Abstract
Background
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to over 170?million cases worldwide with over 33.2?million cases and 594,000 deaths in the US alone as of May 31st, 2021. The pandemic has also created severe shortages of personal protective equipment, particularly of filtering facepiece respirators (FFRs). The Centers for Disease Control and Prevention (CDC) has issued recommendations to help conserve FFRs, as well as crisis standards, including four criteria required for decontamination of the traditionally single use respirators. This review is designed to provide an overview of the current literature on vaporized hydrogen peroxide (vHP), hydrogen peroxide gas plasma (HPGP), and aerosolized hydrogen peroxide (aHP) with respect to each of the four CDC decontamination criteria.
Methods
PubMed and Medrxiv were queried for relevant articles. All articles underwent a title and abstract screen as well as subsequent full text screen by two blinded reviewers if indicated.
Results
Searches yielded 195 papers, of which, 79 were found to be relevant. Of those, 23 papers presented unique findings and 8 additional articles and technical papers were added to provide a comprehensive review. Overall, while there are potential concerns for all 3 decontamination methods, we found that vHP has the most evidence supporting its use in FFR decontamination consistent with CDC recommendation.
Conclusions
Future research is recommended to evaluate biological inactivation and real world fit failures after FFR reuse.
Key Words: N95 Respirator, Filtering Facepiece Respirator, Decontamination, Vaporized Hydrogen Peroxide, Aerosolized Hydrogen Peroxide, COVID-19
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to over 170 million cases worldwide with over 33.2 million cases and 5,94,000 deaths in the US alone as of May 31st, 2021.1 Based on modeling released in March of 2020, the World Health Organization (WHO) estimated that 89 million medical masks would be needed for COVID-19 response each month, requiring an increase in global manufacturing of at least 40 percent.2
Stockpiling by health systems and governments, panic buying by the public, travel and export restrictions, and nationwide lockdowns that created interruptions in an already taxed supply chain further exacerbated shortages of medical supplies, including ventilators, associated consumables, and disinfection supplies. Most notably, personal protective equipment (PPE), specifically filtering facepiece respirators (FFRs), have faced severe shortages.2
In the United States, N95 respirators are the dominant FFR used by healthcare workers and are tested according to National Institute for Occupational Safety and Health (NIOSH) standards. They are designed to form a seal around the wearer's face and provide ≥ 95% filtration efficiency of 0.3 µm non-oily particles.
The Centers for Disease Control and Prevention (CDC) has created several recommendations to conserve N95 respirators, including the use of administrative controls to minimize both the number of individuals who require respiratory protection and patient encounters. The CDC has also developed crisis strategies only to be used when supplies of FFRs cannot match with the rate of use (the “burn rate”). These crisis strategies include the use of respirators beyond their expiration date, reuse of single-use respirators for multiple patient encounters, wearing the respirators for extended wear times (extended use), and prioritizing N95 respirators for personnel at highest risk of contracting an infection, such as those performing aerosol-generating procedures.3 In the event that these crisis strategies are insufficient to meet FFR demand, the CDC has also outlined guidance for the decontamination of traditionally single-use FFRs, specifying that any technique meet the four criteria listed in Table 1 . 3
Table 1.
CDC criteria for evaluation of decontamination methods for FFR
| Criteria 1 | Filtration performance is not affected after each cycle of decontamination. |
| Criteria 2 | Fit performance of the respirator is not affected after each decontamination cycle. |
| Criteria 3 | The method can inactivate viruses and bacteria. |
| Criteria 4 | Off-gassing of decontamination chemicals falls below the permissible range. |
Source: CDC 3
Having previously conducted research on vaporized hydrogen peroxide decontamination of N95 respirators under and FDA contract, The Battelle Memorial Institute was the first company to receive an Emergency Use Authorization from the FDA on February 4th of 2020 (later revoked at the request of The Battelle Memorial Institute) with other companies including STERIS Sterilization Systems (STERIS Corporation, OH, USA) and Advanced Sterilization Products (Advanced Sterilization Products, Irvine, CA, USA) receiving authorization for vaporized and hydrogen peroxide gas plasma decontamination systems beginning in April of 2020.4
In May of 2020, the FDA issued Recommendations for Sponsors Requesting EUAs for Decontamination and Bioburden Reduction Systems for Surgical Masks and Respirators During the Coronavirus Disease 2019 (COVID19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff. 5 These non-binding recommendations outlined three “Tiers” of decontamination, with Tier 1 designed for respirators and surgical masks that will be shared between HCPs, Tier 2 designed for reuse by the same HCP, and Tier 3 designed solely to reduce the bioburden on respirators that will be reused according to CDC reuse guidelines. Each of these has different microbial inactivation guidelines with Tier 1 requiring inactivation against organisms with higher levels of resistance. The Tiers described in the guidelines are summarized in Table 2 . In addition, the FDA requested companies provide information including cycle parameters and monitoring thereof, ability to meet microbial inactivation in accordance with Table 2, compatibility with masks/respirators, number of times they can be decontaminated, and tracking and handling systems for the masks/respirators.5
Table 2.
FDA Recommendations for decontamination EUAs
| Tier | Use | Biological inactivation requirements |
|---|---|---|
| Tier 1 | Decontamination of compatible surgical masks and/or respirators that may be suitable for single- or multiple-users. | ≥ 6 log spore reduction of the most resistant spore for the proposed process OR ≥ 6- og reduction of a Mycobacterium species (eg, M. terrae or M. abscessus). |
| Tier 2 | Decontamination of compatible surgical masks and/or respirators only suitable for single-users. | ≥ 6 log reduction of 3 non-enveloped viruses OR ≥ 6 log reduction of 2 gram-positive and 2 gram-negative vegetative bacteria |
| Tier 3 | Bioburden reduction system only to be used by single users to supplement CDC reuse recommendations. | ≥ 3 log reduction of a non-enveloped virus OR ≥ 3 log reduction of 2 gram-positive and 2 gram-negative vegetative bacteria OR Other evidence demonstrating that the bioburden reduction system will reliably achieve > 3 log reduction in non-enveloped virus or vegetative bacteria, which could include, where appropriate, published scientific literature, and scientific and engineering studies. |
Source: FDA5
A NIOSH-led literature review conducted in April 2020 recommended ultraviolet germicidal irradiation (UVGI), vaporous hydrogen peroxide (vHP), and moist heat as having the most promise as potential methods to decontaminate N95 respirators.3 However, vHP is one of multiple hydrogen peroxide-based decontamination methods. Hydrogen peroxide gas plasma (HPGP) and aerosolized hydrogen peroxide (aHP) have also been suggested as possible techniques for use in PPE decontamination. A brief overview of each method is shown in Table 3 . The aim of this review is to use current literature to evaluate the viability of aHP, vHP, and HGHP as techniques for crisis strategy FFR decontamination based on the four CDC criteria. Our goal is to inform health systems of their options to decontaminate PPE during the COVID-19 pandemic.
Table 3.
Hydrogen peroxide decontamination methods for medical equipment 9,18,23,26,25,27
| Hydrogen peroxide gas plasma (HPGP) | Aerosolized hydrogen peroxide (aHP) | Vaporized hydrogen peroxide (vHP) | |
|---|---|---|---|
| Mechanism of Action | A vacuum is used to vaporize the hydrogen peroxide during the diffusion stage, allowing it to diffuse through the items undergoing decontamination. Radio frequency or electrical energy is then used to generate hydrogen peroxide plasma, creating reactive oxygen species that inactivate bacteria and viruses. The hydrogen peroxide plasma decomposes into water and oxygen and the chamber is brought back to atmospheric pressure. | Hydrogen peroxide, often mixed with <50 ppm Ag cations or peracetic acid, is emitted through a nozzle, producing an aerosol of approximately 8-10 micrometer particles which can interact and decontaminate items in the treatment area. Following exposure, the aerosol can be left to decompose into water and oxygen passively or actively removed by scrubbers if a Peracetic Acid mixture is used. | Consists of 3 phases, often used in multiple cycles. Hydrogen peroxide is vaporized until it saturates the treatment area during the gassing phase, causing microcondensation. Next, a dwell period allows the hydrogen peroxide vapor to decontaminate the items undergoing treatment. Lastly, the aeration phase consists of introducing fresh air into the treatment area to catalyze the breakdown of the hydrogen peroxide vapor to oxygen and water. |
| Hydrogen Peroxide Concentration | 50%-60% H2O2 | 5%-7% H2O2 | 30%-59% H2O2 |
| Time Required to Sterilize | 30 min- 120 min | 2-3 h | 1.5-8 h |
Methods
To conduct this review, PubMed and MedRxiv were searched through October 31, 2020 to capture the most relevant published and pre-publication data. Search terms included “aerosolized hydrogen peroxide,” “vaporized hydrogen peroxide,” “hydrogen peroxide gas plasma,” and “hydrogen peroxide plasma,” “((Decontamination) OR (disinfection)) AND (PPE) AND (Hydrogen Peroxide).” Additional technical documents, systematic reviews, and related articles were hand-selected based on relevance and the need to provide a comprehensive analysis. For any systematic review, all relevant articles cited in the systematic review were separately reviewed to ensure consistency with the systematic review conclusions.
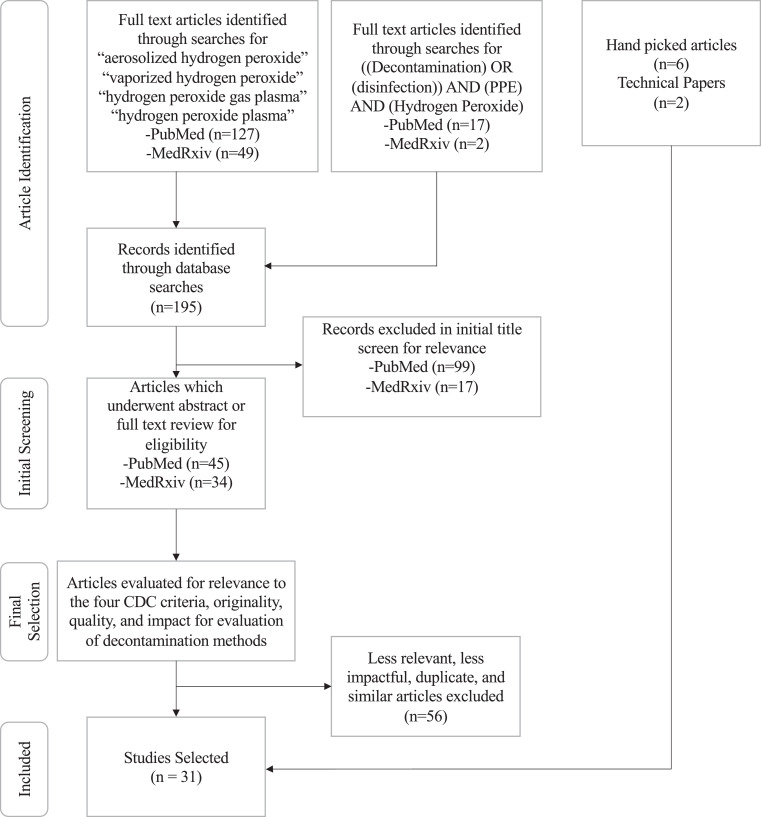
All articles retrieved by the search terms were first reviewed by title, and if they appeared relevant, by abstract. Articles which were relevant after abstract review were reviewed in full, and selections were prioritized based on the quality of the study, relevance to one or more of the CDC criteria described in Table 1, and impact of the findings, with priority going to articles that had undergone peer review. A summary of the process is shown in Figure 1: Overview of Article Selection Process.
Fig 1.
Overview of article selection process.
Results
Ultimately, 31 papers were selected to include in this review. Summaries of the decontamination technology, parameters utilized, and conclusions of these papers are shown in Table 4 , Table 5 , and Table 6 for HPGP, aHP, and vHP respectively.
Table 4.
Summary of HPGP papers included in the review
| Authors/Date | FFR Tested | Decontamination System | Conclusion |
|---|---|---|---|
| Bergman, Viscusi, Heimbuch, Wander, Sambol, Shaffer (2010) | N95 Respirators, Unspecified Model/Manufacturer | STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA), 55- min cycle, (59% H2O2) | Filter performance: three cycles of HPGP had >4% increase in FFR penetration with differing results among respirators stacked in the same decontamination pouches. |
| Chen, Ngan, Manson, Maynes, Borschel, Rotstein, Gu (2020) | 1860, 8210, 9210, (3M, St. Paul, MN) | STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA), Long Cycle (72 min), (59% H2O2) | Filter performance:not affected by single cycle, but demonstrated increased transmission (>1.5%) after third cycle. Fit performance: increased leakage around nose following 5 cycles. |
| Ibáñez-Cervantes, Bravata-Alcántara, Nájera-Cortés, Meneses-Cruz, Delgado-Balbuena, Cruz-Cruz, Durán-Manuel, Cureño-Díaz, Gómez-Zamora, Chávez-Ocaña, Sosa-Hernández, Aguilar-Rojas, Bello-López (2020) | 1860 (3M, St. Paul, MN) | STERRAD 100 NX (Advanced Sterilization Products, Irvine, CA, USA), Standard Cycle (47 min), (59% H2O2) | Microbial inactivation: SARS-CoV-2 was not detected in all assays and A. baumannii and S. aureus were not cultivable after decontamination. |
| Kumar, Kasloff, Leung, Cutts, Strong, Hills, Vazquez-Grande, Rush, Lother, Zarychanski, Krishnan (2020) | 1804, 1860, 1870, 8210 and 9210, (3M, St. Paul, MN), 1054S (Aearo Company, Indianapolis, IN). | STERRAD 100 NX (Advanced Sterilization Products, Irvine, CA, USA), Standard Cycle (47 min), (59% H2O2) | Fit performance: failed fit testing after 2 cycles of HPGP. |
| Peltier, Wang, Hollenbeck, Lanza, Furtado, Cyr, Ellison, Kobayashi (2020) | 1860 and 1860s (3M, St. Paul, MN) | STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA), 55- min cycle, (59% H2O2), STERRAD 100 NX (Advanced Sterilization Products, Irvine, CA, USA), Express Cycle (24 min), (59% H2O2), STERRAD 100 NX (Advanced Sterilization Products, Irvine, CA, USA), Standard Cycle (47 min), 59% H2O2 | Filter performance:not affected (at >90% filtration efficiency) by single cycle, but affected (<90% filtration efficiency) by multiple or long cycles (especially STERRAD 100S and 100 NX standard). Filtration intact for STERRAD 100 NX express after five cycles. |
| Viscusi, Bergman, Eimer, Shaffer (2009) | N95 Respirators, Unspecified Model/Manufacturer | STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA), 55- min cycle, (59% H2O2) | Filter performance:not affected after a single decontamination cycle. |
| Wigginton, Arts, Clack, Fitzsimmons, Gamba, Harrison, LeBar, Lauring, Li, Robers, Rockey, Torreblanca, Young, Anderegg, Cohn, Doyle, Meisenhelder, Raskin, Love, Kaye (2021) | 1860 (3M, St. Paul, MN) | STERRAD 100 NX (Advanced Sterilization Products, Irvine, CA, USA), Express Cycle (24 minutes), (59% H2O2) | Filter performance: decreased performance after 3 cycles of decontamination. Microbial inactivation: inactivation of Phi6, influenza H3N2 and bacteriophage MS2. |
Table 5.
Summary of aHP papers included in the review
| Authors/Date | FFR Tested | Decontamination System | Conclusion |
|---|---|---|---|
| Cadnum, Li, Redmond, John, Pearlmutter and Donskey (2020) | 1860 (3M, St. Paul, MN) | Altapure AP-4 (Altapure, Mequon, WI) 21- min cycle time including 1- min fogging, 5- min dwell, and 15- min of scrubbing. (22% hydrogen peroxide and 4.5% peracetic acid aerosolized into droplets containing 0.88% hydrogen peroxide, 0.18% peracetic acid, and 0.36% acetic acid) | Microbial Inactivation: respirators inoculated with MRSA and bacteriophage MS2 that underwent 1, 2, or 3 treatment cycles showed reductions of >2.1, >3.6, and >6 log10 plaque or colony forming units. |
| Derr, James, Kuny, Patel, Kandel, Field, Beckman, Hockett, Bates, Sutton, Szpara (2020) | 1860, 1870+, 8511, 9211+ (3M, St. Paul, MN), N1125, (Honeywell Safety Products, Smithfield, RI) | Pathogo Curis (Curis Decontamination, Oviedo, FL, USA) 42- min cycle time with an 11 min 43 s aHP infusion and 6 subsequent H2O2 pulses over the following 30 min (7% H2O2 in proprietary Curoxide) | Fit performance: passed fit testing at first, fifth, and 10th rounds of aHP decontamination. Microbial inactivation: viral RNA bacteriophage phi6, HSV-1 and coxsackievirus B3. Safety: H2O2 was less than <0.5 ppm, within permitted levels, after treatment. |
| Fu and Kumar (2012) | Non-FFR Sample Tested | Sterinis SR2 (Now GLOSAIR 400) (GLOSAIR, Advanced Sterilization Products, Irvine, CA, previously Sterinis), (5% H2O2 and Ag Cation, sold as Sterusil) | Microbial Inactivation: aHP achieved less than a 4 log reduction on the biological indicators and test discs containing MRSA, C. difficile, and A. baumannii. Uneven distribution was evident for the aHP system that did not occur in the vHP system it was compared to. |
| Herruzo, Vizcaino, Herruzo (2014) | Non-FFR Sample Tested | GLOSAIR 400 (GLOSAIR, Advanced Sterilization Products, Irvine, CA, previously Sterinis), 35 min infusion and 2-h dwell time, (6% H2O2 and Ag Cation) | Microbial Inactivation: aHP obtained >3 log mean destruction of the 18 clinical isolates with mixed flora containing a range of gram positive and gram negative organisms. |
| John, Raju, Cadnum, Mdes, McClellan, Akkus, Miller, Jennings, Buehler, Li, Redmond, Braskie, Hoyen, Donskey (2020) | 1860 (3M, St. Paul, MN) | Altapure AP-4 (Altapure, Mequon, WI) 21- min cycle time including 1 min of fogging, 5- min dwell, and 15 min of scrubbing. (22% hydrogen peroxide and 4.5% peracetic acid aerosolized into droplets containing 0.88% hydrogen peroxide, 0.18% peracetic acid, and 0.36% acetic acid) | Filter performance: not affected following 5 cycles. Microbial inactivation: G. stearothermophilus spores and bacteriophage MS2. Safety: H2O2 had dissipated to 0.0 ppm within 60 min of off-gassing and the peracetic acid was undetectable within 20 min. |
| Kumar, Kasloff, Leung, Cutts, Strong, Hills, Vazquez-Grande, Rush, Lother, Zarychanski, Krishnan (2020) | 1804, 1860, 1870, 8210 and 9210, (3M, St. Paul, MN), 1054S (Aearo Company, Indianapolis, IN). | STERIS ARD (STERIS Corporation, OH, USA), 1 h cycle time with 10 min of dehumidification, 3 min of conditioning, 30- min dwell time, and 20- min aeration phase. (35% H202 in proprietary VAPROX) | Microbial inactivation: Indiana serotype of vesicular stomatitis virus and SARS-CoV-2. |
| Microbial Inactivation: MRSA, C. difficile spores, bacteriophage MS2, Candida auris, and G. stearothermophilus biological indicator spores all showed a ≥5 log reduction in plaque or colony forming units. Safety: calculated acetic acid exposure was 0.2 ppm for 5 h which is below the NIOSH standard. | Non-FFR Sample Tested | Altapure AP-4 (Altapure, Mequon, WI) 21- min cycle time including 1- min fogging, 5- min dwell, and 15 minutes of scrubbing. (22% hydrogen peroxide and 4.5% peracetic acid aerosolized into droplets containing 0.88% hydrogen peroxide, 0.18% peracetic acid, and 0.36% acetic acid) | All organisms tested showed a ≥5 log CFU or PFU reduction which included methicillin-resistant Staphylococcus aureus, C. difficile spores, the nonenveloped virus bacteriophage MS2, and Candida auris and G. stearothermophilus biological indicator spores. In terms of safety, calculated acetic acid exposure was 0.2 ppm for 5 h which is below the NIOSH standard. |
| Weber and Boyce (2016) | Non-FFR Sample Tested | Review of multiple studies using the GLOSAIR 400 (GLOSAIR, Advanced Sterilization Products, Irvine, CA, previously Sterinis) with varying cycle times, (6% H2O2 and Ag Cation) | Microbial Inactivation: Described studies showing no significant decontamination of M. tuberculosis as well as showing vHP was more effective at inactivating G. stearothermophilus than aHP. |
Table 6.
Summary of vHP papers included in the review8, 9, 10, 11, 12, 14, 15, 16, 18, 19, 20, 21, 26, 27, 29, 30, 31, 32, 34, 36
| Authors (Date) | FFR Tested | Decontamination System | Conclusion |
|---|---|---|---|
| Battelle Memorial Institute (2016) | 1860 (3M, St. Paul, MN) | Bioquell Clarus C (Bioquell, Horsham, PA, USA) 8 h cycle time with a 10- min conditioning phase, 20- min gassing phase, 150- min dwell phase, and a 300- min aeration phase, (35% H2O2) | Filter performance: not affected after 50 cycles. Fit performance: similar fit to untreated models after 20 cycles. |
| Bergman, Viscusi, Heimbuch, Wander, Sambol, Shaffer (2010) | N95 Respirators, Unspecified Model/Manufacturer | Bioquell Room Bio-Decontamination Service utilizing the Clarus R vHP Generator (Bioquell UK Ltd, Andover, UK) 125- min cycle time including a 15- min dwell time with a room concentration of 8g/m3, (30% H2O2) | Filter performance: three cycles of vHP had <4% change in FFR penetration. |
| Chen, Ngan, Manson, Maynes, Borschel, Rotstein, Gu (2020) | 1860, 8210, 9210, (3M, St. Paul, MN) | STERIS V-PRO maX (STERIS Corporation, OH, USA), 28- min non-lumen cycle with 4 H2O2 pulses, (59% H202 in proprietary VAPROX) | Filter performance: filter performance not affected by single cycle, but demonstrated increased transmission (>1.5%) after third cycle. Fit performance: leakage maintained less than or equal to 0.49% up to 3 cycles consistently, and up to 10 cycles. |
| Fischer, Morris, van Doremalen, Sarchette, Matson, Bushmaker, Yinda, Seifert, Gamble, Williamson, Judson, Wit, Lloyd-Smith, Munster (2020) | Filtration Testing: 9211 (3M, St. Paul, MN). Microbial Inactivation: FFR Test Discs from N9504C (Aearo Company, Indianapolis, IN). | Panasonic 15 MCO-19AIC-PT, (PHC Corp. of North America Wood Dale, IL), 10- min cycle at 1,000 ppm H2O2 | Fit performance: No marked reduction of fit based on quantitative fit test after 1 cycle. Microbial inactivation: HCoV-19 nCoV-WA1-2020 strain of SARS-CoV-2. |
| Fu and Kumar (2012) | Non-FFR Sample Tested | Sterinis SR2 (Now GLOSAIR 400) (GLOSAIR, Advanced Sterilization Products, Irvine, CA, previously Sterinis), (30% H2O2) | Microbial Inactivation: vHP achieved a 6 log reduction of Geobacillus stearothermophilus biological indicators. |
| Jatta, Kiefer, Patolia, Pan, Harb, Marr, Baffoe-Bonnie (2021) | 8211 and 9210 (3M, St. Paul, MN) | STERIS V-PRO maX (STERIS Corporation, OH, USA), 28- min non-lumen cycle with 4 H2O2 pulses, (59% H202) | Filter performance: no filter degradation after 10 cycles. |
| Kahnert, Seiler, Stein, Aze, McDonnell and Kaufmann (2005) | Non-FFR Sample Tested | STERIS vHP 1001 (STERIS Corporation, OH, USA), 365-370 min cycle times with conditioning times from 15-20 min and dwell times from 60-105 min (35% H2O2) | Microbial Inactivation: ≥6 log reduction of M. tuberculosis. |
| Kenney, Chan, Kortright, Cintron, Havill, Russi, Epright, Lee, Balcezak, Martinello (2020) | 1870 (3M, St. Paul, MN) | BQ-50, (Bioquell, Horsham, PA, USA), 3.5 h cycle time with a 10- min conditioning phase, 30-40 min gassing phase, 25- min dwell time, and 150- min aeration, (35% H2O2) | Microbial inactivation: Phage phi-6, phage T7, and phage T1. Mask integrity: No visible changes to masks after 5 cycles. |
| Kumar, Kasloff, Leung, Cutts, Strong, Hills, Vazquez-Grande, Rush, Lother, Zarychanski, Krishnan (2020) | 1804, 1860, 1870, 8210 and 9210, (3M, St. Paul, MN), 1054S (Aearo Company, Indianapolis, IN). | STERIS ARD System (STERIS Corporation, OH, USA), 1-h cycle time with 10 min of dehumidification, 3 min of conditioning, 30- min dwell time, and 20- min aeration phase. (35% H2O2 in proprietary VAPROX) | Fit performance: FFRs passed fit test after 10 cycles. |
| Levine, Grady, Block, Hurley, Russo, Peixoto, Frees, Ruiz, Alland | 1860 and 1860s, (3M, St. Paul, MN), 46727 and 46827, (O&M Halyard Health, Alpharetta, GA) | STERIS Victory System (Steris Life Sciences, Mentor, OH, USA). A 3-h dwell time was used with a target concentration of 400 ppm H2O2, (35% H2O2) | Fit performance: passed quantitative fit after 8 cycles. |
| Oral, Wannomae, Connolly, Gardecki, Leung, Muratoglu (2020) | 1860 (3M, St. Paul, MN) | STERIS ARD 1000 (STERIS Corporation, OH, USA), 4.5- h cycle with 3 h of conditioning/dwell and 1.5 hours of off-gassing. (35% H202 in proprietary VAPROX) | Filter performance: not affected after 1 cycle. Fit performance: passed fit test up to 10 cycles. Safety: unable to detect H2O2 4.5 hours after vHP treatment. |
| Peltier, Wang, Hollenbeck, Lanza, Furtado, Cyr, Ellison, Kobayashi (2020) | 1860 and 1860s (3M, St. Paul, MN) | Bioquell Clarus C via Batelle's CCDS (Bioquell, Horsham, PA, USA) 8 h cycle time with a 10- min conditioning phase, 20- min gassing phase, 150- min dwell phase, and a 300-minute aeration phase, (35% H2O2), Unspecified Bioquell Model (Bioquell, Horsham, PA, USA) Variable Cycle Time, (35% H2O2), Unspecified Bioquell Model (Bioquell, Horsham, PA, USA) Variable Cycle Time, (35% H2O2) | Filter performance: maintained filtration ability after 10 cycles of vHP. |
| Pottage, Macken, Walker and Bennett (2012) | Non-FFR Sample Tested | STERRAD ARD 1000 (Steris Ltd, Basingstoke, UK), 750 ppm H2O2 (35% H202 in proprietary VAPROX) | Microbial Inactivation: There was a reduction of ~2.5 log in recoverable MRSA numbers within 20 min of vHP exposure and a ~3 log after 30 min while G. stearothermophilus biological indicators showed a 4 log reduction in 10 min and a 5 log reduction in 30 min. |
| Saini, Sikri, Batra, Kalra, Gautam (2002) | N95 Respirators, Unspecified Model/Manufacturer | SATEJ Plus, (Radiant Innovations, Ahmedabad, India), 10- min run time with 2-h dwell. (6%, 8%, and 10% concentrations of H202 were utilized) | Filter performance: not affected after 15 cycles. Microbial inactivation: B. stearothermophilus (complete inactivation), E. coli (complete inactivation), and M. smegmatis (>7 log reduction). |
| Salter, Kinney, Wallace, Lumley, Heimbuch and Wander (2010) | N95 Respirators, Unspecified Model/Manufacturer | STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA), 55-min cycle, (59% H2O2) | Safety: The residual H2O2 found on respirators after decontamination would pose no significant health hazard. |
| Schwartz, Stiegel, Greeson, Vogel, Thomann, Brown, Sempowski, Alderman, Condreay, Burch, Wolfe, Smith, Lewis (2020) | 1860 (3M, St. Paul, MN) | Bioquell Clarus C, (Bioquell, Horsham, PA, USA) 45- min cycle time with 25- min gas phase and 20- min dwell time, (35% H2O2) | Fit performance: FFRs passed fit test on human subjects after decontamination. Safety: H2O2 was undetectable after 4 h. |
| Smith J, Hanseler H, Welle J, Rattray R, et al. (2020) | 1860, 1870+, 8511 (3M, St. Paul, MN) | Bioquell Z (Andover, Hampshire, UK) 4-h 50- min cycle time with 20-minute gas phase, 60- min dwell time, and 2-h 10- min aeration (30% H2O2) | Fit Performance: Passed quantitative fit testing after 2 cycles of vHP. Microbial Inactivation: vHP achieved a 5 log reduction in SARS-CoV-2 deposited on N95 respirators. |
| Tuladhar, Terpstra, Koopmans and Duizer (2012) | Non-FFR Sample Tested | BONECO 7131 (BONECO North America Corp., Encino, CA) 1-h cycle with 126 ppm H2O2, (12% H2O2) | Microbial Inactivation: vHP decontamination resulted in complete inactivation of all viruses tested, characterized by >4 log reduction in infectious particles for poliovirus, rotavirus, adenovirus and murine norovirus and >2 log reduction for influenza A virus. |
| Weber and Boyce (2016) | Non-FFR Sample Tested | Review paper describing results of multiple vHP systems including Bioquell (Andover, Hampshire, UK) and Sterinis (Now GLOSAIR 400) (GLOSAIR, Advanced Sterilization Products, Irvine, CA) | Microbial Inactivation: MRSA, C. difficile, Vancomycin-resistant Enterococcus, Acinetobacter species, K. pneumoniae, Serratia, aerobic spore bearers, skin flora, coliforms, and a 6 log reduction in C. difficile. |
| Wigginton, Arts, Clack, Fitzsimmons, Gamba, Harrison, LeBar, Lauring, Li, Robers, Rockey, Torreblanca, Young, Anderegg, Cohn, Doyle, Meisenhelder, Raskin, Love, Kaye (2021) | 1860 (3M, St. Paul, MN) | Bioquell Q10 (Bioquell, Horsham, PA, USA) Parametric Cycle which determines settings based on room volume for set ppm (446 ppm H2O2 for gassing 1, 495 ppm for gassing 1, and 490 ppm for the 20- min dwell portion followed by 1-h and 8- min of aeration). A second cycle was used with 135 minutes of gassing (659 ppm H2O2 peak), 150- min dwell time (647 ppm peak), and 80 min of aeration), (35% H2O2) | Filter performance: Retained 99% filtration efficacy after 5 cycles. Microbial inactivation: Complete inactivation of spore indicators as well a >3.8 log inactivation of E. coli. Safety: no H2O2 off-gassing from the N95s after vHP treatment. |
Evaluation of CDC Decontamination Criteria 1: Filtration performance is not affected after each cycle of decontamination.
Hydrogen peroxide gas plasma
A study conducted by Viscusi et al. showed that a single HPGP decontamination cycle with either the STERRAD 100 NX or STERRAD 100S (Advanced Sterilization Products, Irvine, CA, USA) did not significantly affect the filter performance or air resistance of the FFRs, although the longer cycle time in the STERRAD 100S resulted in a non-statistically significant increase in filter penetration when compared with the STERRAD 100 NX.6 A subsequent study by Viscusi et al. using the STERRAD 100S found similar results, with the exception of some slight tarnishing of the nose pieces.7 However, Bergman et al. found that when N95 respirators were subjected to 3 cycles of decontamination using the STERRAD 100S, 25% had filter penetration of >5%, meaning they would no longer meet NIOSH requirements to provide respiratory protection.8
In unpublished data by Wiggington et al., HPGP resulted in decreased filtration efficiency after 3 cycles. This reduction in efficiency was substantially greater than all other decontamination methods trialed in this study, which included dry heat, Bioquell Q10 vHP (Bioquell, Horsham, PA, USA), ethylene oxide, and pulsed xenon UV, both in combination with various methods of heat and humidity as well as with vHP (Wiggington, unpublished Data). Peltier et al. also evaluated filter integrity post-decontamination with either HPGP or vHP and found that while a single short cycle of HPGP did not appear to damage respirator filters, longer or multiple cycles did cause substantial damage. The STERRAD 100S and STERRAD 100NX HPGP units were seen to degrade performance substantially and had the longest cycle times of 55 and 47 minutes respectively.10
An unpublished study by Chen et al. found similar results to Peltier et al., finding that HPGP (STERRAD 100S) treated FFRs were comparable to those treated by vHP (STERIS V-PRO maX, STERIS Corporation, OH, USA) for the first cycle. It should also be noted that after 3 cycles of decontamination, the HPGP treated FFRs experienced increased filter penetration (calibrated based on particle size and temperature, humidity and pressure controlled), whereas the vHP treated respirators were unaffected for 10 cycles (Chen, unpublished Data).
Aerosolized hydrogen peroxide
Saini et al. studied the efficacy of a 7%-8% hydrogen peroxide solution stabilized with silver nitrate using the SATEJ Plus (SATEJ Plus, Ahmedabad, India). After 15 repeated decontamination cycles, no physical tears, deformity, or change in droplet permeability were noted. Upon verification with a scanning electron microscope (SEM), no damage to or change in fiber thickness was observed. In addition, there was no noted change in user comfort.12 John et al. found that with the Altapure AP-4 aHP system with peracetic acid (Altapure, Mequon, WI), both hydrophobicity and filtration efficiency were retained following 5 cycles of decontamination.13
Vaporized hydrogen peroxide
Due to the higher concentrations of H2O2 used with vHP compared to aHP, it has been hypothesized that FFRs may experience greater damage during each decontamination cycle. A technical report produced by the Battelle Memorial Institute using the Bioquell Clarus C (Bioquell, Horsham, PA, USA) vHP system found that the filtration efficiency of 3M 1860 masks were not affected after 50 vHP cycles (Battelle Memorial Institute, Technical Report). Unpublished data by Oral et al. showed a single vHP decontamination cycle in an LTS‐V (STERIS, Mentor, OH, USA) did not affect the filter performance, while Wiggington et al. found that the N95 respirator retained at least 99% of its filtration efficiency after 5 cycles (Wiggington, Oral, unpublished Data). Peltier et al. and Jatta et al. found no filter degradation after 10 cycles of decontamination with the Steris V-PRO maX.10, 15 Bergman et al. found that N95s subjected to 3 cycles of VHP with a Bioquell Clarus R (Bioquell UK Ltd, Andover, UK) had similar filtration efficiencies to controls.8 However, technologies such as vHP cannot be used on all respirators, as respirators containing cellulose may absorb the hydrogen peroxide and impede proper decontamination.
Evaluation of CDC Decontamination Criteria 2: Fit performance of the respirator is not affected after each decontamination cycle.
Hydrogen peroxide gas plasma
Chen et al. assessed leakage via fit testing and found increased leakage around the nose after 5 cycles of HPGP treatment with the STERRAD 100S Long Cycle. They suspected that the generation of reactive oxygen species degraded the polyurethane nose foam across the 3M models tested (1860S, 8210 and 9210). However, this study also found filter failure prior to fit failure, indicating that fit would not be the limiting factor in reuse (Chen, unpublished Data). Kumar et al. noted similar damage after HPGP treatment with the STERRAD 100NX, finding the respirators failed fit testing after 2 cycles (Kumar, unpublished Data).
Aerosolized hydrogen peroxide
Unpublished data by Derr et al. found that the 3M 8511 N95 respirators could successfully pass both qualitative and quantitative fit testing (QLFT and QNFT, respectively) at first, fifth, and tenth rounds of aHP decontamination using the Pathogo Curis system (Curis Decontamination, Oviedo, FL, USA). During the same study, only a single respiratory facepiece failed fit testing due to a broken elastic strap. Additional respiratory models (3M 1860, 1870+, 9211+, and Honeywell N1125) successfully achieved QLFT and QNFT at first, fifth, and tenth rounds with fewer facemasks (Derr, unpublished Data). None of the other articles reviewed evaluated FFR fit performance after aHP decontamination.
Vaporized hydrogen peroxide
The Battelle Memorial Institute Report established that respirators treated with the Bioquell Clarus C vHP unit (Bioquell, Horsham, PA, USA) had similar fit performance to untreated (control) respirators for 20 cycles. Elastic strap degradation began after 30 cycles, resulting in loss of fit unless a substitute strap material was used (Battelle Memorial Institute, Technical Report). STERIS ARD vHP treated masks also passed fit test after 10 cycles of decontamination in a study by Kumar et al. (Kumar, unpublished Data). To expand upon this data, Schwartz et al. attempted to validate this post-decontamination fit in a more realistic setting using 2 human subjects with different facial structures instead of the mannequins used in the Battelle study. They demonstrated a successful seal post-decontamination with the Bioquell Clarus C (Bioquell, Horsham, PA, USA).18 Smith et al. found that N95 respirators decontaminated in the Bioquell Z Vaporizer (Bioquell UK Ltd., Andover, Hamphsire, United Kingdom) showed no significant changes in quantitative fit after 1 or 2 cycles while Fischer et al. found that N95s decontaminated with a Panasonic MCO-19AIC-PT (PHC Corp. of North America Wood Dale, IL) were able to maintain fit comparable to controls for 2 cycles and “acceptable” fit after 3 cycles (Fischer, unpublished Data).19 Other studies were able to achieve a fit after 1, 6-8 (variance was dependent on mask model), or 10 cycles of vHP (Oral, Fischer, unpublished Data).10 , 21 , 22 However, Lieu et al. attempted to verify respirator fit after decontamination and real world use. In their study, healthcare workers wore N95 respirators for 4consecutive hours while minimizing donning and doffing before N95s were decontaminated by vHP, using the STERIS V-PRO maX, Lieu found that the respirators failed post-decontamination fit test after a median of 2 cycles, or 4 cycles if mechanical failure of the mask was excluded. Additionally, they found a wide variation in the number of cycles before failure among different models of respirators.23
Evaluation of CDC Decontamination Criteria 3: The method can inactivate viruses and bacteria.
Hydrogen peroxide gas plasma
Ibañez-Cervantes et al. evaluated hydrogen peroxide plasma's ability to inactivate SARS-CoV-2, Acinetobacter baumannii, and Staphylococcus aureus via STERRAD 100NX sterilization system. An undiluted sample and four serial dilutions (1:10, 1:100, 1:1000, and 1:10000) of the SARS-CoV-2 virus, as well as 106 CFU/50 μL and dilutions of 102, 103, 104, and 105 CFU/50 μL of A. baumannii and S. aureus were tested. After treatment, SARS-CoV-2 was not detectable in any serial dilutions nor were A. baumannii and S. aureus culturable in the tested concentrations.24
Wiggington et al. (unpublished data) studied the efficacy of HPGP using the 24 minute express cycle of the STERRAD 100NX. influenza H3N2, Mouse Coronavirus Murine Hepatitis Virus (MHV), +ssRNA bacteriophage MS2, and dsRNA bacteriophage Phi6 were utilized as surrogates for SARS-CoV-2 due to their common use as viral indicators and similarities in genus or envelope structure. The STERRAD HPGP system showed strong viral inactivation with Phi6, influenza H3N2 and bacteriophage MS2 inactivation of >7.9 log, >3.8 log, and 5.6 log inactivation respectively. Scaling beyond this testing was not possible because of the loss of mask integrity after 3 treatments. (Wigginton, unpublished Data)
Aerosolized hydrogen peroxide
aHP has been shown to be an effective decontamination method against multiple bacteria and viruses. Kumar et al. demonstrated a >5 log reduction in Methicillin Resistant S. aureus (MRSA), Candida auris, Clostridium difficile spores, bacteriophage M2, Candida albicans, and G. stearothermophilus spores using the Altapure system.22 Cadnum et al. found a 6 log reduction in C. difficile spores and 2.1 log reduction in bacteriophage MS2 after a single cycle using the Altapure aHP system.25 Unpublished data by Derr et al. found that FFRs infected with RNA bacteriophage phi6, HSV-1 and Coxsackievirus B3 had no infectious virus remaining in 55 of 58 samples after a cycle of Curis aHP system (Derr, unpublished Data). Saini et al. found a complete inactivation of B. stearothermophilus and E. coli, as well as a 7 log reduction in M. smegmatis colony-forming units following a 10 minute aHP cycle with the Satej Plus Machine.12 John et al. found a 6 log reduction of G. stearothermophilus spores and bacteriophage MS2 using the Altapure system.13
However, some studies have also raised concerns that aHP may not be effective at eliminating some pathogens, particularly Mycobacterium tuberculosis. A systematic review conducted by Weber et al. discussed studies showing no significant decontamination of M. tuberculosis by the Sterinis (GLOSAIR, Advanced Sterilization Products, Irvine, CA, previously Sterinis) aHP system.26 In direct comparisons of vHP and aHP, Weber et al. described how in three tests using Sterinis aHP unit, only 10% of G. stearothermophilus biological indicators were inactivated in the first test and 79% in the subsequent two tests. The vHP tests using a Bioquell QC10 unit inactivated all of the indicators in each of the three tests.26 In a study conducted by Fu et al. that compared the Sterinis aHP system and Bioquell Clarus R (Bioquell, Horsham, PA, USA) vHP system, aHP inactivated <10% of pouched and <15% of unpouched 6 log G. stearothermophilus biological indicators and only one-third of 4 log G. stearothermophilus biological indicators. Additionally, only a 2-5 log reduction of MRSA and 1-4 log reduction of A. baumannii was obtained. C. difficile test discs were inactivated at most locations in the experiment, but spores were recoverable by broth culture at 82% of test locations. When simulated soiling was added, only a 1-3 log reduction was obtained for A. baumannii and MRSA. Fu et al. also noted that decontamination was more effective closer to the aHP unit, indicating uneven distribution of the hydrogen peroxide aerosol.27
Herruzo et al. utilized the GLOSAIR 400 (ASP, Madrid, Spain) system to study the efficacy of aHP against 20 different microorganisms. They found Gram-positive cocci most susceptible with an average of >4.3 log reduction excluding Methicillin Sensitive S. aureus (MSSA) which had a 3.42-3.88 log reduction. This was followed by Gram-negative bacilli with a 3.4 log reduction, yeasts at >3.2 log reduction, and lastly, antibiotic-susceptible Gram-negative bacilli with a 3 log reduction. Notably, there was less than a 3 log reduction in Klebsiella pneumoniae, Serratia marcescens, Proteus mirabilis and only a 1.17 log reduction in Enterobacter cloacae within 1 meter of the hydrogen peroxide generator and less than a 1 log reduction at distances greater than 1 meter from the unit.28
Vaporized hydrogen peroxide
A systematic review conducted by Weber et al. identified numerous other studies showing the Bioquell vHP unit's ability to disinfect hospital surfaces contaminated with MRSA, C. difficile, Vancomycin Resistant Enterococcus (VRE), Acinetobacter species, K. pneumoniae, Serratia, S. Aureus, aerobic spore bearers, skin flora, coliforms, and a 6 log reduction in C. difficile. 26 When Fu et al. tested vHP using the Bioquell Clarus R system, they found that it inactivated 90% of the 6 log and 95% of the 4 log G. stearothermophilus biological indicators. It obtained a >6 log reduction on 55% of A. baumannii, 82% of MRSA, and 100% of C. difficile test discs, with most of the recoverable bacteria requiring a broth culture, likely due to low levels of remaining contamination. When simulated soiling was added, a 4-6 log reduction was still achieved for MRSA and the rate of A. baumannii and C. difficile inactivation were not affected.27 Unpublished data from Wigginton et al. showed that the Bioquell Q10 (Bioquell, Horsham, PA, USA) unit could completely inactivate spore indicators as well obtain a >3.8 log inactivation of E. coli (ATCC 25922). However, it did not achieve the >3 log reductions of the influenza H3N2, Mouse Coronavirus Murine Hepatitis Vitis (MHV), +ssRNA bacteriophage MS2, and dsRNA bacteriophage Phi6, even with the EUA protocol was utilized.5
In regard to activity against SARS-CoV-2, unpublished data by Oral et al. found no detectable infectious virus on N95 respirators after STERIS ARD1000 vHP treatment (STERIS Corporation, OH, USA), corresponding to a >2.6 log reduction (Oral, unpublished Data). Kumar et al. also found a 5.2-6.3 log reduction with no detectable infectious virus after treatment with the STERIS ARD System as well as a >6 log reduction of Vesicular stomatitis virus, Indiana serotype. 6 A study by Kenney et al. showed the successful inactivation of proxies for SARS-CoV-2 such as bacteriophage T1, T7, and Pseudomonas phage phi-6 with the BQ-50 (Bioquell, Horsham, PA) (Kenney, unpublished Data). Fischer et al. achieved inactivation of the HCoV-19 nCoV-WA1-2020 strain of SARS-CoV-2, on N95 respirators using the Panasonic MCO-19AIC-PT vHP (PHC Corp. of North America Wood Dale, IL) systems respectively. (Fischer, unpublished Data). Smith et al. achieved a 5 log reduction in SARS-CoV-2 deposited on N95s using a Bioquell Z Vaporizer. While this did not represent a complete elimination of all viral RNA from the respirators, the authors note the inoculum dose utilized was likely much higher than a healthcare worker would encounter in a clinical environment.19 Kahnert et al. showed that a Steris VHP1001 vHP system (STERIS V-PRO, STERIS Corporation, OH, USA) was effective against M. tuberculosis, resulting in a >6 log reduction of colony forming units.30 In terms of other viral inactivity, Tuladhar et al. demonstrated that using the BONECO 7131 (BONECO North America Corp., Encino, CA) with 12% H2O2 to achieve a 126 ppm concentration of H2O2 achieved greater than 4 log reduction of viability of poliovirus, rotavirus, adenovirus, and murine norovirus 1. They also showed a greater than 2 log reduction of influenza A, the most they were able to show due to their low-titer sample.31
Discussion
Efficacy in decontamination: Pathogen reduction
Studies have demonstrated vHP to be more effective than aHP in viral and bacterial reduction. Fu et al. demonstrated that aHP only inactivated <10% of pouched and <15% of unpouched 6 log G. stearothermophilus biological indicators and only one-third of 4 log biological indicators, in contrast to the Bioquell Clarus R vHP system that inactivated 90% of the 6 log and 95% of the 4 log biological indicators. The STERIS V-PRO maX vHP system achieved better inactivation of test discs of MRSA, A. baumannii, and C. difficile under normal conditions as well as in simulated soil.23 Weber et al. discussed an additional study showing how vHP was more reliably able to inactivate G. stearothermophilus biological indicators than aHP.26
While the ability of vHP to inactivate bacteria and viruses has been well documented, Pottage et al. showed that MRSA is more resistant to vHP (Bioquell Clarus L, Bioquell, Horsham, PA, USA) than the commercially produced G. stearothermophilus biological indicators that are often used as a metric for decontamination. In their study using the Steris vHP system, the G. stearothermophilus indicators showed a 4 log reduction of spores within 10 minutes of vHP exposure and a 5 log reduction over 30 minutes. However, MRSA only sustained a 3 log reduction over the 30 minute treatment.32 Additionally, unpublished data by Wigginton et al. also found that vHP did not completely inactivate S. aureus, obtaining only a >1.6 log - >2.3 reduction log in their study, even though the G. stearothermophilus indicators and E. coli coupons were completely inactivated.5 Although Weber et al. summarized existing literature showing vHP's effectiveness against MRSA, additional studies may be needed to determine the best organisms to use as surrogates to confirm decontamination has occurred.26 vHP and HPGP have been shown to effectively decontaminate N95 masks of SARS-CoV-2 and viral surrogates such as P. phage Phi-6, which has not been shown for aerosolized hydrogen peroxide decontamination methods (Oral, unpublished Data).4 , 5 , 19 , 22 , 24 However, Wigginton noted that there is currently no FDA specified application medium for testing efficacy of viral decontamination and found that the deposition solution they used affected their results.5 The variability in results from different testing methodologies highlights the need for a testing procedure for biological inactivation that will enable comparison across technology types with biologically comparable pathogens applied and cultured in a standardized fashion. In addition to culture method, factors such as temperature, humidity, and inoculation size must be controlled, as Otter et al. found that variations resulted in “large standard deviations between cycles” in their study utilizing the Clarus R (Bioquell UK Ltd., Andover, Hamphsire, United Kingdom).33 Ultimately, it is important that the testing procedures be validated to ensure that they provide an accurate representation of the contamination FFRs will experience in the clinical environment.
Some studies have also shown that aHP is not capable of eliminating some pathogens, including M. tuberculosis, whereas vHP has been shown to achieve a >6 log reduction.26 , 30 No studies investigating HPGP's ability to inactivate M. tuberculosis were found during by our review. Studies have also shown that aHP's efficacy can vary with distance from the unit, something not seen with vHP as it saturates the treatment areas.27 , 28
Integrity of FFRs
N95 respirators have been shown to retain fit after 20 rounds of vHP, while fit testing after aHP decontamination has only been studied for up to 10 rounds of decontamination at the time this review was conducted (Battelle Memorial Institute, Technical Report; Derr, unpublished Data) Additionally, the N95 manufacturer 3M issued a technical bulletin detailing testing that they have performed on 3M branded masks with specific vHP generators, showing that the respirator filtration efficiency and fit were not affected after 10-20 cycles depending on the decontamination system utilized (3M, Technical Report).
However, findings by Lieu et al. raise concerns about respirator fit in the clinical environment compared to the experimental settings in which most tests have been conducted. They found that post-vHP decontamination respirators failed fit testing after a median of 2 cycles, or 4 cycles if mechanical failure of the respirator was excluded. Additionally, they found that a seal check conducted by the wearer prior to fit testing failed to predict fit test failure in 77.8% failures, indicating that healthcare workers likely would not be able to determine when their respirator no longer provided a sufficient seal unless they underwent repeat fit testing.23
This data is in line with previous data showing that multiple respirator donnings can result in fit loss even without decontamination. Degesys et al. found that for dome-shaped masks, fit test failure was associated with a median of 4 shifts (2 shifts for duck-billed), 15 donnings (8 donnings for duck-billed), and 14 hours of wear time (12 hours for duck-billed), suggesting that the strap degradation may not be the limiting factor for reuse as indicated in Battelle Memorial Institute's report (Battelle Memorial Institute, Technical Report).34 The variation in number of donnings and wear time between models before fit failure was also noted by Lieu et al., who suggested that the across the board recommendations for respirator reuse may not sufficiently account for differences between models and brands.23 Levine et al. also found differences between different N95 models, both in the number of times they would pass fit testing post-vHP decontamination with the STERIS Victory (STERIS Corporation, OH, USA), as well as their ability to pass fit test post-decontamination when tested by a different wearer, a scenario which would likely occur if respirators are collected and redistributed to other healthcare workers under Tier 1 usage.21 Recognizing these concerns, the FDA reissued a number of its EUAs, specifying that respirators may only be decontaminated for reuse a maximum of 4 times.35
With respect to HPGP, although much of the data available is not peer reviewed, multiple studies have shown that HPGP may degrade the integrity of the respirators, damaging both the filter and impairing the fit. These studies directly compared HPGP to vHP and showed that vHP maintained its filtration efficiency and fit significantly longer than the HPGP, (Kumar, Chen, unpublished Data).6 As such, aHP and vHP are likely much more viable FFR decontamination options than HPGP.
Safety
aHP has been shown to be safe in domains of residual H2O2 on the surface of the respirator.22 It also offers the advantage of using a lower level of H2O2 than comparable methods, such as vHP systems, most of which concentrations of 30%to59% H2O2. Using the Altapure AP-4 aHP with peracetic acid, John et al. found that H2O2 had dissipated to 0.0 ppm within 60 minutes of off-gassing and the peracetic acid was undetectable within 20 minutes.13 Unpublished data by Derr et al. found that H2O2 was less than <0.5 ppm, within permitted levels, after treatment. However, many aHP solutions contain silver cations or peracetic acid, requiring additional studies to determine if any toxic residues or off-gassing post-decontamination could cause harm to the wearer (Derr, unpublished Data).
In addition to additives, hydrogen peroxide can be hazardous and has a short-term exposure limit (STEL) for hydrogen peroxide of 2 ppm as a 15-minutes time weighted average. One of the purported advantages of aHP is that the lower concentration of H2O2 reduces safety concerns. However, Fu et al. showed that system design considerations such as inadequate door sealing resulted in increased hydrogen peroxide exposure potential.27
Another potential concern about vHP is that the higher concentrations of H2O2 could worsen potential off-gassing from the treated respirators and create a hazard to the wearer. However, data does not appear to support these concerns as Schwartz et al. measured the H2O2 level in the room with off-gassing respirators, finding H2O2 was undetectable after 4 hours.18 Oral et al. also was unable to detect H2O2 4.5 hours after vHP treatment and Wigginton et al. found no H2O2 off-gassing from the N95s after vHP treatment. (Oral, Wigginton, unpublished Data) 5 Salter et al. found that even after 18 hours, the treated respirators retained some H2O2. However, because it is so slow to evaporate, they concluded that the residual amount was unlikely to pose a significant health hazard.36
Conclusion and Additional Research Recommendations
Overall, vHP has the most evidence supporting its efficacy for use in FFR decontamination. It has been shown to be more effective at inactivating bacteria and viruses than aHP, and it does not degrade the filtration efficiency of the respirators as HPGP does. Compared to aHP, FFR filter performance and fit have been validated at more decontamination cycles, indicating the need for further aHP studies for adequate comparison. However, studies have raised significant concerns regarding the ability of respirators to maintain fit in the clinical setting, regardless of decontamination technique, potentially limiting the number of cycles respirators can be reused.
Additionally, lack of consistency in testing of efficacy of microbial inactivation across the technologies makes both comparison between, and real-world validation of function difficult, indicating the need for standardization of testing and validation protocols. As many of these technologies will be utilized to decontaminate equipment in unique spaces such as a closet or hospital room rather than a standardized compartment that would be attached to the machine, the applicability of testing to real application must be validated. This includes testing in or controlling the specific operating conditions, such as compartment material, humidity, temperature, and load volume, in addition to the types of respirators at varying soil levels that the system will eventually decontaminate for reuse.
Furthermore, variables such as cycle time, startup and operational costs, and training are important considerations for any FFR decontamination system, but these are beyond the scope of this review. Importantly, to validate safe reuse, public health surveillance to track COVID-19 transmission rates in healthcare workers using FFRs that have been decontaminated should be conducted.
Footnotes
Conflicts of interest: None to report.
References
- 1.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . News Release; 2020. Shortage of Personal Protective Equipment Endangering Health Workers Worldwide.https://www.who.int/news/item/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide Available at: Accessed April 1, 2020. [Google Scholar]
- 3.Center for Disease Control and PreventionCDC Webpage; 2020. Coronavirus Disease 2019 (COVID-19) Decontamination and Reuse.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: Accessed November 1, 2020. [Google Scholar]
- 4.Food and Drug Administration . FDA Webpage; 2020. Decontamination System EUAs for Personal Protective Equipment.https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/decontamination-system-euas-personal-protective-equipment Available at: Accessed May 5, 2021. [Google Scholar]
- 5.Food and Drug Administration . FDA Guidance; May 2020. Recommendations for Sponsors Requesting EUAs for Decontamination and Bioburden Reduction Systems for Surgical Masks and Respirators During the Coronavirus Disease 2019 (COVID19) Public Health Emergency Guidance for Industry and Food and Drug Administration Staff.https://www.fda.gov/media/138362/download Available at: Accessed May 5, 2021. [Google Scholar]
- 6.Viscusi DJ, King WP, Shaffer RE. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J Int Soc Respir Prot. 2007 https://www.isrp.com/the-isrp-journal/journal-public-abstracts Available at: Accessed July 30, 2021. [Google Scholar]
- 7.Viscusi DJ, Bergman MS, Eimer BC, Shaffer RE. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bergman MS, Viscusi DJ, Heimbuch BK, Wander JD, Sambol AR. Evaluation of multiple (3-Cycle) decontamination processing for filtering facepiece respirators. J Eng Fiber Fabr. 2010;5:33–41. [Google Scholar]
- 9.Wigginton KR, Arts PJ, Clack H, et al. Validation of N95 filtering facepiece respirator decontamination methods available at a large university hospital. medRxiv. 2020 doi: 10.1093/ofid/ofaa610. Unpublished Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltier RE, Wang J, Hollenbeck BL, et al. Addressing decontaminated respirators: some methods appear to damage mask integrity and protective function. Infect Control Hosp Epidemiol. 2020;41:1446–1448. doi: 10.1017/ice.2020.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen PZ, Ngan A, Manson N, et al. Transmission of aerosols through pristine and reprocessed N95 respirators. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.05.14.20094821v1 Available at: Accessed August 24, 2021. [Google Scholar]
- 12.Saini V, Sikri K, Batra SD, Kalra P, Gautam K. Development of a highly effective low-cost vaporized hydrogen peroxide-based method for disinfection of personal protective equipment for their selective reuse during pandemics. Gut Pathog. 2020;12:29. doi: 10.1186/s13099-020-00367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John AR, Raju S, Cadnum JL, et al. Scalable in-hospital decontamination of N95 filtering face-piece respirator with a peracetic acid room disinfection system. Infect Control Hosp Epidemiol. 2021;2:678–687. doi: 10.1017/ice.2020.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oral E, Wannomae KK, Connolly RL, Gardecki JA, Leung HM. Vapor H2O2 sterilization as a decontamination method for the reuse of N95 respirators in the COVID-19 emergency. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.04.11.20062026v2 Available at: Accessed August 24, 2021. [Google Scholar]
- 15.Jatta M, Kiefer C, Patolia H, et al. N95 reprocessing by low temperature sterilization with 59% vaporized hydrogen peroxide during the 2020 COVID-19 pandemic. Am J Infect Control. 2021;49:8–14. doi: 10.1016/j.ajic.2020.06.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar A, Kasloff SB, Leung A, et al. N95 Mask decontamination using standard hospital sterilization technologies. medRxiv. 2020 https://www.medrxiv.org/content/10.1101/2020.04.05.20049346v2 Available at: Accessed August 24, 2021. [Google Scholar]
- 17.Derr TH, James MA, Kuny CV., Kandel P, Beckman MD. Aerosolized hydrogen peroxide decontamination of N95 respirators, with fit-testing and virologic confirmation of suitability for re-use during the covid-19 pandemic. medRxiv. 2020 doi: 10.1128/msphere.00303-22. Unpublished Results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz A, Stiegel M, Greeson N, et al. Decontamination and reuse of N95 Respirators with hydrogen peroxide vapor to address worldwide personal protective equipment shortages during the SARS-CoV-2 (COVID-19) pandemic. Appl Biosaf. 2020 doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JS, Hanseler H, Welle J, et al. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. J Clin Transl Sci. 2020;5:e10. doi: 10.1017/cts.2020.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer R, Morris DH, van Doremalen N, Sarchette S, Matson J. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv. 2020 doi: 10.3201/eid2609.201524. https://www.medrxiv.org/content/10.1101/2020.04.11.20062018v2 Available at: Accessed August 24, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levine C, Grady C, Block T, et al. Use, reuse or discard: quantitatively defined variance in the functional integrity of N95 respirators following vaporized hydrogen peroxide decontamination during the COVID-19 pandemic. J Hosp Infect. 2021;107:50–56. doi: 10.1016/j.jhin.2020.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar JA, Cadnum JL, Jencson AL, Donskey CJ. Efficacy of a multi-purpose high level disinfection cabinet against Candida auris and other health care-associated pathogen. Am J Infect Control. 2020;48:849–850. doi: 10.1016/j.ajic.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 23.Lieu A, Mah J, Zanichelli V, Exantus RC, Longtin Y. Impact of extended use and decontamination with vaporized hydrogen peroxide on N95 respirator fit. Am J Infect Control. 2020;48:1457–1461. doi: 10.1016/j.ajic.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibáñez-Cervantes G, Bravata-Alcántara JC, Nájera-Cortés AS, et al. Disinfection of N95 masks artificially contaminated with SARS-CoV-2 and ESKAPE bacteria using hydrogen peroxide plasma: Impact on the reutilization of disposable devices. Am J Infect Control. 2020;48:1037–1041. doi: 10.1016/j.ajic.2020.06.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cadnum JL, Li DF, Redmond SN, John AR, Pearlmutter B, Donskey CJ. Effectiveness of ultraviolet-c light and a high-level disinfection cabinet for decontamination of n95 respirators. Pathog Immun. 2020;5:52–67. doi: 10.20411/pai.v5i1.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weber DJ, Rutala WA, Anderson DJ, Chen LF, Sickbert-Bennett EE, Boyce JM. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: focus on clinical trials. Am J Infect Control. 2016;44(5 Suppl):e77–e84. doi: 10.1016/j.ajic.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fu TY, Gent P, Kumar V. Efficacy, efficiency and safety aspects of hydrogen peroxide vapour and aerosolized hydrogen peroxide room disinfection systems. J Hosp Infect. 2012;80:199–205. doi: 10.1016/j.jhin.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 28.Herruzo R, Vizcaíno MJ, Herruzo I. Quantifying GlosairTM 400 efficacy for surface disinfection of American Type Culture Collection strains and micro-organisms recently isolated from intensive care unit patients. J Hosp Infect. 2014;87:175–178. doi: 10.1016/j.jhin.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Kenney P, Chan BK, Kortright K, et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. Infect Control Hosp Epidemiol. 2021:1–3. doi: 10.1017/ice.2021.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahnert A, Seiler P, Stein M, Aze B, McDonnell G, Kaufmann SH. Decontamination with vaporized hydrogen peroxide is effective against Mycobacterium tuberculosis. Lett Appl Microbiol. 2005;40:448–452. doi: 10.1111/j.1472-765X.2005.01683.x. [DOI] [PubMed] [Google Scholar]
- 31.Tuladhar E, Terpstra P, Koopmans M, Duizer E. Virucidal efficacy of hydrogen peroxide vapour disinfection. J Hosp Infect. 2012;80:110–115. doi: 10.1016/j.jhin.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 32.Pottage T, Macken S, Walker JT, Bennett AM. Methicillin-resistant Staphylococcus aureus is more resistant to vaporized hydrogen peroxide than commercial Geobacillus stearothermophilus biological indicators. J Hosp Infect. 2012;80:41–45. doi: 10.1016/j.jhin.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Otter JA, French GL. Survival of nosocomial bacteria and spores on surfaces and inactivation by hydrogen peroxide vapor. J Clin Microbiol. 2009;47:205–207. doi: 10.1128/JCM.02004-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Degesys NF, Wang RC, Kwan E, Fahimi J, Noble JA, MC Raven. Correlation between N95 extended use and reuse and fit failure in an emergency department. JAMA - J Am Med Assoc. 2020;324:94–96. doi: 10.1001/jama.2020.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Food and Drug Administration . FDA News Release; 2021. Coronavirus (COVID-19) Update: January 22, 2021.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-january-22-2021 Available at: Accessed February 2, 2021. [Google Scholar]
- 36.Salter WB, Kinney K, Wallace WH, Lumley AE, Heimbuch BK, Wander JD. Analysis of residual chemicals on filtering facepiece respirators after decontamination. J Occup Environ Hyg. 2010;7:437–445. doi: 10.1080/15459624.2010.484794. [DOI] [PMC free article] [PubMed] [Google Scholar]