Abstract
Scientific advancements from 2002 to 2020 for coronaviruses, i.e., SARS-CoV and MERS-CoV outbreaks, could lead towards a better understanding of the exposure to a health crisis. However, data on its transmission routes and persistence in the environment is still in need of the hour. In this review, we discuss the impact of environmental matrices on dealing with the consequences of the global COVID-19 outbreak. We have compiled the most recent data on the epidemiology and pathogenesis of the diseases. The review aims to help researchers and the larger public recognize and deal with the consequences of co-occurring viral indicators for COVID-19 and provide nano-technological perspectives of possible diagnostic and treatment tools for further studies. The review highlights environmental wastes such as hospital wastewater effluents, pathogen-laden waste, pathogen-laden ground/surface water, wastewater sludge residues and discusses their potential remediation technologies, i.e., pathogen-contaminated soil disposal, municipal and medical solid waste collection, recycling, and final disposal. Finally, holistic suggestions to tackle environmental-related issues by the scientific community have been provided, where scientists, consultants may involve in a tiered assessment from the hazard to risk management in the post-COVID-19 world.
Keywords: SARS-CoV-2, COVID-19, Outbreak, Hospital wastewater, Solid waste
1. Introduction
The severe acute respiratory syndrome coronavirus (SARS-CoV) is from the lineage of β -coronaviruses that caused the emergence of previous outbreaks of SARS (2002, China) and the Middle East Respiratory Syndrome virus MERS (2012, Saudi Arabia) [1]. The β-coronaviruses such as SARS-CoV and MERS-CoV can induce infection in the lower respiratory tract, followed by cough and fever, favoring severe respiratory illness in humans [2]. A recent outbreak by the novel SARS-CoV-2 shows similarity to SARS-CoV and MERS-CoV, predominately attacks alveolar epithelial cells of the lung, stimulates peripheral pneumonia, and results in mass mortality [3]. This suggests that the clinical spectrum of SARS-CoV-2 is not confined to peripheral pneumonia but indicates a multiple series of illnesses leading to multiple organ failures such as heart, liver, kidney, gut, or gastrointestinal tract [4,5]. WHO on March 11, 2020, has declared the novel coronavirus (COVID-19) outbreak a global pandemic.
Pathogenic diseases in humans are caused by infectious agents primarily comprised of a virus, fungi, prions, parasites, and a more comprehensive range of unicellular and multicellular eukaryotes and microorganisms. However, vulnerability and occurrences largely depend on pathogenicity and climatic conditions [6]. It is imperative to develop detection methods for (i) enteric viral indicators of human-fecal origin (specifically), which would tell us about the environmental water pollution levels and (ii) specific pathogenic viruses (in this case, SARS-CoV-2). As enteric viruses cannot multiply in the absence of their host cells, therefore, it can be a possible strategy to avoid the discharge of fatal viruses directly into the environment and human food chain [7,8] rather than their disposal via wastewater treatment plants (WWTP).
It is uttermost important to consider and proceed in the process of inactivation of enteric viruses. It has been observed that under different environmental conditions, the enteroviruses and adenoviruses have been detected and isolated from various water sources, including groundwater, seawater, river, and drinking water [9].
At the starting of February 2021, the case is increasing exponentially to 106 million globally and 2.3 million fatalities, rendering it one of the most significant challenges civilization had ever encountered. Indeed, further studies are needed to predict the direct and indirect impact on the water system [10,11]. Furthermore, the prediction of the impact and related technologies to handle future consequences of such problems is vital [12,13].
Often, to detect the relatively low concentration of enteric viruses in environmental samples, many studies have been conducted to focus on concentrating the virus for enhancing the usefulness of detection assays, using molecular- and culturing-based techniques [14,15]. Research focus across global research networks has been developed to harness present technologies for developing risk assessment tools, instrumental in checking the spread of the COVID-19 virus [16,17].
The present review focuses on the importance of pathogenic markers in environmental water and their detection. Like human enteric viruses, pathogenic viruses can be traced in water and wastewater systems to investigate microbial contamination for monitoring and epidemiology and is of paramount importance in a pandemic period like COVID-19. The review further limelight maneuvering nanotechnology-based surveillance, diagnosis, and treatment of SARS-CoV-2 for better and faster results.
2. Pathogenic contamination and associated environmental risks
Due to the various policies adopted by government agencies after the emergence of the COVID-19 outbreak, the positive and negative impact of the virus on the environment has been reported by various authors, as shown in Table 1 . It is evident from literature that temperature and coronavirus transmission have nonlinear relation. Prata et al. (2020) applied a generalized additive model (GAM) was applied to represent the behavior of the growth curve of COVID-19 in the capital cities of Brazil [18]. The GAM curve implied a negative linear relationship between temperatures and daily cumulative confirmed cases of COVID-19 in the range from 16.8° to 27.4 °C.
Table 1.
An overview of the COVID-19 complex impacts (+or -) on multiple exposure areas of the environment across the world.
| Areas/Sectors | Impact on the environment | Findings | References |
|---|---|---|---|
| Air quality | Positive | Reduction of PM2.5, PM10, NO2, and CO concentrations were reduced during the lockdown. | [72] |
| Negative | O3 concentrations increased by around 50% | ||
| Surface water | Negative | The existence of almost all enteric viruses, result in surface water contamination | [73] |
| Negative | Detection of disinfection by products due to excessive disinfectant use | [49,74,75] | |
| Groundwater | Positive | Reduction of As, Se, Pb, and Fe in industrial waste during COVID-19 lockdown. | [76] |
| NO3 and coliform reduced due to the closure of industrial activities including fisheries. | |||
| WWTP's | Negative | SARS-CoV-2 RNA was quantified in wastewater treatment plants in various countries (Australia, China, France, Germany, Israel, Italy, India, Netherland, Japan, Spain, Turkey, and the USA). Leakage and defects in the sewer supply was identified as the transmission mode for SARS. |
[1,3,17,39,52,77] |
| Negative | Detection of disinfection by products due to excessive disinfectant use | [78,79] | |
| Surface Soil contamination | Negative | At least one enteric pathogens were detected in 96% of soil samples. | [80] |
| Agriculture sector | Negative | Negatively impact harvesting, resulting in crop losses and reduce the supply of fruits and vegetables | [81] |
| Solid Waste Management sector | Negative | Improper disposal of medical, household, and other hazardous waste-laden pathogens, thereby threatening to the terrestrial environment | [38,82] |
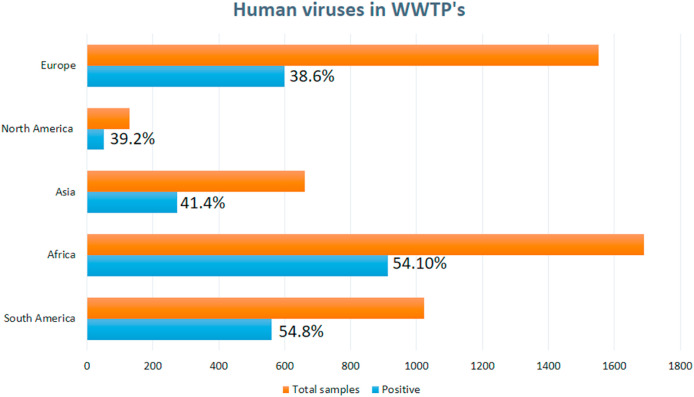
Air qualities are also a viable option for COVID-19 cases worldwide, especially in China, where air quality index (AQI) gone high to 10 units and transmission cases by 5 to 7%. Few cities of Chine show a negative effect on air quality interaction with COVID-19 transmission, attributed to an increase in temperature causes less spread and declining cases. This will help in understanding both aspects during any future pandemic situations [12,19]. Several recent studies have shown that pathogens present in environmental matrices such as landfill leachate [20], soil [21], food (fruit and vegetables) [22], irrigation water [23,24], and sewage sludge [25] are scientist's primary concern. The growing attention of this issue from the last three decades has emerged as an important epidemiological study of wastewater, although its impact and viability remain unsatisfactory despite global efforts. According to a recent study, a variety of viral pathogens are commonly reported in wastewater in various countries over diverse continents, including Africa, North America, Asia, Europe, South America, and Australia. The established independent studies detected the number of samples positive for human viruses, such as Noroviruses (NoVs), Enteroviruses (EVs), Hepatitis A virus (HAV), and Adenoviruses (AdVs) in WWTP's globally is approximately 48.3% (2396/4963). The higher detection rates (samples positive per total samples) were reported in South America (560/1022: 54.8%), followed by Africa (1689/913: 54.1%), Asia (273/660: 41.4%), North America (51/130: 39.2%), and Europe (599/1553: 38.6%) respectively (Fig. 1 ) [26]. A summary of global detection of Noroviruses (NoVs), Enteroviruses (EVs), Hepatitis A virus (HAV), and Adenoviruses (AdVs), SARS-CoV-2 in the collected samples from untreated wastewater from WWTP's using RT-PCR technique are shown in Table 2 .
Fig. 1.
Worldwide detection rates of human viruses (HAV, EVs, NoVs, AVs, and AdVs) with number of positive samples from total samples WWTP's [26].
Table 2.
Global detection of Noroviruses (NoVs), Enteroviruses (EVs), Hepatitis A virus (HAV), and Adenoviruses (AdVs), SARS-CoV-2 in the collected samples from untreated wastewater from WWTP's using RT-PCR technique.
| Country | Amount of sample (mL) | Concentration (copies/L) | Limit of quantification (copies/L) or detection rate | References |
|---|---|---|---|---|
| Australia | 100–200 | 0–120 | – | [42] |
| Bangladesh | 50 | 102–108 | 75% | [83] |
| Brazil | 40 | Qualitative | – | [58] |
| China | 100 | Qualitative | 103 | [41] |
| Canada | 250 | 5.5 × 104–5.5 × 107 | 95% | [84] |
| France | 50 | 0–103 | – | [85] |
| Finland | 2000–5000 | 3.6–4.8 | 2.4–2.6 | [86] |
| Germany | 45 | 2.7 × 103 to 3.7 × 104 | – | [87] |
| Japan | 200–5000 | 0–104 | – | [17] |
| Netherlands | 250 | Qualitative | – | [88] |
| Pakistan | 400 | 103–107 | – | [89] |
| Spain | 200 | 0–5.5 | 4.45 | [50] |
| Switzerland | 500 | Qualitative | – | [48] |
| Turkey | 250 | 0–9.33 × 104 | – | [77] |
| USA | 40 | 0–105 | – | [90] |
| India | 50 | 0–8.05 × 102 | – | [1] |
| Italy | 500 | Qualitative | – | [73] |
3. Viral indicators in wastewater
Human enteric viruses may develop over a hundred forms, and there are rising amounts of diverse emergent strains. Appropriate indicators are needed to evaluate enteric viruses for determining the treatment efficiency of WTTPs and drinking water sources. This would involve measuring indices such as quantitative analysis of viral indicators, pathogenic persistence and abundance, absorption to solid matter, and transference to the marine environment. To tackle the complexity, it is imperative to have trustable markers for detecting human-derived enteric viruses and cost-effective technologies to set-up surveillance via wastewater-based epidemiology (WBE) systems. Farkas et al. (2020) comprehensively reviewed the optimal criteria for detecting human-derived enteric viruses across aquatic environments [27], which will be used as a reference study throughout this manuscript. The criteria for the best possible practices for a viral indicator have been based on (i) detection and quantification of enteric viruses under minimal infrastructural amenities, (ii) specific measurements to human-derived waste, (iii) high concentration levels in wastewater (for easy detection), (iv) persistence in larger aquatic ecosystems, and (v) geographical distribution over time.
A suitable indicator for human-derived fecal pollution would ideally have to be specific, exclusive for human (and not animals), and have to have similar biophysical properties such as size, structure, retention, inactivation, just like common enteric viruses, present throughout the year and ubiquitously in all wastewater-contaminated environments (such as sewers, WTTPs, aquatic ecosystems). Enteric viruses are also remarkably resistant to wastewater treatment [28], contrary to enveloped viruses such as SARS-CoV-2. With the inception of the WBE concept, sampling of viral indicators from sewers, contaminated surface water could serve as a representative of communities, leading to practical action-plans and environmental policymaking during the COVID-19 outbreak [29].
Elevated levels of influenza viruses, coronaviruses, circoviruses, and papillomaviruses have been found in wastewater but not in contaminated environments due to their quick decay in water. Among well-characterized indicators, fecal indicator bacteria (FIB Coliform bacteria, E-coli, Streptococcus, and Enterococcus spp.) are generally used to indicate fecal contamination. Bacteria effectively get killed in WWTPs before their discharge to surface waters. Hence FIB-based water quality monitoring for indirectly indicating viral populations would not be a smart choice. Among enteric viruses, Hepatitis E and A viruses abundantly contaminate urbanized environments [30], while less frequently in developed regions; but they too fall short of the criteria of being human feces-specific viral indicators. Based on analyzing over 120 studies of various potential viral indicators that lack seasonal specificity and are abundantly present in the contaminated environments, Human polyomaviruses (PyVs), Group F mastadenoviruses (AdVs) (adenoviruses associated with gastroenteritis), and Aichi viruses (AiVs) are the best fit for the criteria [31,32].
One of the most potential viral indicators, the plant virus, pepper mild motel virus (PMMoV; Virgaviridae family), has also been shown to be specific to human waste. It is derived from the human diet upon the consumption of peppers (Capsicum spp.) and food products contaminated with the virus and found in contaminated surfaces, groundwater, and potable water [33]. The waterborne disease made scientists again think about the reach of the outbreak of a pandemic. Enteric viruses will come from different human activities like human waste, which use to travel quickly with little decay. Their monitoring as well identification are important parts for accessing the risk management as well as for early control. Individual identification is difficult. Instead, viral metrics can be employed for wastewater evaluation, viral decay, and its transport detection [34,35]. PMMoV belongs to the same genus (Tobamovirus) as the Tobacco Mosiac virus, and several studies (across continents) have individually concluded PMMoV's suitability as a human enteric virus indicator [36,37]. Additionally, the relative safety of the non-pathogenic plant virus makes PMMoV suitable as an enteric virus indicator. PMMoV is noted as the most abundant RNA virus in human feces distributed globally [33,36].
4. Association of coronaviruses with human-derived waste
Although known to be primarily targeting the respiratory system, the previous coronavirus outbreaks of SARS-CoV and MERS-CoV in 2002–2003 and 2012, respectively, indicated transmission through the fecal-oral route, behaving as enteric viruses [38]. The gastrointestinal symptoms and presence of viral RNA in stool in hospitalized COVID-19 patients was studied by at least 15 research groups (until July 2020) in a meta-analysis of more than 6000 patients from various cohorts [17,39]. It was found that viral RNA was found in the stool of 15 to 83% of patients; especially, those admitted to the intensive care unit (ICU) and even in the absence of gastrointestinal symptoms [40,41].
It is essential to have simplified, rapid, inexpensive methods for detecting human enteric viruses and pathogens understudy at the strain-level. As human-transmitted virus strains- AdV, AiV, and PyV are sensible indicators for human feces contamination, and the enteric viral pollution can be assessed based on these viruses and their equivalent animal-associated strains. For instance, SYBR Green quantitative polymerase chain reaction (qPCR) was used for detecting and quantifying distinctive sequences of human, bovine, canine, porcine, and avian AdV genomes depending on their melting temperature in samples of water and sediment [1,33]. Agricultural and human sewage contamination in recreational, groundwater, and drinking water was evaluated by measuring strains of human and porcine AdVs, bovine PyVs, and porcine circovirus [1]. Human AdV qPCR assays were used to exclusively targeted human-specific AdV groups (A-G), mainly (A-F), in multiple studies [27]. Few studies are based on the mutation of SARS-CoV-2 sequences from the sequence GenBank collections. In the USA, next-gen sequencing samples of five-point mutation were found out with clonal impact were seen. It is also reported that SARS-CoV-2 is still mutating in the helicases gene, implying more developments yet to come. Hence it will help in understanding the nature as well as early detection via PCR (RT-qPCR)-based viral research [42,43]. The waterborne AdVF group (mostly type 41) was found to be most widespread in wastewater and the environment. Among polyomaviruses (PyV), the JC and BK strains were exclusive to human waste (compared to animal waste); and are very common in wastewater, and MC PyV was also present in wastewater. Further studies could conclusively determine the association of human-specific AiV (groups A and B) with animal waste, which is abundant in wastewater-contaminated environments [31,44].
Interestingly, some coliphages can be used to differentiate human and animal-derived wastes. Coliphages are relatively well-characterized fecal viral/water pollution indicators [45]. It has been shown that FRNAPH or F + RNA (F-specific RNA coliphage) genogroups II and III are well associated with human sources, while genogroups I and IV are related to non-human sources. These differences among FRNAP genogroups have been exploited for developing protocols to identify the source of fecal contamination [1,46]. Among the phages most specific to humans are those infecting Bacteroides, BG-124 (BacBG124P), RYC-2056 (BacRYC 2056P), GA-17 (BacGA17P) & ARABA-84 (BacARABA84P). However, there have been confounding results with animal wastes, as seen in a study in abattoirs [47]. crAsphage is found to be quite specific to humans; although there are few cases of cross-reactivity with dogs, gulls, poultry, pigs, and bovine feces [48,49], it is in many orders of magnitude less than human sources.
5. The occurrence of SARS-CoV-2 RNA in water
Several studies have been performed on SARS-CoV-2 RNA detection in the wastewater treatment plants (WWTP'S), domestic wastewater, and surface water in Australia, China, France, Germany, Italy, India, Netherland, Japan, Spain, Turkey, and the USA [3,17,39,50]. This led to insights that WBE-based diagnosis is a unique pre-exposure prophylactic measure and monitoring system for COVID-19 in communities worldwide [41]. El-Baz et al. (2020) concluded that WBE is also relevant for COVID-19 drugs detection in wastewater in early stages within a community [51].
Sewage monitoring and WBE practices have been successfully used for poliovirus detection in the past for monitoring the resurfacing of the disease [41] and monitoring narcotic metabolites in communities in China [29]. Thus, wastewater-based surveillance for monitoring CoV's infections is much needed to prevent it in communities. Besides, the safety aspect should be considered in sampling wastewater, containing all PPE with protective sampling kits to avoid exposure to pathogens. In vitro observations demonstrated SARS-CoV-2 virus persistence for 2, 3, and 14 days in hospital wastewater, feces, and urine, respectively, at 20 °C [52]. In another study, the authors reported that SARS-CoV-2 RNA might be released through urine (10%) depending on patients' severity [53]. The persistence of SARS-CoV-2 in wastewater was recently investigated, and it was reported that 1.4–6.5 days at room temperature would cause a 2log10 reduction in virus concentration [41].
6. Prospective of nanotechnology in diagnosing and treatment of COVID-19
Due to the SARS-CoV-2 outbreak, fast and accurate diagnostic tools are instantly required to screen COVID-19 patients and confirm the positive cases [54]. The standard method of diagnosis for SARS-CoV-2 is nucleic acid detection by real-time-PCR using reverse transcription (RT-qPCR) and screened via Computer tomography (CT) scans for lungs [55]. On the other hand, nanotechnology can play a role in producing scalable and inexpensive detection methods. Nano-sensors can potentially be used as a diagnostic tool to detect symptoms at an earlier stage, when viral loads are very low [54]. Optical bio-nano sensors to detect the coronavirus in ~30 min from patients' samples without needing centralized laboratory tests have been proposed; such technologies can potentially track the evolution of viruses and prevent future outbreaks [56].
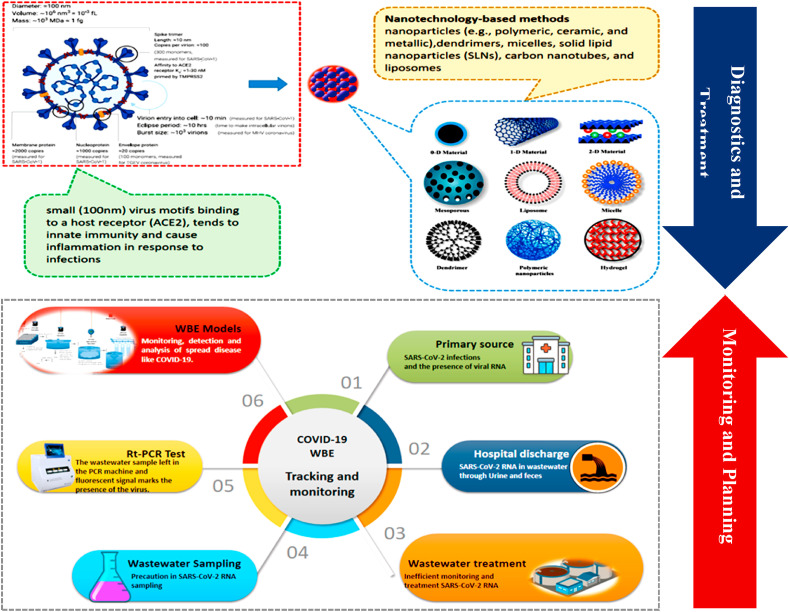
The conventional oxygen therapy by high-flow nasal cannula (HFNC) via ventilator plays a significant role in the SARS-CoV-2 treatment as conventional O2-therapy is not responding well to COVID-19 patients. Recent advancements in Nanotechnology can play a substantial role in the treatment process by nanoparticles [57] that can provide a broad range of promising development of nano-based therapeutic agents or vaccines against several types of coronaviruses (Fig. 2 ). Various photosynthesized nanoparticles possess antiviral and antibacterial activities to reduce the disease risk. Prado et al. (2020) investigated that Gold (Au), Silver (Ag), and Au–Ag bimetallic nanoparticles have antibacterial potential [58]. Ag has previously shown antibacterial activity against E. coli and S. aureus [59,60]. Ag nanoparticles (AgNPs) can interact with the whole virus due to the minimal size (davg = 20 nm) of Ag nanoparticles than the average size of (davg = 120 nm) SARS-CoV-2. The authors highlighted the potential of AgNPs to inhibit the viral entry in host cells, as in the case of the HIV-1 virus, interact with cell receptors, and inhibit the viral replication [60]. Hence, it is expected that the global consumption for healthcare-related nanotechnology is expected to be > 50 tons alone for silver nanoparticles [61].
Fig. 2.
AgNPs for the diagnosis and treatment of SARS-CoV-2 (Adapted from Ref. [62] and WBE of SARS-CoV-2 for surveillance and strategy to tackle COVID-19.
Even nanocomposites are preferable in drug delivery and medicinal applications. For instance, graphene oxide-Silver (GO-Ag) nanocomposites increase the materials' biocompatibility and lessen the toxicological effects of the nanosilver as a metallic nanoparticle [63]. Chen et al. (2016) reported that GO alone inhibited 16% of infection of an enveloped virus such as feline coronavirus (FCoV) but found no antiviral activity against non-enveloped virus-infectious bursal disease virus (IBDV) [64]. Another study showed that GO-Ag restrains 25% of infection by FCoV and 23% by IBDV. The reason is that GO sheets are negatively charged [[65], [66], [67], [68], [69]], they can interact with the positively charged lipid membranes, and Ag can bind to the sulfur groups of the viral proteins to impede the infection. Moreover, nanomaterials applications such as Graphene coated material can be used for medical devices, personal protective equipment, or facemasks to minimize transmission risk [70]. Unfortunately, the facemasks users often wear deficient quality, and even N95 filters have very low protection efficiency against SARS-CoV-2 [71]. Recently Jokanović et al. (2020) concluded that nanosilver masks have significant advantages as they are safe and can have high activity against viruses and bacteria, pertaining to their small size (3–10 nm) [71].
7. Conclusion and future scope
Reforming the healthcare system by governing bodies and investing in awareness programs in controlling infectious diseases proves advantageous. Endorsing the QSS model (Quarantine, shield, and stop) by the governments/health ministry helps resilient infection in the community. Although the report of detection of SARS-CoV-2 in pristine (raw) wastewater revealed that municipal wastewater could be used to monitor the prevalence of SARS-CoV-2 in the community. Besides, it does not provide a clear overview of the presence of viral RNA, which may contribute to the waterborne transmission of the virus. However, more research is required to have a deep insight into exposure assessment about the emission of bioaerosols from the WWTPs, which may pose a severe health concern for wastewater workers. Therefore, a conceptual framework is needed to monitor the SARS-CoV-2 presence and its survival in the air of WWTPs.
Also, investigation on the tools for identifying the infectious SARS-CoV-2 from all corners of environmental, although it needs appropriate safety measures to prevent the risk of transmission. This could be a major challenge to mitigate the likely cause associated with the presence, emergence or transmission of the virus in various environmental matrices. An introspection on informal referral systems, expertise in infection control sections, and suitable control measures for health crises would be necessary. The sense of urgency can be used to readdress the status quo, introducing technologically sophisticated infrastructure and sanitation planning. Also, nanotechnology and wastewater-based epidemiology program could be cutting-edge interdisciplinary solutions and promising tools to diagnose, early detect, and control the spread of the outbreak.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are very thankful and acknowledge to administration of Jazan University, Kingdom of Saudi Arabia. Furthermore, the administration of other universities/institutes, which authors represent. The authors also express their sincere gratitude to the unknown referee for critically reviewing the manuscript and suggesting useful changes.
References
- 1.Kumar M., Patel A.K., Shah A.V., Raval J., Rajpara N., Joshi M., Joshi C.G. First proof of the capability of wastewater surveillance for COVID-19 in India through detection of genetic material of SARS-CoV-2. Sci. Total Environ. 2020;746:141326. doi: 10.1016/j.scitotenv.2020.141326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC Coronavirus Disease 2019 (COVID-19)Centers for Disease Control and Prevention. 2020. [Google Scholar]
- 3.Wu Y.-C., Chen C.-S., Chan Y.-J. The outbreak of COVID-19: an overview. J. Chin. Med. Assoc. 2020;83:217. doi: 10.1097/JCMA.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robba C., Battaglini D., Pelosi P., Rocco P.R.M. Multiple Organ Dysfunction in SARS-CoV-2: MODS-CoV-2 Expert Review of Respiratory Medicine. 2020. pp. 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang B., Bragazzi N.L., Li Q., Tang S., Xiao Y., Wu J. An updated estimation of the risk of transmission of the novel coronavirus (2019-nCov) Infectious Disease Modelling. 2020;5:248–255. doi: 10.1016/j.idm.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tosam M.J., Ambe J.R., Chi Global P.C. In: Socio-cultural Dimensions of Emerging Infectious Diseases in Africa: an Indigenous Response to Deadly Epidemics. Tangwa G.B., Abayomi A., Ujewe S.J., Munung N.S., editors. Springer International Publishing; Cham: 2019. Emerging pathogens, poverty and vulnerability: an ethical analysis; pp. 243–253. [Google Scholar]
- 7.Adelodun B., Ajibade F.O., Tiamiyu A.O., Nwogwu N.A., Ibrahim R.G., Kumar P., Kumar V., Odey G., Yadav K.K., Khan A.H., Cabral-Pinto M.M.S., Kareem K.Y., Bakare H.O., Ajibade T.F., Naveed Q.N., Islam S., Fadare O.O., Choi K.S. Monitoring the presence and persistence of SARS-CoV-2 in water-food-environmental compartments: state of the knowledge and research needs. Environ. Res. 2021;200:111373. doi: 10.1016/j.envres.2021.111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sano D., Amarasiri M., Hata A., Watanabe T., Katayama H. Risk management of viral infectious diseases in wastewater reclamation and reuse. Review Environment International. 2016;91:220–229. doi: 10.1016/j.envint.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fong T.-T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: health risks detection. and Potential Water Quality Assessment Tools. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahmoud A.E.D., Umachandran K., Sawicka B., Mtewa T.K. In: Phytochemistry, the Military and Health. Mtewa A.G., Egbuna C., editors. Elsevier; 2021. Water resources security and management for sustainable communities; pp. 509–522. [Google Scholar]
- 11.Mahmoud A.E.D., Fawzy M. Nanosensors and Nanobiosensors for Monitoring the Environmental Pollutants Waste Recycling Technologies for Nanomaterials Manufacturing. Springer; 2021. pp. 229–246. [Google Scholar]
- 12.Bandala E.R., Kruger B.R., Cesarino I., Leao A.L., Wijesiri B., Goonetilleke A. Impacts of COVID-19 pandemic on the wastewater pathway into surface water. A review Science of The Total Environment. 2021;774:145586. doi: 10.1016/j.scitotenv.2021.145586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sawicka B., Umachandran K., Fawzy M., Mahmoud A.E.D. In: Phytochemistry, the Military and Health. Mtewa A.G., Egbuna C., editors. Elsevier; 2021. Impacts of inorganic/organic pollutants on agroecosystems and eco-friendly solutions; pp. 523–552. [Google Scholar]
- 14.Cashdollar J., Wymer L. Methods for primary concentration of viruses from water samples: a review and meta-analysis of recent studies. J. Appl. Microbiol. 2013;115:1–11. doi: 10.1111/jam.12143. [DOI] [PubMed] [Google Scholar]
- 15.Ikner L.A., Gerba C.P., Bright K.R. Concentration and recovery of viruses from water: a comprehensive review. Food Environmental Virology. 2012;4:41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- 16.Farkas K., Mannion F., Hillary L.S., Malham S.K., Walker D.I. Emerging technologies for the rapid detection of enteric viruses in the aquatic environment. Current Opinion in Environmental Science & Health. 2020;16:1–6. [Google Scholar]
- 17.Haramoto E., Malla B., Thakali O., Kitajima M. First environmental surveillance for the presence of SARS-CoV-2 RNA in wastewater and river water in Japan. Sci. Total Environ. 2020;737:140405. doi: 10.1016/j.scitotenv.2020.140405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prata D.N., Rodrigues W., Bermejo P.H. Temperature significantly changes COVID-19 transmission in (sub)tropical cities of Brazil. Sci. Total Environ. 2020;729:138862. doi: 10.1016/j.scitotenv.2020.138862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C., Zheng W., Huang X., Bell E.W., Zhou X., Zhang Y. Protein Structure and Sequence Re-analysis of 2019-nCoV Genome Does Not Indicate Snakes as its Intermediate Host or the Unique Similarity between its Spike Protein Insertions and HIV-1, bioRxiv : the Preprint Server for Biology. 2020. p. 2020. 2002.2004.933135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohan S.V., Hemalatha M., Kopperi H., Ranjith I., Kumar A.K. SARS-CoV-2 in environmental perspective: occurrence, persistence, surveillance, inactivation and challenges. Chem. Eng. J. 2021;405:126893. doi: 10.1016/j.cej.2020.126893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffan J.J., Derby J.A., Brevik E.C. Soil pathogens that may potentially cause pandemics, including severe acute respiratory syndrome (SARS) coronaviruses. Current Opinion in Environmental Science & Health. 2020;17:35–40. doi: 10.1016/j.coesh.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., Guida M. Persistence of SARS-CoV-2 in the environment and COVID-19 transmission risk from environmental matrices and surfaces. Environ. Pollut. 2020;265:115010. doi: 10.1016/j.envpol.2020.115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Franklin A.B., Bevins S.N. Spillover of SARS-CoV-2 into novel wild hosts in North America: a conceptual model for perpetuation of the pathogen. Sci. Total Environ. 2020;733:139358. doi: 10.1016/j.scitotenv.2020.139358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahmoud A.E.D., Stolle A., Stelter M., Braeutigam P. Abstracts of Papers of the American Chemical Society. Amer Chemical Soc 1155 16th; St, Nw, Washington, DC 20036 USA: 2018. Adsorption technique for organic pollutants using different carbon materials. [Google Scholar]
- 25.Collivignarelli M.C., Collivignarelli C., Carnevale Miino M., Abbà A., Pedrazzani R., Bertanza G. vol. 143. 2020. pp. 196–203. (SARS-CoV-2 in Sewer Systems and Connected Facilities Process Safety and Environmental Protection). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali W., Zhang H., Wang Z., Chang C., Javed A., Ali K., Du W., Niazi N.K., Mao K., Yang Z. Occurrence of various viruses and recent evidence of SARS-CoV-2 in wastewater systems. J. Hazard Mater. 2021;414:125439. doi: 10.1016/j.jhazmat.2021.125439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Farkas K., Walker D.I., Adriaenssens E.M., McDonald J.E., Hillary L.S., Malham S.K., Jones D.L. vol. 181. 2020. p. 115926. (Viral Indicators for Tracking Domestic Wastewater Contamination in the Aquatic Environment Water Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corpuz M.V.A., Buonerba A., Vigliotta G., Zarra T., Ballesteros F., Campiglia P., Belgiorno V., Korshin G., Naddeo V. Viruses in wastewater: occurrence, abundance and detection methods. Sci. Total Environ. 2020;745:140910. doi: 10.1016/j.scitotenv.2020.140910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mao K., Zhang K., Du W., Ali W., Feng X., Zhang H. The potential of wastewater-based epidemiology as surveillance and early warning of infectious disease outbreaks. Current Opinion in Environmental Science & Health. 2020;17:1–7. doi: 10.1016/j.coesh.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iaconelli M., Bonanno Ferraro G., Mancini P., Suffredini E., Veneri C., Ciccaglione A.R., Bruni R., Della Libera S., Bignami F., Brambilla M., De Medici D., Brandtner D., Schembri P., D'Amato S., La Rosa G. vol. 17. 2020. p. 2059. (Nine-Year Nationwide Environmental Surveillance of Hepatitis E Virus in Urban Wastewaters in Italy (2011–2019)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rachmadi A.T., Torrey J.R., Kitajim M. vol. 105. 2016. pp. 456–469. (Human Polyomavirus: Advantages and Limitations as a Human-specific Viral Marker in Aquatic Environments Water Research). [DOI] [PubMed] [Google Scholar]
- 32.Rames E., Roiko A., Stratton H., Macdonald J. vol. 96. 2016. pp. 308–326. (Technical Aspects of Using Human Adenovirus as a Viral Water Quality Indicator Water Research). [DOI] [PubMed] [Google Scholar]
- 33.Symonds E.M., Nguyen K.H., Harwood V.J., Breitbart M. vol. 144. 2018. pp. 1–12. (Pepper Mild Mottle Virus: A Plant Pathogen with a Greater Purpose in (Waste)water Treatment Development and Public Health Management Water Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739:139960. doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan A.H., Tirth V., Fawzy M., Mahmoud A.E.D., Khan N.A., Ahmed S., Ali S.S., Akram M., Hameed L., Islam S., Das G., Roy S., Dehghani M.H. 2021. COVID-19 Transmission, Vulnerability, Persistence and Nanotherapy: a Review Environmental Chemistry Letters. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitajima M., Sassi H.P., Torrey J.R. vol. 1. 2018. p. 19. (Pepper Mild Mottle Virus as a Water Quality Indicator Npj Clean Water). [Google Scholar]
- 37.Tandukar S., Sherchan S.P., Haramoto E. Reduction of human enteric and indicator viruses at a wastewater treatment plant in southern Louisiana. USA Food and Environmental Virology. 2020;12:260–263. doi: 10.1007/s12560-020-09433-1. [DOI] [PubMed] [Google Scholar]
- 38.Zambrano-Monserrate M.A., Ruano M.A., Sanchez-Alcalde L. Indirect effects of COVID-19 on the environment. Sci. Total Environ. 2020;728:138813. doi: 10.1016/j.scitotenv.2020.138813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carraturo F., Del Giudice C., Morelli M., Cerullo V., Libralato G., Galdiero E., Guida M. Persistence of SARS-CoV-2 in the Environment and COVID-19 Transmission Risk from Environmental Matrices and Surfaces Environmental Pollution. 2020. p. 115010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Foladori P., Cutrupi F., Segata N., Manara S., Pinto F., Malpei F., Bruni L., La Rosa G. SARS-CoV-2 from faeces to wastewater treatment: what do we know? A review Science of The Total Environment. 2020;743:140444. doi: 10.1016/j.scitotenv.2020.140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kitajima M., Ahmed W., Bibby K., Carducci A., Gerba C.P., Hamilton K.A., Haramoto E., Rose J.B. SARS-CoV-2 in wastewater: state of the knowledge and research needs. Sci. Total Environ. 2020;739:139076. doi: 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ahmed W., Angel N., Edson J., Bibby K., Bivins A., O'Brien J.W., Choi P.M., Kitajima M., Simpson S.L., Li J., Tscharke B., Verhagen R., Smith W.J.M., Zaugg J., Dierens L., Hugenholtz P., Thomas K.V., Mueller J.F. First confirmed detection of SARS-CoV-2 in untreated wastewater in Australia: a proof of concept for the wastewater surveillance of COVID-19 in the community. Sci. Total Environ. 2020;728:138764. doi: 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D., Yang Y., Huang X., Jiang J., Li M., Zhang X., Ling H., Li J., Liu Y., Li G. 2020. SARS-CoV-2 Spillover into Hospital Outdoor Environments medRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gyawali P., Croucher D., Ahmed W., Devane M., Hewitt J. Evaluation of pepper mild mottle virus as an indicator of human faecal pollution in shellfish and growing waters. Water Res. 2019;154:370–376. doi: 10.1016/j.watres.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 45.McMinn B.R., Ashbolt N.J. vol. 65. 2017. pp. 11–26. (A. Korajkic Bacteriophages as Indicators of Faecal Pollution and Enteric Virus Removal Letters in Applied Microbiology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haramoto E., Kitajima M., Hata A., Torrey J.R., Masago Y., Sano D., Katayama H. A review on recent progress in the detection methods and prevalence of human enteric viruses in water. Water Res. 2018;135:168–186. doi: 10.1016/j.watres.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Wicki M., Auckenthaler A., Felleisen R., Karabulut F., Niederhauser I., Tanner M., Baumgartner A. Assessment of source tracking methods for application in spring water. J. Water Health. 2015;13:473–488. doi: 10.2166/wh.2014.255. [DOI] [PubMed] [Google Scholar]
- 48.Jahn K., Dreifuss D., Topolsky I., Kull A., Ganesanandamoorthy P., Fernandez-Cassi X., Bänziger C., Stachler E., Fuhrmann L., Jablonski K.P. 2021. Detection of SARS-CoV-2 Variants in Switzerland by Genomic Analysis of Wastewater Samples medRxiv. [Google Scholar]
- 49.García-Ávila F., Valdiviezo-Gonzales L., Cadme-Galabay M., Gutiérrez-Ortega H., Altamirano-Cárdenas L., Arévalo C.Z., Flores del Pino L. Considerations on water quality and the use of chlorine in times of SARS-CoV-2 (COVID-19) pandemic in the community Case Studies in Chemical and Environmental Engineering. 2020;2:100049. doi: 10.1016/j.cscee.2020.100049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Randazzo W., Truchado P., Cuevas-Ferrando E., Simón P., Allende A., Sánchez G. vol. 181. 2020. p. 115942. (SARS-CoV-2 RNA in Wastewater Anticipated COVID-19 Occurrence in a Low Prevalence Area Water Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Baz L.M.F., Elwakeel K.Z., Elgarahy A.M. COVID-19 from mysterious enemy to an environmental detection process. a critical review Innovative Infrastructure Solutions. 2020;5:84. [Google Scholar]
- 52.Wang X.-W., Li J.-S., Jin M., Zhen B., Kong Q.-X., Song N., Xiao W.-J., Yin J., Wei W., Wang G.-J., Si B.-y., Guo B.-Z., Liu C., Ou G.-R., Wang M.-N., Fang T.-Y., Chao F.-H., Li J.-W. Study on the resistance of severe acute respiratory syndrome-associated coronavirus. J. Virol Methods. 2005;126:171–177. doi: 10.1016/j.jviromet.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nomoto H., Ishikane M., Katagiri D., Kinoshita N., Nagashima M., Sadamasu K., Yoshimura K. N. Ohmagari Cautious handling of urine from moderate to severe COVID-19 patients. Am. J. Infect. Contr. 2020 doi: 10.1016/j.ajic.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waris A., Ali M., Khan A.U., Ali A. A. Baset Role of nanotechnology in diagnosing and treating COVID-19 during the Pandemic. Int. J. Comput. Vis. 2020;4:65–70. [Google Scholar]
- 55.Yang W., Dang X., Wang Q., Xu M., Zhao Q., Zhou Y., Zhao H., Wang L., Xu Y., Wang J. 2020. Rapid Detection of SARS-CoV-2 Using Reverse Transcription RT-LAMP Method medRxiv. [Google Scholar]
- 56.Zhu X., Wang X., Han L., Chen T., Wang L., Li H., Li S., He L., Fu X., Chen S. 2020. Reverse Transcription Loop-Mediated Isothermal Amplification Combined with Nanoparticles-Based Biosensor for Diagnosis of COVID-19 MedRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahmoud Nanomaterials A.E.D. In: Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications. Kharissova O.V., Martínez L.M.T., Kharisov B.I., editors. Springer International Publishing; Cham: 2020. Green synthesis for water applications; pp. 1–21. [Google Scholar]
- 58.Prado T., Fumian T.M., Mannarino C.F., Maranhão A.G., Siqueira M.M., Miagostovich M.P. vol. 115. Brazil Memórias do Instituto Oswaldo Cruz; Rio de Janeiro: 2020. (Preliminary Results of SARS-CoV-2 Detection in Sewerage System in Niterói Municipality). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gomathi M., Rajkumar P.V., Prakasam A., Ravichandran K. Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resource-Efficient Technologies. 2017;3:280–284. [Google Scholar]
- 60.Sudha A., Jeyakanthan J., Srinivasan P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant. antibacterial and cytotoxic effects Resource-Efficient Technologies. 2017;3:506–515. [Google Scholar]
- 61.Syafiuddin A., Salim M.R., Beng Hong Kueh A., Hadibarata T., Nur H. A review of silver nanoparticles: research trends, global consumption, synthesis, properties, and future challenges. J. Chin. Chem. Soc. 2017;64:732–756. [Google Scholar]
- 62.Vazquez-Munoz R., Lopez-Ribot J.L. Nanotechnology as an alternative to reduce the spread of COVID-19. Challenges. 2020;11:15. [Google Scholar]
- 63.Badr N.B.E., Al-Qahtani K.M., Mahmoud A.E.D. Factorial experimental design for optimizing selenium sorption on Cyperus laevigatus biomass and green-synthesized nano-silver. Alexandria Engineering Journal. 2020;59:5219–5229. [Google Scholar]
- 64.Chen Y.-N., Hsueh Y.-H., Hsieh C.-T., Tzou D.-Y., Chang P.-L. Antiviral activity of graphene–silver nanocomposites against non-enveloped and enveloped viruses. Int. J. Environ. Res. Publ. Health. 2016;13:430. doi: 10.3390/ijerph13040430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmoud A.E.D. Eco-friendly reduction of graphene oxide via agricultural byproducts or aquatic macrophytes Materials. Chemistry and Physics. 2020;253:123336. [Google Scholar]
- 66.Mahmoud A.E.D., Franke M., Stelter M., Braeutigam P. Mechanochemical versus chemical routes for graphitic precursors and their performance in micropollutants removal in water. Powder Technol. 2020;366:629–640. [Google Scholar]
- 67.Cagno V., Andreozzi P., D'Alicarnasso M., Silva P.J., Mueller M., Galloux M., Le Goffic R., Jones S.T., Vallino M. vol. 17. 2018. pp. 195–203. (J. Hodek Broad-Spectrum Non-toxic Antiviral Nanoparticles with a Virucidal Inhibition Mechanism Nature Materials). [DOI] [PubMed] [Google Scholar]
- 68.Mahmoud A.E.D., Stolle A., Stelter Sustainable M. Synthesis of high-surface-area graphite oxide via dry ball milling. ACS Sustain. Chem. Eng. 2018;6:6358–6369. [Google Scholar]
- 69.Mahmoud A.E.D. Graphene-based nanomaterials for the removal of organic pollutants: insights into linear versus nonlinear mathematical models. J. Environ. Manag. 2020;270:110911. doi: 10.1016/j.jenvman.2020.110911. [DOI] [PubMed] [Google Scholar]
- 70.Palmieri V., Papi M. Can graphene take part in the fight against COVID-19? Nano Today. 2020;33:100883. doi: 10.1016/j.nantod.2020.100883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jokanović V., Živković M., Zdravković N. A new approach to extraordinary efficient protection against COVID 19 based on nanotechnology. Stomatol. Glas. Srb. 2020;67:100–109. [Google Scholar]
- 72.Ju M.J., Oh J., Choi Y.-H. Changes in air pollution levels after COVID-19 outbreak in Korea. Sci. Total Environ. 2021;750:141521. doi: 10.1016/j.scitotenv.2020.141521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.La Rosa G., Bonadonna L., Lucentini L., Kenmoe S., Suffredini E. Coronavirus in water environments: occurrence, persistence and concentration methods - A scoping review Water Research. 2020;179:115899. doi: 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bilal M., Nazir M.S., Rasheed T., Parra-Saldivar R., Iqbal H.M.N. Water matrices as potential source of SARS-CoV-2 transmission – an overview from environmental perspective. Case Studies in Chemical and Environmental Engineering. 2020;2:100023. doi: 10.1016/j.cscee.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ganguly R.K., Chakraborty S.K. Integrated approach in municipal solid waste management in COVID-19 pandemic: perspectives of a developing country like India in a global scenario. Case Studies in Chemical and Environmental Engineering. 2021;3:100087. [Google Scholar]
- 76.Selvam S., Jesuraja K., Venkatramanan S., Chung S.Y., Roy P.D., Muthukumar P., Kumar M. Imprints of pandemic lockdown on subsurface water quality in the coastal industrial city of Tuticorin, South India: a revival perspective. Sci. Total Environ. 2020;738:139848. doi: 10.1016/j.scitotenv.2020.139848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kocamemi B.A., Kurt H., Sait A., Sarac F., Saatci A.M., Pakdemirli B. 2020. SARS-CoV-2 Detection in Istanbul Wastewater Treatment Plant Sludges medRxiv. [Google Scholar]
- 78.Wang Y., Yuan Y., Wang Q., Liu C., Zhi Q., Cao J. Changes in air quality related to the control of coronavirus in China: implications for traffic and industrial emissions. Sci. Total Environ. 2020;731:139133. doi: 10.1016/j.scitotenv.2020.139133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Buonerba A., Corpuz M.V.A., Ballesteros F., Choo K.-H., Hasan S.W., Korshin G.V., Belgiorno V., Barceló D., Naddeo V. Coronavirus in water media: analysis, fate, disinfection and epidemiological applications. J. Hazard Mater. 2021;415:125580. doi: 10.1016/j.jhazmat.2021.125580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bauza V., Madadi V., Ocharo R., Nguyen T.H. J.S. Guest Enteric pathogens from water, hands, surface, soil, drainage ditch, and stream exposure points in a low-income neighborhood of Nairobi. Kenya Science of The Total Environment. 2020;709:135344. doi: 10.1016/j.scitotenv.2019.135344. [DOI] [PubMed] [Google Scholar]
- 81.Mishra A., Bruno E., Zilberman D. vol. 754. 2021. p. 142210. (Compound Natural and Human Disasters: Managing Drought and COVID-19 to Sustain Global Agriculture and Food Sectors Science of the Total Environment). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.SanJuan-Reyes S., Gómez-Oliván L.M., Islas-Flores H. COVID-19 in the environment Chemosphere. 2021;263:127973. doi: 10.1016/j.chemosphere.2020.127973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ahmed F., Islam M.A., Kumar M., Hossain M., Bhattacharya P., Islam M.T., Hossen F., Hossain M.S., Islam M.S., Uddin M.M. medRxiv; 2020. First Detection of SARS-CoV-2 Genetic Material in the Vicinity of COVID-19 Isolation Centre through Wastewater Surveillance in Bangladesh. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.D'Aoust P.M., Mercier E., Montpetit D., Jia J.-J., Alexandrov I., Neault N., Baig A.T., Mayne J., Zhang X., Alain T., Langlois M.-A., Servos M.R., MacKenzie M., Figeys D., MacKenzie A.E., Graber T.E., Delatolla R. vol. 188. 2021. p. 116560. (Quantitative Analysis of SARS-CoV-2 RNA from Wastewater Solids in Communities with Low COVID-19 Incidence and Prevalence Water Research). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wurtzer S., Marechal V., Mouchel J.-M., Moulin L. 2020. Time Course Quantitative Detection of SARS-CoV-2 in Parisian Wastewaters Correlates with COVID-19 Confirmed Cases MedRxiv. [Google Scholar]
- 86.Hokajärvi A.-M., Rytkönen A., Tiwari A., Kauppinen A., Oikarinen S., Lehto K.-M., Kankaanpää A., Gunnar T., Al-Hello H., Blomqvist S., Miettinen I.T., Savolainen-Kopra C., Pitkänen T. The detection and stability of the SARS-CoV-2 RNA biomarkers in wastewater influent in Helsinki. Finland Science of The Total Environment. 2021;770:145274. doi: 10.1016/j.scitotenv.2021.145274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westhaus S., Weber F.-A., Schiwy S., Linnemann V., Brinkmann M., Widera M., Greve C., Janke A., Hollert H., Wintgens T., Ciesek S. Detection of SARS-CoV-2 in raw and treated wastewater in Germany – suitability for COVID-19 surveillance and potential transmission risks. Sci. Total Environ. 2021;751:141750. doi: 10.1016/j.scitotenv.2020.141750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Medema G., Heijnen L., Elsinga G., Italiaander R. vol. 7. 2020. pp. 511–516. (A. Brouwer Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in the Netherlands Environmental Science Technology Letters). [DOI] [PubMed] [Google Scholar]
- 89.Sharif S., Ikram A., Khurshid A., Salman M., Mehmood N., Arshad Y., Ahmad J., Angez M., Alam M.M., Rehman L. 2020. Detection of SARs-CoV-2 in Wastewater, Using the Existing Environmental Surveillance Network: an Epidemiological Gateway to an Early Warning for COVID-19 in Communities medRxiv. [Google Scholar]
- 90.Green H., Wilder M., Middleton F.A., Collins M., Fenty A., Gentile K., Kmush B., Zeng T., Larsen D.A. 2020. Quantification of SARS-CoV-2 and Cross-Assembly Phage (crAssphage) from Wastewater to Monitor Coronavirus Transmission within Communities MedRxiv. [Google Scholar]