Abstract
Background and aims
Statin therapy is administered to patients with high cardiovascular risk. These patients are also at risk for severe course of coronavirus disease 2019 (COVID-19). Statins exhibit not only cardioprotective but also immunomodulatory and anti-inflammatory effects. This study performed a systematic review of published evidence regarding statin treatment and COVID-19 related mortality.
Methods
A systematic PubMed/Embase search was performed from February 10, 2020 until March 05, 2021 for studies in COVID-19 patients that reported adjusted hazard or odds ratio for death in statin users versus non-users.
Results
22 studies fulfilled the inclusion criteria and were included in the systematic review. Meta-analysis of 10 studies (n = 41,807, weighted age 56 ± 8 years, men 51%, hypertension 34%, diabetes 21%, statin users 14%) that reported adjusted hazard ratios for mortality in statin users versus non-users showed pooled estimate at 0.65 (95% confidence intervals [CI] 0.53, 0.81). Meta-analysis of 6 studies that reported continuation of statin therapy during hospitalization (58–100% of patients) revealed a pooled hazard ratio of 0.54 (95% CI 0.47, 0.62). Meta-analysis of 12 studies (n = 72,881, weighted age 65 ± 2 years, men 54%, hypertension 66%, diabetes 43%, statin users 30%) that reported adjusted odds ratios for mortality showed pooled estimate at 0.65 (95% CI 0.55, 0.78). Multivariable meta-regression analysis did not reveal any significant association of hazard or odds ratios with anthropometric characteristics or comorbidities.
Conclusions
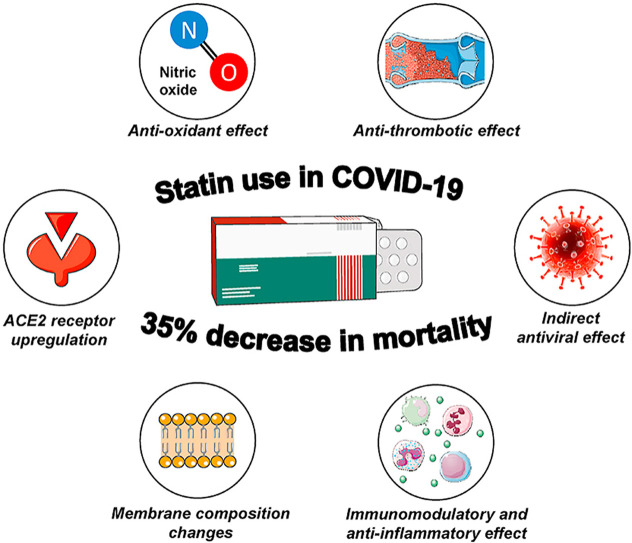
This meta-analysis of retrospective observational studies showed that statin therapy was associated with an about 35% decrease in the adjusted risk of mortality in hospitalized COVID-19 patients.
Keywords: Statins, SARS-CoV-2, Death, COVID-19, Mortality, Meta-analysis
Graphical abstract
1. Introduction
Statins are the most frequently prescribed hypolipidemic agents [1]. Their wide use is strongly supported by their evidence-based cardioprotective effect whereas severe adverse events are extremely rare [1]. Additional beneficial (pleiotropic) effects attributed to their anti-inflammatory and antioxidant properties render these drugs an irreplaceable choice when it comes to lipid lowering and cardioprotection treatment [2].
In the era of coronavirus disease 2019 (COVID-19), the impact and safety of established background therapies of COVID-19 patients have been questioned. It should be noted that statin-treated individuals are more likely to be older and to have cardiovascular risk factors or disease, and thereby more likely to have severe COVID-19 [3]. Statins seem to up-regulate angiotensin-converting enzyme 2, which could possibly enhance COVID-19 transmissibility [4]; on the other hand, these drugs present anti-oxidative, anti-inflammatory, anti-arrhythmic, anti-thrombotic properties and have beneficial effects on endothelial dysfunction, which could be proved protective against fatal respiratory, cardiovascular and thromboembolic complications in COVID-19 [5,6].
Accumulating evidence as presented in the available relevant meta-analyses seems to support a beneficial or at least a neutral effect of statins in COVID-19 patients in terms of mortality [[7], [8], [9], [10], [11], [12], [13]]. However, there is significant methodological heterogeneity (analysis of unadjusted and adjusted risks, simultaneous use of odds and hazard ratios, inclusion of a limited number of studies or non-peer-reviewed studies) which might have accounted for the heterogeneity in the reported outcome [[7], [8], [9], [10], [11], [12], [13]].
The aim of the present study was to perform an updated systematic review of the current literature in order to investigate the impact of statin treatment on the mortality of COVID-19 patients by using strict methodological criteria.
2. Materials and methods
2.1. Search strategy
A systematic review and meta-analysis was performed according to Meta-analysis of Observational Studies in Epidemiology (MOOSE) Guidelines [14]. A systematic search of PubMed and EMBASE databases was performed to identify eligible articles from February 10, 2020 until March 05, 2021 using the following term strategy: (“coronavirus 2019” OR “2019-nCoV” OR “SARS-CoV-2” OR “COVID-19” OR COVID OR COVID19) AND (statin* OR atorvastatin OR rosuvastatin OR simvastatin OR pitavastatin OR pravastatin OR fluvastatin OR lovastatin) AND (mortality OR death OR fatal). Articles were also identified from reference lists of relevant papers and hand search. The study selection was performed independently by 2 investigators (K.G.K and I.G.K). Disagreements were resolved by consensus with a senior author (A.K.).
2.2. Selection criteria
The primary study outcome included the adjusted risk of death in statin users versus non-users with a COVID-19 diagnosis. Eligible studies were full-text peer-reviewed articles in English that: (1) had prospective or retrospective design, (2) included only COVID-19 patients, (3) reported the use of statins among patients at least at baseline, (4) presented the hazard ratio or odds ratio for death in statin users versus non-users which should be adjusted for anthropometric characteristics/comorbidities or calculated in matched groups for such variables. In the case of more than one study from the same dataset, only the most relevant (reporting on the outcome of interest) or the largest one was included.
2.3. Data extraction and risk of bias assessment
Authors of the included studies were contacted by email to obtain additional details not reported in the published paper regarding the continuation of statin use during hospitalization. Three investigators (A.K, K.G.K and I.G.K) extracted independently data concerning study design, main characteristics of included populations and data regarding primary endpoint from included studies where available. The risk of bias was assessed using a combined checklist from Joanna Briggs Institute Critical Appraisal Checklists for Analytical Cross Sectional Studies and for Cohort Studies [15].
2.4. Statistical analysis
Meta-analysis regression was performed using the Stata/SE 11 (Texas) software. Natural logarithms of adjusted hazard ratios or odds ratios and corresponding standard errors were used for the analysis (random-effects meta-analysis). Sensitivity and stratified analyses were performed in order to compensate for the observed methodological heterogeneity among the included studies: (i) analysis of studies reporting in-hospital use of statins, (ii) analysis by excluding one study each time (influence analysis), and (iii) analyses by selecting studies on the criteria of mean age and percentage of males, as well as excluding those performed exclusively in diabetic or intensive care unit patients. Multivariable meta-regression analysis was performed for assessing associations between the natural logarithms of adjusted hazard or odds ratios for death with gender, age, prevalence of diabetes, hypertension, coronary heart disease, and chronic obstructive pulmonary disease or lung disease in general across studies. Mean values of variables for subgroups were combined where feasible in order to get the mean value for the total sample [16]. Median values were converted to mean values [17]. Heterogeneity was tested using I2 statistics. Publication bias was assessed by inspecting funnel plots for primary outcomes in which the ln(hazard ratio) and ln(odds ratio) were plotted against SE, as well as Egger's test (linear regression method) and Begg's test (rank correlation method) [18,19]. Two-sided p values of <0.05 were considered significant.
3. Results
3.1. Included studies
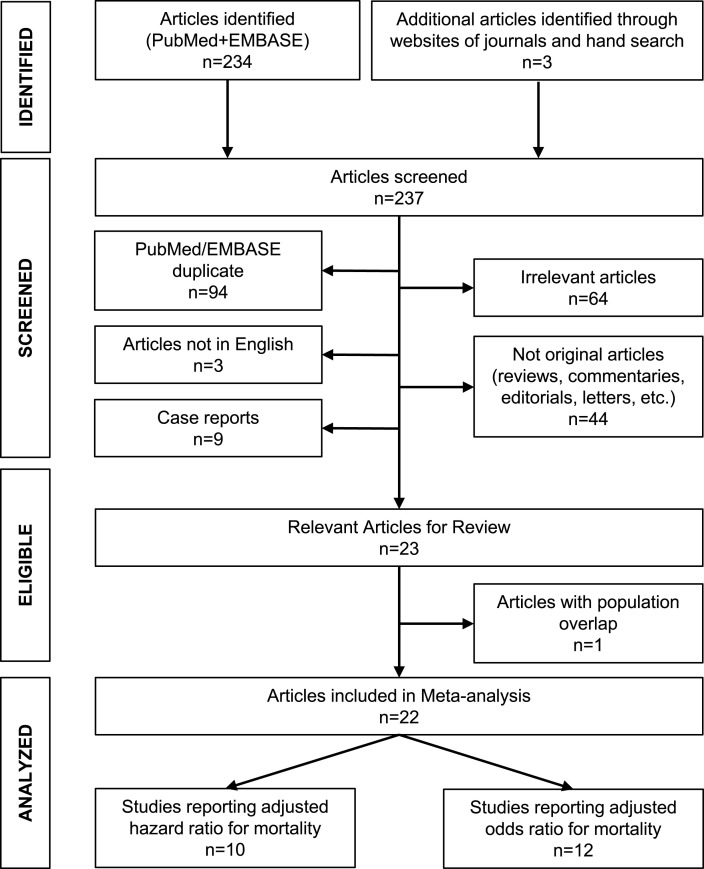
The MOOSE Statement - Reporting Checklist for the present meta-analysis of observational studies is presented in Supplementary Appendix A. Among 237 initially identified articles, 22 studies fulfilled the inclusion criteria and were included in the systematic review (flowchart in Fig. 1 ) [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. The main characteristics of these studies are shown in Table 1 . All the studies were of retrospective observational design and all reported the risk of death associated with statin use adjusted for several confounding factors. Regarding the latter, the most commonly reported included: (i) Demographics: age, gender, body mass index; (ii) Comorbidities: hypertension, diabetes, coronary heart disease; (iii) Medications: angiotensin converting enzyme inhibitors or angiotensin II receptor blockers, antiplatelets, anticoagulants; (iv) Biochemical indices: lipids, creatinine levels. Some of the studies reported the duration of the follow-up, which ranged from a few days to several weeks [20,21,[23], [24], [25], [26],28,32,33,41]. In addition, 2 studies reported that the median length of hospitalization of the non-survivors was about 10 days [27,31], and 1 study reported that the majority of deaths occurred within the first 2 weeks of follow-up [20].
Fig. 1.
Flowchart for study selection.
Table 1.
Main characteristics and findings of included studies.
| Study | N | Country, Setting | Age (mean ± SD) | Males (%) | HTN (%) | DM (%) | CHD (%) | COPD or lung disease (%) | Statin use (%) | Type of statin used (%) | Continuation of statins during COVID-19 (%) | HR/OR adjustment factors | Total sample mortality (%) | LDL-c (mean ± SD, mg/dl) in statin users/non-users | Adjusted HR/OR for mortality (95% CI) (statin users vs non-users) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies reporting adjusted HR | |||||||||||||||
| Lee et al. [20] | 10,448 | S. Korea, Hospitalized | 45 ± 20 | 40 | 21 | 18 | 6 | 14 | 5 | A, R, S, P, other | NR | Demographics Comorbidities |
2 | NR | 0.64 (0.43, 0.95) |
| Peymani et al. [21] | 150 | Iran, Hospitalized | 62 ± 15 | 58 | 29 | 21 | NR | 13 | 50 | A (94), R (3) S (3) |
100 | NR | 13 | NR | 0.92 (0.21, 4.16) |
| Fan et al. [22] | 2,147 | China, Hospitalized | 59 ± 15 | 48 | 33 | 14 | 8 | 2 | 12 | A (65), R (30), other (5) | 100 | Demographics Comorbidities Medications Biochemical indices |
4 | 101 ± 37/98 ± 27 | 0.43 (0.17, 0.91) |
| Butt et al. [23] | 4,842 | Denmark, Emergency department/Hospitalized/Outpatients |
55 ± 24 | 47 | 20 | 9 | 9 | 5 | 17 | A (50), R (7), S (42), P (1) | NR (generally continued) | Demographics Comorbidities Medications |
10 | NR | 0.96 (0.78, 1.18) |
| Masana et al. [24] | 1,162 | Spain, Hospitalized | 73 ± 13 | 60 | 78 | 44 | 25 | 21 | 50 | A, R, other | 58 | Matched population | 23 | 98 ± 26/120 ± 31 | 0.60 (0.39, 0.92) |
| Saeed et al. [25] | 2,266 (2,039 in the final analysis) | USA, Hospitalized, DM type 2 | 68 ± 13 | 52 | 87 | 100 | 36 | 34 | 43 | A (76), R (1), S (18), P (5) | 100 | Demographics Comorbidities Medications Biochemical indices |
32 | NR | 0.51 (0.43, 0.61) |
| Grasselli et al. [26] | 3,988 | Italy, ICU | 63 ± 10 | 80 | 41 | 13 | 13 | 2 | 12 | NR | NR | Demographics Comorbidities Medications |
48 | NR | 0.98 (0.81, 1.20) |
| Rodriguez-Nava et al. [27] | 87 | USA, ICU | 67 ± 13 | 64 | NR | NR | NR | NR | 54 | A (100) | 100 | Demographics Comorbidities Medications |
55 | NR | 0.38 (0.18, 0.77) |
| Zhang et al. [28] | 13,981 | China, Hospitalized | 57 ± 16 | 49 | 35 | 16 | 8 | 1 | 9 | A (83), R (15), other (2) | 100 | Demographics Comorbidities Medications Biochemical indices |
7 | 90 ± 37/94 ± 32 | 0.63 (0.48, 0.84) |
| Lala et al. [29] | 2,736 | USA, Hospitalized | 66 ± 16 | 60 | 39 | 26 | 17 | 6 | 36 | NR | NR | Demographics Comorbidities Medications |
18 | NR | 0.57 (0.47, 0.69) |
| Studies reporting adjusted OR | |||||||||||||||
| Chacko et al. [30] | 255 | USA, Hospitalized | 65 ± 15 | 51 | 73 | 48 | 18 | 13 | 45 | NR | NR | Demographics Comorbidities Medications Biochemical indices |
20 | NR | 0.14 (0.03, 0.61) |
| Nicholson et al. [31] | 1,042 | USA, Hospitalized | 64 ± 16 | 57 | 56 | 43 | 17 | 12 | 49 | NR | NR | NR | 20 | NR | 0.47 (0.24, 0.92) |
| Gupta et al. [32] | 2,626 | USA, Hospitalized | 65 ± 18 | 57 | 54 | 37 | 13 | 18 | 36 | NR | 77 | Demographics Comorbidities Medications |
NR | 82 ± 35/91 ± 37 | 0.49 (0.38, 0.63) |
| Wargny et al. [33] | 2,796 | France, Hospitalized, DM type 2 | 70 ± 13 | 64 | 76 | 100 | NR | 10 | 46 | NR | NR | Demographics Comorbidities Medications Biochemical indices |
21 | NR | 1.42 (1.00, 2.02) |
| Oh et al. [34] | 7,780 | S. Korea, Hospitalized | NR | NR | NR | NR | NR | NR | 17 | NR | NR | NR | 3 | NR | 0.74 (0.52, 1.05) |
| Mitacchione et al. [35] | 290 | Italy, Hospitalized | 71 ± 13 | 68 | 71 | 33 | 27 | 9 | 50 | A (49), R (16), S (30), other (5) | NR (generally continued) | Matched population | 27 | NR | 0.90 (0.54, 1.51) |
| Rosenthal et al. [36] | 35,302 | USA, Hospitalized | 64 ± 18 | 53 | 66 | 40.5 | 9.4 | 21 | 40 | NR | NR | NR (known confounders) | 20 | NR | 0.60 (0.56, 0.65) |
| Bifulco et al. [37] | 541 | Italy, Hospitalized | 65 ± 14 | 63 | 51 | 24 | NR | 13 | 22 | NR | NR | Demographics Comorbidities Biochemical indices |
23 | 84 ± 40/105 ± 38 | 0.75 (0.26, 2.17) |
| Mallow et al. [38] | 21,676 | USA, Hospitalized | 65 ± 17 | 53 | 68 | 42 | 8 | 21 | 25 | NR | 100 | Demographics Comorbidities Medications Biochemical indices |
23 | NR | 0.54 (0.49, 0.60) |
| Song et al. [39] | 249 | USA, Hospitalized | 63 ± 17 | 57 | 49 | 33 | NR | 16 | 49 | NR | NR (generally continued) | Demographics Comorbidities |
17 | NR | 0.88 (0.37, 2.08) |
| Daniels et al. [40] | 170 | USA, Hospitalized | 59 ± 20 | 58 | 44 | 20 | NR | 4 | 27 | NR | NR | Demographics Comorbidities |
13 | NR | 0.45 (0.11, 1.87) |
| De Spiegeleer et al. [41] | 154 | Belgium, Nursing home residents |
86 ± 7 | 33 | 25 | 18 | NR | NR | 20 | A, R, S, P, other | NR (generally continued) | Demographics Comorbidities |
NR | NR | 0.51 (0.14, 1.35) |
A, atorvastatin; CI, confidence intervals; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; LDL-c, low-density lipoprotein cholesterol; DM, diabetes mellitus; HR, hazard ratio; HTN, arterial hypertension; ICU, intensive care unit; NR, not reported; OR, odds ratio; P, pitavastatin; R, rosuvastatin; S, simvastatin.
3.2. Studies reporting hazard ratios
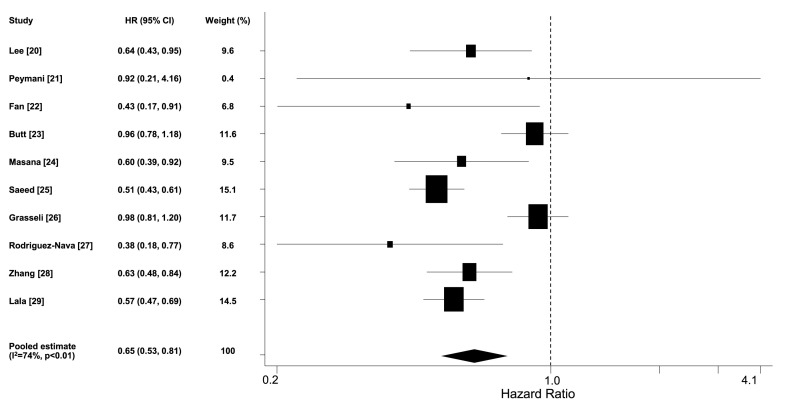
Ten studies (n = 41,807, weighted age 56 ± 8 years, men 51%, hypertension 34%, diabetes 21%, coronary heart disease 11%, statin users 14%) reported the adjusted hazard ratio for death in statin users versus non-users [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29]]. The pooled estimate of hazard ratio was 0.65 (95% confidence intervals [CI] 0.53, 0.81) (Fig. 2 ). By removing one study which included both outpatients and hospitalized patients [23], the pooled estimate of hazard ratio from studies that included only hospitalized patients was 0.62 (95% CI 0.50, 0.76). Six studies (n = 19,793) reported the continuation of statin use during hospitalization (5 of them in 100% of patients under such treatment [21,22,25,27,28] and 1 in 58% [24]), whereas this information was not available for the rest studies. Meta-analysis of these 6 studies revealed a pooled hazard ratio of 0.54 (95% CI 0.47, 0.62). The results were the same when the analysis included the 5 studies reporting continuation of statin use in 100% of patients (pooled hazard ratio 0.54 [95% CI 0.46, 0.62]) [21,22,25,27,28]. Sensitivity analysis by excluding one study each time confirmed that the pooled estimate was consistent among studies with balanced weight (Fig. 1A, Supplementary Appendix B). Additional sensitivity and stratified analyses are presented in Table 1, Supplementary Appendix B. No publication bias was identified (p = 0.93 and 0.62 for Begg's and Egger's test respectively; Begg's funnel plot is presented in Fig. 2A, Supplementary Appendix B).
Fig. 2.
Forest plot of adjusted hazard ratios for death in statin users versus non-users among COVID-19 patients.
Multivariable meta-regression analysis did not reveal any significant association of hazard ratios for death with gender, age, prevalence of diabetes, hypertension, coronary heart disease and chronic obstructive pulmonary disease or lung disease across the included studies (all p = NS). All but one studies were assigned high quality scores (Table 2, Supplementary Appendix B).
3.3. Studies reporting odds ratios
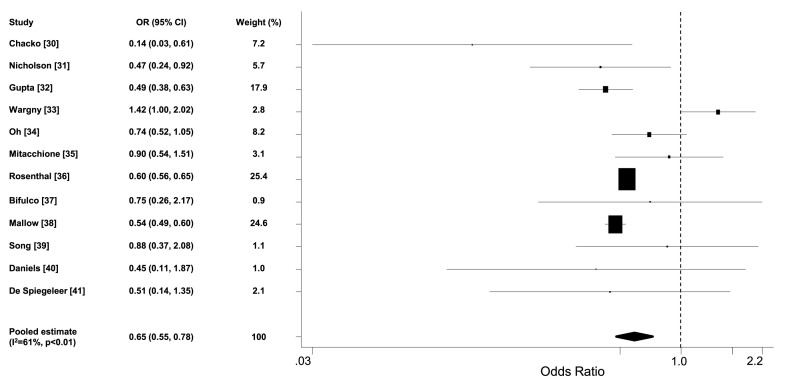
Twelve studies (n = 72,881, weighted age 65 ± 2 years, men 54%, hypertension 66%, diabetes 43%, statin users 30%) reported the adjusted odds ratio for death in statin users versus non-users [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. The pooled estimate of odds ratio was 0.65 (95% CI 0.55, 0.78) (Fig. 3 ). Sensitivity analysis by excluding one study each time confirmed that the pooled estimate was consistent and not driven by specific studies (Fig. 1B, Supplementary Appendix B). Additional sensitivity and stratified analyses are presented in Table 1, Supplementary Appendix B. No publication bias was identified (p = 0.95 and 0.67 for Begg's and Egger's test respectively; Begg's funnel plot is presented in Fig. 2B, Supplementary Appendix B). Multivariable meta-regression analysis did not reveal any significant associations of the odds ratios for death with gender, age, prevalence of diabetes, hypertension and chronic obstructive pulmonary disease or lung disease across the included studies (all p = NS). Prevalence of coronary heart disease was not included in the previous analysis due to insufficient observations. Half of the studies were assigned high quality scores (Table 2, Supplementary Appendix B).
Fig. 3.
Forest plot of adjusted odds ratios for death in statin users versus non-users among COVID-19 patients.
4. Discussion
This meta-analysis of retrospective observational studies showed that statin therapy was associated with an about 35% decrease in the adjusted risk of COVID-19 related mortality. Statin users compared to non-users showed a pooled estimate of hazard or odds ratio for death at about 0.65 after adjustment for confounders.
Cardiovascular risk factors and established cardiovascular disease constitute risk factors for severe COVID-19 [3]. Statin users are in general patients with adverse cardiovascular profile and thereby at higher risk for an adverse course of COVID-19. Moreover, acute cardiac injury, including myocardial ischemia, has been reported among the complications of COVID-19 [42]. Therefore, the effect of statins on mortality should be examined by taking into account the adverse cardiovascular background of these patients. Despite the heterogeneity in the populations’ characteristics across the included studies, the pooled estimate of the adjusted mortality risk was similar either when using hazard ratios or odds ratios, all of which suggest a beneficial effect of statins in COVID-19. This was also supported by the meta-regression analysis, which failed to show any significant associations between the risk reduction with statins and anthropometric characteristics or comorbidities. Indeed, all studies showed reduced or trend for reduced risk of mortality with statin use [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32],[34], [35], [36], [37], [38], [39], [40], [41]], apart from a single study which included only diabetic patients and showed increased mortality in statin users [33]. In a large nationwide whole-population analysis in England, patients with diabetes were at higher risk for COVID-19 related mortality than non-diabetes patients [43]. Increased COVID-19 related mortality in these patients was associated with cardiovascular and renal complications of diabetes, as well as with glycaemic control and obesity [44]. However, statin use again was independently associated with decreased COVID-19 related mortality (adjusted hazard ratio 0.72) in type 2 diabetics [44].
Continuation of statin use in hospitalized patients with severe COVID-19 raises several concerns, mainly due to drug interactions (i.e. with macrolides) and transaminase elevations which are common in COVID-19 and are attributed either to the virus itself or to specific therapies i.e. remdesivir [45]. In addition, it seems that statins up-regulate angiotensin-converting enzyme 2 receptor, which might facilitate SARS-CoV-2 cell entry [4]. However, liver injury from statins is very rare, including COVID-19 patients [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41]]. Moreover, statins exert several beneficial pathophysiological effects. Increased levels of angiotensin-converting enzyme 2 receptor lead to increased degradation of angiotensin II to angiotensin 1-7 which exerts vasodilatory, anti-proliferative, anti-inflammatory, anti-thrombotic and anti-hypertrophic effects, and which in turn alleviate major pathophysiological disorders of SARS-CoV-2 infection, including acute lung injury, endotheliitis and thromboinflammation [46]. In addition, statins exert immunomodulatory effects by stabilization of the myeloid differentiation primary response 88 (MyD88) during hypoxia and stress, leading to mitigation of the NF‐κB induced hyper-inflammatory activation during severe SARS-CoV-2 infection [47,48]. Other effects of statins, including membrane composition changes, antiviral activity, anti-oxidant and anti-thrombotic effects, might also have a protective role in severe COVID-19 [6,49,50]. Indeed, based on data from a few studies, the difference in LDL-cholesterol levels between statin users and non-users was only moderate, suggesting that the protective effect of statins might be over and beyond that of cholesterol lowering.
The importance of the in-hospital continuation of statin therapy has been addressed in non-COVID-19 patients with acute myocardial infarction [51]. Specifically, it has been shown that new or continued treatment with a statin in the first 24 h of admission for acute myocardial infarction was associated with a decreased risk of mortality compared with no statin use, whereas discontinuation of statin treatment was associated with a slightly increased risk of mortality [51]. The findings of this meta-analysis support the in-hospital continuation of statin use in COVID-19 patients since in sensitivity analysis including the studies in which statin therapy was continued in the majority of patients, the decrease in the mortality risk was highly significant. Not surprisingly, clinical guidelines now recommend that outpatient statin treatment should be continued in hospitalized COVID-19 patients [52].
The findings of this study should be interpreted in light of some limitations. The current evidence is exclusively derived from observational retrospective studies. Prospective randomized studies are lacking and difficult to obtain. Despite the source of evidence, there seems to be consistency in the key findings across the included studies either examining hazard or odds ratios in terms of lower mortality in statin users. Moreover, the inclusion of studies that reported adjusted risks allowed reasonable methodological quality. However, important details such as the duration of the follow-up or of the hospitalization, the time point of the death event during the hospitalization, and the occurrence of other complications not directly related to COVID-19, were not homogeneously reported or not even available among the included studies. Moreover, stratified analyses based on patients’ characteristics i.e., in hypertensive or diabetic patients would be desirable but this was not feasible. Nevertheless, meta-regression analyses on the association between the risk reduction with statin use and gender distribution, mean age, prevalence of diabetes, hypertension, coronary heart disease, and lung disease across the included studies did not reveal any significant effect. Another issue is that despite the fact that most studies were assigned a low risk of bias, a significant proportion was characterized by an unclear grading regarding the inclusion criteria and the exposure measurement. However, the respective grading criteria were rather strict. First, exclusively polymerase chain reaction-based diagnosis of COVID-19, which was used as inclusion criterion, was unclear in many studies but this reflects real clinical practice. Other criteria for diagnosis such as imaging or other laboratory tests might have been used but these probably regarded only a minority of patients and not the whole study sample. Second, the exposure was examined in terms of statin use both at baseline and during the follow-up. The studies included patients already on statins who either continued or discontinued the statin treatment after admission. Several studies did not report the continuation rate of statin use during the hospitalization and were thus characterized as unclear. In addition, exact details regarding the duration of statin administration prior to hospitalization were not available.
The added value of the present meta-analysis lies on the (i) inclusion of updated evidence which is rapidly accumulating, and (ii) use of strict methodological criteria and sensitivity analyses. Previous relevant meta-analyses have provided heterogeneous results regarding the beneficial effect of statins on outcome in COVID-19 patients [[7], [8], [9], [10], [11], [12], [13]]. However, major methodological issues (inclusion of not peer reviewed studies, use of unadjusted and adjusted ratios, mixed use of odds and hazard ratios, definition of mixed outcome) should be taken into account [[7], [8], [9], [10], [11], [12], [13]]. The current meta-analysis examined only the adjusted risk with statin use and this was performed separately for odds and hazard ratios and solely for the outcome of death. In addition, a separate analysis was performed for studies that reported in-hospital use of statins. It should be highlighted that in all the present analyses, the outcome was consistent and in favour of statin use.
In conclusion, this meta-analysis suggests that statin use is associated with lower risk of COVID-19 mortality after adjustment for anthropometric variables and comorbidities. The exact pathophysiological mechanisms through which statins might impact the natural history of SARS-CoV-2 infection are unclear, but possibly include immunomodulatory and anti-inflammatory effects apart from their established cardioprotective action. Additional data from prospective studies are needed to confirm these findings.
Financial support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Anastasios Kollias: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Visualization, Writing – original draft. Konstantinos G. Kyriakoulis: Data curation, Investigation, Methodology, Project administration, Resources, Software, Visualization, Writing – original draft. Ioannis G. Kyriakoulis: Data curation, Resources, Software, Visualization. Thomas Nitsotolis: Investigation, Resources, Writing – original draft. Garyphallia Poulakou: Supervision, Writing – review & editing. George S. Stergiou: Supervision, Writing – review & editing. Konstantinos Syrigos: Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors are grateful to the corresponding authors of the studies who provided additional useful information.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.atherosclerosis.2021.06.911.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mach F., Baigent C., Catapano A.L., Koskinas K.C., Casula M., Badimon L., et al. ESC Scientific Document Group ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020. 2019;41:111–188. doi: 10.1093/eurheartj/ehz455. [DOI] [PubMed] [Google Scholar]
- 2.Davignon J. Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39–43. doi: 10.1161/01.CIR.0000131517.20177.5a. [DOI] [PubMed] [Google Scholar]
- 3.Izcovich A., Ragusa M.A., Tortosa F., Lavena Marzio M.A., Agnoletti C., et al. Prognostic factors for severity and mortality in patients infected with COVID-19: a systematic review. PloS One. 2020;15 doi: 10.1371/journal.pone.0241955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y.H., Wang Q.X., Zhou J.W., Chu X.M., Man Y.L., Liu P., et al. Effects of rosuvastatin on expression of angiotensin-converting enzyme 2 after vascular balloon injury in rats. J Geriatr Cardiol. 2013;10:151–158. doi: 10.3969/j.issn.1671-5411.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barkas F., Milionis H., Anastasiou G., Liberopoulos E. Statins and PCSK9 inhibitors: what is their role in coronavirus disease 2019? Med. Hypotheses. 2020;146 doi: 10.1016/j.mehy.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganjali S., Bianconi V., Penson P.E., Pirro M., Banach M., Watts G.F., et al. Commentary: statins, COVID-19, and coronary artery disease: killing two birds with one stone. Metabolism. 2020;113:154375. doi: 10.1016/j.metabol.2020.154375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am. J. Cardiol. 2020;134:153–155. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabet Metab Syndr. 2020;14:1613–1615. doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hariyanto T.I., Kurniawan A. Statin and outcomes of coronavirus disease 2019 (COVID-19): a systematic review, meta-analysis, and meta-regression. Nutr. Metabol. Cardiovasc. Dis. 2021 doi: 10.1016/j.numecd.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheen A.J. Statins and clinical outcomes with COVID-19: meta-analyses of observational studies. Diabetes Metab. 2020 doi: 10.1016/j.diabet.2020.101220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vahedian-Azimi A., Mohammadi S.M., Beni F.H., Banach M., Guest P.C., Jamialahmadi T., et al. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: a systematic review and meta-analysis. Arch. Med. Sci. 2021:17. doi: 10.5114/aoms/132950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pal R., Banerjee M., Yadav U., Bhattacharjee S. Statin use and clinical outcomes in patients with COVID-19: an updated systematic review and meta-analysis. Postgrad. Med. 2021 doi: 10.1136/postgradmedj-2020-139172. postgradmedj-2020-139172. [DOI] [PubMed] [Google Scholar]
- 13.Permana H., Huang I., Purwiga A., Kusumawardhani N.Y., Sihite T.A., Martanto E., et al. In-hospital use of statins is associated with a reduced risk of mortality in coronavirus-2019 (COVID-19): systematic review and meta-analysis. Pharmacol. Rep. 2021:1–12. doi: 10.1007/s43440-021-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. J. Am. Med. Assoc. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 15.The Joanna Briggs Institute critical appraisal tools for use in JBI systematic reviews checklist for analytical Cross sectional studies and for cohort studies. 2021. https://jbi.global/critical-appraisal-tools
- 16.StatsToDo: combine means and SDs into one group program. 2020. https://www.statstodo.com/CombineMeansSDs_Pgm.php
- 17.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 19.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee H.Y., Ahn J., Park J., Kyung Kang C., Won S.H., et al. Korean society of hypertension, national committee for clinical management of emerging infectious diseases. Beneficial effect of statins in COVID-19-related outcomes-brief report: a national population-based cohort study. Arterioscler. Thromb. Vasc. Biol. 2021;41:e175–e182. doi: 10.1161/ATVBAHA.120.315551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peymani P., Dehesh T., Aligolighasemabadi F., Sadeghdoust M., Kotfis K., Ahmadi M., et al. Statins in patients with COVID-19: a retrospective cohort study in Iranian COVID-19 patients. Transl Med Commun. 2021;6(1):3. doi: 10.1186/s41231-021-00082-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y., Guo T., Yan F., Gong M., Zhang X.A., Li C., et al. Association of statin use with the in-hospital outcomes of 2019-coronavirus disease patients: a retrospective study. Front. Med. 2020;7:584870. doi: 10.3389/fmed.2020.584870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butt J.H., Gerds T.A., Schou M., Kragholm K., Phelps M., Havers-Borgersen E., et al. Association between statin use and outcomes in patients with coronavirus disease 2019 (COVID-19): a nationwide cohort study. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-044421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masana L., Correig E., Rodríguez-Borjabad C., Anoro E., Arroyo J.A., Jericó C., Pedragosa A., et al. Group OBOTSR Effect of statin therapy on SARS-CoV-2 infection-related. Eur Heart J Cardiovasc Pharmacother. 2020 doi: 10.1093/ehjcvp/pvaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saeed O., Castagna F., Agalliu I., Xue X., Patel S.R., Rochlani Y., et al. Statin use and in-hospital mortality in patients with diabetes mellitus and COVID-19. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.120.018475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grasselli G., Greco M., Zanella A., Albano G., Antonelli M., Bellani G., et al. COVID-19 lombardy ICU network. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern Med. 2020;180:1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit. Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y., et al. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metabol. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lala A., Johnson K.W., Januzzi J.L., Russak A.J., Paranjpe I., Richter F., et al. Mount sinai COVID informatics center. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J. Am. Coll. Cardiol. 2020 Aug 4;76(5):533–546. doi: 10.1016/j.jacc.2020.06.007. Epub 2020 Jun 8. PMID: 32517963; PMCID: PMC7279721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chacko S.R., DeJoy R., 3rd, Lo K.B., Albano J., Peterson E., Bhargav R., et al. Association of pre-admission statin use with reduced in-hospital mortality in COVID-19. Am. J. Med. Sci. 2021;S0002–9629(21) doi: 10.1016/j.amjms.2021.03.001. 00089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicholson C.J., Wooster L., Sigurslid H.H., Li R.H., Jiang W., Tian W., et al. Estimating risk of mechanical ventilation and in-hospital mortality among adult COVID-19 patients admitted to Mass General Brigham: the VICE and DICE scores. EClinicalMedicine. 2021;33 doi: 10.1016/j.eclinm.2021.100765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat. Commun. 2021;12:1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wargny M., Potier L., Gourdy P., Pichelin M., Amadou C., Benhamou P.Y., et al. CORONADO investigators. Predictors of hospital discharge and mortality in patients with diabetes and COVID-19: updated results from the nationwide CORONADO study. Diabetologia. 2021;64:778–794. doi: 10.1007/s00125-020-05351-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh T.K., Song I.A., Jeon Y.T. Statin therapy and the risk of COVID-19: a cohort study of the national health insurance service in South Korea. J. Personalized Med. 2021;11:116. doi: 10.3390/jpm11020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitacchione G., Schiavone M., Curnis A., Arca M., Antinori S., Gasperetti A., et al. Impact of prior statin use on clinical outcomes in COVID-19 patients: data from tertiary referral hospitals during COVID-19 pandemic in Italy. J Clin Lipidol. 2020 doi: 10.1016/j.jacl.2020.12.008. S1933-2874(20)30345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenthal N., Cao Z., Gundrum J., Sianis J., Safo S. Risk factors associated with in-hospital mortality in a US national sample of patients with COVID-19. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.29058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bifulco M., Ciccarelli M., Bruzzese D., Dipasquale A., Lania A.G., Mazziotti G., et al. The benefit of statins in SARS-CoV-2 patients: further metabolic and prospective clinical studies are needed. Endocrine. 2020:1–3. doi: 10.1007/s12020-020-02550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mallow P.J., Belk K.W., Topmiller M., Hooker E.A. Outcomes of hospitalized COVID-19 patients by risk factors: results from a United States hospital claims database. J Health Econ Outcomes Res. 2020;7:165–174. doi: 10.36469/jheor.2020.17331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song S.L., Hays S.B., Panton C.E., Mylona E.K., Kalligeros M., Shehadeh F., et al. Statin use is associated with decreased risk of invasive mechanical ventilation in COVID-19 patients: a preliminary study. Pathogens. 2020;9:759. doi: 10.3390/pathogens9090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Daniels L.B., Sitapati A.M., Zhang J., Zou J., Bui Q.M., Ren J., et al. Relation of statin use prior to admission to severity and recovery among COVID-19 inpatients. Am. J. Cardiol. 2020;136:149–155. doi: 10.1016/j.amjcard.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Spiegeleer A., Bronselaer A., Teo J.T., Byttebier G., De Tré G., Belmans L., et al. The effects of ARBs, ACEis, and statins on clinical outcomes of COVID-19 infection among nursing home residents. J. Am. Med. Dir. Assoc. 2020;21:909–914.e2. doi: 10.1016/j.jamda.2020.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kollias A., Kyriakoulis K.G., Destounis A., Stergiou G.S., Syrigos K. Cardiac injury and prognosis in COVID-19: methodological considerations and updated meta-analysis. J. Infect. 2020;81:e181–e182. doi: 10.1016/j.jinf.2020.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabet Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabet Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertolini A., van de Peppel I.P., Bodewes F.A.J.A., Moshage H., Fantin A., Farinati F., et al. Abnormal liver function tests in patients with COVID-19: relevance and potential pathogenesis. Hepatology. 2020;72:1864–1872. doi: 10.1002/hep.31480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar R., Lee M.H., Mickael C., Kassa B., Pasha Q., Tuder R., et al. Pathophysiology and potential future therapeutic targets using preclinical models of COVID-19. ERJ Open Res. 2020;6 doi: 10.1183/23120541.00405-2020. 00405-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. mBio. 2015;6 doi: 10.1128/mBio.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuan X., Deng Y., Guo X., Shang J., Zhu D., Liu H. Atorvastatin attenuates myocardial remodeling induced by chronic intermittent hypoxia in rats: partly involvement of TLR-4/MYD88 pathway. Biochem. Biophys. Res. Commun. 2014;446:292–297. doi: 10.1016/j.bbrc.2014.02.091. [DOI] [PubMed] [Google Scholar]
- 49.Glende J., Schwegmann-Wessels C., Al-Falah M., Pfefferle S., Qu X., Deng H., et al. Importance of cholesterol-rich membrane microdomains in the interaction of the S protein of SARS-coronavirus with the cellular receptor angiotensin-converting enzyme 2. Virology. 2008;381:215–221. doi: 10.1016/j.virol.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reiner Ž., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T., et al. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch. Med. Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fonarow G.C., Wright R.S., Spencer F.A., Fredrick P.D., Dong W., Every N., et al. National Registry of Myocardial Infarction 4 Investigators. Effect of statin use within the first 24 hours of admission for acute myocardial infarction on early morbidity and mortality. Am. J. Cardiol. 2005;96:611–616. doi: 10.1016/j.amjcard.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 52.Iqbal Z., Ho J.H., Adam S., France M., Syed A., Neely D., et al. Heart UK's Medical Scientific and Research Committee. Managing hyperlipidaemia in patients with COVID-19 and during its pandemic: an expert panel position statement from HEART UK. Atherosclerosis. 2020;313:126–136. doi: 10.1016/j.atherosclerosis.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.