |
Survival Analysis of sPD-L1 and PD-L1
Among 190 patients, there were 111 deaths during a median follow-up time of 13 months (range: 3–38 months), where the one-third-year OS was 70.0% and 9.8%, respectively.
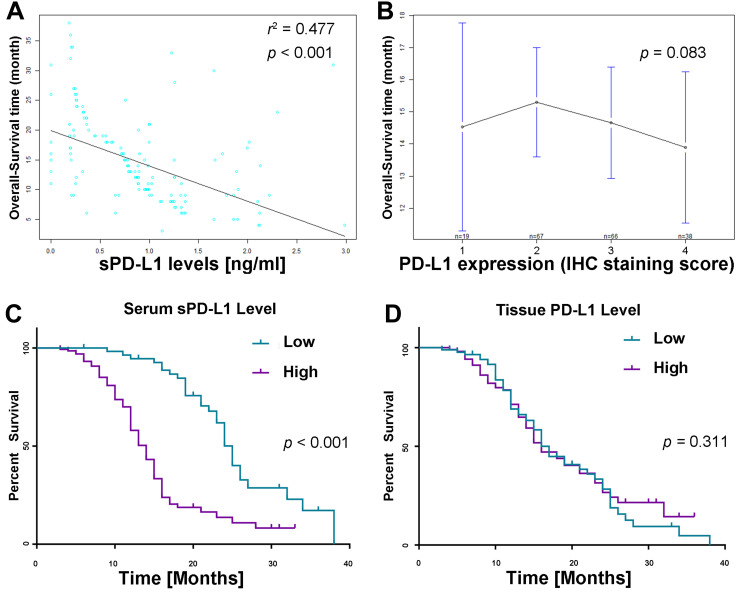
Firstly, we observed that patients’ survival time highly correlated with serum sPD-L1 concentration (p<0.001, Figure 6A). However, the mean OS in different PD-L1 expression groups (scoring 1, 2, 3 or 4) was 14.5, 15.3, 14.7, and 13.9 months, respectively, indicating no significant difference in OS between different PD-L1 expression groups (p=0.803, Figure 6B). Next, Kaplan–Meier curve analysis revealed significantly impaired OS in patients with locally advanced or metastatic ESCC with sPD-L1 serum concentration above the cut-off value of 0.63 ng/mL (Figure 6C). Median OS of sPD-L1-high patients was lower than in patients with low sPD-L1 level (12 months vs 21 months, p < 0.001). However, we did not observe strongly impaired OS between ESCC patients with low and high tissue PD-L1 expression levels (p=0.89, Figure 6D). Median OS of low or high PD-L1 expression patients was 14 and 13 months, respectively (p=0.311).
Figure 6.
Survival analysis according to sPD-L1 and PD-L1. (A) Correlation between sPD-L1 level and OS. Each dot represents one ESCC patient (r2=0.477, p<0.001). (B) Mean OS did not differ significantly between different tissue PD-L1 staining levels (p=0.083). (C) Subgroup analysis of OS according to serum sPD-L1 concentration (p<0.001). (D) Subgroup analysis of OS according to tissue PD-L1 expression level (p=0.311).
sPD-L1 but Not PD-L1 is an Independent Prognostic Factor of OS in Advanced ESCC
After univariate Cox-regression analysis, variables of gender, ECOG performance score, lymph node metastasis, distant metastasis, sPD-L1, NLR, CRP, ALB, and GLB were entered into the multivariate Cox-regression analysis. However, tissue PD-L1 expression level was not found to be significant (p=0.898). We adjusted for the possible factors related to ESCC patients’ overall survival, including radiotherapy record, subsequent therapy record, age at diagnosis, BMI, tumor location, pathology grade, and T classification. Eventually, multivariate analysis demonstrated that HRs were significantly higher for the factors of female patients, lymph node metastasis, sPD-L1 high group, ECOG performance score ≥2, and NLR≥6.60 (Table 3). While sPD-L1 was still an independent prognostic factor, lymph node metastasis was found to be non-significant after adjustment for possible confounders.
Table 3.
Prognostic Factors for Overall-Survival
| Factors | Univariate Analysis | Multivariate Analysis (Non-Adjusted) | Multivariate Analysis (Adjusted***) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p* | HR | 95% CI | p** | HR | 95% CI | p** | |
| Age (years) | |||||||||
| <68 | |||||||||
| ≥68 | 1.02 | 0.64–1.63 | 0.932 | ||||||
| Gender | |||||||||
| Male | |||||||||
| Female | 1.11 | 1.42–3.13 | <0.001 | 1.02 | 0.63–1.65 | 0.933 | 1.08 | 0.63–1.83 | 0.788 |
| BMI (kg/m2) | |||||||||
| <25 | |||||||||
| ≥25 | 0.66 | 0.40–1.09 | 0.102 | ||||||
| ECOG PS | |||||||||
| 0–1 | |||||||||
| ≥2 | 2.16 | 1.41–3.30 | <0.001 | 1.88 | 1.22–2.90 | 0.004 | 1.81 | 1.15–2.85 | 0.010 |
| Tumor location | |||||||||
| Upper | |||||||||
| Middle | 0.65 | 0.37–1.16 | 0.144 | ||||||
| Lower | 0.83 | 0.47–1.46 | 0.508 | ||||||
| Overlapping | 1.07 | 0.51–2.25 | 0.860 | ||||||
| Pathology grade | |||||||||
| Well | |||||||||
| Moderately | 0.75 | 0.27–2.08 | 0.578 | ||||||
| Poorly | 0.85 | 0.30–2.36 | 0.750 | ||||||
| T classification | |||||||||
| T2 | |||||||||
| T3 | 0.69 | 0.42–1.13 | 0.144 | ||||||
| T4 | 0.76 | 0.46–1.25 | 0.278 | ||||||
| LN metastasis | |||||||||
| Absent | |||||||||
| Present | 2.07 | 1.17–3.67 | 0.013 | 1.98 | 1.08–3.63 | 0.028 | 1.87 | 0.97–3.61 | 0.063 |
| Distant metastasis | |||||||||
| Absent | |||||||||
| Present | 1.56 | 1.07–2.27 | 0.021 | 1.43 | 0.97–2.11 | 0.069 | 1.48 | 0.88–2.48 | 0.137 |
| sPD-L1 | |||||||||
| Low | |||||||||
| High | 3.96 | 2.51–6.26 | <0.001 | 3.71 | 2.05–6.71 | < 0.001 | 4.06 | 2.12–7.79 | < 0.001 |
| PD-L1 | |||||||||
| Low | |||||||||
| High | 0.98 | 0.67–1.42 | 0.898 | ||||||
| NLR | |||||||||
| <6.60 | |||||||||
| ≥6.60 | 1.57 | 1.03–2.31 | 0.036 | 1.72 | 1.08–2.74 | 0.021 | 1.69 | 1.05–2.70 | 0.030 |
| PLR | |||||||||
| <145 | |||||||||
| ≥145 | 1.29 | 0.75–2.23 | 0.360 | ||||||
| CRP (mg/L) | |||||||||
| <5.21 | |||||||||
| ≥5.21 | 1.55 | 1.02–2.36 | 0.038 | 1.29 | 0.84–2.00 | 0.2502 | 1.14 | 0.72–1.83 | 0.573 |
| ALB (g/L) | |||||||||
| <40.0 | |||||||||
| ≥40.0 | 1.39 | 0.95–2.02 | 0.093 | 1.27 | 0.85–1.90 | 0.251 | 1.24 | 0.80–1.92 | 0.337 |
| GLB (g/L) | |||||||||
| <25.7 | |||||||||
| ≥25.7 | 0.67 | 0.45–0.99 | 0.042 | 0.95 | 0.63–1.42 | 0.796 | 0.92 | 0.60–1.40 | 0.688 |
| LDH (U/L) | |||||||||
| <212 | |||||||||
| ≥212 | 0.76 | 0.51–1.14 | 0.182 | ||||||
Notes: *p<0.1 was considered significant in Univariate analysis. **p<0.05 was considered significant in Multivariate analysis.
Abbreviations: HR, hazard ratio; 95% CI, 95% confidence intervals; BMI, body mass index; ECOG PS, Eastern Cooperative Oncology Group performance score; LN metastasis, lymph node metastasis; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; CRP, C-reactive protein; ALB, albumin; GLB, globulin; LDH, lactate dehydrogenase.
Discussion
We conducted this study to evaluate the association of pre-treatment serum sPD-L1 concentration with clinicopathological characteristics and prognosis in patients with locally advanced or metastatic ESCC. Only a few reports have assessed the clinical role of sPD-L1 as a biomarker of prognosis in patients with malignancies,13–15 and to the best of our knowledge, the clinical significance of serum sPD-L1 level in ESCC and direct comparison of the expression between tissue and serum PD-L1 has not been demonstrated.
In this study, we assessed tissue PD-L1 expression and serum sPD-L1 concentration using matched tissue and pre-treatment serum samples from ESCC patients and made several novel discoveries. First, we assessed sPD-L1 concentration in 190 ESCC patients before treatment and in 58 healthy controls. We found that sPD-L1 levels were significantly elevated in patients with locally advanced or metastatic ESCC. sPD-L1 is released from PD-L1-positive cells, which binds to receptor of PD-1, and was not associated with tissue PD-L1 expression level, and the mechanisms behind it need to be further explored. Interestingly, sPD-L1 concentration was highly elevated in female patients and in patients with high level of GLB, it suggest this may have something to do with the lifestyle of both men and women, as well as their hormone levels. The specific mechanism needs to be further explored and studied, however, we did not observe any association with local disease progression factors, including lymph node metastasis, distant metastasis, poor pathologic grade, and impaired ECOG performance status. Second, we assessed tissue PD-L1 expression in ESCC, and divided the ESCC cohort into the PD-L1-high and PD-L1-low groups based on IHC staining intensity. Eventually, there were 45.3% (n=86) patients in the PD-L1-low group (staining score 1 or 2) and 54.7% (n=104) in the PD-L1-high group (staining score 3 or 4). No factors correlated with tissue PD-L1 expression level in this study. Third, we evaluated association of sPD-L1 concentration and tissue PD-L1 expression level, but we did not observe significant correlation. It was consistent with the results of a previous study in gastric cancer.16 Finally, the multivariate Cox regression analysis clearly showed that high level of sPD-L1 but not tissue PD-L1 expression was an independent prognostic factor of overall survival in patients with locally advanced or metastatic ESCC.
The prognostic role of IHC-evaluated PD-L1 expression has been reported in many tumor types, including ESCC.17,18 The majority of studies has demonstrated that PD-L1 expression was associated with impaired OS. Several studies investigated PD-L1 expression level in patients with ESCC. Recently, He et al reported that positive PD-L1 expression was a favorable predictor in ESCC patients with I–II stage disease or without lymph node metastasis, but not in patients with stage III disease or without lymph node metastasis.19 Given the advanced stage of ESCC in our study, we conducted the survival analysis to investigate whether lymph node metastasis status could influence the prognostic value of tissue PD-L1 expression, but we found no differences in survival between high and low PD-L1 tissue expression irrespective of the presence of lymph node metastasis (Supplementary Figure 2). Similar results were found between patients with specific T grades (T2, T3, or T4), pathologic grades (well, moderately, or poorly differentiation), or distant metastasis status (metastasis or non-metastasis). Jiang et al recently reported that high PD-L1 expression was associated with a favorable prognosis in patients with ESCC undergoing postoperative adjuvant radiotherapy,20 while Wang et al reported that high PD-L1 expression on tumor cells was linked with impaired OS in ESCC patients.21 These results suggest that PD-L1 expression in ESCC is highly heterogeneous, and survival benefit may also correlate with specific clinicopathological characteristics or treatment measures.
PD-L1 is broadly expressed on the membrane or cytoplasm of various types of cells, including tumor cells, T cells, B cells, dendritic cells, macrophages, and even bronchial epithelial cells.16,22,23 Recent studies have demonstrated the existence of soluble PD-L1 released from PD-L1 positive cells in human serum. In addition, Takeuchi et al reported that sPD-L1 in plasma of patients with NSCLC also had PD-1 binding capacity.12 Furthermore, Shigemori et al found that a high level of PD-L1 expression was associated with a lower density of CD3- and CD8-positive Tumor-infiltrating cell (TILs) in gastric cancer, while sPD-L1 concentration in serum had no significant association with the density of TILs in the tumor microenvironment.16 It was suggested that PD-L1 but not sPD-L1 could modulate host tumor immunity in primary tumor location.
Several studies have demonstrated the feasibility of circulating molecular or hematologic markers as biomarkers of OS in patients with malignancies. Sven H. Loosen reported that circulating levels of osteopontin could predict patients’ outcomes after resection of colorectal liver metastases.24 Cho et al evaluated the NLR in 621 patients and showed that high NLR was an independent poor prognostic factor in head and neck cancer treated with radiotherapy.25 Recently, studies also demonstrated that sPD-L1 could act as a biomarker of survival in patients with cancer. Shigemori et al evaluated sPD-L1 levels in serum samples before treatment in 180 gastric cancer patients who underwent surgery, and reported that high sPD-L1 significantly impaired the OS in gastric cancer patients.16 More recently, Bian et al quantified circulating sPD-L1 concentration in 59 patients with pancreatic adenocarcinoma (PDAC) and showed that elevated level of serum sPD-L1 was also an independent prognostic factor in PDAC patients.10 Likewise, Ha et al reported that high level of sPD-L1 was one of the independent poor prognostic factors in patients with advanced biliary tract cancer who received palliative chemotherapy.26 For immunotherapy, Costantini et al demonstrated that plasma sPD-L1 level at baseline was associated with clinical outcome in NSCLC patients treated with nivolumab (one of the PD-1 blockers).27 These studies revealed that monitoring the concentration of serum sPD-L1 might be helpful for predicting survival in subgroups of patients and subsequently improve their treatment.
In our study, we aimed to assess the prognostic value of serum sPD-L1 in patients with locally advanced or metastatic ESCC who received cytotoxic combination chemotherapy. In total, 190 eligible patients were enrolled, and chemotherapy regimens were restricted to docetaxel plus nedaplatin, a conventional chemotherapy regimen for advanced ESCC. First, our results showed that sPD-L1 level was significantly elevated in patients with advanced ESCC patients compared with healthy controls, and interestingly, it was more obvious in female than in male patients. Next, we made a direct association analysis of the clinical burden, and we found no correlation between tissue and serum PD-L1. This result was in accordance with the previous report by Tsunehiko Shigemori in gastric cancer patients. Several studies showed that serum sPD-L1 in cancer patients might originate from PD-L1 released during pro-tumor inflammatory responses, antitumor immune responses, and intrinsic activities in tumor cells. These sources might influence the circulating sPD-L1 levels in ESCC patients. Then, we conducted KM survival and Cox regression analyses and demonstrated that high sPD-L1 concentration significantly impaired the OS and acted as an independent prognostic factor in patients with locally advanced or metastatic ESCC after adjustment for possible confounders.
Our study showed that serum sPD-L1 concentration did not correlate with major clinicopathological characteristics or hematologic markers including lymph node metastasis, distant metastasis, tumor pathologic grade, T grade, ECOG performance status, NLR, and PLR (Supplementary Figure 3). Therefore, our findings suggest that sPD-L1 does not reflect the local disease development in the tumor’s primary location, distant metastasis, or host hematologic characteristics. On the other hand, previous evidence suggested that PD-L1 could suppress the activation of T cells and decrease the density of T cells infiltrating the tumor microenvironment. sPD-L1 was also associated with immune suppression and host-immune damage through inhibiting T cell activation in non-small cell lung carcinoma (NSCLC), aggressive renal cell carcinoma, and aggressive diffuse large-B cell lymphoma.28–30 However, the role of sPD-L1 in the host immune system in ESCC patients needs to be confirmed by further research. Limitations of the present study included the single-center, single-arm design, and the relatively small sample size. Therefore, a more accurate and effective prognostic model based on sPD-L1 might be developed for individual prognosis of clinical outcome in ESCC patients.
Conclusion
In conclusion, our study demonstrated that sPD-L1 concentration in serum did not correlate with tissue PD-L1 expression. Serum sPD-L1 but not tissue PD-L1 expression level might be used as a prognostic biomarker in patients with locally advanced or metastatic ESCC.
Acknowledgments
Thank you for the sincere cooperation between creators. The creators report no clashes of intrigued related to this work. Thanks to LetPub for its touch on the language and grammar of articles.
Disclosure
The authors declare no conflict of interest. This study was approved by Ethics Committee of Affiliated Hospital of Shandong University of Traditional Chinese Medicine. The healthy volunteers provided informed consent to participate in this study, and that it was conducted in accordance with the Declaration of Helsinki.
References
- 1.Glenn TF. Esophageal cancer. Facts, figures, and screening. Gastroenterol Nurs. 2001;24:271–275. doi: 10.1097/00001610-200111000-00002 [DOI] [PubMed] [Google Scholar]
- 2.Wei WQ, Chen ZF, He YT, et al. Long-term follow-up of a community assignment, one-time endoscopic screening study of esophageal cancer in China. J Clin Oncol. 2015;33:1951–1957. doi: 10.1200/jco.2014.58.0423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 4.Herskovic A, Russell W, Liptay M, Fidler MJ, Al-Sarraf M. Esophageal carcinoma advances in treatment results for locally advanced disease: review. Ann Oncol. 2012;23:1095–1103. doi: 10.1093/annonc/mdr433 [DOI] [PubMed] [Google Scholar]
- 5.Hirano H, Kato K. Systemic treatment of advanced esophageal squamous cell carcinoma: chemotherapy, molecular-targeting therapy and immunotherapy. Jpn J Clin Oncol. 2019;49:412–420. doi: 10.1093/jjco/hyz034 [DOI] [PubMed] [Google Scholar]
- 6.Kajiura S, Hosokawa A, Yoshita H, et al. Phase I study of docetaxel plus nedaplatin in patients with metastatic or recurrent esophageal squamous cell carcinoma after cisplatin plus 5-fluorouracil treatment. Am J Clin Oncol. 2016;39:13–17. doi: 10.1097/coc.0000000000000018 [DOI] [PubMed] [Google Scholar]
- 7.Yang PW, Huang PM, Yong LS, et al. Circulating interleukin-6 is associated with prognosis and genetic polymorphisms of MIR608 in patients with esophageal squamous cell carcinoma. Ann Surg Oncol. 2018;25:2449–2456. doi: 10.1245/s10434-018-6532-4 [DOI] [PubMed] [Google Scholar]
- 8.Bradley CA. Pembrolizumab improves OS across PD-L1 subgroups. Nat Rev Clin Oncol. 2019;16:403. doi: 10.1038/s41571-019-0213-5 [DOI] [PubMed] [Google Scholar]
- 9.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian B, Fanale D, Dusetti N, et al. Prognostic significance of circulating PD-1, PD-L1, pan-BTN3As, BTN3A1 and BTLA in patients with pancreatic adenocarcinoma. Oncoimmunology. 2019;8:e1561120. doi: 10.1080/2162402x.2018.1561120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shen H, Ji Y, Zhou D, Zhang Y. Soluble programmed death-ligand 1 are highly expressed in peripheral T-cell lymphoma: a biomarker for prognosis. Hematology (Amsterdam, Netherlands). 2019;24:392–398. doi: 10.1080/16078454.2019.1590965 [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi M, Doi T, Obayashi K, et al. Soluble PD-L1 with PD-1-binding capacity exists in the plasma of patients with non-small cell lung cancer. Immunol Lett. 2018;196:155–160. doi: 10.1016/j.imlet.2018.01.007 [DOI] [PubMed] [Google Scholar]
- 13.Chang B, Huang T, Wei H, et al. The correlation and prognostic value of serum levels of soluble programmed death protein 1 (sPD-1) and soluble programmed death-ligand 1 (sPD-L1) in patients with hepatocellular carcinoma. Cancer Immunol Immunother. 2019;68:353–363. doi: 10.1007/s00262-018-2271-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cubillos-Zapata C, Martinez-Garcia MA, Campos-Rodriguez F, et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur Respir J. 2019;53:1801298. doi: 10.1183/13993003.01298-2018 [DOI] [PubMed] [Google Scholar]
- 15.Kushlinskii NE, Gershtein ES, Morozov AA, et al. Soluble ligand of the immune checkpoint receptor (sPD-L1) in blood serum of patients with renal cell carcinoma. Bull Exp Biol Med. 2019;166:353–357. doi: 10.1007/s10517-019-04349-8 [DOI] [PubMed] [Google Scholar]
- 16.Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26:876–883. doi: 10.1245/s10434-018-07112-x [DOI] [PubMed] [Google Scholar]
- 17.Hsieh CC, Hsu HS, Li AF, Chen YJ. Clinical relevance of PD-L1 and PD-L2 overexpression in patients with esophageal squamous cell carcinoma. J Thorac Dis. 2018;10:4433–4444. doi: 10.21037/jtd.2018.06.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rong L, Liu Y, Hui Z, et al. PD-L1 expression and its clinicopathological correlation in advanced esophageal squamous cell carcinoma in a Chinese population. Diagn Pathol. 2019;14:6. doi: 10.1186/s13000-019-0778-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo W, Zhang F, Shao F, et al. PD-L1 expression on tumor cells associated with favorable prognosis in surgically resected esophageal squamous cell carcinoma. Hum Pathol. 2019;84:291–298. doi: 10.1016/j.humpath.2018.09.014 [DOI] [PubMed] [Google Scholar]
- 20.Jiang C, Zhu Y, Tang S, et al. High PD-L1 expression is associated with a favorable prognosis in patients with esophageal squamous cell carcinoma undergoing postoperative adjuvant radiotherapy. Oncol Lett. 2019;17:1626–1634. doi: 10.3892/ol.2018.9747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu F, Xu L, Wang Q, An G, Feng G, Liu F. Clinicopathological and prognostic value of programmed death ligand-1 (PD-L1) in renal cell carcinoma: a meta-analysis. Int J Clin Exp Med. 2015;8:14595–14603. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer. 2016;19:42–52. doi: 10.1007/s10120-014-0440-5 [DOI] [PubMed] [Google Scholar]
- 23.Spranger S, Spaapen RM, Zha Y, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5:200ra116. doi: 10.1126/scitranslmed.3006504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loosen SH, Heise D, Dejong CH, Roy S, Tacke F. Circulating levels of osteopontin predict patients’ outcome after resection of colorectal liver metastases. J Clin Med. 2018;7:390. doi: 10.3390/jcm7110390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho Y, Kim JW, Yoon HI, Lee CG, Keum KC, Lee IJ. The prognostic significance of neutrophil-to-lymphocyte ratio in head and neck cancer patients treated with radiotherapy. J Clin Med. 2018;7:512. doi: 10.3390/jcm7120512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ha H, Nam AR, Bang JH, et al. Soluble programmed death-ligand 1 (sPDL1) and neutrophil-to-lymphocyte ratio (NLR) predicts survival in advanced biliary tract cancer patients treated with palliative chemotherapy. Oncotarget. 2016;7:76604–76612. doi: 10.18632/oncotarget.12810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Costantini A, Julie C, Dumenil C, et al. Predictive role of plasmatic biomarkers in advanced non-small cell lung cancer treated by nivolumab. Oncoimmunology. 2018;7:e1452581. doi: 10.1080/2162402x.2018.1452581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frigola X, Inman BA, Lohse CM, et al. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin Cancer Res. 2011;17:1915–1923. doi: 10.1158/1078-0432.ccr-10-0250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossille D, Gressier M, Damotte D, et al. High level of soluble programmed cell death ligand 1 in blood impacts overall survival in aggressive diffuse large B-Cell lymphoma: results from a French multicenter clinical trial. Leukemia. 2014;28:2367–2375. doi: 10.1038/leu.2014.137 [DOI] [PubMed] [Google Scholar]
- 30.Zhao J, Zhang P, Wang J, et al. Plasma levels of soluble programmed death ligand-1 may be associated with overall survival in nonsmall cell lung cancer patients receiving thoracic radiotherapy. Medicine. 2017;96:e6102. doi: 10.1097/md.0000000000006102 [DOI] [PMC free article] [PubMed] [Google Scholar]