Abstract
Background
COVID-19 is a new disease that appeared in December 2019. Millions of people have been infected and died from this infection. Until today, the pathophysiology and treatment of this infection remain unknown, but a lot of studies are trying to solve the mystery. The trail of inflammation remains the most convincing, especially the Interleukin 6 (IL-6) which could play an important role in a reaction cascade leading to a cytokine storm. According to studies, although few in number, the Tociluzimab (TCZ), which is an anti-IL6, could prevent or even suppress this storm, leading to a less severe clinical state of the disease and a faster recovery. This could decrease the use of oxygen, avoid the risk of intubation and mortality.
Patients and methods
This single-center retrospective observational case review brought together 557 COVID-19 seriously ill patients (pulmonary involvement> 25% + SatO2AA <90%) admitted to the intensive care unit of our university hospital from March 1st, 2020 to February 28th, 2021. They were divided into 2 groups a Tociluzimab group (TCZ group) and a Non Tociluzimab group (NON TCZ) to facilitate the comparison. The aim of the study was to compare the length of hospital stay, the use of mechanical ventilation and the mortality in the TCZ group versus the NON TCZ group.
Results
The average age of our patients was 62,05 years (±13.51) and 62.61 years (±16.33) respectively in the TCZ versus NON TCZ group. 76 (76%) were men while 24 were women (24%) in the TCZ group; and there was 313 (68.49%) men and 144 (31.51%) women in the NON TCZ group. Their average BMI was 28 kg/m2 (±4.52) in the TCZ group versus 27.89 kg/m2 (±4.73) in the NON TCZ group. Among them, the TCZ group included 38 (38%) diabetic patients, 38 hypertensive (38%), 12 heart disease (12%) and 2 chronic renal failure (2%), while the NON TCZ group regrouped 35 (7.65%) diabetics, 33 (7.22%) hypertensive, 12 heart disease (2.67%), and 5 chronic renal failure (1.09%) patients. The mean time to consultation of patients was almost similar in the two groups: 8.86 (±7,28) days for TCZ and 8.83 (±7,03) days for NON TCZ group. The mean length of ICU hospital stay was 9 days (4,94) for the TCZ group and 8,75 days (4,73) for the other one. The saturation at admission was at 74.92% (10.45) for the TCZ group ranging from 40% to 92%, and at 73,56% for the NON TCZ group. Lung damage from COVID-19 was extensive in 12%, severe in 32%, and critical in 56% of TCZ group enrolled cases. Meanwhile it was extensive in 23.63%, severe in 41,35%, and critical in 35,01% of the NON TCZ group. The biological findings found average of white blood cells at 12256/12082 e/mm3, lymphocytes at 761/842 e/mm3, CRP at 181/199 mg/L, ferritin at 1747/528 μg/L, and fibrinogen at 6.92/6.27 g/L for the TCZ group versus NON TCZ group. Medical care was based on isolation, oxygenotherapy, azithromycin, vitamin C, zinc, vitamin D, salicylic acid, dexamethasone followed with methylprednisolone, and anticoagulation for all hospitalized patients. The TCZ group received at least 1 course of Tociluzimab dosed at 400 mg (2 patients received 2 doses and 1 patient received 3 doses). The indication of a Tociluzimab course in our department was based on a set of arguments: an increase in oxygen requirements, a progression of lesions on chest-computed tomography and an increase in inflammation markers including IL-6, CRP, ferritin, fibrinogen, and a decrease in the percentage of lymphocytes. The invasive mechanical ventilation was indicated for 4 (4%) patients in the TCZ group versus 192 (42,01%) in the NON TCZ. Among the 100 patients included in our cohort in the TCZ group, 40% died in intensive care unit and 60% had a favorable evolution with a decrease of the biological markers of inflammation. However, in the NON TCZ group, 197 (43,10%) passed away.
Conclusion
The use of Tociluzimab in ICU patients with severe COVID-19 pneumonia did not contribute to a significant difference in the reduction of hospital stay. However, the invasive mechanical ventilation was less needed in patients receiving Tociluzimab than the others. Moreover, there was a mortality benefit associated with the use of Tociluzimab, but only before 10 days of hospitalization.
Keywords: COVID-19, Cytokine storm, Interleukin-6, Tocilizumab, Morocco, Case series
Highlights
-
•
Several studies have shown the benefits of Tocilizumab in the prevention of cytokine storm.
-
•
The aims of the study is to describe the use of Tocilizumab, within our department, in the prevention and treatment of cytokine storms in patients with severe form of SARS-CoV-2 infection. Also, based on length of hospital stay, risk of intubation and mortality.
-
•
Descriptive statistics and percentages were used to describe our study population. Chi square or Fisher's exact tests and T test were used to compare the patients' data. Survival was estimated using Kaplan-Meir method.
-
•
Ferritin, Interleukine 6, and CRP are markers of inflammation which make it possible to detect patients at risk.
1. Introduction
Coronavirus disease or SARS-CoV-2 infection first appeared in December 2019 in Wuhan, Hubei province, China.
This new disease is known for its highly inflammatory effect, which can progress in severe cases to cardiovascular collapse, multisystem failure or even death [1]. In fact, the severe form of patients infected with SARS-CoV-2 is characterized by an overproduction of pro-inflammatory cytokines causing thunderstorms [2].
Interleukin 6 (IL6), interleukin 2 (IL 2), ferritin, fibrinogen and lymphocytes play a crucial role in this reaction. Several Chinese studies suggest that IL-6 is one of the most important inflammatory cytokines involved in these reaction cascades demanded by SARS-CoV-2 infection [3].
This will make Tocilizumab, a humanized monoclonal antibody against the interleukin 6, an agent highly recommended for critically ill patients with IL 6 > 3 times normal.
It is true that the actual data on the effectiveness of Tocilizumab are still limited, but the early identification of these patients with cytokine storms allows for better management [4].
In this retrospective observational study, we aim to describe the use of Tocilizumab, within our department, in the prevention and treatment of cytokine storms in patients with severe form of SARS-CoV-2 infection. The study is also based on length of hospital stay, risk of intubation and mortality. The findings of this study were reported according to the PROCESS guidelines [5].
2. Materials and methods
This is a mono-centric retrospective comparative observational study carried out in the intensive care unit of Mohammed VI University Hospital in Oujda.
It includes all patients (557 patients) hospitalized in intensive care infected with COVID-19. They were seriously ill (pulmonary involvement> 25% + SatO2AA <90%) from March 1st, 2020 to February 28th, 2021.
These 557 patients were divided into 2 groups: a first group of 100 patients who received at least one dose of Tociluzimab (TCZ GROUP); and a second group including the remaining 457 patients who received no dose of TCZ (NON TCZ group). (Fig. 1).
Fig. 1.
Distribution of study patients.
The aim of the study was to compare the length of hospital stay, the use of mechanical ventilation and the mortality in the TCZ group versus the NON TCZ group.
Epidemiological, clinical, para-clinical, therapeutic and evolutionary data were gathered using an operating sheet including the various variables collected from patient's medical records. Then, they were computerized and analyzed using SPSS software.
Descriptive statistics and percentages were used to describe our study population. Chi square or Fisher's exact tests and T test were used to compare the patients' data. Survival was estimated using Kaplan-Meier method. A pValue < .05 was considered statistically significant.
Access to patient data has been authorized by the Mohammed VI University Hospital. In view of the retrospective design of this study, the requirement for patient consent was disregarded.
Data anonymity has been respected in accordance with national and international guidelines.
The IL-6 assay was not available in our laboratories at the start of the pandemic.
The indication for administration of TCZ in the first patients has only been made on the basis of clinical symptoms associated with the analysis of the level of ferritin, fibrinogen and CRP.
Only patients included in the study from February 2021 were able to benefit from an IL-6 assay.
The laboratory results of the patients were evaluated on D-7 following treatment with TCZ.
The duration of hospitalization, use of mechanical ventilation and mortality were compared to D-21 of the day of hospitalization.
This case series has been reported in line with the PROCESS Guidelines.
3. Results: (Table 1)
Table 1.
Patients characteristics.
| Characteristics | TCZ group |
NON TCZ group |
All patients |
|---|---|---|---|
| (N = 100) | (N = 457) | (N = 557) | |
| Median age (SD) | 62,05 (13,51) | 62,61 (16,33) | 62,51 (15,85) |
| Male sex – n°(%) | 76 (76%) | 313 (68,49%) | 389 (69,83%) |
| Median BMI – (SD) | 28 (4,52) | 27,89 (4,73) | 27,82 (4,69) |
| Median time from symptoms onset to admission – day (SD) | 10,94 (5,62) | – | – |
| Hypertension n°(%) | 38 (38%) | 35 (7,64%) | 73 (13,1%) |
| Diabetes n°(%) | 38 (38%) | 33 (7,22%) | 71 (12,74%) |
| Heart condition n°(%) | 12 (12%) | 12 (2,62%) | 24 (4,3%) |
| Chronic renal failure n°(%) | 2 (2%) | 5 (1,09%) | 7 (1,25%) |
| Oxygenotherapy - n°(%) | 97 (97%) | 383 (83,8%) | 480 (86,17%) |
| Median onset saturation in % | 74,92% | 73,56% | 73,81% |
| Onset saturation – n° (%) | |||
| 80–90% | 41 (41%) | 142 (31,07%) | 183 (32,85%) |
| 70–80% | 30 (30%) | 117 (25,6%) | 147 (26,39%) |
| 60–70% | 19 (19%) | 64 (14%) | 83 (14,9%) |
| 60-50% | 6 (6%) | 34 (7,43%) | 40 (7,18%) |
| <50% | 2 (2%) | 39 (8,53%) | 41 (7,36%) |
| Thoracic Scan | |||
| - 25–50% | 12 (12%) | 108 (23,63%) | 120 (21,54%) |
| - 50–75% | 32 (32%) | 189 (41,35%) | 221 (39,67%) |
| - >75% | 56 (56%) | 160 (35,01%) | 216 (38,77%) |
| Median laboratory values n°(SD) | – | – | – |
| White cells | 12256 (5286,69) | 12082 | 12144 |
| (6186) | (6031) | ||
| Lymphocyte count | 761 (356,52) | 842 (623) | 827 (585,38) |
| C-reactive protein level | 181 (85,95) | 199 (96) | 196 (95,19) |
| Ferritin serum level | 1747 (1085,1) | 528 (310) | 747 (712,85) |
| Fibrinogen serum level | 6,92 (1,64) | 6,27 (1,86) | 6,38 (1,83) |
| Serum interleukine-6 level ** | 189,57 (92,19) | – | – |
| Median hospital stay – day (SD) | 9 (4,94) | 8,75 (4,73) | 9,05 (7,19) |
| Survivors – n°(%) | 60 (60%) | 260 (56,89%) | 320 (57,45%) |
| Non-survivors – n°(%) | 40 (40%) | 197 (43,10%) | 237 (42,54%) |
| Still hospitalized at day 21 | 5 (5%) | 180 (39,38%) | 185 (33,21%) |
| Still intubated at day 21 | 4 (4%) | 164 (35,88%) | 168 (30,16%) |
| Still alive at day 21 | 64 (64%) | 271 (59,29%) | 347 (60,14%) |
Between March 1st, 2020 and February 28th, 2021, 604 patients were hospitalized in intensive care: 47 (7.78%) patients had lung involvement <25% and had SatO2AA> 90% and were not included in our study, 100 (16.55%) received at least one dose of TCZ (TCZ group), and 457 (75.66%) received none (NON TCZ group).
3.1. Epidemiological characteristics
In the TCZ group, we had 76 (76%) men and 24 (24%) women. The majority of them were elderly with an average age of 62.05 years (±13.51).
In the NON TCZ group, we had 313 (68.49%) men and 144 (31.51%) women. Their average age was 62.61 years (±16.33).
The mean BMI was 28 kg/m2 (±4.52) in the TCZ group versus 27.89 kg/m2 (±4.73) in the NON TCZ group.
3.2. Hospital stay and medical history
Regarding the history of our patients, the TCZ group included 38 (38%) diabetic patients, 38 hypertensive (38%), 12 heart disease (12%) and 2 chronic renal failure patients (2%).
In the NON TCZ group, there were 35 (7.65%) diabetics, 33 (7.22%) hypertensive, 12 heart disease (2.67%), and 5 chronic renal failure patients (1.09%).
The mean time to consultation of patients was almost similar in the two groups: 8.86 (±7,28) days for TCZ and 8.83 (±7,03) days for NON TCZ.
The mean length of ICU hospital stay was 9 days (4,94) for the TCZ group and 8,75 days (4,73) for the other one.
3.3. Clinical et radiological findings
The saturation at admission was at 74.92% (10.45) for the TCZ group ranging from 40% to 92%, and at 73,56% for the NON TCZ group.
Regarding the TCZ group, 97 (97%) patients required oxygen supplementation which consisted of 12 (12%) nasal canula, 60 (60%) high concentration masks, 19 (19%) non invasive ventilation (NIV), 4 (4%) high flow nasal canula treatment and 2 mechanical ventilation (2%).
In the TCZ group, thoracic CT found a moderate damage in 12 patients (12%), a severe one in 32 patients (32%) and a critical one in 56 patients (56%).
While in the NON TCZ group, the lung damage from COVID-19 was moderate in 108 cases, (23.63%) severe in 189 (41.35%) cases and critical in 160 cases (35.01%).
3.4. The biological findings
In the TCZ group the patients had an average of white blood cells at 12256 e/mm3 (±5286.69), lymphocytes at 761 e/mm3 (±356.52), a CRP at 181 mg/L (±85.95), a ferritin at 1747 μg/L (±1085.1) and a fibrinogen at 6.92 g/L (±1.64).
In the non-TCZ group, the biological assessment was slightly less disturbed with white blood cells which averaged 12082 e/mm3 (±6186), lymphocytes at 842 e/mm3 (±623), ferritin at 528 μg/L (±310) and fibrinogen at 6.27 g/L (±1.86). Only CRP was higher in this group at 199 mg/L (±96).
3.5. Medical care
In our series, 100 (16.55%) patients received a course of Tociluzimab dosed at 400 mg. Five patients out of these received 2 doses (400 mg 1 week apart) and 2 patients received 3 doses (400 mg 1 week apart).
In addition to Tociluzimab, all patients received treatment with Vitamin C (2 g/d), Zinc (45 mg 2cp/d), Vitamin D (25000IU/wk), acetylsalicylic acid 160 mg/d, an anticoagulation at curative dose, dexamethasone (6 mg/d) and a possible antibiotic therapy, depending on the clinical and biological state, based on tazocillin (4 g/6h) variconazole (200mg/12h) and amikacin 2 g/d or else: ceftriaxone (2 g/d) and ciprofloxacin (200mg/12h).
The indication of a Tociluzimab course in our department was based on a set of arguments: an increase in oxygen requirements, a progression of lesions on chest-computed tomography and an increase in inflammation markers including IL-6, CRP, ferritin, fibrinogen, and a decrease in the percentage of lymphocytes.
3.6. Evolution
There were 40 deaths (40%) in the TCZ group, versus 197 (43.10%) in the NON TCZ group.
In the TCZ group, the course of the 60 survivors was marked by recovery and discharge from hospital.
Indeed, we noticed a spectacular improvement in their clinical symptoms and their laboratory results 7 days after the administration of the 1st dose of TCZ.
These patients saw their ferritin number decrease by more than - 48.42% of its initial value. After 7 days, only 3 patients had an increase in their ferritin (+103.74%), and these were patients to whom TCZ had been administered on D11, D15 and D18 of the onset of symptoms.
One patient of these received a second dose of TCZ and improved with a decrease in ferritin of −38.08%.
As for the CRP of D7, in 58 patients, it fell by −75.72% on average.
In the survivor group, the TCZ had been administered on average on D7 of the onset of symptoms (min: D2; max: D15). (Fig. 2, Fig. 3).
Fig. 2.
Ferritin and CPR levels of the survivors.
Fig. 3.
Ferritin and CPR levels of the non-survivors.
In the 40 patients who passed away, the course was pejorative, marked by an ARDS and death (40% of cases). We noticed in this series, in which ferritinemia had mainly increased by + 124.06%, that the administration of TCZ took place on average on D12 of the onset of symptoms (min: D3; max: D21).
In this group, 97 (97%) patients required oxygen supplementation which consisted of 12 (12%) nasal canula, 24 (24%) masks, 36 (36%) high concentration masks, 19 (19%) NIV and 4 (4%) hight flow nasal cannula. Use of mechanical ventilation occurred in only 2 patients on admission and in 2 cases during hospitalization in patients who received TCZ (4%).
4. Discussion
SARS-CoV-2 replicates in pulmonary alveolar epithelial cells. Once the virions are released, they will either infect neighbouring cells or be captured and destroyed by macrophages, dendritic cells and neutrophils [6].
During this time, damaged epithelial cells produce alarmins, which will lead to the release of pro-inflammatory cytokines, increased permeability of the alveolar vessels and cell recruitment to the site of infection. This will cause the pathological activation of IL-6, IL-1, TNF and other pro-inflammatory cytokines [7].
Another feature of this cytokine release syndrome is the modification of iron homeostasis and hyperferritinemia, as indicated by elevated serum ferritin levels [8].
In COVID-19 patients, elevation of ferritin was correlated with a poor prognosis [8], as it was an indication of haemolytic events caused by hypercoagulation and hemphagocytosis.
Several studies have shown a strong correlation between serum IL-6 levels and future respiratory failure [[9], [10], [11]]. Even moderately elevated IL-6 levels above 80 pg/mL have been shown to be sufficient to identify patients infected with COVID-19 at high risk for respiratory failure [12].
Since IL-6 overexpression appears to be associated with severe COVID-19 findings, anti-IL-6 receptor antibodies targeting the host's exacerbated inflammatory immune response may provide a vital approach in preventing cytokine release syndrome [7].
In a non-randomized clinical trial, 21 patients with severe or critical COVID-19 were treated with a single dose of Tocilizumab, in addition to routine treatment [13]. 90% of patients have recovered, suggesting that anti-IL-6 could be a potent potential rescue therapy in the management of the acute respiratory distress syndrome of COVID-19 and in inhibiting the outcome of the disease.
More unfavourable [14], after using Tocilizumab, the first change in laboratory tests was seen in % lymphocytes, which increased during the first day. CRP dropped sharply one day after Tocilizumab. Two days after Tocilizumab, IL-6 strongly decreased, but ferritin and d-dimer decreased slightly [14].
In our study of ICU hospital patients with severe COVID-19 pneumonia, we did not find that administration of TCZ contributed to a significant difference in the reduction in hospital stay (<0.001).
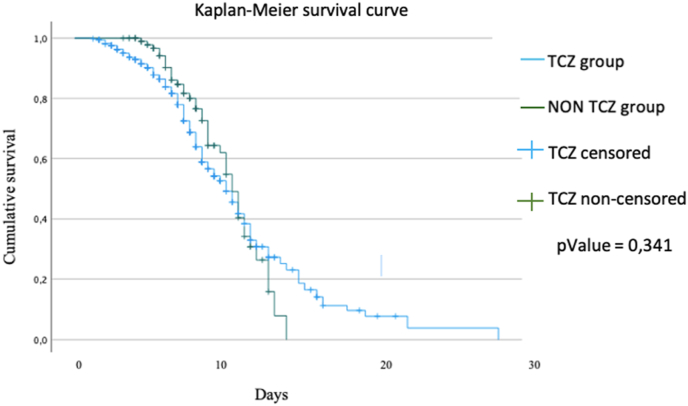
However, there was a mortality benefit associated with the use of TCZ, but only before 10 days of hospitalization (<0.001). Once these 10 days were exceeded, the use or not of TCZ had no effect on the mortality (p = .341) (Fig. 4).
Fig. 4.
Kaplan-Meier survival curve.
Clinical improvement was effective in patients who received their 1st dose of TCZ within D7 of the onset of symptoms.
We observed a decrease in the use of intubation in the TCZ group (p < .001), and a decrease in the number of patients still intubated on D21 (Table 2).
Table 2.
Conclusion of analysis study.
| TCZ group | NON TCZ group | pValue | |
|---|---|---|---|
| ICU stay (days) | 9 | 8,75 | <0,001 |
| Invasive mechanical ventilation – nb (%) | 4 (4%) | 192 (42,01%) | <0,001 |
| Still hospitalized at day 21 -nb (%) | 5 (5%) | 180 (39,38%) | <0,001 |
| Mortality – nb (%) | 40 (40%) | 197 (43,10%) | 0,341 |
In addition, we found that after the administration of the first dose of Tociluzimab, the CRP and ferritin values dropped significantly, and the lymphocyte count increased (p.<.0001).
In the patients in whom we dosed Interleukin-6, we noticed a decrease in its value. (p < .001).
On the imaging side, the radio-clinical comparison found that the severity of the respiratory distress and the risk of intubation were consistent with the degree of parenchymal involvement associated with SARS-Cov-2 (p < .0001).
In addition, we found that the greater the parenchymal involvement is, the greater the risk of mortality (p < .001).
5. Conclusion
The recommendations for the administration of Tociluzimab are said to be based on an increase in oxygen requirements, progression of lesions on chest-computed tomography and an increase in inflammation markers including IL-6, CRP, ferritin, fibrinogen, and a decrease in the percentage of lymphocytes.
Early and adequate use of Tociluzimab during COVID-19 infection for critically ill patients would offer a better prognosis. The correct timing of the administration of TCZ would be before the onset of the cytokine storm because it would prevent it, and thus prevent its complications.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Sources of funding
This research was not funded
Ethical approval
This is a retrospective case series that does not require a formal ethical committee approval. Data were anonymously registered in our database. Access to data was approved by the head of the department.
Consent
This is a retrospective study.
Author statement contribution
Dr. Abdelilah El Rhalete and Dr. Inas Rhazi: are principal investigators that collected and analyzed data, wrote the manuscript and prepared the final draft for the submission.
Dr. Amine Bensaid, Dr. Kaouini abderrahim, Dr Essaad Ounci, Dr Choukri Bahouh, Dr Soufiane Diass, Dr Mohammed Maarad, Dr Mohammed Aabdi: participated in patients’ management.
Prof. Naima Abda and Prof. Zineb Alami: collaborated in the project.
Prof. Brahim Housni and Prof. Houssam Bkiyer: supervised the research project and approved the final draft for publication.
All authors approved the final version of the manuscript.
Registration of research studies
Researchregistry6573.
Guarantor
Prof. Brahim Housni.
Dr Abdelilah El Rhalete and Dr Inas Rhazi.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
We would like to thank the medical and nursing teams of Mohammed VI University Hospital for their significant involvement in the management of the patients included in our study.
References
- 1.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., Zhou M., Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaur S., Bansal Y., Kumar R., Bansal G. A panoramic review of IL‐6: structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020;28(5):115327. doi: 10.1016/j.bmc.2020.115327. [DOI] [PubMed] [Google Scholar]
- 4.Kaly L., Rosner I. Tocilizumab—a novel therapy for non-organ-specific autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2012;26:157–165. doi: 10.1016/j.berh.2012.01.001. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 5.Agha R.A., Sohrabi C., Mathew G., Franchi T., Kerwan A., O'Neill N for the PROCESS Group The PROCESS 2020 guideline: updating consensus preferred reporting of CasE series in surgery (PROCESS) guidelines. Int. J. Surg. 2020;84:231–235. doi: 10.1016/j.ijsu.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Gubernatorova E.O., Gorshkova E.A., Polinova A.I., Drutskaya M.S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sackett K., Cunderlik M., Sahni N., Killeen A.A., Olson A.P. Extreme hyperferritinemia: causes and impact on diagnostic reasoning. Am. J. Clin. Pathol. 2016;145:646–650. doi: 10.1093/ajcp/aqw053. [DOI] [PubMed] [Google Scholar]
- 8.Chen G., Wu D., Guo W., Cao Y., Huang D., Wang H. Clinical and immunologic features in severe and moderate Coronavirus Disease 2019. J. Clin. Invest. 2020;130(5):2620–2629. doi: 10.1172/JCI137244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coomes E.A., medRxiv Haghbayan H. 2020. Interleukin-6 in COVID-19: A Systematic Review and Meta-Analysis. March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X., Zhao B., Qu Y., Chen Y., Xiong J., Feng Y., Men D., Huang Q., Liu Y., Yang B., Ding J. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 2020 April;71(8):1937–1942. doi: 10.1093/cid/ciaa449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020 May;55(5):105954. doi: 10.1016/j.ijantimicag.2020.105954. Epub 2020 Mar 29. PMID: 32234467; PMCID: PMC7118634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herold Tobias, Jurinovic Vindi, Arnreich Chiara, Hellmuth Johannes C., von Bergwelt-Baildon Michael, Klein Matthias, Weinberger Tobias. medRxiv. 2020 doi: 10.1101/2020.04.01.20047381. 04.01.20047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu X., Han M., Li T., Sun W., Wang D., Fu B. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keske Ş., Tekin S., Sait B., İrkören P., Kapmaz M., Çimen C., Uğur S., Çelebi İ., Bakır V.O., Palaoğlu E., Şentürk E., Çağlayan B., Çakar N., Tabak L., Ergönül Ö. Appropriate use of tocilizumab in COVID-19 infection. Int. J. Infect. Dis. 2020 Oct;99:338–343. doi: 10.1016/j.ijid.2020.07.036. Epub 2020 Jul 26. PMID: 32726724; PMCID: PMC7382959. [DOI] [PMC free article] [PubMed] [Google Scholar]