Abstract
Fundamental modulation of energy metabolism in immune cells is increasingly being recognized for the ability to impart important changes in cellular properties. In homeostasis, cells of the innate immune system, such as monocytes, macrophages and dendritic cells (DCs), are enabled to respond rapidly to various forms of acute cellular and environmental stress, such as pathogens. In chronic stress milieus, these cells may undergo a re-programming, thereby triggering processes that may instigate tissue damage and failure of resolution. In settings of metabolic dysfunction, moieties such as excess sugars (glucose, fructose and sucrose) accumulate in the tissues and may form advanced glycation end products (AGEs), which are signaling ligands for the receptor for advanced glycation end products (RAGE). In addition, cellular accumulation of cholesterol species such as that occurring upon macrophage engulfment of dead/dying cells, presents these cells with a major challenge to metabolize/efflux excess cholesterol. RAGE contributes to reduced expression and activities of molecules mediating cholesterol efflux. This Review chronicles examples of the roles that sugars and cholesterol, via RAGE, play in immune cells in instigation of maladaptive cellular signaling and the mediation of chronic cellular stress. At this time, emerging roles for the ligand-RAGE axis in metabolism-mediated modulation of inflammatory signaling in immune cells are being unearthed and add to the growing body of factors underlying pathological immunometabolism.
Keywords: receptor for advanced glycation end products (RAGE), sugar metabolism, cholesterol metabolism, interferon signaling, diabetes, immune cell, monocyte/macrophage, dendritic cell
INTRODUCTION
The Receptor for Advanced Glycation End Products (RAGE) was born from the quest to discern how sugar (glucose)-modified molecules contribute to the pathogenesis of diabetic complications. In first experiments in cultured endothelial cells testing D-glucose-modified albumin (AGE-albumin), as well as other backbone proteins, it was discovered that the AGE-modified proteins, but not the backbone (non-AGE’d proteins), bound to a member of the immunoglobulin superfamily, RAGE [1]. From this discovery, the simultaneous expansion of the biology of AGEs and that of RAGE highlighted novel roles for AGEs beyond diabetes. Furthermore, in-depth study of RAGE uncovered its role as a multi-ligand receptor. These discoveries led to the identification of roles for RAGE in immunometabolism, with implications for disorders such as atherosclerosis, cardiovascular disease and obesity. These introductory sections, to follow, briefly summarize the ligands of RAGE; the body of the Review focuses on examples of settings in which RAGE has been shown to modulate immunometabolic functions.
AGEs
AGEs are a heterogeneous group of structures that include both fluorescent and non-fluorescent forms [2,3]. Although AGEs have traditionally been measured by biochemical methods, more recently, “skin autofluorescence” has been proposed as a means to estimate AGE content through non-invasive technology [4,5]. Beyond innate in vivo production of AGEs driven by multiple factors, including high levels of blood glucose, food/oral-derived AGEs are considered to be an exogenous source of these species; upon uptake through the gastrointestinal tract, up to 10–30% may become part of a subject’s total AGE burden [6–8]. The balance of the orally-derived AGEs may reach the colon, in which case they might interact with the bacterial microbiota [9]. Although more work needs to be done in this area, studies in human subjects have suggested the possibility that dietary restriction of AGEs might beneficially affect the composition of the gut microbiome and reduce metabolic dysfunction [10,11].
In addition to roles for AGEs in diabetes and diabetic complications, more recent evidence has linked AGEs such as carboxymethyllysine (CML)-AGE to obesity. For example, in the PANACEA study (Physical Activity, Nutrition, Alcohol, Cessation of smoking, Eating out of home in relation to Anthropometry), a sub-cohort of EPIC study (European Prospective Investigation into Cancer and Nutrition), specific common AGEs (CML, carboxyethyllysine (CEL), and Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine (MG-H1) were measured in about 200 foods and a number of factors, particularly body weight, were tracked. It was reported that in European adults, over five years, higher levels of dietary AGEs were associated with marginally greater weight gain [12]. In distinct studies, adipose tissue depots retrieved from obese vs lean subjects displayed higher levels of CML-AGEs, in parallel with higher expression of RAGE [13]. It was reported that in parallel with higher adipose tissue CML-AGE levels, plasma of the obese vs lean subjects contained lower levels of the CML-AGEs; the authors demonstrated that in obesity, higher RAGE expression in the fat tissue “trapped” the CML-AGEs. Furthermore, lower levels of CML-AGE in plasma correlated with higher degrees of insulin resistance. It was notable that these overall conclusions were strengthened by experiments in mice devoid of Ager (Ager is the gene encoding RAGE) in that study [13].
Although AGEs were the first of the RAGE ligands to be discovered, studies from multiple laboratories have illustrated that RAGE is a multi-ligand receptor. Although the implications of AGE-RAGE interaction in immunometabolism are becoming increasingly apparent, experiments have elucidated that the non-AGE ligands of RAGE also play roles in immune and metabolic pathologies.
Non-AGE Ligands of RAGE
In addition to AGEs, RAGE is a receptor for multiple members of the S100/calgranulin family; although the first S100s to be identified as RAGE ligands were S100A12 and S100B, a number of other members of this family have been shown to bind and signal through RAGE [14,15]. In addition, high-mobility group box 1 (HMGB1), lysophosphatidic acid (LPA) and oligomeric forms of amyloid beta peptide (Aβ) and islet amyloid polypeptide (IAPP) are also RAGE ligands [16–19]. Among other identified ligands of RAGE are MAC-1, C1q and phosphatidylserine (PS) [20–22].
The RAGE extracellular domains consist of one Variable (V)-type immunoglobulin (Ig) domain followed by two constant (C)-type Ig domains (C1 and C2); although V or VC1 binds to many of the ligand families, the binding sites on the V-domain are heterogeneous and are spatially distinct. In addition, it is established that some of the RAGE ligands may bind solely at the extracellular C1 and C2-type Ig domains [15,23–25]. On account of this diversity of ligand binding to discrete sites on the extracellular domains and that the story of RAGE pathobiological roles in chronic disease is not limited to “one ligand to one disease”, it is plausible that putative therapeutic agents targeting discrete regions on these extracellular domains may display limited efficacy if they do not capture or sequester the biological effects of key mediating ligands in each disease setting.
As noted above, this review will present examples of RAGE roles in immunometabolic perturbations through the lenses of sugars and cholesterol, as key examples by which such metabolic species intersect with the immune system through RAGE.
GLUCOSE, FRUCTOSE AND SUCROSE: INTERFACING WITH THE RAGE AXIS
A common means to test the effects of d-glucose or other sugars on gene expression properties in immune cells, such as macrophages or dendritic cells (DCs), is the exposure of these cells to diabetes-relevant levels of d-glucose (15–25 mM d-glucose) vs homeostatic, non-diabetic levels of d-glucose (5 mM). To study the effects of RAGE on the response to varied glucose conditions, murine bone marrow derived macrophages (BMDMs) were isolated and incubated with diabetes-relevant high levels of glucose (25 mM d-glucose) or euglycemic levels (5 mM). Incubation of BMDMs exerted significant effects on expression of inflammatory mediators; compared to d-glucose (5 mM), treatment of these cells with high glucose resulted in significant upregulation of mRNA transcripts encoding Il1b, Tnf, Nos2 and Ccr7 in wild-type cells; these effects were significantly mitigated in BMDMs retrieved from Ager null mice [26]. In contrast, high glucose resulted in downregulation of Egr1 and Ccl2 in wild-type but not Ager null BMDMs. Whereas high glucose downregulated mRNA transcripts for Arg1 and Il10 in wild-type BMDMs, these effects were significantly attenuated by deletion of BMDM Ager and, in fact, in high glucose conditions, BMDMs devoid of Ager displayed even higher levels of these two key mediators linked to anti-inflammatory/tissue repair processes [26] (Figure 1). These findings indicate the pleiotropy of RAGE-dependent actions driven by glucose in immune cells such as macrophages, derived from the bone marrow. Whereas expression of RAGE results in upregulation of certain “pro-inflammatory” mediators, others, such as Ccl2, were downregulated by RAGE in BMDMs. Although these studies did not probe how direct metabolic effects contributed to or were consequences of RAGE actions by glucose, others studies have addressed the roles of distinct sugars in immune cells, such as DCs.
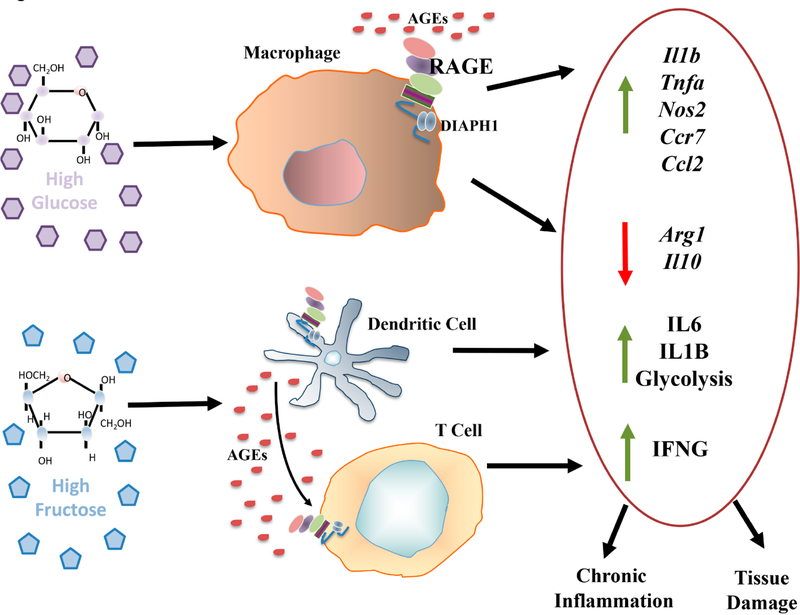
Figure 1. Effect of glucose and fructose on inflammation in murine bone marrow derived macrophages and human dendritic cells and T cells.
The sugars glucose and fructose exert significant effects on immune cells. In murine bone marrow-derived macrophages (top), incubation with diabetes-relevant levels of d-glucose (25 mM) vs non-diabetes relevant levels of d-glucose (5 mM) resulted in upregulation of the indicated pro-inflammatory genes, in parallel with downregulation of resolution-provoking genes; these processes were traced to RAGE [26]. In other studies, human peripheral blood-derived DCs incubated with high levels of d-fructose but not d-glucose, increased production of IL1B and IL6 (bottom). Co-incubation of d-fructose-treated DCs with T cells increased T cell production of IFNG. These experiments traced the effects of d-fructose-derived inflammation to RAGE [27].
In in vitro studies, incubation of human peripheral blood-derived DCs with high levels of d-fructose, but not d-glucose (15 mM), resulted in production of IL6 and IL1B [27]. When T cells were incubated with the d-fructose-treated DCs, production of Interferon (IFN)-gamma (G) (IFNG) was enhanced. The underlying mechanisms were traced to high fructose-mediated AGE generation, which, via RAGE, mediated these effects. In the acute setting (24 h), these effects were not dependent on metabolic changes as there was no evidence of increased glycolysis [27]. However, when DCs were chronically exposed to high fructose (72 h), Seahorse analyzer studies revealed a shift towards glycolysis that was not observed when the cells were treated with glucose. When the authors compared AGE formation and its inflammatory potential driven by glucose vs fructose, their studies suggested that fructose-derived AGEs, via RAGE, were more inflammatory than those stimulated by high glucose [27]. Collectively, these findings implicated fructose-derived AGEs, via RAGE, in upregulation of proinflammatory pathways in human DCs (Figure 1).
The dietary effects of distinct sugars, sucrose and starch (the latter, polymeric carbohydrate consisting of numerous glucose units joined by glycosidic bonds (polymers)), were tested in vivo for their potential effects on hypothalamic inflammation. Microglia, the resident “macrophages” of the central nervous system, play key roles in modulation of properties in surrounding cells in the environment, such as neurons and astrocytes [28]. To test the potential distinct effects of fats vs sugars on hypothalamic inflammation, mice were fed various diets as follows: Diet 1: normal chow (high carbohydrate (64.3%) (wheat and corn), low fat 16.7%); Diet 2: high carbohydrate/high fat (25.5% [sucrose], 58% fat); Diet 3: (high carbohydrate/high fat (19.4% [starch], 61.9% fat); Diet 4: low carbohydrate/high fat (2.2% carbohydrate, 78.7% fat); and Diet 5: low carbohydrate/high fat (1.7% carbohydrate, 92.8% fat). Among the numerous endpoints studied, the authors concluded that only diets high in the carbohydrates (sucrose or starch), but not high fat alone, mediated microglial reactivity and they showed that these effects were due to diet-driven generation of AGEs, acting via RAGE [29]. In parallel, examination of metabolic factors in the mice devoid of Ager and fed the high carbohydrate-containing diet revealed that obesity was reduced, as was glucose intolerance, suggesting that AGEs, at least in part via RAGE, mediated these pathological factors in the hypothalami of the mice [29].
LIGAND-RAGE AXIS: CLEARANCE OF DEAD CELLS, AUTOPHAGY AND MITOPHAGY
Roles for the ligand-RAGE axis in the clearance of dead cells has been considered, as the underlying mechanisms and consequences directly impact metabolic pathways. Examples of such findings in the context of the RAGE axis continue to accrue. The clearance of apoptotic cells, or efferocytosis, is a multi-step process triggered by caspase-dependent apoptosis. This process is essential for the removal of dead/dying cells, both in development and in the maintenance of tissue homeostasis in the adult state, particularly upon imposition of perturbations in which the accumulation of dead/dying cells and their elaborated potentially toxic mediators are maladaptive in the cellular milieu [30,31]. Among the numerous “eat-me” signals on dead/dying cells is the cell surface expression of phosphatidylserine (PS), which was shown to interact with RAGE [22,32,33]. Mice devoid of Ager demonstrated a reduced ability to engulf apoptotic neutrophils and thymocytes [33]. In addition to experiments testing genetic deletion of Ager, other studies used sRAGE or anti-RAGE antibodies to show decreased phagocytosis of apoptotic cells and in gain-of-function experiments, using overexpression strategies in human embryonic kidney cells (HEK cells), an increased ability to engulf apoptotic cells was illustrated [33]. The specific mechanism was traced to binding of RAGE to PS by use of a solid-state ELISA and an adhesion assay.
Others demonstrated analogous findings; surface plasmon resonance (SPR) was used to show that sRAGE bound to PS (KD = 563 nM) [22]. In addition, fluorescence resonance energy transfer (FRET) also confirmed the interaction. In vivo studies were also performed in which lung injury was induced by lipopolysaccharide (LPS); in the Ager null mice, there were increased numbers of apoptotic neutrophils in the bronchoalveolar lavage fluid (BAL), suggesting reduced clearance in the absence of RAGE [22]. Those authors also proposed that these findings present interesting insights into how the balance between circulating sRAGE levels and the membrane RAGE (mRAGE) might be crucial in determining the overall effects of RAGE in the clearance of apoptotic cells. As it is well established that levels of sRAGE may vary in human subjects depending on presence or absence of diseases in which RAGE ligands accumulate [34–37], such findings may be important in determining the potential (or not) for the clearance of apoptotic cells, which, themselves, may augment inflammatory responses. Importantly, in addition to RAGE, numerous receptors that recognize PS have been identified [38]. Certainly, such versatility provides for flexibility in discrete organs and, likely, in the setting of distinct perturbants and triggers of severe cellular stress and apoptosis. If and how RAGE may be called upon for roles in these processes remains to be fully elucidated.
Increasing evidence has provided insight into the metabolic reprogramming of innate immune cells, including myeloid cells, in processes spanning glycolysis and oxidative phosphorylation [39]. In microglia, the ligand-RAGE axis has also been linked to mitophagy in responses to chronic stress. In a study by Zhang and colleagues [40], chronic stress induced by intermittent electric foot shocks and noise stress, which was administered two hours/day for 15 consecutive days, resulted in increased cytoplasmic translocations of RAGE ligand HMGB1 and upregulation of RAGE. In the rostral ventrolateral medulla (RVLM), mitochondria and oxidative stress, with consequent proinflammatory polarization of microglia, were reduced in the RVLM of stressed mice devoid of microglia Ager. HMGB1-RAGE impaired the late stages of mitophagy flux in these cells, which was associated with increased neuroinflammation (NF-kB pathway) and increased blood pressure in the stressed mice; these processes were relieved by microglia Ager deletion or by delivery of siRNAs to reduce expression of Hmgb1 in the RVLM [40].
Mitophagy is thought to be intricately related to the inflammasome [41], which is known to regulate a distinct form of cell death, known as pyroptosis, which has been studied in the context of RAGE [42]. Pyroptosis is a form of programmed lytic death in immune cells consequent to infection [43]. Once triggered, the purpose of pyroptosis is to eliminate the infected cells, which, in turn, leads to initiation of inflammatory responses [44]. As in other forms of acute responses to stress, however, overactivation of these mechanisms may damage the host. The canonical inflammasome pathway mediates these responses, leading to maturation and activation of IL1beta and IL18 [43,44]. In a murine model of sepsis, RAGE ligand HMGB1 derived from neutrophil extracellular traps (NETs), acting via RAGE and dynamin-dependent signaling, and the downstream molecular pathways, drove macrophage pyroptosis through cathepsin B (CatB) release from ruptured lysosomes, followed by the formation of the pyroptosome and caspase-1 activation [45].
In the case of phagocytes and the engulfment of dead cells, a large burden of metabolic species is immediately presented to the cell. In a recent review, Doran and colleagues identified three examples of mechanisms by which the surge in lipids might be handled by the engulfing phagocyte [46]. First, the free cholesterol may be esterified by acyl-CoA cholesterol acyltransferase (ACAT) into cholesterol esters; consequent to this step, the species may be sequestered in neutral lipid droplets or effluxed from the cell, the latter in an effort to reduce the intracellular accumulation. Cholesterol efflux transporters, such as ATP-binding cassette transporter A1 (ABCA1) may be recruited for this effort. Second, the fatty acids derived from the apoptotic cells may undergo fatty acid oxidation via the mitochondria; this results in the expression of IL10 (resolution pathway). Third, the binding of the apoptotic cells to PS-expressing efferocyte receptors may lead to the upregulation of glucose transporter 1 (GLUT1 or SLC2A1) and serum/glucocorticoid regulated kinase 1 (SGK1), which is required for GLUT1 transport to the plasma membrane [46], thereby regulating glucose metabolism. The latter findings implicate the process of glycolysis in the response to acquired metabolite loads upon the engulfment of dead/dying cells. In this context, glycolysis in macrophages has been suggested to link to pro-inflammatory mechanisms [47–49]. The specific effects of RAGE on glycolysis in macrophages have yet to be fully explored; rather, a number of studies have suggested that the products of glycolysis may generate RAGE ligand methylglyoxal-modified species [50,51]. A number of studies have linked RAGE to cholesterol metabolism and will be considered in the sections to follow.
RAGE AND CHOLESTEROL METABOLISM
RAGE has been implicated in downregulation of cholesterol transporters. Such effects, if not accompanied by opposing processes implicated in cholesterol metabolism, would have the effect of increasing cholesterol accumulation in macrophages [52–54]. These questions in the context of RAGE have been examined in a number of research studies, as detailed below.
In human macrophages, the effects of exposure to AGE-BSA were detected using microarray analyses followed by PCR studies. In those cells, treatment with AGE-BSA resulted in a reduction in the mRNA of the ATP-binding cassette transporter G1 (ABCG1), but no effects on the transporter ABCA1 were shown [55]. Treatment with anti-RAGE antibodies reduced the downregulation of ABCG1, thereby implicating RAGE as a mediator of these effects. In in vitro studies to determine the putative effects on cholesterol efflux to high density lipoprotein (HDL) or Apolipoprotein A1 (ApoA1), it was shown that AGE-BSA reduced cholesterol efflux to HDL but not to ApoA1. Although the regulation of ABCG1 (and ABCA1) has been extensively linked to the Liver X Receptors (LXRs) [56], AGE-BSA did not reduce the mRNA levels of the genes linked to LXRs and had no effect on the binding of nuclear proteins to the LXR response element when compared with treatment with BSA (non-glycated) [56].
A distinct study examined the effect of a different RAGE ligand, S100B, on expression of cholesterol transporters using human THP1 monocyte/macrophage-like cells and human monocytes [57]. In those studies, the authors showed that treatment of THP1 cells with S100B resulted in 2–3-fold reduction in ABCA1 and ABCG1 mRNA and that by Western blotting, the levels of ABCA1 protein were also significantly attenuated by S100B. These effects of S100B-mediated suppression of these transporters was attenuated by anti-RAGE antibodies. It was shown that the suppressive effects of S100B were reversed by treatment with ligands of the LXR [57]. Furthermore, the authors isolated peripheral blood mononuclear cells (PBMCs) from diabetic patients and reported that the mRNA levels of ABCA1 and ABCG1 were reduced by about 3-fold in the cells from type 1 and type 2 diabetic patients vs non-diabetic control subjects [57]. In terms of molecular regulation by S100B, although the RAGE ligand had no effect on ABCA1 mRNA stability, it was shown that the effects of S100B on the ABCA1 transporter were dependent on LXR [57].
As implied by the studies discussed immediately above, in addition to reduced levels of the cholesterol transporters ABCA1 and ABCG1 in diabetes, it has been reported that serum cholesterol efflux capacity and reverse cholesterol transport (RCT), the functional indices of the biological effects of these transporters, are reduced in diabetes [58,59]. These findings are suggested to have pathological relevance, as inferred from the documented inverse relationship between cholesterol efflux capacity and carotid-intima thickness, a well-established surrogate marker of atherosclerosis [60,61]. Daffu and colleagues tested the hypothesis that RAGE contributed to these processes in diabetes. In macrophages grown in diabetes-relevant vs euglycemic levels of d-glucose, macrophage cholesterol efflux to ApoA1 and HDL were both reduced and, in vivo, RCT to plasma, liver and feces was significantly reduced in diabetic macrophages, at least in part via RAGE [62]. In vitro, RAGE ligands suppressed macrophage levels of ABCG1 and ABCA1; interestingly, these processes were independent of LXR-dependent mechanisms, but were dependent on peroxisome proliferator-activated receptor-γ (PPARG)-responsive promoter elements [62]. To determine if RAGE contributed to downregulation of these cholesterol transporters in diabetic macrophages in murine atherosclerotic plaques, laser capture microdissection of CD68-expressing macrophages was performed on atherosclerotic plaques of diabetic mice devoid of Ldlr expressing or devoid of both the Ldlr and Ager. In this setting, macrophages devoid of Ager displayed higher levels of Abca1, Abcg1 and Pparg mRNA transcripts vs those macrophages from diabetic mice expressing Ager; these effects of deletion of Ager were independent of glycemia [62]. Hence, those studies provided insight into roles for RAGE in diabetes-associated suppression of macrophage cholesterol efflux and RCT.
These studies were recently extended to the study of in vivo-derived AGEs. Albumin was isolated from patients with type 1 and type 2 diabetes (T1D and T2D, respectively) and from non-diabetic control subjects. Macrophages were retrieved from Ager null and wild-type mice, or human THP1 cells were subjected to treatment with silencing RNAs to reduce the expression of AGER [63]. Compared to wild-type murine macrophages and control-treated THP1 cells, cholesterol efflux was reduced in response to albumin from T1D or T2D patients vs control albumin [63]; in parallel with increased intracellular lipid content. These effects of the human diabetic albumin were reduced in macrophages lacking Ager; in that setting, cholesterol efflux and lipid staining were higher and lower, respectively, when compared to the control RAGE-expressing cells [63]. These latter studies are important as they used in vivo-isolated vs in vitro-prepared AGEs; nevertheless, they indicate that the in vitro-prepared material behaves analogously to that of AGEs obtained directly from diabetic subjects.
In a distinct study, the effects of AGEs on macrophage lipid content and underlying mechanisms were examined. It had been previously shown that the AGE-RAGE axis was implicated in the accumulation of lipid droplets accumulation in the aortas of high fat-fed diabetic Goto Kakisaki (GK) rats [64]. As it was also previously reported that AGEs promote foam cell formation [65,66], the role of AGE-RAGE interaction in cholesterol uptake, synthesis and efflux of cholesterol in macrophages was addressed in distinct studies. To comprehensively test this pathway, THP1 cells differentiated into macrophages were treated with AGEs alone or in the presence of modified forms of LDL [67]. The authors reported that AGEs plus modified LDL increased lipid accumulation and cholesterol esters in macrophages, and decreased HDL-mediated efflux of cholesterol through regulation of CD36, scavenger receptors A1, HMG-CoA-reductase, ACAT1 and ABCG1; processes which were reversed in the presence of anti-RAGE antibodies [67].
Collectively, these studies highlight roles for RAGE in regulation of macrophage cholesterol content; processes which appear to be linked to increased uptake and esterification and to downregulation of molecules that are important for efflux of cholesterol from these cells.
Together, these changes in intracellular cholesterol content, particularly in diabetes, may exert significant impact on inflammatory and pro-atherogenic mechanisms.
RAGE AND ATHEROSCLEROSIS: SUGARS & LIPIDS
Among the complications of diabetes is the development of accelerated atherosclerosis and cardiovascular complications; evidence from long-term clinical studies in T1D and T2D suggest that reduction in glucose levels contributes to reduction of cardiovascular complications [68,69]. However, the protection imbued by rigorous control of hyperglycemia, especially in T2D is not complete and, in fact, may be harmful (due in part to hypoglycemic episodes) [70]. In T1D, after a period of approximately 15 years, the beneficial effects of strict control of hyperglycemia may begin to wane, and usher in a next phase of “metabolic amnesia” [71]. These considerations suggest that while high levels of glucose are key contributing factors, there remain distinct contributors that affect long-term cardiovascular complications.
Studies in human atherosclerosis have illustrated that diabetes is associated with higher expression of lesional RAGE, higher macrophage content and evidence of increased inflammatory and oxidative stress [72,73]. Experiments from multiple groups have suggested that diabetes-accelerated atherosclerosis in mouse models may be mitigated by deletion of Ager (globally or in bone marrow-derived cells) [74–76] or by pharmacological agents directed to RAGE ligands and RAGE, such as sRAGE and anti-RAGE antibodies [77–79]. To follow in the sections below are examples of studies linking RAGE, sugars and lipids to immunometabolic consequences in atherosclerosis.
As discussed above, diabetes accelerates atherosclerosis in human subjects and in animal models. Using RAGE-directed antibodies for imaging, prominent RAGE expression was localized to the diabetic atherosclerotic plaques in mice and in pigs [80,81]. As in the clinical studies in T1D, the question was asked, what happens to RAGE expression and atherosclerosis in diabetic mice devoid of Apoe and subjected to insulin treatment to lower blood glucose? In that study, Apoe null mice were rendered T1D-like diabetic with streptozotocin; after 6 weeks of established diabetes, insulin pellets vs vehicle were implanted in the diabetic mice for an additional 15 weeks. In parallel with decreased glucose levels, lesional RAGE expression and lesion size were reduced by treatment with insulin and macrophage content was significantly reduced in the lesions, as measured by immunoreactivity with Mac-3 [82]. Of note, however, cholesterol levels were not reported in that study; hence, the effects of insulin and reduced hyperglycemia might have resulted, at least in part, through reductions in hypercholesterolemia.
In a distinct study, this issue was directly addressed in studies in murine models of atherosclerosis. To mimic transient intermittent hyperglycemia (TIH), a common metabolic feature observed in human subjects, which has been associated with cardiovascular disease [83,84], TIH was induced through repeated injections of glucose through the peritoneal cavity in Apoe null mice. Although TIH accelerated atherosclerosis in the glucose-treated mice, there was no effect on plasma cholesterol, thereby de-coupling the effects of glucose vs cholesterol on atherosclerosis [85]. The mechanisms were traced to promotion of myelopoiesis with increased circulating monocytes (especially the Ly6CHI subset), and were linked to the RAGE ligand S100A8/A9) and to RAGE itself using genetically modified mice and bone marrow [85]. In neutrophils, the increased uptake of glucose uptake via GLUT enhanced glycolysis in these cells, thereby triggering the production of RAGE ligand S100A8/A9, which was linked directly to atherosclerosis through myeloid-specific deletion of Slc2a1 (GLUT1) [85]. Hence, these studies linked glucose, glycolysis, S100A8/A9 and RAGE in a cholesterol-independent manner to acceleration of atherosclerosis in mice devoid of Apoe.
Diabetes is also associated with increased vascular calcification; coronary artery calcification has been associated with increased cardiovascular morbidity and mortality [86]. RAGE ligand S100A9 was linked to calcification mechanisms through high glucose treatment of macrophages. When human primary macrophages were cultured in the presence of high glucose conditions, the secretion of RAGE ligand S100A9 and expression of RAGE in those cells was noted to increase [87]. Recombinant S100A9 was employed to directly test its effects on macrophages; S100A9 induced expression of osteogenic factors and the production of extracellular vesicles that contained high calcific potential (on account of high alkaline phosphatase activity); this was prevented by blockade of RAGE or silencing RNAs targeting S100a9. In vivo, streptozotocin-induced T1D-like mice devoid of Apoe and S100a9 or diabetic mice treated with siRNAs targeted against S100a9 encapsulated in lipid nanoparticles displayed decreased atherosclerosis and microcalcifications in the atherosclerotic plaques [87]. Those authors also tested human carotid plaques and showed that the S100A9-RAGE axis associated with osteogenic activity and microcalcification [87]. Collectively, these studies directly linked high glucose exposure in macrophages to induction of pro-calcific mechanisms linked to atherosclerotic plaque microcalcification.
Finally, multiple studies have illustrated that the regression of established atherosclerosis is impaired by diabetes [88–91]. When roles for RAGE were explored in regression of diabetic atherosclerosis, exciting findings emerged that linked diabetes to interferon signaling with consequent effects on macrophage inflammation, glycolysis and cholesterol metabolism, as follows [92]. Regression of atherosclerosis was explored through a model in which transplantation of aortic arches from diabetic, Western diet-fed mice devoid of the Ldlr were transplanted into Ager null vs wild-type diabetic recipient mice; transfer of the arches into the normolipidemic yet diabetic environment accelerated regression of atherosclerosis. When the arches were transplanted into diabetic Ager null mice, regression of atherosclerosis was accelerated, as evidenced by reduced macrophage content, lesion size and Oil Red O content [92]. To dissect underlying mechanisms, RNA-sequencing experiments were performed on macrophages isolated from the donor arches pre-transplant (CD45.2) and from the macrophages retrieved from the regressing plaques in the recipient mice (CD45.1). Analyses revealed that the CD45.1 macrophages retrieved from recipients devoid of Ager revealed downregulation of Interferon Regulatory Factor 7 (IRF7) and upregulation of glycolysis pathway compared to the macrophages (CD45.1) from the wild-type mice recipients [92]. Immunohistochemistry studies performed on both human and murine diabetic atherosclerotic plaques colocalized IRF7 and macrophages. Experiments were performed in BMDMs; those studies revealed that RAGE ligands upregulated expression of Irf7 mRNA, and in BMDMs exposed to a cholesterol-enriched medium, knockdown of Irf7 modulated expression of genes linked to inflammation and cholesterol metabolism. Specifically, knockdown of Irf7 increased expression of genes linked to cholesterol efflux (Abca1 and Abcg1); increased expression of Nr1h2 (LXRβ) and Nr1h3 (LXRα); and downregulated expression of Cd36 compared to control siRNAs. In terms of inflammatory pathways, knockdown of Irf7 upregulated Arg1 and Il10; and significantly downregulated Tnfa, Nos2, Il6, and Ccl2 [92] (Figure 2). Although the potential effects of RAGE and, possibly, IRF7 on glycolysis in macrophages (based on the analysis of the RNA-sequencing studies) were not directly studied in that work, the overall “glycolysis” pathway was found to be increased in recipient macrophages devoid of Ager in the atherosclerosis regression environment. Hence, such findings form the basis of future investigation to explore RAGE/IRF7 links to glycolysis—cause and/or effect?
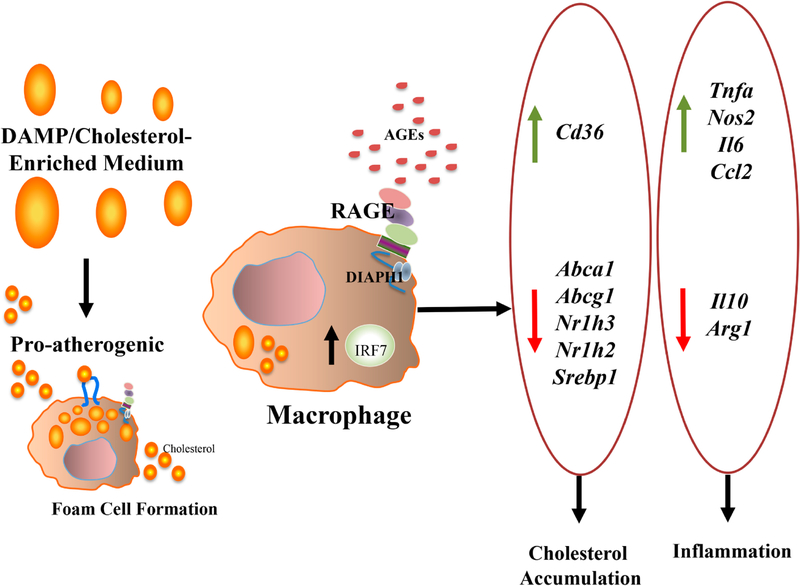
Figure 2. Effect of DAMPs and cholesterol-enriched medium on gene expression in murine bone marrow derived macrophages through IRF7.
IRF7 is linked to regulation of cholesterol- and inflammation-modulating genes in macrophages. In murine bone marrow derived macrophages, incubation with DAMP-enriched cholesterol-enriched medium resulted in increased expression of Abca1, Abcg1, Nr1h2 (LXRβ) and Nr1h3 (LXRα), and decreased expression of Cd36; regulation of these factors was reversed by knockdown of Irf7. Furthermore, with respect to inflammation, siRNA-knockdown of Irf7 upregulated Arg1 and Il10, and significantly downregulated Tnfa, Nos2, Il6, and Ccl2 in bone marrow derived macrophages [92]. In those studies, it was shown that RAGE ligands upregulate Irf7 in macrophages.
RAGE AND OBESITY: EVIDENCE FOR ROLES FOR ENERGY EXPENDITURE AND INSULIN RESISTANCE
Even in the absence of diabetes, obese adipose tissue from human subjects and animal models fed a high fat diet (HFD) displays higher levels of AGE, RAGE and other ligands, such as HMGB1 [93]. Consistent with important roles for RAGE in the response to HFD feeding in mice, it was reported that mice globally devoid of Ager, when fed a HFD, displayed less weight gain, in parallel with higher energy expenditure and reduced adipose tissue inflammation (reduced macrophage and reduced F4/80+/CD11C+ cells in epididymal adipose tissue (eWAT)) [93]. In these mice devoid of Ager, insulin tolerance testing and hyperinsulinemic euglycemic clamp studies illustrated that the Ager null mice were remarkably more insulin sensitive. Ager deficiency had no effect on genetic forms of diabetes induced by obesity, such as in animals with disruption of melancortin signaling [93].
In the search for underlying mechanisms, mice bearing adipocyte-specific deletion of Ager (brown and white adipocytes) displayed significant protection from HFD feeding and from cold challenge. Energy expenditure was significantly higher in the HFD-fed adipocyte Ager-deleted animals vs floxed mice controls (Adipoq cre recombinase) [94], in a manner independent of changes in food intake or physical activity. In parallel, insulin and glucose tolerance was improved in the adipocyte-Ager-deleted mice and eWAT displayed lower expression of pro-inflammatory genes compared to the wild-type control mice [94]. RAGE-dependent mechanisms were mediated by ligand-RAGE-induced suppression of protein kinase A (PKA)-mediated phosphorylation of targets, hormone-sensitive lipase and p38 mitogen-activated protein kinase, upon β-adrenergic receptor stimulation, which culminated in reduced expression of UCP1 [94].
These investigations regarding the RAGE axis were extended to human subjects with obesity or morbid obesity, in which case subcutaneous and omental adipose tissues (SAT and OAT, respectively) were obtained at the time of bariatric surgery and experiments were performed to determine if there associations between AGER mRNA expression and markers of immunometabolic perturbations. In SAT but not OAT, AGER mRNA expression significantly and positively correlated with CD68 mRNA (marker of macrophages) and with PPARG [95]. Strikingly, in SAT but not in OAT, only AGER expression, but not expression of CD68 or PPARG, was significantly associated with HOMA-IR (homeostatic model assessment for insulin resistance) [95]. In SAT and OAT, no differences in AGE content were identified, thereby indicating that the expression pattern of AGER mRNA and associations with immunometabolic markers were independent of AGE ligand levels in these adipose tissue depots [95].
Taken together, in the setting of cardiometabolic disorders, evidence supports that the ligand-RAGE axis impacts metabolism in macrophages and other myeloid cells, at least in part through the effects of glucose and cholesterol species.
SUMMARY AND PERSPECTIVES
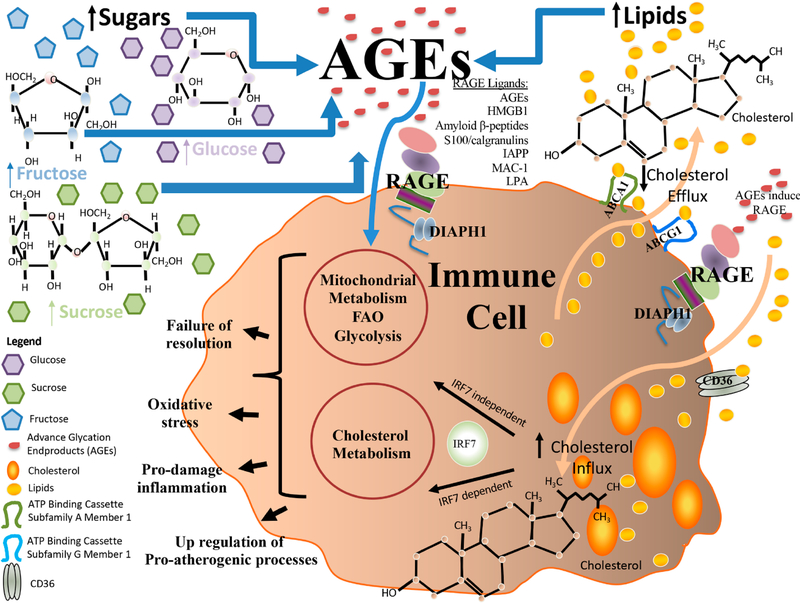
Recent insights into immunometabolism have illustrated the integration of the inextricably linked processes regulating inflammatory (such as pro- vs anti-inflammatory/resolution responses) and metabolic pathways (such as glycolysis, amino acid metabolism, oxidative phosphorylation, fatty acid oxidation and mitochondrial processes that generate both ATP and, in certain cases, excess oxygen free radicals) (Figure 3). In the context of the RAGE pathway, evidence supports that numerous types of sugars, glucose, sucrose and fructose, for example, may form advanced species such as AGEs that trigger RAGE activities in vitro and in vivo. In addition, observations from a number of groups support that macrophage RAGE is important for cholesterol metabolism, at least in part through regulation of genes linked to downregulation of cholesterol efflux, such as Abca1 and Abcg1; processes which appear to be independent of the LXRs. Recent findings linked RAGE to IRF7 and regulation of cholesterol transporters in macrophages may be the heretofore unrecognized connecting mechanism and deserves further experimental attention (Figure 3). Collectively, these findings unveil that there are multiple future directions; a few of these are considered below.
Figure 3. RAGE and Roles in Immunometabolism: an evolving biology in the sphere of sugars, lipids and cholesterol.
RAGE plays numerous roles in immunometabolism and research has unveiled that a number distinct biochemical species drive these responses. For example, studies have identified roles for sugars such as sucrose, fructose and glucose in the production of AGEs, which, via RAGE, may exert numerous downstream consequences, such as exacerbation of pro-damage inflammation; failure of resolution of inflammation; oxidative stress and up-regulation of atherogenic processes. Such downstream consequences affect the properties of immune cells, such as macrophages and DCs. In addition, it has been shown that RAGE expression in immune cells, such as macrophages, plays important roles in the handling of cholesterol, such as in cellular uptake, esterification and, as shown in numerous studies, in efflux, at least in part through RAGE ligand-mediated downregulation of cholesterol transporters Abca1 and Abcg1. In this context, the increased level of intracellular lipid species may, particularly in pro-atherogenic or diabetic environments, lead to oxidation of these moieties, thereby further exacerbating cellular stress and dysfunction. In addition, recent insights have uncovered roles for RAGE in regulation of IRF7, which, based on the functions of IRF7, highlights a molecular bridge for these pathways in regulation of RAGE ligand-dependent cholesterol metabolism and inflammation.
RAGE is a Damage Associated Molecular Pattern (DAMP) receptor; DAMPs are recognized as the released products of distressed or damaged cells that themselves may inherently trigger immune responses [96]. Examples of these include AGEs, HMGB1, multiple members of the S100/calgranulin family, amyloid beta peptide and DNA [96]. What is also well-appreciated is that many of these molecules, in addition to their ability to bind to RAGE, may also interact with other DAMP receptors, particularly the toll like receptors (TLRs), such as TLR2, TLR4, and TLR9. TLRs have been associated with integration with interferon signaling pathways; recent discoveries linking RAGE ligands to regulation of Irf7 in macrophages further strengthen the molecular bridges between these two innate immune signaling effector pathways. Critical to discerning the safety and predicted effectiveness of blockade of RAGE in chronic disease will be its cooperation with or antagonism of TLR pathways. It is predicted that the cellular selection of TLR and/or RAGE is likely to be cell type specific, context specific and, likely, mediated by the distinct stimulating DAMPs.
Earlier work in liver injury suggested that RAGE and TLRs might have distinct functions; compared to wild-type mice undergoing 85% (massive hepatectomy), mice devoid of Myd88 (downstream signaling effector for some of the TLRs) displayed reduced survival. In contrast, mice devoid of Ager displayed higher survival compared to the wild-type or Myd88 null mice. However, mice devoid of both Ager and Myd88 succumbed rapidly, consistent with the survival patterns experienced by the wild-type or the Myd88 null mice [97]. Such data suggest that at least in that setting, MYD88 plays protective functions in the response to liver injury, whereas RAGE acts to blunt survival responses. Since the double-null mice behave more similarly to the mice devoid of Myd88, it may be intuited that TLRs are fully essential for immediate and innate survival responses in that setting [97]. If and how RAGE vs TLR modulated immune cell metabolism in massive hepatectomy was not addressed in that work, but forms the basis for future investigations.
The recent discovery of links between RAGE and interferon pathways bears implications beyond regulation of macrophage inflammation and cholesterol metabolism. IRF7, in particular, has been described as the master regulator of type 1 interferon responses, which are essential for rapid response to multiple types of viral infections [98]. Like RAGE, IRFs such as IRF7 are complex as well; a recent study showed that IRF7 regulated pro-fibrotic responses through transforming growth factor beta pathways [99]; concepts which might well extend a molecular and metabolic bridge between RAGE and IRF7 to cardiovascular diseases and to diseases of the diabetic kidney, which bear strong links to RAGE signaling [100]. In the context of viral infections, dysregulation of interferon pathways has been suggested as one component of the host maladaptive responses to SARS-CoV-2 infection [101–104]. Given the multiple and diverse DAMP ligands of RAGE that are likely to be produced and released in SARS-CoV-2 infection [105], by immune cells and endogenous lung epithelial cells, it will be very interesting to discover if and how RAGE might contribute to interferon dysregulation in the infected lung.
In this context, a recent study directly tested the effects of glucose and glycolytic metabolism on macrophage properties in SARS-CoV-2 infection [106]. In that work, it was shown that SARS-CoV-2 stimulates the production of mitochondrial reactive oxygen species production in macrophages, which results in the stabilization of hypoxia-inducible factor-1α (HIF-1α) and the promotion of glycolysis [106]. The effects of HIF-1α in monocyte metabolism upon SARS-CoV-2 infection cause direct effects on inhibition of T cell responses with consequent reductions in epithelial cell survival [106]. Hence, future studies might focus on the potential roles for RAGE, glucose, metabolism and altered monocyte and immune responses upon SARS-CoV-2 infection in order to discern if, and by what mechanisms, RAGE might contribute to pathobiological responses to this infection.
Finally, with respect to therapeutic opportunities, it is well-established that the cytoplasmic domain of RAGE is essential for RAGE-dependent signaling [107]. The cytoplasmic domain of RAGE binds to distinct intracellular molecules [108,109]; among the best-characterized of these is the interaction of the RAGE cytoplasmic domain with Diaphanous-1 or DIAPH1, a member of the formin family [110,111] (Figure 3). Formins have particular characteristics that render their potential roles in RAGE signaling quite plausible; DIAPH1 is an effector of RhoGTPase signaling; formins play key roles in the actin cytoskeleton, thereby implicating them in cellular migration; and formins may regulate gene expression, at least in part through serum response factors (SRFs), which have been previously linked to RAGE [112]. Structural biology studies unveiled the precise amino acids in the cytoplasmic tail of RAGE (human, R5/Q6), which were required for binding to the Formin Homology 1 domain (FH1 domain) of DIAPH1 [23,113]. Based on this knowledge, small molecule antagonists of this interaction are now under study and development as putative novel antagonists of RAGE signaling [114]. If and to what degree such modulation might re-set adaptive immunometabolic roles for RAGE in chronic disease settings remains to be dissected. If successful, novel therapeutic agents for disorders of metabolism, such as diabetes, obesity, atherosclerosis and cardiovascular disease, among others, may be introduced for clinical trial assessment.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the expert assistance of Ms. Latoya Woods of the Diabetes Research Program in the preparation of this manuscript.
FUNDING
These studies were supported by U.S. Public Health Service grants 1R01HL132516, 1P01HL131481, 1R01DK109675 (to AMS and RR), 1P01HL146367 (to AMS, RR and AS) and the American Heart Association grant 17SFRN33520045 (to AMS).
Footnotes
CONFLICTS OF INTEREST
AMS, AS, MBM and RR are inventors on awarded (US 9,353,3078, US 9364472) and pending (US 16/094270, Europe 177864360.0) patent applications assigned to NYU Grossman Medical Center.
REFERENCES
- 1.Ramasamy R, Yan SF, Schmidt AM. Advanced glycation endproducts: from precursors to RAGE: round and round we go. Amino acids. 2012;42(4):115–161. doi: 10.1007/s00726-010-0773-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamagishi S. Advanced glycation end products and receptor-oxidative stress system in diabetic vascular complications. Ther Apher Dial. 2009;13(6):534–9. doi: 10.1111/j.1744-9987.2009.00775.x [DOI] [PubMed] [Google Scholar]
- 3.Slade DJ, Subramanian V, Fuhrmann J, Thompson PR. Chemical and biological methods to detect post-translational modifications of arginine. Biopolymers. 2014;101(2):133–43. doi: 10.1002/bip.22256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fokkens BT, Smit AJ. Skin fluorescence as a clinical tool for non-invasive assessment of advanced glycation and long-term complications of diabetes. Glycoconj J 2016;33(4):527–35. doi: 10.1007/s10719-016-9683-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Moura Semedo C, Webb M, Waller H, Khunti K, Davies M. Skin autofluorescence, a non-invasive marker of advanced glycation end products: clinical relevance and limitations. Postgrad Med J 2017;93(1099):289–94. doi: 10.1136/postgradmedj-2016-134579 [DOI] [PubMed] [Google Scholar]
- 6.Sharma C, Kaur A, Thind SS, Singh B, Raina S. Advanced glycation End-products (AGEs): an emerging concern for processed food industries. J Food Sci Technol 2015;52(12):7561–76. doi: 10.1007/s13197-015-1851-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OʼBrien J, Morrissey PA. Nutritional and toxicological aspects of the Maillard browning reaction in foods. Crit Rev Food Sci Nutr 1989;28(3):211–48. doi: 10.1080/10408398909527499 [DOI] [PubMed] [Google Scholar]
- 8.Koschinsky T, He CJ, Mitsuhashi T, Bucala R, Liu C, Buenting C, et al. Orally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathy. Proc Nat Acad Sci U S A. 1997;94(12):6474–9. doi: 10.1073/pnas.94.12.6474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borrelli RC, Fogliano V. Bread crust melanoidins as potential prebiotic ingredients. Mol Nutr Food Res. 2005;49(7):673–8. doi: 10.1002/mnfr.200500011 [DOI] [PubMed] [Google Scholar]
- 10.Yacoub R, Nugent M, Cai W, Nadkarni GN, Chaves LD, Abyad S, et al. Advanced glycation end products dietary restriction effects on bacterial gut microbiota in peritoneal dialysis patients; a randomized open label controlled trial. PLoS One. 2017;12(9):e0184789. doi: 10.1371/journal.pone.0184789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baye E, Kiriakova V, Uribarri J, Moran LJ, de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci Rep 2017;7(1):2266. doi: 10.1038/s41598-017-02268-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordova R, Knaze V, Viallon V, Rust P, Schalkwijk CG, Weiderpass E, et al. Dietary intake of advanced glycation end products (AGEs) and changes in body weight in European adults. Eur J Nutr 2020;59(7):2893–904. doi: 10.1007/s00394-019-02129-8 [DOI] [PubMed] [Google Scholar]
- 13.Gaens KH, Goossens GH, Niessen PM, van Greevenbroek MM, van der Kallen CJ, Niessen HW, et al. Nε-(carboxymethyl)lysine-receptor for advanced glycation end product axis is a key modulator of obesity-induced dysregulation of adipokine expression and insulin resistance. Arterioscler Thromb Vasc Biol 2014;34(6):1199–208. doi: 10.1161/atvbaha.113.302281 [DOI] [PubMed] [Google Scholar]
- 14.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6 [DOI] [PubMed] [Google Scholar]
- 15.Leclerc E, Fritz G, Vetter SW, Heizmann CW. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta 2009;1793(6):993–1007. doi: 10.1016/j.bbamcr.2008.11.016 [DOI] [PubMed] [Google Scholar]
- 16.Taguchi A, Blood DC, del Toro G, Canet A, Lee DC, Qu W, et al. Blockade of RAGE-amphoterin signalling suppresses tumour growth and metastases. Nature. 2000;405(6784):354–60. doi: 10.1038/35012626 [DOI] [PubMed] [Google Scholar]
- 17.Yan SD, Chen X, Fu J, Chen M, Zhu H, Roher A, et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimerʼs disease. Nature. 1996;382(6593):685–91. doi: 10.1038/382685a0 [DOI] [PubMed] [Google Scholar]
- 18.Abedini A, Cao P, Plesner A, Zhang J, He M, Derk J, et al. RAGE binds preamyloid IAPP intermediates and mediates pancreatic β cell proteotoxicity. J Clin Invest 2018;128(2):682–98. doi: 10.1172/jci85210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rai V, Touré F, Chitayat S, Pei R, Song F, Li Q, et al. Lysophosphatidic acid targets vascular and oncogenic pathways via RAGE signaling. J Exp Med 2012;209(13):2339–50. doi: 10.1084/jem.20120873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J 2007;26(4):1129–39. doi: 10.1038/sj.emboj.7601552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma W, Rai V, Hudson BI, Song F, Schmidt AM, Barile GR. RAGE binds C1q and enhances C1q-mediated phagocytosis. Cell Immunol 2012;274(1–2):72–82. doi: 10.1016/j.cellimm.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 22.He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, et al. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep 2011;12(4):358–64. doi: 10.1038/embor.2011.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue J, Manigrasso M, Scalabrin M, Rai V, Reverdatto S, Burz DS, et al. Change in the Molecular Dimension of a RAGE-Ligand Complex Triggers RAGE Signaling. Structure. 2016;24(9):1509–22. doi: 10.1016/j.str.2016.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park H, Adsit FG, Boyington JC. The 1.5 Å crystal structure of human receptor for advanced glycation endproducts (RAGE) ectodomains reveals unique features determining ligand binding. J Biol Chem 2010;285(52):40762–70. doi: 10.1074/jbc.M110.169276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch M, Chitayat S, Dattilo BM, Schiefner A, Diez J, Chazin WJ, et al. Structural basis for ligand recognition and activation of RAGE. Structure. 2010;18(10):1342–52. doi: 10.1016/j.str.2010.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.López-Díez R, Shen X, Daffu G, Khursheed M, Hu J, Song F, et al. Ager Deletion Enhances Ischemic Muscle Inflammation, Angiogenesis, and Blood Flow Recovery in Diabetic Mice. Arterioscler thromb vas biol 2017;37(8):1536–47. doi: 10.1161/atvbaha.117.309714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaiswal N, Agrawal S, Agrawal A. High fructose-induced metabolic changes enhance inflammation in human dendritic cells. Clin Exp Immunol 2019;197(2):237–49. doi: 10.1111/cei.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker FR, Nilsson M, Jones K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr Drug Targets. 2013;14(11):1262–76. doi: 10.2174/13894501113149990208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y, Bielohuby M, Fleming T, Grabner GF, Foppen E, Bernhard W, et al. Dietary sugars, not lipids, drive hypothalamic inflammation. Mol Metab 2017;6(8):897–908. doi: 10.1016/j.molmet.2017.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henson PM, Hume DA. Apoptotic cell removal in development and tissue homeostasis. Trends Immunol 2006;27(5):244–50. doi: 10.1016/j.it.2006.03.005 [DOI] [PubMed] [Google Scholar]
- 31.Gheibi Hayat SM, Bianconi V, Pirro M, Sahebkar A. Efferocytosis: molecular mechanisms and pathophysiological perspectives. Immunol Cell Biol 2019;97(2):124–33. doi: 10.1111/imcb.12206 [DOI] [PubMed] [Google Scholar]
- 32.Banerjee S, Friggeri A, Liu G, Abraham E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J Leukoc biol 2010;88(5):973–9. doi: 10.1189/jlb.0510262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friggeri A, Banerjee S, Biswas S, de Freitas A, Liu G, Bierhaus A, et al. Participation of the receptor for advanced glycation end products in efferocytosis. J Immunol 2011;186(11):6191–8. doi: 10.4049/jimmunol.1004134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen LJ, Flyvbjerg A, Bjerre M. Soluble Receptor for Advanced Glycation End Product: A Biomarker for Acute Coronary Syndrome. BioMed Res Int 2015;2015:815942. doi: 10.1155/2015/815942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yonchuk JG, Silverman EK, Bowler RP, Agustí A, Lomas DA, Miller BE, et al. Circulating soluble receptor for advanced glycation end products (sRAGE) as a biomarker of emphysema and the RAGE axis in the lung. Am J Respir Crit Care Med 2015;192(7):785–92. doi: 10.1164/rccm.201501-0137PP [DOI] [PubMed] [Google Scholar]
- 36.Schmidt AM. Soluble RAGEs—Prospects for treating & tracking metabolic and inflammatory disease. Vasc Pharmacol 2015;72:1–8. doi: 10.1016/j.vph.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steenbeke M, De Bruyne S, De Buyzere M, Lapauw B, Speeckaert R, Petrovic M, et al. The role of soluble receptor for advanced glycation end-products (sRAGE) in the general population and patients with diabetes mellitus with a focus on renal function and overall outcome. Crit Rev Clin Lab Sci 2020;58(2):113–30. doi: 10.1080/10408363.2020.1791045 [DOI] [PubMed] [Google Scholar]
- 38.Naeini MB, Bianconi V, Pirro M, Sahebkar A. The role of phosphatidylserine recognition receptors in multiple biological functions. Cell Mol Biol Lett 2020;25:23. doi: 10.1186/s11658-020-00214-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gkikas I, Palikaras K, Tavernarakis N. The Role of Mitophagy in Innate Immunity. Front Immunol 2018;9:1283. doi: 10.3389/fimmu.2018.01283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang S, Hu L, Jiang J, Li H, Wu Q, Ooi K, et al. HMGB1/RAGE axis mediates stress-induced RVLM neuroinflammation in mice via impairing mitophagy flux in microglia. J Neuroinflammation. 2020;17(1):15. doi: 10.1186/s12974-019-1673-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuk JM, Silwal P, Jo EK. Inflammasome and Mitophagy Connection in Health and Disease. Int J Mol Sci 2020;21(13). doi: 10.3390/ijms21134714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wallach D, Kang TB, Dillon CP, Green DR. Programmed necrosis in inflammation: Toward identification of the effector molecules. Science. 2016;352(6281):aaf2154. doi: 10.1126/science.aaf2154 [DOI] [PubMed] [Google Scholar]
- 43.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol 2017;17(3):151–64. doi: 10.1038/nri.2016.147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfalzgraff A, Weindl G. Intracellular Lipopolysaccharide Sensing as a Potential Therapeutic Target for Sepsis. Trends Pharmacol Sci 2019;40(3):187–97. doi: 10.1016/j.tips.2019.01.001 [DOI] [PubMed] [Google Scholar]
- 45.Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis 2018;9(6):597. doi: 10.1038/s41419-018-0538-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doran AC, Yurdagul A Jr, Tabas I. Efferocytosis in health and disease. Nat Rev Immunol 2020;20(4):254–67. doi: 10.1038/s41577-019-0240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 2002;23(11):549–55. doi: 10.1016/s1471-4906(02)02302-5 [DOI] [PubMed] [Google Scholar]
- 48.El Kasmi KC, Stenmark KR. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol 2015;27(4):267–75. doi: 10.1016/j.smim.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Curi R, de Siqueira Mendes R, de Campos Crispin LA, Norata GD, Sampaio SC, Newsholme P. A past and present overview of macrophage metabolism and functional outcomes. Clin Sci 2017;131(12):1329–42. doi: 10.1042/cs20170220 [DOI] [PubMed] [Google Scholar]
- 50.Wetzels S, Wouters K, Schalkwijk CG, Vanmierlo T, Hendriks JJ. Methylglyoxal-Derived Advanced Glycation Endproducts in Multiple Sclerosis. Int J Mol Sci 2017;18(2). doi: 10.3390/ijms18020421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wetzels S, Vanmierlo T, Scheijen J, van Horssen J, Amor S, Somers V, et al. Methylglyoxal-Derived Advanced Glycation Endproducts Accumulate in Multiple Sclerosis Lesions. Front Immunol 2019;10:855. doi: 10.3389/fimmu.2019.00855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang N, Westerterp M. ABC Transporters, Cholesterol Efflux, and Implications for Cardiovascular Diseases. Adv Exp Med Biol 2020;1276:67–83. doi: 10.1007/978-981-15-6082-8_6 [DOI] [PubMed] [Google Scholar]
- 53.Davis W Jr, Tew KD. ATP-binding cassette transporter-2 (ABCA2) as a therapeutic target. Biochem Pharmacol 2018;151:188–200. doi: 10.1016/j.bcp.2017.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen WJ, Azhar S, Kraemer FB. SR-B1: A Unique Multifunctional Receptor for Cholesterol Influx and Efflux. Annu Rev Physiol 2018;80:95–116. doi: 10.1146/annurev-physiol-021317-121550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isoda K, Folco EJ, Shimizu K, Libby P. AGE-BSA decreases ABCG1 expression and reduces macrophage cholesterol efflux to HDL. Atherosclerosis. 2007;192(2):298–304. doi: 10.1016/j.atherosclerosis.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 56.Uehara Y, Miura S, von Eckardstein A, Abe S, Fujii A, Matsuo Y, et al. Unsaturated fatty acids suppress the expression of the ATP-binding cassette transporter G1 (ABCG1) and ABCA1 genes via an LXR/RXR responsive element. Atherosclerosis. 2007;191(1):11–21. doi: 10.1016/j.atherosclerosis.2006.04.018 [DOI] [PubMed] [Google Scholar]
- 57.Kumar P, Raghavan S, Shanmugam G, Shanmugam N. Ligation of RAGE with ligand S100B attenuates ABCA1 expression in monocytes. Metabolism. 2013;62(8):1149–58. doi: 10.1016/j.metabol.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 58.Shiu SW, Zhou H, Wong Y, Tan KC. Endothelial lipase and reverse cholesterol transport in type 2 diabetes mellitus. J Diabetes Investig 2010;1(3):111–6. doi: 10.1111/j.2040-1124.2010.00016.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou H, Shiu SW, Wong Y, Tan KC. Impaired serum capacity to induce cholesterol efflux is associated with endothelial dysfunction in type 2 diabetes mellitus. Diabetes Vasc Dis Res 2009;6(4):238–43. doi: 10.1177/1479164109344934 [DOI] [PubMed] [Google Scholar]
- 60.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 2011;364(2):127–35. doi: 10.1056/NEJMoa1001689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rohatgi A, Khera A, Berry JD, Givens EG, Ayers CR, Wedin KE, et al. HDL cholesterol efflux capacity and incident cardiovascular events. N Engl J Med 2014;371(25):2383–93. doi: 10.1056/NEJMoa1409065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Daffu G, Shen X, Senatus L, Thiagarajan D, Abedini A, Hurtado Del Pozo C, et al. RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes. 2015;64(12):4046–60. doi: 10.2337/db15-0575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Machado-Lima A, López-Díez R, Iborra RT, Pinto RS, Daffu G, Shen X, et al. RAGE Mediates Cholesterol Efflux Impairment in Macrophages Caused by Human Advanced Glycated Albumin. Int J Mol Sci 2020;21(19). doi: 10.3390/ijms21197265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng B, Xu L, Wang H, Yan X, Xue J, Liu F, et al. Atorvastatin exerts its anti-atherosclerotic effects by targeting the receptor for advanced glycation end products. Biochim Biophys Acta 2011;1812(9):1130–7. doi: 10.1016/j.bbadis.2011.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan Y, Zhao L, Chen Y, Moorhead JF, Varghese Z, Powis SH, et al. Advanced glycation end products (AGEs) increase human mesangial foam cell formation by increasing Golgi SCAP glycosylation in vitro. Am J Physiol Renal Physiol 2011;301(1):F236–43. doi: 10.1152/ajprenal.00646.2010 [DOI] [PubMed] [Google Scholar]
- 66.Wang XQ, Yang K, He YS, Lu L, Shen WF. Receptor mediated elevation in FABP4 levels by advanced glycation end products induces cholesterol and triacylglycerol accumulation in THP-1 macrophages. Lipids. 2011;46(6):479–86. doi: 10.1007/s11745-011-3542-4 [DOI] [PubMed] [Google Scholar]
- 67.Xu L, Wang YR, Li PC, Feng B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis 2016;15(1):161. doi: 10.1186/s12944-016-0334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 69.Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005;353(25):2643–53. doi: 10.1056/NEJMoa052187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Action to Control Cardiovascular Risk in Diabetes Study Group, Gerstein HC, Miller ME, Byington RP, Goff DC Jr., Bigger JT, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358(24):2545–59. doi: 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lachin JM, Nathan DM, Zinman B, Bebu I. Comment on Miller and Orchard: Understanding Metabolic Memory: A Tale of Two Studies. Diabetes 2020;69:291–299. Diabetes. 2020;69(7):e7–8. doi: 10.2337/db20-0311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, et al. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol 2004;24(7):1266–71. doi: 10.1161/01.Atv.0000131783.74034.97 [DOI] [PubMed] [Google Scholar]
- 73.Cipollone F, Iezzi A, Fazia M, Zucchelli M, Pini B, Cuccurullo C, et al. The receptor RAGE as a progression factor amplifying arachidonate-dependent inflammatory and proteolytic response in human atherosclerotic plaques: role of glycemic control. Circulation. 2003;108(9):1070–7. doi: 10.1161/01.Cir.0000086014.80477.0d [DOI] [PubMed] [Google Scholar]
- 74.Bu DX, Rai V, Shen X, Rosario R, Lu Y, DʼAgati V, et al. Activation of the ROCK1 branch of the transforming growth factor-beta pathway contributes to RAGE-dependent acceleration of atherosclerosis in diabetic ApoE-null mice. Circ Res 2010;106(6):1040–51. doi: 10.1161/circresaha.109.201103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soro-Paavonen A, Watson AM, Li J, Paavonen K, Koitka A, Calkin AC, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57(9):2461–9. doi: 10.2337/db07-1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koulis C, Kanellakis P, Pickering RJ, Tsorotes D, Murphy AJ, Gray SP, et al. Role of bone-marrow- and non-bone-marrow-derived receptor for advanced glycation end-products (RAGE) in a mouse model of diabetes-associated atherosclerosis. Clin Sci 2014;127(7):485–97. doi: 10.1042/cs20140045 [DOI] [PubMed] [Google Scholar]
- 77.Park L, Raman KG, Lee KJ, Lu Y, Ferran LJ Jr., Chow WS, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med 1998;4(9):1025–31. doi: 10.1038/2012 [DOI] [PubMed] [Google Scholar]
- 78.Bucciarelli LG, Wendt T, Qu W, Lu Y, Lalla E, Rong LL, et al. RAGE blockade stabilizes established atherosclerosis in diabetic apolipoprotein E-null mice. Circulation. 2002;106(22):2827–35. doi: 10.1161/01.cir.0000039325.03698.36 [DOI] [PubMed] [Google Scholar]
- 79.Bro S, Flyvbjerg A, Binder CJ, Bang CA, Denner L, Olgaard K, et al. A neutralizing antibody against receptor for advanced glycation end products (RAGE) reduces atherosclerosis in uremic mice. Atherosclerosis. 2008;201(2):274–80. doi: 10.1016/j.atherosclerosis.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 80.Tekabe Y, Luma J, Einstein AJ, Sedlar M, Li Q, Schmidt AM, et al. A novel monoclonal antibody for RAGE-directed imaging identifies accelerated atherosclerosis in diabetes. J Nucl Med 2010;51(1):92–7. doi: 10.2967/jnumed.109.064659 [DOI] [PubMed] [Google Scholar]
- 81.Johnson LL, Tekabe Y, Kollaros M, Eng G, Bhatia K, Li C, et al. Imaging RAGE expression in atherosclerotic plaques in hyperlipidemic pigs. EJNMMI Res 2014;4:26. doi: 10.1186/s13550-014-0026-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tekabe Y, Kollaros M, Li Q, Zhang G, Li C, Schmidt AM, et al. Beneficial Effect of Glucose Control on Atherosclerosis Progression in Diabetic ApoE(−/−) Mice: Shown by Rage Directed Imaging. International journal of molecular imaging. 2014;2014:695391. doi: 10.1155/2014/695391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ning F, Zhang L, Dekker JM, Onat A, Stehouwer CD, Yudkin JS, et al. Development of coronary heart disease and ischemic stroke in relation to fasting and 2-hour plasma glucose levels in the normal range. Cardiovasc Diabetol 2012;11:76. doi: 10.1186/1475-2840-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DECODE Study Group; The European Diabetes Epidemiology Group. Glucose tolerance and cardiovascular mortality: comparison of fasting and 2-hour diagnostic criteria. Arch Intern Med 2001;161(3):397–405. doi: 10.1001/archinte.161.3.397 [DOI] [PubMed] [Google Scholar]
- 85.Flynn MC, Kraakman MJ, Tikellis C, Lee MKS, Hanssen NMJ, Kammoun HL, et al. Transient Intermittent Hyperglycemia Accelerates Atherosclerosis by Promoting Myelopoiesis. Circ Res 2020;127(7):877–92. doi: 10.1161/circresaha.120.316653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, et al. Coronary Artery Calcification and Risk of Cardiovascular Disease and Death Among Patients With Chronic Kidney Disease. JAMA Cardiol 2017;2(6):635–43. doi: 10.1001/jamacardio.2017.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawakami R, Katsuki S, Travers R, Romero DC, Becker-Greene D, Passos LSA, et al. S100A9-RAGE Axis Accelerates Formation of Macrophage-Mediated Extracellular Vesicle Microcalcification in Diabetes Mellitus. Arterioscler thromb Vasc Biol 2020;40(8):1838–53. doi: 10.1161/atvbaha.118.314087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan C, Hu J, Parathath S, Grauer L, Cassella CB, Bagdasarov S, et al. Human Aldose Reductase Expression Prevents Atherosclerosis Regression in Diabetic Mice. Diabetes. 2018;67(9):1880–91. doi: 10.2337/db18-0156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Distel E, Barrett TJ, Chung K, Girgis NM, Parathath S, Essau CC, et al. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res 2014;115(9):759–69. doi: 10.1161/circresaha.115.304164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gaudreault N, Kumar N, Olivas VR, Eberlé D, Stephens K, Raffai RL. Hyperglycemia impairs atherosclerosis regression in mice. Am J Pathol 2013;183(6):1981–92. doi: 10.1016/j.ajpath.2013.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Parathath S, Grauer L, Huang LS, Sanson M, Distel E, Goldberg IJ, et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60(6):1759–69. doi: 10.2337/db10-0778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Senatus L, López-Díez R, Egaña-Gorroño L, Liu J, Hu J, Daffu G, et al. RAGE impairs murine diabetic atherosclerosis regression and implicates IRF7 in macrophage inflammation and cholesterol metabolism. JCI insight 2020;5(13):e137289. doi: 10.1172/jci.insight.137289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, et al. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63(6):1948–65. doi: 10.2337/db13-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hurtado del Pozo C, Ruiz HH, Arivazhagan L, Aranda JF, Shim C, Daya P, et al. A Receptor of the Immunoglobulin Superfamily Regulates Adaptive Thermogenesis. Cell Rep 2019;28(3):773–91.e7. 10.1016/j.celrep.2019.06.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ruiz HH, Nguyen A, Wang C, He L, Li H, Hallowell P, et al. AGE/RAGE/DIAPH1 axis is associated with immunometabolic markers and risk of insulin resistance in subcutaneous but not omental adipose tissue in human obesity. Int J Obes. Forthcoming 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol 2020;20(2):95–112. doi: 10.1038/s41577-019-0215-7 [DOI] [PubMed] [Google Scholar]
- 97.Zeng S, Zhang QY, Huang J, Vedantham S, Rosario R, Ananthakrishnan R, et al. Opposing roles of RAGE and Myd88 signaling in extensive liver resection. FASEB J 2012;26(2):882–93. doi: 10.1096/fj.11-192997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434(7034):772–7. doi: 10.1038/nature03464 [DOI] [PubMed] [Google Scholar]
- 99.Wu M, Skaug B, Bi X, Mills T, Salazar G, Zhou X, et al. Interferon regulatory factor 7 (IRF7) represents a link between inflammation and fibrosis in the pathogenesis of systemic sclerosis. Ann Rheum Dis 2019;78(11):1583–91. doi: 10.1136/annrheumdis-2019-215208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sanajou D, Ghorbani Haghjo A, Argani H, Aslani S. AGE-RAGE axis blockade in diabetic nephropathy: Current status and future directions. European journal of pharmacology. 2018;833:158–64. doi: 10.1016/j.ejphar.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 101.Wei L, Ming S, Zou B, Wu Y, Hong Z, Li Z, et al. Viral Invasion and Type I Interferon Response Characterize the Immunophenotypes During Covid-19 Infection. Available from: https://ssrncom/abstract=3564998. Accessed 2021 May 31.
- 102.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020;181(5):1036–45.e9. doi: 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Trouillet-Assant S, Viel S, Gaymard A, Pons S, Richard JC, Perret M, et al. Type I IFN immunoprofiling in COVID-19 patients. J Allergy Clin Immunol 2020;146(1):206–8.e2. doi: 10.1016/j.jaci.2020.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–24. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roy D, Ramasamy R, Schmidt AM. Journey to a Receptor for Advanced Glycation End Products Connection in Severe Acute Respiratory Syndrome Coronavirus 2 Infection: With Stops Along the Way in the Lung, Heart, Blood Vessels, and Adipose Tissue. Arterioscler Thromb Vasc Biol 2020;41(2):Atvbaha120315527. doi: 10.1161/atvbaha.120.315527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Codo AC, Davanzo GG, Monteiro LB, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab 2020;32(3):437–46.e5. doi: 10.1016/j.cmet.2020.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Harja E, Bu DX, Hudson BI, Chang JS, Shen X, Hallam K, et al. Vascular and inflammatory stresses mediate atherosclerosis via RAGE and its ligands in apoE−/− mice. J Clin Invest 2008;118(1):183–94. doi: 10.1172/jci32703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sakaguchi M, Kinoshita R, Putranto EW, Ruma IMW, Sumardika IW, Youyi C, et al. Signal Diversity of Receptor for Advanced Glycation End Products. Acta Med Okayama. 2017;71(6):459–65. doi: 10.18926/amo/55582 [DOI] [PubMed] [Google Scholar]
- 109.Yan Z, Luo H, Xie B, Tian T, Li S, Chen Z, et al. Targeting adaptor protein SLP76 of RAGE as a therapeutic approach for lethal sepsis. Nat commun 2021;12(1):308. doi: 10.1038/s41467-020-20577-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hudson BI, Kalea AZ, Del Mar Arriero M, Harja E, Boulanger E, D’Agati V, et al. Interaction of the RAGE cytoplasmic domain with diaphanous-1 is required for ligand-stimulated cellular migration through activation of Rac1 and Cdc42. J Biol Chem 2008;283(49):34457–68. doi: 10.1074/jbc.M801465200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Young KG, Copeland JW. Formins in cell signaling. Biochim Biophys Acta 2010;1803(2):183–90. doi: 10.1016/j.bbamcr.2008.09.017 [DOI] [PubMed] [Google Scholar]
- 112.OʼShea KM, Ananthakrishnan R, Li Q, Quadri N, Thiagarajan D, Sreejit G, et al. The Formin, DIAPH1, is a Key Modulator of Myocardial Ischemia/Reperfusion Injury. EBioMedicine. 2017;26:165–74. doi: 10.1016/j.ebiom.2017.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rai V, Maldonado AY, Burz DS, Reverdatto S, Yan SF, Schmidt AM, et al. Signal transduction in receptor for advanced glycation end products (RAGE): solution structure of C-terminal rage (ctRAGE) and its binding to mDia1. J Biol Chem 2012;287(7):5133–44. doi: 10.1074/jbc.M111.277731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, et al. Small Molecule Inhibition of Ligand-Stimulated RAGE-DIAPH1 Signal Transduction. Sci Rep 2016;6:22450. doi: 10.1038/srep22450 [DOI] [PMC free article] [PubMed] [Google Scholar]