Abstract
Background & aims
To investigate the association between the parameters used in nutritional screening assessment (body mass index [BMI], unintentional weight loss [WL] and reduced food intake) and clinical outcomes in non-critically ill, hospitalized coronavirus disease 2019 (COVID-19) patients.
Methods
This was a prospective multicenter real-life study carried out during the first pandemic wave in 11 Italian Hospitals. In total, 1391 patients were included. The primary end-point was a composite of in-hospital mortality or admission to ICU, whichever came first. The key secondary end-point was in-hospital mortality.
Results
Multivariable models were based on 1183 patients with complete data. Reduced self-reported food intake before hospitalization and/or expected by physicians in the next days since admission was found to have a negative prognostic impact for both the primary and secondary end-point (P < .001 for both). No association with BMI and WL was observed. Other predictors of outcomes were age and presence of multiple comorbidities. A significant interaction between obesity and multi-morbidity (≥2) was detected. Obesity was found to be a risk factor for composite end-point (HR = 1.36 [95%CI, 1.03–1.80]; P = .031) and a protective factor against in-hospital mortality (HR = 0.32 [95%CI, 0.20–0.51]; P < .001) in patients with and without multiple comorbidities, respectively. Secondary analysis (patients, N = 829), further adjusted for high C-reactive protein (>21 mg/dL) and LDH (>430 mU/mL) levels yielded consistent findings.
Conclusions
Reduced self-reported food intake before hospitalization and/or expected by physicians in the next days since admission was associated with negative clinical outcomes in non-critically ill, hospitalized COVID-19 patients. This simple and easily obtainable parameter may be useful to identify patients at highest risk of poor prognosis, who may benefit from prompt nutritional support. The presence of comorbidities could be the key factor, which may determine the protective or harmful role of a high body mass index in COVID-19.
Keywords: Coronavirus disease 2019, Nutritional risk, Malnutrition, Food intake, Obesity, Mortality
1. Introduction
The ongoing global pandemic of coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), leads affected patients to develop a complex clinical spectrum, which usually involves the respiratory tract [1] and requires hospitalization for the most severe cases [2], even in a sub-intensive or intensive care unit (ICU) [3,4].
SARS-CoV-2 infection causes poor clinical outcomes and higher mortality among elderly patients [5] and in those with comorbidities such as diabetes, hypertension, chronic kidney disease, cancer, cardiovascular disease and chronic obstructive pulmonary disease [6].
Recently, the presence of malnutrition in COVID-19 patients has been detected being a prognostic factor as powerful as older age and comorbidities [7], and associated with prolonged hospitalization [8], disease progression and worse prognosis [9]. Based on the available data, hospitalized patients requiring intensive care hospitalization range from 5% to 10%, with greater prevalence among elderly patients [10], whose nutritional status is more frequently impaired [11].
On the other hand, several studies from different countries observed a strong association between high body mass index (BMI) and the increased risk of severe disease and worse prognosis among COVID-19 patients [12]. Particularly, a large prospective cohort study showed that obesity increases the risk of mortality in hospitalized COVID-19 patients [13]. This appears to be in contrast with the NutritionDay multinational survey, in which a protective role of overweight in hospitalized patients was detected. Accordingly, it was speculated that a high BMI could be a surrogate of nutritional reserves, which may be helpful to counteract the acute stress conditions associated with hospitalization [14].
Early nutritional screening in COVID-19 patients may be effective for planning an optimal nutritional support [15], as highlighted by previous protocols [16]. However, nutritional screening tools present several intrinsic limitations, which could affect their reliability in this patient's population. This applies also to those recommended for systematic use in the hospital setting, such as the Nutritional Risk Screening 2002 tool (NRS-2002) and the Malnutrition Universal Screening Tool (MUST) [17]. Both tools rely on the identification of low BMI, weight loss, reduced food intake (recent or expected) and disease severity. Therefore, given the inflammatory background associated with COVID-19 [18] and the potential difficulties in providing nutritional support [16], most patients would be rated at high nutritional risk, particularly with the use of NRS-2002, which assigns additional risk to older age, thus making nutritional screening unnecessary or scantily informative.
To date, particularly during the first pandemic wave, no large multicenter studies have been carried out among non-critically ill COVID-19 patients to assess the independent association between the parameters commonly used in nutritional screening assessment and clinical outcomes.
Therefore, the here presented prospective multicenter real-life NUTRI-COVID19 study was designed and conducted in 11 Italian hospitals.
2. Materials and methods
2.1. Ethics
The protocol of the NUTRI-COVID-19 study has been approved by local Institutional Ethics Committees and written informed consent was obtained from every patient.
2.2. Participants
Study participants were consecutive adult non-critically ill COVID-19 patients (positive result on real-time reverse-transcriptase–polymerase-chain-reaction [RT-PCR] assay of nasopharyngeal swab) admitted to 11 Italian hospitals (April–July 2020; see Appendix 1).
2.3. Assessments
In addition to demographic information, the following data were collected within 48 h since admission:
-
1.
Major comorbidities - chronic obstructive pulmonary disease, coronary heart disease, diabetes, hypertension, dyslipidemia, cancer, and chronic kidney failure. Multi-morbidity was defined by a number of comorbidities ≥2.
-
2.
Presence of severe pneumonia - diagnosed using the criteria of the American Thoracic Society and Infectious Diseases Society of America [19].
-
3.
Anthropometry - body weight (to the nearest 0.1 kg) and height (to the nearest 0.5 cm) were measured and body mass index (BMI) was calculated (weight [kg]/height [m]) [20]. Then, patients were stratified in the following BMI categories: <20.0, 20.0 to 24.9 (reference), 25.0 to 29.9, and ≥30.0 kg/m2. The occurrence of unintentional weight loss in the previous month (yes, no [reference] or unknown) was also investigated.
-
4.
Food intake - patients were inquired about the reduction of food intake in the previous 3–5 days (yes or no) and the expected impairment of intake in the 3–5 days following the assessment was rated (yes or no) by the investigating physicians. Accordingly, patients were divided in ‘reduced food categories’ as follows: reduced self-reported food intake before hospitalization and expected by physicians in the next days since admission (before and after), reduced self-reported food intake before hospitalization or expected by physicians in the next days since admission (before or after), and none (reference).
-
5.
Biochemistry (optional assessment) - in some study sites C-reactive protein (CRP) and lactate dehydrogenase (LDH), as markers of disease severity, were evaluated in fasting conditions.
2.4. Outcome ascertainment
The primary end-point was a composite of in-hospital mortality or admission to ICU, whichever came first. The key secondary end-point was in-hospital mortality (including death in ICU). Patient survival was defined as the time between the date of enrolment and the date of death or admission to ICU or discharge.
2.5. Statistical analysis
Continuous variables were presented as means and standard deviations (SDs) or medians and interquartile ranges (IQRs). Categorical variables were described as counts and percentages. We compared survival and event-free survival for a series of candidate risk factors using the log rank test and Cox regression. We computed Huber-White robust standard errors to account for intra-Center correlation. We report the hazard ratio (HR) and its 95% confidence interval (95%CI). We fitted a multivariable Cox model including a series of predefined variable (age, sex, BMI, comorbidities, weight loss and intakes). A second model was fitted on a subgroup of patients who had CRP and LDH available. We computed the Harrell's statistic for model discrimination. We assessed the clinically relevant interaction of BMI and multiple comorbidities and fitted separate models for patients with and without multiple comorbidities. We plotted survival curves using the Kaplan Meier method.
We used the STATA software (release 16.1, Stata Corporation, College Station, TX, USA) for all analyses. A two-sided p level of <0.05 was considered as statistically significant.
3. Results
In total, 1391 patients were included (Internal Medicine, 68.1%; Infectious diseases, 19.1%; Pneumology, 11.8%). The demographic, clinical and nutritional characteristics of the study cohort are reported in Table 1 .
Table 1.
Descriptive statistics of the study population.
| Feature | Patients with data available (N) | Descriptive statistics |
|---|---|---|
| Female, N (%) | 1391 | 560 (40.3) |
|
Age (years), Median (IQR) <70, N (%) ≥70, N (%) |
1391 | 77 (65.0–85.0) 567 (40.8) 824 (59.2) |
|
Body mass index (kg/m2), Median (IQR) <20.0, N (%) 20.0–24.9, N (%) 25.0–29.9, N (%) ≥30.0, N (%) |
1322 | 24.7 (22.5–27.6) 105 (7.9) 543 (41.1) 459 (34.7) 215 (16.3) |
|
Weight loss in the previous month, N (%) No Yes Unknown |
1241 | 654 (52.7) 154 (12.4) 433 (34.9) |
|
Reduced food intake, N (%) No Before or after assessment Before and after assessment |
1391 | 407 (29.3) 357 (25.7) 627 (45.1) |
| COPD, N (%) | 1320 | 193 (14.6) |
| Coronary heart disease, N (%) | 1320 | 350 (26.5) |
| Diabetes, N (%) | 1320 | 338 (25.6) |
| Hypertension, N (%) | 1318 | 789 (59.9) |
| Dyslipidemia, N (%) | 1314 | 306 (23.3) |
| Cancer, N (%) | 1319 | 168 (12.7) |
| Chronic kidney disease, N (%) | 1321 | 178 (13.5) |
|
Number of comorbidities, Median (IQR) >2, N (%) |
1312 | 2 9(1–3) 689 (52.5) |
|
Lactate dehydrogenase (mU/mL), Median (IQR) >430 mU/mL, N (%) |
869 | 316 (242–429) 217 (25.0) |
|
C-reactive protein (mg/dL), Median (IQR) >21 mg/dL, N (%) |
939 | 9.9 (3.65–20.6) 234 (24.9) |
| Severe pneumoniaa, N (%) | 1391 | 1173 (84.3) |
Abbreviations:COPD, chronic obstructive pulmonary disease; IQR, interquartile range.
According to the criteria of the American Thoracic Society and Infectious Diseases Society of America [23].
After a median follow-up of 18 days [25th-75th, 10–31], a primary composite end-point event occurred in 454 patients and 362 patients had died.
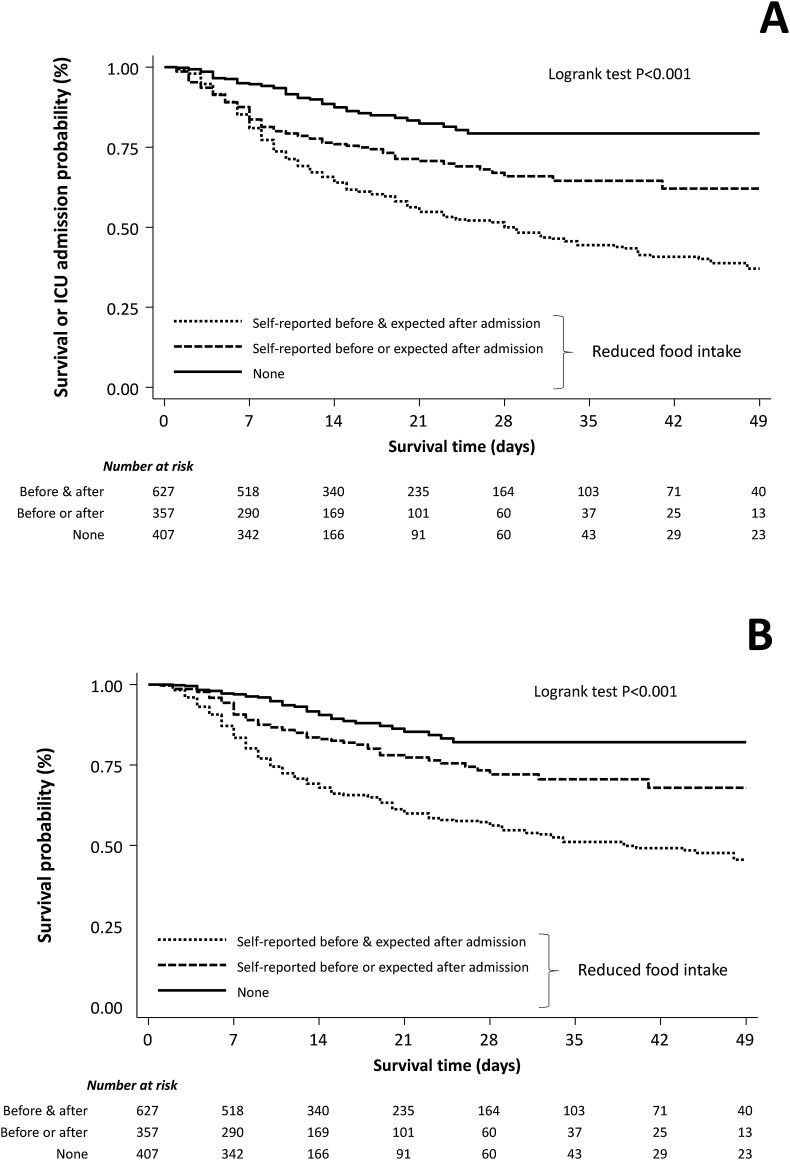
Among nutritional parameters, only reduced food intake was associated (P < .001) with both primary and secondary end-points at the univariable analysis (Fig. 1 ). Compared to patients with preserved food intake, patients with reduced self-reported food intake before hospitalization and/or expected by physicians in the next days since admission had a 3.5- and 2-fold increase risk of death or admission to ICU, respectively (HR = 3.59 [95%CI, 2.01–6.43], p < .001 and HR = 2.18 [95%CI, 1.47–3.23], p < .001). A similar increase in the risk of death was observed (HR = 3.95 [95%CI 2.31–6.76], p < .001 and HR 1.99 [95%CI 1.27–3.13], p = .003).
Fig. 1.
Cumulative event-free survival (Panel A; composite of in-hospital mortality or admission to ICU) and survival (Panel B) curves of reduced food intake in the study cohort.
Multivariable models were based on 1183 patients with complete data (events, N = 345; deaths, N = 278) and reduced self-reported food intake before hospitalization and/or expected by physicians in the next days since admission was found to have a negative prognostic impact for both primary and secondary end-points (Table 2 ). Other predictors of outcome were age and presence of multiple comorbidities. A significant association between food intake categories and markers of disease severity (CRP and LDH) was observed (P < .001 for all). Secondary analysis (patients, N = 829; events, N = 242; deaths, N = 200), further adjusted for high (upper quartile of the distribution) CRP (>21 mg/dL) and LDH (>430 mU/mL) levels yielded consistent findings, with increased risk associated also with high LDH levels.
Table 2.
Predictors of composite end-point (in-hospital mortality or admission to ICU) and in-hospital mortality (risk estimates by Cox's regression).
| Variables | Composite end-point Model p < .001, Harrell's C = 0.68 |
Mortality Model p < .001, Harrell's C = 0.75 |
||
|---|---|---|---|---|
| HR (95%CI) | P-value | HR (95%CI) | P-value | |
| Female gender | 0.84 (0.69–1.02) | 0.086 | 0.92 (0.72–1.18) | 0.51 |
| Age ≥70 years | 2.37 (1.79–3.14) | <0.001 | 5.20 (3.97–6.82) | <0.001 |
|
Body mass index (kg/m2) <20.0 20.0–24.9 25.0–29.9 ≥30.0 |
0.97 (0.63–1.49) 1 (reference) 1.10 (0.81–1.49) 1.20 (0.98–1.48) |
0.37 0.89 0.56 0.079 |
0.91 (0.51–1.62) 1 (reference) 1.02 (0.66–1.59) 0.98 (0.75–1.27) |
0.97 0.75 0.91 0.86 |
|
Weight loss in the previous month No Yes Unknown |
1 (reference) 0.68 (0.31–1.49) 0.84 (0.37–1.92) |
0.54 0.34 0.69 |
1 (reference) 0.79 (0.36–1.74) 0.92 (0.40–2.11) |
0.82 0.57 0.85 |
|
Reduced food intake No Self-reported before or expected after admission Self-reported before & expected after admission |
1 (reference) 2.24 (1.42–3.54) 3.21 (2.14–4.81) |
<0.001 0.001 <0.001 |
1 (reference) 1.97 (1.25–3.11) 3.20 (1.90–5.38) |
<0.001 0.004 <0.001 |
| Number of comorbidities >2 | 1.33 (1.02–1.73) | 0.033 | 1.75 (1.36–2.25) | <0.001 |
Abbreviations: HR, hazard ratio; 95%CI, 95% confidence interval.
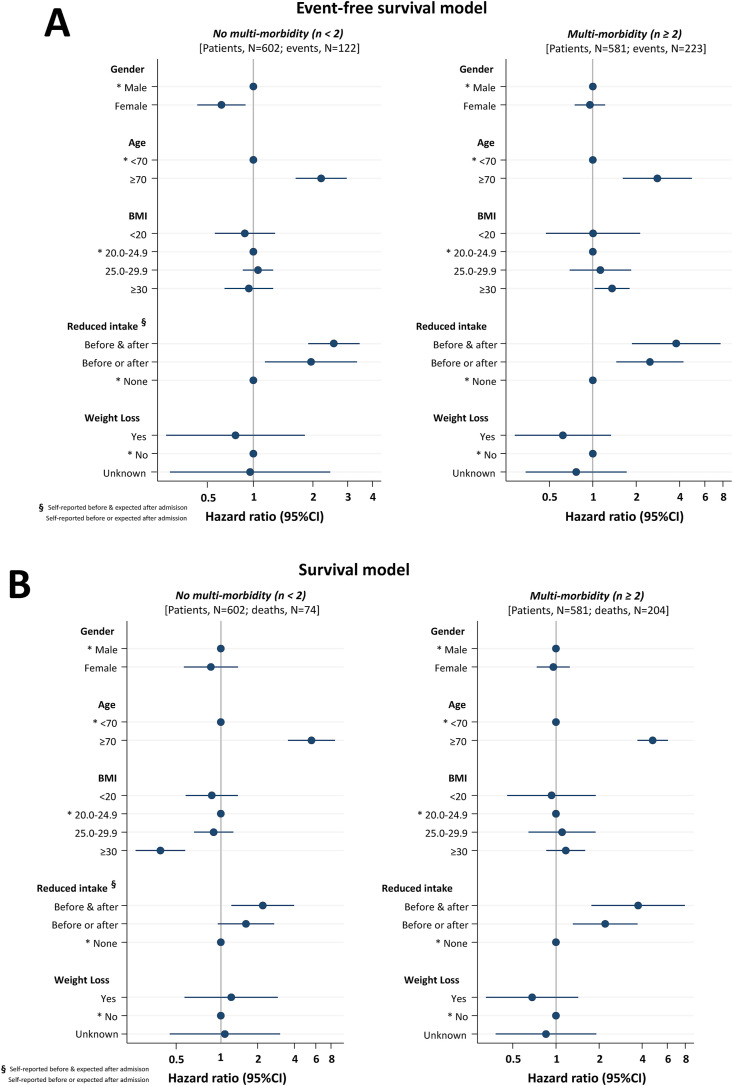
However, for both end-points we detected a significant interaction (P < .001) between BMI and the number of comorbidities. Accordingly, the original models were refitted on patients with and without multi-morbidity. In all models, reduced food intake and higher age remained associated with negative outcome (Fig. 2 ). Interestingly, obesity (BMI≥30.0 kg/m2) was found to be a risk factor for composite end-point (HR = 1.36 [95%CI, 1.03–1.80]; P = .031) and a protective factor against in-hospital mortality (HR = 0.32 [95%CI, 0.20–0.51]; P < .001) in patients with and without multiple comorbidities, respectively. Female gender was also associated with lower risk of composite end-point in absence of multiple comorbidities. Further adjustment for CRP and LDH yielded consistent findings.
Fig. 2.
Cox's multivariable risk models of composite outcome (Panel A) and in-hospital mortality (Panel B) in multi-morbidity strata (number of comorbidities <2 [left panel] vs. ≥2 [right panel]).
4. Discussion
In the present multicentre prospective real-life study, we investigated the association between the major nutritional screening parameters and clinical outcomes in non-critically ill, hospitalized COVID-19 patients. We found that reduced self-reported food intake before admission and/or expected by the physicians during the first days after hospital admission was an independent determinant of a negative outcome.
Although some previous investigations evaluated the impact of nutritional risk in COVID-19 patients [15,21], we focused our analysis on the single parameters involved, due to the intrinsic limitations of nutritional screening scores in this patient's population, particularly during the first pandemic wave, which was characterized by a massive health management emergency, although important pressures were observed also in subsequent surges.
In general, according to the available literature, the prevalence of malnutrition and nutritional risk is very high among COVID-19 patients and is associated with worse clinical outcomes [[22], [23], [24], [25]]. Nutritional risk scores often include parameters like age and disease severity, which increase the probability to detect the risk in acute diseases like COVID-19 and are strongly associated with negative outcomes [26,27]. In addition, the commonly used nutritional parameters (i.e., BMI and weight loss) could be misleading or not reliable in hospitalized COVID-19 patients [16,28]. For this reason, modified screening procedures have also been arbitrarily proposed [16,25,28].
Weight loss was reported only by 12% of our sample at hospital admission and it was unknown in more than one third of patients. Moreover, during the first pandemic wave and the subsequent epidemic peaks, systematic weight and height measurement could result hard or even impossible to perform, due to the usual lack of scales and in consideration of the hygienic precautions required and the associated safety concerns. This may limit the accuracy of nutritional assessment and force to rely on estimated or referred anthropometric values. For the above reasons, we were not able to apply the Global Leadership Initiative on Malnutrition (GLIM) criteria [29] for the proper diagnosis of malnutrition.
Several studies reported that obesity is a predictor of negative prognosis as it increases the risk of ICU admission and death in COVID-19 [12]. Overweight and obesity have been reported as frequent conditions in the general hospital population [14] and in COVID-19 patients [30], and our data are in line with this evidence, as we detected a prevalence of around 35% and 16%, respectively. However, large hospital population studies such as the NutritionDay multinational survey, showed that overweight is associated with a more favorable outcome [14]. Interestingly, in our cohort, obesity in combination with 2 or more comorbidities was associated with increased mortality, while in the presence of less than 2 comorbidities it represented a protective prognostic factor. As already stated, a high BMI per-se, without the negative consequences of an overt metabolic syndrome and an increased low-grade inflammatory background [31], could be a protective condition, as nutritional reserves could play a key role in counteracting acute stress reactions in hospitalized patients [14]. On the other hand, complicated obesity may worsen the clinical conditions of COVID-19 patients with comorbidities, as shown by the available literature [12]. Only less than 8% of our sample presented a BMI <20 kg/m2 at hospital admission. Hence, nutritional risk would have been determined mainly by age and disease severity. Nonetheless, obesity per-se may mask the risk of malnutrition but increased risk could be determined by concomitant sarcopenia [32]. Nowadays, the assessment of body composition represents a cornerstone in nutritional science, due to its prognostic value in different clinical conditions [33]. Schiaffino et al. observed that low muscle mass detected by CT scans was independently associated with in-hospital mortality and ICU admission among COVID-19 patients [34]. On the contrary, a retrospective study observed that bioimpedance analysis does not appear to bring further predictive value than nutritional risk screening alone [35]. Despite of the undoubtable value of body composition analysis, it should be acknowledged that it was very hard to perform during the first pandemic wave, due to the emergency scenario and that it may still not be easily available in all the hospitals facing the COVID-19 epidemic.
The most relevant result of our study was the independent correlation between reduced self-reported food intake before admission and/or expected by the physicians during the first days after hospital admission and mortality. Inadequate food intake has been shown to be independently associated with greater infections risk, more frequent complications, prolonged hospitalization, and worse clinical outcomes in hospitalized patients [36,37]. This implies that, like in the general inpatients population [38], personalized nutritional support is potentially beneficial to improve clinical outcomes and prognosis in hospitalized COVID-19 patients at nutritional risk [28,39,40]. To our knowledge, the association explored herein had not been investigated in COVID-19 before, although it was already reported that hospitalized non-ICU patients satisfying their calorie-protein requirements were more frequently discharged than those who did not reach their nutritional targets [28]. Moreover, it was recently shown that early caloric deficit may independently contribute to worsening survival in mechanically ventilated COVID-19 patients [41].
Due to the observational nature of our study, it should be obviously still clarified whether reduced food intake represents a cause or a consequence of disease severity, but it is certainly an easily obtainable parameter, which could be useful in the daily clinical practice to identify high-risk patients who may benefit from prompt nutritional support. Interestingly, food intake reduction was associated with markers of disease severity, such as CRP and LDH serum concentration. However, in line with previous evidences [36,37], in our study the association between self-reported and/or expected food intake reduction and clinical outcomes resulted independent of some of the acknowledged prognostic factors in COVID-19, i.e. age, number of comorbidities, CRP and LDH. Hence, it may also be interesting to assess this variable in future intervention trials, even if they are objectively difficult to perform in COVID-19, particularly due to the ethical issues related to a possible arm including intentionally undernourished patients.
A further potential limitation of our study could be the inclusion bias associated with the setting of care. Our population consisted of particularly severe patients, being the Institutions involved relevant referral hospitals in their territories.
From a methodological point of view, we are aware of the intrinsic limitations of the parameters considered and that nutritional assessment, including food intake estimation, should be systematically repeated during hospitalization. Ideally, GLIM criteria [29] should have been taken in to consideration for the proper diagnosis of malnutrition.
However, it was not possible to overcome the above mentioned limitations in our study, mainly due to the emergency scenario.
On the other hand, to the best of our knowledge, this was the largest prospective multicenter study which investigated the independent impact of different nutritional parameters on clinical outcomes in COVID-19 patients.
5. Conclusion
This prospective multicenter real-life study showed that reduced self-reported food intake before and/or expected by the physicians after hospital admission, was independently associated with negative clinical outcomes in non-critically ill, hospitalized COVID-19 patients and reinforces the hypothesis that early provision of adequate nutrition may be crucial for the successful management of the disease. Although adequately designed intervention trials would be necessary to ascertain whether it represents a cause or a consequence of disease severity, food intake reduction is a simple and easily obtainable parameter, which may be useful to identify COVID-19 patients at highest risk of poor prognosis, who may benefit from prompt nutritional support. Finally, the study also suggested that the presence of comorbidities could be the key factor, which may determine the protective or harmful role of a high BMI in COVID-19.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Caccialanza, Cereda and Klersy had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Caccialanza is chief investigator and acts as guarantor for this work.
Concept and design: Caccialanza, Cereda, Klersy.
Acquisition, analysis, or interpretation of data: Caccialanza, Formisano, Klersy, Ferrari, Demontis, Mascheroni, Masi, Crotti, Lobascio, Cerutti, Orlandoni, Dalla Costa, Redaelli, Fabbri, Malesci, Corrao, Bordandini, Cereda and the other members of the NUTRI-COVID19 Collaborative Working Group.
Drafting of the manuscript: Caccialanza, Formisano, Klersy, Cereda.
Critical revision of the manuscript for important intellectual content: Demontis, Mascheroni, Cerutti, Orlandoni, Dalla Costa, Redaelli, Fabbri, Malesci, Corrao, Bordandini.
Statistical analysis: Klersy, Ferretti.
Administrative, technical, or material support: Ferrari, Masi, Crotti, Lobascio.
Supervision: Caccialanza.
Other - Research facilitator responsible for data collection from participants: Ferrari.
Other - Trial management: the NUTRI-COVID19 Collaborative Working Group.
Conflicts of interest
None of the authors has conflicts of interest to disclose.
Acknowledgments
We are infinitively grateful to all the employees of the participating Institutions for their tremendous and courageous efforts in struggling against the current tragic clinical and social COVID-19 emergency.
Contributor Information
NUTRI-COVID19 Collaborative Working Group:
Riccardo Caccialanza, Elena Formisano, Catherine Klersy, Virginia Ferretti, Alessandra Ferrari, Sara Masi, Silvia Crotti, Federica Lobascio, Emanuele Cereda, Raffaele Bruno, Carlo Maurizio Montecucco, Angelo Guido Corsico, Mirko Belliato, Antonio Di Sabatino, Serena Ludovisi, Laura Bogliolo, Francesca Mariani, Chiara Muggia, Gabriele Croce, Chiara Barteselli, Jacopo Mambella, Francesco Di Terlizzi, Cloè Dalla Costa, Elena Lenta, Emanuela Nigro, Annalisa Mascheroni, Elisa Merelli, Alessandro Maria Misotti, Andrea de Monte, Elena Redaelli, Laura Iorio Laura, Paola Rossi, Nadia Cerutti, Flavia Favareto, Elisa Pisocri, Manuela Cimorelli, Paolo Orlandoni, Claudia Venturini, Alessandra Fabbri, Salvatore Vaccaro, Simona Bodecchi, Elisa Monzali, Alberto Malesci, Vincenzo Craviotto, Paolo Dario Omodei, Paoletta Preatoni, Manuela Pastore, Leonardo Da Rio, Stefania Demontis, Cecilia Ivaldi, Elsa Sferrazzo, Lorenzina Arieta, Erika Natta, Salvatore Corrao, Raffaella Mollaci Bocchio, Lorella Bordandini, Francesco Palmese, and Alessandro Graziani
APPENDIX 1.
The NUTRI-COVID19 Collaborative Working Group is composed by the following individuals:
-
-
Fondazione IRCCS Policlinico San Matteo, Pavia, Italy: Riccardo Caccialanza, Elena Formisano, Catherine Klersy, Virginia Ferretti, Alessandra Ferrari, Sara Masi, Silvia Crotti, Federica Lobascio, Emanuele Cereda, Raffaele Bruno, Carlo Maurizio Montecucco, Angelo Guido Corsico, Mirko Belliato, Antonio Di Sabatino, Serena Ludovisi, Laura Bogliolo, Francesca Mariani, Chiara Muggia, Gabriele Croce, Chiara Barteselli, Jacopo Mambella, Francesco Di Terlizzi.
-
-
Michele e Pietro Ferrero Hospital, Verduno (Cuneo), Italy: Cloè Dalla Costa, Elena Lenta, Emanuela Nigro.
-
-
ASST Melegnano-Martesana, Melegnano (MI), Italy: Annalisa Mascheroni, Elisa Merelli, Alessandro Maria Misotti, Andrea de Monte.
-
-
ASST Lecco, Lecco Hospital, Lecco, Italy: Elena Redaelli, Laura Iorio Laura, Paola Rossi.
-
-
ASST Pavia, Pavia, Italy: Nadia Cerutti, Flavia Favareto, Elisa Pisocri, Manuela Cimorelli.
-
-
IRCCS-INRCA, Ancona, Italy: Paolo Orlandoni, Claudia Venturini.
-
-
Local Health Unit-IRCCS of Reggio Emilia, Reggio Emilia, Italy: Alessandra Fabbri, Salvatore Vaccaro, Simona Bodecchi, Elisa Monzali.
-
-
Humanitas Research Hospital and Humanitas University, Rozzano, Milano, Italy: Alberto Malesci, Vincenzo Craviotto, Paolo Dario Omodei, Paoletta Preatoni, Manuela Pastore, Leonardo Da Rio.
-
-
Giovanni Borea Civil Hospital, Sanremo, Italy: Stefania Demontis, Cecilia Ivaldi, Elsa Sferrazzo, Lorenzina Arieta, Erika Natta.
-
-
National Relevance and High Specialization Hospital Trust ARNAS Civico, Di Cristina, Benfratelli, Palermo, Italy: Salvatore Corrao, Raffaella Mollaci Bocchio.
-
-
AUSL della Romagna, S.Maria delle Croci Hospital, Ravenna, Italy: Lorella Bordandini, Francesco Palmese, Alessandro Graziani.
References
- 1.Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlin D.A., Gulick R.M., Martinez F.J. Severe covid-19. N Engl J Med. 2020 Dec 17;383(25):2451–2460. doi: 10.1056/NEJMcp2009575. [DOI] [PubMed] [Google Scholar]
- 3.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region. Italy. JAMA. 2020 Apr 28;323(16):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solinas C., Perra L., Aiello M., Migliori E., Petrosillo N. A critical evaluation of glucocorticoids in the management of severe COVID-19. Cytokine Growth Factor Rev. 2020 Aug;54:8–23. doi: 10.1016/j.cytogfr.2020.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang S.-J., Jung S.I. Age-related morbidity and mortality among patients with COVID-19. Infect Chemother. 2020 Jun;52(2):154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahid Z., Kalayanamitra R., McClafferty B., Kepko D., Ramgobin D., Patel R., et al. COVID-19 and older adults: what we know. J Am Geriatr Soc. 2020 May;68(5):926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abadía Otero J., Briongos Figuero L.S., Gabella Mattín M., Usategui Martín I., Cubero Morais P., Cuellar Olmedo L., et al. The nutritional status of the elderly patient infected with COVID-19: the forgotten risk factor? Curr Med Res Opin. 2021 Feb 16:1–9. doi: 10.1080/03007995.2021.1882414. [DOI] [PubMed] [Google Scholar]
- 8.Yu Y., Ye J., Chen M., Jiang C., Lin W., Lu Y., et al. Malnutrition prolongs the hospitalization of patients with COVID-19 infection: a clinical epidemiological analysis. J Nutr Health Aging. 2021;25(3):369–373. doi: 10.1007/s12603-020-1541-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverio R., Gonçalves D.C., Andrade M.F., Seelaender M. Coronavirus disease 2019 (COVID-19) and nutritional status: the missing link? Adv Nutr. 2020 Sep 25:nmaa125. doi: 10.1093/advances/nmaa125. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covino M., De Matteis G., Burzo M.L., Russo A., Forte E., Carnicelli A., et al. GEMELLI AGAINST COVID-19 Group Predicting in-hospital mortality in COVID-19 older patients with specifically developed scores. J Am Geriatr Soc. 2021 Jan;69(1):37–43. doi: 10.1111/jgs.16956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dent E., Hoogendijk E.O., Visvanathan R., Wright O.R.L. Malnutrition screening and assessment in hospitalised older people: a review. J Nutr Health Aging. 2019;23(5):431–441. doi: 10.1007/s12603-019-1176-z. [DOI] [PubMed] [Google Scholar]
- 12.Huang Y., Lu Y., Huang Y.-M., Wang M., Ling W., Sui Y., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020 Dec;113:154378. doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czernichow S., Beeker N., Rives-Lange C., Guerot E., Diehl J.-L., Katsahian S., et al. AP-HP/universities/INSERM COVID-19 research collaboration and AP-HP covid CDR initiative. Obesity doubles mortality in patients hospitalized for severe acute respiratory syndrome coronavirus 2 in paris hospitals, France: a cohort study on 5,795 patients. Obes Silver Spring Md. 2020 Dec;28(12):2282–2289. doi: 10.1002/oby.23014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cereda E., Klersy C., Hiesmayr M., Schindler K., Singer P., Laviano A., et al. NutritionDay Survey Collaborators Body mass index, age and in-hospital mortality: the NutritionDay multinational survey. Clin Nutr. 2017 Jun;36(3):839–847. doi: 10.1016/j.clnu.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Bedock D., Bel Lassen P., Mathian A., Moreau P., Couffignal J., Ciangura C., et al. Prevalence and severity of malnutrition in hospitalized COVID-19 patients. Clin Nutr. 2020 Dec;40:214–219. doi: 10.1016/j.clnesp.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caccialanza R., Laviano A., Lobascio F., Montagna E., Bruno R., Ludovisi S., et al. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020 Jun;74:110835. doi: 10.1016/j.nut.2020.110835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barazzoni R., Bischoff S.C., Breda J., Wickramasinghe K., Krznaric Z., Nitzan D., et al. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin Nutr. 2020 Jun;39(6):1631–1638. doi: 10.1016/j.clnu.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ombrello M.J., Schulert G.S. COVID-19 and cytokine storm syndrome: are there lessons from macrophage activation syndrome? Transl Res. 2021 Mar 5;(21):52–59. doi: 10.1016/j.trsl.2021.03.002. S1931-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Metlay J.P., Waterer G.W., Long A.C., Anzueto A., Brozek J., Crothers K., et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am J Respir Crit Care Med. 2019;200:e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization Physical status: the use and interpretation of anthropometry. Report of a WHO Expert Committee. World Health Organ Tech Rep Ser. 1995;854:1–452. [PubMed] [Google Scholar]
- 21.Li G., Zhou C.-L., Ba Y.-M., Wang Y.-M., Song B., Cheng X.-B., et al. Nutritional risk and therapy for severe and critical COVID-19 patients: a multicenter retrospective observational study. Clin Nutr. 2021;40:2154–2161. doi: 10.1016/j.clnu.2020.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larrazabal R.B., Perez B.M.B., Masamayor E.M.I., Chiu H.H.C., Palileo-Villanueva L.A.M. The prevalence of malnutrition and analysis of related factors among adult patients with the Coronavirus Disease 2019 (COVID 19) in a tertiary government hospital: the MalnutriCoV study. Clin Nutr. 2021 Apr;42:98–104. doi: 10.1016/j.clnesp.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu A., Cong J., Wang Q., Mei Y., Peng Y., Zhou M., et al. Risk of malnutrition is common in patients with coronavirus disease 2019 (COVID-19) in wuhan, China: a cross-sectional study. J Nutr. 2021;151:1591–1596. doi: 10.1093/jn/nxab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva D.F.O., Lima S.C.V.C., Sena-Evangelista K.C.M., Marchioni D.M., Cobucci R.N., Andrade FB de. Nutritional risk screening tools for older adults with COVID-19: a systematic review. Nutrients. 2020 Sep 27;(10):12. doi: 10.3390/nu12102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pironi L., Sasdelli A.S., Ravaioli F., Baracco B., Battaiola C., Bocedi G., et al. Malnutrition and nutritional therapy in patients with SARS-CoV-2 disease. Clin Nutr. 2021 Mar;40(3):1330–1337. doi: 10.1016/j.clnu.2020.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Biswas M., Rahaman S., Biswas T.K., Haque Z., Ibrahim B. Association of sex, age, and comorbidities with mortality in COVID-19 patients: a systematic review and meta-analysis. Intervirology. 2020 Dec 9:1–12. doi: 10.1159/000512592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yanez N.D., Weiss N.S., Romand J.-A., Treggiari M.M. COVID-19 mortality risk for older men and women. BMC Publ Health. 2020 Nov 19;20(1):1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Formisano E., Di Maio P., Ivaldi C., Sferrazzo E., Arieta L., Bongiovanni S., et al. Nutritional therapy for patients with coronavirus disease 2019 (COVID-19): practical protocol from a single center highly affected by an outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Nutrition. 2021 Feb;82:111048. doi: 10.1016/j.nut.2020.111048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cederholm T., Jensen G.L., Correia M.I.T.D., Gonzalez M.C., Fukushima R., Higashiguchi T., et al. GLIM Core Leadership Committee. GLIM Working Group GLIM criteria for the diagnosis of malnutrition - a consensus report from the global clinical nutrition community. Clin Nutr. 2019;38:1–9. doi: 10.1016/j.clnu.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhu X., Yang L., Huang K. COVID-19 and obesity: epidemiology, pathogenesis and treatment. Diabetes, Metab Syndrome Obes Targets Ther. 2020 Dec 14;13:4953–4959. doi: 10.2147/DMSO.S285197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Leeuw A.J.M., Oude Luttikhuis M.A.M., Wellen A.C., Müller C., Calkhoven C.F. Obesity and its impact on COVID-19. J Mol Med (Berl) 2021 Apr 6:1–17. doi: 10.1007/s00109-021-02072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barazzoni R., Bischoff S., Boirie Y., Busetto L., Cederholm T., Dicker D., et al. Sarcopenic obesity: time to meet the challenge. Obes Facts. 2018;11(4):294–305. doi: 10.1159/000490361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sheean P., Gonzalez M.C., Prado C.M., McKeever L., Hall A.M., Braunschweig C.A. American society for parenteral and enteral nutrition clinical guidelines: the validity of body composition assessment in clinical populations. J Parenter Enter Nutr. 2020 Jan;44(1):12–43. doi: 10.1002/jpen.1669. [DOI] [PubMed] [Google Scholar]
- 34.Schiaffino S., Albano D., Cozzi A., Messina C., Arioli R., Bnà C., et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology. 2021 Mar 16:204141. doi: 10.1148/radiol.2021204141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Giorno R., Quarenghi M., Stefanelli K., Capelli S., Giagulli A., Quarleri L., et al. Nutritional risk screening and body composition in COVID-19 patients hospitalized in an internal medicine ward. Int J Gen Med. 2020;13:1643–1651. doi: 10.2147/IJGM.S286484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thibault R., Makhlouf A.-M., Kossovsky M.P., Iavindrasana J., Chikhi M., Meyer R., et al. Healthcare-associated infections are associated with insufficient dietary intake: an observational cross-sectional study. PloS One. 2015;10(4) doi: 10.1371/journal.pone.0123695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiesmayr M., Schindler K., Pernicka E., Schuh C., Schoeniger-Hekele A., Bauer P., et al. NutritionDay Audit Team Decreased food intake is a risk factor for mortality in hospitalised patients: the NutritionDay survey 2006. Clin Nutr. 2009 Oct;28(5):484–491. doi: 10.1016/j.clnu.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 38.Schuetz P., Fehr R., Baechli V., Geiser M., Deiss M., Gomes F., et al. Individualised nutritional support in medical inpatients at nutritional risk: a randomised clinical trial. Lancet. 2019 Jun 8;393(10188):2312–2321. doi: 10.1016/S0140-6736(18)32776-4. [DOI] [PubMed] [Google Scholar]
- 39.Laviano A., Koverech A., Zanetti M. Nutrition support in the time of SARS-CoV-2 (COVID-19) Nutrition. 2020 Jun;74:110834. doi: 10.1016/j.nut.2020.110834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fernández-Quintela A., Milton-Laskibar I., Trepiana J., Gómez-Zorita S., Kajarabille N., Léniz A., et al. Key aspects in nutritional management of COVID-19 patients. J Clin Med. 2020 Aug 10;9(8) doi: 10.3390/jcm9082589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cereda E., Guzzardella A., Klersy C., Belliato M., Pellegrini A., Sciutti F., et al. Early caloric deficit is associated with a higher risk of death in invasive ventilated COVID-19 patients. Clin Nutr. 2021 doi: 10.1016/j.clnu.2021.02.020. S0261-5614(21)00094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]